Abstract
Purpose
Electroanatomical mapping (EAM) could increase cardiac magnetic resonance imaging (CMR) sensitivity in detecting ventricular scar. Possible bias may be scar over-estimation due to inadequate tissue contact. Aim of the study is to evaluate contact-force monitoring influence during EAM, in patients with idiopathic right ventricular arrhythmias.
Methods
20 pts (13 M; 43 ± 12 y) with idiopathic right ventricular outflow tract (RVOT) arrhythmias and no structural abnormalities were submitted to Smarttouch catheter Carto3 EAM. Native maps included points collected without considering contact-force. EAM scar was defined as area ≥1 cm2 including at least 3 adjacent points with signal amplitude (bipolar <0.5 mV, unipolar 3,5 mV), surrounded by low-voltage border zone. EAM were re-evaluated offline, removing points collected with contact force <5 g. Finally, contact force-corrected maps were compared to the native ones.
Results
An EAM was created for each patient (345 ± 85 points). After removing poor contact points, a mean of 149 ± 60 points was collected. The percentage of false scar, collected during contact force blinded mapping compared to total volume, was 6.0 ± 5.2% for bipolar scar and 7.1 ± 5.9% for unipolar scar, respectively. No EAM scar was present after poor contact points removal. Right ventricular areas analysis revealed a greater number of points with contact force < 5 g acquired in free wall, where reduced mean bipolar and unipolar voltage were recorded.
Conclusions
To date this is the first work conducted on structurally normal hearts in which contact-force significantly increases EAM accuracy, avoiding “false scar” related to non-adequate contact between catheter and tissue.
Keywords: Right ventricular map, Ventricular scar, Cardiac magnetic resonance, Contact force
1. Introduction
In the last decades electroanatomic three-dimensional mapping (EAM) has become increasingly widespread, mostly because of its potential to facilitate complex supraventricular and ventricular arrhythmias ablation. Three-dimensional mapping systems are required in some electrophysiological procedures in order to allow non-fluoroscopic catheter navigation, reconstruction of electrophysiological mechanisms, particularly of complex arrhythmias, and to facilitate catheter ablation [1].
Improved anatomical resolution of 3D mapping systems allowed to significantly reduce radiation exposure during electrophysiological procedures, providing an accurate non-fluoroscopic catheters navigation and reducing radiation-related risks both for patients and clinicians [2]. Moreover, EAM was tested for its potential to access structural abnormalities of ventricular myocardium in different heart diseases [3]. Bipolar and unipolar voltage mapping (VM) have been shown to be a useful tool in ventricular scar identification [4]. It has been observed that bipolar VM through electroanatomic 3D mapping may further increase the sensitivity of cardiac magnetic resonance imaging (CMR) to detect ventricular scar [5]. However, a possible bias of bipolar VM may be an over-estimation of a scar region related to non-adequate contact between the mapping catheter and the tissue.
Nowadays several types of mapping catheters are available. Multipolar catheters with shorter inter-electrode spacing are the most accurate [6,7] but catheter with contact force sensor improves monitoring of catheter-tissue contact force [8].
The most common cutoff thresholds applied for scar identification were obtained from relatively small series of subjects without structural heart disease. Typically, the 5th percentiles of bipolar and unipolar voltages in normal hearts were arbitrarily used as cutoffs for the definition of scar [9,10]. Several studies compared CMR data and EAM voltage for the detection of left ventricular (LV) scar [11,12], however, a real comparison between right ventricle mapping and scar has not been described in individuals without structural heart disease. Evidence supporting the impact of contact forceCF) on EAM for right ventricular scar detection was provided by a study conducted in a group of patients affected by tetralogy of Fallot [13]. However, experiences evaluating the role of CF in EAM in normal hearts are lacking.
Aim of the study is to evaluate the influence of contact force monitoring during right ventricular bipolar VM in a group of patients with idiopathic right ventricular (RV) arrhythmias and no evidence of structural heart disease at CMR.
2. Methods
2.1. Study population
From April 2016 to November 2018 consecutive adult patients with idiopathic RV outflow tract arrhythmias without evidence of structural heart disease at delayed-enhancement CMR scar imaging, were enrolled and submitted to SmartTouch catheter Carto3 EAM and subsequently to RV premature beats ablation by means of an activation map [14]. All procedure were performed in Policlinico Casilino laboratories by experienced electrophysiologist. Only adult patients (≥18 years) of age were included.
Idiopathic right ventricular outflow tract premature ventricular beats were defined basing on the following criteria: normal resting ECG except for RV outflow tract arrhythmias; absence of late potentials on signal-averaged ECG; normal dimension and function (global and regional) of the left and right ventricular chambers, determined by echocardiography and confirmed by CMR and no evidence of late gadolinium enhancement at CMR. Indication for ablation was the presence of disabling symptoms due to premature ventricular complexes (PVC). All patients underwent CMR before electrophysiological study and ablation. The study was approved by the institutional review board and all patients gave their written informed consent to study participation.
2.2. Cardiac magnetic resonance
All patients underwent late gadolinium enhancement CMR on a 1.5-T clinical system (Philips Achieva, Best, the Netherlands) with a 5-channel phased array cardiac coil. A 3-dimensional, inversion recovery–prepared, ECG-gated, respiration-navigated gradient-echo pulse sequence with fat saturation was used. Late gadolinium enhancement imaging was started 10 min after intravenous injection of gadobutrol (Gadovist, Bayer) at a dose of 0.2 mmol/kg with an inversion recovery turbo field echo T1 weighted sequence. Conventional breathhold T1 weighted fast-spin echo images were acquired in the same short-axis views (10-mm-slice thickness, no gap, size 1.25 × 1.25 × 2.5 mm) and long-axis view.
Left and right ventricular end-diastolic, end-systolic volumes and ejection fraction were obtained from the short-axis views (View Forum Software). For quantitative reporting and comparison with EAM findings, the RV was divided into 5 regions: the outflow tract, the posterior/inferior wall (i.e., including both the inferior and posterior segments), the free wall, the apex, and the septal wall. All measurements were performed by fully blinded operators (C.L. and M.D.R) (radiologist and cardiologist with level-3experience basing on Cardiovascular Magnetic Resonance Society Criteria).
2.3. Electro-anatomical mapping
All patients, gave their written informed consent to electrophysiological study and ablative procedure. Electrophysiological procedures were performed in fasting state after mild sedation. Right ventricular EAM was performed by the CARTO 3 system (Biosense-Webster, Inc., Diamond Bar, CA, USA). Mapping points were sampled with a 8 F 3.5 mm irrigated tip SmartTouch catheter (Biosense-Webster, Inc.), in order to generate an accurate RV EAM. Before starting points acquisition, the contact-force sensing system was calibrated to 0 g, when fluoroscopy and signals detection was consistent with absence of contact. Details of contact-force sensing technology have been previously described [1]. All points were acquired in sinus rhythm and no long sheaths were used to optimize catheter stability. During points’ acquisition, operators were blinded to the contact force information. High-density right ventricular mapping was obtained in sinus rhythm (reference channel: QRS complex), by sampling at least 300 points evenly distributed. The voltage maps were edited, setting the point density (fill threshold) at 5 mm. Intracavitary points were manually removed as reported elsewhere [16]. During point’s acquisition, as previously described [6] adequate catheter contact was confirmed by concordance of catheter tip motion and the cardiac silhouettes on fluoroscopy.
In addition, other conventional indirect contact information were used:
- The signal had to meet the 3 stability criteria automatically detected by the CARTO 3 system regarding cycle length, local activation time and beat-to-beat difference in the location of the catheter (<2%, <3 m/s, and <3 mm, respectively);
- Both bipolar and unipolar signals were simultaneously acquired through the analysis of local electrograms (in particular the shape of the unipolar electrograms) in order to confirm true catheter contact;
- In the low voltages areas, at least 3 additional points were acquired at the same site, to confirm the reproducibility of the voltage measurement. According to previous studies, “EAM scar” was defined as an area ≥1 cm2 including at least 3 adjacent points with bipolar signal amplitude <0.5 mV, surrounded by a border zone with reduced signal amplitude (0.5–1.5 mV) and ≥2 cm2 unipolar signal amplitude <3.5 mV surrounded by a border zone with reduced signal amplitude (3.5–5.5 mV) [15,16]. Low voltages areas with an extension less than 1 cm2 were not considered. Five different RV areas were identified: the right ventricular outflow tract (RVOT), the posterior/inferior wall (including both the inferior and posterior segments), the free wall, the apex, and the septal wall. A dedicated CARTO 3 software was used to measure the extension of low-voltage areas. Extension of low voltage RV areas, calculated as the ratio between low voltage area and total RV area, were reported as well. Scar border zone was further analysed by manual re-navigation of the catheter in the same site, in order to confirm the reproducibility of the voltage measurements. Electroanatomic maps were obtained (native maps) and analysed separately by 2 expert electrophysiologists blinded to the results of CMR.
After the procedures, an offline re-analysis of the native maps was performed. For all points the contact-force of the catheter over the tissue was re-evaluated. It was defined a cut off of 5 g, as previously reported [13] and all points collected with a contact force lower than 5 g were eliminated from the maps. The contact-force corrected maps were further re-analysed to detect scar areas.
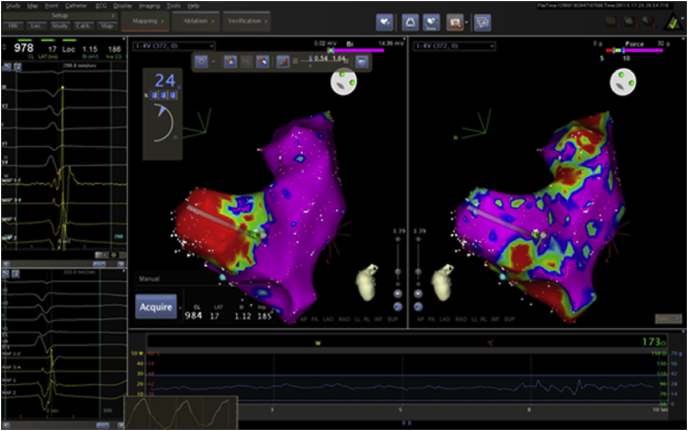
Force maps could be created using a 8 F 3.5 mm irrigated tip SmartTouch catheter (Biosense-Webster, Inc). It was than possible to reconstruct colour coded force maps, in fact each acquired point represents the value of the applied force to the catheter tip in real-time.
The force map allows viewing the catheter’s contact areas with the tissue. This could be a useful tool in all the phases of electrophysiological procedures: during mapping, as it ensures anatomical/electrical reconstructions faithful to reality; during ablation, to visualize the low-contact areas and the homogeneous distribution of acquired points (Fig. 1).
Fig. 1.
Bipolar map and Force map.
Right ventricle was divided, as mentioned before, in five areas (RVOT, posterior-inferior wall, free wall, septal wall and apex), in order to analyze contact force and voltage results in every single area.
2.4. Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and categorical variables are reported as number (%). Kolmogorov-Smirnov test was used to verify normal distribution of continuous variables. Differences across groups were assessed with unpaired Student’s t-test, Mann-Whitney U test, 1-way analysis of variance, and Fisher’s Exact test, as appropriate.
Subgroup analyses were conducted with ANOVA repeated measures with log link function and with a post hoc pairwise comparisons with Sidak correction for multiple comparisons.
All probability (p) values reported are 2-sided, and a p value of <0.05 was considered statistically significant. Statistical analysis was performed with the STATA 11.1 statistical package (Stata Corporation, College Station, TX, USA).
3. Results
Twenty consecutive patients (13 males, mean age 43 ± 12 years) with idiopathic right ventricular arrhythmias were included in the study. Baseline patients’ characteristics are summarized in Table 1 There was no evidence of structural heart disease, according to echocardiographic and CMR examination.
Table 1.
Patients baseline characteristics and imaging data.
| Baseline Characteristics | |
|---|---|
| Male Gender, n (%) | 13 (65%) |
| Age, years | 43 ± 12 |
| Hypertension, n (%) | 2 (10%) |
| Type 2 diabetes mellitus, n (%) | 1 (5%) |
| Dyslipidemia, n (%) | 1 (5%) |
| Echo LVEF, % | 61 ± 3.7 |
| CMR LVEF, % | 58 ± 7.6 |
| CMR RVEF, % | 57 ± 4.2 |
| CMR LVEDV, ml | 131.4 ± 13.1 |
| CMR RVEDV, ml | 122.8 ± 29.2 |
Values expressed as n (%), mean ± standard deviation.
CMR = Cardiac magnetic resonance LVEF = Left ventricle ejection fraction. RVEF = Right ventricle ejection fraction. LVEDV = left ventricular end diastolic volume. RVEDV = right ventricular end diastolic volume.
An accurate right ventricular voltage map was created for each patient collecting a mean of 345 ± 85 points: native maps. Mean right ventricular volume at electro-anatomical mapping was 124.3 ± 24.7 ml. Applying conventional bipolar voltage scale and unipolar voltage scale to right ventricle maps, bipolar scar areas and unipolar scar areas were identified. Bipolar scar areas were present in 18 subjects (90%) whereas unipolar scars were present in 19 subjects (95%) (Table 2). Mean surface was 8.3 ± 7.6 cm2 for bipolar scar and 12.5 ± 10.9 cm2 for unipolar scar. The percentage of scar areas compared to the total right ventricular surface was 6.0 ± 5.2% for bipolar scar and 7.1 ± 5.9% for unipolar scar. Detailed data on unipolar and bipolar scars location are shown in Table 2.
Table 2.
Scar areas in native bipolar and unipolar right ventricular voltage map (contact-force blinded).
| Scar area bipolar (cmˆ2) | % Area scar bipolar/Total RV Area | Scar area unipolar (cmˆ2) | % Area scar unipolar/Total RV Area | Scar position (bipolar map) | Scar position (unipolar map) | |
|---|---|---|---|---|---|---|
| 1 | 0 | 0% | 7 | 4.2% | 0 | Free wall |
| 2 | 11.4 | 7% | 8.6 | 5% | Free wall | Free wall |
| 3 | 9 | 6% | 4.3 | 2.5% | Free wall | Free wall |
| 4 | 5.1 | 5% | 15 | 12% | Free wall | Free wall |
| 5 | 27.1 | 15% | 30.4 | 14.7% | Free wall | Free wall and Septum |
| 6 | 3.7 | 2.3% | 1.1 | 0.6% | Posterior wall | Posterior wall |
| 7 | 7.8 | 5.3% | 17 | 11% | Free wall | Free wall |
| 8 | 2 | 1.0% | 5.6 | 2.7% | Apex | Inferior wall |
| 9 | 2.5 | 1.4% | 4.8 | 2.8% | Free wall | Free wall |
| 10 | 0 | 0% | 0 | 0% | 0 | 0 |
| 11 | 4.2 | 2.6% | 5 | 3% | Posterior/inferior wall | Posterior/inferior wall |
| 12 | 34.4 | 16.5% | 45.1 | 18.0% | Free wall | Free wall |
| 13 | 15 | 13.6% | 39.3 | 22.0% | Free wall | Free wall |
| 14 | 18.1 | 10.6% | 33.8 | 20% | Free wall | Free wall |
| 15 | 4 | 2.7% | 20.6 | 14.2% | Free wall | Inferior wall |
| 16 | 5 | 1.6% | 5.7 | 3.3% | Apex | Posterior wall |
| 17 | 9 | 4.7% | 12.2 | 6.4% | Free wall | Free wall |
| 18 | 5.4 | 4.2% | 5.9 | 6.1% | Free wall | Free wall |
| 19 | 12.1 | 5.1% | 29.9 | 12.4% | Free wall | Posterior/inferior wall |
| 20 | 2.8 | 2.3% | 6.2 | 4.4% | Inferior wall | Apex |
Values expressed as cm2 for areas and % of area with significant scar compared to the total RV area. RV = Right ventricle.
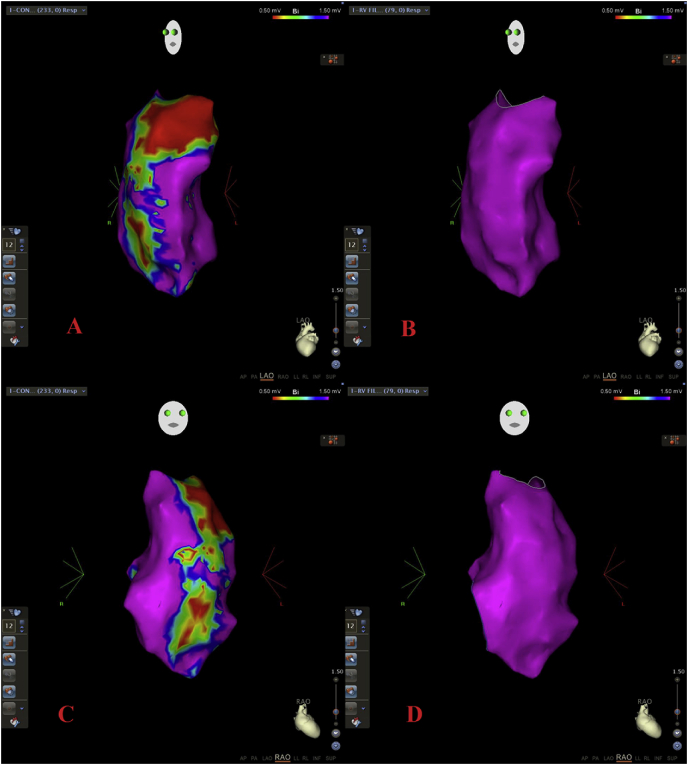
After exclusion of points with contact force lower than 5 g, a mean of 149 ± 60 points were collected as contact-force corrected maps, where no significant bipolar and unipolar scar areas were found any more. Fig. 2 highlights false scar areas in LAO and RAO orientation when contact force sensing was not considered.
Fig. 2.
LAO orientation bipolar mapping of a right ventricle without (panel A; native map) and with contact force cut-off of 5 g (panel B; contact-force corrected map). RAO orientation bipolar mapping of a right ventricle without (panel C; native map) and with contact force cut-off of 5 g (panel D; contact-force corrected map).
Results of voltage mapping in the five different areas of the right ventricle are shown in Table 3.
Table 3.
Mean bipolar voltage and mean unipolar voltage with standard deviation in different right ventricular areas. In the last column data are compared with a post hoc pairwise comparisons with Sidak correction (only significant comparison are reported in the table).
| RVOT | Post-inf wall | Free wall | Septal wall | Apex | P | |
|---|---|---|---|---|---|---|
| Mean contact force (g) | 6.8 ± 1.4∗ | 6.4 ± 2.1 | 3.9 ± 0.8∗∗ | 6.4 ± 2.0 | 5.3 ± 1.3 | ∗ = 0.015 vs Apex ∗∗ < 0.001 vs RVOT ∗∗ = 0.03 vs Post-inf wall ∗∗ = 0.002 vs Septal Wall ∗∗ = 0.003 vs Apex |
| Mean bipolar voltage (V) | 3.5 ± 0.5 | 3.3 ± 0.9 | 2.4 ± 0.5∗ | 4.2 ± 1.6 | 3.7 ± 1.8 | ∗ < 0.001 vs RVOT ∗ = 0.003 vs Post-inf wall ∗ = 0.001 vs Septal wall ∗ = 0.046 vs Apex |
| Mean unipolar voltage (V) | 5.3 ± 1.4∗ | 6.4 ± 1.9 | 5.4 ± 1.4∗∗ | 8.0 ± 2.3 | 7.3 ± 1.8 | ∗ < 0.001 vs Septal wall ∗ = 0.01 vs Apex ∗∗ = 0.01 vs Septal wall ∗∗ = 0.017 vs Apex |
After exclusion of points with contact force lower than 5 g, a minimum of 25 points in every area were collected. Right ventricular free wall resulted to be the region in which a prevalence of poor contact force was observed. In the same region, false scar areas were prevalent, when compared to other right ventricular areas. Poor contact force during mapping reflects itself in a reduced mean bipolar and unipolar voltage and was prevalent in right ventricular free wall, as well.
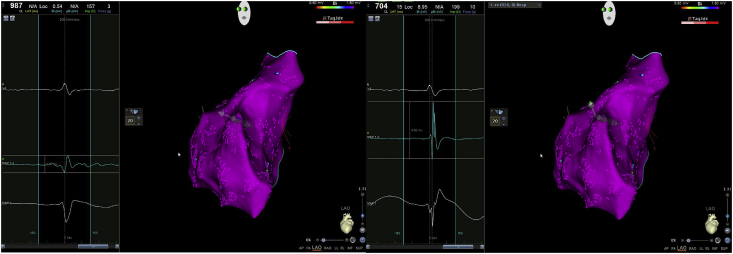
In Fig. 3 two different electrograms in the same RV location are presented (the distance between the two points is less than 1 mm). In panel A the ventricular electrogram is collected with a contact force of 3 g, with a bipolar voltage (MAP1-2) of 0,54 mV: local electrogram looks like a low voltage “far field” signal. In panel B the electrogram is acquired with a contact force of 10 g: the local bipolar voltage is 8.95 mV and the local signal is very well defined with multiple sharp deflections.
Fig. 3.
: two local bipolar electrograms acquired in sinus rhythm in the same right ventricular site with different contact-force. In panel A a low voltage electrogram (see MAP 1–2) was acquired with a poor contact-force. In panel B the local bipolar electrogram is 8.95 mV with a contact force of 10 g. See the text for other descriptions.
4. Discussion
Electro-anatomic 3D mapping has the potential to investigate the underlying ventricular arrhythmic substrate, identifying damaged myocardial areas and/or scars. From the early report of Marchlinski et al. [18] in arrhythmogenic right ventricular cardiomyopathy, the promising role of this catheter-based technique has been confirmed in different clinical conditions [[19], [20], [21]]. Moreover, it has been proposed that VM in the contest of EAM could be as accurate as CMR for ventricular scar detection in experimental models of dilated cardiomyopathy [22], myocardial infarction [23], and in patients with postinfarction ventricular arrhythmias [24]. However, a possible bias of EAM can be the overestimation of low voltage areas and scar areas due to inadequate contact of mapping catheter tip to myocardial tissue. Many published experiences about voltage mapping through EAM have been carried out without specific technologies testing the catheter contact over the tissue [[16], [17], [18]]. Several contact surrogates have been used, in order to minimize overestimation of low voltage and scar areas, but a direct contact confirmation with dedicated catheters in the setting of EAM is still lacking.
Since its introduction, contact-force monitoring technology became increasingly common in electrophysiology, because of its safety and efficacy in the ablative procedures. Moreover, the possibility to collect points in the maps only when an adequate contact between the catheter tip and the tissue is confirmed, could theoretically increase voltage maps accuracy, avoiding false scar identification. Our study seems to confirm the last hypothesis. In our experience, conducted in 20 subjects with structurally normal hearts, the 3D VM post-processing provided evidence for the importance of contact-force sensing. When native maps are considered, a great number of electro-anatomical scars (false scars) were identified. It is interesting to underline that points included in the native maps were acquired according to the standard precautions of the previous experiences, involving fluoroscopy, quality of signals and conventional catheter stability parameters of the CARTO system. This suggests that it could not be possible to trust standard precautions as effective surrogates of good mapping catheter contact over the tissue. With post-processing off-line adjustments, after eliminating points acquired with non-adequate contact (contact force < 5 g), all scars disappeared. A complete correlation between contact-force corrected maps and CMR was observed, without any significant scar detected by both techniques.
The importance of contact-force in VM was supported by a previous experience in fourteen patients with tetralogy of Fallot [13]. In this experience, the exclusion of points acquired with contact force lower than 5 g was correlated to high specificity and sensitivity levels for scars detection [13].
Our study further supports these findings even in a population with structurally normal hearts, but are in apparent discordance with Letsas et al. [25] who studied a series of patients with idiopathic right ventricular outflow tract arrhythmias and normal CMR findings with high density multipolar electroanatomical mapping. A decapolar catheter was used for mapping and low voltage areas were confirmed by a contact force sensing SmartTouch catheter (Biosense Webster). Despite the absence of significant alterations at CMR, low bipolar voltages areas were identified in the majority of patients [25]. Unfortunately, detailed correlation analysis between multipolar mapping and point by point mapping with SmartTouch catheter are not provided by authors. However it is to note that Letsas et al. [25] applied different mapping criteria and, for instance, considered as low bipolar electrograms signals with amplitude below 1 mV. Multipolar catheters are currently considered as a standard of care in substrate mapping [26,27]. However, multipolar systems use signal quality indicators which are merely contact surrogates. Further studies are required in order to compare single catheter provided with contact force technologies versus multipolar catheters approach in the EAM field.
In our study, we demonstrated that the possibility of detecting false scar is strongly reduced using a bipolar ventricular mapping with a contact force sensing upper than 5 g. A good ventricular voltage could be obtained in patients with idiopathic right ventricular arrhythmias and no evidence of structural heart disease at CMR. These observations provide evidence for the importance of achieving a good contact during mapping. Operators should be aware about this and catheter maneuverability should be addressed to this goal. Sometimes, gentle catheter rotation is sufficient to increase contact force. Bidirectional steerable catheters, as well as the use of long sheaths or steerable long sheaths may be helpful tools to optimize contact force.
The analysis of the force native maps (Table 3) showed that the prevalent areas of poor catheter contact were located in correspondence of the right ventricular free wall and it was confirmed by pairwise comparisons with Sidak correction for each area: free wall is the most difficult area in which to guarantee adequate specificity without the contact force. In fact, the native bipolar voltage maps (not corrected for the contact), showed higher prevalence of “false” scars in this area. It is not new that the right ventricular free wall is sometimes difficult to achieve, providing good catheter stability. Particular attention should be paid when mapping this area, in order to avoid false positives. Contact-force may be very useful, and a good contact confirmation represents a crucial tool to detect the presence of a scar. The possibility to have live information about the contact force of the catheter over myocardium may help the efficacy and safety of VM.
5. Study limitations
A possible limitation of our experience could be the use of CMR as a perfect gold standard to rule out RV scars. However, we must consider that our study population characteristics with normal clinical findings, no evidence of RV structural and functional abnormalities at the echocardiography and completely normal ECG except from the right ventricular outflow tract premature beats, seem to confirm that an underestimation of a right ventricular scar at CMR has to be considered a very remote possibility.
Our study included a relatively small sample of patients. However, such a limitation seems to be, at least in part, overcome by the relevant number of points collected in the single maps.
Another issue to consider is that the electrogram (EGM) amplitude depends not only on the contact force but also on the orientation of the recording dipole relative to the direction of the activation wavefront. This could be particularly true when mapping is performed with a relatively large electrode like the tip of the Smarttouch catheter. However we should consider that this could be very important in experimental models, but it is hardly applicable in clinical settings, particularly in ventricular substrate mapping studies.
All maps were performed in sinus rhythm without use of ventricular pacing. This issue could have influenced our scar detection.
Another possible limitation is related to the density of points in the different RV areas. In fact, after removal of poor contact points, the number of collected points significantly decreased. However, a minimum value of 25 points was recorded in a given area (RV free wall), allowing a density of points sufficient to perform a substrate analysis in that region.
6. Conclusions
To date this is the first experience on structurally normal hearts in which contact-force revealed to significantly increase the bipolar and unipolar VM accuracy. This is particularly true in some ventricular areas like right ventricular free wall, where is more difficult to reach a good and stable catheter contact over the tissue. Only points when a good contact is confirmed should be considered for mapping analysis. Previous published experiences about 3D VM should be revisited and confirmed with the present technologies.
Author contribution
Luigi Sciarra, contribute with Conceptualization, Data curation, Formal analysis, Investigation and writing the main text. Zefferino Palamà, contribute with Conceptualization, Data curation, Formal analysis, Investigation and writing the main text. Martina Nesti, contribute with Conceptualization, Data curation, Formal analysis, Investigation and writing the main text. Elena Cavarretta, contribute with Conceptualization, Data curation, Formal analysis, Investigation and writing the main text. Antonio Scarà; and contribute with Conceptualization, Data curation, Formal analysis, Investigation and writing the main text. Chiara Lanzillo contribute with Conceptualization, Data curation, Formal analysis, Investigation and writing the main text. Mauro Di Roma, contribute with Software analysis, Data curation Investigation. Ermenegildo De Ruvo, contribute with Software analysis, Data curation Investigation. Antonio Gianluca Robles, contribute with Software analysis, Data curation Investigation. Lucia De Luca and contribute with Software analysis, Data curation Investigation. Domenico Grieco contribute with Software analysis, Data curation ad investigation. Mariano Rillo, contribute with Writing – review & editing Supervision. Silvio Romano, contribute with Writing – review & editing and Supervision. Renata Petroni, contribute with Writing – review & editing and Supervision. Maria Penco and contribute with Writing – review & editing and Supervision. Leonardo Calò; contribute with Writing – review & editing Supervision.
Funding
No funding research received.
Declaration of competing interest
None.
Data supporting the results can be found in datasets in Policlinico Casilino Rome Italy.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Sciarra L., Dottori S., De Ruvo E., De Luca L., Pitrone P., Rebecchi M., Zuccaro L., Lanzillo C., Minati M., Lioy E., Calò L. The new electroanatomical Carto3 mapping system: three-dimensional right ventricular fast anatomical map resolution in comparison to magnetic resonance image. J Cardiovasc Med. 2011 Jun;12(6):434–435. doi: 10.2459/JCM.0b013e3283429342. [DOI] [PubMed] [Google Scholar]
- 2.Haegeli L.M., Mohsen M., Wolber T., Brunckhorst C., On C.J., Duru F. Feasibility of zero or near zero fluoroscopy during catheter ablation procedures. Cardiol J. 2018 Apr 3 doi: 10.5603/CJ.a2018.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sramko M., Hoogendoorn J.C., Glashan C.A. Advancement in cardiac imaging for treatment of ventricular arrhythmias in structural heart disease. Europace. 2019 Mar 1;21(3):383–403. doi: 10.1093/europace/euy150. [DOI] [PubMed] [Google Scholar]
- 4.Spears D.A., Suszko A.M., Dalvi R. Relationship of bipolar and unipolar electrogram voltage to scar transmurality and composition derived by magnetic resonance imaging in patients with nonischemic cardiomyopathy undergoing VT ablation. Heart Rhythm. 2012 Nov;9(11):1837–1846. doi: 10.1016/j.hrthm.2012.07.022. Epub 2012 Jul 27. [DOI] [PubMed] [Google Scholar]
- 5.Santangeli P., Hamilton-Craig C., Dello Russo A., Pieroni M., Casella M., Pelargonio G., Di Biase L., Smaldone C., Bartoletti S., Narducci M.L., Tondo C., Bellocci F., Natale A. Imaging of scar in patients with ventricular arrhythmias of right ventricular origin: cardiac magnetic resonance versus electroanatomic mapping. J Cardiovasc Electrophysiol. 2011 Dec;22(12):1359–1366. doi: 10.1111/j.1540-8167.2011.02127.x. [DOI] [PubMed] [Google Scholar]
- 6.Berte B., Relan J., Sacher F. Impact of electrode type on mapping of scar-related VT. J Cardiovasc Electrophysiol. 2015. Nov;26(11):1213–1223. doi: 10.1111/jce.12761. Epub 2015 Sep. 10. [DOI] [PubMed] [Google Scholar]
- 7.Tschabrunn C.M., Roujol S., Dorman N.C., Nezafat R., Josephson M.E., Anter E. High-resolution mapping of ventricular scar: comparison between single and multielectrode catheters. Circ Arrhythm Electrophysiol. 2016 Jun;9(6) doi: 10.1161/CIRCEP.115.003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okumura Y., Johnson S.B., Bunch T.J., Henz B.D., O’Brien C.J., Packer D.L. A systematical analysis of in vivo contact forces on virtual catheter tip/tissue surface contact during cardiac mapping and intervention. J Cardiovasc Electrophysiol. 2008;19:632–640. doi: 10.1111/j.1540-8167.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- 9.Botker H.E., Lassen J.F., Hermansen F. Electromechanical mapping for detection of myocardial viability in patients with ischemic cardiomyopathy. Circulation. 2001;103:1631–1637. doi: 10.1161/01.cir.103.12.1631. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs S., Hendel R.C., Baim D.S. Comparison of endocardial electromechanical mapping with radionuclide perfusion imaging to assess myocardial viability and severity of myocardial ischemia in angina pectoris. Am J Cardiol. 2001;87:874–880. doi: 10.1016/s0002-9149(00)01529-0. [DOI] [PubMed] [Google Scholar]
- 11.Codreanu A.1, Odille F., Aliot E., Marie P.Y., Magnin-Poull I., Andronache M., Mandry D., Djaballah W., Régent D., Felblinger J., de Chillou C. Electroanatomic characterization of post-infarct scars comparison with 3-dimensional myocardial scar reconstruction based on magnetic resonance imaging. J Am Coll Cardiol. 2008 Sep 2;52(10):839–842. doi: 10.1016/j.jacc.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 12.Thajudeen A., Jackman W.M., Stewart B. Correlation of scar in cardiac MRI and high-resolution contact mapping of left ventricle in a chronic infarct model. Pacing Clin Electrophysiol. 2015 Jun;38(6):663–674. doi: 10.1111/pace.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teijeira-Fernandez E., Cochet H., Bourier F. Influence of contact force on voltage mapping: a combined magnetic resonance imaging and electroanatomic mapping study in patients with tetralogy of Fallot. Heart Rhythm. 2018 Aug;15(8):1198–1205. doi: 10.1016/j.hrthm.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Calvo N., Jongbloed M., Zeppenfeld K. Radiofrequency catheter ablation of idiopathic right ventricular outflow tract arrhythmias. Indian Pacing Electrophysiol J. 2013 Jan-Feb;13(1):14–33. doi: 10.1016/s0972-6292(16)30585-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesel L., Sacher F., Komatsu Y. Characterization of contact force during endocardial and epicardial ventricular mapping. Circ Arrhythm Electrophysiol. 2014;7:1168–1173. doi: 10.1161/CIRCEP.113.001219. [DOI] [PubMed] [Google Scholar]
- 16.Corrado D., Basso C., Leoni L. Three-dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2005 Jun 14;111(23):3042–3050. doi: 10.1161/CIRCULATIONAHA.104.486977. [DOI] [PubMed] [Google Scholar]
- 17.Polin G.M., Haqqani H., Tzou W. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm. 2011;8:76–83. doi: 10.1016/j.hrthm.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 18.Marchlinski F.E., Zado E., Dixit S. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation. 2004 Oct 19;110(16):2293–2298. doi: 10.1161/01.CIR.0000145154.02436.90. [DOI] [PubMed] [Google Scholar]
- 19.Migliore F., Zorzi A., Silvano M. Prognostic value of endocardial voltage mapping in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Arrhythm Electrophysiol. 2013 Feb;6(1):167–176. doi: 10.1161/CIRCEP.111.974881. Epub 2013 Feb 7. [DOI] [PubMed] [Google Scholar]
- 20.Fukuzawa K., Yoshida A., Kubo Endocardial substrate mapping for monomorphic ventricular tachycardia ablation in ischemic and non-ischemic cardiomyopathy. Kobe J Med Sci. 2008 Jul 18;54(2):E122–E135. [PubMed] [Google Scholar]
- 21.Soto-Becerra R., Bazan V., Bautista W. Ventricular tachycardia in the setting of chagasic cardiomyopathy: use of voltage mapping to characterize endoepicardial nonischemic scar distribution. Circ Arrhythm Electrophysiol. 2017 Nov;10(11) doi: 10.1161/CIRCEP.116.004950. [DOI] [PubMed] [Google Scholar]
- 22.Psaltis P.J., Carbone A., Leong D.P. Assessment of myocardial fibrosis by endoventricular electromechanical mapping in experimental nonischemic cardiomyopathy. Int J Cardiovasc Imag. 2011 Jan;27(1):25–37. doi: 10.1007/s10554-010-9657-5. [DOI] [PubMed] [Google Scholar]
- 23.Pavo N., Jakab A., Emmert M.Y. Comparison of NOGA endocardial mapping and cardiac magnetic resonance imaging for determining infarct size and infarct transmurality for intramyocardial injection therapy using experimental data. PloS One. 2014 Nov 19;9(11) doi: 10.1371/journal.pone.0113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desjardins B., Crawford T., Good E. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009 May;6(5):644–651. doi: 10.1016/j.hrthm.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letsas K.P., Efredmidis M., Vlachos K. Right ventricular outflow tract low-voltage areas identify the site of origin of idiopathic ventricular arrhythmias: a high-density mapping study. J Cardiovasc Electrophysiol. 2019:1–8. doi: 10.1111/jce.14155. [DOI] [PubMed] [Google Scholar]
- 26.Jiang R., Beaser A.D., Aziz Z. High-density grid catheter for detailed mapping of sinus rhythm and scar-related ventricular tachycardia: comparison with a linear duodecapolar catheter. JACC Clin Electrophysiol. 2020 Mar;6(3):311–323. doi: 10.1016/j.jacep.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Maagh P., Christoph A., Dopp H. High-density mapping in ventricular tachycardia ablation: a PentaRay® study. Cardiol Res. 2017 Dec;8(6):293–303. doi: 10.14740/cr636w. [DOI] [PMC free article] [PubMed] [Google Scholar]