Abstract
Lung Ultra-Sound (LUS) can be very helpful at the diagnostic stage of COVID-19 pneumonia. We describe four clinical cases that summarize other helpful employment of LUS during the management of severe COVID-19 pneumonia with lung failure. LUS, together with clinical signs and arterial blood gases values, assists in guiding prompt clinical management of potential worsening of conditions. The monitoring of size and signs of aeration of consolidations is an important adjuvant in evaluating clinical evolution. The monitoring of LUS patterns can guide the management of non-invasive ventilation as well as the timing of CPAP weaning.
Keywords: Severe COVID-19 disease, Pneumonia, Lung ultrasound, Non-invasive ventilation
Abbreviations: ABG, Arterial Blood Gas; COVID-19, coronavirus disease 2019; CPAP, Positive Airway Pressure; HRTC, High-Resolution Computed Tomography; LUS, Lung Ultra Sound; P/F, ratio between arterial oxygen partial pressure (PaO2) and inspired oxygen fraction (FiO2); PEEP, Positive End Expiratory Pressure
1. Introduction
COVID-19 disease can evolve into a severe form with acute hypoxemic lung failure [1], about a week after illness onset [2]. The management of this phase is based on its early detection, administration of antiviral therapy (e.g. Remdesivir®) and/or immunomodulatory therapies (with cytokines inhibitors -e.g. Tocilizumab®- and steroids) [3], and on an optimal respiratory support, preferring non-invasive ventilation but not delaying the invasive support when required [4].
Lung Ultra-Sound (LUS) was already known to be helpful and accurate in diagnosing pneumonic alveolar-interstitial syndrome [5] and in the clinical management of critically ill patients [6]. In the specific context of COVID-19 pandemic, LUS has been well employed in the diagnostic stage [7], and during the invasive ventilation support to monitor the evolution of aeration [8].
LUS features of COVID-19 pneumonia correspond with that obtained with the gold standard Computed Tomography, although the sensitivity and specificity remain to be determined [9]. The injured lung patterns vary from mild to severe interstitial pattern (patchy single to confluent B lines, waterfall or light beam [10], white lung), to alveolar involvement with small and large consolidations that suggest hypo-ventilated or non-ventilated parenchyma. Air-bronchogram expresses the aeration entity. Pleural effusion is uncommon. Lesions are generally distributed to the posterior, inferior and peripheral portions of the lung.
We describe the management of four patients with COVID-19 pneumonia, confirmed by specific nasopharyngeal swab and by HRCT, admitted into our Sub-Intensive Care Unit, for underling the LUS added value to decision-making processes concerning medical treatment and to the management of non-invasive ventilation. All patients were admitted into our Sub-Intensive Care Unit for COVID-19 disease, from February to May 2020. Written informed consent was obtained from all patient. The sonographic examination was performed by a single experienced operator using a convex multifrequency transducer. Fourteen lung areas (3 posterior, 2 lateral and 2 anterior) were scanned per patient and was employed a lung severity score (LUS score) divided in three values (0 normal, 1 discontinuous pleural lines with B lines, 2 small consolidations or white lung, 3 large consolidations), with a minimum score of zero and maximum score of 42 [8]. Oxygen therapy is distinguished as low support when <6 l/min and high support when ≥6 l/min. SpO2 target of any ventilation support was ≥95%. Clinical and laboratory data of patients are showed in Table 1, Table 2.
Table 1.
Symptoms and clinical parameters at admission.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Symptoms | Dyspnea malaise dry cough anosmia |
asthenia fever dyspnea diarrhoea |
Dyspnea, fever | fever |
| Comorbidities | No | No | smoker, previous breast cancer | heart disease |
| Body mass Index | 31 | 29 | 27 | 20 |
| Temperature (°C) | 39 | 37.6 | 37 | 39 |
| Heart Rate HR (beats per minutes) | 75 | 77 | 96 | 77 |
| Blood Pressure (mmHg) | 110/80 | 120/75 | 110/80 | 129/70 |
| Respiratory Rate (breaths per minute) | 24 | 18 | 35 | 21 |
| Peripheral Oxygen Saturation (SpO2) | 97% | 95% | 88% | 96% |
| P/F ratio | 348 with low oxygen support | 311 with low oxygen support | 176 with high oxygen support | 184 with low oxygen flow support |
Table 2.
Laboratory Test results.
| Test | Haemoglobin | White blood cell | Lymphocytes | LDH | CRP | Ferritin | d-dimer | IL-6 |
|---|---|---|---|---|---|---|---|---|
| n.v. | 13-16 gr/dl | 4-10 cellx103/L | 1-4 cellx103/L | 135–225 U/L |
<5 mg/l | 30-400 mcg/L | <500 μg/L | <5.9 pg/ml |
| Case 1 | 15.8 | 9.2 | 0.95 | 253 | 6.8 | 1883 | 1609 | 488 |
| Case 2 | 12.2 | 3.05 | 0.63 | 235 | 6.3 | 262 | 862 | 32 |
| Case 3 | 12.3 | 7.81 | 1.1 | 352 | 66.5 | 671 | n.a. | 5.2 |
| Case 4 | 13.9 | 3.4 | 0.61 | 237 | 70 | 443 | 901 | 43.2 |
n.v: normal value; n.a: not available; CRP: C-reactive Protein.
1.1. Case 1: LUS aiding in decision-making
A 51-year-old man was admitted with two days history of symptoms. HRCT showed areas of crazy paving at both lungs. Ratio between arterial oxygen partial pressure and inspired oxygen fraction (FiO2) (P/F ratio) was 348 with low oxygen support. He was commenced on Hydroxychloroquine and thromboprophylaxis. Seven days after admission (day 9 since illness onset), LUS showed an evolution with diffuse B lines [Fig. 1a] and large consolidations at the lower lobes [Fig. 1b, Fig. 1cb–c]. LUS score of 31.
Fig. 1a.
Confluent B lines at the mid-axillary lines (“light beams”).
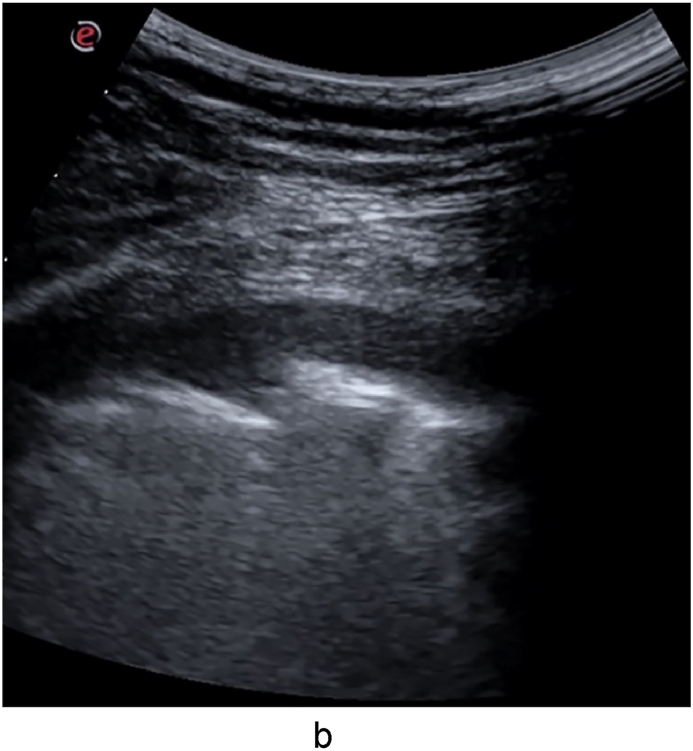
Fig. 1b.
Large hypoechoic and irregularly bordered consolidation in the left lower lobe (day 9).
Fig. 1c.
Large consolidation with partial irregular air-bronchogram (hyperechoic punctiform or linear images) on the left edge, in the right lower lobe (day 9).
Two days later the clinical features worsened, showing a P/F of 182.
The patient quickly received Tocilizumab and Positive Airway Pressure (CPAP) using a face mask (PEEP of 10–12 cmH20 and FiO2 up to 70%). CPAP was continued for 48 hours (stable P/F of 200), then delivered three-hours cycles/day (in the morning and in the afternoon) and all the night, with a gradual reduction of PEEP and FiO2 levels until its discontinuation.
Five days later (day 16), LUS showed wide appearance of air-bronchogram [Fig. 1d - Fig. 1e]. LUS score of 16. After two days, SpO2 above 95% on free air was obtained.
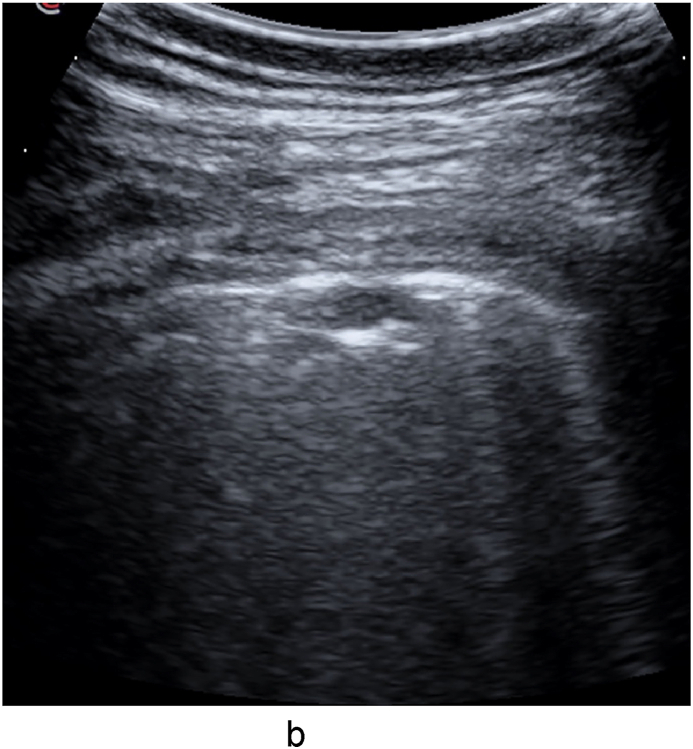
Fig. 1d.
Large consolidation with complete air-bronchogram on the left edge and a clear border, in the right lower lobe (day 16).
Fig. 1e.
Large consolidation with complete air-bronchogram, in the left lower lobe (day 16).
1.2. Considerations
LUS assisted in detecting early signs of worsening of lung pattern, and with worsening of P/F ratio, it helped in prompting decision making for the administration of immunomodulatory therapy and non-invasive ventilation support.
1.3. Case 2: LUS in guiding CPAP weaning
A 64-year-old woman was admitted with 8 days. HRTC showed a diffuse ground-glass pattern. P/F ratio was 311 with low oxygen support. She was commenced on Hydroxychloroquine and thromboprophylaxis. Two days later (day 10 since illness onset) the patient worsened, with P/F of 100. Helmet CPAP (PEEP of 12 cmH20 and FiO2 up to 70%) was administered and Dexamethasone (20 mg q24h for 5 days) was prescribed. Four days later (day 14), the patient was shifted to high oxygen flow achieving a P/F of 240, but she was still complaining of dyspnea. LUS revealed diffuse interstitial involvement [Fig. 2a], with a large consolidation at the right lower lobe [Fig. 2b]. Pleural effusion with lobar atelectasis was found at the left lower lobe [Fig. 2c]. LUS score of 22 with pleural effusion.
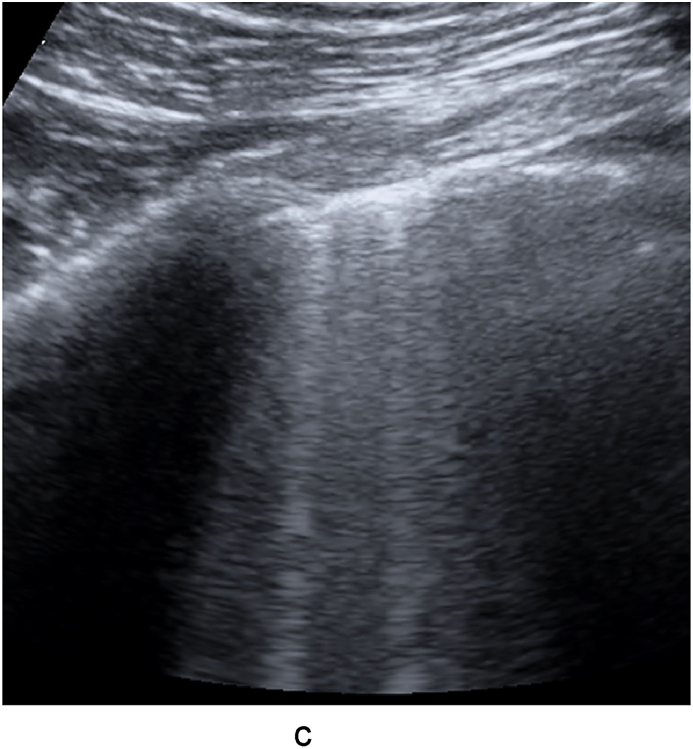
Fig. 2a.
Diffuse irregular pleural line, B lines and small consolidations.
Fig. 2b.
Large consolidation at the paravertebral line (L 54 mm x T 24 mm), in the right lower lobe (day 14).
Fig. 2c.
Pleural effusion in the left lower lobe.
CPAP was re-started, divided into three-hours cycles/day (in the morning, afternoon and evening).
After four days (day 18), the LUS examination showed B lines reduction and broader signs of air-bronchogram within the consolidations [Fig. 2d]. LUS score 16 with pleural effusion.
Fig. 2d.
Large consolidation with air-bronchograms (L 48 mm x T 18 mm), in the right lower lobe (day 18).
CPAP was continued for two days more, de-escalating the PEEP until discontinuation. Seven days later, LUS showed marked improvements with a minimal pleural effusion. LUS score of 8.
1.4. Considerations
LUS findings combined with clinical signs and P/F values, guided non-invasive ventilation weaning through intermittent CPAP de-escalation plan and PEEP titration.
1.5. Case 3: LUS for monitoring of consolidations
A 68-year-old woman was admitted with a seven days history of symptoms. HRCT showed bilateral ground-glass with consolidations at the lower lobes (embolism was excluded). P/F was 176 under high oxygen support.
She was started on helmet CPAP (PEEP of 10–14 cmH20 and FiO2 up to 60%); thromboprophylaxis and Dexamethasone (20 mg q24h for 5 days) were administered. After six days (day 13 after onset of symptoms), LUS highlighted the persistence of large consolidations at the lower lobes (right consolidation measured 90 × 33mm, the left 42 × 30 mm) [Fig. 3a Fig. 3b]. LUS score of 26.
Fig. 3a.
Large consolidation (Longitudinal 90 mm x Transverse 33 mm) at the paravertebral line, in the right lower lobe.
Fig. 3b.
Consolidation at the mid axillary line(L 42 mm x T 30mm), in the left lower lobe.
CPAP support continued, stopping it during meals. After four days (day 17), LUS revealed air-bronchogram signs within consolidations associated to a concurrent clinical and P/F improvement (P/F 276). LUS score of 24. Eight days after (day 25), the patient progressively became eupneic, consolidations showed reduction in size (right consolidation 66 × 21mm, left 24 × 30 mm) and increase in air-bronchogram (Fig. 3c - Fig. 3d). LUS score of 18.
Fig. 3c.
Smaller consolidation (L 66mmx T21mm) with more air-bronchograms at the paravertebral line, in the right lower lobe.
Fig. 3d.
Smaller consolidation (L 24 mm x T 30mm) at the mid-axillary line, in the left lower lobe.
1.6. Considerations
Monitoring of appearance of air-bronchogram and reduction in the extension of consolidations, confirmed evolution of re-aeration that correlates to disease progression and recovery.
1.7. Case 4: B lines pattern in guiding CPAP
A 70-year-old man presented with a four days history of fever. HRCT showed patchy ground glass. P/F was 184 with low oxygen flow. Hydroxychloroquine and thromboprophylaxis were started.
Four days later (day 8 since illness onset), the condition worsened showing a P/F of 174. On LUS, diffuse B lines with white lung at the bases [Fig. 4a] and small consolidations at the apices were visible [Fig. 4b]. LUS score of 25.
Fig. 4a.
Coalescent B lines with thickening of pleural line and irregular pattern, in the left lower lobe.
Fig. 4b.
B lines and small consolations in the right apex.
Tocilizumab was administered and the patient was supported with CPAP (PEEP 10–12.5 cmH20, FiO2 up to 70%). Seven days after (day 15), diffuse B lines and patchy white lung pattern persisted, without appearance of consolidations (LUS score of 21) [Fig. 4c – Fig. 4d] and stable P/F. PEEP was titrated until CPAP discontinuation and the patient's full recovery.
Fig. 4c.
Confluent B lines in the apex.
Fig. 4d.
Confluent B lines in the lower left lobe.
1.8. Considerations
B-lines persistence, without worsening of degree (stable LUS score) with a stable P/F ratio, supported the decision to continue non-invasive ventilation and guided titration of the PEEP.
2. Discussion
We purposed four main applications of LUS during the management of severe COVID-19 pneumonia in the Sub-Intensive Respiratory Unit.
Case series on LUS employment in COVID-19 disease, have primarily explored the diagnostic phase and the intensive care phase [9,[11], [12], [13]]. A monitoring role of LUS in the lung failure of COVID-19 in phase of Sub-Intensive care, is less investigated although its crucial role has been suggested [14].
Based on the evidence that an early intervention is the key to success, the first case underlines the crucial monitoring role of LUS, that integrates clinical signs, vital parameters and arterial blood gases values, to allow early detection of the evolution to severe pneumonia. Indicatively, a first examination should be performed within seven days from the illness onset or in case of clinical worsening.
In the second and fourth case, we showed the use of ultrasound to help in guiding the management of non-invasive ventilation, with CPAP helping to avoid intubation [15].
In COVID-19 pneumonia, hypoxia is due to ventilation-perfusion mismatch or intrapulmonary shunting [16], attempts at finding the optimal respiratory treatment protocol are widely discussed [17]. CT scan can detect and quantify the changes in the degree of pulmonary aeration but also LUS has been applied for this purpose in critically ill patients with ARDS [18], and it has been proposed for this evaluation in COVID-19 pneumonia [19]. Despite its high performance, for close monitoring of COVID-19 pneumonia patients, CT scan monitoring is unadvisable, due to the intrinsic biological risk, both in terms of infection control and increased radiation to the patient, while LUS looks like a compelling alternative and has been described to have high rates of sensitivity and specificity in assisting the clinician during diagnosis of the acute respiratory phase [20]. An algorithm has been proposed to combine ultrasound features of mild to severe patters, with respiratory rate and oxygenation index, to determining the requirement for intubation of COVID-19 patients with acute respiratory failure [13]. During mechanical ventilatory support and its weaning [21], LUS examination showed lung re-aeration mostly by the disappearance of B lines, as guide to the PEEP titration [22], and an ultrasound re-aeration score is validated [23]. The reduction in B lines, the resolution of consolidations or air-bronchogram reappearance suggest an improvement of respiratory gas exchange.
The monitoring of LUS features of aeration could guide CPAP treatment, by adjusting CPAP weaning time. This is useful to improve the patient's compliance to non-invasive ventilation, preventing the alveolar de-recruitment after CPAP suspension and leading to a gradual reduction in PEEP values. This requires serial LUS examinations, almost every 2–3 days, preferably not during CPAP support.
The third case underlines the utility of LUS in a qualitative monitoring of size of consolidations, and presence/absence of air-bronchogram. Clinical recovery usually comes before the complete resolution of lesions but the occurrence and enhancement of the air-bronchogram and the progressive shrinking of consolidations are signs of the improvement. The bidimensional shape of consolidations has a partial correspondence to their volume but it may suggest their evolution as well as may represent common lesions [5]. We suggest this assessment of consolidations every 4–7 days.
We chose one [8] of the proposed severity lung scores for COVID-19 pneumonia [24,25] which divides the lungs in 14 areas examined. We found it useful to allowing a better anatomical localization of pulmonary lesions when the back of patient is explorable, because the lesions have a typical posterior and gravitational position. However, the known LUS assessment in 12 regions remains as well accurate [26].
Combining the severity score with information regarding the presence of pleural effusion, atelectasis and a qualitative evaluation of consolidations, could give relevant information about illness evolution.
LUS evaluation has however some limitations in this context. Ultrasound lacks the panoramic vision, consolidations cannot rule out the coexistence of embolisms [27], although it has been described that Contrast-Enhanced Ultrasound may be of assistance [28].
Furthermore, LUS is operator dependent and it still lacks of a standardized anatomic evaluation as well as an ultrasound re-aeration score is not validated for non-invasive ventilation and LUS may underestimated lung recruitment. Prospective data with larger sample size that can be related to outcomes and management will be useful to explore these purposes of LUS employment in non-invasive ventilation therapy.
3. Conclusions
In severe COVID-19 pneumonia, LUS findings combined to clinical and arterial blood gases parameters, may be an important aid, in supporting the decision-making process, prompting timely intervention and tailoring non-invasive ventilation therapy.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Dr. Massimo Migani L. Guidotti Hospital Mutoko Zimbabwe.
References
- 1.Berlin D.A., Gulick R.M., Martinez F.J. Severe covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMcp2009575. https://doi: 10.1056/NEJMcp2009575 [DOI] [PubMed] [Google Scholar]
- 2.Siddaqi H.K., Mehra M.R. COVID-19 Illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. https://doi: 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Rhodes A., Cheng A.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19) J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.12839. https://doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 4.Alhazzani W., Moller M.H., Arabi Y.M. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit. Care Med. 2020;48(6):e440–e469. doi: 10.1097/CCM.0000000000004363. https://doi: 10.1007/s00134-020-06022-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtestein D.A. Ultrasound in the management of thoracic disease. Crit. Care Med. 2007;35(5):250–261. doi: 10.1097/01.CCM.0000260674.60761.85. https://doi: 10.1097/01 [DOI] [PubMed] [Google Scholar]
- 6.Arbelot C., Ferrari F., Bouhemad B., Rouby J.J. Lung ultrasound in acute respiratory distress syndrome and acute lung injury. Curr. Opin. Crit. Care. 2008;14(1):70–74. doi: 10.1097/MCC.0b013e3282f43d05. https://doi: 10.1097/MCC.0b013e3282f43d05 [DOI] [PubMed] [Google Scholar]
- 7.Volpicelli G., Lamorte A., Villén T. What's new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med. 2020;4:1–4. doi: 10.1007/s00134-020-06048-9. https://doi: 10.1007/s00134-020-06048-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soldati G., Smargiassi A., Inchingolo R. Proposal for international standardization of the use of lung ultrasound for patients with COVID-19. J. Ultrasound Med. 2020;39(7):1413–1419. doi: 10.1002/jum.15285. https://doi: 10.1002/jum.15285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Q.Y., Wang X.T., Zhang L.N. Chinese Cricital Care Ultrasound Study. Findings of lung ultrasonography of novel coronavirus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46(5):849–850. doi: 10.1007/s00134-020-05996-6. https://doi: 10.1007/s00134-020-05996-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpicelli G., Gargani L. Sonographic signs and patterns of COVID-19 Pneumonia. Ultrasound J. 2020;12(1):22. doi: 10.1186/s13089-020-00171-w. https://doi: 10.1186/s13089-020-00171-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y., Wang S., Zhang Y. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical novel coronavirus pneumonia (COVID-19) SSRN. 2020 doi: 10.2139/ssrn.3544750. [DOI] [Google Scholar]
- 12.Lomoro P., Verde F., Zerboni F. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020 doi: 10.1016/j.ejro.2020.100231. https://doi: 10.1016/j.ejro.2020.100231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denault A.Y., Delisle S., Canty D., Royse A., Royse C. A proposed lung ultrasound and phenotypic algorithm for the care of COVID-19 patients with acute respiratory failure. Can J Anesth/J Can Anesth. 2020;21:1–12. doi: 10.1007/s12630-020-01704-6. https://doi: 10.1097/MCC.0b013e3282f43d05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith M.J., Hayward S.A., Innes S.M., Miller A.S.C. Point-of-care lung ultrasound in patients with COVID-19–a narrative review. Anaesthesia. 2020 doi: 10.1111/anae.15082. https://doi: 10.1111/anae.15082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oranger M., Bermejo J.G., Dacosta-Noble P. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two period retrospective case-control study. Eur. Respir. J. 2020 doi: 10.1183/13993003.01692-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobin J.M. Basing respiratory management of coronavirus on physiological principles. Am. J. Respir. Crit. Care Med. 2020:1–9. doi: 10.1164/rccm.202004-1076ED. https://doi: 10.1164/rccm.202004-1076ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L., Chiumello D., Camporota L. COVID-19 pneumonia: different respiratory treatments for differents phenotypes? Intensive Care Med. 2020:1–4. doi: 10.1007/s00134-020-06033-2. https://doi: 10.1007/s00134-020-06033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouhemad B., Brisson H., Rouby J.J. Bedside Ultrasound assessement of positive end-expiratory pressure-induced lung recruitment. Am. J. Respir. Crit. Care Med. 2011;183:341–347. doi: 10.1164/rccm.201003-0369OC. https://doi: 10.1164/rccm.201003-0369OC [DOI] [PubMed] [Google Scholar]
- 19.Covissar D., Gibson L.E., Chang M.G. Application of lung ultrasound during the coronavirus disease 2019 pandemic: a narrative review. Anesth. Analg. 2020;131(2):345–350. doi: 10.1213/ANE.0000000000004929. http://doi: 10.1213/ANE.0000000000004929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtenstein D., Mezière G G. Relevance of lung ultrasound in the diagnosis of acute respiratory failure. The BLUE protocol. Chest. 2008;134(1):117–125. doi: 10.1378/chest.07-2800. https://doi: 10.1378/chest.07-2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayo P., Volpicelli G., Lerolle N., Dchreiber A. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med. 2016;42(7):1107–1117. doi: 10.1007/s00134-016-4245-3. https://doi: 10.1007/s00134-016-4245-3 [DOI] [PubMed] [Google Scholar]
- 22.Bouhemad B., Brisson H., Le-Guen M., Arbelot C. Bedside Ultrasound assessement of positive end-expiratory pressure-induced lung recruitment. Am. J. Respir. Crit. Care Med. 2011;183(3):341–347. doi: 10.1164/rccm.201003-0369OC. https://doi: 10.1097/CCM.0000000000003340 [DOI] [PubMed] [Google Scholar]
- 23.Bouhemad B., Liu Z.H., Arbelot C e C. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit. Care Med. 2010;38(1):84–92. doi: 10.1097/CCM.0b013e3181b08cdb. https://doi: 10.1097/CCM.0b013e3181b08cdb [DOI] [PubMed] [Google Scholar]
- 24.Prima linea covid-19 ecografia in urgenza. 2020. www.simeu.it
- 25.Nilam G.S., Nathason R., Proud K., Restrepo M. FOCUSED LUNG & CARDIAC POCUS EXAM in COVID-19, south TX veterans health care system and university of Texas health san antonio. http://www.chestnet.org/
- 26.Volpicelli G., Elbarbary M., Blaivas M. International liaison committee on lung ultrasound (ILC-LUS) for international consensus conference on lung ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 27.Zotzmann V., Lang C.N., Bamberg F., Bode C., Staudacher D.L. Are subpleural consolidations indicators for segmental pulmonary embolism in COVID-19? Intensive Care Med. 2020;46(6):1109–1110. doi: 10.1007/s00134-020-06044-z. https://doi: 10.1007/s00134-020-06044-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soldati G., Giannasi G., Smargiassi A., Inchingolo R., Demi L. Contrast-Enhanced Ultrasound in patients with COVID-19: pneumonia, acute respiratory distress syndrome, or something else? J. Ultrasound Med. 2020;10 doi: 10.1002/jum.15338. [DOI] [PMC free article] [PubMed] [Google Scholar]