Graphical abstract

Keywords: COVID-19, Therapeutics, Drug interaction, Antiviral drugs, Health and safety
Abbreviations: NLRP3, (Nucleotide Oligomerization Domain)-like receptor protein 3 (NLRP3) Inflammasome activation; MERS-CoV, Middle East Respiratory Syndrome Corona Virus; SARS-CoV, Severe Acute Respiratory Syndrome Corona Virus, RpRd, RNA-dependent RNA polymerase; CQ, Chloroquine; HCQ, hydroxychloroquine; US-FDA, Food and Drug Administration; ACE2, Angiotensin-converting enzyme 2; EC50, Half maximal Effective concentration; WHO, World Health Organization; Mpro, Main Protease Enzymes; 3ClPro, 3-Chymotrypsin-like Protease; HA, Hemagglutinin; Hb, Haemoglobin; CC50, Half-maximal Cytotoxic Concentration; EC50, Half-maximal Effective Concentration; RpRd, RNA-dependent RNA polymerase; QTc, the Corrected interval between the wave of Q and T
Abstract
The disease caused by viral pneumonia called severe acute respiratory syndrome coronavirus type-2 (SARS-CoV-2) declared by the World Health Organization is a global pandemic that the world has witnessed since the last Ebola epidemic, SARS and MERS viruses. Many chemical compounds with antiviral activity are currently undergoing clinical investigation in order to find treatments for SARS-CoV-2 infected patients. On-going drug-drug interaction examinations on new, existing, and repurposed antiviral drugs are yet to provide adequate safety, toxicological, and effective monitoring protocols. This review presents an overview of direct and indirect antiviral drugs, antibiotics, and immune-stimulants used in the management of SARS-CoV-2. It also seeks to outline the recent development of drugs with anti-coronavirus effects; their mono and combination therapy in managing the disease vis-à-vis their biological sources and chemistry. Co-administration of these drugs and their interactions were discussed to provide significant insight into how adequate monitoring of patients towards effective health management could be achieved.
1. Introduction
Since time immemorial, there has been a periodic recurrence of viral outbreaks at both epidemic and pandemic levels. In the recent past were severe acute respiratory syndrome coronavirus (SARS-CoV), middle east respiratory syndrome coronavirus (MERS-CoV), Ebola, and Lassa virus to mention the least. In December 2019, a history of virus outbreak repeated itself through the emergence of a novel coronavirus disease (2019-nCoV) called severe acute respiratory syndrome coronavirus (SARS-CoV-2) which was first discovered in Wuhan, Hubei Province of the People’s Republic of China. The virus has been reportedly transmitted from animal to man and causing severe respiratory disorders [1], [2]. The spread of this virus has caused global health challenges and huge economic losses by which reason the World Health Organization declared it a pandemic and threat to human existence. The virulence of the disease was initially not understood at the emergence until the increasing rate of spread of the virus with 80 cases of death and more than 4,000 cases confirmed in 5 days [3]. Scientific investigations showed that the newly discovered coronavirus has some definitive similarities with bat-linked genomes that were discovered in late 2002 and 2012 (reported as Severe Acute Respiratory Syndrome (SARS-CoV) and Middle East Respiratory Syndrome (MERS-CoV) respectively), transmitted to human from civet cats and dromedary camels respectively [4]. To forestall the transmission, measures taken by the Chinese Government include immediate shutdown and restriction of movement placed on dwellers at the host centres of the disease even during the famous Chinese Annual Spring Festival in China.
The Chinese health authorities examined case fatality indices of pneumonia among the victims in Wuhan city and an in-depth protein sequencing, eukaryotic cell culturing and characterization of air-way secreted fluid called Bronchoalveolar Lavage (BAL) revealed a unique Beta-coronavirus with close to 79% and 50% respective structural similarities with SARS-CoV 2002 and MERS-CoV 2012 [5]. Based on the established practice of virus naming, the World Health Organization (WHO) and the International Committee on Virus Taxonomy renamed this novel strain of 2019-nCoV to SARS-CoV-2 to portray the phylogenetic origins of the virus [6], [7], [8]. This review seeks to outline the recent development of drugs with anti-coronavirus effects; their mono and combination therapy in managing the disease vis-à-vis their biological sources and chemistry. Co-administration of these drugs and their interactions were discussed to provide significant insight for adequate monitoring of patients towards effective health management.
2. Review methodology
This review focuses on reported curative antiviral drugs, broad-spectrum antibiotics and their chemistry, active components from botanical sources as well as possible clinical trials and safety information. Post- SARS-CoV-2 health-related issues that may arise due to drug toxicity and interaction were discussed with precautionary measures suggested. Scientific articles and reports were sourced across many high impact journals and filtered with relevant keywords on drug agents for COVID-19.
The World Health Organization situation reports on COVID-19, PubMed, NCBI, Research Gate COVID-19, and Google search filters as well as other scientific databases on SARS-CoV-2 related information were carefully accessed towards the identification of the current medicinal agents that are being used in the treatment and management of the virus. Publications and scientific related articles that are under preprints, peer review, editorial comments, letters, and personal opinions were employed accordingly as stated in the reference section. Also, chemical structures were drawn using Chem-Draw Ultra 8.0 (Cambridge Soft, 100 Cambridge Park Drive, Cambridge, MA 02140) [9]
3. Active medicinal components and SARS-CoV-2
Herein, we discussed some direct and indirect antivirals, immuno-stimulants, antibiotics, natural medicinal agents, and other repurposed viable agents both traditional and orthodox with a natural and synthetic basis that are currently being investigated for novel coronavirus treatment. Some of these compounds are currently being subjected to clinical trials and have demonstrated preliminary activity against the virus. Notably, they have been reported in the 6th edition of Guidelines for the Prevention, Diagnosis, and Treatment of Novel Coronavirus-induced Pneumonia issued by the National Health Commission (NHC) of the People’s Republic of China for tentative treatment of coronavirus infections. These compounds include Ribavirin, Lopinavir-ritonavir, Chloroquine, Favipiravir, and Arbidol [10]. Other drugs that are still undergoing clinical trials with promising activity include Remdesivir, Atazanavir, Presatovir, Carmofur, Emetine dihydrochloride, Omacetaxince (Homoharringtonine), Azithromycin Ivermectin, Colchicine, TMPRSS2, and Interferon α, were also discussed. The clinical features of these drugs and their mechanisms of action/target, EC50, and CC50 were reviewed and summarized in Table 1 .
Table 1.
Summary of some clinical features involving drugs reviewed in the present work against SARS-COV-2.
| Drugs | Mechanisms of Action/Targets | Some clinical features |
EC50 (µM) | CC50 (µM) | References | ||||
|---|---|---|---|---|---|---|---|---|---|
| aClinical Trial ID | Interventions | Control | Study Design (Phase/Location /Enrolment No.) | Summarized Outcomes | |||||
| Combined Therapies | |||||||||
| Lopinavir-Ritonavir | Lopinavir and ritonavir areprotease inhibitors, which block viral replication, Ritonavir is a CYP3A inhibitor which functions primarily to reduce the metabolism of lopinavir, thereby boosting lopinavir levels. | NCT04343768 | Hydroxychloroquine + Lopinavir/Ritonavir + Interferon Beta-1A (in first group) and + Interferon Beta-1B (in second group) | Hydroxychloroquine + Lopinavir/Ritonavir | Randomized (Completed/Iran /60) | No results on clinical outcomes with the full protocol as provided by WHO and National clinical trial on outcome measures but the phases completed. | 26.63 | [26], [58], [149] | |
| Ribavirin | Ribavirin, a guanosine analogue inhibits viral RNA polymerase and mRNA capping. | NCT04276688 | Lopinavir/ritonavir 400 mg/100 mg twice daily for 14 days + Ribavirin 400 mg twice daily for 14 days + Interferon Beta-1B 0.25 mg subcutaneous injection alternate day for 3 days | Lopinavir/ritonavir 400 mg/100 mg twice daily for 14 days | Randomized (Completed/ HongKong/127) | Three combinations were safe and superior to Lopinavir–Ritonavir alone and shortening virus shedding, alleviating symptoms, and facilitating discharge of patients with mild to moderate COVID-19 | 109.50 | >400 | [150], [32], [151] |
| Arbidol | Arbidol inhibits by interfering with multiple steps of the virus replication cycle. The stages of SARS-CoV-2 replication targeted by arbidol is during the virus entry process, the post-entry stages, or the entire process of infection (Full-time). Arbidol efficiently blocked both viral entry and post-entry stages. | NCT04476719 | Atafenovir 200 mg KAPSUL (Capsules containing 207.009 mg Umifenovir hydrochloride monohydrate equivalent to 200 mg Umifenovir hydrochloride. | Arbidol 100 mg KAPSUL (Capsules containing 103.504 mg Umifenovir hydrochloride monohydrate equivalent to 100 mg Umifenovir hydrochloride | Open-label, Randomized, Single oral dose, two-period, cross over. (Phase 1/Turkey /18) | To assess the bioequivalence of atafenovir 200 mg Kapsul In comparison with Arbidol 100 mg Kapsul in healthy male subjects under fasting conditions alongside with ethical protocols and strong confidence intervals evaluation. | 4.11 | 31.79 | [32] |
| Direct antiviral (Monotherapy) | |||||||||
| Favipiravir | RNA-dependent RNA polymerase (RdRp) inhibitor. Blocking the replication of other RNA viruses. Activated into its phosphoribosylated form (favipiravir-RTP) in cells, which then inhibits viral RNA polymerase activity | NCT04336904 | Day 1: 1800 mg, BID; Day 2 – 14: 600 mg, TID | Placebo Dosage: Day 1: 1800 mg, BID; Day 2 and thereafter: 600 mg, TID, for a maximum of 14 days. | Multi-centre, randomized, double-blind, placebo-controlled (1:1) Phase 3 (Italy/100) | This is a multi-centre, randomized, double-blind, placebo-controlled (1:1) clinical study to explore the efficacy and safety of Favipiravir in the treatment of adult subjects with COVID-19-moderate type. | 61.88 | >400 | [18], [150] |
| Remdesivir | Remdesivir is a nucleotide analogue with the triphosphate form i.e., RDV-TP being used as a substrate for many viral RNA-dependent RNA polymerase (RdRp) complexes. RDV-TP has been reported to inhibit the viral RNA synthesis via a specific mechanism of delayed chain termination for coronaviruses including SARS-CoV-2 RdRp. It has been observed that RDV-TP specifically resembles Adenosine triphosphate (ATP) molecule and competes with the nucleotide during the viral RNA synthesis. | NCT04292899 | Standard of care with and without mechanical ventilation + Remdesivir Administered as an IV for 5 and 10 days separately (RDV 200 mg Day 1 and 100 mg from Day 2–5 or 2–10 according to the grouping) | Standard of care | Randomized completed (USA/4891) | The magnitude of benefit cannot be determined because there was no placebo control, however, the most common adverse events noticed were nausea worsening respiratory failure, elevated alanine aminotransferase level, and constipation. | 0.77 | >100 | [151], [152] |
| Atazanavir | Atazanavir is of high interest because of its bioavailability within the respiratory tract. ATV could dock in the active site of SARS-CoV-2 Mpro, with greater strength even than Lopinavir. ATV inhibits SARS-CoV-2 replication, alone or in combination with ritonavir (RTV) in Vero cells, human pulmonary epithelial cell line, and primary monocytes, impairing virus-induced enhancement of IL-6 and TNF-α levels. | NCT04452565 | NA-831 (60 mg orally twice a day for one day, followed by 30 mg once a day for four consecutive days (Five days in total)) and Atazanavir (400 mg orally twice a day for one day, followed by 200 mg daily for four consecutive days (five days total)) | NA-831 60 mg orally twice a day for one day, followed by 30 mg once a day for four consecutive days (Five days in total) | Randomized Controlled Phase 2/3 (USA/525) | Currently recruiting in the Phase 2/3 trial to evaluate four treatment strategies for non-critically ill hospitalized participants(not requiring ICU admission and/or mechanical ventilation)with SARS CoV-2 infection, in which participants will receive NA-831 or Atazanavir with or without Dexamethasone. | [153] | ||
| Indirect antiviral | |||||||||
| Hydroxychloroquine (HCQ) | Hydroxychloroquine exhibits antiviral potency by inhibiting virus entry into host cells. The pathway can be related to post-translation alteration of newly synthesized proteins via glycosylation inhibition | NCT04328285 | HCQ 200 mg: 2 tablets on the evening of Day 1 and 2 tablets on the morning at Day 2 and 1 tablet once daily afterwards | Placebo of HCQ, 2 tablets on the evening on Day 1, and 2 tablets on the morning at Day 2 and 1 tablet once daily afterwards. | Randomized, double-blinded, placebo-controlled. Phase 3 (France/ 600) | In a placebo-controlled trial, lopinavir is being compared to hydroxychloroquine, which is used to treat malaria, rheumatoid arthritis, and lupus erythematosus, as a preventative treatment for COVID-19 in exposed health-care workers with the primary outcome being the occurrence of the infection (NCT04328285) | 0.72 | [52], [154] | |
| Omacetaxine (Homoharringtonine) | No clear mechanism of action recorded against SARS-CoV-2 but its actions in other viruses implicates ribosomal bond to prevent protein translation | N/A | N/A | N/A | N/A | Clinical investigation is required to ascertain its mechanism of action and the ideal dose against SARS-Co-2 | 2.10 | 59.75 | [58], [73], [127] |
| Emetine dihydrochloride | Prevention of DNA and RNA replication in Vero E6 cells. | A broad-spectrum antiviral drug with in vitro activity against MERS-CoV at an EC50 of 0.34 µM | Used in combination with Remdesivir and the nucleoside analogue GS-5734 to exhibit synergistic inhibitory effect against SARS-CoV-2 replication | Use for the treatment of generality of viral infection. | 0.50 (Reduction in viral RNA) 0.46 (Reduction in infectious viruses) | 56.46 | [58], [155] | ||
| Presatovir | Inhibition of fusion intercellular mediated by the F protein | NCT02135614 | Randomize, double-blind, placebo-controlled studies Phase 2b clinical trial | Used against the respiratory syncytial virus (RSV) resistance development and treatment | [156] | ||||
| Chloroquine (CQ) | Chloroquine as Hydroxychloroquine earlier described exhibits its antiviral potency equally by inhibiting virus entry into host cells. The pathway can be related to post-translation alteration of newly synthesized proteins via glycosylation inhibition Chloroquine is also proposed to inhibit the glycosylation of ACE2 receptor chains, thus limiting ligand recognition of these receptors, rendering the viral spike protein unable to mediate cell entry. | NCT04342650 | CQ 450 mg twice daily (3 tablets of 150 mg, every 12 h) on day 1, followed by CQ 450 mg once daily (3 tablets of 150 mg) from D2 to D5. Oral administration. | 150 mg placebo tablets (oral) | Randomized completed (Brazil/152) | The preliminary findings from the Clinical trial suggest that a higher dosage of CQ in COVID-19 should be carefully administered because of safety concerns regarding QTc prolongation and its potential safety hazards | 1.13 | >100 | [151], [154], [157], [158] |
| Antibiotics (combined therapies) | |||||||||
| Azithromycin | Azithromycin in combination therapy with hydroxychloroquine Inhibits endosomal acidification via early endosomal pathway | NCT04329832 | Azithromycin 500 mg on day 1 plus 250 mg daily on days 2–5 (maybe administered intravenously per clinician preference). If the patient has already received azithromycin before randomization (no more than 2 days), the prior doses will count toward the 5-day total. | N/A | Randomized Phase 2 (USA/85) | A formalized protocol for trials with Bayesian statistical evidence approached currently ongoing. Statistical approach contributes to the network of meta-analyses of therapeutics of COVID-19 | 2.12 | [53], [86], [158] | |
| Ivermectin | The hypothesized mechanism of action is through inhibiting IMPα/β1-mediated nuclear import of viral proteins by dissociation of the heterodimer which is the same for other RNA viruses. | NCT04343092 | Ivermectin 12 mg /weekly) + Hydroxychloroquine 400 mg/daily + azithromycin 500 mg daily (Ivermectin 0.2 mg /kg (single dose at once = 2 tablets of 6 mg/weekly) | N/A | Pilot Randomized completed (Iraq/100) | This study showed that adding IVM to HCQ and AZT had a better cure rate and shorter time to stay in the hospital compared with controls but it was relatively safe without observable safety signals. results are needed to be validated in a larger prospective follow up study | [104] | ||
| Colchicine | Microtubule polymerization inhibitor by binding with the beta-tubulin subunit to prevent it from assembling (Tubulin-colchicine complex). Also, it inhibits Monosodium urate (MSU), as well as the inhibitory effect on neutrophil functions. | NCT04392141 | Oral administration of Colchicine + Herbal Phenolic Monoterpene Fractions | N/A | Randomized Phase 2 (Iran/200) | No significant results yet but it’s an ongoing oral administration with some herbal phenolic monoterpene fractions will be added to standard treatment in patients with COVID-19. | [159] | ||
| Immuno-stimulants | |||||||||
| Transmembrane Protease Serine2 (TMPRSS2) inhibitor | The inhibitors block viral activity by preventing TMPRSS2′s action on S protein processing (inhibit SARs-CoV-2 entry into the host cell). | NCT04509999 | Bicalutamide 150 mg oral tablet daily at 1:1 randomization for up to 4 weeks | Standard of care and placebo | Randomized Phase 3 (USA/100) | The study is an ongoing clinical trial that proposes to test bicalutamide at 150 mg oral daily dosing in a double-blind placebo-controlled randomized trial in male patients with early symptomatic COVID-19 disease | [160] | ||
| Interferon (IFNs) | Type I and III IFNs are broad-spectrum antivirals both direct inhibitory effects on viral replication in the upper airway, reducing viral spread to the lungs and transmission, and supporting an immune response to clear virus infection. | NCT04389645 | IP-10 in CDS protocol. Dynamic IP-10 measurements in hospitalized COVID-19 positive patients | N/A | ObservationalCompleted (Israel/52) | The longitudinal and real-time IP-10 measurements could help with personalizing immunomodulatory treatment regimens for COVID-19 patients and may support better patient outcomes. | [161], [162], [163] | ||
| Teicoplanin and other glycoprotein drugs (Dalbavancin, oritavancin, and telavancin) | Teicoplanin exhibits antiviral activity in the early stage of the viral life cycle of viruses such as Ebola virus, MERS-CoV, and SARS-CoV via inhibition of the low-pH cleavage of the viral spike protein by host cell’s cathepsin L and cathepsin B in the late endosomes thereby preventing the release of viral RNA and replication. | NCT04492501 | Assessing efficacy and safety of standard treatment including steroids, Remedisvir, Tocilizumab, mesenchymal stem cell therapy therapeutic plasma exchange in addition to standard treatment as well in combination with convalescent Plasma with other investigational treatments inlined with standard treatment Operational Definitions | Interventional retrospective case-control, single centre-based cohort study | An open-label Phase II Non-randomized (Pakistan/600) | Investigators will use different investigational treatment as mono or in combination to see mortality and morbidity benefit based on the limited evidence available so far. These investigational modalities include Therapeutic plasma exchange (TPE), Convalescent Plasma (CP), Remdesivir, Tocilizumab, and Mesenchymal stem cell (MSC) therapy in addition to standard supportive treatment. | 8.78 concentration reached for a daily dose of 400 mg which is higher than 1.66 μM to inhibit 50% of viruses (IC50) in vitro | [159], [163] | |
3.1. Ribavirin
This is an FDA approved compound usually referred to as synthetic guanosine analogue with antiviral potential (Fig. 1 a). It is used as a blend with other antiviral drugs and in most cases with interferon-alpha (IFN-a) for the treatment of several viral infections such as chronic hepatitis C virus, viral hemorrhagic fever, and respiratory syncytial virus [11]. Ribavirin was first commercialized in the early 1980s for the treatment of respiratory syncytial virus in children which makes it a premier and standard antiviral agent over the newly developed drugs. Aside from being regarded as a broad-spectrum antiviral agent against DNA and RNA, which can obscure the production of viral messenger RNA binding RNA-dependent RNA polymerase (RpRd). It is also a prodrug that metabolizes into nucleoside analogues blocking viral RNA and viral mRNA capping [12]. Ribavirin was found to be effective against Crimean-Congo hemorrhagic fever, Hantavirus infection, Lassa fever, and Venezuelan hemorrhagic fever. Meanwhile, promising results had emanated previously about the blend of ribavirin with Interferon-alpha for the treatment of MERS-CoV [13]. Conflicting data have equally been reported for patients with MERS-CoV infection that were treated with a blend of ribavirin and two forms of interferon-a; IFN-a 2b and IFN-b1 [14]. It can affect haemoglobin (Hb) counts of the red blood cells which is an undesirable side effect in patients with respiratory disorders; a feature that reduces its potential as a potent antiviral agent against coronavirus infection. However, during the SARS-CoV outbreak in 2003, few countries including China and Canada successfully administered high doses of a blend of ribavirin with antibiotics and hormone against the virus [15]. A possible combination of ribavirin with lopinavir-ritonavir has proven to be potent against SARS-CoV-2 [16].
Fig. 1.
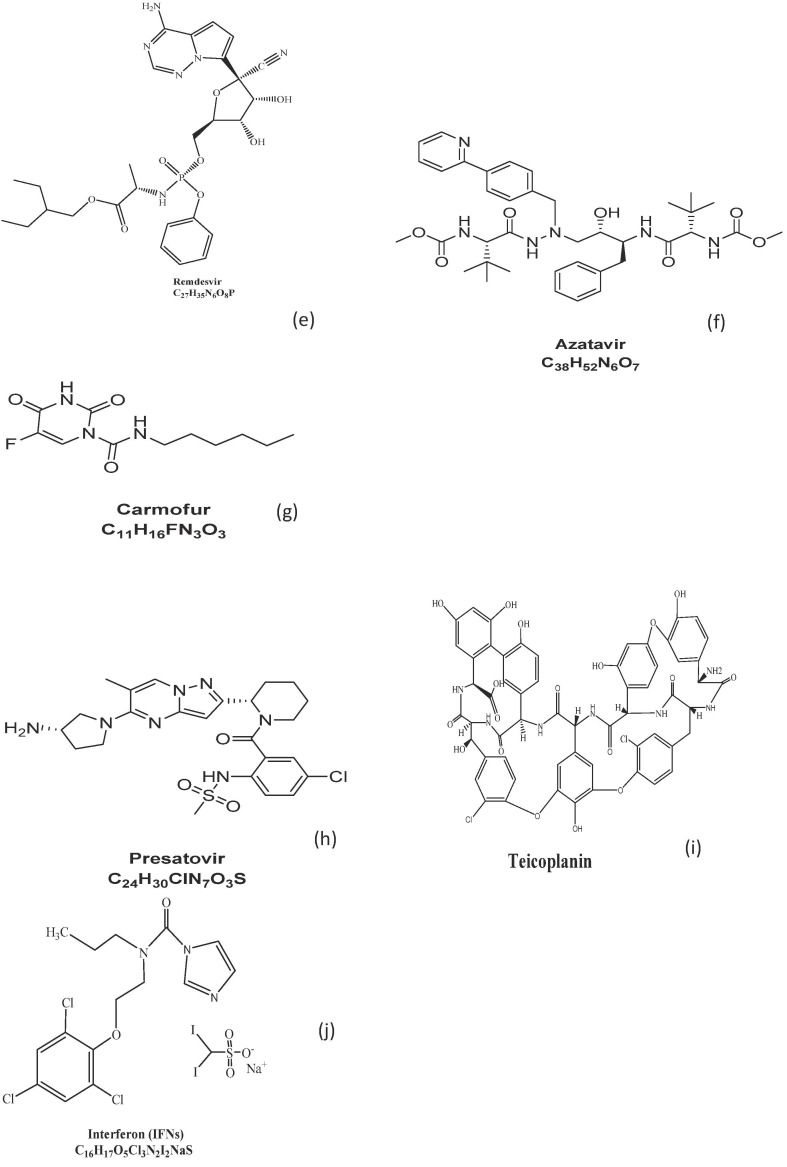
(a-j): Chemical structures of some reported SARS-COV-2 Drug.
3.2. Favipiravir
This is a newly discovered RNA-dependent RNA polymerase (RdRp) inhibitor similar to ribavirin earlier discussed (Fig. 1b). Apart from its anti-influenza viral activity, clinical experiments have revealed that it is capable of blocking the replication of flavi-, alpha-, filo-, bunya-, arena-, noro-, and other RNA viruses [17]. Favipiravir when in cells is transformed into an active phospho-ribosylated (F-RTP), a form that is recognized by viral RNA polymerase as a substrate thus inhibiting its activity in patients [18]. This further suggests the possible antiviral efficiency of Favipiravir against SARS-CoV-2 to which end the Clinical Medical Research Centre of the National Infectious Diseases in collaboration with Third People’s Hospital of Shenzhen conducted a clinical trial on 14th February 2020 and obtained promising results. The preliminary results showed that the antiviral efficacy of Favipiravir is more than that of Lopinavir-ritonavir blend [19].
3.3. Arbidol
Arbidol, known as “Umifenovir” (Fig. 1c) is an active antiviral agent that has greatly been used for the treatment of the influenza virus. For decades, it has been an effective agent, with no reported side effect, commonly administered in China and Russia to prevent severe pneumonia and cytokine dysregulation associated with viral infections [20]. It is a broad-spectrum antiviral agent with both in-vitro and in-vivo inhibitory potentials against various infectious diseases such as influenza, hepatitis B virus (HBV), hepatitis C virus (HCV), Hantaan virus, and other pathogenic human respiratory viruses. Aside from enhancing the immune response of host cells, Arbidol actively inhibits the binding of viral cell walls with the membrane of target cells, thus preventing its entry [21]. Arbidol has now been nominated as a first-aid therapy against novel coronavirus and studies are ongoing towards further optimization [22], [23], [24].
3.4. Lopinavir-ritonavir
This is a medication usually administered in combination with other antiviral drugs such as ribavirin for treating Human Immunodeficiency Virus HIV [25]. It (Fig. 1d) belongs to a class of protease inhibitors capable of targeting SARS-CoV non-structural protein 3Clpro [16]. Chu et al. [26] discovered that lopinavir-ritonavir combination therapy with ribavirin demonstrated anti-SARS-CoV activity in-vitro and also in clinical studies when used to treat patients under a non-randomized clinical trial. Less SARS patients died after receiving a dose of the combination compared with those in the control group who received doses of blends of ribavirin and Corticosteroids. When MERS-CoV emerged, intensive investigations into potential antiviral compounds identified lopinavir as active against the virus in vitro [27]. Its drug-drug therapy does not come without a minor side effect of diarrhoea. Nevertheless, it has been recommended as an effective anti-SARS-CoV-2 agent in China [16]. Further investigations are ongoing by some Chinese researchers andclinicians into the therapeutic efficacy of Lopinavir-ritonavir.
3.5. Remdesivir
Remdesivir is one of the most promising antiviral drugs tested for the treatment of coronavirus infection. It is a phosphoramidite prodrug of adenosine nucleotide [28] with a broad-spectrum in-vitro antiviral activity against a wide range of RNA viruses like Ebola virus, respiratory syncytial virus, Nipah virus, Hendra virus, Marburg virus, pathogenic viruses such as MERS-CoV and SARS-CoV and bat CoV strains [29], [30]. Although it is a nucleotide analogue, Remdesivir has shown to inhibit viral RNA replication, prematurely terminate viral RNA transcription by targeting viral RNA-dependent RNA polymerase, and evade viral exoribonuclease proofreading [31]. To date, pharmacokinetic and clinical detail on Remdesivir is still obscure and critical investigations are ongoing. Markedly, concerns of antiviral resistance against its usage have been studied [31]. Very recently, the antiviral activity of Remdesivir was examined at an early stage after virus entry into Vero E6 cells detailing its mechanism of action as a nucleotide [32]. The EC90 results were observed to be 1.76 μM, suggesting its potency against the virus. In a mouse model study on SARS-CoV-2 pathogenesis, prophylactic and therapeutic activity [32] observed that early administration of the drug reduced lung viral load by an order of magnitude greater than 2 on day 4 or 5 post-infection, restrained the disease and improved respiratory function. More so, in a tissue culture model, a biologically important in-vitro model of pulmonary infection, Remdesivir displayed low half-maximal effective concentrations (EC50s) of 0.069 and 0.074 μM for SARS-CoV and MERS-COV respectively which revealed its effectiveness against a wide range of highly divergent coronaviruses like the endemic CoVs hCoV-OC43 and hCoV-229E within the sub-micromolar EC50 [29], [33]. In another animal model examination, a rhesus monkey model on the Ebola virus, 10 mg/kg dose administered daily for consecutive 12 days inhibits the Ebola virus replication and protects all infected animals against infection [34]. Based on the successful clinical experience of Remdesivir therapy on Ebola virus patient [35] as well as on the first SARS-CoV-2 confirmed case in Washington, United States of America (with a negative swab at day 1 after treatment) has suggested that Remdesivir is a promising antiviral therapy for COVID-19 treatment. The molecular mass of Remdesivir is 602.6 g/mol with a chemical formula of C27H35O8P (Fig. 1e).
3.6. Atazanavir
This is another anti-retroviral FDA approved medication commonly used for HIV treatment and prevention. Atazanavir is distinguished from other protease inhibitors in that it can only be administered one daily rather than requiring multiple doses per day and has lesser effects on the patient’s lipid profile [36], [37]. As an azapeptide HIV-1 protease inhibitor, atazanavir binds to the protease active site and inhibits the enzyme activity. Owing to its antiviral activity [38] released a drug-target interaction deep learning model (denoted as MT-DTI model) result of atazanavir showing an inhibitory potency Kd of 94.94 nM against SARS-CoV-2 3C-like protease. This protease enzyme is also found in coronaviruses due to its cleavage potentials to the coronavirus polyprotein at different conserved sites. Atazanavir (Fig. 1f) has a molecular weight of 704 g/mol.
3.7. Carmofur
Carmofur is a promising candidate drug that has shown to inhibit SARS-CoV-2 main protease (Mpro) enzymes responsible for many coronaviruses with a half-maximal effective concentration (EC50) of 24.30 μM and a half-maximal inhibitory concentration (IC50) of 1.82 μM, showing its ability to inhibit viral replication in cells [39]. It is a derivative of fluorouracil, an antineoplastic agent that has demonstrated clinical benefit as a cancer therapy but reported to induce leukoencephalopathy. Carmofur (Fig. 1g) with the general formula of C11H16FN3O3S and a mass number of 257 g/mol, is an orally administered anticancer drug, verified by FDA and potent for usage in the treatment of breast, colorectal and other types of solid cancer [40]. It is less toxic, inhibiting human acid ceramidase (AC), and has been utilized clinically for several years [41]. An in-vitro study has affirmed the potency of carmofur in terminating AC activities and the spread of cancer cells with a median effective concentration of 2965 nM [42]. Further studies revealed that the inhibition of fatty acid amide hydrolase (FAAH) and N-acylethanolamine acid amidase (NAAA) by carmofur as well as its therapeutic activity against several inflammation-related diseases makes it a potential therapeutic candidate in the treatment of SARS-CoV-2 infections [43]. This was further corroborated by a recent investigation, which confirmed the efficacy of carmofur in inhibiting SARS-CoV-2 main protease and broader effectiveness against the virus via an interaction between the crystal structure of the virus main protease and carmofur which could modify the catalytic (Cys145) properties of SARS-CoV-2 [40].
3.8. Presatovir
Presatovir is an off-label drug currently undergoing trials for its activity against coronavirus disease owing to its activity against the respiratory syncytial virus (RSV), a causative agent of lower respiratory tract infections. Presatovir is regarded as a candidate RSV fusion (F) protein inhibitor due to the pyrazolo[1,5-a] pyrimidine series of compounds containing a piperidine ring at 2-position of the pyrazolo[1,5-a] pyrimidine scaffold [44]. It has a general formula C24 H30ClN7O3S and a mass number of 532 g/mol (Fig. 1h).
3.9. Chloroquine and hydroxychloroquine
Chloroquine, a synthetic succedaneum of the quinine alkaloid isolated from Cinchona tree bark. It is a well-known antimalarial drug that was introduced into medicine in the 1940s (Fig. 2 ). It is a widely used antimalarial agent that was discovered to possess a broad-spectrum antiviral capacity [45]. Chloroquine, having a long history, is considered safe to inhibit parasitaemia levels in mosquitoes. Besides, it is widely used for the treatment of patients with malaria cases with mild and transitory side-effects. To date, many people currently use these two drugs; Chloroquine (CQ) and Hydroxychloroquine (HCQ). A derivative of CQ and HCQ which is usually administered orally to treat malaria can also be applied to treat symptoms of rheumatoid arthritis, systemic and discoid lupus erythematosus, pemphigus, lichen planus, sarcoidosis, scleroderma polymyositis, and porphyria cutanea tarda. Chloroquine was found also to be an effective antiviral agent that inhibits SARS-CoV infection and spread [14]. CQ and HCQ are both off-label drugs and have been proposed as a promising antiviral therapy for the treatment of COVID-19 patients especially in severe condition [46]. To assess the efficacy of CQ and HCQ in coronavirus infected patients, many compelling in-vitro and in-vivo studies, including clinical trials in different animal cells, viruses, and infected humans have been conducted [47], [48], [49]. Significantly, over eighty clinical trials of CQ and HCQ as well in their combination were reported worldwide. CQ alters the terminal glycosylation of the angiotensin-converting enzyme 2 (ACE2) receptor thereby suppressing SARS-CoV-2 S-protein binding and significantly reduces the viral replication by interfering with the fusion process of the virus [32]. In an in-vitro experiment conducted to investigate the elicit antiviral activity of CQ against SARS-CoV-2, a low micromolar dose of it inhibits the virus with half-maximal effective concentration (EC50) of 1.13 µM and a half-cytotoxic concentration (CC50) greater than 100 µM [32]. Several researchers across countries such as China, USA, Germany, UK, and many others have stepped up further investigations into the optimization of CQ as one of the most effective antiviral drugs to treat SARS-CoV-2. The results obtained from more than 100 patients demonstrated CQ as superior to inhibit exacerbation of pneumonia, improve lung imaging findings, promote virus-negative conversion, and shorten the disease course [50]. Another research investigation has demonstrated the potential CQ and HCQ as a target regimen and effective antiviral therapy for COVID-19 treatment. CQ acts by increasing endosomal pH, preventing virus-cell fusion, altering protein degradation pathways through acidic hydrolases in the lysosomes, macromolecule synthesis in the endosomes, and post-translational modifications in the Golgi apparatus [51].
Fig. 2.
Cinchonia spp, botanical source, and some isolated chemical compounds.
In a physiologically-based model in-vitro study of CQ and HCQ on SARS-CoV-2 Vero cells of some infected patients, an oral loading dose of 400 mg twice daily at day 1 followed by an oral maintenance dose of 200 mg twice daily for 4 days was recommended [52]. The results of the authors further displayed half-maximal effective concentration (EC50s) of 0.72% and 5.47% μM for HCQ and CQ respectively. Additionally, it has been reported that the effect of HCQ on COVID-19 patients could be significantly improved with azithromycin [53]. At variance, a report document detailed that a long-term and combined usage of CQ or HCQ with any other drugs (such as azithromycin) induce QTc interval prolongation besides other severe side-effects like arrhythmogenic and cardiac problems, QRS widening, and negative entropy. CQ and HCQ are notable for inhibiting the P-glycoprotein transport system (in gut luminal and blood-brain barrier endothelial cells), which in turn increase cyclosporine and digoxin levels and ultimately to a more severe complicated result. Since the mode of actions of CQ and HCQ are identical, their activity on the virus may probably be the same. In a multinational registry analysis conducted on SARS-CoV-2 patients from 671 hospitals in six continents, who were placed on CQ, HCQ, the combination of antibiotics or without combination as a macrolide, there was a strong indication that CQ and HCQ when used alone or with antibiotics as drug regimens were associated with an increased frequency of ventricular arrhythmias and mortality rates in hospital among SARS-CoV-2 patients [54]. Although, these should not negate the ongoing HCQ clinical trials in managing the COVID-19. Adequate safety evaluation is therefore required before the recommendation of CQ and HCQ as candidate therapies for COVID-19 management.
3.10. Emetine dihydrochloride
Emetine dihydrochloride, principally from ipecac root, has been used extensively as an anti-protozoan approved drug for amoebiasis treatment (Fig. 3 ). It blocks ribosomal protein synthesis by inhibiting the movement of ribosomes along mRNA and inhibits DNA replication in early S-phase [55]. Owing to its antiviral activity against both RNA and DNA viruses, Khandelwal et al. [56] reported that emetine demonstrated a significant antiviral activity capable of fighting against four serotypes of dengue virus and inhibiting viral infection. Very recently, in an in-vitro model study, emetine was observed to inhibit MERS-CoV and HCoV-OC43 with EC50 of 0.16 and 0.34 μM respectively within a sub-micromolar range [57]. In another in-vitro study carried out to investigate the antiviral activity of several chemical compounds against SARS-CoV-2 in Vero E6 cells, emetine demonstrated the lowest half-maximal effective concentrations (EC50) of 0.46 μM, showing its ability to inhibit coronavirus replication. The authors further detailed that a synergistic administration of emetine (0.195 μM) and Remesdivir (at 6.25 μM) may result in 64.9% viral inhibition, supporting that combination therapy may help to reduce (EC50) of the compound below the therapeutic plasma concentrations and provide clinical benefit [58]. SARS-CoV-2 in Vero E6 cell with the compound demonstrated a half-maximal effective concentration (EC50) of 2.55 μM showing its potency against the virus [58].
Fig. 3.
Carapichea ipecacuanha, botanical source, and some isolated chemical compounds.
3.11. Teicoplanin and other glycoprotein drugs (Dalbavancin, oritavancin, and telavancin)
Teicoplanin was previously known as Teichomycin A2 because it was co-purified together with moenomycin-like teichomycin A1 that was isolated from a soil sample in India in 1978 [59], [60], [61]. It acts as a glycopeptide that binds with the D-ala-D-ala terminus of the lipid (II) in the peptidoglycan to cause bacterial cell deaths and is being used in many nations to combat infections and multiple drug antibiotics resistance pathogens such as Staphylococcus aureus [60]. The complexity of Teicoplanin is that it is a mixture of different derivatives of closely related compounds and possibly be differentiated based on their length, the aliphatic chains, and its possible mechanism. The chemistry, biosynthesis alongside the gene-phylogenetic studies has been discussed [61], [62], [63], [64]. Teicoplanin is an antibiotic (Fig. 1i) that is capable of inhibiting methicillin-resistant pathogens and is currently being used in the management and treatment of SARS-CoV-2 patients in the world. The mechanism revealed that teicoplanin inhibits the host cell's cathepsin L and cathepsin B and is attached to the viral glycoprotein thereby unfolding the receptor-binding domain subsequently released in the cytoplasm of the host cells. These glycopeptides have previously shown inhibiting action on some human viruses in the coronavirus family including Ebola, MERS-CoV, influenza, hepatitis C virus, HIV, and also on SARS-CoV-2 virus [65], [66]. Similar studies also indicated the potent role of teicoplanin and its derivatives (vancomycin, dalbavancin, oritavancin, and telavancin) as novel inhibitors of cathepsin L-dependent viruses even in Coronaviridae family [67], [68], [69]. It was reported that teicoplanin inhibited SARS-CoV-2 in a dose-dependent manner when tested on HIV-luc/2019-nCoV-S pseudovirus and the inhibitory concentration of 1.66 uM which is potent to suppress the entry of the SARSCoV-2 virus in different types of cells alongside with some -gram-positive bacterial infection [70]. Teicoplanin has a safe history and advantage as an antibacterial regimen. It is usually administered intramuscularly or by intravenous injection. Based on previous clinical studies carried out on 1300 patients [71], teicoplanin showed about 90 percent effective rates and compatibility for adult, children and the elderly when administered as a mono- or combined antibacterial therapy. Although, lower rates were achieved among patients with fever, diabetes, malignant diseases and other immune-compromised disorders with possible nephron and ototoxicity. Teicoplanin can be used in the prevention and treatment of serious infections caused by gram-positive bacteria [72]. It has been reported that a combination of teicoplanin and ciprofloxacin is more effective in relieving respiratory tract infections [59]. However, adequate monitoring and clinical observations must be ensured when prescribing teicoplanin with patients who have a history of vancomycin hypersensitivity.
3.12. Azithromycin
Among several potential drugs tested against SARS-CoV-2, Azithromycin is a broad-spectrum antibiotics macrolide that is currently in use for the management of infected patients in many countries based on an open-label non-randomized clinical trial [53], [73]. It is a well-known brand and a 15-membered ring macrolide (azalide) antibiotic (Fig. 4 ). This drug 9-deoxy-9a-aza-9a-methyl-9a-homoerythomycin (C38H72N2O12) is different from Erythromycin with the presence of a methyl-substituted nitrogen atom in the macrolide ring with PKA values of 8.1 and 8.8 respectively [74]. Azithromycin is a unique broad-spectrum antibiotic owing to its rapid and effective tissue and serum penetration in addition to its potency against gram-positive and gram-negative bacteria. Azithromycin has found application in the treatment of fungal infections, respiratory tract infections, viral infections, inflammations and many other immunomodulatory disorders [75], [76], [77]. Many ongoing clinical trials are also seeing the potentials of azithromycin combined with chloroquine in managing SARS- CoV-2 while some reports have expressed concerns over prolonged QTc and safety of administration among other underlying side effects as diabetes, heart-related diseases, mental illnesses among others. [78], [79], [80], [81], [82], [83], [84] The administration of azithromycin on different patients showed no significant evidence that it cures COVID-19 but could only suppress bacterial infections in the host. As earlier pointed out that in combination with chloroquine, azithromycin causes a significant improvement in patients with malaria without any indication of risk due to QTc prolongation above that of chloroquine. [85], [86], [87] The addition of zinc sulphate alongside hydroxychloroquine and azithromycin played an important role in the increased number of SARS-CoV-2 patients being discharged therefore zinc sulphate can also be used as prophylaxis [88].
Fig. 4.
Chemical structure of Azithromycin from Erythromycin A.
In Morocco, it was reported that concomitant administration of hydroxychloroquine and azithromycin increased the number of cured SARS-CoV-2 patients and decreased mortality rates [89]. Azithromycin was also reported to be compatible with breastfeeding coronavirus nursing mothers and during pregnancy but with caution and adequate monitoring [90]. Notably, treatment with azithromycin combined with hydroxychloroquine showed a more prolonged QTc than treatment with only azithromycin [91]. An insight into the pharmacokinetics of hydroxychloroquine and azithromycin combination therapy explains the effect of this combination as a function of their accumulation on the lysosomal cells in the local concentration via ion-trapping mediation. The need for further clinical studies to possibly unravel the safety and risk benefits is therefore essential [92]. Furthermore, Bayesian application with statistical analysis on the effects of combining hydroxychloroquine and azithromycin also yielded viral load reduction [93]. Azithromycin and bee derived products (such as honey) were suggested especially among high-risk SARS-CoV-2 frontline health workers as prophylactic treatment; further investigations into some of the compounds in the bee products are suggested [94].
3.13. Ivermectin
Ivermectin is an anti-parasitic broad-spectrum drug that was reported to inhibit the interaction between HIV-1 replicates [95]. Chemically, the compound reveals two subsets: the 1st subset possesses an olefinic bond at C-22 while the 2nd subset bond is hydrated with a hydroxyl (–OH) group at position 23 known as 22, 23-dihydro derivative of avermectin B1 which is a macrolide lactone produced by actinomycetes Streptomyces avermitilis [96] (Fig. 5 ). Utilizations of ivermectin are numerous and potent to combat other viruses that have been fully documented [97], [98], [99], [100]. This broad-spectrum activity on viruses is due to the importin alpha/beta-1 during infection [101], [102]. Quest for drugs to combat this novel coronavirus revealed a possible role for ivermectin and importin alpha/beta-1 during infection as a signal-dependent nucleocytoplasmic shuttling of the SARs-CoV nucleocapsid protein mediated nuclear import [103], [104], [105], [106]. In Australia, the antiviral activity of ivermectin on SARS-CoV-2 infected cells in an in-vitro study revealed a 93 percent reduction in the viral RNA present in the supernatant and 99.80 percent reduction in cell-associated with viral RNA. It was observed that ivermectin treatment led to a rapid reduction in viral RNA load by about 5000-fold in 48 hrs [104]. The pharmacokinetic perspective of this drug was analysed based on the pharmacokinetic data from clinically relevant and excessive dosing studies available; It was indicated that the SARS-CoV-2 inhibitory concentrations are not likely to be attainable in humans and that a single dosage is practically inappropriate because of the infected cells which were exposed at different concentrations negate the pharmacokinetics findings of some previously used doses pooled in the treatment regimen of ivermectin [107] and therefore suggests a need for further clinical trials to determine the best level of inhibitory concentration. It was reported that the combination of ivermectin and hydroxychloroquine could work synergistically with adequate clinical monitoring when administered [108]. In a screening of 1,408 patients gathered from 3 continents and carefully matched with age, gender/ethnicity, comorbidities, and illness severity score group consideration; it was concluded that administration of ivermectin on COVID-19 patients decreased death rates and reduced the length of hospital admission with the hope that an ivermectin drug used for filarial worm treatment (a neglected tropical disease) would be potent enough to combat the current SARS-CoV-2 alongside with clinical trials on the standard practices [109]. However, some researchers suspected possible toxicity with the use of ivermectin but recommended careful consideration of risk-benefit ratios and clinical trials to fully understand the pharmacokinetics and safety of administration [110], [111]. It was demonstrated that both ivermectin and chloroquine are capable to inhibit replication of the SARS-CoV-2 in-vitro with a suggestion that a combination of these drugs could be effective especially in malaria-endemic regions where chloroquine is still an effective drug to combat Plasmodium vivax blood-stage therapeutic [112].
Fig. 5.
Streptomyces avermitilis, bacterial source, Ivermectin, and some isolated chemical compounds.
3.14. Colchicine, (-) -N-5,6,7,9-tetrahydro 1, 2, 3, 10- tetra methoxy-9-oxybenzone-7-yl (s)- acetamide
This is an iso-quinoline alkaloid originated from Colchicum autumnale belonging to the family of Liliaceae [113] (Fig. 6 ). Traditionally, it has been used in gout management, Mediterranean fever, liver cirrhosis, chronic myelocytic leukaemia, hepatic disorders, cardiovascular diseases, and also in potential anticancer drugs [114], [115]. The biosynthesis of colchicine involves mainly phenylalanine and tyrosine. It is a very toxic antitumor drug and has been reported in patients with kidney and liver failure, however, derivatives such as dem-colchicine, trimethyl-colchicine acid methyl-ester, 2-dimethyl thiocolchicine, 3-dimethyl thio-colchicine and the biosynthetic colchicine’s which are less toxic are used as anti-leukaemia agents [116], [117]. Colchicine has anti-inflammatory effects on the IL-1 and IL--6 axes which further prolong neutrophils and macrophages actions as well as the multiple cellular actions in the assembly of the nucleotide-binding domain and leucine-rich repeat protein inflammasome [118]. It is a non-selective inhibitor of Nucleotide Oligomerization Domain)-like receptor protein 3 (NLRP3) inflammasome that plays a huge role in anti-inflammatory diseases and binds to unpolymerized tubulin heterodimers, forming a stable complex that can effectively inhibit microtubule dynamics upon binding to microtubules ends [118], [119]. In addition to this, colchicine plays an important role in managing patients with SARS-CoV-2 especially patients with myocardial infarction related cases even at acute and chronic phase periods [120]. Colchicine was recommended possibly on its clinical trial to see if it could assist in the clinical management of SARS-CoV-2 patients and reduce the inflammation caused by the virus [121]. According to [122], severe acute respiratory infections can cause pulmonary and systemic inflammation among SARS-CoV-2 patients and could lead to cardiac injury, heart failure, and respiratory complications. Significant evidence was reported that colchicine could ameliorate the effect of inflammation and hyper inflammation activity on SARS-CoV-2 patients and that it could be used as a supporting therapy with possible clinical trials suggested [123]. In another study, there was a significant finding on the physicochemical properties, chemical properties, and the mechanism of action and the conclusion that it may not be beneficial to patients since it has effects of increasing cytosolic pH and by implication might increase the rate of acute respiratory distress syndrome and multiple organ failure among SARS-CoV-2 patients [124]. In Israel, experimental studies were conducted using real-world data, a conclusion was reached that colchicine could be utilized based on the NLRP3 inflammasome which can be activated and triggered by different SARS-CoV-2 proteins and therefore could lead to severe adult respiratory distress syndrome. [125]. Colchicine clinical trials should be done carefully among patients with the underlying heart and other myocardial disorders towards saving more lives.
Fig. 6.
Colchicum spp, botanical source, and some isolated chemical compounds.
3.15. Omacetaxine (Homoharringtonine)
Homoharringtonine is a traditional antiviral compound that is effective for reducing viral load at about 0.05 or 0.2 mg/kg doses, inhibiting vesicular stomatitis virus at 50 nM, new castle virus at 100 nM, and porcine epidemic diarrhoea virus at 150 nM [126]. It is a Cephalotaxus fortunei derived plant alkaloid (Fig. 7 ) with antitumor activity capable of inhibiting the first cycle of the elongation phase of eukaryotic translation [127], [128]. The semi-synthetic form of homorringtonine is called Omacetaxine [73], [129] which is responsible for the treatment of chronic myeloid leukaemia refractory to tyrosine kinase inhibitors [130]. The anti-SARS-CoV-2 effect of homoharringtonine was recently examined while it has been reported to inhibit the virus with EC50 value 2.10 µM [58]. A maximal plasma concentration of 36 ng/mL (0.066 µM) obtained on day 11 from a pharmacokinetic study conducted by Nemunaitis et al., [131] with 1.25 mg/m2 omaxacetine administered subcutaneously every 12 h (twice daily) on patients with solid tumours and hematologic malignancies revealed a much lower EC50 than the concentration against SARS-CoV-2 virus in-vitro.
Fig. 7.
Cephalotaxus fortune, botanical source, and some isolated chemical compounds.
3.16. Transmembrane Protease Serine2 (TMPRSS2) inhibitor
Camostat Mesylate, Flumadin, Nafamostat, Trasylol which are Transmembrane Protease Serine-2 (TMPRSS2), and other synthetic inhibitors used in managing prostate cancer have been expressed in the epithelial cells of specific tissues including those in the autodigestive tract [128]. Previous reports revealed that the Coronaviridae family including influenza virus have also utilized TMPRSS2 for viral entry via the hemagglutinin protein (HA) attached to angiotensin-converting enzyme (ACE2) being expressed on respiratory epithelial cells [132], [133], [134], [135], [136]. Hoffmann et al. [137] conducted an in-vitro study using Camostat mesylate, a protease inhibitor and observed a partial inhibition at the entry stage of the SARS-CoV-2 virus in the epithelial lung cells. This suggests that protease inhibitors could combat the novel 2019 coronavirus. Furthermore, the authors argued that Nafamostat mesylate offered more antiviral protection than camostat mesylate (Foipan) which is in use in Japan to treat SARS-CoV-2 infected patients. They also proposed that Nafamostat mesylate should be subjected to clinical investigations to evaluate its curative activity and strength to inhibit SARS-CoV-2 and further recommended that camostat mesylate, Nafamostat, and bromhexine hydrochloride (BHH) be considered for inhibition of TMPRSS2 in the control of COVID-19 [138]. Interestingly, in a large cohort study especially on African-American SARS-CoV-2 male patients diagnosed with Diabetes mellitus and Asthma using their sputum cells, the result showed that there was a higher expression of ACE2 and TMPRSS2 in diabetics patients with lower expression of ACE2 and TMPRSS2 in asthmatic patients. This suggests further investigation into the effective regimen among people with diabetes and asthma COVID-19 patients with effective monitoring including its role in the lung, renal, heart, and neurones of patients with SARS-CoV-2 [139], [140], [141]. Bestle et al. [142] found out that TMPRSS2, as well as FURIN protease enzymes which are abundant in the respiratory tract, could activate their surface glycoprotein and this suggests that they are potential drugs in the treatment of SARS-CoV-2.
3.17. Interferon (IFNs)
Interferon- a and b are naturally broad-spectrum antiviral active agents (Fig. 1j). The administration of IFNs can be used as prophylaxis as well as early therapy based on the principle of “supplement to compensate”. Type-1 IFNs suppress chronic hepatitis B and C viral infections. The antiviral effect of interferon b (IFN- b) is still unknown however, it has an immune-modulating activity of improving the conditions of marmosets infected with MERS-CoV [143], [144]. To assess the efficacy of interferon on SARS-CoV-2, several reports have demonstrated the antiviral effect with lambda Interferon (IFN-γ), a Type III interferon sharing low homology with Type-1 IFNs and IL-10, which has shown more potency against a variety of viruses including SARS-CoV and MERS-CoV [145]. IFN-γ therapy was recently proposed to possess an antiviral immune-modulating activity for reducing viral load and hyper-inflammation; it also prevents mass tissue damage in the lung. Besides, a pegylated IFNb-γ which is readily in existence as the only therapeutic agent has also been shown to possess an effective safety profile in human [146].
4. Projection and human health risk
It was noticed that there are different dosage regimens, drug combination trials, and skill-based experience treatment by clinicians on different patients with an underlying medical health history. This suggests complications in many developing and low-income countries with little or no quality standard health coverage in managing the post-SARS-CoV-2 health crisis. There is a need for more research and investigations into the lead drugs that could cure and manage the confirmed cases with the expectation of producing an effective and accessible vaccine to curtail the virus spread. Also, adequate monitoring of dosage regimen and drug combination, as well as ethical consideration, should be ensured while dealing with COVID-19 potential experimental drugs. Although, it is a pandemic situation, human rights to life including choice of drugs, allergy, pains and other difficult challenges and quality laboratory investigations before clinical trials should be considered. Safe health record-keeping, monitoring, toxicity check, dosage formulation, dug-drug interaction, mechanism of action, and side effect are paramount especially as post-COVID health-related management is concerned. However, it was advised that couples should avoid pregnancy during the pandemic and in-vitro fertilization should be carefully examined to prevent the potential threat to developing embryos [147] in spite no evidence of SARS-CoV-2 virus in semen of males recovering from the novel coronavirus after 30 days of diagnosis [148]. This also suggests quality and adequate examination of new-born babies during this pandemic to ensure they are not carriers of the COVID-19 virus. Furthermore, unprotected sexual related activities should be cautioned while it is hoped there would be scientific investigations into the protective measures during sexual intercourse.
Governments and health organizations should continually ensure access to a clean environment, clean water, quality life, housing, and basic amenities to meet the Global Health Security Agenda (GHSA), which could help alleviate some potential human health risks, especially among developing countries.
5. Conclusion and recommendations
This review has highlighted reported curative antiviral drugs and broad-spectrum antibiotics (both traditional and orthodox) used in the treatment of SARS-CoV-2; their chemistry, botanical sources, and active components as well as possible clinical trials and safety information. However, there is still a need for continued investigation of herbal medicines and isolation of compounds that could completely inhibit the virus. A full understanding of their mechanisms of action which would help in drug development needs to be known. Further in-depth studies on vaccines and molecular investigations into potential RNA targets should be a crucial priority. Best practices for virus prevention which include observing personal hygiene, following health guidelines by a way of physical distancing, and social responsibility among people are important in alleviating the coronavirus spread.
6. Funding*
This review article did not receive any grant from funding agencies.
7. Authors’ contribution*
All authors contributed equally and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the painstaking efforts of the anonymous reviewers toward the enhanced improvement of this work.
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X.i., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease (COVID-2019) situation reports. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports 30th April 2020.
- 3.NHC,2020 [https://health.nhcgov.com/yourenvironment/publichealth/coronavirus/nhc-coronavirus-news/].
- 4.World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Available from: https://www.who.int/newsroom/factsheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov) (Accessed 2019).
- 5.Lu R., Zhao X., Li J., Niu P., Yang B.o., Wu H., Wang W., Song H., Huang B., Zhu N.a., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L.i., Chen J., Meng Y., Wang J.i., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorbalenya A.E., Baker S.C., Babic R.S., de Groot R.J., Drosten C., Gulyaeva A.A., et al. Severe acute respiratory syndrome-related coronavirus: the species and its viruses a statement of the Coronavirus Study Group. BioRxiv. 2020 doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- 7.Jarvis L.M. Drug firms mobilize to combat the coronavirus outbreak. C&EN. 2020;98:5. [Google Scholar]
- 8.Nature News. Coronavirus latest: death toll passes 2,000. Available from: https://www.nature.com/articles/d41586-020-00154-w (accessed on 20th Feb 2020).
- 9.ChemDraw Ultra 9.0. CambridgeSoft, 100 CambridgePark Drive, Cambridge, MA 02140. www. cambridgesoft.com. See Web site for pricing options.
- 10.NHC, 2020 [https://health.nhcgov.com/yourenvironment/publichealth/coronavirus/nhc-coronavirus-news/].
- 11.Laguno M., Murillas J., Blanco J.L., Martínez E., Miquel R., Sánchez-Tapias J.M., Bargallo X., García-Criado A., Lazzari E.d., Larrousse M., León A., Loncá M., Milinkovic A., Gatell J.M., Mallolas J. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for treatment of HIV/HCV co-infected patients. AIDS. 2004;18(13):27–36. doi: 10.1097/00002030-200409030-00003. [DOI] [PubMed] [Google Scholar]
- 12.Goswami B.B., Borek E., Sharma O.K., Fujitaki J., Smith R.A. The broad-spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem. Bioph. Res. Co. 1979;89(3):830–836. doi: 10.1016/0006-291x(79)91853-9. [DOI] [PubMed] [Google Scholar]
- 13.Falzarano D., De Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P., Brining D., Bushmaker T., Martellaro C., Baseler L., Benecke A.G., Katze M.G., Munster V.J., Feldmann H. Treatment with interferon-2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat. Med. 2013;19:13131317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2(1):69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnard N.D., Cohen J., Jenkins D.J.A., Turner-McGrievy G., Gloede L., Jaster B., Seidl K., Green A.A., Talpers S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777–1783. doi: 10.2337/dc06-0606. [DOI] [PubMed] [Google Scholar]
- 16.Wei L., Hai-Liang Z., Yongtao D. Effective Chemicals against Novel Coronavirus (COVID-19) in China. Curr. Top Med Chem. 2020;20(8):603–605. doi: 10.2174/1568026620999200305145032. PMID: 32133962. [DOI] [PubMed] [Google Scholar]
- 17.Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Japan Acad. Ser. B: Phys. Biol. Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao J., Tian Z., Yang X.u. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BST. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 20.Boriskin Y., Leneva I., Pecheur E.-I., Polyak S. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008;15(10):997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 21.Blaising J., Polyak S.J., Pécheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antiviral Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su B., Wang Y., Zhou R., Jiang T., Zhang H., Li Z., Liu A., Shao Y., Hua W., Zhang T., Wu H., He S., Dai L., Sun L. Efficacy and tolerability of lopinavir/ritonavir- and efavirenz-based initial antiretroviral therapy in HIV-1- infected patients in a tertiary care hospital in Beijing, China. Front. Pharmacol. 2019;10:1472. doi: 10.3389/fphar.2019.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 24.Lin L., Li T.S. Interpretation of “guidelines for the Diagnosis and treatment of novel coronavirus (2019-nCoV) infection by the national health commission (trial version 5)”. Zhonghua Yixue Zazhi. 2020;100:E001. doi: 10.3760/cma.j.issn.0376-2491.2020.0001. [DOI] [PubMed] [Google Scholar]
- 25.Ping Xu, Huang Jianping, Fan Zhao, Huang Wendi, Qi Minghua, Lin Xuwen, Song Weidong, Yi Li. Arbidol/IFN-a2b therapy for patients with coronavirus disease, a retrospective multicenter cohort study. Microbes and Infection. 2019;22(2020):200–205. doi: 10.1016/j.micinf.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., Kao R.Y.T., Poon L.L.M., Wong C.L.P., Guan Y., Peiris J.S.M., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Wilde Adriaan H., Jochmans Dirk, Posthuma Clara C., Zevenhoven-Dobbe Jessika C., van Nieuwkoop Stefan, Bestebroer Theo M., van den Hoogen Bernadette G., Neyts Johan, Snijder Eric J. Screening of an FDA-approved compound Library identifies four small-molecule inhibitors of middle east respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58(8):4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel D., Hui H.C., Doerffler E., Clarke M.O., Chun K., Zhang L. Discovery and Synthesis of a phosphoramidite prodrug of a pyrrolo[2,1—f][triazin-4-amino]adenine-nucleoside (GS-5374)for the treatment of Ebola and Emerging virus. J. Med. Chem. 2017;60:1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 29.Sheahan Timothy P., Sims Amy C., Graham Rachel L., Menachery Vineet D., Gralinski Lisa E., Case James B., Leist Sarah R., Pyrc Krzysztof, Feng Joy Y., Trantcheva Iva, Bannister Roy, Park Yeojin, Babusis Darius, Clarke Michael O., Mackman Richard L., Spahn Jamie E., Palmiotti Christopher A., Siegel Dustin, Ray Adrian S., Cihlar Tomas, Jordan Robert, Denison Mark R., Baric Ralph S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396):eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Morales Alfonso J., Gallego Viviana, Escalera-Antezana Juan Pablo, Méndez Claudio A., Zambrano Lysien I., Franco-Paredes Carlos, Suárez Jose A., Rodriguez-Enciso Hernan D., Balbin-Ramon Graciela Josefina, Savio-Larriera Eduardo, Risquez Alejandro, Cimerman Sergio. COVID-19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med. Infect. Dis. 2020;35:101613. doi: 10.1016/j.tmaid.2020.101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus susceptibility to the Antiviral Remedsvir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.0021-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Manli, Cao Ruiyuan, Zhang Leike, Yang Xinglou, Liu Jia, Xu Mingyue, Shi Zhengli, Hu Zhihong, Zhong Wu, Xiao Gengfu. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown Ariane J., Won John J., Graham Rachel L., Dinnon Kenneth H., III, Sims Amy C., Feng Joy Y., Cihlar Tomas, Denison Mark R., Baric Ralph S., Sheahan Timothy P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.T.K. Warren, R. Jordan, M.K. Lo, A.S. Ray, Rl. Mackman, V. Soloveva, Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys 531 (2016) 381-385. [DOI] [PMC free article] [PubMed]
- 35.Jacobs M, Rodger A, Bell DJ, Bhagani S, Cropley I, Filipe A. Ebola virus relapse causing meningoencephalitis: A case report 338 (2016) 498–503. [DOI] [PMC free article] [PubMed]
- 36.Croom Katherine F., Dhillon Sohita, Keam Susan J. Atazanavir: a review of its use in the management of HIV-1 infection. Drugs. 2009;69(8):1107–1140. doi: 10.2165/00003495-200969080-00009. [DOI] [PubMed] [Google Scholar]
- 37.Von Hentig N. Atazanavir/ritonavir: a review of its use in HIV therapy. Drugs Today. 2008;44(2):103. doi: 10.1358/dot.2008.44.2.1137107. [DOI] [PubMed] [Google Scholar]
- 38.Ram Beck Bo, Shin Bonggun, Choi Yoonjung, Park Sungsoo, Kang Keunsoo. Predicting commercially available antiviral drugs that may act on the novel coronavirus (2019-nCoV), Wuhan, China through a drug-target interaction deep learning model. Computational Struct. Biotechnol. J. 2020;18:784–790. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin Zhenming, Zhao Yao, Sun Yuan, Zhang Bing, Wang Haofeng, Wu Yan, Zhu Yan, Zhu Chen, Hu Tianyu, Du Xiaoyu, Duan Yinkai, Yu Jing, Yang Xiaobao, Yang Xiuna, Yang Kailin, Liu Xiang, Guddat Luke W., Xiao Gengfu, Zhang Leike, Yang Haitao, Rao Zihe. Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat. Struct. Mol. Biol. 2020;27(6):529–532. doi: 10.1038/s41594-020-0440-6. [DOI] [PubMed] [Google Scholar]
- 40.Jin Zhenming, Xiaoyu Du., Yechun Xu., Deng Yongqiang, Liu Meiqin, Zhao Yao, Zhang Bing, Li Xiaofeng, Zhang Leike, Peng Chao, Duan Yinkai, Jing Yu., Wang Lin, Yang Kailin, Liu Fengjiang, Jiang Rendi, Yang Xinglou, You Tian, Liu Xiaoce, Yang Xiuna, Bai Fang, Liu Hong, Liu Xiang, Guddat Luke W., Wenqing Xu., Xiao Gengfu, Qin Chengfeng, Shi Zhengli, Jiang Hualiang, Rao Zihe, Yang Haitao. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 41.Taguchi T. Review of a new antimetabolic agent 1-hexylcarbamoyl-5-fluorouracil (HCFU) Recent Results. Cancer Res. 1980;70:125–132. doi: 10.1007/978-3-642-81392-4_13. [DOI] [PubMed] [Google Scholar]
- 42.Realini N., Solorzano C., Pagliuca C., Pizzirani D., Armirotti A., Luciani R., et al. Discovery of highly potent acid ceramidase inhibitors with in vitro tumour chemosensitizing activity. Sci. Rep. 2013;3:1035. doi: 10.1038/srep01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu K., Xiu Y., Zhou P., Qiu Y., Li Y. A new use for an old drug: carmofur attenuates lipopolysaccharide (LPS)-induced acute lung injury via inhibition of FAAH and NAAA activities. Front. Pharmacol. 2019;10:818. doi: 10.3389/fphar.2019.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamaguchi-Sasaki Toru, Tamura Yunoshin, Ogata Yuya, Kawaguchi Takanori, Kurosaka Jun, Sugaya Yutaka, Iwakiri Kanako, Busujima Tsuyoshi, Takahashi Ryo, Ueda-Yonemoto Naoko, Tanigawa Eiji, Abe-Kumasaka Tomoko, Sugiyama Hiroyuki, Kanuma Kosuke. Design and synthesis of 2-(1-Alkylaminoalkyl)pyrazolo[1,5-a]pyrimidines as new respiratory syncytial virus fusion protein inhibitors. Chem. Pharm. Bull. 2020;68(4):345–362. doi: 10.1248/cpb.c19-00895. [DOI] [PubMed] [Google Scholar]
- 45.Savarino Andrea, Di Trani Livia, Donatelli Isabella, Cauda Roberto, Cassone Antonio. New insights into the antiviral effects of chloroquine. Lancet. Infect. Dis. 2006;6(2):67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colson P., Rolain J.M., Raoult D. Chloroquine for 2019 novel coronavirus SARS-CoV-2. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Ma X., Yu F., Liu J., Zou F., Pan T., Zhang H. Teicoplanin potently blocks the cell entry of 2019nCoV [Preprint] Microbiology. 2020 doi: 10.1101/2020.02.05.935387. [DOI] [Google Scholar]
- 48.Chan K.W., Wong V.T., Tang S.C.W. COVID-19: an update on the epidemiological, clinical, preventive, and therapeutic evidence and guidelines of integrative Chinese Western Medicine for the Management of the novel coronavirus. Am. J. Chin. Med. 2019;2020:1–26. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 49.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A Systematic Review on the Efficacy and Safety of CHlorquine for the Treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. S0883-9441(20)30390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao J., Tain Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in the treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends in. 2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 51.Rainsford, D. Kim, M.C. Powanda, M.W. Whitehouse, Novel Natural Products: Therapeutic Effects in Pain, Arthritis, and Gastro-intestinal Diseases, Progress in Drug Research 2015 ISBN 978-3-0348-0927-6. [PubMed]
- 52.Yao X., Ye F., Zhang M., Cui C., Huang B., Nui P. In-vitro antiviral activity and Projection of Optimized dosing design of the Hydroxychloroquine for the treatment of Severe acute respiratory syndrome coronavirus2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gautret Philippe, Lagier Jean-Christophe, Parola Philippe, Hoang Van Thuan, Meddeb Line, Mailhe Morgane, Doudier Barbara, Courjon Johan, Giordanengo Valérie, Vieira Vera Esteves, Tissot Dupont Hervé, Honoré Stéphane, Colson Philippe, Chabrière Eric, La Scola Bernard, Rolain Jean-Marc, Brouqui Philippe, Raoult Didier. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Mandeep R.M., Sapan S.D., Frank R., Amit N.P. Hydroxy chloroquine or chloroquine with or without a Macrolide for treatment of COVID-19: a multinational Registry. The Lancet. 2020:1–10. doi: 10.1016/S0140-6736(20)31180-6. [DOI] [Google Scholar]
- 55.Low J.S.Y., Chen K.C., Wu K.X., Ml Ng, Chu J.J.H. Antiviral activity of Emetine dihydrochloride against dengue virus infection. J. Antivirals Antiretroviral. 2009:162–171. [Google Scholar]
- 56.Khandelwal Nitin, Chander Yogesh, Rawat Krishan Dutt, Riyesh Thachamvally, Nishanth Chikkahonnaiah, Sharma Shalini, Jindal Naresh, Tripathi Bhupendra N., Barua Sanjay, Kumar Naveen. Emetine inhibits replication of RNA and DNA viruses without generating drug-resistant virus variants. Antiviral Res. 2017;144:196–204. doi: 10.1016/j.antiviral.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Shen Y., MacLean A.B., Aishwarya G., Rukiye N., Isaac J.K., Cameron R.G., Alex P., Russell W.M., Masatoshi I., Mir A.H., Suming H., Kazuhiko I., Jörg B. Erratum for Shen et al., “Identification of a Novel Enhancer/Chromatin Opening Element Associated With High-Level γ-Globin Gene Expression”. Mol Cell Biol. 2019;39(11):e00168–e00219. doi: 10.1128/MCB.00168-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choy Ka-Tim, Wong Alvina Yin-Lam, Kaewpreedee Prathanporn, Sia Sin Fun, Chen Dongdong, Hui Kenrie Pui Yan, Chu Daniel Ka Wing, Chan Michael Chi Wai, Cheung Peter Pak-Hang, Huang Xuhui, Peiris Malik, Yen Hui-Ling. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim Byung-Keun, Kim Jung-Hyun, Sohn Kyoung-Hee, Kim Ju-Young, Chang Yoon-Seok, Kim Sae-Hoon. Incidence of teicoplanin adverse drug reactions among patients with vancomycin-associated adverse drug reactions and its risk factors. Korean J. Intern. Med. 2020;35(3):714–722. doi: 10.3904/kjim.2018.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parenti F., Pagani Kineosporia H. A new genus of the order actinomycetales. Int. J. Systematic Evolutionary Microbiol. 1978;28(3) [Google Scholar]
- 61.Butler Mark S, Hansford Karl A, Blaskovich Mark A T, Halai Reena, Cooper Matthew A. Glycopeptide antibiotics: back to the future. J. Antibiot. 2014;67(9):631–644. doi: 10.1038/ja.2014.111. [DOI] [PubMed] [Google Scholar]
- 62.Jung Joonil, Xu Kexiang, Lessing Derek, Bonini Nancy M. Preventing Ataxin-3 protein cleavage mitigates degeneration in a Drosophila model of SCA3. Hum. Mol. Genet. 2009;18(24):4843–4852. doi: 10.1093/hmg/ddp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcone Giorgia Letizia, Binda Elisa, Berini Francesca, Marinelli Flavia. Old and new glycopeptide antibiotics: From product to gene and back in the post-genomic era. Biotechnol. Adv. 2018;36(2):534–554. doi: 10.1016/j.biotechadv.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Yushchuk O., Ostash B., Truman A.W., Marinelli F., Fedorenko V. Teicoplanin biosynthesis: unraveling the interplay of structural, regulatory, and resistance genes. Appl. Microbiol. Biotechnol. 2020;104(8):3279–3291. doi: 10.1007/s00253-020-10436-y. [DOI] [PubMed] [Google Scholar]
- 65.Baron Sophie Alexandra, Devaux Christian, Colson Philippe, Raoult Didier, Rolain Jean-Marc. Teicoplanin: an alternative drug for the treatment of COVID-19? Int. J. Antimicrob. Agents. 2020;55(4):105944. doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takeda Y., Matsumoto K., Watanabe E., Kanazawa N., Fukamizu T., Shigemi A., Yokoyama Y., Ikawa K., Morikawa N. Pharmacokinetic/pharmacodynamic analysis of teicoplanin in patients with MRSA infections. Clin. Pharmacol.: Adv. Appl. 2016;15 doi: 10.2147/CPAA.S96143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Ying, Yuikawa Naoya, Nakatsuka Hiroki, Maekawa Hiromi, Harashima Satoshi, Nakanishi Yoichi, Kaneko Yoshinobu. Core regulatory components of the PHO pathway are conserved in the methylotrophic yeast Hansenula polymorpha. Curr Genet. 2016;62(3):595–605. doi: 10.1007/s00294-016-0565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malabarba Adriano, Goldstein Beth P. Origin, structure, and activity in vitro and in vivo of dalbavancin. J. Antimicrob. Chemother. 2005;55(suppl_2):ii15–ii20. doi: 10.1093/jac/dki005. [DOI] [PubMed] [Google Scholar]
- 69.Jean Shio-Shin, Lee Ping-Ing, Hsueh Po-Ren. Treatment options for COVID-19: the reality and challenges. J. Microbiol. Immunol. Infect. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J., Ma X., Yu F., Liu J., Zou F., Pan T., Zhang H. Teicoplanin potently blocks the cell entry of 2019-nCoV [Preprint] Microbiology. 2020 doi: 10.1101/2020.02.05.935387. [DOI] [Google Scholar]
- 71.Campoli-Richards Deborah M., Brogden Rex N., Faulds Diana. Teicoplanin: a review of its antibacterial activity, pharmacokinetic properties and therapeutic potential. Drugs. 1990;40(3):449–486. doi: 10.2165/00003495-199040030-00007. [DOI] [PubMed] [Google Scholar]
- 72.F. Khamesipour, S.M. Hashemian, P. Tabarsi, A.A. Velayati, A Review of Teicoplanin Used in the Prevention and Treatment of Serious Infections Caused by Gram-Positive Bacteria and Compared Its Effects with Some Other Antibiotics. 8 (2015) 9. DOI: 10.13005/bpj/641. [DOI]
- 73.Andersen P.I., Krpina K., Ianevski A., Shtaida N., Jo E., Yang J., Koit S., Tenson T., Hukkanen V., Anthonsen M.W., Bjoras M., Evander M., Windisch M.P., Zusinaite E., Kainov D.E. Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine. Viruses. 2019 doi: 10.3390/v11100964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.H. Lode, K. Borner, P. Koeppe, T. Schaberg Azithromycin—Review of key chemical, pharmacokinetics and microbiological features 8 (1996) DOI: 10.1093/jac/37.suppl_c.1. [DOI] [PubMed]
- 75.McCarty James M. Azithromycin (Zithromax®) Infectious Dis. Obstetrics Gynecol. 1996;4(4):215–220. doi: 10.1155/S1064744996000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jelić D., Antolović R. From erythromycin to azithromycin and new potential ribosome binding antimicrobials. Antibiotics. 2016;5(3):29. doi: 10.3390/antibiotics5030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.F. Valentin, Azithromycin and COVID-19 History and Review, 2020, DOI: 10.13140/RG.2.2.33299.63524.
- 78.Bessière Francis, Roccia Hugo, Delinière Antoine, Charrière Rome, Chevalier Philippe, Argaud Laurent, Cour Martin. Assessment of QT intervals in a case series of patients with Coronavirus Disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5(9):1067. doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choudhary R., Sharma A.K. Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance. New Microbes New Infections. 2020;35:100684. doi: 10.1016/j.nmni.2020.100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Funck-Brentano C., Salem J.E., Nguyen L.S., Drici M.D., Roden D.M. Response to the editorial “COVID-19 in patients with cardiovascular diseases”. Arch. Cardiovascular Diseases. 2020 doi: 10.1016/j.acvd.2020.04.001. S1875213620300929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gbinigie Kome, Frie Kerstin. Should azithromycin be used to treat COVID-19? A rapid review. BJGP Open. 2020;4(2) doi: 10.3399/bjgpopen20X101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Juurlink David N. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192(17):E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mercuro Nicholas J., Yen Christina F., Shim David J., Maher Timothy R., McCoy Christopher M., Zimetbaum Peter J., Gold Howard S. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saleh Moussa, Gabriels James, Chang David, Soo Kim Beom, Mansoor Amtul, Mahmood Eitezaz, Makker Parth, Ismail Haisam, Goldner Bruce, Willner Jonathan, Beldner Stuart, Mitra Raman, John Roy, Chinitz Jason, Skipitaris Nicholas, Mountantonakis Stavros, Epstein Laurence M. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ: Arrhythmia Electrophysiol. 2020;13(6) doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andreani Julien, Le Bideau Marion, Duflot Isabelle, Jardot Priscilla, Rolland Clara, Boxberger Manon, Wurtz Nathalie, Rolain Jean-Marc, Colson Philippe, La Scola Bernard, Raoult Didier. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Damle Bharat, Vourvahis Manoli, Wang Erjian, Leaney Joanne, Corrigan Brian. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID‐19. Clin. Pharmacol. Ther. 2020;108(2):201–211. doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lane J.C.E., Weaver J., Kostk K., Duarte-Salles T., Abrahao M.T.F., Alghoul H., Alser O., Alshammari T.M., Biedermann P., Burn E., Casajust P., Conover M., Culhane A.C., Davydov A., DuVall S.L., Dymshyts D., Fernández Bertolín S., Fišter K., Hardin J., Prieto-Alhambra D. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study [Preprint] Rheumatology. 2020 doi: 10.1101/2020.04.08.20054551. [DOI] [Google Scholar]
- 88.Carlucci P., Ahuja T., Petrilli C.M., Rajagopalan H., Jones S., Rahimian J. Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: Outcomes in hospitalized COVID-19 patients [Preprint] Infectious Diseases. 2020 doi: 10.1101/2020.05.02.20080036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Addi R.A., Benksim A., Amine M., Cherkaoui M. African exemplary steps in fighting against COVID-19: A Moroccan example. Bull. Environ. Pharmacol. Life Sci. 2020;9(6) 6-119:1-7. [Google Scholar]
- 90.Keskin-Arslan E, Kaplan YC, Koren G. Use of azithromycin during pregnancy and breastfeeding: A coronavirus pandemic (COVID-19) update. 2020;10. Motherisk Int J 2020;1:12.
- 91.Ramireddy A., Chugh H.S., Reinier K., Ebinger J., Park E., Thompson M., Cingolani E., Cheng S., Marban E., Albert C., Chugh S.S. Experience with hydroxychloroquine and azithromycin in the COVID-19 pandemic: implications for QT interval monitoring [Preprint] Cardiovascular Med. 2020 doi: 10.1101/2020.04.22.20075671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Derendorf Hartmut. Excessive lysosomal ion-trapping of hydroxychloroquine and azithromycin. Int. J. Antimicrob. Agents. 2020;55(6):106007. doi: 10.1016/j.ijantimicag.2020.106007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hulme O.J., Wagenmakers E.J., Damkier P., Madelung C.F., Siebner H.R., Helweg-Larsen J., Gronau Q., Benfield T.L., Madsen K.H. A Bayesian reanalysis of the effects of hydroxychloroquine and azithromycin on the viral carriage in patients with COVID-19 [Preprint] Infectious Diseases (except HIV/AIDS) 2020 doi: 10.1101/2020.03.31.20048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Block J. High-risk COVID-19SARS COV-2: potential intervention at multiple points in the COVID-19 disease process via prophylactic treatment with azithromycin or bee derived products [Preprint] Med. Pharmacol. 2020 [Google Scholar]
- 95.Wagstaff K.M., et al. An AlphaScreen(R)-based assay for high-throughput screening for specific inhibitors of nuclear import. J. Biomol. 2011;16(2):192–200. doi: 10.1177/1087057110390360. [DOI] [PubMed] [Google Scholar]
- 96.Campbell L.D., Jacobsen I., Eggum B.O., Just A. A study of the variability of the endogenous energy output by adult roosters and a determination of the available energy of nine different feedstuffs. J. Sci. Food Agric. 1983;34(3):221–226. doi: 10.1002/jsfa.2740340304. [DOI] [PubMed] [Google Scholar]
- 97.Mastrangelo E., et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J. Antimicrob. Chemother. 2012;67(8):1884–1894. doi: 10.1093/jac/dks147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tay M.Y., et al. Nuclear localization of dengue virus (DENV) 1–4 nonstructural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antivir. Res. 2013;99(3):301–306. doi: 10.1016/j.antiviral.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 99.Götz Veronika, Magar Linda, Dornfeld Dominik, Giese Sebastian, Pohlmann Anne, Höper Dirk, Kong Byung-Whi, Jans David A., Beer Martin, Haller Otto, Schwemmle Martin. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep. 2016;6(1) doi: 10.1038/srep23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tu Y.F., Chien C.S., Yarmishyn A.A., Lin Y.Y., Luo Y.H., Lin Y.T., Lai W.Y., Yang D.M., Chou S.J., Yang Y.P., Wang M.L., Chiou S.H. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caly L., Wagstaff K.M., Jans D.A. Nuclear trafficking of proteins from RNA viruses: a potential target for anti-virals. AntivirRes. 2012;95:202–206. doi: 10.1016/j.antiviral.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 102.Jans D.A., Martin A.J., Wagstaff K.M. Inhibitors of nuclear transport. Curr. Opin. Cell Biol. 2019;58:50–60. doi: 10.1016/j.ceb.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Wulan W.N., et al. Nucleocytoplasmic transport of nucleocapsid proteins of enveloped RNA viruses. Front. Microbiol. 2015;6:553. doi: 10.3389/fmicb.2015.00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Caly Leon, Druce Julian D., Catton Mike G., Jans David A., Wagstaff Kylie M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O. Perišić, Recognition of potential Covid-19 drug treatments through the study of existing protein-drug and protein-protein structures: an analysis of kinetically active residues (n.d.) 18. [DOI] [PMC free article] [PubMed]
- 106.Wu Renyi, Wang Lujing, Kuo Hsiao-Chen Dina, Shannar Ahmad, Peter Rebecca, Chou Pochung Jordan, Li Shanyi, Hudlikar Rasika, Liu Xia, Liu Zhigang, Poiani George J., Amorosa Louis, Brunetti Luigi, Kong Ah-Ng. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 2020;6(3):56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Momekov G., Momekova D. Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view [Preprint] Infect. Dis. (except HIV/AIDS) 2020 doi: 10.1101/2020.04.11.20061804. [DOI] [Google Scholar]
- 108.Patrì Angela, Fabbrocini Gabriella. Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment? J. Am. Acad. Dermatol. 2020;82(6):e221. doi: 10.1016/j.jaad.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaccour C., Hammann F., Ramón-García S., Rabinovich N.R. Ivermectin and Novel Coronavirus Disease (COVID-19): keeping rigor in times of urgency. Am. J. Tropical Med. Hygiene. 2020 doi: 10.4269/ajtmh.20-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vora Agam, Arora V.K., Behera D., Tripathy Surya Kant. White paper on Ivermectin as a potential therapy for COVID-19. Indian J. Tuberculosis. 2020;67(3):448–451. doi: 10.1016/j.ijtb.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmith Virginia D., Zhou Jie (Jessie), Lohmer Lauren R.L. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID‐19. Clin. Pharmacol. Ther. 2020;108(4):762–765. doi: 10.1002/cpt.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vanachayangkul P., Im-erbsin R., Tungtaeng A., Kodchakorn C., Roth A., Adams J., Chaisati C., Saingam P., Sciotti R.J., Reichard G.A., Nolan C.K., Pybus B.S., Black C.C., Lugo L.A., Wegner M.D., Smith P.L., Wojnarski M., Vesely B.A., Kobylinski K.C. Safety, pharmacokinetics, and liver-stage Plasmodium cynomolgi effect of high-dose ivermectin and chloroquine in Rhesus Macaques [Preprint] Pharmacol. Toxicol. 2020 doi: 10.1101/2020.04.27.065409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muzaffar A, Brossi A. Chemistry of colchicine. New York., 1996;5. [DOI] [PubMed]
- 114.Dasgeb B., Kornreich D., McGuinn K., Okon L., Brownell I., Sackett D.L. Colchicine: an ancient drug with novel applications. Br. J. Dermatol. 2018;178(2):350–356. doi: 10.1111/bjd.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kurek Joanna. Cytotoxicity. InTech; 2018. [DOI] [Google Scholar]
- 116.Leung Ying Ying, Yao Hui Laura Li, Kraus Virginia B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015;45(3):341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sapra Sameer, Bhalla Yashika, Nandani Sahil, Sharma, Singh Gagandip, Nepali Kunal, Budhiraja Abhishek, Dhar Kanaya L. Colchicine and its various physicochemical and biological aspects. Med. Chem. Res. 2013;22(2):531–547. doi: 10.1007/s00044-012-0077-z. [DOI] [Google Scholar]
- 118.Misawa Takuma, Takahama Michihiro, Kozaki Tatsuya, Lee Hanna, Zou Jian, Saitoh Tatsuya, Akira Shizuo. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14(5):454–460. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 119.Perricone Carlo, Triggianese Paola, Bartoloni Elena, Cafaro Giacomo, Bonifacio Angelo F., Bursi Roberto, Perricone Roberto, Gerli Roberto. The anti-viral facet of anti-rheumatic drugs: lessons from COVID-19. J. Autoimmun. 2020;111:102468. doi: 10.1016/j.jaut.2020.102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deftereos Spyridon G., Siasos Gerasimos, Giannopoulos Georgios, Vrachatis Dimitrios A., Angelidis Christos, Giotaki Sotiria G., Gargalianos Panagiotis, Giamarellou Helen, Gogos Charalampos, Daikos Georgios, Lazanas Marios, Lagiou Pagona, Saroglou Georgios, Sipsas Nikolaos, Tsiodras Sotirios, Chatzigeorgiou Dimitrios, Moussas Nikolaos, Kotanidou Anastasia, Koulouris Nikolaos, Oikonomou Evangelos, Kaoukis Andreas, Kossyvakis Charalampos, Raisakis Konstantinos, Fountoulaki Katerina, Comis Mihalis, Tsiachris Dimitrios, Sarri Eleni, Theodorakis Andreas, Martinez-Dolz Luis, Sanz-Sánchez Jorge, Reimers Bernhard, Stefanini Giulio G., Cleman Michael, Filippou Dimitrios, Olympios Christoforos D., Pyrgakis Vlasios N., Goudevenos John, Hahalis George, Kolettis Theofilos M., Iliodromitis Efstathios, Tousoulis Dimitrios, Stefanadis Christodoulos. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): Rationale and study design. Hellenic J. Cardiol. 2020;61(1):42–45. doi: 10.1016/j.hjc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vitiello A., Ferrara F., Pelliccia C., Granata G., La Porta R. Cytokine storm and colchicine potential role fighting SARS-CoV-2 pneumonia. Italian J. Med. 2020 doi: 10.4081/itjm.2020.1284. [DOI] [Google Scholar]
- 122.Chen Tao, Wu Di, Chen Huilong, Yan Weiming, Yang Danlei, Chen Guang, Ma Ke, Xu Dong, Yu Haijing, Wang Hongwu, Wang Tao, Guo Wei, Chen Jia, Ding Chen, Zhang Xiaoping, Huang Jiaquan, Han Meifang, Li Shusheng, Luo Xiaoping, Zhao Jianping, Ning Qin. Clinical characteristics of 113 deceased patients with coronavirus disease 2019. Retrospective Study. 2020:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keikhaei Bijan, Bahadoram Mohammad, Rajaei Elham, Alikhani Kosar, Helalinasab Ammar. Possible ameliorative effect of colchicine on the prevention of cytokine storm and its associated hyper-inflammation in patients with COVID-19. J. Prev. Epidemiol. 2020;5(1):4. doi: 10.34172/jpe.2020.02. [DOI] [Google Scholar]
- 124.Cumhur Cure Medine, Kucuk Adem, Cure Erkan. Colchicine may not be effective in COVID-19 infection; it may even be harmful? Clin. Rheumatol. 2020;39(7):2101–2102. doi: 10.1007/s10067-020-05144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gendelman Omer, Amital Howard, Bragazzi Nicola Luigi, Watad Abdulla, Chodick Gabriel. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: insights from a large healthcare database analysis. Autoimmun. Rev. 2020;19(7):102566. doi: 10.1016/j.autrev.2020.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dong Lingbo, Bettinger Pete, Qin Huiyan, Liu Zhaogang. Reflections on the number of independent solutions for forest spatial harvest scheduling problems: a case of simulated annealing. Silva Fenn. 2018;52(1) doi: 10.14214/sf.7803. [DOI] [Google Scholar]
- 127.Jin J., Jiang D.Z., Mai W.Y., Meng H.T., Qian W.B., Tong H.Y., Huang J., Mao I.P., Tong Y., Wang, et al. Homoharringtonine in Combination with cytarabine and aclarubicin resulted in a high complete remission rate after the first induction therapy in a patient with denovo acute myeloid leukaemia. Leukemia. 2006;20(1361–1367) doi: 10.1038/SJ.ley.2404287. [DOI] [PubMed] [Google Scholar]
- 128.Shuqing Lu. Wang jianmin Homoharringtonine and omacetaxine for myeloid haematological malignancies. J. Haematol. Oncol. 2014;7(2) doi: 10.1186/1756-8722-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.FDA 2012 [https://www.usa.gov/federal-agencies/food-and-drug-administration] Assessed.
- 130.Dong Hui-Jun, Wang Zhao-Hua, Meng Wen, Li Cui-Cui, Hu Yan-Xin, Zhou Lei, Wang Xiao-Jia. The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses. 2018;10(11):601. doi: 10.3390/v10110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nemunaitis J., Mita A., Stephenson J., Mita M.M., Sarantopoulos J., PadmanabhanIyer S., et al. Pharmacokinetic study of omacetaxine mepesuccinate administered subcutaneously to patients with advanced solid and hematologic tumours. Canc. Chemother. Pharmacol. 2013;71(1):35–41. doi: 10.1007/s00280-012-1963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin B., Ferguson C., White J.T., Wang S., Vessella R., True L.D., et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59(17):4180–4184. [PubMed] [Google Scholar]
- 133.Azouz N.P., Klingler A.M., Rothenberg M.E. Alpha 1 antitrypsin is an inhibitor of the SARS-CoV2–priming protease TMPRSS2 [Preprint] Microbiology. 2020 doi: 10.1101/2020.05.04.077826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bittmann Stefan. COVID 19: camostat and the role of serine protease entry inhibitor TMPRSS2. J. Regenerative Biol. Med. 2020 doi: 10.37191/Mapsci-2582-385X-2(2)-020. [DOI] [Google Scholar]
- 135.Liu Y., Chan W., Wang Z., Hur J., Xie J., Yu H., He Y. Ontological and bioinformatics analysis of anti-coronavirus drugs and their implication for drug repurposing against COVID-19 [Preprint] Med. Pharmacol. 2020 [Google Scholar]
- 136.Stopsack Konrad H., Mucci Lorelei A., Antonarakis Emmanuel S., Nelson Peter S., Kantoff Philip W. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov. 2020;10(6):779–782. doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrobial Agents Chemotherapy. 2020 doi: 10.1128/AAC.00754-20. AAC.00754-20, aac; AAC.00754-20v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.K. Sonawane, S.S. Barale, M.J. Dhanavade, S.R. Waghmare, N.H. Nadaf, S.A. Kamble, A.A. Mohammed, A.M. Makandar, P.M. Fandilolu, A.S. Dound, N.M. Naik, Homology Modeling and Docking Studies of TMPRSS2 with Experimentally Known Inhibitors Camostat Mesylate, Nafamostat, and Bromhexine Hydrochloride to Control SARS-Coronavirus-2, 2020 [Preprint]. 10.26434/chemrxiv.12162360.v1. [DOI] [PMC free article] [PubMed]
- 139.Gill D., Arvanitis M., Carter P., Hernandez Cordero A.I., Jo B., Karhunen V., Larsson S.C., Li X., Lockhart S.M., Mason A.M., Pashos E., Sah A., Tan V., Zuber V., Bosse Y., Fahle S., Hao K., Jiang T., Joubert P., Burgess S. ACE inhibition and cardiometabolic risk factors, lung ACE2 and TMPRSS2 gene expression, and plasma ACE2 levels: a Mendelian randomization study [Preprint] Genetic Genomic Med. 2020 doi: 10.1101/2020.04.10.20059121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jankun J. COVID-19 pandemic; Transmembrane Protease Serine 2 (TMPRSS2) inhibitors as potential therapeutics for SARS-CoV-2 coronavirus. 6. UTJMS. 2020;7:1–5. [Google Scholar]
- 141.Russo R., Andolfo I., Lasorsa V.A., Iolascon A., Capasso M. Genetic analysis of the novel SARS-CoV-2 host receptor TMPRSS2 in different populations [Preprint] Genetics. 2020 doi: 10.1101/2020.04.23.057190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bestle D., Heindl M.R., Limburg H., van T.V.L., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O., Rohde C., Becker S., Klenk H.D., Garten W., Steinmetzer T., Böttcher-Friebertshäuser E. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets [Preprint] Microbiology. 2020 doi: 10.1101/2020.04.15.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chan J.F.W., Yao Y., Yeung M.L., Deng W., Bao L., Jia L., et al. Treatment with Lopinavir/ritonavir or interferon-Beta1b improves the outcome of MERS-CoV infection in a no human primate model of the common marmoset. J. Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zumla A., Chan J.F., Azhar E.L., Hui D.S., Yuen K.Y. Coronavirus drug discovery and therapeutics options. Nat. Rev. Drugs Discovery. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Prokunina-Olsson L., Noémie A., Ruth E.D., Joan E.D., Jeffrey S.G., Rune H., Sergei V.K., Helen M.L., Thomas R.O.B., Charlotte O., Olusegun O.O., Helen P., Deanna M.S., Nancy C.R., Andreas W., Ivan Z. COVID-19 and emerging viral infections: the case for interferon lambda. J. Exp. Med. 2020;217(5):e20200653. doi: 10.1084/jem.2020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Andreakos E., Sotirios T. COVID 19: Lambda interferon against viral load and hyperinflammation. Mol. Med. 2020;12(6):e12465. doi: 10.15252/emmm.202012465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ashray N., Bhide A., Chakarborty P., Colaco S., Mishra A., Chhabria K., Jolly M.K., Modi D. Single-cell RNA-seq identifies cell subsets in human placenta that highly expresses factors to drive pathogenesis of SARS-CoV-2 [Preprint] Life Sci. 2020 doi: 10.20944/preprints202005.0195.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pan F., Xiao X., Guo J., Song Y., Li H., Patel D.P., Spivak A.M., Alukal J.P., Zhang X., Xiong C., Li P.S., Hotaling J.M. No evidence of SARS-CoV-2 in the semen of males recovering from COVID-19. Fertil. Steril. 2020 doi: 10.1016/j. S0015028220303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Irvani Seyed Sina Naghibi, Golmohammadi Maryam, Pourhoseingholi Mohamad Amin, Shokouhi Shervin, Darazam Ilad Alavi. Effectiveness of Interferon Beta 1a, compared to Interferon Beta 1b and the usual therapeutic regimen to treat adults with moderate to severe COVID-19: structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1) doi: 10.1186/s13063-020-04382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tan Emily L.C., Ooi Eng Eong, Lin Chin-Yo, Tan Hwee Cheng, Ling Ai Ee, Lim Bing, Stanton Lawrence W. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10(4):581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ivan Fan-Ngai Hung, Kwok-Cheung Lung, Eugene Yuk-Keung Tso, Raymond Liu, Tom Wai-Hin Chung, Man-Yee Chu, Yuk-Yung Ng, Jenny Lo, et al, Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial, Lancet 395 (2020) https://doi.org/10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed]
- 152.Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S., Chakraborty C. Probable molecular mechanism of remdesivir for the treatment of COVID-19. Arch. Med. Res. (Elsevier) 2020 doi: 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fintelman R.N., Sacramento C.Q., Lima C.R., et al. Atazanavir inhibits SARSCoV-2 replication and pro-inflammatory cytokine production. BioRxiv. 2020 doi: 10.1101/2020.04.04.020925. [DOI] [Google Scholar]
- 154.Rolain Jean-Marc, Colson Philippe, Raoult Didier. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents. 2007;30(4):297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shen Liang, Niu Junwei, Wang Chunhua, Huang Baoying, Wang Wenling, Zhu Na, Deng Yao, Wang Huijuan, Ye Fei, Cen Shan, Tan Wenjie, Gallagher Tom. High-throughput screening and identification of potent broad-spectrum inhibitors of coronaviruses. J. Virol. 2019;93(12) doi: 10.1128/JVI.00023-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Beigel J.H., Nam H.H., Adams P.L., et al. Advances in respiratory virus therapeutics - a meeting report from the 6th Antiviral Group conference. Antiviral Res. 2019;167:45–67. doi: 10.1016/j.antiviral.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Devaux Christian A., Rolain Jean-Marc, Colson Philippe, Raoult Didier. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55(5):105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brown Samuel M., Peltan Ithan D., Webb Brandon, Kumar Naresh, Starr Nathan, Grissom Colin, Buckel Whitney R., Srivastava Raj, Harris Estelle S., Leither Lindsay M., Johnson Stacy A., Paine Robert, III, Greene Tom. Hydroxychloroquine versus Azithromycin for Hospitalized Patients with Suspected or Confirmed COVID-19 (HAHPS). Protocol for a Pragmatic, Open-Label, Active Comparator Trial. Annals ATS. 2020;17(8):1008–1015. doi: 10.1513/AnnalsATS.202004-309SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schlesinger Naomi, Firestein Bonnie L., Brunetti Luigi. Colchicine in COVID-19: an old drug, new use. Curr. Pharmacol Rep. 2020;6(4):137–145. doi: 10.1007/s40495-020-00225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ragia Georgia, Manolopoulos Vangelis G. Inhibition of SARS-CoV-2 entry through the ACE2/TMPRSS2 pathway: a promising approach for uncovering early COVID-19 drug therapies. Eur. J. Clin. Pharmacol. 2020;76(12):1623–1630. doi: 10.1007/s00228-020-02963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park Annsea, Iwasaki Akiko. Type I and Type III Interferons – induction, signaling, evasion, and application to Combat COVID-19. Cell Host Microbe. 2020;27(6):870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.I.G. Faiq, M. Sabeeh, M.S. Hend, D.D. Basim, M. Marwan, AlMashhadani, et al., Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management (Pilot Trial) medRxiv preprint, DOI: https://doi.org/10.1101/2020.07.07.20145979, doi: medRxiv preprint.
- 163.Zhou Nan, Pan Ting, Zhang Junsong, Li Qianwen, Zhang Xue, Bai Chuan, Huang Feng, Peng Tao, Zhang Jianhua, Liu Chao, Tao Liang, Zhang Hui. Glycopeptide Antibiotics Potently Inhibit Cathepsin L in the Late Endosome/Lysosome and Block the Entry of Ebola Virus, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) J. Biol. Chem. 2016;291(17):9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]