Abstract
The netrins form a family of laminin-related proteins which were first described as modulators of cell migration and axonal guidance during fetal development. Netrin-1 is the most extensively studied member of this family and, since its discovery, non-neural roles have been associated with it. Together with its receptors, DCC/neogenin and UNC5, netrin-1 has been shown to be involved in the regulation of angiogenesis, organogenesis, cancer and inflammation. An NF-κB-dependent truncated isoform of netrin-1 has also been shown to be produced in endothelial and some types of cancer cells, which both accumulates in and affects the function of the nucleus. In atherosclerosis, conflicting roles for netrin-1 have been reported on plaque progression via its receptor UNC5b. Whereas endothelial-derived netrin-1 inhibits chemotaxis of leukocytes and reduces the migration of monocytes to the atherosclerotic plaque, netrin-1 expressed by macrophages within the plaque plays a pro-atherogenic role, promoting cell survival, recruiting smooth muscle cells and inhibiting foam cell egress to the lymphatic system. In contrast, there is evidence that netrin-1 promotes macrophage differentiation to an alternative activated phenotype and induces expression of IL-4 and IL-13, while downregulate expression of IL-6 and COX-2. Further work is needed to elucidate the precise roles of the two isoforms of netrin-1 in different cell types in the context of atherosclerosis, and its potential as a putative novel therapeutic target in this disease.
Keywords: Pathophysiology, atherosclerosis, cardiology, cell biology/structural biology, cell signalling/signal transduction, mechanism of atherosclerosis/growth factors, vascular biology
Introduction
Atherosclerosis, the principal pathophysiology underlying ischaemic heart disease and stroke, is caused by progressive accumulation of cholesterol in the arterial wall. In recent years, it has become clear that there is an important inflammatory component to atherosclerosis, which progresses over a long period of time. Atherosclerosis is initially triggered by an accumulation of low-density lipoproteins (LDL) in the arterial wall, between the endothelium and the tunica intima, and mostly in artery segments where endothelial integrity is compromised, such as curvatures or branching points, due to local turbulence of blood flow.1 Once inside the arterial wall, LDL undergoes a chain of chemical and enzymatic reactions which ultimately results in an oxidized form of LDL, changing the biological function of its membrane phospholipids and creating degradation products, which promote oxidative stress.2 Oxidized LDL (oxLDL) activates the endothelium and induces the expression of chemokines and adhesion molecules, such as MCP-1 and VCAM-1, leading to the recruitment of leukocytes, especially monocytes.3 In the plaque, monocytes differentiate into macrophages and recognise oxLDL as a pathogen-associated molecular pattern, through its scavenger and toll-like receptors. This recognition leads to macrophage shifting into a pro-inflammatory phenotype, increasing the expression of pro-inflammatory cytokines and ultimately promoting atherogenesis.4 Macrophages take up cholesterol from the sub-intimal layer and transform into foam cells, losing their ability to egress to the lymphatic system and contributing to the development of necrotic plaque core and thinning of the fibrous cap, the plaque thereby becoming more vulnerable to erosion or rupture with resultant superadded arterial thrombosis giving rise to cardiovascular events.5 Netrins and semaphorins have been studied as strong candidates for causing the loss of mobility observed in foam cells.6,7 Nonetheless, more research is required to completely understand how these molecules modulate atherosclerosis.
Recent research has focussed on novel anti-inflammatory approaches which may offer benefits over and above standard cardiovascular therapies such as lipid-lowering and anti-platelet drugs. In this review, we will focus on the role of netrin-1 in normal physiology and in the modulation of inflammation, and we will discuss specifically how this may relate to the pathophysiology of inflammation in atherosclerosis and how this may pave the way to novel therapeutic approaches in this disease.
The netrin family of proteins
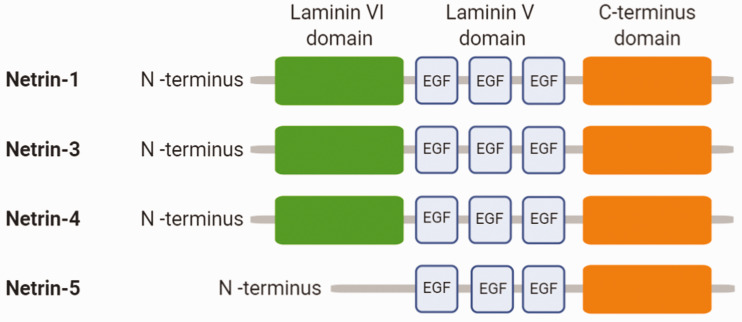
Netrins are a family of laminin-like proteins, first described in the early 1990s as axonal guidance cues during embryonic development. In humans, four different netrins have been described (netrin-1, -3, -4 and -5: Figure 1), presenting a secondary structure which is highly conserved between species. Netrin-1 to -4 are composed of one N-terminal domain VI, a positively charged C-terminal domain and three laminin V-type epidermal growth factor (EGF) domains with the same characteristic number and spacing of Cys residues as observed in laminins.8,9 Unlike the structures of netrin-1, -3, and -4, full length netrin-5 comprises three V-type EGF and the C-terminal domains but lacks the N-terminal laminin VI domain (Figure 1).10 While netrin-1 and 3 present similar V- and VI-type domains to those observed in laminin-γ1, netrin-4 amino-terminal domains are most similar to the amino terminus of the laminin-β1 chain1.11,12 The C-terminal domain is the part which varies the greatest between species. The C-terminal sequences are rich in basic amino acids, which are binding sites for membrane glucolipids and proteoglycans, thereby allowing interaction with cell surface components and extracellular matrix.9
Figure 1.
Schematic structure of the netrin family members.
Most of the members of the netrin family are secreted proteins and they are bifunctional, acting as both short- and long-range signals.13 They bind to different receptors due to the different homologies between them.
Although netrins were first defined due to their role in the development of the central nervous system, more recently other physiological and pathological roles have been ascribed to these proteins. There is evidence that netrins play important roles in different types of cancer and in the morphogenesis of various tissues;14 nonetheless, the only netrin that has been described in the cardiovascular system to date is netrin-1, and therefore we will concentrate on this family member in this review.
Netrin-1
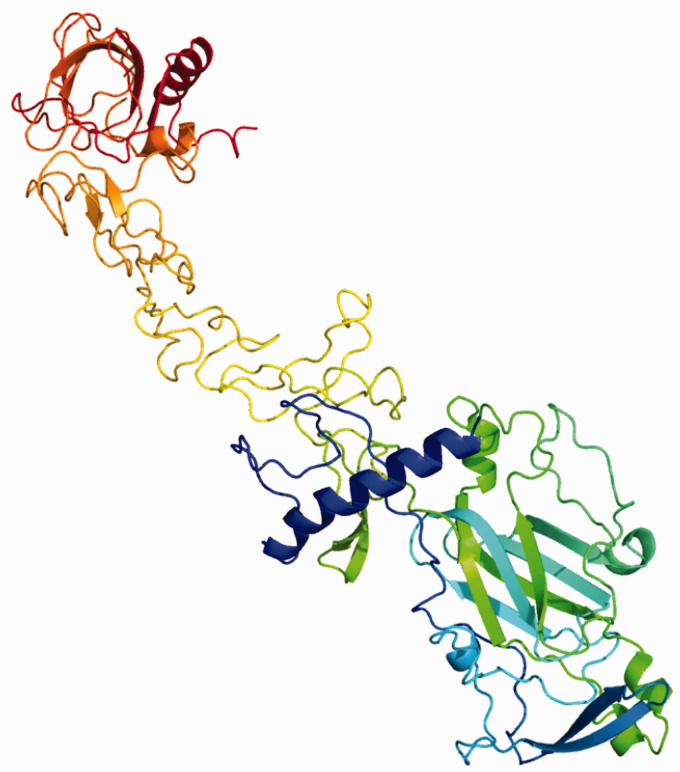
Netrin-1 is the most extensively studied of all the netrins. In humans, it is encoded by the gene NTN1 in chromosome 17 and encodes a highly conserved 604 amino acid protein. It is expressed by different types of cells and can be found in most tissues. Relative quantification of mRNA shows high expression of netrin-1 in brain, heart, kidney and lungs, and low expression in the liver, intestine and spleen.15Netrin-1 possesses two receptor-binding sites in the two ends of an elongated flower-shaped structure (Figure 2), which interact with two different receptor molecules.16
Figure 2.
Predicted unbound netrin-1 tertiary structure generated by Phyre2 software, using the human netrin-1 UniProt sequence. It has a flower-shaped structure, formed by the N-terminus on the bottom right hand-side of the Figure (green and blue motifs), followed by three V-type epidermal growth factor domains and the C-terminus at the top (red motif).
The functions of netrin-1 were initially identified as mediation of axonal orientation, axonal outgrowth and neuronal migration.17 The ability of netrin-1 to repel neuronal cells makes it a potential candidate for the regulation of inflammatory cell migration. Netrin-1 has been identified as having roles in angiogenesis,18 tumorigenesis,19 organogenesis20 and inflammation, suggesting that it regulates cell migration in a broad context, as described below.
Netrin-1 has been reported as an angiogenic agent in both physiological and pathological contexts, acting either as promoter or as inhibitor of angiogenesis. It is well established that netrin-1 participates in both angiogenesis and morphogenesis of the vasculature during fetal life and as well as in adult homeostasis. Similar to what is observed in axon formation, the specific effects of netrin-1 depend on which receptors it binds to. Park et al. showed that netrin-1 not only promotes angiogenesis itself, but also enhances the angiogenic activity of vascular endothelial growth factor (VEGF) both in vitro and in vivo.18Pro-angiogenic effects were also observed in pathological angiogenesis, where it promoted the creation of new vessels in conditions such as cancer. Shimizu et al. and Akino et al. respectively showed that netrin-1 promotes the creation of new vessels in glioblastoma and paediatric medulloblastoma, leading to a more aggressive pathology.21,22
Netrin-1 has also been classified as an oncogene due to its various roles in tumorgenesis, including inhibition of macrophage recruitment, promotion of cell survival, stimulation of invasiveness and of pathological angiogenesis. Several groups have shown that netrin-1 is upregulated in glioblastoma, medulloblastoma and other malignancies, and its overexpression leads to poorer prognosis.21,22 The ability of netrin-1 to inhibit apoptosis is related to binding to its specific receptors, which trigger apoptosis in the absence of the ligand. Cancers producing excessive amounts of netrin-1 will therefore have their apoptosis program inhibited. This interaction between netrin-1 and its receptors will be described in more detail in the following section.
In addition to axonal guidance and angiogenesis, the role of netrin-1 and its receptors in organogenesis have been studied in terms of its regulation of migration, differentiation and branching morphogenesis. The importance of this molecule in the development of tissues, such as lungs, pancreas or mammary glands, has been revealed in the last couple of decades.20,23,24 Dalvin et al. showed that netrin-1 controls epithelial-mesenchymal interaction, which is a key process in lung organogenesis.23 Furthermore, De Breuck et al. demonstrated that netrin-1 is involved in pancreatic morphogenesis and tissue remodelling in vivo.24 Together, these data show that netrin-1 and its receptors play an important role in the morphogenesis of branched tissues.
As mentioned above, there is evidence that netrin-1 mediates inflammation due to its effect on immune cells, having a role in their recruitment and motility, as well as in their stimulation and production of cytokines. Nonetheless, it is controversial whether a high expression of netrin-1 is beneficial or detrimental. Some studies show that netrin-1 has a protective effect in pathological models. In vivo work performed by Mirakaj et al. showed that expression of netrin-1 was repressed in acute inflammation in lung injury and peritonitis. Moreover, this group showed that treatment with exogenous netrin-1 dampened the inflammation and reduced tissue damage.25,26 In accordance with this, Grenz et al. demonstrated that acute kidney injury was aggravated in a mouse model with partial netrin-1 deficiency, and treatment with exogenous netrin-1 attenuated the effects observed, reducing tissue injury.27 In contrast, Mediero et al. have proposed a pro-inflammatory role for netrin-1, which is required for the development of particle-induced osteolysis, hypothesising that it leads to the recruitment, retention and enhancement of osteoclast differentiation and activity.28 Van Gils and colleagues have provided evidence that netrin-1 promotes atherosclerosis due to its effect on inhibiting the emigration of macrophages and increasing recruitment of smooth muscle cells to the plaque.29 Ramkhelawon et al. showed that adipose tissue produces high amounts of netrin-1, promoting macrophage retention, chronic inflammation and insulin resistance.30 The pro- and anti-inflammatory effects of netrin-1 therefore appear to depend on the model being studied.
Netrin-1 receptors
The function of netrin-1 in regulating neuronal navigation has been associated with two main types of receptors, termed dependence receptors: DCC (Deleted in Colorectal Carcinoma) and its orthologue neogenin, and UNC5 (UNCoordinated protein 5) receptors (UNC5a-d). These are receptors that play a dual role triggering different cellular responses in the presence or absence of ligand. On the one hand cell proliferation, migration and survival are promoted in the presence of netrin-1 whilst, on the other hand, apoptosis is induced in the absence of the ligand. Moreover, relative expression levels of both families of receptors will mediate the attraction or repulsion effects observed in neuronal development, which will be further explored below.31,32
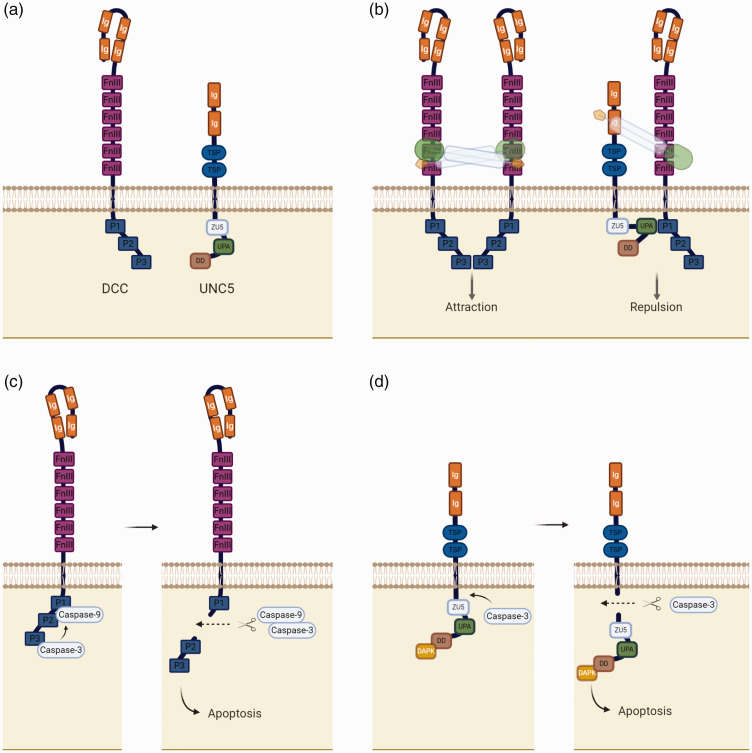
DCC and neogenin are receptor homologues which share nearly half of their amino acid identity and display the same secondary structure.33 They are single-pass transmembrane receptors with an extensive extracellular region, which comprises four immunoglobulin (Ig)-like domains in a horseshoe configuration,34 followed by six fibronectin type III domains (FnIII). The intracellular portion of the receptor contains three conserved motifs, which are responsible for signalling (P1, P2 and P3) (Figure 3(a)).35 Biochemical studies suggest that FnIII4, FnIII5 and FnIII6 are the DCC domains responsible for the binding of netrins.36,37
Figure 3.
Netrin-1 dependence receptors. (a) Schematic structure of DCC and UNC5 cytoplasmic and extracellular domains. (b) Netrin-1 binding to DCC-DCC and DCC-UNC5 complexes, promoting neuronal attraction and repulsion respectively. (c) Apoptosis promoted by DCC receptor in the absence of netrin-1. (d) Apoptosis promoted by UNC5 receptor in the absence of netrin-1.
UNC5 receptors are also single-pass transmembrane receptors with an extracellular region consisting of two Ig-like motifs, followed by two thrombospondin I modules (TSP).38 On the cytosolic side, UNC5 receptors have a long tail which consists of a ZU5 domain, a DCC-binding motif (UPA) and a death domain (DD) (Figure 3(a)).39 Both Ig-like domains may be responsible for the primary binding of netrins and it is suggested that the second motif (Ig2) contributes more to the binding than Ig1.36
Different signalling pathways have been proposed for these receptors and receptor complexes. In the development of the central nervous system, netrin-1 exerts a bifunctional effect, depending on which combination of receptors it binds to. Netrin-1 can promote attraction if it binds to a DCC-DCC complex, leading to the activation of several cascades of events which promote directional axonal outgrowth. The opposite effect occurs, namely repulsion, when netrin-1 binds exclusively to an UNC5 receptor or UNC5 and DCC simultaneously, promoting short- and long-range repulsion respectively (Figure 3(b)).32,40
DCC was originally characterised as a suppressor of colorectal cancer when a pronounced reduction of its expression was observed in several colorectal carcinoma cell lines, due to a deletion in chromosome 18q21.41 Many studies have established the importance of DCC/neogenin and netrin-1 in different pathologies. For example, Reyes-Mugica et al. and Koren et al. showed that loss of DCC expression is associated with poor prognosis in patients with glioma and breast cancer respectively.42,43 In the immune system, the DCC/neogenin receptor family mediates a pro-migration response to netrin-1. Boneschansker et al. reported that netrin-1 plays an important role inducing migration of leukocytes via its receptor neogenin. Netrin-1 is an inducer of CD4+ T cells, and its interactions with neogenin augmented the migration of these leukocytes in an in vitro model. The same group also reported that neogenin-expressing T cells are recruited in pro-inflammatory environments where netrin-1 is expressed. Using a humanized SCID mouse model engrafted with human skin, injection of netrin-1 led to an increase in T cell infiltration compared to vehicle injection.44
Apoptosis and cell survival have also been identified as downstream effects dependent on netrin-1 and its receptors. Mehlen et al. showed that, in the absence of netrin-1, DCC induces apoptosis. Moreover, this receptor is a caspase substrate which loses its pro-apoptotic effect if there is a mutation at the site where caspase-3 cleaves DCC (Figure 3(c)).45 Cleavage of UNC5 by caspase-3 similarly induces cell apoptosis, through its intracellular death domain (Figure 3(d)). However, contrary to that which is observed in DCC, mutation of the caspase-3 cleavage site of UNC5 does not inhibit apoptosis; on the other hand, deletion of the myristoylation signal peptide, which is localized in the C-terminus of the receptor, prevents its induction of apoptosis.31
Netrin-1 has been shown to play a key role in modulating the migration of leukocytes through its UNC5b receptor.15 Although the precise signalling pathway is not yet understood, there is strong evidence that this event is an important modulator in the recruitment of leukocytes and inflammation; this will be described further below, in the context of the role of netrin-1 in atherosclerosis.
CD146, an adhesion molecule expressed by endothelial cells, mostly in the vasculature, has also been described as a netrin-1 receptor. The molecular mechanisms associated with this receptor are angiogenesis, vessel permeability and leukocyte transendothelial migration.46 CD146 has been described as a pro-angiogenic mediator, counterbalancing the UNC5b anti-angiogenic signal. Further studies are needed to clarify which signalling pathways it utilises in leukocytes to modulate their migration.
Down syndrome cell adhesion molecule (DSCAM) has more recently been proposed as a novel receptor for netrin-1. Its collaboration with DCC has been shown to be essential for commissural axons to grow towards and across the midline.47 There is no evidence to date that this receptor plays a role in cardiovascular disease, and therefore it will not be explored further in this review.
Netrin-1 isoforms
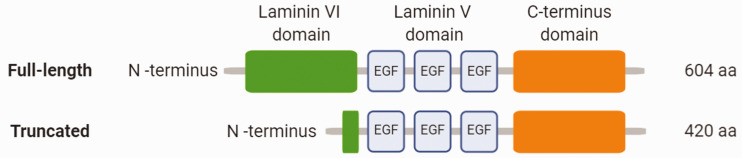
In the last decade, a number of studies have shown the existence of two isoforms of netrin-1 (Figure 4), which may help to explain some of the apparently conflicting effects reported for this protein.19,48,49Delloye-Bourgeois et al. first described a new netrin-1 isoform which is truncated and abundantly expressed in a neuroblastoma cell line model.19 This isoform is produced by an alternative promoter, localised in intron I of the netrin-1 DNA sequence, which is NF-κB dependent.48 They reported that the C-terminus of netrin-1 mediates its nucleolar localisation and the N-terminus prevents it, the latter instead promoting its secretion from the cell. As a result, the truncated netrin-1, which lacks part of the N-terminal domain, stays localised within the nuclei.19 Truncated netrin-1 has been shown to be expressed in a range of types of cancer, and high expression of this isoform appears to associate with poorer patient prognosis.19,49 This truncated isoform affects nucleolar function; for instance, in cancer cells, overexpression of this isoform promotes ribosome synthesis, tumour growth and cell proliferation.19
Figure 4.
Schematic structure of netrin-1 isoforms.
Passacquale et al. showed that endothelial cells also express the truncated isoform of netrin-1. TNF-α treatment disturbs the balance of expression between the two isoforms, promoting upregulation of the truncated one and downregulation of the secreted (full length) isoform. They also demonstrated that the two isoforms are regulated differently in endothelial cells. As previously described by Delloye-Bourgeois et al. in neuroblastoma cells, the truncated isoform promoter was found to be NF-κB dependent, so that treating endothelial cells with an NF-κB inhibitor supressed TNF-α-induced expression of the truncated isoform, both at the transcriptional and protein levels. They also showed that the induction of secreted full-length netrin-1 is NF-κB-independent, but on the other hand is dependent on histone H3 acetylation, which in turn could be induced by aspirin treatment of endothelial cells.48
The effects of the nucleolar isoform in other cell types, such as leukocytes, has not been determined as yet. Further work is required to clarify whether this isoform is expressed in inflammatory conditions, such as atherosclerosis, and what its contribution to the modulation of such inflammatory pathologies might be.
Netrin-1 in atherosclerosis
As described earlier, atherosclerosis is a chronic inflammatory disease that progresses over many years and often is only detected when the patient develops symptoms such as myocardial ischaemia. Monocytes are recruited to the arterial wall in areas of developing atherosclerotic plaque, where they differentiate into macrophages and take up oxLDL, becoming immobile foam cells, increasing the cellular burden and instability within the plaque.
The reported effects of netrin-1 in atherosclerosis have been conflicting, having been shown to play both pro- and anti-inflammatory roles in the development of the disease. This conflicting evidence may be explained by the temporal and spatial expression of netrin-1: endothelial-derived netrin-1 has been reported as playing a protective role, whilst netrin-1 secreted within the atherosclerotic plaque by macrophages appears to exert a pro-atherogenic effect. These are described in detail below.
Endothelial-derived netrin-1
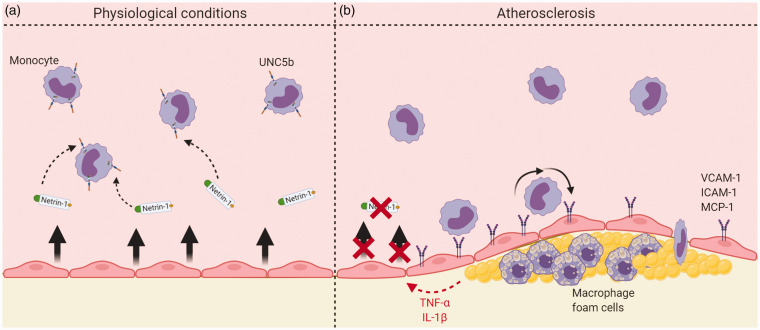
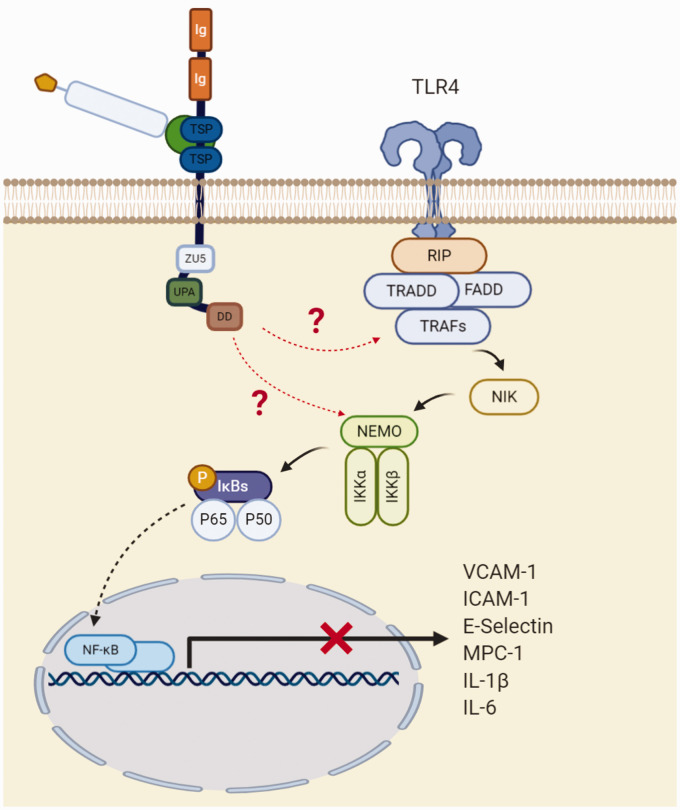
Endothelial cells secrete full length netrin-1, leading to a reduction of monocyte chemotaxis into the arterial wall (Figure 5(a)), as first demonstrated by Passacquale et al. In ApoE–/– mice, they found that UNC5b blockade inhibited the effects of netrin-1 with a consequent increase in accumulation of monocytes in the brachiocephalic artery.48 Furthermore, Lin et al. and Bruikman et al. showed that netrin-1 reduces the expression of adhesion molecules induced by the pro-inflammatory cytokine TNF-α. In an in vitro model of human aortic endothelial cells stimulated with TNF-α, a significant reduction in the expression of VCAM-1, ICAM-1 and E-selectin was observed when cells were co-treated with netrin-1, and high concentrations of netrin-1 completely abolished the expression of these adhesion molecules.50 Significant downregulation of ICAM-1, IL-6 and MCP-1 has also been observed in endothelial cells from patients with underlying atherosclerosis, this effect being abolished by UNC5b blockade.51 Furthermore, Lin et al. also showed that netrin-1 supresses endothelial-derived cytokine production induced by TNF-α in a concentration-dependent manner (Figures 5(b) and 6). They demonstrated that netrin-1 selectively suppresses toll-like receptor 4 (TLR4) and inhibits the NF- κB pathway by suppressing TNF-α-induced IKK and IκBα activation in the cytoplasm. They also showed that netrin-1 affects the nuclear NF- κB subunit p65 by reducing its accumulation in the nuclei. While stimulation with TNF-α led to an increase of approximately 3.4 fold of p65 in the nuclei compared with basal levels, treatment with 200 and 400 ng/ml of netrin-1 reduced its nuclear accumulation by 25% and 50%, respectively. Moreover, netrin-1 suppressed NF-κB promoter activation in endothelial cells after TNF-α stimulation: TNF-α increased the promoter activity by 70-fold, and this was supressed by 30% and 60% after treatment with 200 and 400 ng/ml of netrin-1 respectively.50
Figure 5.
Overview of netrin-1 effects in the context of normal physiology and atherosclerosis. (a) Endothelial-derived netrin-1 interacts with UNC5b receptors on the monocyte surface, causing chemorepulsion. (b) In atherosclerosis, pro-inflammatory cytokines act on endothelial cells to downregulate expression of endothelial-derived netrin-1, as well as upregulate endothelial cell adhesion molecules, the resultant effect of both being increased monocyte recruitment to the plaque.
Figure 6.
Proposed model for the protective role of netrin-1 in endothelium. In the absence of netrin-1, pro-inflammatory stimuli induce the TLR4 cascade leading to transmigration of NF-κB to the nucleus, promoting expression of pro-inflammatory cytokines and adhesion molecules. The protective effects of netrin-1 are believed to be exerted through inhibition of IKK activation and IκB phosphorylation and degradation, via the UNC5b receptor, thereby preventing nuclear translocation. Image adapted from Mehlen et al.45
From a different perspective, Passacquale et al. showed that pro-inflammatory stimuli cause downregulation of endothelial-derived netrin-1 expression.48 Thus, it would be expected that the protective effect of netrin-1 will decrease as the atherosclerotic process proceeds, thereby further accelerating its progression. Indeed, Bruikman and colleagues showed that netrin-1 plasma levels are inversely correlated with arterial wall inflammation as well as total plaque volume.51
Macrophage-derived netrin-1
Macrophages can also express netrin-1.15,29,30,52 Nonetheless, what effects macrophage-derived netrin-1 may exert on the ability of macrophages to migrate in response to chemokines or on modulation of their phenotype remain entirely unclear. Tadagavadi et al. and Komatsuzaki et al. reported, in terms of netrin-1 receptors, that only UNC5b is expressed in monocytes, granulocytes and lymphocytes, using immunohistochemistry and quantitative RT-PCR.15,53 We will therefore concentrate on the possible effects of netrin-1 on macrophages on the basis that it interacts with UNC5b only.
Ly et al. showed that, in infection and pro-inflammatory models, the levels of netrin-1 decrease and the recruitment of leukocytes to the site is promoted. They hypothesised that netrin-1 is an immunomodulator, which keeps leukocyte influx in check, preventing aberrant tissue destruction. This inhibitory effect on migration was verified in vitro and in vivo in response to a range of chemoattractive stimuli, whilst not affecting other functions, for example the production of superoxide.15 Moreover, Taylor et al. demonstrated that netrin-1 is also capable of inhibiting macrophage chemotaxis to non-chemokine attractants.52 This group showed that the chemotaxis generated by the complement component C5a can be partially inhibited by netrin-1, through UNC5b.
In contrast, other workers describe netrin-1 as a pro-inflammatory mediator. Van Gils and colleagues showed that netrin-1 can promote atherosclerosis, inhibiting the migration of the foam cells to the lymph nodes, becoming trapped in the atherosclerotic plaque and thereby contributing to its development and instability. In accordance to what was reported in cancer cell models, they also reported that netrin-1 promotes cell survival in the atherosclerotic plaque. Deficiency of netrin-1 in the macrophages reduced atherosclerosis and allowed egress of foam cells to the lymphatic system.29
Similarly, Ramkhelawon et al. showed that netrin-1 promotes the retention of macrophages in adipose tissue, enhancing metabolic dysfunction and chronic inflammation. In an experiment where mutant mice, whose macrophages do not express netrin-1, and wild-type mice were fed a high fat diet, they found that recruitment of macrophages to the adipose tissue was similar in both groups. However, on the 14th day, the retention of macrophages was significantly lower in the mutant mice compared with the wild-types, consistent with the hypothesis that netrin-1 traps macrophages in the site and promotes chronic inflammation. Furthermore, adipose tissue macrophages isolated from the mutant mice exhibited lower mRNA expression of pro-inflammatory markers and cytokines and a higher expression of M2 macrophage markers, compared with the wild-type.30
Regarding macrophage phenotypes, Ranganathan et al. and Mao et al. showed the opposite to what was observed in the Ramkhelawon et al. study. In their experiments, they found that higher expression of netrin-1 is protective, enhancing the shifting of macrophage phenotype towards M2, with concomitant upregulation of anti-inflammatory markers such as IL-4 and IL-13 and downregulation of IL-6 and cyxlooxygenase-2 (COX-2).54,55 Both groups showed that netrin-1 activates anti-inflammatory pathways that are known to regulate macrophage polarization, such as peroxisome proliferator-activated receptors (PPAR). PPAR mediate a variety of inflammatory mechanisms, which include repressing the activities of pro-inflammatory transcription factors such as NF- κB or STATs.56Netrin-1 led to a decrease of in M1 polarization and cytokine production induced by IFN-γ and suppressed ischaemia-reperfusion injury, effects which were inhibited by PPAR antagonists.54 Further work by Ranganathan et al. suggests that netrin-1 regulates inflammation and cell migration through suppression of NF- κB, leading to downregulation of COX-2-mediated PGE2 and thromboxane A2 production, both in vitro and in vivo.57
The current data suggest that the immunomodulatory effects of netrin-1 depend on which cell type expresses it and in which environment. Macrophage-derived netrin-1 in the plaque may be detrimental because it stops macrophage egress to lymph nodes, whereas endothelial-derived (systemic) netrin-1 may be essential to suppress levels of inflammation.
Conclusion
Netrin-1 plays different roles in a range of pathologies, and its effects vary depending on which type of cells it acts upon and which receptors it binds to. In the context of atherosclerosis, its effects may be both pro- and anti-inflammatory, the balance depending on where it is produced (endothelial versus macrophage-derived), their relative production, and the relative expression of the truncated versus the full length isoform. Despite netrin-1 isoforms having been studied and characterised in endothelial cells, information is currently lacking as to how their expression varies in macrophages under different inflammatory conditions. To fully understand the relevance of netrin-1 in atherosclerosis, it will be important for expression of its isoforms to also be characterised in the different macrophage phenotypes. As described previously, endothelial-derived netrin-1 expression is suppressed under inflammatory conditons,48 but further work is needed to establish what effect restoration of endothelial-derived netrin-1 expression would have on foam cell function and motility within the plaque.
Whether the netrin-1 system presents a worthwhile therapeutic target in atherosclerosis remains unclear. It may be that netrin-1 delivery would have to be targeted in order to exert a useful therapeutic effect. Further work is needed to address this question and to further elucidate the precise roles played by the different isoforms of netrin-1 in the pathophysiology of atherosclerosis.
Acknowledgements
None.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a King’s British Heart Foundation Centre for Excellence Award [RE/18/2/34213]. V.C. is supported by British Heart Foundation PhD Studentship [FS/18/69/33884].
Ethical approval: None required.
Guarantor: Both authors act as guarantors for the manuscript.
Contributorship: Vasco Claro performed the literature search. Both authors wrote the paper.
ORCID iD: Albert Ferro https://orcid.org/0000-0002-5486-9145
References
- 1.Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol 2010; 21: 305–311. [DOI] [PubMed] [Google Scholar]
- 2.Zhong S, Li L, Shen X, et al. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic Biol Med 2019; 144: 266–278. [DOI] [PubMed] [Google Scholar]
- 3.Bochkov VN, Oskolkova OV, Birukov KG, et al. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal 2010; 12: 1009–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobryshev YV, Ivanova EA, Chistiakov DA, et al. Macrophages and their role in atherosclerosis: Pathophysiology and transcriptome analysis. Biomed Res Int 2016; 2016: 9582430–9582413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011; 145: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinstein J, Ramkhelawon B. Netrins & semaphorins: novel regulators of the immune response. Biochim Biophys Acta Mol Basis Dis 2017; 1863: 3183–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013; 13: 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol 2004; 16: 572–579. [DOI] [PubMed] [Google Scholar]
- 9.Kappler J, Franken S, Junghans U, et al. Glycosaminoglycan-binding properties and secondary structure of the C-terminus of netrin-1. Biochem Biophys Res Commun 2000; 271: 287–291. [DOI] [PubMed] [Google Scholar]
- 10.Yamagishi S, Yamada K, Sawada M, et al. Netrin-5 is highly expressed in neurogenic regions of the adult brain. Front Cell Neurosci 2015; 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Copeland NG, Gilbert DJ, et al. Netrin-3, a mouse homolog of human NTN2L, is highly expressed in sensory ganglia and shows differential binding to netrin receptors. J Neurosci 1999; 19: 4938–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin Y, Sanes JR, Miner JH. Identification and expression of mouse netrin-4. Mech Dev 2000; 96: 115–119. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy TE. Cellular mechanisms of netrin function: long-range and short-range actions. Biochem Cell Biol 2000; 78: 569–575. [PubMed] [Google Scholar]
- 14.Bruikman CS, Zhang H, Kemper AM, et al. Netrin family: role for protein isoforms in cancer. J Nucleic Acids 2019; 2019: 3947123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ly NP, Komatsuzaki K, Fraser IP, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci USA 2005; 102: 14729–14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu K, Wu Z, Renier N, et al. Structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science 2014; 344: 1275–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy TE, Serafini T, de la Torre JR, et al. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 1994; 78: 425–435. [DOI] [PubMed] [Google Scholar]
- 18.Park KW, Crouse D, Lee M, et al. The axonal attractant netrin-1 is an angiogenic factor. Proc Natl Acad Sci Usa 2004; 101: 16210–16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delloye-Bourgeois C, Goldschneider D, Paradisi A, et al. Nucleolar localization of a netrin-1 isoform enhances tumor cell proliferation. Sci Signal 2012; 5: ra57. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan K, Strickland P, Valdes A, et al. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell 2003; 4: 371–382. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu A, Nakayama H, Wang P, et al. Netrin-1 promotes glioblastoma cell invasiveness and angiogenesis by multiple pathways including activation of RhoA, cathepsin B, and cAMP-response element-binding protein. J Biol Chem 2013; 288: 2210–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akino T, Han X, Nakayama H, et al. Netrin-1 promotes medulloblastoma cell invasiveness and angiogenesis, and demonstrates elevated expression in tumor tissue and urine of patients with pediatric medulloblastoma. Cancer Res 2014; 74: 3716–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalvin S, Anselmo MA, Prodhan P, et al. Expression of netrin-1 and its two receptors DCC and UNC5H2 in the developing mouse lung. Gene Expr Patterns 2003; 3: 279–283. [DOI] [PubMed] [Google Scholar]
- 24.De Breuck S, Lardon J, Rooman I, et al. Netrin-1 expression in fetal and regenerating rat pancreas and its effect on the migration of human pancreatic duct and porcine islet precursor cells. Diabetologia 2003; 46: 926–933. [DOI] [PubMed] [Google Scholar]
- 25.Mirakaj V, Thix CA, Laucher S, et al. Netrin-1 dampens pulmonary inflammation during acute lung injury. Am J Respir Crit Care Med 2010; 181: 815–824. [DOI] [PubMed] [Google Scholar]
- 26.Mirakaj V, Gatidou D, Potzsch C, et al. Netrin-1 signaling dampens inflammatory peritonitis. J Immunol 2011; 186: 549–555. [DOI] [PubMed] [Google Scholar]
- 27.Grenz A, Dalton JH, Bauerle JD, et al. Partial netrin-1 deficiency aggravates acute kidney injury. PLoS One 2011; 6: e14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mediero A, Ramkhelawon B, Wilder T, et al. Netrin-1 is highly expressed and required in inflammatory infiltrates in wear particle-induced osteolysis. Ann Rheum Dis 2016; 75: 1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Gils JM, Derby MC, Fernandes LR, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol 2012; 13: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramkhelawon B, Hennessy EJ, Ménager M, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nat Med 2014; 20: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llambi F, Causeret F, Bloch-Gallego E, et al. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. Embo J 2001; 20: 2715–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyer NP, Gupton SL. Revisiting netrin-1: one who guides (axons). Front Cell Neurosci 2018; 12: Article221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson NH, Key B. Neogenin: one receptor, many functions. Int J Biochem Cell Biol 2007; 39: 874–878. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q, Sun X, Zhou XH, et al. N-terminal horseshoe conformation of DCC is functionally required for axon guidance and might be shared by other neural receptors. J Cell Sci 2013; 126: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolodziej PA, Timpe LC, Mitchell KJ, et al. Frazzled encodes a drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 1996; 87: 197–204. [DOI] [PubMed] [Google Scholar]
- 36.Kruger RP, Lee J, Li W, et al. Mapping netrin receptor binding reveals domains of Unc5 regulating its tyrosine phosphorylation. J Neurosci 2004; 24: 10826–10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finci LI, Krüger N, Sun X, et al. The crystal structure of netrin-1 in complex with DCC reveals the bifunctionality of netrin-1 as a guidance cue. Neuron 2014; 83: 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong K, Hinck L, Nishiyama M, et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 1999; 97: 927–941. [DOI] [PubMed] [Google Scholar]
- 39.Wang R, Wei Z, Jin H, et al. Autoinhibition of UNC5b revealed by the cytoplasmic domain structure of the receptor. Mol Cell 2009; 33: 692–703. [DOI] [PubMed] [Google Scholar]
- 40.Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol 2007; 8: 296–306. [DOI] [PubMed] [Google Scholar]
- 41.Fearon ER, Cho KR, Nigro JM, et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 1990; 247: 49–56. [DOI] [PubMed] [Google Scholar]
- 42.Reyes-Mugica M, Rieger-Christ K, Ohgaki H, et al. Loss of DCC expression and glioma progression. Cancer Res 1997; 57: 382–386. [PubMed] [Google Scholar]
- 43.Koren R, Dekel Y, Sherman E, et al. The expression of DCC protein in female breast cancer. Breast Cancer Res Treat 2003; 80: 215–220. [DOI] [PubMed] [Google Scholar]
- 44.Boneschansker L, Nakayama H, Eisenga M, et al. Netrin-1 augments chemokinesis in CD4+ T cells in vitro and elicits a proinflammatory response in vivo. J Immunol 2016; 197: 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehlen P, Rabizadeh S, Snipas SJ, et al. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 1998; 395: 801–804. [DOI] [PubMed] [Google Scholar]
- 46.Leroyer AS, Blin MG, Bachelier R, et al. CD146 (cluster of differentiation 146). Arterioscler Thromb Vasc Biol 2019; [DOI] [PubMed] [Google Scholar]
- 47.Ly A, Nikolaev A, Suresh G, et al. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell 2008; 133: 1241–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passacquale G, Phinikaridou A, Warboys C, et al. Aspirin-induced histone acetylation in endothelial cells enhances synthesis of the secreted isoform of netrin-1 thus inhibiting monocyte vascular infiltration. Br J Pharmacol 2015; 172: 3548–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harter PN, Zinke J, Scholz A, et al. Netrin-1 expression is an independent prognostic factor for poor patient survival in brain metastases. PLoS One 2014; 9: e92311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin Z, Jin J, Bai W, et al. Netrin-1 prevents the attachment of monocytes to endothelial cells via an anti-inflammatory effect. Mol Immunol 2018; 103: 166–172. [DOI] [PubMed] [Google Scholar]
- 51.Bruikman CS, Vreeken D, Hoogeveen RM, et al. Netrin-1 and the grade of atherosclerosis are inversely correlated in humans. ATVB 2020; 40: 462–472. [DOI] [PubMed] [Google Scholar]
- 52.Taylor L, Brodermann MH, McCaffary D, et al. Netrin-1 reduces monocyte and macrophage chemotaxis towards the complement component C5a. PLoS One 2016; 11: e0160685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia–reperfusion injury. J Immunol 2010; 185: 3750–3758. [DOI] [PubMed] [Google Scholar]
- 54.Ranganathan PV, Jayakumar C, Ramesh G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am J Physiol Renal Physiol 2013; 304: F948–F957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mao X, Xing H, Mao A, et al. Netrin-1 attenuates cardiac ischemia reperfusion injury and generates alternatively activated macrophages. Inflammation 2014; 37: 573–580. [DOI] [PubMed] [Google Scholar]
- 56.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol 2002; 2: 748–759. [DOI] [PubMed] [Google Scholar]
- 57.Ranganathan PV, Jayakumar C, Mohamed R, et al. Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 production. Kidney Int 2013; 83: 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]