Abstract
Background
Oesophageal changes and injuries were recorded after atrial fibrillation(AF) ablation procedures. The reduction of power in the posterior left atrial(LA) wall(closest to the oesophagus) and the monitoring of temperature in the oesophagus(OE) reduced oesophageal injuries. The intracardiac-echocardiography(ICE) with a Cartosound module provides two-dimensional imaging (2D) to assess detailed cardiac anatomy and its relationship with the OE. The aim of this study was to highlight the safety and feasibility of 3D-reconstruction of the oesophageal course in left atrial catheter ablation(CA) procedures without OE temperature probe or quadripolar catheter to guide ICE OE reconstruction.
Methods
180 patients(PT) underwent left atrial ablation. AF ablation were 125(69.5%); incisional left atrial tachycardias(IAFL) were 37(20.6%); left atrial tachycardias(LAT) were 19(10.6%). The LA and pulmonary vein anatomies were rendered by traditional electroanatomic mapping(EAM) and merged with an ICE anatomic map. In 109 PT ICE imaging was used to create a geometry of the OE(group A). A quadripolar catheter was used in 71 PT to show OE course associated to ICE(group B).
Results
Ablation energy delivery was performed outside the broadest OE anatomy borders. The duration of procedures was longer in group B vs group A Fluoroscopy time was lower in Group A than Group B(Group A 7 ± 3.2 vs 19.2 ± 2.4 min; p < 0.01).
Conclusions
OE monitoring with ICE is safe and feasible. Oesophageal anatomy is complex and variable. Many PT will have a broad oesophageal boundary, which increases the risk of untoward thermal injury during posterior LA ablation. ICE with 3D construction of the OE enhances border detection of the OE, and as such, should decrease the risk of oesophageal injury by improving avoidance strategies without intra-oesophageal catheter visualization.
Keywords: Cartosound, Intracardiac echocardiography, Oesophageal fistula, Oesophageal imaging, Left atrial catheter ablation
1. Introduction
Oesophageal changes and injuries were recorded after AF ablation procedures [[1], [2], [3]]. This complication is related to the OE position [1]. The OE is immediately contiguous to the left atrial posterior wall (LAPW) and it has a variable relationship to the left and right pulmonary veins [[4], [5], [6]]. Atrio-oesophageal fistula (AOF) is a devastating complication that may well be under-reported; it has an incidence reported to be between 0.05 and 1.3% [[7], [8], [9]]. Possible risk factors of fistula formation are general anesthesia, left atrium (LA) to OE distance, the anatomical position of OE concerning LA, the use of nasogastric tubes, the magnitude and duration of local tissue heating and the thickness and the character of intervening connective tissue [10]. The temperature probe is the most used tool to prevent AOF. When it is not optimally positioned anatomically, it provides a false sense of security of ablation safety, and energy delivery may be delivered to vulnerable regions of the OE [7]. Real-time imaging, such as the use of temperature probes and diagnostic characters, is useful to assess the general oesophageal location but does not adequately define anatomic variation, such as in the presence of hiatal hernia [11]. Continuous monitoring of luminal temperature does not serve as a means to prevent injury, as even the small rises in intraluminal temperatures that are often delayed can reflect extensive extraluminal heating. The utility of these devices would greatly decrease with distance from the catheter, leaving uncovered regions of the OE vulnerable [1,7]. Despite the application of this preventative measure, cases of atrial-oesophageal fistulas have been reported [7]. Using ICE with an electronic phased array transducer catheter provides 2D imaging and 3D reconstruction merged on a Carto system to assess the detailed cardiac 3D anatomy of the LA and shows a tri-dimensional oesophageal course [12]. This study aimed to highlight the safety and feasibility of 3D reconstruction of the oesophageal course in LA catheter ablation procedures to improve anatomical OE details and reduce complications that are OE-related without OE temperature probe. We compared the OE reconstruction with a quadripolar catheter positioned into OE.
2. Methods
2.1. Studied population
One eighty hundred patients (PT) underwent LA ablation. All consecutive patients were prospectively enrolled. The baseline demographics of all population are listed in Table 1. One-hundred-twenty-five PT underwent AF ablation (69.5%), 37 PT IAL ablation (20.6%), 19 PT LAT ablation. The IAL PT underwent a previous cardiac surgery; twenty-six PT performed mitral valve replacement. Seven PT performed mechanical mitral valve implantation. Four patients underwent biologic mitral valve implantation. The LA surgical approach was “the extended vertical transatrial septal approach to the mitral valve”, described as first by Guiradon in 1991 [13]. The averages age was 65 ± 9.3 years. 80% of all population was male. 57.2% had arterial hypertension being treated with antihypertensive drugs. The LA and pulmonary vein anatomies were rendered by traditional EAM and merged with an ICE anatomic map. The duration of procedure referred to the beginning of the procedure, from the admission of the patient in the EP laboratory to the end of the procedure, after 20 min over the last RF delivery. One hundred nine PT performed OE monitoring with ICE (group A). In 71 PT a quadripolar catheter was positioned into OE until its tip height was approximately 1–2 cm under the lowest part of the LA using fluoroscopy (group B). A standard quadripolar catheter coated with lubrificant, is nasally inserted into the OE under fluoroscopy guidance. In group B the course of OE was performed using ICE and quadripolar catheter shown on EAM. The electrode catheter is kept within the OE during the entire CA procedure, and the electrode location is continuously displayed and monitored. The PT were divided into two groups to verify the feasibility of OE reconstruction without a reference (quadripolar catheter) in OE during OE reconstruction using ICE. In all PT the ICE was used during CA procedures for transseptal puncture, LA anatomical reconstruction, monitoring pericardial effusion, and visualize OE. The integration of ICE imaging was used to create a geometry of the OE in both groups. All PT used a proton pump inhibitor drug for two weeks after the CA procedure. All of the procedures were performed under conscious sedation using bolus administration of fentanyl and midazolam. A written informed consent was obtained. All work was in compliance with the Declaration of Helsinki.
Table 1.
.Biometric data of the study population. BSA: body surface area; CAD: coronary artery disease, LAV: left atrial volume; LAVi: left atrial volume indexed by BSA; LVEF: Left ventricle ejection fraction.
| Biometric data of Study Population | Total (n. 180) | Group a (n. 109) | Group b (n.71) | p value |
|---|---|---|---|---|
| Age years (mean/st. dev.) | 62.2 ± 5.9 | 63.2 ± 9.4 | 61.1 ± 2.6 | NS |
| BSA (mean/st. dev.) | 2.2 ± 0.5 | 2.2 ± 0.4 | 2.19 ± 0.6 | NS |
| Male (n/%) | 144/80 | 87/80 | 57/80.2 | NS |
| Hypertension (n/%) | 97/53.9 | 62/57 | 35/49.3 | NS |
| Diabetes (n/%) | 52/28.9 | 30/28 | 22/31.1 | NS |
| Smokers (n/%) | 57/31.7 | 35/32 | 22/31.1 | NS |
| CAD (n/%) | 64/35.6 | 39/36 | 25/35.2 | NS |
| Dyslipidemia (n/%) | 80/44.5 | 48/44 | 32/45.1 | NS |
| LVEF %(mean/st. dev.) | 64.8 ± 12.6 | 65.1 ± 13 | 64.5 ± 12.2 | NS |
| LAV ml (mean/st. dev.) | 54.9 ± 6.9 | 55.3 ± 7.6 | 54.4 ± 6.2 | NS |
| LAVi ml/m2 (mean/st. dev.) | 29.3 ± 4.9 | 29.7 ± 5.4 | 28.9 ± 4.3 | NS |
| Antiarrhytmics drugs (n/%) | 147/81.7 | 89/82 | 58/81.7 | NS |
| Atrial fibrillation (n/%) | 125/69.5 | 76/70 | 49/60 | NS |
| Incisional atrial tachycardia (n/%) | 37/20.6 | 22/20 | 15/21.1 | NS |
| Left atrial tachycardia (n/%) | 19/10.6 | 11/10 | 8/11.2 | NS |
2.2. Catheter positioning, mapping and ablation procedure
After standard femoral access, a 3.5-mm externally irrigated radiofrequency (RF) ablation catheter (Thermocool SF; Biosense-Webster, Thermocool Smart touch Inc., Diamond Bar, CA) was advanced into the inferior vena cava (IVC) under EAM system guidance (CARTO 3, Biosense-Webster, Inc., Diamond Bar, CA). Three-dimensional (3D) geometric contours of the IVC were created by sweeping the catheter tip while advancing it up until the initial appearance of atrial electrograms that denoted the junction between the IVC and the right atrium (RA). Any difficulties in catheter advancement were overcome with a geometric acquisition of the trajectory of the catheter through the venous system. Ultimately, the catheter was advanced to the RA to delineate its border. Once the 3D shell of the RA was obtained, two quadripolar catheters were advanced into the RA and positioned respectively in the His bundle region and the right ventricle (RV) using the right and left anterior oblique views of the EAM system. In the same manner, a decapolar deflectable catheter was advanced into the distal coronary sinus, where required. ICE was used to provide information on the individual pulmonary vein anatomy, such as the number of PVs, the presence of common ostia and of additional PVs, which may influence the ablation strategy in anatomical based isolation procedures. The anatomy of LA was then reconstructed using an ICE and CARTOSOUND module (Soundstar, Biosense Webster Inc, Diamond Bar, CA). Briefly sequential 2D ICE contours were acquired and used to create a 3D map of the LA. ICE CARTOSOUND map was merged on CARTO EAM. Besides the ICE imaging was used to guide transseptal catheterization, assist positioning of the mapping and ablation catheters at the pulmonary veins (PV) ostium, measure PV ostial flow before and after RF lesions, and monitor for possible complications. PV isolation was the only AF ablation strategy. Ablation lesions are created at 30 ± 5 W (irrigation at 17 ml/min, the maximum temperature of 33 ± 3 °C) for 60–90 s. Energy delivery was reduced near OE to 25 ± 5 W. The region was not retargeted for a minimum of 2 min. Antiarrhythmic drugs were stopped two days before the CA.
2.3. Oesophageal reconstruction
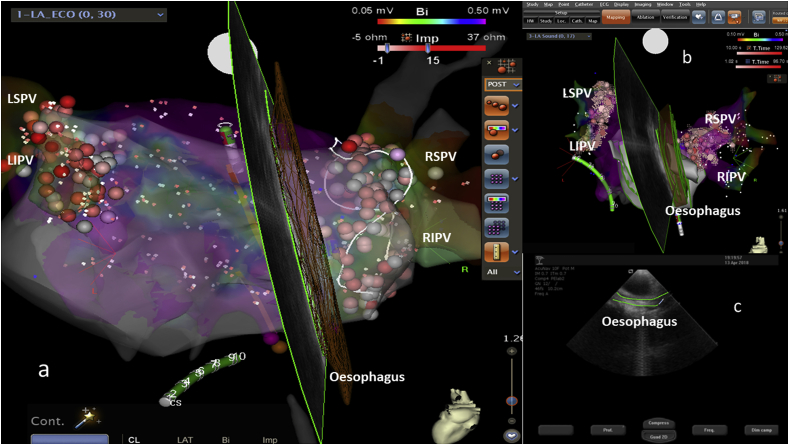
The SoundStar® catheter was inserted into the femoral vein through an 11-Fr introducer sheath and advanced into the RA. To image and monitor the OE and the LAPW oesophageal contiguous wall region, the transducer was placed into the middle/high RA and rotated clockwise to scan between the mid/lower right and left PV Ostia during the ablation procedure. ICE imaging was used to identify the location of the OE relative to the LAPW (Fig. 1 a-c). The course of the OE was then reconstructed using ICE with a CARTOSOUND module (Siemens, Acuson Cypress PLUS – Fig. 1c). Briefly, sequential 2D ICE contours were acquired and used to create a 3D map of the atria with the OE (Fig. 2). Using EAM, the anterior and posterior oesophageal surfaces were marked on EAM. The ICE catheter was further torqued clockwise with oesophageal surface rendering every 2–3 mm until the medial border was identified. The oesophageal map was created before transseptal left atrial access to minimize echo artifacts from the left atrial sheaths and catheters within the LA. Oesophageal imaging features were evaluated, and position and relationship with LAPW was noted. The location of the OE based upon reference to the posterior LA was leftward, midline and rightward. The time necessary to reconstruct OE using ICE was 5 ± 1.6 min.
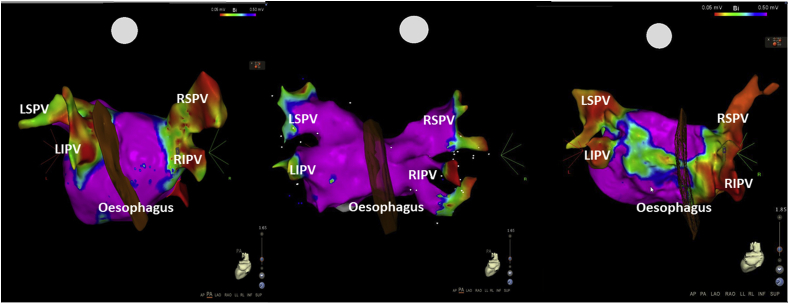
Fig. 1.
EAM with pulmonary veins and OE. The locations of the OE to the posterior left atrium was leftward in the left image, midline in the central image and rightward in the right image. RIPV: right inferior pulmonary vein. RSPV: right superior pulmonary vein. LIPV: left inferior pulmonary vein. LSPV: left superior pulmonary vein.
Fig. 2.
a–b) EAM with pulmonary veins and OE. The OE was interpolated with an ultrasound beam. CS: coronary sinus catheter, painted green. c) 2D ultrasound beam with a green tracked OE during ICE acquisition.
2.4. Statistical analysis
Continuous data were expressed as mean ± standard deviation. Analyses were performed using SPSS 20 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
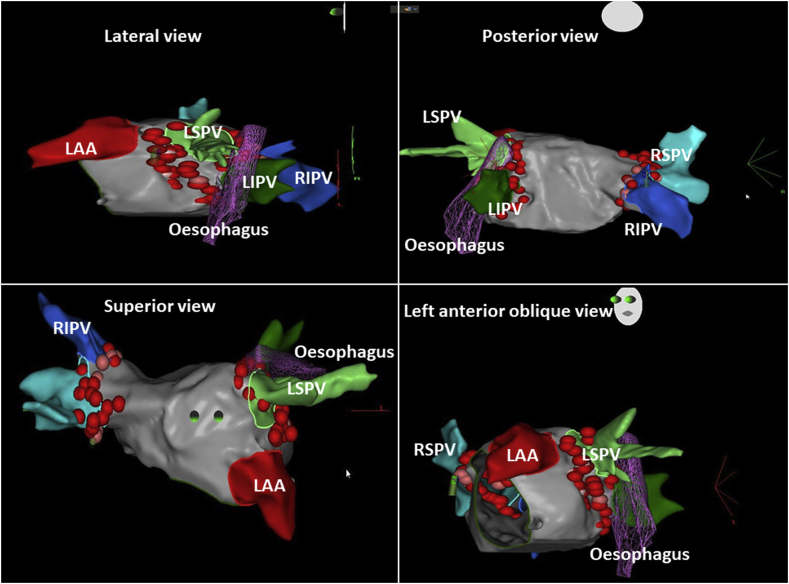
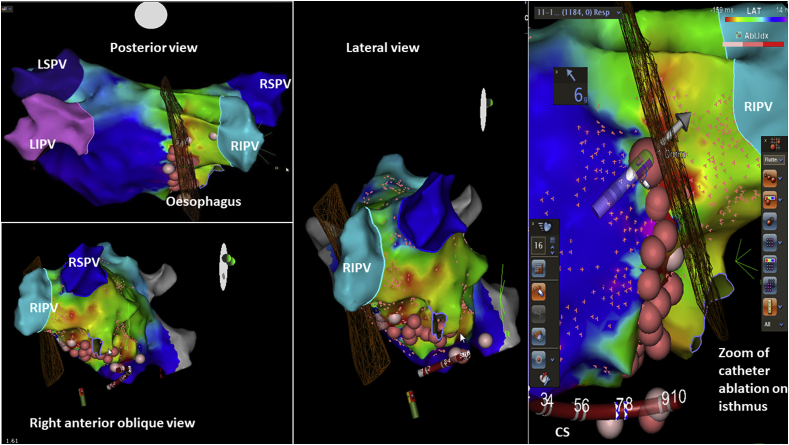
No differences were shown between two groups regarding OE damage. In group B ICE showed the course of quadripolar catheter on the OE. The duration of CA procedures was longer in group B than group A (Group A: 258.2 ± 20 min vs Group B 385.6 ± 19.2 min) due to movements of PT with a quadripolar catheter into OE. Fluoroscopy time was lower in Group A than Group B (Group A 7 ± 3.2 vs 19.2 ± 2.4 min; p < 0.01). In the overall population studied the location of the OE to the posterior LA was leftward in 11.9%, midline in 53.2% and rightward in 34.8% (Fig. 1). Ablation energy delivery was performed outside the greatest OE anatomy borders. Among those with 12-month follow-up, 67.9% were AF-free, 89.7% were IAFL-free and 90% were LAT-free. No PT developed atrial-oesophageal fistula or complications related to the OE. The OE position was not related to sex, hiatal hernia, drugs, smoke or left atrial dimension. One patient had a left inferior pulmonary vein level with the ten o’clock of the circumference of the vein, as a principal trigger of AF; the OE of this patient had a very particular course on the leftward side between the inferior course of the superior left pulmonary vein and superior course of the inferior pulmonary vein, at the ten o’clock visualization of the vein (Fig. 3). The course was posterior to the superior left pulmonary vein wall and lateral to the inferior left pulmonary vein. Another case described was a left isthmus precisely posterior to the oesophageal course (Fig. 4).
Fig. 3.
.Particular course of the OE. The OE course of this patient had a very particular course on the leftward side between the left pulmonary veins. RSPV: right superior pulmonary vein. LIPV: left inferior pulmonary vein. LSPV: left superior pulmonary vein. LAA: left atrial appendage.
Fig. 4.
.The left critical isthmus was precisely posterior to the oesophageal course. RSPV: right superior pulmonary vein. LIPV: left inferior pulmonary vein. LSPV: left superior pulmonary vein. CS: coronary sinus catheter.
4. Discussion
The oesophageal anatomy is complex and variable [5,6]. 3D ICE/Cartosound imaging can add important spatial/anatomical relationships between the LAPW with PV Ostia and the OE, which enhances the capability of modifying ablation lesions in proximity to the OE by adjusting the power, temperature and duration of lesion application [[12], [13], [14], [15], [16]]. ICE with 3D construction of the OE enhances border detection of the OE, and as such, should decrease the risk of oesophageal injury by reducing the power of radiofrequency [12]. As an alternative, the use of an intra-oesophageal temperature probe may increase the risk of oesophageal damage, possibly by fixing the OE into position and even pressing the anterior oesophageal wall against the left-atrial posterior wall [17]. ICE 3D mapping for understanding oesophageal characteristics has many potential advantages during left atrial ablation [12,18]. First, it is a real-time assessment of the OE during the ablative procedure, it reproduces not a static image of the OE but a live monitoring of the position and course of the OE [16]. Ultrasound imaging of the OE depends on whether the OE is collapsed or distended [19]. A variety of oesophageal imaging features – including whether filled with gas, fluid or both, movement within its segment or variably collapsed lumen, may identify the OE with real-time 2D ICE and a fixed 3D Cartosound reconstruction, while monitoring during the LA ablation procedure [17,20,21]. We did not use general anesthesia, so once the procedure begins, the oesophageal location and anatomical characteristics should be relatively recognizable, by swallowing the patient during the visualization of the OE along its entire journey [22]. The use of the OE temperature probe or catheter, to identify the OE borders, is sometimes associated with OE injuries and increased fluoroscopy duration during CA procedures [23,24]. The metal thermistor of the electrode in OE may interact with the RF current and may act as secondary antennas during RF application [24]. Besides, in our studied population the catheter in OE determined discomfort, nausea and movements of the patients that could affect the accuracy of the merged EAM/ICE map and prolong CA duration procedure. Furthermore, ICE 3D OE mapping can be directly integrated into the 3D map of the LA and pulmonary veins and provides fluoroless, real-time localization of the OE during left atrial ablation [11,12]. In our electrophysiological (EP) laboratory, all medical staff use the X-ray explosion only for transseptal puncture; so ICE OE monitoring represents a tool that is useful to improve ‘a near-zero fluoroscopy’ LA approach [19]. The prolonged interventional procedures have added to stochastics effects also the deterministic effects. During ablation the prolonged fluoroscopic guidance exposed the patients to significant levels of radiation exposure because of these potential risks and the linear relationship between radiation dose and increased risk of future malignancy. The radiation exposure must be “as low as reasonably achievable” (ALARA) [22]. Obese patients receive more than twice the effective radiation dose of normal-weight patients. This translates to over 2.5 times the attributable lifetime risk of all-cancer mortality for obese patients compared with normal-weight patients. However, the physician, nursing, and technical staff are exposed to x-rays daily. Potential negative effects of direct radiation exposure include cataracts and malignancies [22]. Variability of the oesophageal course was shown in our cases. Some PT had an oblique oesophageal course, from the right inferior pulmonary vein to the left superior pulmonary vein named in a midline position. As ablation strategies continue to evolve, a more precise understanding of oesophageal position and anatomy will be of increasing importance, particularly if longer or more extensive posterior left atrial ablation is required [24]. As mentioned previously, we believe that understanding the oesophageal anatomy itself is as important as knowing the location, as reported in Fig. 1, Fig. 4, to avoid oesophageal damage [19]. In fact, as an alternative, the positioning of the oesophageal temperature probe tip can be adjusted to meet the level of the ablation catheter electrode based on the fluoroscopic imaging [25]. Lateral displacement using a temperature probe tip, can exaggerate the distance between the lesion site and the probe location in the oesophageal lumen and may limit its accuracy and sensitivity in monitoring the temperature change during the RF delivered at the LAPW-oesophageal region [7,11]. In this observational study the use of OE visualization with combined use of ICE and quadripolar catheter into OE, increased the duration of procedures and fluoroscopy exposure without any reduction of OE injuries. In our EP laboratory, the use of ICE with a Cartosound module improved the safety of left atrial CA procedures. It added the continuous monitoring of pericardium, anatomical reconstruction, transseptal puncture. Moreover, the use of ICE allows real-time visualization of the OE course with a reduction of fluoroscopy exposure.
4.1. Limitations
At present, the 3D ICE technology is not true real-time imaging [11]. 3D ICE was performed with a 2D transducer using a multiple scan approach for sequential data acquisition, gated to ECG and respiration. This methodology is limited by the need for multiple image sampling producing long data acquisition times and some image artifacts. Besides, as compared to the real-time 2D ICE, 3D ICE has a low spatiotemporal resolution and a slow imaging acquisition rate. To overcome these limitations, real-time 3D ICE with the further miniaturization of the matrix-array transducer and the use of an advanced integrated circuit will be required.
5. Conclusions
OE monitoring with ICE is safe and feasible. The oesophageal anatomy is complex and variable. Many PT will have a broad oesophageal boundary, which increases the risk of untoward thermal injury during posterior left atrial ablation. The traditional tools to assess the OE using an intraluminal catheter and 3D mapping, or fluoroscopy with visual assessment of an intra-oesophageal temperature problem, often grossly underestimate the true margins of the OE and can prolong the CA procedures. ICE with 3D construction of the OE enhances border detection of the OE, and as such, should reduce the risk of oesophageal injury by improving avoidance strategies and by real-time oesophageal visualization without fluoroscopy use for real-time OE monitoring.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank Stefano Pizzini, Giuliano Cerruto, Nicola Zaurino and Martina Costantini (Biosense Webster Italy, Johnson and Johnson Medical, Milan, Italy) for technical support during catheter ablation and Ilaria Corsi, Mirko Iannoto, Michele Lenzini, Donatella Maramai, Anna Missere and Sabrina Tozzi and all sanitary staff of the EP Laboratory for Nursing during CA procedures.
A written informed consent was obtained. All work was in compliance with the Declaration of Helsinki.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Dagres N., Anastasiou-Nana M. Prevention of atrial–esophageal fistula after catheter ablation of atrial fibrillation. Curr Opin Cardiol. 2011;26:1–5. doi: 10.1097/HCO.0b013e328341387d. [DOI] [PubMed] [Google Scholar]
- 2.Halm U., Gaspar T., Zachäus M. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol. Mar 2010;105(3):551–556. doi: 10.1038/ajg.2009.625. [DOI] [PubMed] [Google Scholar]
- 3.Di Biase L., Dodig M., Saliba W. Capsule endoscopy in examination of esophagus for lesions after radiofrequency catheter ablation: a potential tool to select patients with increased risk of complications. J Cardiovasc Electrophysiol. Aug 2010;21(8):839–844. doi: 10.1111/j.1540-8167.2010.01732.x. 1. [DOI] [PubMed] [Google Scholar]
- 4.Macedo P.G., Kapa S., Mears J.A. Correlative anatomy for the electrophysiologist: ablation for atrial fibrillation. Part II: regional anatomy of the atria and relevance to damage of adjacent structures during AF ablation. J Cardiovasc Electrophysiol. 2010;21:829–836. doi: 10.1111/j.1540-8167.2010.01730.x. [DOI] [PubMed] [Google Scholar]
- 5.Gillinov A.M., Pettersson G., Rice T.W. Esophageal injury during radiofrequency ablation for atrial fibrillation. J Thorac Cardiovasc Surg. 2001;122:1239–1240. doi: 10.1067/mtc.2001.118041. [DOI] [PubMed] [Google Scholar]
- 6.Mohr F.W., Fabicius A.M., Falk V. Curative treatment of atrial fibrillation with intraoperative radiofrequency ablation: short-term and midterm results. J Thorac Cardiovasc Surg. 2002;123:919–927. doi: 10.1067/mtc.2002.120730. [DOI] [PubMed] [Google Scholar]
- 7.Ren J.-F., Lin D., Marchlinski F.E., Callans D.J., Patel V. Esophageal imaging and strategies for avoiding injury during left atrial ablation for atrial fibrillation. Heart Rhythm. Oct 2006;3(10):1156–1161. doi: 10.1016/j.hrthm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Cappato R., Calkins H., Chen S.A. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. Feb 2010;3(1):32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 9.Ghia K.K., Chugh A., Good E. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Intervent Card Electrophysiol. 2009;24(1):33–36. doi: 10.1007/s10840-008-9307-1. [DOI] [PubMed] [Google Scholar]
- 10.Cappato R., Calkins H., Chen S.A. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol. May 2009;12(19):53. doi: 10.1016/j.jacc.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Jared Bunch T., May Heidi T., Crandall Brian G. Intracardiac ultrasound for esophageal anatomic assessment and localization during left atrial ablation for atrial fibrillation. J Cardiovasc Electrophysiol Jan. 2013;24(1):33–39. doi: 10.1111/j.1540-8167.2012.02441.x. [DOI] [PubMed] [Google Scholar]
- 12.Ren Jian-Fang, Francis E., Marchlinski Utility of intracardiac echocardiography in left heart ablation for tachyarrhythmias. Echocardiography. May 2007;24(5):533–540. doi: 10.1111/j.1540-8175.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- 13.Guiradon G.M., Ofiesh J.G., Kaushik R. Extended vertical transatrial septal approach to the mitral valve. Ann Thorac Surg. 1991;52:1058–1062. doi: 10.1016/0003-4975(91)91281-y. [DOI] [PubMed] [Google Scholar]
- 14.Wang S.L., Ooi C.G., Siu C.W. Endocardial visualization of esophageal-left atrial anatomic relationship by three-dimensional multidetector computed tomography “navigator imaging. Pacing Clin Electrophysiol. 2006;29:502–508. doi: 10.1111/j.1540-8159.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 15.Pollak S.J., Monir G., Chernoby M.S., Elenberger C.D. Novel imaging techniques of the esophagus enhancing safety of left atrial ablation. J Cardiovasc Electrophysiol. 2005;16:244–248. doi: 10.1046/j.1540-8167.2005.40560.x. [DOI] [PubMed] [Google Scholar]
- 16.Piorkowski C., Hindricks G., Schreiber D. Electroanatomic reconstruction of the left atrium, pulmonary veins, and esophagus compared with the “true anatomy” on multislice computed tomography in patients undergoing catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3:317–327. doi: 10.1016/j.hrthm.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 17.Martinek M., Bencsik G., Aichinger J. Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets, and esophageal visualization. J Cardiovasc Electrophysiol Jul. 2009;20(7):726–733. doi: 10.1111/j.1540-8167.2008.01426.x. https://search.crossref.org/?q=Martinek+M%2C+Bencsik+G%2C+Aichinger+J+et+al.+Esophageal+damage+during+radiofrequency+ablation+of+atrial+fibrillation%3A+impact+of+energy+settings%2C+lesion+sets%2C+and+esophageal+visualization.+J+Cardiovasc+Electrophysiol+Jul+2009%3B20%287%29%3A726-733 [DOI] [PubMed] [Google Scholar]
- 18.Kottkamp H., Piorkowski C., Tanner H. Topographic variability of the esophageal left atrial relation influencing ablation lines in patients with atrial fibrillation. J Cardiovasc Electrophysiol. Feb 2005;16(2):146–150. doi: 10.1046/j.1540-8167.2005.40604.x. [DOI] [PubMed] [Google Scholar]
- 19.Sherzer A.I., Feigenblum D.Y., Kulkarni S. Continuous nonfluoroscopic localization of the esophagus during radiofrequency catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:157–160. doi: 10.1111/j.1540-8167.2006.00674.x. [DOI] [PubMed] [Google Scholar]
- 20.Ren Jian-Fang, Callans David J., Marchlinski Francis E., Stiffler Jeffrey A., Sadek Mouhannad, Supple Gregory E. 3D intracardiac echocardiography/cartosound™ imaging of esophagus guided left atrial posterior wall ablation for atrial fibrillation. J Cardiovasc Electrophysiol Feb. 2007;18(2):157–160. [Google Scholar]
- 21.Pappone C., Oral H., Santinelli V. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. Jun 8 2004;109(22):2724–2726. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 22.Vivek Y., Morales G., Ahmed H. Catheter ablation of atrial fibrillation without the use of fluoroscopy. Heart Rhythm. 2010;7:1644–1653. doi: 10.1016/j.hrthm.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Dar T., Yarlagadda B., Alkhatib C. Esophageal laceration related to mechanical trauma from a General Purpose (esophageal/rectal) temperature probe introducer sheath during atrial fibrillation ablation. J Atr Fibrillation. 2018 Feb;10(5):1878. doi: 10.4022/jafib.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller P., Dietrich J.W., Halbfass P., Aly A. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm. 2015 Jul;1(7):1464–1469. doi: 10.1016/j.hrthm.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Kobza R., Schoenenberger A.W., Erne P. Esophagus imaging for catheter ablation of atrial fibrillation: comparison of two methods with showing of esophageal movement. J Intervent Card Electrophysiol. 2009;26:159–164. doi: 10.1007/s10840-009-9434-3. [DOI] [PubMed] [Google Scholar]