Abstract
Objective
To analyze whether there is an association between the use glucocorticoids at high doses, and the evolution of saturation/fraction of inspired oxygen (SAFI) or time to discharge, in patients hospitalized with COVID-19.
Methods
This was an observational study on a cohort of 418 patients admitted to three regional hospitals in Catalonia, Spain. As primary outcomes, we studied the evolution of SAFI in the first 48 h of treatment and the time to discharge. The results were compared between patients treated and untreated with glucocorticoids (methylprednisolone 1 mg/kg/day o dexamethasone 20–40 mg/day) through sub-cohort analyses matched for multiple clinical and prognostic factors, as well as through Cox multivariate models adjusted for prognostic factors. The simultaneous use of different treatments for COVID-19 was taken into account, both in sub-cohorts matching and in COX regression.
Results
There were 187 patients treated with glucocorticoids; of these, 25 patients could be matched with an equivalent number of control patients. In the analysis of these matched sub-cohorts, no significant difference was observed in time to discharge (log-rank: p = 0.291) or the increment in SAFI at 48 h of treatment (glucocorticoides: −0.04; controls: +0.37; p = 0.095). Multivariate models using Cox regression showed a significantly longer time to discharge in patients treated with glucocorticoids (hazard ratio: 7.26; 95% IC: 3.30–15.95).
Conclusions
We have not found improvement in respiratory function or time until discharge, associated with the use of glucocorticoids at high doses.
Keywords: Glucocorticoids, Methylprednisolone, Dexamethasone, COVID-19, SARS-CoV-2, Coronavirus
Abstract
Objetivo
Analizar si existe asociación entre el uso de glucocorticoides a dosis altas y la evolución de la SAFI (saturación/fracción inspirada de oxígeno) o el tiempo hasta el alta, en pacientes hospitalizados por COVID-19.
Métodos
Estudio observacional sobre una cohorte de 418 pacientes ingresados en 3 hospitales comarcales de Cataluña (España). Como resultados primarios se estudiaron la evolución de la SAFI en las primeras 48 h de tratamiento y el tiempo hasta el alta. Los resultados se compararon entre pacientes tratados y no tratados con glucocorticoides (metilprednisolona 1-2 mg/kg/día o dexametasona 20-40 mg/día), mediante el análisis de subcohortes emparejadas por múltiples factores clínicos y pronósticos, así como mediante modelos multivariantes de Cox, ajustados por diversos factores pronósticos. El uso simultáneo de diferentes tratamientos para la COVID-19 fue tenido en cuenta, tanto en el emparejamiento de subcohortes como en la regresión de Cox.
Resultados
Hubo 187 pacientes con glucocorticoides; de ellos, 25 pacientes pudieron ser emparejados con un número equivalente de pacientes control. En las subcohortes emparejadas, no se apreció diferencia en el tiempo hasta el alta (log-rank: p = 0,291), ni en el cambio en la SAFI a las 48 h desde la basal (glucocorticoides: −0,04; controles: +0,37; p = 0,095). Los modelos multivariantes mediante regresión de Cox mostraron un tiempo hasta el alta significativamente más largo en pacientes tratados con glucocorticoides (hazard ratio: 7,26; IC 95%: 3,30-15,95).
Conclusiones
No hemos encontrado mejoría en la función respiratoria o tiempo hasta el alta, asociado al uso de glucocorticoides a dosis altas.
Palabras clave: Glucocorticoides, Metilprednisolona, Dexametasona, COVID-19, SARS-CoV-2, Coronavirus
Introduction
In December 2019, an epidemic outbreak associated with a new coronavirus (SARS-CoV-2) with the clinical manifestations being mainly respiratory was reported in Wuhan (China).1 The extent of the outbreak reached such magnitude that the WHO declared it to be a pandemic on 12 March 2020.2 Although the mortality rate in those affected (around 2% among medically treated patients)3 appears to be overestimated due to the underdiagnosis of affected individuals with mild symptoms, the extent of the pandemic means that the search for effective treatments is now the top priority.
Various pharmacological agents have been put forward as potential treatments, based on theoretical considerations, in vitro studies, or the results of clinical trials conducted on other related viruses. However, current evidence has not confirmed the presence or absence of benefit of these treatments and even warns of the possible risks or adverse effects associated with their use.4, 5
Glucocorticoids have been considered as a potential treatment, based on the experience of their use in acute respiratory distress syndrome (ARDS),6, 7 influenza infection8 and infections by other similar coronaviruses such as severe acute respiratory syndrome (SARS-CoV) or the Middle East respiratory syndrome (MERS-CoV),9, 10, 11, 12, 13 even when clear benefit from their use has not been demonstrated in these pathologies. Their powerful anti-inflammatory action is said to account for its beneficial effect, especially in the hyperinflammatory state (“cytokine storm”) associated with COVID-19.14, 15 However, the results available to date from observational studies are disparate and difficult to interpret, since the majority of studies that have a comparative group have not matched patients based on relevant clinical and prognostic characteristics, and neither have they sufficiently adjusted their analyses for these characteristics.16, 17, 18, 19
Observational studies with more robust methodology have used the Propensity Score as a matching technique.20, 21 However, this technique does not serve to match groups based on variables that change over time, such as the progressive introduction of multiple drugs against COVID-19, which has happened frequently in hospitalised patients during the epidemic wave.
Furthermore, although various clinical trials are underway, the only preliminary results published so far are those of the RECOVERY clinical trial22. Based on these results, the use of low-dose dexamethasone (6 mg/day) is associated with an improvement in survival in the most severely ill patients. However, many questions remain to be answered about the use of glucocorticoids in this disease, and while time passes until there are more results from clinical trials, observational studies are a significant source of evidence.
In this study we set out to analyse the effect of high-dose steroid treatment (1−2 mg/kg/day of methylprednisolone or 20–40 mg/day of dexamethasone) in a cohort of patients with steroid treatment and patients without steroid treatment, strongly matched by prognostic and clinical factors.
Methodology
This observational study was carried out on a cohort of 418 patients admitted to the hospitals of the Consorci Sanitari de l’Alt Penedès and Garraf (CSAPG), Catalonia, Spain. These are three regional hospitals, which have 275 acute-care beds available, and attend to a reference population of 247,357 inhabitants of the Alt Penedès and Garraf regions. This cohort was designed to be able to study the efficacy of the different drugs used in COVID-19, allowing the study by case matching.
Data were collected from all patients with a clinical condition compatible with COVID-19 disease, from 12 March to 2 May 2020, from the time of admission to discharge, or up to a maximum of 30 days of hospitalisation. Patients without lung involvement and those with a negative PCR for SARS-CoV-2 in a sample obtained by nasal swab were excluded.
The data were collected from the electronic medical history by the COVID-19 researchers group of CSAPG. The data collected included sociodemographic data, previous diseases, chronic treatment, symptoms of disease presentation, vital signs and clinical evolution each day of hospitalisation, including the need for oxygen therapy, the fraction of inspired oxygen (FiO2) and the oxygen administration system (nasal cannula, venturi oxygen mask, reservoir mask, invasive or non-invasive mechanical ventilation). All treatments used during hospitalisation, and all analyses and chest x-rays were logged. The researchers responsible for data collection collected the data using a structured form created in Open Clinic® (Copyright© OpenClinica LLC and collaborators, Waltham, MA, USA), following a common procedure, for which they had been previously trained. Quality controls were established during the data collection process and the errors detected were corrected, with investigator-retraining as and when necessary.
Glucocorticoid treatment was considered as an exposure variable. According to the hospital protocol, the glucocorticoids were indicated in moderate or severe pneumonia in two possible treatment regimens: (a) intravenous methylprednisolone 1–2 mg/kg/day, 3–5 days; (b) dexamethasone bolus 20–40 mg/day 3–5 days. For the purposes of this study, a patient was considered glucocorticoid exposed if he had received 3 doses of either of the drugs, and unexposed if he had not received any.
The primary variables used for the efficacy analysis were time to discharge and SF [saturation (%)/FiO2 (%)] at 48 h after the start of treatment. The secondary variables were SF analysed at 72 h and 96 h from the start of treatment and mortality.
A double strategy was used in the statistical analysis: (1) analysis of subcohorts paired by confounding factors and (2) analysis of unpaired subcohorts, adjusted by confounding factors.
As part of the first strategy, a subcohort of glucocorticoid-treated patients and a matched subcohort of non-glucocorticoid-treated patients (1:1 matching ratio) were formed. The patients were paired using the following prognostic markers, which were identified in bivariate analyses and multivariate models performed as an initial step: gender, age, obesity, heart failure, chronic renal failure, and sleep apnea-hypopnea syndrome (SAHS). Follow-up for the study drug treated patients started the day they took the first dose of the drug. Follow-up for each of the control patients started on the day during hospitalisation when the SF, vital signs (blood pressure, heart rate and temperature), radiological involvement and CRP were similar to those of the patient with whom they were paired. For the study drug treated patient, the baseline CRP was that obtained on the day follow-up started, or if that was not available, on the day prior to starting the treatment. Likewise, the baseline radiological involvement was that shown on the day treatment was started, or from another imaging result up to a maximum of 2 days prior to the start of treatment. At no time was patient-pairing based on data obtained after the start of steroid treatment, nor at the beginning of the follow-up time in the case of the control patients. Missing data regarding radiological involvement were imputed as follows: the radiological involvement for the days between 2 identical x-rays was assumed to be the same as that of the two x-rays obtained (e.g. if a patient had an x-ray with 3-quadrant involvement on day 1 and another x-ray with 3-quadrant involvement on day 6, then it was assumed that all the days in between also had 3-quadrant involvement). This maximum interpolation allowed, was six days of separation between X-rays. Missing data was not imputed for other variables. The patient who received the study treatment and the control patient were only paired together if they any other treatments they had received for COVID-19 were the same, including hydroxychloroquine, lopinavir/ritonavir, interferon and azithromycin. A 3-day margin was tolerated for the start of other treatments, between the study patient and their control patient. In previous independent analyses, the use of tocilizumab showed no association to the main results of the study in our sample, so this drug was not included in matching the comparison subcohorts.
For pairing, a first step was performed using computing brute force algorithms, which identified all the possible controls in the database, for each of the patients who received the study treatment. In this first step, controls were chosen with the same sex and obesity status (“yes” vs. “no”, according to the medical history), the same radiological involvement (number of quadrants affected in anteroposterior x-ray: from 0 to 4) and an age difference no greater than 15 years. The control could have an SF from 1.1 points lower to 2 points higher than the treated patient and a CRP from 6 mg/dL lower to 4 mg/dL higher than the treated patient. Once the potential controls were identified, pairing was then refined, choosing those most similar in terms of SF, blood pressure, heart rate and CRP, using the Propensity Score.
The pairing success was verified by a comparison of means or percentages between groups. A different trend in patient progress (improvement in one group and worsening in another) was ruled out by checking that the difference between the SF on day 1 of the analysis, and the day prior to entering the analysis, was similar. In the matched subcohorts, the SF was studied at 48, 72 and 96 h, using Student’s t-test for independent samples and time to discharge using the log-rank test. In the SF analyses, patients with palliative sedation were excluded, because SF is not related to disease severity in these patients. In the analyses of time to discharge, deceased patients were excluded.
In the unpaired subcohort analysis (second analysis strategy), the effect of glucocorticoids was analysed in a subcohort which included all patients who had been treated with hydroxychloroquine and lopinavir/ritonavir, and excluded those treated with azithromycin or other drugs with uneven distribution between the glucocorticoid-exposed subgroup and the non-glucocorticoid-exposed subgroup. In the analysis of this subcohort, the total time of hospitalisation from day one of admission was included, and patients were considered as having been exposed to glucocorticoids if they had received them at any time during hospitalisation (at least three doses). Time to discharge (excluding deceased patients) and mortality were studied using Cox regression models, adjusted for the following covariates, which were selected both for their statistical association with the outcome and for their clinical relevance in the opinion of the investigators: sex, age, obesity, heart failure, chronic renal failure, SAHS, baseline saturation in the emergency room, CRP in the emergency room, and involved quadrants in the emergency X-ray. The radiological involvement was introduced in the model in a discrete way, with 5 values (from 0 to 4 involved quadrants), age, CRP and saturation were entered in the linear model as numerical variables, while the rest of the factors were treated dichotomously (presence vs. absence of the factor).
For the statistical analysis, R software, version 3.6.1 (R Project for Statistical Computing) and IBM SPSS statistics, version 26 were used.
The research ethics committee of the Bellvitge University Hospital reviewed the study and accepted the waiver of the patient’s informed consent subject to it being an observational and ambispective study, based on the review of clinical data, and with patients’ personal data being anonymised when published (ref. PR252/20).
Results
Of 464 consecutive patients with a clinical diagnosis of COVID-19 and pulmonary involvement, admitted between 12 March and 2 May 2020, 46 were excluded due to a negative rt-PCR for SARS-CoV-2. Of the 418 patients included in the analyses, 238 (56.9%) were males and 180 (43.1%) were females; the mean age of the sample was 65.4 years (SD 16.6 years) and the median follow-up was 9.5 days (IQR 7 days).
In total, 164 (39.2%) patients received methylprednisolone treatment and 23 (5%) patients received dexamethasone. 346 (82.8%) patients received treatment with both hydroxychloroquine and lopinavir/ritonavir during hospitalisation. 79 patients (18.9%) died within the first 30 days of hospitalisation.
The characteristics of the paired subcohorts are shown in Table 1 and the characteristics of the unpaired subcohort are shown in Table 2 . Table 3 shows the mean change in saturation, FiO2 and SF compared to the baseline at 48, 72 and 96 h after treatment in the paired subcohorts.
Table 1.
Baseline characteristics of patients treated with glucocorticoids and their paired controls.
| Paired subcohorts (total patients)a |
Paired subcohorts (surviving patients)a |
|||||
|---|---|---|---|---|---|---|
| Glucocorticoids (n = 25) | Control (n = 25) | p | Glucocorticoids (n = 20) | Control (n = 20) | p | |
| Age (years) | 69.84 | 68.76 | 0.796 | 67.70 | 68.10 | 0.932 |
| Males (n) | 20 | 20 | 1.000 | 16 | 15 | 1.000 |
| Obesity (n) | 1 | 1 | 1.000 | 1 | 1 | 1.000 |
| CHF (n) | 0 | 0 | – | 0 | 0 | – |
| CKD (n) | 6 | 6 | 1.000 | 4 | 5 | 1.000 |
| SAHS (n) | 2 | 3 | 0.637 | 0 | 2 | 0.487 |
| Saturation (%) | 94.8 | 95.0 | 0.788 | 94.8 | 94.8 | 0.971 |
| Systolic BP (mmHg) | 127.5 | 125.5 | 0.636 | 126.7 | 123.5 | 0.488 |
| Diastolic BP (mmHg) | 73.9 | 71.8 | 0.512 | 74.1 | 71.4 | 0.384 |
| HR (bpm) | 75.0 | 77.1 | 0.370 | 73.9 | 76.7 | 0.321 |
| Temperature (°C) | 36.4 | 36.6 | 0.214 | 36.4 | 36.6 | 0.351 |
| SFb | 3.1 | 3.2 | 0.673 | 3.1 | 3.2 | 0.649 |
| SF tendencyc | 0.1 | 0.0 | 0.386 | 0.1 | 0.1 | 0.495 |
| X-ray involvementd | 2.3 | 2.3 | 0.987 | 2.4 | 2.3 | 0.466 |
| CRP (mg/dL) | 10.3 | 10.3 | 0.946 | 10.7 | 10.5 | 0.938 |
| Urea | 44.8 (n = 15) | 42.0 (n = 17) | 0.744 | 45.3 (n = 11) | 42.2 (n = 12) | 0.644 |
| Hydroxychloroquine (n) | 23 | 23 | 1.000 | 19 | 18 | 1.000 |
| Lop/Rit (n) | 21 | 21 | 1.000 | 18 | 18 | 1.000 |
| Interferon (n) | 2 | 2 | 1.000 | 1 | 1 | 1.000 |
| Tocilizumab (n) | 6 | 2 | 0.247 | 5 | 2 | 0.407 |
| Azithromycin (n) | 18 | 20 | 0.742 | 13 | 17 | 0.273 |
HR: heart rate; CHF: congestive heart failure; CKD: chronic kidney disease; Lop/Rit: lopinavir-ritonavir; BP: blood pressure; CRP: C-reactive protein; SF: saturation (%)/fraction of inspired O2 (%); SAHS: sleep apnea-hypopnea syndrome.
The total of paired patients were used in the calculations of lung function in the following 96 h. Surviving patients were used for the calculations related to time to discharge.
Maximum value of 4.76, corresponding to 100% saturation with 21% FiO2.
Change in SF with respect to the day prior to the start of the follow-up period.
Number of affected quadrants on an anteroposterior chest x-ray. Range: 0–4 (0: no involvement; 4: involvement of upper and lower lobes of both lungs).
Table 2.
Baseline characteristics of the subcohorts of patients treated with hydroxychloroquine/lopinavir-ritonavir and patients with additional glucocorticoid treatment.
| HCL/LOP (n = 63) | HCL/LOP/COR (n = 63) | p | |
|---|---|---|---|
| Age (years) | 57.2 | 63.7 | 0.015 |
| Males (n) | 35 (52.2%) | 46 (68.7%) | 0.052 |
| Obesity (n) | 12 (17.6%) | 13 (19.4%) | 0.793 |
| CHF (n) | 3 (4.4%) | 4 (6.0%) | 0.718 |
| CKD (n) | 4 (5.9%) | 9 (13.4%) | 0.137 |
| SAHS (n) | 5 (7.4%) | 7 (10.4%) | 0.528 |
| Baseline saturation (%) | 93.7 | 91.1 | 0.037 |
| X-ray involvementa | 2.13 | 2.13 | 0.962 |
| CRP (mg/dL) | 99.4 | 150.2 | 0.035 |
| Urea | 33.2 (n = 38) | 43.9 (n = 47) | 0.026 |
| Hydroxychloroquine | 63 (100%) | 63 (100%) | – |
| Lopinavir-Ritonavir | 63 (100%) | 63 (100%) | – |
| Interferon | 12 (17.6%) | 22 (32.8%) | 0.042 |
| Tocilizumab | 7 (10.3%) | 25 (37.3%) | <0.001 |
| Glucocorticoids | 0 | 76 (100%) | – |
| Azithromycin | 0 | 0 | – |
HCL: hydroxychloroquine; COR: glucocorticoids; CHF: congestive heart failure; CKD: chronic kidney disease; LOP: lopinavir-ritonavir; CRP: C-reactive protein; SAHS: sleep apnea-hypopnea syndrome.
Number of affected quadrants on an anteroposterior chest X-ray. Range: 0–4 (0: no involvement; 4: involvement of upper and lower lobes of both lungs).
Table 3.
Increase in respiratory function parameters, with respect to the first day of follow-up, in patients treated with glucocorticoids and paired controls.
| Glucocorticoids | Control | p | |
|---|---|---|---|
| Saturation increment | |||
| 48 h | 0.20 (n = 25) | 0.65 (n = 25) | 0.507 |
| 72 h | 0.67 (n = 21) | 0.46 (n = 21) | 0.813 |
| 96 h | 0.64 (n = 20) | 0.52 (n = 20) | 0.904 |
| FiO2 increment | |||
| 48 h | 3.67 (n = 25) | −2.87 (n = 25) | 0.149 |
| 72 h | 2.65 (n = 24) | −0.98 (n = 21) | 0.587 |
| 96 h | 2.90 (n = 20) | −2.98 (n = 20) | 0.403 |
| SF increment | |||
| 48 h | −0.04 (n = 25) | 0.37 (n = 25) | 0.095 |
| 72 h | 0.01 (n = 20) | 0.28 (n = 21) | 0.351 |
| 96 h | 0.13 (n = 20) | 0.43 (n = 17) | 0.359 |
FiO2: inspired fraction of oxygen; SF: saturation/inspired fraction of Oxygen.
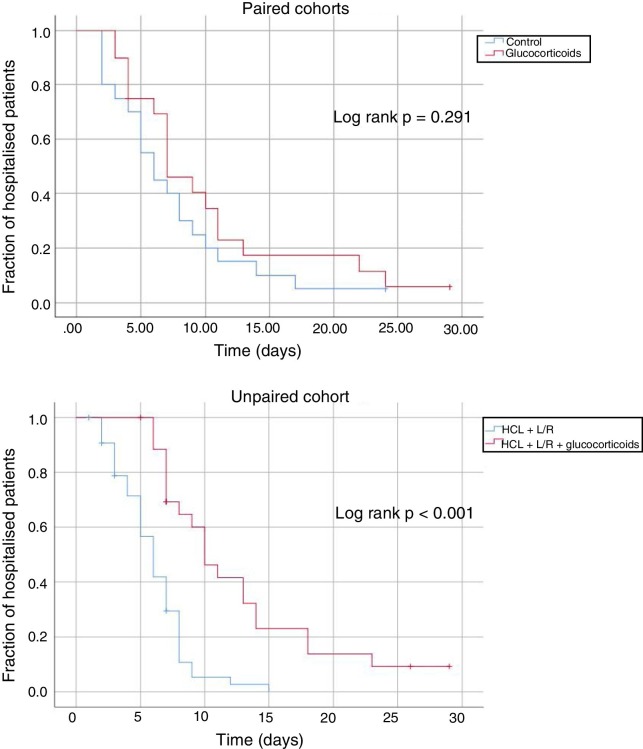
In the paired cohorts, the mean time to discharge of the patients who did not die was 10.3 days (95% CI: 6.9–13.7 days) in the case of the patients treated with glucocorticoids, and 7.5 days (95% CI: 5.1–9.9 days) in the case of the control patients. In the unpaired cohort, this time was 12.0 days (95% CI: 10.0–16.0 days) for patients treated with glucocorticoids and 7.0 days for those not treated with glucocorticoids (95% CI: 6.0–8.0). Fig. 1 shows the unadjusted Kaplan-Meier comparison curves and log-rank test, for time to discharge, in the subcohorts studied. After adjustment of the model for the rest of the confounders was taken into consideration, the multivariate Cox regression models showed that glucocorticoid treatment was associated with longer hospitalisation (hazard ratio 7.26; 95% CI: 3.30–15, 95).
Fig. 1.
Kaplan-Meier comparison curves and log-rank test of the different subcohorts of patients studied.
HCL: hydroxychloroquine; L/R: lopinavir-ritonavir.
There were 6 deaths (8.1%) in the unpaired control subcohort (patients treated with hydroxychloroquine and lopinavir/ritonavir), and 10 deaths (13.2%) among the patients who received additional glucocorticoid therapy. We consider this number of events insufficient to draw conclusions.
Discussion
Our study has not shown a benefit associated with the use of high-dose glucocorticoids in terms of respiratory function (SF) or time to discharge. In fact, hospitalisation was longer in the group treated with glucocorticoids in the unpaired subcohort analyses.
Our results coincide with those reported by Lu et al.20 and with those of Yuan et al.,21 who also found no benefit in the use of corticosteroids in samples of similar size and with adjusted comparison groups (Propensity Score). Lu et al. found an excess of mortality in critically ill patients with the increase in the steroid dose (4% for each increase of 10 mg of hydrocortisone), while the results of Yuan et al. showed a negative effect on recovery from lung damage in patients without severe pneumonia.
In contrast, Chroboczek et al.,23 in a study adjusted by relevant confounding factors, found that steroid therapy decreased the risk of intubation. Unfortunately, the publication does not report the type and regimen of the corticosteroid therapy. Along the same lines, a small study previously published in this journal by Callejas et al. shows a significant advantage of survival of the patient groups treated with glucocorticoids compared to the control group treated with tocilizumab.24 It must be said that the small sample size and the scarce number of events in this study (the control group had 9 patients) leads us to assume that they did not adjust for confounding variables, which obstructs the generalisation and interpretation of the results.
Preliminary results of the RECOVERY clinical trial have recently been published.22 This trial looks at the use of low-dose dexamethasone (6 mg/day) in the survival of patients with COVID-19. According to these preliminary data, treatment with 6 mg daily of dexamethasone would be associated with longer survival, especially in critically ill patients. We have not been able to analyse the mortality in our study, due to the low number of events in our sample. However, it seems that the positive result of the RECOVERY trial is discordant with our results which showed no benefit in the use of glucocorticoids in terms of lung function or time to discharge. Aside from the difference in the primary endpoints, a number of factors can explain this discrepancy. The most commonly used corticosteroid in our trial was methylprednisolone (78% in the paired subcohort and 98% in the unpaired subcohort) and, more importantly, the glucocorticoids in our cohort were not used at a low dose, but at a high dose (dose 3–6 times higher in the case of dexamethasone and a dose equivalent to 2.2 times higher in the case of methylprednisolone). As the authors themselves point out in the publication of the results of the RECOVERY trial, it is possible that the use of high doses of glucocorticoids is more harmful than beneficial, and therefore may be related to complications that could prolong hospitalisation, for example, as stated in the observational study by Lu et al.20 Finally, the patients who most benefited from treatment in the RECOVERY trial were the intubated critically ill patients, with the beneficial effects being more modest in patients with oxygen therapy, and nonexistent in mild patients. Our paired subcohorts only included one pair of intubated patients, with the rest of the patients with mild disease (as can be seen in the average SF of both groups), so it may have been difficult to observe a benefit therein.22
Given the observational nature of our study, the existence of residual confusion cannot be ruled out, mainly due to the tendency to administer glucocorticoids to patients at higher risk or with more severe disease. However, we feel that such confusion was unlikely or minor due to the exhaustive matching method used and the verification of the comparability of the groups. Furthermore, an imbalance between groups, with respect to the initially matched characteristics, can arise from the loss of patients after pairing, leading to the appearance of residual confusion. In our study, this does not affect the primary outcomes (SF at 48 h and time to discharge), since there was no loss of patients after pairing; however, it may affect the secondary outcomes, since the SF data are incomplete after 72 h follow-up.
In addition to its observational design, our study is limited by its small sample size, which creates statistical power problems, especially in the case of some of the most interesting outcomes, such as mortality, which could not be studied in this sample. Additionally, the use of secondary data, obtained from the medical history, might have lead to information biases. However, given that the primary variables are quantitative parameters, which are influenced minimally by the observer or their expertise in obtaining measurements, and given that these parameters are routinely collected in clinical practice and hospital management, we consider the existence of a relevant bias of this type to be unlikely. In any case, the sample size and the observational nature of our study make it necessary to wait for the confirmation which can come via the results of randomised clinical trials in which high doses of glucocorticoids have been used.
In conclusion, in this observational study we have not found evidence of a clinical benefit from the use of glucocorticoids at high doses in patients hospitalised for COVID-19. The use of high-dose glucocorticoids could lengthen the time of hospitalisation in these patients.
Financing
This study is funded by the Consorci Sanitari de l'Alt Penedès i Garraf.
Conflict of interest
None.
Footnotes
Please cite this article as: Rodríguez-Molinero A, Pérez-López C, Gálvez-Barrón C, Miñarro A, Rodríguez Gullello EA, Collado Pérez I, et al. Asociación entre el tratamiento esteroideo a dosis alta, la función respiratoria y el tiempo hasta el alta en pacientes con COVID-19: Estudio de cohortes. Med Clin (Barc). 2020;156:7–12.
Appendix A. COVID-19 Researchers Group of the Consorci Sanitari de l’Alt Penedès i Garraf (CSAPG)
Alberti Casas, Anna PhD, MD; Avalos Garcia, Jose L MD; Borrego Ruiz, Manel BS, Campo Pisa, Pedro L; Capielo Fornerino, Ana M. MD; Dapena, María Dolores; MD; Fenollosa Artes, Andreu MD; Gris Ambros, Clara MD; Hernandez Martinez, Lourdes MD; López, Gabriela F.; Martín Puig, Mireia MD; Martínez, Sergi MD; Macho Pérez, Oscar MD; Molina Hinojosa, José C. MD; Peramiquel Fonollosa, Laura MD; Pisani Zambrano, Italo G. MD; Rives, Juan P. MD; Robles, Maria Teresa MD; Sabria Bach, Enric MD; Sanchez Rodriguez, Yris M. MD; Segura Martín, Maria del Mar RN; Tremosa Llurba, Gemma MD; Ventosa Gili, Ester MD; Venturini Cabanellas, Florencia I. MD; Vidal Meler, Natàlia RN.
References
- 1.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General’s opening remarks at the media briefing on COVID-19. 11 March 2020 [Internet]. [Cited 20 May 2020]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-.11-march-2020.
- 3.Di Gennaro F., Pizzol D., Marotta C., Antunes M., Racalbuto V., Veronese N. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int J Environ Res Public Health. 2020;17:2690. doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tu Y.-F., Chien C.-S., Yarmishyn A.A., Lin Y.-Y., Luo Y.-H., Lin Y.-Y. A review of SARS-CoV-2 and the ongoing clinical trials. Int J Mol Sci. 2020;21:2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol Sci. 2020;41:363–382. doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis S.R., Pritchard M.W., Thomas C.M., Smith A.F. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD004477.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 8.Lansbury L., Rodrigo C., Leonardi-Bee J., Nguyen-Van-Tam J., Lim W.S. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev. 2019;2 doi: 10.1002/14651858.CD010406.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui D.S. Epidemic and emerging coronaviruses (Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome) Clin Chest Med. 2017;38:71–86. doi: 10.1016/j.ccm.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith J.F., Antonio G.E., Kumta S.M., Hui D.S.C., Wong J.K.T., Joynt G.M. Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology. 2005;235:168–175. doi: 10.1148/radiol.2351040100. [DOI] [PubMed] [Google Scholar]
- 11.Sung J.J.Y., Wu A., Joynt G.M., Yuen K.Y., Lee N., Chan P.K.S. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee N., Allen Chan K.C., Hui D.S., Ng E.K.O., Wu A., Chiu R.W.K. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arabi Y.M., Arifi A.A., Balkhy H.H., Najm H., Aldawood A.S., Ghabashi A. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. 2014;160:389–397. doi: 10.7326/M13-2486. [DOI] [PubMed] [Google Scholar]
- 14.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quartuccio L., Semerano L., Benucci M., Boissier M.-C., de Vita S. Urgent avenues in the treatment of COVID-19: targeting downstream inflammation to prevent catastrophic syndrome. Joint Bone Spine. 2020;87:191–193. doi: 10.1016/j.jbspin.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Jiang W., He Q., Wang C., Wang B., Zhou P. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.06.20032342. [DOI] [Google Scholar]
- 17.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 19.Zha L., Li S., Pan L., Tefsen B., Li Y., French N. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X., Chen T., Wang Y., Wang J., Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24:241. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan M., Xu X., Xia D., Tao Z., Yin W., Tan W. Effects of corticosteroid treatment for non-severe COVID-19 pneumonia: a propensity score-based analysis [published online ahead of print, 2020 Jun 2] Shock. 2020 doi: 10.1097/SHK.0000000000001574. [DOI] [PubMed] [Google Scholar]
- 22.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chroboczek T., Lacoste M., Wackenheim C., Challan-Belval T., Amar B., Boisson T. Corticosteroids in patients with COVID-19: what about the control group? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callejas Rubio J.L., Luna Del Castillo J.D., de la Hera Fernández J., Guirao Arrabal E., Colmenero Ruiz M., Ortego Centeno N. Effectiveness of corticoid pulses inpatients with cytokine storm syndrome induced by SARS-CoV-2 infection. MedClin (Barc) 2020 Aug 8;155:159–161. doi: 10.1016/j.medcle.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]