Graphical abstract
Keywords: Lung cancer, SARS‐CoV‐2, COVID-19, Outpatient management, Survey
Highlights
-
•
Survey to evaluate the clinical management of NSCLC patients during the COVID-19 pandemic.
-
•
Delay of lung cancer diagnosis and a dramatic decrease of patients’ accrual within clinical trials.
-
•
Major changes in the treatment management of elderly population.
-
•
Major changes in the selection of second line treatments.
-
•
Telemedicine as a valid support to facilitate patient-healthcare interactions.
Abstract
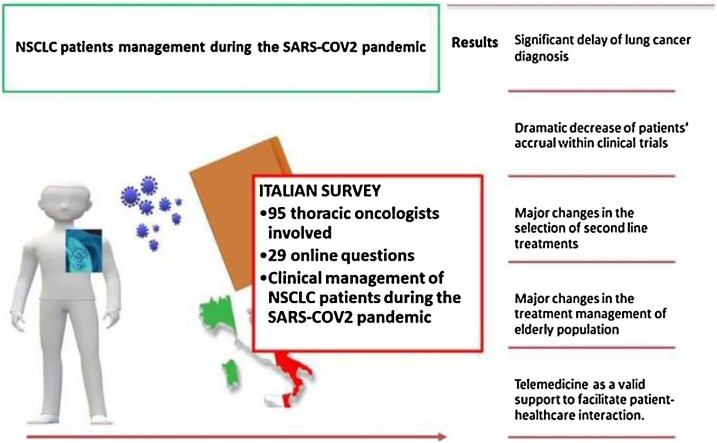
This study investigated the clinical management of non small cell lung cancer (NSCLC) patients during the first wave of coronavirus disease 2019 (COVID-19) outbreak in Italy. A 29-questions survey was sent to 95 Italian thoracic oncologists, with 77 % of them declaring significant changes in the outpatients management and treatment. The results of this survey pointed out a significant delay of lung cancer diagnosis along with a relevant reduction of patients’ accrual within clinical trials. Telemedicine emerged as a valid support for patient-healthcare interactions. Therapeutic indications followed the guidelines for adjuvant chemotherapy and concurrent chemo-radiation. Clinical indications to first-line therapies were largely confirmed, while major changes regarded the selection of second line treatment options as well as the management of elderly population.
This work may represent a valid source of information to improve the clinical management of NSCLC patients during second wave of COVID-19 pandemic.
1. Introduction
At the end of 2019, a novel viral pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was reported at Wuhan, China (Guan et al., 2020). Since then, the related coronavirus disease 2019 (COVID-19) syndrome, has progressively involved countries outside China leading the World Health Organization (2020) to declare the state of pandemic on 11th March 2020. Since the initial detection of the virus, more than 48 million cases and more than 1.200.000 deaths from COVID-19 have been confirmed worldwide (WHO website, 2020). Italy has been one of the most affected countries with, as of 7th November 2020, 809.000 confirmed COVID-19 cases and 48.000 deaths, according to the National Institute of Health (ISS,‘Istituto Superiore di Sanità, 2020) data (ISS website, 2020).
In this challenging situation, the oncological community was called to protect cancer patients, considered one of the most vulnerable population, due to coexisting chronic diseases, overall poor health status, type of infection and systemic immunosuppressive condition caused by both cancer and anticancer therapies (Kuderer et al., 2020). In this regard, the Italian Association of Medical Oncology (AIOM) has proposed specific recommendations to optimize the clinical management of patients and to regulate the access of both patients and caregivers to the hospitals, in order to encourage a careful evaluation of risk/benefit ratio case by case and, ultimately, minimize the risk of infection (AIOM website). Accumulating evidence suggest that lung cancer patients are more susceptible to infection since they are usually elderly and smokers, with poor nutritional status and compromised lung function. Several studies have already shown a higher risk of COVID-19 related complications in non-small cell lung cancer (NSCLC) patients, often related to their immunocompromised status (Rogado et al., 2020). Based on these evidences, the European Society for Medical Oncology (ESMO) has also provided management and treatment recommendations for NSCLC patients adapted to the COVID-19 era (Passaro et al., 2020). In this complex landscape, characterized by the recent occurrence of a second pandemic wave worldwide, where the emergency evolution continues to be uncertain and clinical decisions persistently difficult, sharing opinions and behaviors is of paramount importance to find new common “horizons” in the clinical management of NSCLC, and ultimately ensure, the best care to our patients. Based on these considerations, here we report the results of a national Italian survey carried out on April 2020, aiming to evaluate the clinical management of NSCLC patients and ultimately provide a reliable picture of real-word practice during the emergency of COVID-19.
2. Materials and methods
An online (Google_ form) survey (Supplementary Material) was developed by a group of thoracic oncologists working at the San Luigi Gonzaga Hospital, Orbassano (Turin), and distributed to different thoracic oncologists, across the different Italian regions. The online answers were systematically collected from the 12th of April 2020, until the 2nd of May 2020.
The current survey aimed to investigate the impact of COVID-19 outbreak on the attitudes and practice of thoracic oncologists in their clinical management of NSCLC patients.
The survey was confidential and anonymous and included four different sections, exploring the following topics: general questions about demographic and employment details of respondents (Q1-Q4); outpatient management of NSCLC patients (Q5-Q10); therapeutic decisions from the pandemic declaration to the following four weeks (March 11th -April 11th 2020) (Q11-Q21); clinical management of NSCLC patients with suspected or confirmed COVID-19 infection (Q22−23). Twenty-nine questions have been drawn up with multiple and single answers admitted for 17 and 12 questions, respectively. Descriptive statistics were used to summarize the data. Categorical variables were summarized as counts and percentages.
3. Results
The online survey was distributed to 95 thoracic oncologists from all over Italian Regions. A total of 79 responses were received, with an overall response rate of 83 %. The majority of responders (60) usually manage between 150–200 new cases of NSCLC patients per year. The general characteristics of responders are shown in Supplementary table.
3.1. Outpatient/Day hospital management of NSCLC patients
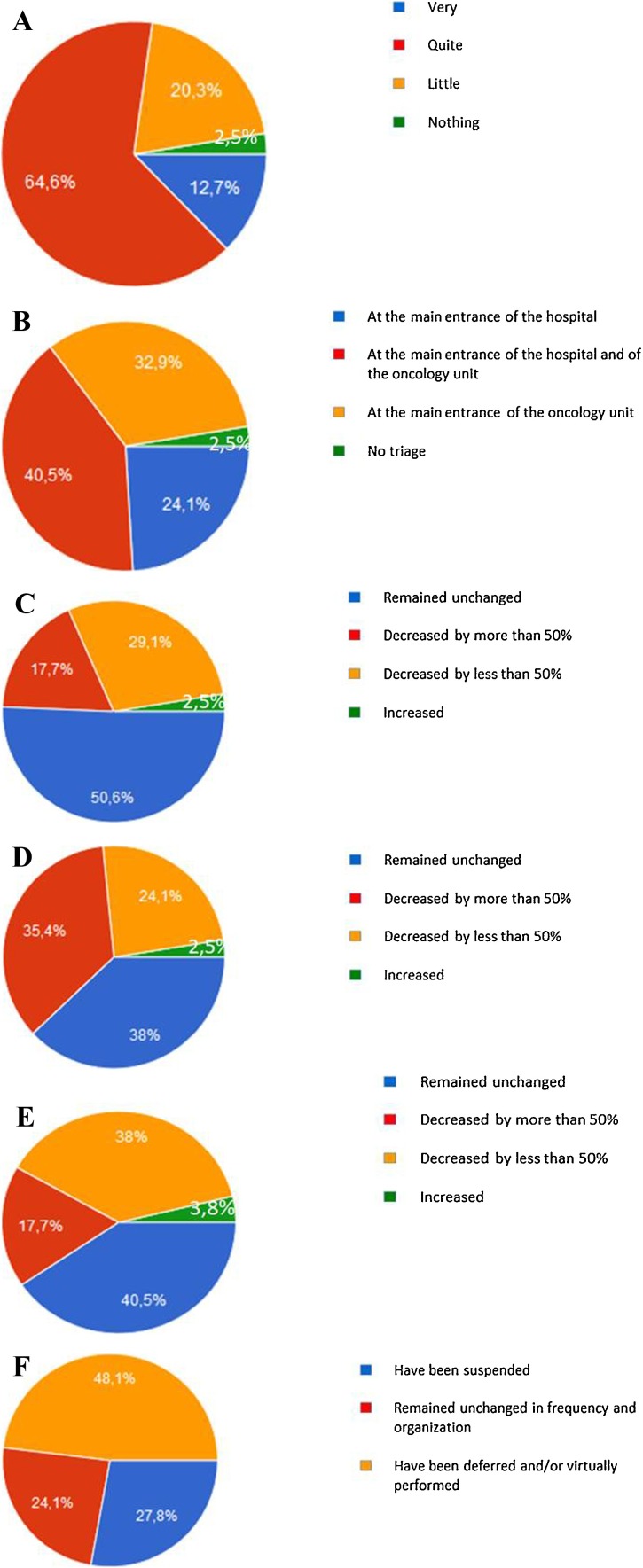
The majority of oncologists (77.3 %) declared a significant change in the outpatient/day hospital management of NSCLC patients during the COVID-19 emergency (Fig. 1 A). In most cases (97.5 %), triage of patients, always including body temperature measurement, was regularly performed before entering the hospital and/or the Medical Oncology Unit. (Fig. 1B). As compared to the pre−COVID-19 emergency period, the number of consultations in case of suspected NSCLC diagnosis decreased in about half of cases (46.8 %), with a major reduction when considering the number of patients coming from the emergency department, as reported in about 60 % of cases (Fig. 1C and D). As consequence, the total number of patients with any stage, newly diagnosed NSCLC, within the observational period, was reported to be lower than the pre-pandemic era by the 55.7 % of oncologists (Fig. 1E). Interestingly, multidisciplinary tumor boards were delayed or performed virtually in about half of cases (48.1 %) (Fig. 1F).
Fig. 1.
Schematic representation of the responses to the following survey’s questions: A Has the outpatient/Day Hospital organization for the management of NSCLC patients changed? B Where NSCLC patients underwent triage (body temperature measurement, questions about epidemiological status and clinical conditions)?. C Did the the number of consultations in patients with suspected NSCLC diagnosis vary?. D Did the number of consultations in patients with suspected NSCLC diagnosis coming from emergency department vary?. E Did the number of patients with newly diagnosed of NSCLC vary?. F Has multidisciplinary tumor boards for the discussion of NSCLC patients management been manteined?.
3.2. Treatment of NSCLC patients
3.2.1. Early and locally advanced disease
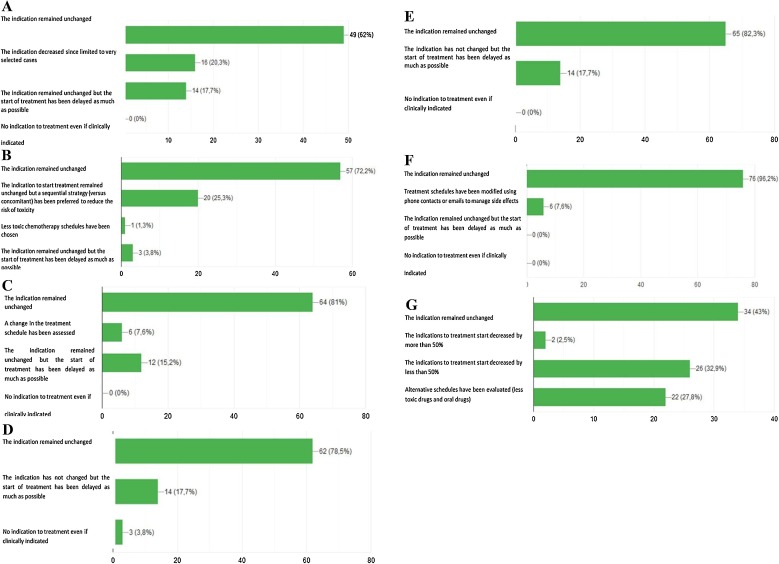
We asked physicians to consider their therapeutic indications for patients with stage I-III NSCLC, from the pandemic declaration to the following four weeks (March 11th -April 11th 2020) in comparison to the pre−COVID-19 period. As for chemotherapy administrated with adjuvant intent, clinical therapeutic indications did not change for the majority of responders (62 %) (Fig. 2 A). Likewise, for the treatment of unresectable locally advanced disease, most of the Italian thoracic oncologists (72.2 %) confirmed, in accordance with available guidelines, the unchanged indication to concomitant chemoradiation. However, a significant subgroup (25.3 %) of colleagues declared their preference for the sequential strategy, because they consider this approach safer in terms of pulmonary toxicity (Fig. 2B).
Fig. 2.
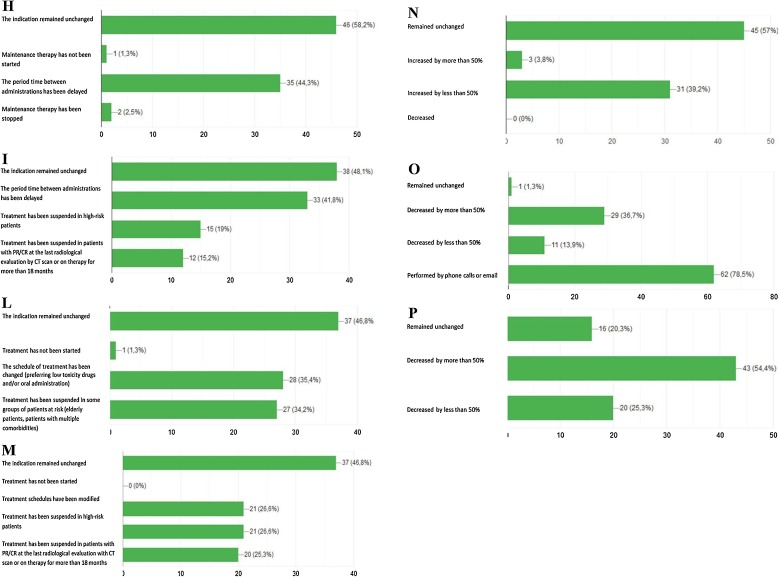
Schematic representation of the responses to the following survey’s questions: A Has the indication to adjuvant treatment been modified for surgically resected NSCLC patients? B Has the indication to concurrent chemo-radiotherapy been modified for NSCLC patients with unresectable locally advanced disease?. C Has the indication to first-line chemotherapy been modified for patients with metastatic NSCLC?. D Has the indication to first-line chemo-immunotherapy been modified for patients with metastatic NSCLC?. E Has the indication to first-line single agent immunotherapy been modified for patients with metastatic NSCLC?. F Has the indication to first-line targeted therapies been modified for patients with metastatic NSCLC? G Has treatment indication to first line chemotherapy or immunotherapy been modified in elderly patients (over 75 years) with non-oncogene addicted metastatic NSCLC?. H Has treatment indication to manteinance therapy benn modifed for patients with metastatic NSCLC candidate to start/continue maintenance chemotherapy (pemetrexed). I Has treatment indication for metastatic NSCLC patients undergoing first line immunotherapy been modified?. L Has treatment indication to second line chemotherapy been modified fro metastatic NSCLC patients?. M Has treatment indication to second line immunotherapy been modified for metastatic NSCLC patients?. N Has the indication to interrupt active treatment and start best supportive care been modified in metastatic NSCLC patients?. O Has follow-up management for NSCLC patients been modified?. P Has NSCLC patients’ accrual in ongoing clinical trials been modified?.
3.2.2. First-line therapy
Physicians reported their clinical indications to the different first line treatment options during the COVID-19 pandemic. As regards chemotherapy, the majority of them (81 %) declared that neither treatment recommendations were modified nor ongoing therapies were suspended. (Fig. 2C). Similarly, clinical indications to first-line immunotherapy alone or in combination with chemotherapy remained unchanged for 78.5 % and 82.3 % of responders, respectively (Fig. 2D,E). However, the 17.7 % of them declared to have delayed the beginning of immune-based treatments as far as possible (Fig. 2D,E). As for frontline tyrosine kinase inhibitors (TKIs), almost all responders (96.2 %) confirmed that their clinical indication did not change, while only 7.6 % modified the therapeutic schedule of TKI administration (Fig. 2F). In elderly patients (over 75 years) with non-oncogene-addicted metastatic NSCLC and potentially candidate to first line therapy (chemotherapy or immunotherapy), therapeutic indications were subjected to relevant changes in the majority of cases, with about 33 % of colleagues declaring a significant decrease of treatment prescription and 27.8 % of them preferring alternative, less toxic schedules, or oral drugs, in order to reduce hospital accesses, and consequently risk of infection (Fig. 2G). Maintenance therapy with pemetrexed has been regularly administered from the majority of oncologists, but free-interval between administrations has been frequently prolonged by one week (44.3 % of cases) (Fig. 2H). Similarly, as regards first-line immunotherapy, a significant percentage of oncologists declared to have prolonged the free-interval between treatment administrations (42 %), to have suspended ongoing therapy in high-risk patients (19 %), and to have definitively discontinued treatment in case of partial or complete response confirmed at the last computed tomography (CT) as well as in cases of long-term disease control (> 18 months of treatment) (15.2 %) (Fig. 2I).
3.2.3. Second-line therapy
The management of second line treatment, including either chemotherapy or immunotherapy was subjected to relevant changes for most of responders (59 %), with about half of them declaring their preference for alternative schedules/drugs characterized by low toxicity profile and/or oral administration. In the remaining cases, treatment was discontinued in high-risk subgroups, like elderly or patients with multiple comorbidities. As for second line immunotherapy, schedule of treatment has been modified or suspended in high risk patients in 27 % of cases and 25 % of oncologists declared to have discontinued treatment in case of partial or complete response confirmed at the last CT scan or in case of achievement of more than 18 months of treatment (Fig. 2L,M). About 40 % of oncologists declared as the tendency to discontinue any active second-line treatment in favor of best supportive care (BSC) was slightly increased during the first wave of COVID-19 pandemic (Fig. 2N). For patients in follow up (out of any active treatment), a telemedicine approach through phone calls or emails was preferred by 78.5 % of responders. Finally, as regards clinical cancer research during the COVID-19 outbreak, the majority of oncologists (79.7 %) declared that the accrual of lung cancer patients within clinical trials decreased by more than 50 % (Fig. 2O,P).
3.3. Clinical management of NSCLC patients with suspected COVID-19
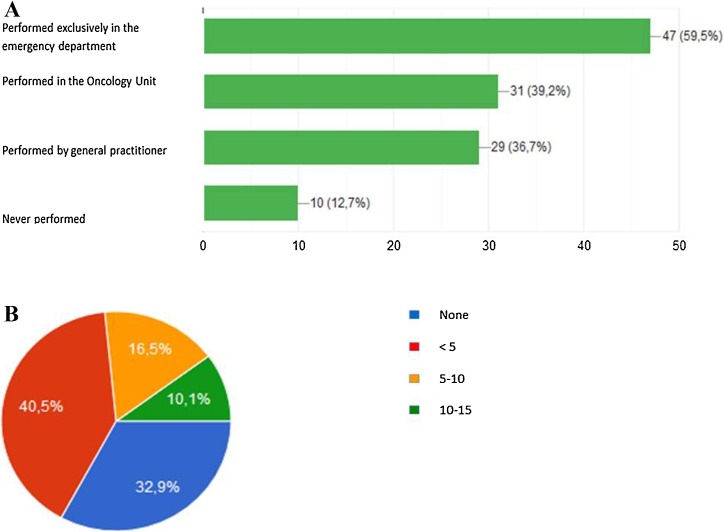
In case of suspected COVID-19 infection in NSCLC patients, in the majority of cases the nasopharyngeal swab was performed at the Emergency Department (60 %), followed by the Medical Oncology Unit (39 %) or general practitioners (37 %) (Fig. 3 A). The majority of oncologists (73 %) declared to have had less than 5 NSCLC patients or none affected by COVID-19 infection at their Institution, within the observational period (Fig. 3B). The characteristics of COVID-19 infection management and outcomes in NSCLC patients were reported in Table 1 .
Fig. 3.
Schematic representation of the responses to the following survey's questions: A In case of suspected COVID-19 infection in NSCLC patients, where the nasopharyngeal swab has been performed?. B How many NSCLC patients have been positive for COVID-19 infection in your Institution considering the period from the pandemic declaration to the following four weeks (March 11th-April 11th 2020)?.
Table 1.
Characteristics of NSCLC patients with suspected/confirmed COVID-19 infection.
| Characteristics | Number (%) |
|---|---|
| Mean age of patients: | |
| <50 years | 1 (1.9 %) |
| 50−60 years | 8 (15.7 %) |
| 60−70 years | 29 (56.8 %) |
| >70 years | 13 (25.5 %) |
| Patients treated with hidroxycloroquine +/-antiviral therapy: | |
| None | 8 (15.7 %) |
| <20 % | 12 (23.5 %) |
| 20−50% | 7 (13.7 %) |
| >50 % | 17 (33.3 %) |
| All patients | 7 (13.7 %) |
| Patients who have achieved recovery/stability: | |
| None | 5 (10.2 %) |
| <20 % | 7 (14.3 %) |
| 20−50% | 11 (22.4 %) |
| >50 % | 17 (34.7 %) |
| All patients | 9 (18.4 %) |
4. Discussion
In general, the results of this survey highlighted as both management and treatment strategies of NSCLC patients have been profoundly modified after the COVID-19 outbreak. Particularly, the reduction of the total number of novel NSCLC diagnoses, partially ascribed to the significant decrease of patients with suspected lung cancer coming from the emergency department, certainly represents one of the most relevant aspects to be adequately addressed. A recent paper, published by Dinmohamed et al., showed also a significant decrease of cancer diagnoses in the Netherlands, when compared to the pre−COVID-19 emergency period (Dinmohamed et al., 2020). Specifically, between the 6th of January 2019 and the 12th of April 2020 the relative change in lung cancer diagnoses was reported to be around 30–40 %. This evidence has been partially explained by the lower access of individuals with non-specific cancer symptoms to their general practitioner, leading to a subsequent delay of clinical investigations. Furthermore, several hospitals primarily involved in the COVID-19 emergency, have had postponed diagnostic evaluation, while national screening programs for early diagnosis of breast, colorectal and cervical cancers have been temporarily halted. The ministerial decrees, which placed Italy under COVID-19 lockdown, forced all Regions to temporally suspend both first level examinations and screening programs, with potential relevant consequences in terms of health and social costs, which will likely emerge in the upcoming months. Despite the emergency status, the majority of Italian medical oncologists maintained their clinical indications to adjuvant treatments and to concurrent chemo-radiotherapy for NSCLC patients with resected and locally advanced disease, respectively. These data reflect the recommendations published by ESMO with three grades of priority (high, medium and low) based on the magnitude of overall survival (OS) gain and improvement of quality of life (QoL) (Passaro et al., 2020). Particularly in the early stages, the high priority is related to the administration of adjuvant chemotherapy in T3/4 N2 for young (<65 years) and fit patients. Similarly, concomitant or sequential chemo-radiotherapy for unresectable NSCLC stage III has been prescribed without delay. For metastatic patients, there was a general consensus among the interviewed oncologists to not delay first-line therapies. ESMO guidelines also highlighted the high priority for therapeutic options in the metastatic setting to maintain survival benefit, along with cancer-related symptoms and QoL control. The final decision should be performed at single patient level, taking into account different factors, like patients’ clinical conditions (age, comorbidities, symptoms), disease’s pathological/molecular characteristics and treatments implications, including the number of hospital accesses required by treatment schedules, carefully balancing both predictable and unpredictable side effects. Despite the risks of toxicity associated with immune-checkpoint inhibitors were not well known during the first wave of COVID-19 emergency, a large fraction of oncologists declared to have spread the free-interval between treatment administrations both in first, and second line setting; to have suspended ongoing therapy in high-risk patients and to have definitively discontinued treatment in case of long-term clinical benefit. In this context, some preliminary evidence suggested that the frequency of severe illness and hospitalization was higher in cancer patients treated by immunotherapy, but the low number of patients included limited any definitive conclusions or changes in our current treatment practice (Robilotti et al., 2020). Moreover, a recent paper evaluated the impact of the programmed death-1 (PD-1) blockade on the severity of COVID-19 infection in NSCLC patients. The authors pointed out that PD-1 inhibition was not associated with an increased risk of severity of COVID-19 infection in patients with lung cancers (Luo et al., 2020). In the upcoming months, preclinical and clinical data will likely help oncologists to clarify the link between COVID-19 infection and possible worse outcome for NSCLC patients treated with immunotherapy. Another relevant aspect emerging from this survey regards the clinical management of elderly population, almost characterized by a significant reduction of first-line treatment prescriptions, including a therapeutic shift to alternative, less toxic schedules or oral drugs, in order to minimize their access to Oncology Departments. A multidisciplinary assessment by the Comprehensive Geriatric Assessment (CGA) emerged as an important tool during this emergency, to plan both medical and social health care for every patient (Passiglia et al., 2020). As for the follow-up visits, this survey pointed out that most of oncologists performed their clinical consultations by mails, phone call and/or web virtual meetings. This strategy has been supported by the AIOM statement, recommending a telemedicine follow-up approach in order to perform a quick triage of the clinical condition, and allowing the examination of laboratory and/or imaging exams. Another major issue regards clinical research activities during the COVID-19 pandemic, characterized by a dramatic decrease of patients’ accrual within clinical trials, with a relevant impact on the quality of care to be offered to our patients. Similarly, the management of patients already enrolled within a clinical trial may have undergone changes during this emergency time. Therefore, to face this risk, the Food and Drug Administration (FDA) (2020), the European Medicines Agency (EMA) (2020) and the Italian Medicines Agency have issued special guidance for the conduction of clinical trials during the COVID-19 emergency (Agenzia Italiana del Farmaco (AIFA), 2020).
Finally, this survey showed a small snapshot of NSCLC patients with COVID-19 infection. Most of oncologists had less than 5 patients infected by COVID-19, generally treated with hydroxychloroquine and antiviral therapy. Several evidences have showed a proportion of asymptomatic COVID-19 patients that play a critical role as source of transmission to cancer patient (Bai et al., 2020). Therefore, it is important to consider NSCLC patients as a specific population to be priority tested due to the increased risk of complications caused by older age, significant cardiovascular and respiratory comorbidities and smoking-related lung damage. In this pandemic scenario, the use of liquid biopsy might be implemented in order to reduce the risks of COVID-19 infection for NSCLC patients by limiting their access to the oncology departments (Rolfo et al., 2020) The proposed diagnostic approach might be practice changing even in the upcoming months when COVID-19 outbreak will be under control.
5. Conclusions
In conclusion, this survey provides a real-word “picture” of the clinical management of NSCLC patients in Italy during first wave of COVID-19 emergency. Although the majority of Italian thoracic oncologists have proven to follow both national and international treatment guidelines even during this difficult period, however major concerns regarded the selection of second or further lines of treatment as well as the clinical management of elderly population, characterized by a potentially unfavorable risk/benefit ratio. The most relevant issues emerging from this survey included the significant delay of lung cancer diagnosis and the dramatic decrease of patients’ accrual within clinical trials, while telemedicine demonstrated to be a valid support to facilitate patient-healthcare interactions.
These data should be keep in mind in the upcoming months, characterized by the second wave of COVID-19 pandemic, in order to provide right solutions to the emerging criticisms and ultimately improve the worldwide clinical management of NSCLC patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
Novello S declared speaker bureau/advisor’s fee from Eli Lilly, MSD, Roche, BMS, Takeda, Pfizer, Astra Zeneca and Boehringer Ingelheim.
Passiglia F declared consultant’s fee from MSD, Boehringer Ingelheim and AstraZeneca.
Bironzo P declared consultant’s fee from MSD, BMS, AstraZeneca, BeiGene and Roche.
Capelletto E declared consultant’s fee from MSD, AstraZeneca, Boehringer Ingelheim.
Other co-authors have no COI to declare.
CRediT authorship contribution statement
Valentina Bertaglia: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing - original draft. Maria Lucia Reale: Data curation, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing - original draft. Paolo Bironzo: Investigation, Validation, Visualization. Erica Palesandro: Investigation, Validation, Visualization. Annapaola Mariniello: Investigation, Validation, Visualization. Gianmarco Leone: Investigation, Validation, Visualization. Fabrizio Tabbò: Investigation, Validation, Visualization. Maristella Bungaro: Investigation, Validation, Visualization. Marco Audisio: Investigation, Validation, Visualization. Simonetta Rapetti: Investigation, Validation, Visualization. Rosario Francesco Di Stefano: Investigation, Validation, Visualization. Simona Carnio: Investigation, Validation, Visualization. Elisa Artusio: Investigation, Validation, Visualization. Enrica Capelletto: Investigation, Validation, Visualization. Paola Sperone: Investigation, Validation, Visualization. Francesco Passiglia: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - review & editing. Silvia Novello: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - review & editing.
Biographies
Dr. Valentina Bertaglia (MD, PhD) is a consultant at Thoracic Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on thoracic malignancies.
Dr. Reale Maria Lucia (MD) is a Medical Oncologist at Thoracic Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on thoracic malignancies.
Dr. Paolo Bironzo (MD) obtained specialisation in Medical Oncology in 2017 at the University of Torino. He is a PhD student on Complex Systems for Life Science at University of Turin. He was appointed assistant professor at the Department of Oncology of the University of Torino in 2018. His research is mainly focused on lung cancer and malignant pleural mesothelioma. He is scientific secretary of the Italian Association of Medical Oncology (AIOM) Clinical Practice Guidelines on Malignant Pleural Mesothelioma. He is author of several papers on peer-reviewed journals.
Dr. Erica Palesandro (MD) is a Medical Oncologist with clinical and research focus on genito-urinary malignancies.
Dr. Annapaola Mariniello (MD) is a Medical Oncologist at the Thoracic Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on thoracic malignancies. She is PhD student in Complex Systems for Quantitative Biomedicine within the Oncology Department at the University of Torino.
Dr Gianmarco Leone (MD) is a Medical Doctor resident at Medical Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on genito-urinary malignancies.
Dr Fabrizio Tabbò (MD, PhD) is a Medical Doctor resident at the Thoracic Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on thoracic malignancies.
Dr Maristella Bungaro (MD) is a Medical Doctor resident at the Thoracic Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on thoracic malignancies.
Dr Marco Audisio (MD) is a Medical Doctor resident in Medical Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and researc focus on gastro-intestinal malignancies.
Dr Simonetta Rapetti (MD) is a Pulmonologist at San Luigi Hospital, Orbassano (Turin) with clinical focus on thoracic malignancies.
Dr Rosario Francesco Di Stefano (MD) is a Medical Oncologist at Medical Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on genito-urinary malignancies.
Dr Simona Carnio (MD) is a Medical Oncologist at Thoracic Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on thoracic malignancies and palliative care.
Dr Elisa Artusio (MD) is a Medical Oncologist at Thoracic Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical focus on thoracic malignancies.
Dr Enrica Capelletto (MD) is a Pulmonologist at Thoracic Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on thoracic malignancies. She is currently PhD student in Biomedical Science and Oncology within the Oncology Department at the University of Torino
Dr Paola Sperone (MD) is a Medical Oncologist at Medical Oncology Unit of San Luigi Hospital, Orbassano (Turin) with clinical and research focus on breast cancer.
Francesco Passiglia (MD) is Assistant Professor at the Department of Oncology of the University of Turin, Italy. He is consultant at the Thoracic Oncology Unit of San Luigi Hospital, Orbassano (Turin). Since October 2019 he is the scientific secretary of the Italian Association of Medical Oncology (AIOM) Lung Cancer Clinical Practice Guidelines. He is member of international scientific societies including the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), the International Association for the Study of Lung Cancer (IASLC). He is author or co-author of over 60 publications in peer-reviewed journals.
Prof.ssa Silvia Novello (MD, PhD) is Full Professor of Medical Oncology in the Oncology Department at San Luigi Hospital in Orbassano, Italy, part of the University of Turin. She is head of the Thoracic Oncology Unit at the San Luigi Hospital, Orbassano (Turin), where she also tutors medical students and Postgraduate students in Respiratory Medicine and Medical Oncology. Prof. Novello’s research interests include thoracic malignancies, primary prevention, gender differences in lung cancer, basic, translational and clinical applied research on lung cancer, including pharmacogenomics. She is involved as PI in many International and national controlled clinical trials evaluating new approaches in diagnosis and lung cancer therapy. From July 2012 until 2016, Prof Novello has been a Member of the Board of Directors of the International Association for the Study of Lung Cancer and since October 2016 Member of the Board of Directors of the Italian Association of Medical Oncology, Secretary of the EORTC Lung Cancer Group and member of several scientific societies including the American Society of Clinical Oncology, American Thoracic Society and the European Society of Medical Oncology. Currently, she the President of WALCE (Women Against Lung Cancer in Europe), a non profit European Association founded in 2006 in Turin, Italy, part of the scientific Committee of LuCe (Lung cancer Europe) and also member of the Scientific Committee of Bonnie J Addario Lung Cancer Foundation and Member of the Scientific Committee of ICAPEM (Investigación sobre Cáncer de Pulmón en Mujeres). She is the author or co-author of over 200 publications in peer-reviewed journals.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.critrevonc.2020.103189.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Agenzia Italiana del Farmaco (AIFA). Available: https://www. aifa. gov. it/ documents/ 20142/ 871583/ Comunicato_ gestione_ studi_ clinici_ in_ emergenza_ COVID- 19_ EN_ 12. 03. 2020. pdf/ ee1f33e3- bb3e- 9ce9-2a93- b33e88eea94 (accessed 20 August 2020).
- Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinmohamed A.G., Visser O., Verhoeven R.H.A. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;6:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency (EMA). Available: https://www. ema. europa. eu/ en/ news/ guidance- sponsors- how- manage- clinical- trials- during- covid- 19- pandemic (accessed 20 August 2020).
- Food and Drug Administration (FDA). Available: https://www. fda. gov/ media/ 136238/ download (accessed 20 August 2020).
- Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istituto Superiore di Sanità. November 7, 2020 https://www.epicentro.iss.it/coronavirus/sars-cov-2-dashboard. (accessed 7 November 2020).
- Kuderer N.M., Choueiri T.K., Shah D.P. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Rizvi H., Egger J.V. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;8:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaro A., Addeo A., Von Garnier C. ESMO management and treatment adapted recommendations in the COVID-19 era: lung cancer. ESMO Open. 2020;5(Suppl3):e000820. doi: 10.1136/esmoopen-2020-000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passiglia F., Pilotto S., Facchinetti F. Treatment of advanced non-small-cell lung cancer: the 2019 AIOM (Italian Association of Medical Oncology) clinical practice guidelines. Crit. Rev. Oncol. Hematol. 2020;146 doi: 10.1016/j.critrevonc.2019.102858. [DOI] [PubMed] [Google Scholar]
- Robilotti E.V., Babady N.E., Mead P.A. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogado J., Pangua C., Serrano-Montero G. Covid-19 and lung cancer: a greater fatality rate? Lung Cancer. 2020;146:19–22. doi: 10.1016/j.lungcan.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfo C., Russo A., de Miguel-Pérez D. Speeding tumor genotyping during the SARS-CoV-2 outbreak through liquid biopsy. Cancer. 2020 doi: 10.1002/cncr.32983. doi: 10.1002/cncr.32983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Novel Coronavirus — China.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.