Abstract
Middle ear osteoma is an extremely rare benign tumor of the middle ear. Due to its very slow growth rate and benign nature, osteoma of the middle ear can be found incidentally without causing any symptoms. The most common clinical signs are conductive hearing loss, the sense of fullness in the ear, tinnitus, and otorrhea. Small-sized osteomas can be misdiagnosed as otosclerosis without showing any signs other than conductive hearing loss. When the mass becomes very large, and symptoms caused by the tumor increase, treatment also becomes difficult. In this paper, we report a case of middle ear osteoma causing conductive hearing loss and effusion due to the effect of pressure on the middle ear ossicles and the Eustachian tube. We also present a review of the pertinent literature.
Keywords: Middle ear lesion, Middle ear osteoma, Conductive hearing loss, Temporal bone osteoma
1. Introduction
Osteomas are slow growing, benign tumors that are made of mature bone. In otorhinolaryngologic practice, the most common region of origin for these tumors is the paranasal sinuses with a point prevalence of 3% (Georgalas et al., 2011). Osteomas occur more often in men, with male-to-female ratio ranging from 1.3:1.0 to 1.5:1.0 (Georgalas et al., 2011).
Osteoma of the temporal bone is usually a single, unilateral tumor. It is most commonly found in the external ear canal and mastoid (Beale and Phelps, 1987; Shimizu et al., 2003). However, osteoma may also arise in the squamous part, internal auditory canal, glenoid fossa, Eustachian tube, petrous apex, or styloid process (Milroy et al., 1989; Greinwald and Simko, 1998; Kim et al., 2006; Yoon et al., 2014). Middle ear osteoma (MEO) is extremely rare (Viswanatha, 2011; Silver et al., 1993). The first description of MEO is attributed to Thomas in 1964 (Thomas, 1964). Here, we report a case of MEO that is causing unilateral middle ear effusion with Eustachian tube obliteration; we also present a review of the relevant literature (Table 1).
Table 1.
Reported osteomas of the middle ear since 2014.
| Author/year | Gender | Age | Side | Initial symptoms | Origin | Treatment | consequences |
|---|---|---|---|---|---|---|---|
| Chang/2014 | F | 14 | R | Aural fullnes and CHL | Promontory | Surgical removing + oosiculoplasty | Persistent CHL |
| Toro/2014 | F | 33 | L | CHL | promontory | Exploration but removal declined | Persistent CHL |
| Curtis/2014 | F | 49 | R | CHL + facial palsy + tinnitus | floor of middle ear | Surgical removing + ossiculoplasty | Facial palsy partially improved |
| Abouzayd/2015 | F | 56 | L | CHL | Posterior epitympanum | Surgical removing + oosiculoplasty | Reduced ABG |
| Molher/2018 | M | 23 | R | MHL | Fallopian canal | Watchful wait | Persistent hearing loss |
| Hamid/2018 | M | 7 | L | CHL | Promontory | Watchful wait | Persistent CHL |
| Gülşen/2019 | M | 21 | R | CHL + tinnitus | Promontory | Endoscopic surgery | Full recovery |
| This study/2019 | F | 25 | L | CHL and aural fullnes EOM |
Promontory | Surgical removing + ossiculoplasty | Reduced ABG |
M = male, F = female, R = right, L = left, CHL = conductive hearing loss, SSHL = sensroineural hearing loss, MHL = mixt hearing loss, ABG = air-bone gap, EOM = otitis media with effusion, (9.1%) showed mixed-type hearing loss, and four (9.1%) cases reported tinnitus. Some cases reported other symptoms, such as vestibular dysfunction, facial palsy, and aural fullness (Table 2) (Hornigold et al., 2003; McDonald and Vrabec, 1997; Ito et al., 1990).
2. Case report
Written informed consent was obtained from the patient for publication of this case report, and for any accompanying data.
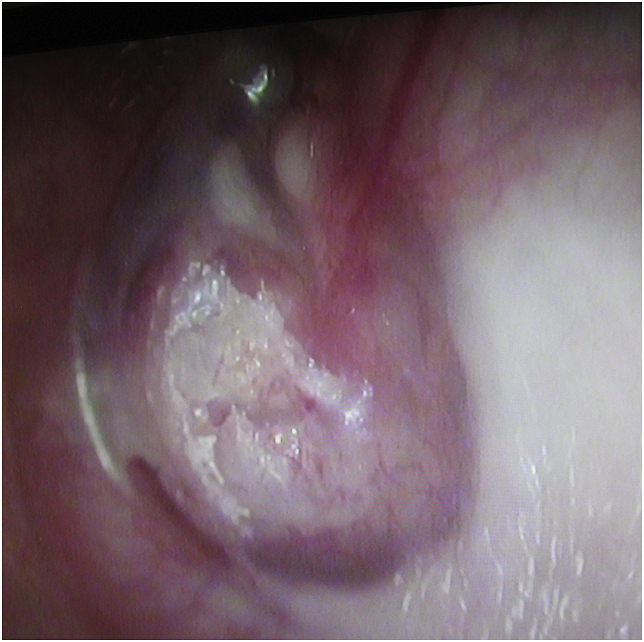
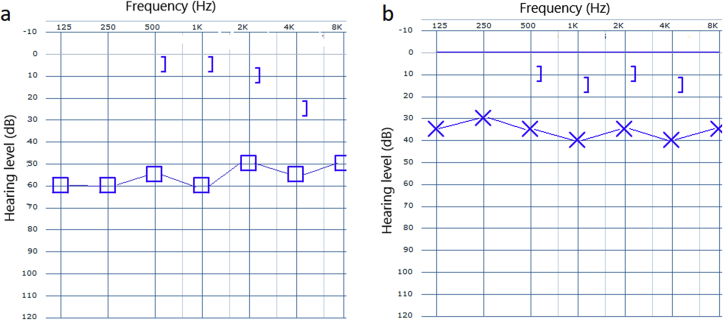
A 25-year-old female patient presented to our ENT clinic with complaints of increased hearing loss and fullness in her left ear. Her medical history revealed that her symptoms had been increasing for 7–8 years. We learned that a ventilation tube was applied to her left ear 6 years ago with the diagnosis of unilateral otitis media with effusion, but her complaints did not regress after surgery. Unfortunately, the patient did not provide any medical report or audiologic test result to suggest a clear picture of her auditory function before and after ventilation tube insertion. Considering the statement that the patient did not benefit from the operation, we predicted that the hearing loss at the time was not only due to effusion, meaning the existing pathology has been contributing to the hearing loss since then. The patient did not have any other otorhinolaryngologic disease. There was no family history of a similar disease. There was no other audiological or vestibular symptoms or signs. Physical examination revealed a white, rough mass in the left middle ear, perforating the anterior upper and lower quadrants of the eardrum and slightly expanding into the ear canal (Fig. 1). The mass pushed the inferior part of the manubrium to the posterior. The remaining part of the pars tensa was slightly vascularized and dull. The Weber test was left lateralized, and the Rinne test of the left ear was negative with a 512 Hz tuning fork. Pure tone audiometry showed a conductive hearing loss on the left side (pure tone threshold: 55 dB with a 45 dB air–bone gap) (Fig. 2a).
Fig. 1.
Otoendoscopic view shows an irregular white mass at the anterior part of the eardrum.
Fig. 2.
Graphs showing: (a) pre-operative pure tone audiometry result of the left ear, 55 dB air-conduction threshold with 45 dB air–bone gap; (b) pure tone audiometry result of the left ear after removal of the MEO and tympanoplasty, 37 dB air-conduction threshold with a 25 dB air–bone gap.
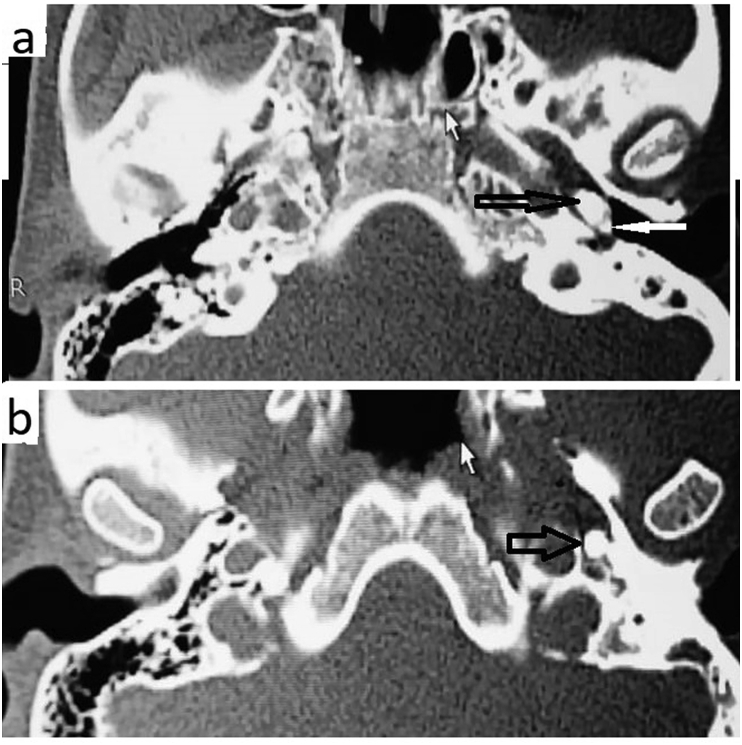
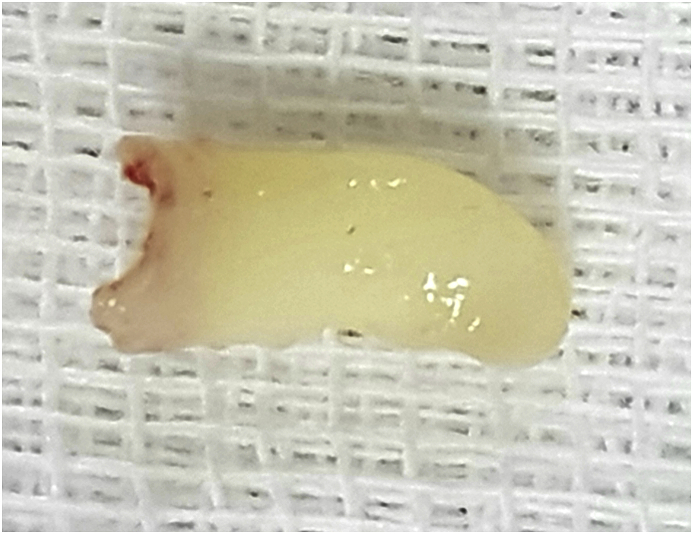
Temporal bone computed tomography (CT) was performed for differential diagnosis. The CT scan revealed a hyperdense homogeneous mass in the left middle ear, affecting the mesotympanum and the hypotympanum. The mass was totally calcified, with a density of 1265 HU (HU: Hounsfield unit). This calcified mass appeared to be irregularly-shaped (Fig. 3a) and involved the promontory, all of the three ossicles and the upper part of the Eustachian tube (Fig. 3b). The diagnosis of middle ear osteoma (MEO) was made, and the patient decided to undergo surgical treatment. Middle-ear exploration with microscopic retroauricular approach showed a bony mass in the middle ear, filling the hypotympanum, and partially filling the mesotympanum and protympanum, extending into the Eustachian tube, so it took the shape of the Eustachian tube (Fig. 4). The tumor completely occluded the Eustachian tube and caused effusion in the middle ear. The osteoma was pushing the inferior part of the malleus to the back part of the middle ear. The inferior part of the long crus of the incus and the lenticular process were eroded. No involvement of the adjacent middle ear structures, such as the stapes footplate, facial nerve, and round window, was observed. However, the osteoma was too large to be removed without damaging the adjacent structures. The incudomalleolar joint was separated and the malleus was pushed to the epitympanum. The superior and posterior portions of the osteoma were reduced with a diamond drill until the remainder became removable (Fig. 4). Ossiculoplasty was performed using a bone graft obtained from the suprameatal spine. Histopathological examination of the specimen showed normal bone tissue structures surrounded by fragments of lamellar bone, which is diagnostic for osteoma.
Fig. 3.
Axial computed tomographic images showing an osseous lesion in the left middle ear space (a) and the connection of the tumor to the malleus. The image b shows the osteoma inside the Eustachian tube. The black arrow indicates the osteoma. The white arrow indicates the malleus.
Fig. 4.
Photograph showing the remainder anterior part of the carved MEO. It seen that the part extending into the Eustachian tube has taken the shape of it.
There were no peri or post-operative complications during or after surgery. The postoperative second month physical and audiologic examinations were satisfactory (Fig. 2b).
3. Discussion
Osteomas of the temporal bone are rare tumors of unknown origin (Li et al., 2012). Thomas has suggested a genetic etiology in two cases occurring in siblings (Thomas, 1964). Kaplan et al. (1994) suggested that trauma and muscle traction may be the reason for this type of tumor. Alternatively, some osteomas are thought to inflammatory in origin (Shimizu et al., 2003; Harley and Berkowitz, 1997). However, the exact reason for the occurrence of osteoma is unknown.
Osteomas most commonly originate in the external auditory canal, arising from the tympanic bone (Yamasoba et al., 1990; Cho et al., 2005). MEO and inner ear osteoma are extremely rare (Tamir et al., 2015). In 2014, Yoon et al. (2014) published a literature review of 32 studies reporting on 36 cases of MEO, 2 of which they reported on. We also performed a literature search of the following databases on November 11, 2019: PubMed, Google Scholar, and Web of Science. We found that only 9 other cases have been reported since Yoon et al. published their findings in 2014 (Toro et al., 2014; Abouzayd and Seghir, 2015; Hamid et al., 2018; Curtis et al., 2014; Gülşen, 2019; Molher et al., 2018), and reported the 46th case of MEO (Table 1). Unfortunately, we were unable to obtain the full text of one paper in which 2 cases of MEO were reported.
Typically, MEO is unilateral. While it can be seen in people of any age, predominantly, it is seen in adolescents and young adults (Unal et al., 2000). Thus, 45% (20 of the 44 cases) of the participants in the available literature, including ours, ranged in age from 15 to 33. Unlike osteomas in other regions, male predominance is more significant in MEO, with 1:1.6 (Table 2) (Yoon et al., 2014; Chekhonina and Feigin, 1977; Cremers, 1985).
Table 2.
Demographic and clinical characteristics of the all reported and available middle ear osteomas.
| Middle ear osteoma | N | % |
|---|---|---|
| Total number | 44 | 100% |
| Male | 27 | 61,3% |
| Female | 17 | 38,6% |
| Age | 5–57 | |
| Median | 28 | |
| Mean | 28,5 | |
| Initial symptoms | ||
| Conductive hearing loss | 32 | 72,7% |
| Otitis media | 7 | 15,9% |
| Assymptomatic (incidental) | 6 | 13,6% |
| Tinnitus | 4 | 9,1% |
| Mixed hearing loss | 4 | 9,1% |
| Dizziness and vertigo | 2 | 4,5% |
| Aural fullnes | 1 | 2,3% |
| Facial palsy | 1 | 2,3% |
| Origin | ||
| Promontory | 17 | 38,6% |
| Ossicles | 8 | 18,1% |
| Epitympanum | 6 | 13,6% |
| Pyramidal process | 4 | 9,1% |
| Hypotympanum | 3 | 6,8% |
| Lateral semicircular canal | 3 | 6,8% |
| Facial nerve canal | 2 | 4,5% |
| Cochleariform process | 1 | 2,3% |
| Annulus | 1 | 2,3% |
| Aditus | 1 | 2,3% |
| Treatmen | ||
| Surgical treatment | 40 | 90,9% |
| Conservative management | 4 | 9,1% |
In our review of the current body of literature, we found that conductive hearing loss (72.7%) is the most frequent initial symptom. The second most common symptom was otitis media (may be acute, chronic or secretory) (Table 2), which may be due to Eustachian dysfunction and/or damaged tympanic membrane. Our patient also had a history of otitis media with effusion, seemingly due to Eustachian tube obstruction. We have seen that the osteoma originating from the promontory filled almost the entire tympanic cavity and extended into the Eustachian tube, even taking the shape of the Eustachian tube (Fig. 2). We assumed that the patient’s hearing loss was primarily due to the mass effect of the osteoma, which was pushing the inferior part of the malleus to the back. A 45 dB gap is too great to be explained by effusion alone. However, it is very difficult to distinguish how much of the hearing loss is related to effusion and how much is due to the mass effect.
On the other hand, MEO may be quite asymptomatic if there is no direct or indirect connection to the ossicular chain eardrum, oval and round windows, Eustachian tube or inner ear (Britt et al., 2000). Of the 44 cases reported, 6 (13.6%) were asymptomatic and found incidentally. Four patients.
These signs and symptoms were most likely due to the mass effect of the tumor, with ossicular chain fixation, tympanic membrane pressure, and obliteration of the round window (Yoon et al., 2014; Britt et al., 2000; Lefantzis, 2012).
The promontory, middle ear ossicles, and epitympanum were the most common sites of origin of MEOs (Table 2) (Jo et al., 2013; Jang and Cho, 2009; Ramadan, 1994). In most cases, computed tomography (CT) and/or exploratory tympanotomy was required for a definitive diagnosis. In a few cases, an osteoma was recognizable on an otoscopic examination due to expansion or perforation of the ear drum. In our case, the diagnosis was not difficult, as the mass perforated the eardrum and was visible in the otoscopic examination. However, we performed a CT scan to confirm the diagnosis and to evaluate the size and extent of the mass in order to discuss treatment options.
A CT scan of the temporal bone is the preferred diagnostic modality in cases of suspected osteomas, as it provides additional information on the oval window, roof of the tympanic cavity, cochlea, facial nerve, and middle ear ossicles. It can also shed light on other alterations that may not be clinically suspected (Gülşen, 2019). Histopathological confirmation is possible following surgical treatment. The typical histopathological features of osteomas are a few osteocytes or lacunae, including an excess number of fibrovascular channels, covered by lamellar bone tissue (Gülşen, 2019).
In the differential diagnosis of MEOs, the following should be considered: osteoid osteomas, fenestral otospongiosis, ossifying hemangiomas, benign osteoblastomas, otosclerosis, ossifying fibromas, fibrous dysplasia, osteochondromas, chondromas, calcified meningiomas, isolated eosinophilic granulomas, giant cell tumors, and malignant lesions (e.g., osteosarcoma sand osteoblastic metastasis) (Gülşen, 2019; Ben-Yaakov et al., 2006). Some previous studies indicated that an MEO may mimic otosclerosis when it does not affect the eardrum (Gülşen, 2019; Molher et al., 2018; Ramdoo et al., 2011). In the present review, of the 44 cases, 40 patients underwent surgical treatment, and only 4 patients were managed conservatively (Table 1, Table 2). (Yoon et al., 2014; Hamid et al., 2018; Gülşen, 2019; Molher et al., 2018; Barbosa et al., 2007). Depending on the localization and size of the mass as well as the surgeon’s preference, microscopic retroauricular or endaural and endoscopic transcanal approaches can be used to remove the MEO. In the literature, most surgeons used a microscopic retroauricular approach and a diamond burr to mobilize and remove the MEO. Some surgeons reported that they used curettes or a bone chisel (Yoon et al., 2014; Gülşen, 2019).
Some authors have argued that asymptomatic MEOs can be managed without surgery. However, others advocate that even asymptomatic cases should be treated surgically, as treatment becomes difficult, and risks increase along with the tumor volume (Yoon et al., 2014). Curtis et al. (2014) reported a case of surgically treated MEO with facial palsy at House–Brackmann grade IV. They reported that the facial palsy improved just up to grade II after surgery (Curtis et al., 2014). Chang et al. (2014) reported that conductive hearing loss persisted, despite successful removal of the MEO and reconstruction of the ossicular chain, and stated that this was due to obliteration and loss of flexibility of the round window (Chang et al., 2014; Glasscock et al., 1987). Molher et al. (2018) described a case of a large sized osteoma, which originated from the promontory and grew up to the fallopian canal. They reported that surgical removal was denied due to the very high risk of facial nerve and inner ear damage (Molher et al., 2018). In our case, in the early stages of the disease, the MEO apparently caused effusion by closing the Eustachian tube. By the time the patient was admitted to our hospital, the mass was very large. The surgery lasted 3 h and was not without risks. As the mass was so large, it had to be drilled in the middle ear to enable safely removing. Small-sized middle ear osteomas can be removed by endoscopic approach (Gülşen, 2019). In our case, due to the large size of the osteoma, microscopic surgery using a retroauricular approach was preferred.
4. Conclusion
In conclusion, MEOs are extremely rare and occur mainly at a young age, with male predominance. The most common initial symptom is conductive hearing loss. However, an MEO may not cause any symptoms in the initial stage when the mass is small. Although some authors contend that asymptomatic cases can be followed conservatively, we believe that the tumor should be removed before it becomes too large and difficult to manage.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Declaration of competing interest
The authors have no have conflict of interest and financial obligations to disclose related to this manuscript.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
References
- Abouzayd M., Seghir A. Epitympanic osteoma of the middle ear: a case report and literature review. Rev. Laryngol. Otol. Rhinol. 2015;136(2):81–83. [PubMed] [Google Scholar]
- Barbosa C.V., Rocba Santos M.A., Becker H.M.G. Osteoma of the middle ear. Rev. Bras. Otorrinolaringol. 2007;73:719. doi: 10.1016/S1808-8694(15)30137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale D.J., Phelps P.D. Osteomas of the temporal bone: a report of three cases. Clin. Radiol. 1987;38:67–69. doi: 10.1016/s0009-9260(87)80411-7. [DOI] [PubMed] [Google Scholar]
- Ben-Yaakov A., Wohlgelernter J., Gross M. Osteoma of the lateral semicircular canal. Acta Otolaryngol. 2006;126:1005–1007. doi: 10.1080/00016480500527292. [DOI] [PubMed] [Google Scholar]
- Britt J.C., Hood R.J., Hashisaki G.T. Round window obliteration byosteoma of the middle ear. Otolaryngol. Head Neck Surg. 2000;123:514–515. doi: 10.1067/mhn.2000.106195. [DOI] [PubMed] [Google Scholar]
- Chang T.S., Lai W.S., Kuo C.Y., Wang C.H. Osteoma of the middle ear. Ear Nose Throat J. 2014 Oct-Nov;93(10-11):E43. doi: 10.1177/014556131409310-1104. [DOI] [PubMed] [Google Scholar]
- Chekhonina E.M., Feigin G.A. Bilateral osteomas of the tympanic cavities. Vestn. Otorinolaringol. 1977;4:80–81. [PubMed] [Google Scholar]
- Cho Y.S., Kim J.H., Hong S.H. A huge osteoma of the middle ear. Int. J. Pediatr. Otorhinolaryngol. 2005;69:1569–1574. doi: 10.1016/j.ijporl.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Cremers C.W. Osteoma of the middle ear. J. Laryngol. Otol. 1985;99:383–386. doi: 10.1017/s0022215100096882. [DOI] [PubMed] [Google Scholar]
- Curtis K., Bance M., Carter M., Hong P. Middle ear osteoma causing progressive facial nerve weakness: a case report. J. Med. Case Rep. 2014;18:310. doi: 10.1186/1752-1947-8-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgalas C., Goudakos J., Fokkens W.J. Osteoma of the skull base and sinuses. Otolaryngol. Clin. 2011;44:875–890. doi: 10.1016/j.otc.2011.06.008. vii. [DOI] [PubMed] [Google Scholar]
- Glasscock M.E., III, McKennan K.X., Levine S.C. Osteoma of the middle ear: a case report. Otolaryngol. Head Neck Surg. 1987;97:64–65. doi: 10.1177/019459988709700111. [DOI] [PubMed] [Google Scholar]
- Greinwald J.H., Jr., Simko E.J. Diagnosis and management of middle ear osteomas: a case report and literature review. Ear Nose Throat J. 1998;77(13–136):138–139. [PubMed] [Google Scholar]
- Gülşen S. Promontory osteoma mimicking otosclerosis: case report. J. Laryngol. Otol. 2019 Sep 27:1–3. doi: 10.1017/S0022215119002007. [DOI] [PubMed] [Google Scholar]
- Hamid Ossama, Abdelhamid Amr Ossama, Taha Togan. Middle ear osteoma: case report and review of literature. J. Otolaryngol. ENT Res. 2018;10(6):325–326. [Google Scholar]
- Harley E.H., Berkowitz R.G. Osteoma of the middle ear. Ann. Otol. Rhinol. Laryngol. 1997;106:714–715. doi: 10.1177/000348949710600819. [DOI] [PubMed] [Google Scholar]
- Hornigold R., Pearch J.B., Gleeson J.M. An osteoma of the middle ear presenting with the Tulio phenomenon. Skull Base. 2003;13:113–117. doi: 10.1055/s-2003-820567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Tanaka H., Hirano M. Osteoma of the middle ear. J. Laryngol. Otol. 1990;104:803–806. doi: 10.1017/s0022215100113945. [DOI] [PubMed] [Google Scholar]
- Jang C.H., Cho Y.B. Osteoma of the incus with congenital cholesteatoma: a case report. Auris Nasus Larynx. 2009;36:349–352. doi: 10.1016/j.anl.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Jo S.Y., Park H.J., Cho Y.B. A case of incudal osteoma accompanied by primary acquired cholesteatoma. Kor. J. Otorhinolaryngol. Head Neck Surg. 2013;56:306–309. [Google Scholar]
- Kaplan I., Calderón S., Buchner A. Peripheral osteoma of the mandible: a study of 10 new cases and analysis of the literature. J. Oral maxillofacial Surg. 1994;52:467–470. doi: 10.1016/0278-2391(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Kim C.W., Oh S.J., Kang J.M. Multiple osteomas in the middle ear. Eur. Arch. Oto-Rhino-Laryngol. 2006;263:1151–1154. doi: 10.1007/s00405-006-0123-x. [DOI] [PubMed] [Google Scholar]
- Lefantzis D. Bilateral middle ear osteomas. Otorhinolaryngol. Head Neck Surg. 2012;47:42–45. [Google Scholar]
- Li Y., Li Q., Gong S. Multiple osteomas in middle ear. Case Rep. Otolaryngol. 2012;2012:1–3. doi: 10.1155/2012/685932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald K.R., Vrabec J.T. Synchronous middle ear osteoma and adenoma. Ear Nose Throat J. 1997;76:866–869. [PubMed] [Google Scholar]
- Milroy C.M., Phelps P.D., Michaels L. Osteoma of the incus. J. Otolaryngol. 1989;18:226–822. [PubMed] [Google Scholar]
- Molher J., Pujol E.M.D., Zounon A.D.S., Darrouzet V., Bonnard D. Middle ear osteoma causing mixed hearing loss: a case report. J. Int. Adv. Otol. 2018 Dec;14(3):493–496. doi: 10.5152/iao.2018.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan H.H. Osteoma of the malleus. Am. J. Otol. 1994;15:807–809. [PubMed] [Google Scholar]
- Ramdoo K., Dale O.T., Heredman R.C. Malleo-incudal osteoma: an unexpected finding during surgery for presumed otosclerosis. J. Laryngol. Otol. 2011;125:968–969. doi: 10.1017/S002221511100168X. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kosuke O., Yuichi M. Osteoma of the malleus: a case report and literature review. Am. J. Otol. 2003;24:239–241. doi: 10.1016/s0196-0709(03)00023-1. [DOI] [PubMed] [Google Scholar]
- Silver F.M., Orobello P.W., Jr., Mangal A. Asymptomatic osteomas of the middle ear. Am. J. Otol. 1993;14:189–190. [PubMed] [Google Scholar]
- Tamir S.O., Cyna-Gorse F., Sterkers O. Internal auditory canal osteoma: case report and review of the literature. Ear Nose Throat J. 2015 Jun;94(6):E23–E25. doi: 10.1177/014556131509400616. [DOI] [PubMed] [Google Scholar]
- Thomas R. Familial osteoma of the middle ear. J. Laryngol. Otol. 1964;78:805–807. doi: 10.1017/s0022215100062794. [DOI] [PubMed] [Google Scholar]
- Toro P.C., Castillo A.C., Martínez R.M. Middle ear promontory osteoma. Am. J. Otolaryngol. 2014;35(5):626–627. doi: 10.1016/j.amjoto.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Unal O.F., Tosun F., Yetiser S. Osteoma of the middle ear. Int. J. Pediatr. Otorhinolaryngol. 2000;15:193–195. doi: 10.1016/s0165-5876(00)00286-x. [DOI] [PubMed] [Google Scholar]
- Viswanatha B. Characteristics of osteoma of the temporal bone in young adolescents. Ear Nose Throat J. 2011 Feb;90(2):72–79. doi: 10.1177/014556131109000207. [DOI] [PubMed] [Google Scholar]
- Yamasoba T., Harada T., Okuno T. Osteoma of the middle ear. Report of a case. Arch. Otolaryngol. Head Neck Surg. 1990;116:1214–1216. doi: 10.1001/archotol.1990.01870100108025. [DOI] [PubMed] [Google Scholar]
- Yoon Y.S., Yoon Y.J., Lee E.J. Incidentally detected middle ear osteoma: two case reports and literature review. Am. J. Otolaryngol. 2014;35:524–528. doi: 10.1016/j.amjoto.2014.03.010. [DOI] [PubMed] [Google Scholar]