Abstract
Purpose
The aim of this study was to investigate the association of dry eye disease (DED)–related signs and symptoms with two tear function tests.
Methods
This was a clinic-based, cross-sectional study with recruitment of consecutive participants. Schirmer test (ST), tear strip meniscometry (SM), and fluorescein tear breakup time were measured and corneal staining score was examined in outpatients at three clinics. Seven subjective symptoms were assessed by interview, including dryness, irritation, pain, lacrimation, fatigue, blurring, and photophobia. Statistical analyses included regression analysis and comparison tests.
Results
The mean age of the 210 participants was 61.2 ± 15.2 years (range, 12–91 years), with 135 women (64.3%) in the cohort. The mean ST value was 12.9 ± 9.3 (0–35) mm, and SM was 2.5 ± 1.6 (0–10) mm, with no difference between women and men. SM values were lower in the presence of irritation (P = 0.046) and photophobia (P = 0.011). Regression analysis revealed ST and SM values were strongly correlated (β = 0.255, P < 0.001). SM was significantly correlated with breakup time (β = 0.149, P = 0.032), whereas there was no correlation between ST and DED-related signs and symptoms.
Conclusions
SM was correlated with DED-related symptoms and breakup time, whereas ST was not. A low SM value could be an alternative clinical parameter to determine tear film–oriented therapy.
Translational Relevance
Tear strip meniscometry could be a useful tear function examination on a routine clinical basis since it is a 5-second noninvasive procedure and associated with subjective symptoms and the value of the conventional Schirmer test.
Keywords: dry eye, strip meniscometry, Schirmer test, tear breakup time
Introduction
A recent consensus report from the Asia Dry Eye Society1 stated the following agreed-on definition of dry eye: “Dry eye is a multifactorial disease characterized by unstable tear film causing a variety of symptoms and/or visual impairment, potentially accompanied by ocular surface damage.” It also emphasized tear film–oriented therapy for dry eye disease (DED) and the diagnostic significance of tear breakup time (BUT) to confirm tear film instability.2,3 BUT is basic information for the diagnosis of DED, and short BUT-type DED may lead to a deterioration of visual function4,5 as observed in Japanese office workers.6,7 Yokoi et al.8 classified breakup patterns to facilitate tear film–oriented therapy by selecting the appropriate eye drops for the five types of breakup patterns they described.
The tear function test is an essential examination for the diagnosis of DED and to determine the therapeutic strategy. The Schirmer test (ST), the most common test of tear secretion, is performed by inserting a strip of filter paper into the lower conjunctival cul-de-sac for 5 minutes to absorb tears produced by basic and reflex tearing.9 The ST does have some disadvantages, including irritation and conjunctival epithelial damage, and it is comparatively time-consuming. Tear strip meniscometry (SM) is an alternative to the ST, first introduced in 2006 to measure aqueous availability at the lower tear meniscus.10 As described in detail previously,10,11 in SM, the wetted length is indicated by a blue-stained line along the tear absorption path in the round-tipped strip (Fig. 1). When the strip is immersed in tears in the lower lateral tear meniscus, the tears enter the groove and turn the indicator blue. SM values had a statistically significant linear correlation with ST values, BUT, corneal staining score, and tear meniscus height measurement by anterior optical coherence tomography (OCT).11 SM has been used for numerous clinical and experimental studies,12–21 including in veterinary medicine22,23 and in a study where medical personnel self-examined seven times per day, revealing a diurnal variation in human tear meniscus volume.24
Figure 1.
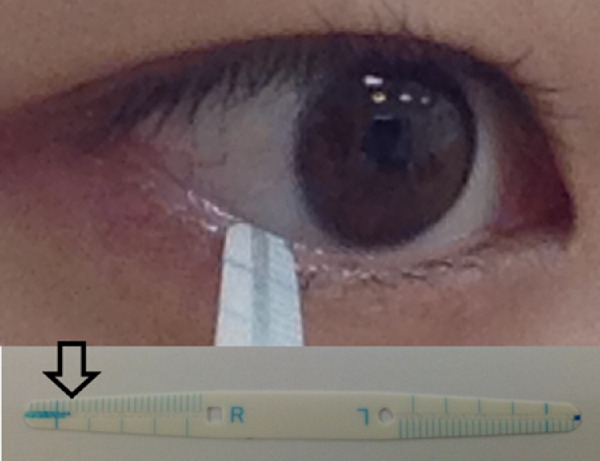
Tear SM. Aqueous availability in the lower meniscus is measured by a round-tipped strip. Wetted length is indicated by a blue-stained line along the tear absorption path (arrow).
DED practitioners often encounter discrepancies between signs and symptoms of DED,25,26 and SM could be a suitable alternative clinical indicator to ST if SM were associated with any signs and symptoms of DED. This study aimed to investigate the association of DED-related parameters with two tear function tests, ST and SM, to explore the clinical applicability of SM. The novelty of the present study includes a comparison between ST and SM with respect to DED-related symptoms and other ocular surface tests. Furthermore, a strength of the study is that it had a sample size double that of previous studies.10,11
Methods
Study Participants and Institutional Review Board Approval
The present hospital-based cross-sectional study was conducted at Otake Clinic Moon View Eye Center (Kanagawa, Japan), Komoro Kosei General Hospital (Nagano, Japan), and Tsukuba Central Hospital (Ibaraki, Japan). The study was carried out in accordance with the Declaration of Helsinki and approved by the institutional review boards and ethics committees of Kanagawa Medical Association, Komoro Kosei General Hospital, and Tsukuba Central Hospital. Informed consent was obtained from all participants.
Inclusion and Exclusion Criteria
The study recruited consecutive patients from the participating clinics and hospitals between April 2015 and May 2020. The inclusion criteria were outpatients with best-corrected visual acuity better than 20/30 in both eyes. The exclusion criteria were any ocular surgery within the previous month and any acute eye disease within the previous week. Consequently, the final study cohort predominantly comprised individuals visiting their clinic for a regular vision check, floaters, or a mild, unidentified ocular symptom.
Ophthalmologic Examinations
During the examination, temperature and humidity of the examination room were adjusted to 25°C to 27°C and 50% to 60%, respectively. The ophthalmologic examination was carried out according to work by Yokoi et al.8 and described elsewhere.27 Briefly, the fluorescein tear break-up time (FTBUT) was measured as the time interval between blink and the appearance of the first dark spot in the cornea. According to standardized DED evaluation,3 the fluorescein staining of tears was strictly monitored with no change in the subject's aqueous tear volume, after placing two drops of saline solution with a fluorescein test strip (Showa Yakuhin Co., Tokyo, Japan). The strip was applied gently to the central lower lid margin. We then asked the patient to close the eye gently and briskly open the eye after several natural blinks. The investigator determined the starting point of eye opening as well as confirmed the reproducibility of FTBUT by three successive observations, and the mean value was used for the current analyses.
A corneal staining score was used to determine corneal epitheliopathy, graded at 0 to 2 for severity and area. ST was performed without topical anesthesia. Strips of filter paper (Whatman No. 41; Showa Yakuhin Kako, Tokyo, Japan) were placed for 5 minutes at the temporal lower conjunctival fornix. The length of the wetted filter paper was recorded (mm). ST was performed at least 5 minutes after SM. The SM testing was performed by a certified orthoptist and board-certified ophthalmologist using SMTube (Echo Electricity Co., Ltd., Fukushima, Japan), which is a single-use strip dedicated to SM measurements. The examiner gently immersed the tip of the strip into the tear meniscus on the lateral side of the lower lid without touching the ocular surface and kept it in place for 5 seconds. The resting tear was rapidly absorbed into the column of the strip due to capillary action. The blue-colored indicator dye, initially impregnated at both ends of the strip, allowed clear visualization of the tear-wetting along the column. Values of the wetted length were read with the aid of the scale marks (mm) printed on the strip and recorded. The sensitivity and specificity of SM have been reported previously,11 and when the cutoff value of the SM result was set at ≤4 mm, the sensitivity and specificity of the SM procedure were 83.52% and 58.16%, respectively. Taken together with other investigations, the cutoff value of SM in the diagnosis of dry eye has been suggested at 4 or 5 mm.10,11,15
Patient Interviews for DE-Related Symptoms
Patients were asked questions to determine the presence or absence of seven common DE-related symptoms: dryness, irritation, pain, lacrimation, eye fatigue, blurring, and photophobia. These questions were selected from items on the Dry Eye–Related Quality-of-Life Score questionnaire28 and based on the seven most prevalent symptoms of DE in patients who had visited the Dry Eye Clinic in the Department of Ophthalmology at Keio University Hospital in 2014.
Statistical Analysis
Where appropriate, data are given as the mean ± SD. We analyzed the data from the right eye for BUT, corneal staining score, ST, and SM. Comparison of symptoms and ocular surface parameters between male and female participants, comparison of ST and SM in evaluating the presence or absence of ocular surface symptoms, and comparison of ST and SM with respect to phakic status and topical medication were made using the t-test for ocular surface parameters, ST values, and SM values and the χ2 test for the other parameters. To identify which ophthalmic parameters and symptoms were correlated with the tear function value, regression analysis and unpaired t-test were performed with potential symptoms, including ST and SM used as dependent variables, while demographic (age and sex) and ophthalmic parameters (BUT, SPK score, and symptoms) were used as independent variables. All analyses were performed using StatFlex (Atech, Osaka, Japan), with P < 0.05 being considered statistically significant.
Results
The mean age of the 210 participants was 61.2 ± 15.2 (range, 12–91) years, with 135 females (64.3%) in the cohort. The results of comparison of symptoms and ocular surface parameters between male and female participants are shown in Table 1. Mucin secretagogues were more often prescribed and BUT was shorter among females, whereas other ocular surface parameters and the prevalence of symptoms were similar in both sexes. The measured value of ST was 12.9 ± 9.3 (0–35) mm, and SM was 2.5 ± 1.6 (0–10) mm, with no difference between females and males.
Table 1.
Comparison of Symptoms and Ocular Surface Parameters Between Male and Female Participants
| Characteristic | All | Male | Female | P Value |
|---|---|---|---|---|
| Prevalence of symptoms (%) | ||||
| Dryness | 24.8 | 22.6 | 25.9 | 0.600 |
| Irritation | 23.8 | 17.3 | 27.4 | 0.101 |
| Pain | 13.8 | 9.3 | 16.3 | 0.161 |
| Lacrimation | 6.7 | 5.3 | 7.4 | 0.564 |
| Fatigue | 41.0 | 36.0 | 43.7 | 0.277 |
| Blurring | 32.4 | 36.0 | 30.4 | 0.403 |
| Photophobia | 27.6 | 25.3 | 28.9 | 0.581 |
| Ocular surface parameters (mean ± standard deviation) | ||||
| ST (mm) | 12.9 ± 9.3 | 12.1 ± 8.4 | 13.4 ± 9.8 | 0.311 |
| SM (mm) | 2.5 ± 1.6 | 2.5 ± 1.7 | 2.4 ± 1.5 | 0.755 |
| Corneal staining score | 0.43 ± 0.66 | 0.36 ± 0.58 | 0.47 ± 0.70 | 0.240 |
| BUT (s) | 3.1 ± 2.0 | 3.6 ± 2.0 | 2.7 ± 2.0 | <0.001a |
| Topical medication and phakic status (%) | ||||
| Topical medication (any) (n = 98) | 41.9 | 34.7 | 45.9 | 0.113 |
| Hyaluronate (n = 32) | 15.3 | 20.0 | 12.7 | 0.152 |
| Mucin secretagogue (n = 40) | 18.7 | 9.3 | 23.9 | 0.010a |
| Steroid (n = 5) | 2.4 | 1.3 | 3.0 | 0.458 |
| Glaucoma (n = 5) | 6.7 | 9.3 | 5.3 | 0.248 |
| IOL (n = 27) | 12.9 | 12.0 | 13.3 | 0.782 |
Eyedrops were hyaluronate (0.1% hyaluronate), mucin secretagogue (3% diquafosol; 2% rebamipide), steroid (prescribed as combination of 0.1% fluorometholone and 0.1% hyaluronate), 0.1% pranoprofen, and antiglaucoma (0.005% latanoprost; 0.004% travoprost, 0.5% timolol; 2% carteolol; mixed combination of 1% dorzolamide and 0.5% timolol; 0.4% ripasudil). IOL, intraocular lens.
P < 0.05, male versus female, χ2 test or t-test as appropriate.
The results of comparison of ST and SM in evaluating the presence or absence of ocular surface symptoms revealed the SM value was lower in the presence of irritation (P = 0.046) and photophobia (P = 0.011; Table 2). ST values were similar between the presence and absence of DED-related symptoms. The results of comparison of ST and SM with respect to phakic status and topical medication demonstrated the ST value was lower in participants prescribed with hyaluronate (P = 0.026) and mucin secretagogue (P = 0.007; Table 3). SM values were lower in participants prescribed with hyaluronate (P = 0.020). There was no difference in ST and SM values between nonglaucoma and glaucoma, as well as between phakic and pseudophakic eye groups.
Table 2.
Comparison of ST and SM in Evaluating the Presence or Absence of Ocular Surface Symptoms
| Characteristic | ST (mm) | SM (mm) | ||||
|---|---|---|---|---|---|---|
| Symptoms | Present | Absent | P Value | Present | Absent | P Value |
| Dryness | 13.1 ± 9.7 | 12.9 ± 9.2 | 0.454 | 3.0 ± 2.0 | 3.1 ± 2.0 | 0.392 |
| Irritation | 13.0 ± 9.2 | 13.0 ± 9.2 | 0.494 | 2.6 ± 1.8 | 3.2 ± 2.1 | 0.046a |
| Pain | 13.6 ± 9.4 | 12.9 ± 9.3 | 0.341 | 2.6 ± 2.1 | 3.1 ± 2.0 | 0.131 |
| Lacrimation | 15.1 ± 9.1 | 12.8 ± 9.3 | 0.207 | 2.7 ± 1.6 | 3.1 ± 2.1 | 0.238 |
| Fatigue | 13.1 ± 9.7 | 12.9 ± 9.4 | 0.442 | 3.2 ± 1.9 | 2.9 ± 2.1 | 0.191 |
| Blurring | 13.7 ± 9.1 | 12.7 ± 9.4 | 0.233 | 2.7 ± 1.6 | 3.2 ± 2.2 | 0.092 |
| Photophobia | 12.6 ± 9.7 | 13.1 ± 9.3 | 0.587 | 2.5 ±1.9 | 3.2 ± 2.1 | 0.011a |
Values are presented as mean ± standard deviation.
P < 0.05, unpaired t-test.
Table 3.
Comparison of ST and SM With Respect to Phakic Status and Topical Medication
| Characteristic | ST (mm) | P Value | SM (mm) | P Value |
|---|---|---|---|---|
| IOL (OD) (±) (n = 183/27) | 13.1 ± 9.4/12.0 ± 8.8 | 0.551 | 2.5 ± 1.6/2.2 ± 1.4 | 0.262 |
| No medication (n = 122) | 14.0 ± 9.8 | — | 2.6 ± 1.8 | — |
| Hyaluronate (n = 32) | 10.1 ± 8.2 | 0.026a | 2.0 ± 1.2 | 0.020a |
| Mucin secretagogue (n = 40) | 10.0 ± 7.2 | 0.007a | 2.3 ± 1.2 | 0.235 |
| Glaucoma (n = 14) | 11.4 ± 7.0 | 0.227 | 2.3 ± 1.7 | 0.468 |
Data are mean ± standard deviation. —, XXX.
P < 0.05, phakic versus IOL or versus no medication, unpaired t-test.
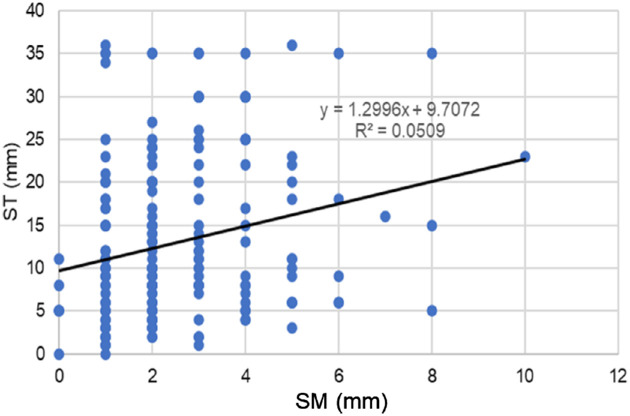
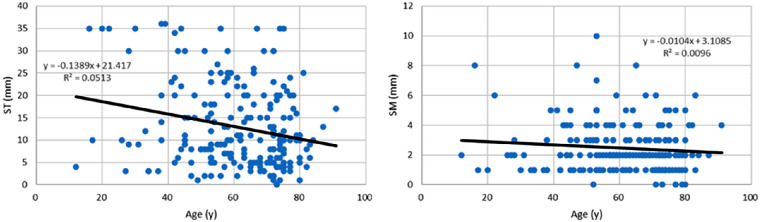
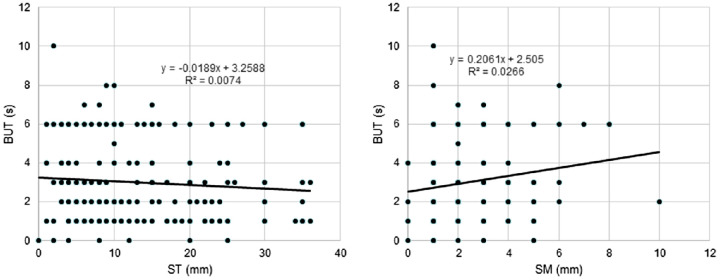
Regression analysis revealed ST and SM values were correlated (β = 0.255, P < 0.001; Table 4, Fig. 2). Age (β = ─0.229, P < 0.001) was correlated with ST (Fig. 3), but sex was not correlated with either ST or SM. SM was significantly correlated with BUT (β = 0.149, P = 0.032; Fig. 4), whereas there was no correlation between ST and DED-related signs and symptoms. ST and SM in short (≤5 mm, n = 161) and normal BUT (>5 mm, n = 49) groups were 13.1 ± 9.3 mm and 12.2 ± 9.4 mm (P = 0.556) for ST and 2.3 ± 1.4 mm and 3.0 ± 2.0 mm (P = 0.018) for SM.
Table 4.
Correlation Between Tear Function Tests and Ocular Surface Parameters
| ST | SM | |||
|---|---|---|---|---|
| Characteristic | β | P Value | β | P Value |
| Linear regression analysis | ||||
| Age | –0.229 (–0.361 to –0.097) | 0.001 | –0.065 (–0.201 to 0.071) | 0.336 |
| Sex | –0.067 (–0.201 to 0.067) | 0.339 | 0.062 (–0.074 to 0.198) | 0.360 |
| Ocular surface parameter | ||||
| BUT | –0.026 (–0.163 to 0.111) | 0.717 | 0.149 (0.012 to 0.286) | 0.031 |
| Corneal staining score | –0.076 (–0.207 to 0.055) | 0.272 | –0.050 (–0.183 to 0.083) | 0.460 |
| ST | — | — | 0.255 (0.118 to 0.392) | <0.001 |
| SM | 0.255 (0.122 to 0.388) | <0.001a | — | — |
| Multiple regression analysis | ||||
| Age | –0.201 (–0.333 to –0.069) | 0.003 | –0.060 (–0.196 to 0.075) | 0.383 |
| Sex | –0.066 (–0.200 to 0.067) | 0.329 | –0.008 (–0.144 to 0.128) | 0.906 |
| BUT | –0.099 (–0.236 to 0.037) | 0.154 | 0.191 (0.054 to 0.328) | 0.006 |
| Corneal staining score | –0.060 (–0.191 to 0.070) | 0.363 | 0.019 (–0.113 to 0.152) | 0.768 |
| ST | — | — | 0.228 (0.092 to 0.365) | 0.001 |
| SM | 0.223 (0.090 to 0.355) | 0.001 | — | — |
The 95% confidence interval is in parentheses.
P < 0.05, standardized partial regression coefficient.
Figure 2.
The correlation between the ST and SM values. The scatterplot of ST and SM values reveals a significant correlation (P < 0.001).
Figure 3.
Scatterplots of ST values (left), SM values (right), and age. ST was correlated with age (P = 0.001), and SM was not (P = 0.336).
Figure 4.
Scatterplots of ST values (left), SM values (right), and tear BUT. SM was correlated with BUT (P = 0.031), but ST was not (P = 0.717). Many symbols in the right panel are overlapping, and the number of data may not seem to match the number of participants.
Discussion
The present study investigated the association of SM with DED-related symptoms and revealed a significant relation with irritation and photophobia. The association of SM values with BUT could also contribute to explaining patients’ complaints in the clinical setting. A low SM value could be an additional clinical parameter to determine the prescription and nonmedical management of patients, although subjective symptoms may also be affected by corneal sensitivity, environment, climate, and various other factors29–32 in addition to ocular surface status. The present study was powered by a sufficient sample size and a wide range of patient ages, and it could contribute to a better understanding of tear film–oriented management of DED.
The minimally invasive and relatively quick SM examination minimizes reflex tearing, and the present study indicates tear meniscus volume was not age dependent, in contrast to ST. This is supported by previous investigations. Mishima et al.,33 using fluorophotometry to observe the decay of fluorescein instilled in the lower conjunctival cul-de-sac under a slit-lamp microscope, suggested that age dependency of ST may be due to decreased reflex tearing. Wang et al.34 measured upper and lower tear menisci, noninvasive tear breakup time, and ST using anterior OCT and found age was not correlated with estimated lower meniscus volume. In contrast, ST was not related to any parameters of tear menisci, volumes, or BUT, and ST was weakly correlated with age. No difference was demonstrated between females and males in another anterior OCT study.35 The current study is consistent with these previous studies, suggesting that SM values can be considered a good approximation to the tear meniscus parameters typically obtained by OCT. It is noteworthy that SM may be further advantageous and practical, since SM is much easier and less costly to perform, and can be readily performed at any clinic.
SM results showed a significant correlation with BUT values (P = 0.032), whereas ST values were not correlated with other ocular surface parameters. Significant correlations between BUT and tear meniscus parameters have been suggested previously.11,36,37 Based on OCT observation,34 BUT results were correlated with lower tear meniscus height and tear meniscus area measured during “normal” blinking (basal secretion) but not during “delayed” blinking (reflex secretion). The current findings are consistent with these reports, suggesting that SM results could reflect the tear meniscus parameters in association with tear film stability. Glaucoma medication and phakic status were not correlated with SM values, being consistent with previous reports on ST.38,39
Mucin secretagogue was more often prescribed for cases with low ST values but not with low SM values. Physicians are currently more familiar with ST than SM and may prioritize the prescription of eye drops for DED based on ST as an established examination. Another reason might be the range of measured values (ST, 0–35; ST, 0–10, in the present study), making determining a clinical cutoff value easier in ST than SM.
The current study demonstrated SM was correlated with certain DED-related parameters that ST was not, suggesting SM could be potentially useful for DED management. However, SM should be improved in several aspects. Compared with ST, clinical data to achieve availability, usefulness, and applicability are lacking with SM. Additionally, SM would become a more popular and reliable method if the technique were more accurate, stable, and standardized, especially for the cases with deformed lower eyelid, floppy eyelid syndrome, and ectropion. Finally, it should be noted that SM values do not directly quantify tear secretion since SM values represent a combination of freshly secreted tears and tears stored in the fornix.40
This study has several limitations. First, selection bias was not excluded since ST and SM were indicated for patients with suspected DED. Nevertheless, patients may have been suitably enrolled in the current study since the clinical results were reasonable, BUT was lower in females, and there was a similar prevalence of symptoms in both sexes. Although SM was correlated with other tests and symptoms in a clinic-based study with consecutive subjects, the results may not be conclusive due to a recruitment bias and participant heterogeneity, with some using dry eye medications and glaucoma medications involving several classes and combinations of glaucoma therapy. The small heterogeneous groups studied here may be partly responsible for the lack of correlation between SM and tested parameters. Second, SM measurements show diurnal variation24 and may vary depending upon season, climate, environment, medications, and systemic comorbidities. Therefore, further investigations are warranted to confirm the clinical applicability of SM. Third, the result of the regression analysis between SM and TBUT was nominal. Nevertheless, the current results may have clinical relevance since ST did not exhibit any significant results even though tear function was measured with ST and SM at the same session, and despite both ST and SM being vulnerable to numerous local and systemic factors and the environment. The difference in SM values between the presence and absence of symptoms was minimal. Although the clinical significance is somewhat limited, further larger studies are warranted to confirm the current results. Finally, DED-related examinations would be necessary to confirm the present results, including tear osmolarity, corneal sensitivity, visual function, and DED symptoms based on questionnaires. Additionally, a comparison of SM and automated tests, such as the noninvasive TFBUT and meniscometry with anterior OCT,11,15 would enhance the clinical potential of SM.
Acknowledgments
The authors thank Keiichi Miyasaka, PhD, for informative comments during the revision of the manuscript and Hiroshi Otake, MD, Tsutomu Sakai, MD, Aya Ohira, MD, and Miko Arai, CO, for help with data collection.
Disclosure: K. Negishi, None; M. Ayaki, None; M. Uchino, None; K. Takei, None; K. Tsubota, Tsubota Laboratory (P)
References
- 1. Tsubota K, Yokoi N, Watanabe H, et al.. A New perspective on dry eye classification: proposal by the Asia Dry Eye Society. Eye Contact Lens. 2020; 46(Suppl 1): S2–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kojima T, Dogru M, Kawashima M, Nakamura S, Tsubota K. Advances in the diagnosis and treatment of dry eye [published online January 29, 2020]. Prog Retin Eye Res. [DOI] [PubMed] [Google Scholar]
- 3. Yokoi N, Georgiev GA.. Tear film–oriented diagnosis and tear film–oriented therapy for dry eye based on tear film dynamics. Invest Ophthalmol Vis Sci. 2018; 59(14): DES13–DES22. [DOI] [PubMed] [Google Scholar]
- 4. Yokoi N, Uchino M, Uchino Y, et al.. Importance of tear film instability in dry eye disease in office workers using visual display terminals: the Osaka Study. Am J Ophthalmol. 2015; 159(4): 748–754. [DOI] [PubMed] [Google Scholar]
- 5. Uchino M, Yokoi N, Uchino Y, et al.. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013; 156(4): 759–766. [DOI] [PubMed] [Google Scholar]
- 6. Kaido M, Toda I, Oobayashi T, Kawashima M, Katada Y, Tsubota K. Reducing short-wavelength-blue light in dry eye patients with unstable tear film improves performance on tests of visual acuity. PLoS One 2016; 11: e0152936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koh S. Irregular astigmatism and higher-order aberrations in eyes with dry eye disease. Invest Ophthalmol Vis Sci. 2018; 59(14): DES36–DES40. [DOI] [PubMed] [Google Scholar]
- 8. Yokoi N, Georgiev GA, Kato H, et al. Classification of fluorescein breakup patterns: a novel method of differential diagnosis for dry eye. Am J Ophthalmol. 2017; 180: 72–85. [DOI] [PubMed] [Google Scholar]
- 9. Cho P, Yap M. Schirmer test, I: a review. Optom Vis Sci. 1993; 70: 152–156. [DOI] [PubMed] [Google Scholar]
- 10. Dogru M, Ishida K, Matsumoto Y, et al.. Strip meniscometry: a new and simple method of tear meniscus evaluation. Invest Ophthalmol Vis Sci. 2006; 47: 1895–1901. [DOI] [PubMed] [Google Scholar]
- 11. Ibrahim OMA, Dogru M, Ward SK, et al.. The efficacy, sensitivity, and specificity of strip meniscometry in conjunction with tear function tests in the assessment of tear meniscus. Invest Ophthalmol Vis Sci. 2011; 52: 2194–2198. [DOI] [PubMed] [Google Scholar]
- 12. Kojima T, Matsumoto Y, Ibrahim OMA, et al.. Effect of controlled adverse chamber environment exposure on tear functions in silicon hydrogel and hydrogel soft contact lens wearers. Invest Ophthalmol Vis Sci. 2011; 52: 8811–8817. [DOI] [PubMed] [Google Scholar]
- 13. Sano K, Kawashima M, Ikeura K, Arita R, Tsubota K. Abdominal breathing increases tear secretion in healthy women. Ocul Surf. 2015; 13: 82–87. [DOI] [PubMed] [Google Scholar]
- 14. Ishikawa S, Takeuchi M, Kato N. The combination of strip meniscometry and dry eye-related quality-of-life score is useful for dry eye screening during health checkup: cross-sectional study. Medicine (Baltimore). 2018; 97: e12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shinzawa M, Dogru M, Miyasaka K, Shimazaki J, Sekiryu T. Application of CASIA SS-1000 optical coherence tomography tear meniscus imaging in testing the efficacy of new strip meniscometry in dry eye diagnosis. Eye Contact Lens. 2018; 44(suppl 1): S44–S49. [DOI] [PubMed] [Google Scholar]
- 16. Lee KW, Kim JY, Chin HS, Seo KY, Kim TI, Jung JW. Assessment of the tear meniscus by strip meniscometry and keratograph in patients with dry eye disease according to the presence of meibomian gland dysfunction. Cornea. 2017; 36: 189–195. [DOI] [PubMed] [Google Scholar]
- 17. Shinzawa M, Dogru M, Miyasaka K, Kojima T, Tsubota K. The application of strip meniscometry to the evaluation of tear volume in mice. Invest Ophthalmol Vis Sci. 2019; 60: 2088–2091. [DOI] [PubMed] [Google Scholar]
- 18. Alshammeri S, Madden L, Hagan S, et al.. Strip meniscometry tube: a rapid method for assessing aqueous deficient dry eye. Clin Exp Optom. 2020; 103(4): 469–473. [DOI] [PubMed] [Google Scholar]
- 19. Ishikawa S, Shoji T, Yamada N, et al.. Efficacy of strip meniscometry for detecting lacrimal obstructive diseases among patients with epiphora. Transl Vis Sci Technol. 2019; 8(6): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osawa I, Esaka Y, Kojima T, Simsek C, Kudo H, Dogru M. Feasibility of strip meniscometry for tear volume evaluation in lacrimal passage obstruction. Diagnostics (Basel). 2020; 10(4): 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sagara H, Sekiryu T, Imaizumi K, Shintake H, Sugiyama U, Maehara H. Impact of tear metrics on the reliability of perimetry in patients with dry eye. PLoS One. 2019; 14(9): e0222467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyasaka K, Kazama Y, Iwashita H, Wakaiki S, Saito A. A novel strip meniscometry method for measuring aqueous tear volume in dogs: clinical correlations with the Schirmer tear and phenol red thread tests. Vet Ophthalmol. 2019; 22(6): 864–871. [DOI] [PubMed] [Google Scholar]
- 23. Rajaei SM, Ansari Mood M, Asadi F, et al.. Strip meniscometry in dogs, cats, and rabbits. Vet Ophthalmol. 2018; 21(2): 210–213. [DOI] [PubMed] [Google Scholar]
- 24. Ayaki M, Tachi N, Hashimoto Y, Kawashima M, Tsubota K, Negishi K. Diurnal variation of human tear meniscus volume measured with tear strip meniscometry self-examination. PLoS One. 2019; 14(4): e0215922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pflugfelder SC, Tseng SC, Sanabria O, et al.. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea 1998; 17: 38–56. [DOI] [PubMed] [Google Scholar]
- 26. Tesón M, Calonge M, Fernández I, Stern ME, González-García MJ. Characterization by Belmonte's gas esthesiometer of mechanical, chemical, and thermal corneal sensitivity thresholds in a normal population. Invest Ophthalmol Vis Sci. 2012; 53: 3154–3160. [DOI] [PubMed] [Google Scholar]
- 27. Ayaki M, Negishi K, Kawashima M, Uchino M, Kaido M, Tsubota K. Age is a determining factor of dry eye–related signs and symptoms. Diagnostics (Basel). 2020; 10(4): 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakane Y, Yamaguchi M, Yokoi N, et al.. Development and validation of the dry eye–related quality-of-life score questionnaire. JAMA Ophthalmol. 2013; 131: 1331–1338. [DOI] [PubMed] [Google Scholar]
- 29. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al.. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014; 157: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Uchino M, Nishiwaki Y, Michikawa T, et al.. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011; 118: 2361–2367. [DOI] [PubMed] [Google Scholar]
- 31. American Academy of Ophthalmology. Cornea/External Disease Panel Preferred Practice Pattern Guidelines: Dry Eye Syndrome 5–6. San Francisco, CA: American Academy of Ophthalmology; 2013. [Google Scholar]
- 32. Ayaki M, Kawashima M, Uchino M, Tsubota K, Negishi K. Possible association between subtypes of dry eye disease and seasonal variation. Clin Ophthalmol. 2017; 11: 1769–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mishima S, Gasset A, Klyce SD Jr, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol Vis Sci. 1966; 5(3): 264–276. [PubMed] [Google Scholar]
- 34. Wang J, Palakuru JR, Aquavella JV. Correlations among upper and lower tear menisci, noninvasive tear break-up time, and the Schirmer test. Am J Ophthalmol. 2008; 145(5): 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shen M, Wang J, Tao A, et al. Diurnal variation of upper and lower tear menisci. Am J Ophthalmol. 2008; 145(5): 801–806. [DOI] [PubMed] [Google Scholar]
- 36. Golding TR, Bruce AS, Mainstone JC. Relationship between tear-meniscus parameters and tear-film breakup. Cornea. 1997; 16(6): 649–661. [PubMed] [Google Scholar]
- 37. Mainstone JC, Bruce AS, Golding TR. Tear meniscus measurement in the diagnosis of dry eye. Curr Eye Res. 1996; 15(6): 653–661. [DOI] [PubMed] [Google Scholar]
- 38. Ra S, Ayaki M, Tsubota K, Negishi K. Dry eye, sleep quality, and mood status in glaucoma patients receiving prostaglandin monotherapy were comparable with those in non-glaucoma subjects. PLoS One. 2017; 12(11): e0188534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park DH, Chung JK, Seo DR, Lee SJ. Clinical effects and safety of 3% diquafosol ophthalmic solution for patients with dry eye after cataract surgery: a randomized controlled trial. Am J Ophthalmol. 2016; 163: 122–131. [DOI] [PubMed] [Google Scholar]
- 40. Huang Y, Sheha H, Tseng SCG. Conjunctivochalasis interferes with tear flow from fornix to tear meniscus. Ophthalmology. 2013; 120: 1681–1687. [DOI] [PubMed] [Google Scholar]