Abstract
Objective
To investigate the role of the periotic mesenchyme (POM) in the development of sensory cells of developing auditory epithelium.
Methods
Developing auditory epithelium with or without periotic mesenchyme was isolated from mice at embryonic days 11.5 (E11.5), E12.5 and E13.5, respectively, and cultured in vitro to an equivalent of E18.5’s epithelium in vivo. Then, the explants were co-stained with antibodies targeting myosin VIIA, Sox2 and BrdU.
Results
More hair cells in E11.5 + 7 DIV, E12.5 + 6 DIV and E13.5 + 5 DIV auditory epithelia were found upon culture with POM (225.90 ± 62.44, 476.94 ± 100.81, and 1386.60 ± 202.38, respectively) compared with the non-POM group (68.17 ± 23.74, 205.00 ± 44.23, and 1266.80 ± 38.84, respectively). Moreover, regardless of developmental stage, the mesenchymal tissue increased the amount of cochlear sensory cells as well as the ratio of differentiated hair cells to total sensory cells.
Conclusions
The periotic mesenchyme promotes the development of cochlear sensory cells, and its effect depends on the developmental stage of the auditory epithelium.
Keywords: Inner ear, Cochlea, Sensory cells, Mesenchyme, Development
Abbreviations: POM, Periotic mesenchyme
1. Introduction
Sensory hair cells that contain mechanotranducing hair bundles extending into the inner ear’s endolymph are important in hearing and balance. Inner ear generation requires multiple cell fate determination and morphogenetic processes to follow specific temporal and spatial patterns and attain its mature shape at E18.5 in mice (Fuchs and Tucker, 2015; Driver et al., 2017). Various inducing signals from the adjacent mesenchyme and the developing hindbrain highly contribute to regulating inner ear development (Ladher, 2017). Indeed, the mesenchyme is necessary for the proliferation and differentiation of mouse inner ear’s precursor cells into hair cells (Huh et al., 2015). These signals begin as the surface ectoderm beside the anterior hindbrain thickens to form the otic placode. The periotic mesenchyme (POM) also exerts an important regulatory effect on spiral ganglion neuron development (Coate et al., 2012; Fritzsch et al., 2015; Brooks et al., 2020). However, the mechanisms and developmental timing of events determining the cell fate during inner ear formation and how they are related to the POM are largely unknown.
Single-cell in vitro studies suggested that precursor cell differentiation into hair cells requires the POM, as its presence in the culture system induces cell growth and proliferation (Doetzlhofer et al., 2004). However, the absence of the typical stereoscopic microenvironment and related regulatory factors prevents cells from completely expressing the necessary gene products required for differentiation, including factors involved in Notch signaling. The Notch pathway has a key function in inner ear’s hair cell differentiation, and disturbing this signaling cascade could lead to chimerism in the morphology and function of individual sensory precursor cells (Munnamalai and Fekete, 2016; Shaya et al., 2017). The expression of Sox2, which is considered a presumptive cochlear sensory cell marker, also greatly affects sensory cell differentiation (Bok et al., 2007). Various reports also indicates a link between the Notch pathway and Sox2 amounts in inner ear development in both mice (Dabdoub et al., 2008; Pan et al., 2013; Liu et al., 2018) and chicken (Neves et al., 2011). Meanwhile, the roles of the adjacent mesenchyme in these signaling pathways, as well as the subsequent sensory cell development, have not been fully clarified.
In this study, auditory epithelia were isolated from embryonic mice and cultured with or without POM to investigate the effects of the mesenchymal tissue on cochlear sensory epithelium differentiation. We found that Sox2 expression in sensory epithelium was upregulated by culture with POM compared with samples cultured without POM. In addition, the sensory epithelium cultured with POM had a higher trans-differential ratio from precursors to hair cells and showed more hair cells subsequently. These results suggest that POM promotes sensory epithelium morphogenesis, and induces hair cell differentiation and formation.
2. Materials and methods
2.1. Mice
Cochlear sensory epithelial specimens were initially obtained from embryos of pregnant C57BL/6J mice under anesthesia at E11.5, E12.5, and E13.5, respectively, as previously described (Liu et al., 2011). The day of vaginal plug detection was considered E0.5. The mice were provided by the Laboratory Animal Center at the Fudan University Medical College. Experiments involving animals had approval from the Animal Ethical Committee of Yijishan Hospital, and followed current national and international guidelines.
2.2. Tissue dissection and culture
Embryos were collected after euthanasia. Under a dissecting microscope, the otocyst was exposed, the surrounding tissues were separated, and the vestibular membrane was cut off, to completely expose the cochlear auditory epithelium. Then, the whole auditory epithelium was carefully separated with a special tungsten needle. In this case, a thin layer of the mesenchymal tissue adjacent to the auditory epithelium was preserved. Alternatively, the whole developing auditory epithelium was completely dissociated from the mesenchyme by the special tungsten needle after incubation at 37 °C for 20 min with 0.25 mg/L Thermolysin (Invitrogen, USA). The isolated primary cochlear sensory epithelia with or without periotic mesenchyme were carefully placed on poly-l-ornithine-treated (Sigma, USA) coverslips in 35-mm dishes containing 100 μl DMEM/high glucose with 10% fetal calf serum (FCS) for 30 min on ice followed by 2 h of incubation at 37 °C. After washing, these explants underwent culture in 1.5 ml DMEM/F12 medium (mixed 1:1) without serum but containing N2 and B27 (Invitrogen). The culture continued until epithelia likened those of E18.5 in vivo (termed E11.5 + 7 DIV, E12.5 + 6 DIV, and E13.5 + 5 DIV, respectively). The explants were then harvested, fixed overnight with 4% paraformaldehyde, and prepared for immunochemical staining and quantitative analysis.
2.3. Immunohistochemistry
To evaluate the expression levels of inner ear cell differentiation markers, immunohistochemistry was performed with polyclonal anti-myosin VIIA (Proteus Biosciences, CA), Sox2 (Y-17) goat polyclonal IgG (Santa Cruz, USA), and monoclonal anti-bromodeoxyuridine (BrdU; Sigma) antibodies. Staining was carried out as reported in a previous study (Liu et al., 2011). In brief, nonspecific binding sites on the cover slip-bound cells were blocked (0.5 h) with a buffer containing 0.1% TritonX-100, 1% BSA (w/v), and 5% (v/v) heat-inactivated goat serum in PBS (PBT1). This was followed by incubation at 4 °C with respective primary antibodies diluted in PBT1 (1:200) overnight. To evaluate proliferation using BrdU labeling, DNA in samples was denatured with 2 N HCl in 0.1% TritonX-100 (30 min). The samples were washed twice with PBT1, and further washed with PBT2 (PBT1 with serum omitted) at ambient. Then, incubation was performed for 1 h with tetra-methyl rhodamine (TRITC)-linked goat anti-mouse (Jackson ImmunoResearch, USA) and Alexa Fluor 488-linked donkey anti-rabbit/goat IgG (Molecular Probes, USA) antibodies (1:200 in PBS). After three washes with PBS for 15 min each, a Leica SP5 confocal microscope or a Nikon FluoView system was employed for analysis, and images were processed using Photoshop. Negative controls were run with no primary antibody.
2.4. Quantitation of Sox2-and myosin VIIA-positive cells
Sox2 is a presumptive cochlear sensory cell marker (Bok et al., 2007) found in developing hair cells and supporting cells (Kiernan et al., 2005; Liu et al., 2018). In contrast, myosin VIIA represents a biomarker of differentiated hair cells. To evaluate POM’s effects on the amounts and ratio of hair- and presumptive sensory-cells, cells staining for Sox2-and myosin VIIA were assessed in the primary sensory epithelia cultured with and without POM, respectively.
2.5. Cell proliferation
Prior to incubating the proliferating cells with the BrdU antibody described above, 6 μg/ml of BrdU was added to the media of developing auditory epithelia cultured with or without POM for the whole culture process. This chemical is known to be incorporated into actively proliferating cells, and can subsequently be immunochemically detected. Double-immunofluorescent staining for BrdU and myosin VIIA was carried out for identifying newly formed hair cells.
2.6. Statistical analysis
The amounts of Sox2-, myosin VIIA-, and BrdU-positive cells were compared by the Student’s t-test. Data are mean ± standard error of the mean (SEM). P < 0.05 indicated statistical significance.
3. Results
3.1. The mesenchymal tissue is necessary for the cochlear prosensory formation
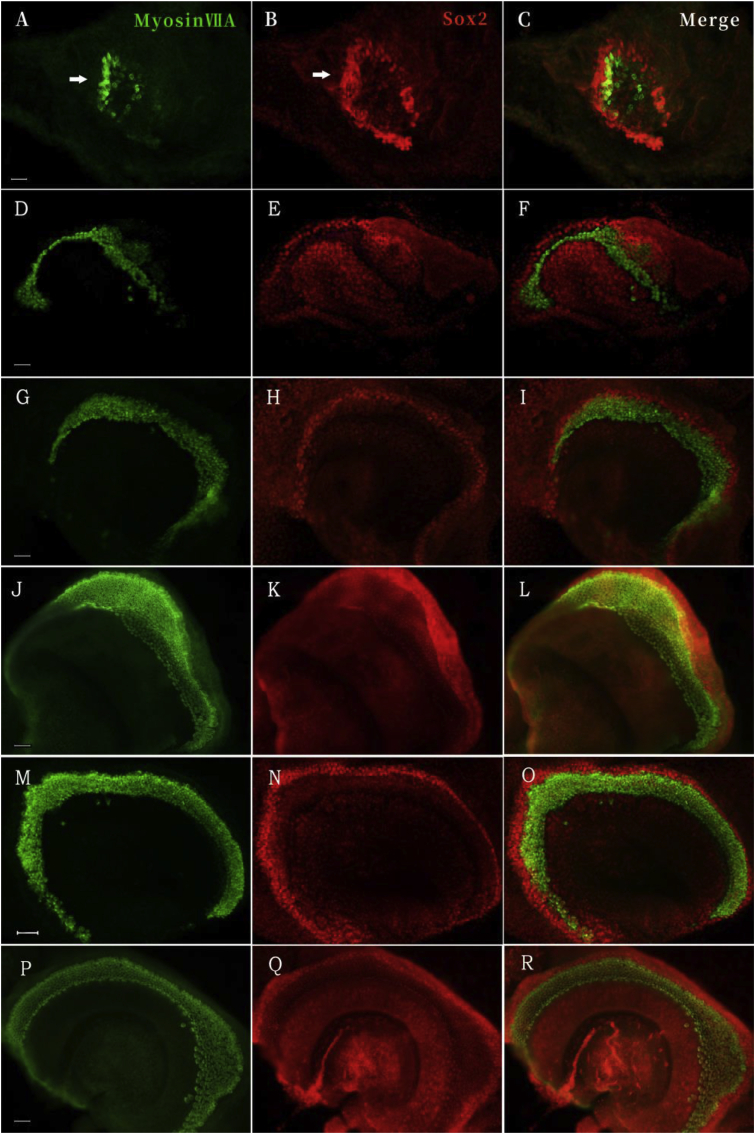
After the cultures reached a stage resembling the developing cochlea at E18.5 in vivo (E11.5 + 7 DIV, E12.5 + 6 DIV, and E13.5 + 5 DIV, respectively), all sensory epithelia appeared to be morphologically normal and healthy (Fig. 1). To determine the effect of the adjacent mesenchyme tissue on the development of the cochlear epithelium’s sensory cells, we co-stained the explants for Sox2 and myosin VIIA. In the cultured cochlear sensory epithelia, both the differentiated hair cells and supporting cells expressed Sox2, which supports the findings of previous studies that Sox2 was expressed in prosensory cells and subsequently in immature hair cells and the developing supporting cells before ultimately becoming restricted only to the supporting cells in the auditory epithelia (Kiernan et al., 2005; Bok et al., 2007; Liu et al., 2018). Further, the developing auditory epithelia cultured with POM showed more cells expressing Sox2 compared with explants cultured without POM. Moreover, the ratio of myosin VIIA-expressing cells to the total number of Sox2-expressing cells was also increased in auditory epithelia cultured with POM compared with the non-POM group (Fig. 2).
Fig. 1.
Representative images of myosin VIIA- and Sox2-positive cells in the cochlear sensory epithelial explants isolated at the specified developmental stages. Myosin VIIA (green staining) and Sox2 (red staining) protein expression were evaluated in the E11.5+7DIV cochlear sensory epithelial explants (arrows) without (A–C) and with (D–F) mesenchyme tissue. A similar analysis was performed for the E12.5 + 6 DIV explants without (G–I) and with (J–L) mesenchyme tissue, as well as the E13.5 + 5 DIV explants without (M–O) and with (P–R) POM. The magnification of each image was not equal. All scale bars in this figure represent 50 μm.
Fig. 2.
Box and whisker plot highlighting the ratio of myosin VIIA-positive hair cells to total Sox2-positive sensory cells in cochlear sensory epithelium explants isolated at the specified developmental stage cultured with and without mesenchyme.○ represents outlier.
3.2. The mesenchymal tissue promotes hair cell formation at different development stages
To evaluate cellular differentiation and the localizations of various protein markers, immunohistochemical staining was performed. In this analysis, more myosin VIIA-expressing cochlear epithelial cells were found in E11.5 + 7 DIV, E12.5 + 6 DIV, and E13.5 + 5 DIV explants, respectively, after culture with POM (225.90 ± 62.44, 476.94 ± 100.81, and 1386.60 ± 202.38, respectively) compared with cells cultured without POM (68.17 ± 23.74, 205.00 ± 44.23, and 1266.80 ± 38.84). Thus, the mesenchymal tissue significantly increased the amount of differentiated hair cells, and also caused an overall cellular alignment and patterning at E11.5 + 7 DIV, and E12.5 + 6 DIV (P < 0.01). Meanwhile, there were no obvious differences in development between the auditory epithelia cultured with or without POM at E13.5 + 5 DIV (P = 0.633).
3.3. Sensory epithelial cell proliferation is not significantly affected by the mesenchyme in culture
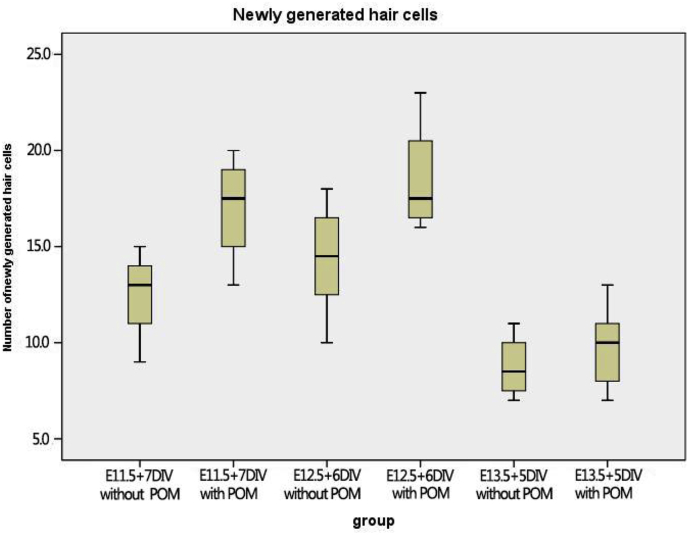
In order to further clarify the mechanism by which POM promotes hair cell formation and to determine the source of hair cells, 6 μg/ml BrdU was added during the culture of the developing cochlear sensory epithelia, with or without POM. We then quantitatively analyzed cell proliferation by co-staining the cells with BrdU and myosin VIIA, thus highlighting newly formed hair cells (Fig. 3). Notably, the number of new hair cells was not significantly affected by the presence of POM during the culture of sensory epithelia at E11.5 + 7 DIV, E12.5 + 6 DIV, and E13.5 + 5 DIV, respectively (Fig. 4). This is an intriguing phenomenon, which deserves further investigation.
Fig. 3.
Representative images of newborn hair cells (white arrows) double-labeled with myosin VIIA (green staining) and BrdU (red staining) at E12.5 + 6 DIV. Scale bar: 10 μm.
Fig. 4.
Box and whisker plot showing the number of newly generated hair cells in cochlear sensory epithelium explants isolated at the specified developmental stage cultured with and without mesenchyme.
4. Discussion
The periotic mesenchyme derives from embryonic mesodermal cells, while the inner ear’s sensory epithelial cells are produced by the neuroectoderm. The POM generates signals inducing otic placode formation and controlling patterning and differentiation in sensory epithelial cells (Hu et al., 2015; Driver et al., 2017). Inner ear development begins with ectoderm thickening on both sides of the developing hindbrain, for otic placode formation. Then, the otic placode closes, forming the otocyst. It is during this stage that otocyst’s axial patterning is mostly found, a process essential for optimal generation of multiple sensory structures in the inner ear, e.g., the cochlea. Significant changes occur at E12.5, and the cochlea acquires a more elaborate shape consisting of a turn at E13.5 in mice. Moreover, about 85% of sensory cells whose fate has been determined to differentiate into cochlear hair cells have left the cell cycle by E13.5 (Morsli et al., 1998; Alsina and Whitfield, 2017). All of these indicate that E13.5 is the critical time point in the development of cochlear hair cells in mice.
Increasing evidence shows that inductive signals play a significant role in initiating otic placode thickening, vesicle formation, and otocyst morphogenesis as well as cellular differentiation within the cochlear prosensory domain (Chen and Streit, 2013; Anwar et al., 2017). Indeed, forced mesenchymal-to-epithelial transition (MET) appears to mediate hair cell development, as highlighted by increased formation of cells that show swift permeability to FM1-43, a staining reagent that has been used to identify the time at which developing hair cells start showing transduction channel function (Hu and Corwin, 2007). While research to elucidate the mechanisms underlying these inductive signals is ongoing, the role of the adjacent mesenchyme, a possible origin of cochlear-related inductive signals, in sensory development and related downstream signaling pathways is largely unknown.
In addition, the influence of the mesenchyme in early-stage differentiation and patterning in the Organ of Corti remains also unclear. In this study, our data showed that the POM does affect auditory epithelial cells, and these effects appear to be dependent on the developmental stage of the Organ of Corti. As shown above, at E11.5 after culture without mesenchymal tissue, the auditory epithelium struggled to survive in vitro, regardless of the presence or absence of serum in culture media. In addition, at E13.5 the auditory epithelium was only slightly affected by the mesenchymal tissue. These data support a previous study showing that POM has almost no influence on the cellular differentiation and development patterning of the auditory epithelium at E13.5 (Montcouquiol and Kelley, 2003).
Furthermore, the above results also indicated that at E12.5, the auditory epithelium could achieve an ideal developmental state in serum-free culture conditions, even in the absence of the mesenchyme. It is well known that these cells have not differentiated into hair cells at this stage (Doetzlhofer et al., 2006), and serum-freein vitro culture conditions allow them to maintain their current cellular identity and morphology. These culture methods have removed all of the auditory epithelial mesenchyme and surrounding tissues, and therefore all the local inductive signals, which could then be added back to the cultures separately or in combination to evaluate the effects of each intervention factor. Thus, the culture of E12.5 mouse auditory epithelium could be used as a model system to explore the developmental mechanisms underlying hair cell differentiation. This study firstly explored such a model for the development of cochlear sensor cells.
Our findings indicate that in early development, the POM adjacent to the cochlear sensory epithelium facilitates proliferation in sensory precursor cells and promotes their differentiation into hair cells. These data suggest that the mesenchymal tissue in the local environment plays an active role in inducing essential processes during cochlear hair cell development. While the detailed mechanisms involved in POM-mediated proliferation and differentiation require further investigation, the downstream signaling cascades are likely related to the known Sox2 and Notch pathways, as Sox2 expression was altered in POM-treated cultures, as shown above. This study enhances our understanding of the factors involved in cochlear sensory epithelium morphogenesis.
5. Conclusion
The periotic mesenchyme promotes cochlear sensory cells development, and its influence depends on stage of the auditory epithelium development.
Acknowledgements
The authors thank Huawei Li, Wen Li, Yan Chen, Wei Li, and Yali Zhou for their excellent technical support. Huawei Li conceived and directed the study. This work was supported by the Chinese National Natural Science Foundation of China (grant number 81371089). The authors report no conflicts of interest relevant to this study.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Jingjiang Huang, Email: zhongliang19832006@163.com.
Na Zuo, Email: 1369504429@qq.com.
Cheng Wu, Email: 22494978@qq.com.
Peipei Chen, Email: 18225906862@163.com.
Jun Ma, Email: mj20071342@163.com.
Chuanxi Wang, Email: 15212239815@163.com.
Wenyan Li, Email: wenyan_li2000@126.com.
Shaofeng Liu, Email: liusf_cn@163.com.
References
- Alsina B., Whitfield T.T. Sculpting the labyrinth: morphogenesis of the developing inner ear. Semin. Cell Dev. Biol. 2017;65:47–59. doi: 10.1016/j.semcdb.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Anwar M., Tambalo M., Ranganathan R., Grocott T., Streit A. A gene network regulated by FGF signalling during ear development. Sci. Rep. 2017;7(1):6162. doi: 10.1038/s41598-017-05472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J., Chang W., Wu D.K. Patterning and morphogenesis of the vertebrate inner ear. Int. J. Dev. Biol. 2007;51(6–7):521–533. doi: 10.1387/ijdb.072381jb. [DOI] [PubMed] [Google Scholar]
- Brooks P.M., Rose K.P., MacRae M.L., Rangoussis K.M., Gurjar M., Hertzano R. Pou3f4-expressing otic mesenchyme cells promote spi ral ganglion neuron survival in the postnatal mouse cochlea. J. Comp. Neurol. 2020 doi: 10.1002/cne.24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Streit A. Induction of the inner ear: stepwise specification of otic fate from multipotent progenitors. Hear. Res. 2013;297:3–12. doi: 10.1016/j.heares.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Coate T.M., Raft S., Zhao X., Ryan A.K., Crenshaw E.B., 3rd, Kelley M.W. Otic mesenchyme cells regulate spiral ganglion axon fasciculation through a Pou3f4/EphA4 signaling pathway. Neuron. 2012;73:49–63. doi: 10.1016/j.neuron.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A., Puligilla C., Jones J.M., Fritzsch B., Cheah K.S., Pevny L.H. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. U. S. A. 2008;105(47):18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A., White P.M., Johnson J.E., Segil N., Groves A.K. In vitro growth and differentiation of mammalian sensory hair cell progenitors: a requirement for EGF and periotic mesenchyme. Dev. Biol. 2004;272(2):432–447. doi: 10.1016/j.ydbio.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A., White P., Lee Y.S., Groves A., Segil N. Prospective identification and purification of hair cell and supporting cell progenitors from the embryonic cochlea. Brain Res. 2006;1091(1):282–288. doi: 10.1016/j.brainres.2006.02.071. [DOI] [PubMed] [Google Scholar]
- Driver E.C., Northrop A., Kelley M.W. Cell migration, intercalation and growth regulate mammalian cochlear extension. Development (Camb.) 2017;144(20):3766–3776. doi: 10.1242/dev.151761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B., Pan N., Jahan I., Elliott K.L. Inner ear development: building a spiral ganglion and an organ of Corti out of unspecified ectoderm. Cell Tissue Res. 2015;361(1):7–24. doi: 10.1007/s00441-014-2031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J.C., Tucker A.S. Development and integration of the ear. Curr. Top. Dev. Biol. 2015;115:213–232. doi: 10.1016/bs.ctdb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Hu Z., Corwin J.T. Inner ear hair cells produced in vitro by a mesenchymal-to-epithelial transition. Proc. Natl. Acad. Sci. U. S. A. 2007;104(42):16675–16680. doi: 10.1073/pnas.0704576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh S.H., Warchol M.E., Ornitz D.M. Cochlear progenitor number is controlled through mesenchymal FGF receptor signaling. eLife. 2015;4 doi: 10.7554/eLife.05921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan A.E., Pelling A.L., Leung K.K., Tang A.S., Bell D.M., Tease C. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434(7036):1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Ladher R.K. Changing shape and shaping change: inducing the inner ear. Semin. Cell Dev. Biol. 2017;65:39–46. doi: 10.1016/j.semcdb.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Liu S., Li W., Chen Y., Lin Q., Wang Z., Li H. Mouse auditory organ development required bone morphogenetic protein signaling. Neuroreport. 2011;22(8):396–401. doi: 10.1097/WNR.0b013e328346c10f. https://doi:10.1097/WNR.0b013e328346c10f 2011. [DOI] [PubMed] [Google Scholar]
- Liu S., Wang Y., Lu Y., Li W., Liu W., Ma J. The key transcription factor expression in the developing vestibular and auditory sensory organs: a comprehensive comparison of spatial and temporal patterns. Neural Plast. 2018;2018:7513258. doi: 10.1155/2018/7513258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol M., Kelley M.W. Planar and vertical signals control cellular differentiation and patterning in the mammalian cochlea. J. Neurosci. 2003;23(28):9469–9478. doi: 10.1523/JNEUROSCI.23-28-09469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsli H., Choo D., Ryan A., Johnson R., Wu D.K. Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 1998;18(9):3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. https://doi:10.1523/JNEUROSCI.18-09-03327.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnamalai V., Fekete D.M. Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner. Development (Camb.) 2016;143(21):4003–4015. doi: 10.1242/dev.139469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J., Parada C., Chamizo M., Giráldez F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development (Camb.) 2011;138(4):735–744. doi: 10.1242/dev.060657. [DOI] [PubMed] [Google Scholar]
- Pan W., Jin Y., Chen J., Rottier R.J., Steel K.P., Kiernan A.E. Ectopic expression of activated Notch or SOX2 reveals similar and unique roles in the development of the sensory cell progenitors in the mammalian inner ear. J. Neurosci. 2013;33(41):16146–16157. doi: 10.1523/JNEUROSCI.3150-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaya O., Binshtok U., Hersch M., Rivkin D., Weinreb S., Amir-Zilberstein L. Cell-cell contact area affects Notch signaling and Notch-dependent patterning. Dev. Cell. 2017;40(5):505–511. doi: 10.1016/j.devcel.2017.02.009. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]