Abstract
Primary squamous cell carcinoma of salivary gland (SCG) is an extremely rare type of malignant salivary gland tumor, which in turn results in scarcity of data available regarding both its treatment and associated genetic alterations. A retrospective analysis of 12 patients with primary SCG was conducted, along with analysis of the association between treatment, clinical/pathological characteristics, and outcomes. Most patients (8) were staged IVa, with the majority of them (10) having G3 fast growing cancer. Local and systemic recurrence were reported in only three out of nine parotid cases (0 out of 2 submandibular SCGs). In two out of eight patients local relapse occurred after integrated treatment, while recurrence occurred in two out of three patients undergoing exclusive surgery. Five patients eventually died. Treatment of resectable disease must be aggressive and multimodal, with achievement of loco-regional control in order to reduce rate of recurrence and improve outcomes. Metastatic disease would require a therapeutic strategy tailored to the molecular profile in order to improve the currently disappointing results.
Keywords: Primary squamous cell carcinoma, salivary gland neoplasms, parotid gland, submandibular gland, parotidectomy, sialoadenectomy
Introduction
Malignant tumors of the salivary glands (SGMTs) are uncommon, accounting for less than 5% of all head and neck cancers.1 SGMTs are a heterogeneous spectrum of diseases showing distinct molecular, pathological, and clinical features.2 The clinical-pathological spectrum ranges from low grade tumors to high grade ones with undifferentiated phenotypes.3 Slow growing and/or low grade histotypes are characterized by a prolonged clinical course with prevalent local recurrence and latent distant metastases.4 On the other hand, malignant poorly differentiated SGMTs showed high mortality rate, frequent spread to lymph nodes, nerve impairment, and chemo-resistant profile.5–7 Despite the low incidence the molecular landscape is strongly under investigation, allowing for the identification of several targets to act on.8 These include the epidermal growth factor receptor family (ErbB1/EGFR and ErbB2), androgen receptor, NOTCH pathway, tumor mutational burden, PTCH-1/SMO, and BRAF V600 mutation and the checkpoint receptor PD-L1.9–18
Squamous cells carcinoma of the major salivary glands (SCG) accounts just for 1.6% (0.9–4.7) of all SGMTs, with a higher incidence in the parotid gland.19,20 Submandibular SCG is very rare (about 2% of the tumors) but it is highly aggressive, with poor outcome.21 Due to how rare primitive SCGs are, metastases from a primary carcinoma of head and neck or from regional cutaneous malignancies must be ruled out as well as direct invasion of salivary gland from carcinomas of the external ear/preauricular skin.22 Only few data are available about the genetic alterations in SCG tumors.2 Wnt/β-catenin signaling demonstrates a relevant role in the carcinogenesis of rapidly growing, aggressive SCGs.23
Clinical trials based on a small and unselected patients population with SGMTs failed to provide conclusive results due to the heterogeneity of pathological histotypes which leads to a varied response to treatment.10,24–26 Our knowledge on safety and efficacy of target therapy comes from case reports, small retrospective studies and case studies only. Moreover, only few data with disappointing results are available regarding treatment of SCGs resulting in a lack of gold standard strategies for both localized and advanced SCGs.21,22,27–37 In this scenario, it is reasonable to assume for expert recommendations to be based on small series of patients with SCG.
This retrospective study shows the Authors’ experience in treating a small series of patients with this rare tumor and aims to highlight the efficacy of a complex, multimodal strategy based on local guidelines in tackling SCGs management.7
Patients and methods
A retrospective study of patients with primary squamous cell carcinoma of the major salivary glands was conducted. The study was approved by the Scientific Commission of the department of Radiological, Oncological and Pathological Science of Sapienza University of Rome. All the patients were discussed at baseline and in every decision-making step by the local multidisciplinary team between 2009 and 2019.
Inclusion criteria were histologically confirmed squamous cell carcinoma of salivary gland primary localized in parotid, submandibular or sublingual gland. Patients with distant metastasis or recurrent disease at the initial staging were included. Patients who had non-squamous salivary gland carcinomas or hybrid salivary gland tumors or other concomitant/previous malignant disease including cutaneous malignancy were not included in the study, as well as patients with squamous cell carcinoma of head and neck.38 Patients who received treatment in the past were also excluded.
Data including age, gender, tumor location, Eastern Cooperative Oncology Group performance status (ECOG PS), comorbidities, history of tobacco smoking and alcohol abuse, symptoms and histology were collected retrospectively. Primary tumor extension and nodal involvement were staged and reclassified when appropriate, according to the TNM system based on 8th edition of the American Joint Committee on Cancer (AJCC).39 Imaging for diagnosis and staging were reviewed. All the histological sections were viewed by two experienced independent pathologist [CDG;BC] according to histological type, tumor grade, surgical margin status. Tumoral proliferative index was evaluated by means of ki-67 immunohistochemical staining (IHC) and expressed as percentage of nuclear positivity. Tumors were classified as low or high proliferative (0%–49% and ⩾50% ki-67 positive tumor cells respectively). For each case histochemical (PAS and PAS-diastase resistant) and IHC stainings (CK-5, CK AE1/AE3, p63, p40, androgen receptor, GATA, and HER2) were examined.
Primary treatment data were collected based on surgical technique, regional lymph node dissection and its level, indication to monolateral or bilateral node dissection, chemo/radiotherapy for inoperable locally advanced disease, any neo-adjuvant treatment, adjuvant treatment, treatment for metastatic disease. Table 1 resumes the clinical recommendations of the “Sapienza” unit which were followed by the authors.
Table 1.
Clinical recommendations, Head and Neck Unit.
| 1) Baseline symptoms evaluation | Recommended |
| 2) EUS-FNAB | Recommended |
| 3) EUS-FNAC | Recommended |
| 4) Ultrasound imaging | Recommended |
| 5) Contrast-enhanced magnetic resonance imaging | Recommended |
| 6) Contrast-enhanced computed tomography | Recommended |
| 7) Positron emission tomography | Recommended only in metastatic setting |
| 8) Immunochemistry (CK, p63, GATA3, androgen receptor) | Recommended |
| 9) Radical parotidectomy en block with infiltrated structures modified Radical Neck dissection (level Ib-V) | Recommended in parotid gland carcinoma |
| 10) Sialoadenectomy en block with infiltrated structures Modified radical neck dissection (livel I-V) | Recommended in sub-mandibular carcinoma |
| 11) Adjuvant radiotherapy +/- concomitant chemotherapy (IMRT) | Recommended (in case of T3-T4, high grade, close/positive margin, perineural invasion, Nodal status positive, ENE+) |
| 12) Neoadjuvant chemotherapy | Not recommended TPF schedule Considered Carboplatin-Paclitaxel schedule |
| 13) Molecular profiling in metastatic setting:EGFR expression, RAS mutation, PDL1 expression, NOTCH mutation | Recommended for personalized therapy |
EUS-FNAB: endoscopic ultrasound guided-fine needle aspiration biopsy; EUS-FNAC: endoscopic ultrasound guided-fine needle aspiration cytology; ENE: extranodal extension.
After definitive treatment patients underwent periodic follow up visits.40 Sites of both local and distant recurrences were recorded. Disease free survival (DFS) was defined as the time in months from the end of definitive treatment until the occurrence of either progression/death or the date of last follow up. Overall survival (OS) was defined as the time in months from diagnosis to the date of either death or last follow up. In metastatic setting, progression free survival (PFS) in months was evaluated from the date of metastatic disease diagnosis to the date of disease progression.
Results
A total of 12 patients were included in this study. Patients and treatment features are described in Table 2. Median age was 70 years (range 30–87), two female patients with parotid SCG had an early onset at 30 and 35 years respectively; one more female patient had the first diagnosis at 43 years. ECOG PS was 0 and 1 in four and eight patients, respectively. Only five patients reported a smoking history. Simultaneous alcohol abuse was not recorded. Parotid and submandibular glands were reported as the primary tumor location in nine and three patients, respectively.
Table 2.
Clinicopathological characteristics and outcomes.
| All patients N 12 | |
|---|---|
| Age (years) | |
| ⩾70 | 6 |
| <70 | 6 |
| Median age (range) | 70 (30–87) |
| Gender | |
| Male | 5 |
| Female | 7 |
| ECOG PS baseline | |
| 0 | 4 |
| 1 | 8 |
| Risk factors | |
| Smoking history (SH) | 5 |
| Alcool abuse | 0 |
| Missing | 2 |
| Comorbidity | 7 |
| Tumor location | |
| Parotid | 9 |
| Submandibular glands | 3 |
| Clinical T stage (All) | |
| 2 | 1 |
| 3 | 2 |
| 4a | 8 |
| 4b | 1 |
| Clinical N stage (all) | |
| 0 | 4 |
| 1 | 4 |
| 2 | 4 |
| Clinical M (all) | |
| M0 | 11 |
| M1 | 1 |
| Clinical stage | |
| II | 1 |
| III | 1 |
| Iva | 8 |
| IVb | 1 |
| IVc | 1 |
| Histology | |
| Squamous | 12 |
| Grading | |
| 2 | 1 |
| 3 | 10 |
| 1 | 1 |
| Neoadjuvant chemotherapy | 3 |
| First line chemotherapy | 1 |
| Surgery | |
| Parotidectomy | 9 |
| Sialoadenectomy | 2 |
| Neck dissection | 11 |
| Adjuvant radiotherapy | 6 |
| Adjuvant chemo/radiotherapy | 2 |
| Median DFS (range) | 13 (2–120) |
| Median OS (range) | 13 (4–120) |
| Lost to Follow up | 2 |
| Local and distant progression after surgery and adj treatment | 2 |
| Local progression after surgery | 1 |
| Distant progression after surgery | 1 |
ECOG PS: eastern cooperative oncology group performance status.
Clinical findings
The majority of patients with SCG presented with locally advanced stage (n = 9). Findings included single painless parotid/submandibular mass, swelling, variable degree of tethering to surrounding structures and VII cranial nerve deficit up to complete paralysis. One patient with primary submandibular cancer had metastasis on diagnosis. Early onset was associated with aggressive disease, with worse clinical course in patients under 40 years of age. As shown in Table 3, young patients are the ones to most commonly experience a rapid disease progression and death despite the multimodal treatment provided (Table 3).
Table 3.
Case series: clinicopathological information and outcomes.
| Patients | Age | Primary tumor | Clinical stage | G | Neoadj therapy | Surgery | R | Nodal status | Adj Therapy | DFS/PFS | OS | Follow up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 78 | Parotid | III | 3 | Yes | Total Parotidectomy + SND | 0 | 0 | RT | 20 | 20 | NEDa |
| Case 2 | 35 | Parotid | IVA | 3 | Yes | Radical Parotidectomy+ RMND | 0 | + | RTCT | 4 | 5 | DODb |
| Case 3 | 30 | Parotid | IVA | 3 | No | Radical Parotidectomy + RMND | 0 | + | RT | 10 | 11 | DOD |
| Case 4 | 87 | Parotid | IVA | 3 | No | Radical Parotidectomy + RMND | 0 | + | RT | − | − | LF |
| Case 5 | 80 | Parotid | II | 2 | No | Radical Parotidectomy + RMND | 0 | 0 | No* | 30 | 31 | DOD |
| Case 6 | 55 | Parotid | IVa | 3 | No | Radical Parotidectomy + RMND | 0 | + | RTCT | 120 | 120 | NED |
| Case 7 | 85 | Parotid | IVa | 3 | No | Radical Parotidectomy + RMND | 0 | + | RT | 24 | 24 | NED |
| Case 8 | 43 | Parotid | IVb | 1 | No | Radical parotidectomy + RMND | 0 | 0 | No | 5 | 6 | DOD |
| Case 9 | 57 | Parotid | IVa | 3 | No | Radical Parotidectomy + RMND | 0 | + | RT | 4 | 4 | LF |
| Case 10 | 75 | Submandibular | IVc | 3 | No | / | / | / | No | 2 | 4 | DOD |
| Case 11 | 80 | Submandibular | IVa | 3 | No | Sialoadenectomy + RMND | 0 | 0 | RT | 21 | 21 | NED |
| Case 12 | 65 | Submandibular | IVa | 3 | Yes | Sialoadenectomy + RMND | 0 | 0 | No* | 13 | 13 | NED |
NED: no evidence of disease; DOD: death of disease; LF: lost in follow up; R: residual disease; DFS: disease free survival; OS: overall survival; Adj: adjuvant; SND: simplified neck dissection; RMND: modified radical neck dissection; RT: radiotherapy; RTCT: radiochemotherapy.
Patient refused adjuvant treatment.
Imaging and diagnostic tools
Ultrasound was first used to investigate swelling masses in parotid and submandibular area. Baseline head and neck magnetic resonance imaging (MRI) with contrast allowed to define size, involvement of adjacent structures and regional lymph nodes in all cases and allowed evaluation of cancer resectability (Figure 1). Total body Computed Tomography (CT) with contrast was performed in all cases. The only one patient presenting with distant metastasis (case 8) underwent positron emission tomography (PET)-CT to confirm the metastatic setting before undergoing systemic therapy.
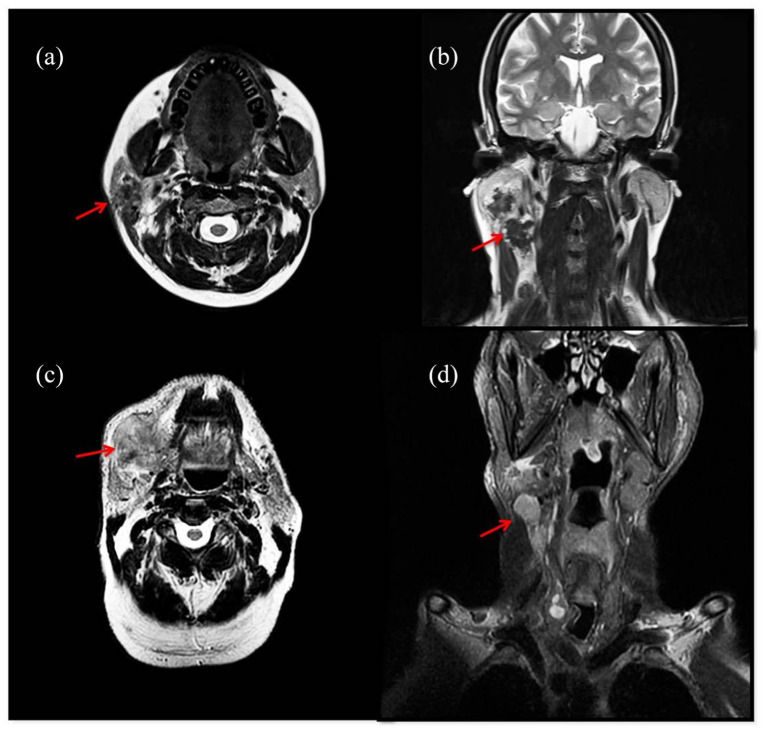
Figure 1.
MRI images of a 35-year-old patient with squamous cell carcinoma of the right parotid gland (Case 2). Axial T2-weighted image shows ill-defined lesion involving superficial lobe of the right parotid (a). The lesion is inseparable from anterior aspect of sternocleidomastoid muscle (a). Coronal T2 weighted image shows enlarged ipsilateral lymph nodes (b). MRI images of a 75-year-old patient with squamous cell carcinoma of the right submandibular gland (Case 10). Axial T2-weighted image shows an ill-defined mass with diffuse invasive growth involving right submandibular space (c). The lesion is inseparable from ioglossus muscle and ipsilateral parotid gland (c). Coronal T2 fat-sat weighted image shows enlarged ipsilateral lymph nodes (d).
Fine-needle aspiration biopsy (EUS–FNAB) was performed in 9/12 cases; three patients (cases 1, 3, 5) underwent radical parotidectomy after a superficial parotidectomy which was carried out in other centers.
In two patients fine needle citology (EUS-FNAC) was performed prior to biopsy: in one case (case 10) the cytological result was pleomorphic adenoma, while in the other one (case 12) the cytology resulted negative for malignancy. In both cases a subsequent biopsy was performed to define the diagnosis.
Pathological findings
Pathological review confirmed the squamous histotype in all of the 12 cases with 10 poorly differentiated (G3), one moderately differentiated (G2) and one well differentiated (G1) carcinomas. There were no lymphatic nor venous invasion, while perineural invasion was described in three patients. There were no PAS positive neoplastic cells in any of the cases. The IHC profile of all the tumors was: diffuse and strong reactivity for CK-5, CK AE1/AE, p40 and negative for androgen, GATA and HER2. Ten out of 12 squamous carcinomas showed a high proliferation index (⩾70%) (Figure 2). No correlation was found between tumor differentiation, kinetics, and clinical outcomes.
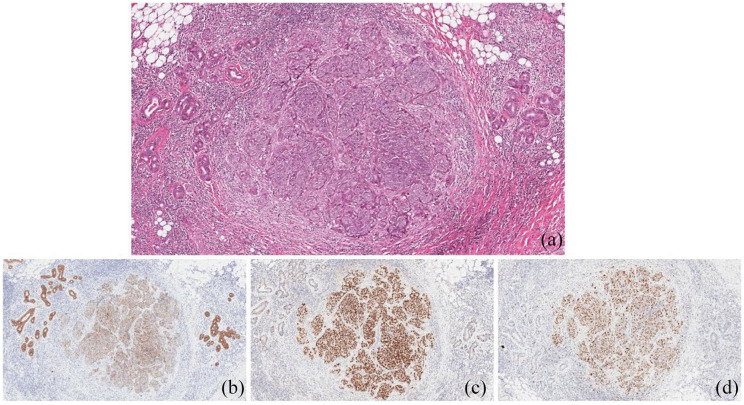
Figure 2.
Primary squamous carcinoma of the parotid gland (Case 1). Poorly differentiated (G3) squamous carcinoma infiltrating the serous salivary gland tissue (a. Hematoxylin eosin stain). The neoplastic cells were positive to the immunohistochemical stains for CK AE1/AE3 (b) and p63 (c). The tumor proliferative index evaluated by Ki-67 was 70% (d) original magnification 10X.
Surgery
All patients with parotid tumors (n = 9) underwent surgery. Total parotidectomy and monolateral selective neck dissection (level Ib-IV) was done in only one patient after neoadjuvant treatment (case 1). In the remaining eight patients parotidectomy required en bloc resection with sacrifice of contiguous anatomical structures in order to achieve oncological radicality including partial mandibulectomy (2), excision of the overlying skin (5) masseter excision, (1) zygomatic arch resection (1) and mastoidectomy (1). Surgery was completed with modified radical neck dissection (Level Ib–V) (case 2–8). In all these cases the reconstructive phase was carried out in the same operative session. Facial nerve preservation was possible in two patients; resection of facial nerve was performed both in case of preoperative impairment due to direct cancer infiltration and when no clear cleavage plane between cancer and nerve was detected intraoperatively, regardless of preoperative deficit. Nerve reconstruction was performed with different techniques in four patients, while one patient underwent static procedures only. In two patients with submandibular squamous cell carcinoma, radical sialodenectomy with partial mandibulectomy and radical modified neck dissection (Level I–V) was performed (cases 11, 12).
Negative surgical margins were achieved in all patients (11/11). A patient with metastatic disease at the time of diagnosis was excluded for loco-regional treatment and treated with first line chemotherapy (case 10).
Adjuvant radiotherapy
Radiation therapy following surgery was planned in all patients with no evidence of distant metastasis on diagnosis (n = 11). Eight out of those 11 patients underwent adjuvant radiotherapy, with (n = 2) or without (n = 6) concomitant chemotherapy. One patient did not receive radiotherapy because of poor clinical conditions and two patients refused the planned adjuvant treatment.
Intensity modulated radiotherapy (IMRT) technique was employed for all of the patients. Based on surgery and clinical-pathological findings, a dose of 66–70 Gy (2 Gy/fraction) was delivered to the high risk target volume and a dose of 50 Gy (2 Gy/fraction) to the elective target volume. Chemotherapy consisted of two courses of cisplatin infusion (100 mg/m2), on days 1 and 22 of the radiotherapy course. Adjuvant treatment was well tolerated. Mild (G1) to moderate (G2) toxicity was recorded, including dysphagia, mucositis, xerostomia, dysgeusia, and dermatitis.
Neoadjuvant treatment
One patient (case 12) with locally advanced SCG of submandibular gland with massive soft palate invasion, staged IVa, received induction chemotherapy according to TPF schedule (cisplatin 75 mg, 5-fluoruracile 800 mg/die from day 1 to day 4, docetaxel 75 mg/mq every 21 days) planned for two cycles.41 After the first cycle of induction chemotherapy acute renal failure occurred and therapy was stopped. Nevertheless, the patient obtained partial response to treatment and underwent radical surgery once the toxicities settled after TPF discontinuation. He refused adjuvant treatment but was disease free at the follow-up 13 months after surgery.
Another patient (case 2) underwent the same scheduled treatment for a locally advanced parotid tumor. Minimal tumor progression occurred after only one cycle of induction chemotherapy which caused early discontinuation of treatment; radical parotidectomy with excision of the overlying skin, and neck dissection were then performed with negative surgical margin. Subsequently the patient underwent adjuvant radiotherapy. Despite this, local and distant early progression were noted at follow up after 4 months. Considering the rapid clinical deterioration, palliative treatment was provided. Only one patient (case 1) obtained a complete clinical and pathological response after induction chemotherapy, according to the scheme carboplatin area under the curve (AUC) 5, paclitaxel 175 mg/mq every 21 days for two cycles. A previous incomplete partial resection was performed in another cancer center showing squamous carcinoma of parotid gland arising from an area of epidermoid metaplasia. Baseline staging after incomplete tumorectomy was stage III. Total parotidectomy and neck dissection were performed prior to adjuvant radiotherapy. This patient was disease free at 20 months follow up.
Treatment for metastatic disease
Just one patient (case 10) with SCG of submandibular gland presented with nodal and lung metastases. Two cycles of first line chemotherapy were administered according to the EXTREME scheme which included Cisplatin 75 mg/mq on day 1 and 5-Fluoruracile 800 mg/die/mq from day 1 to day 5, every 21 days associated with the chimeric monoclonal autoantibody cetuximab (EGFR inhibitor) at the loading dose of 400 mg/mq for the first cycle and then at the standard dose of 250 mg/mq weekly. Cetuximab was used off-label.42 Due to disease progression after 2 months, chemotherapy was definitively suspended and palliative treatment was provided for the rapid decline of physical condition.
Clinical outcome
Progression of disease occurred in four patients. One patient with SCG to the parotid (case 2), who received induction chemotherapy, surgery and adjuvant concomitant chemo/radiotherapy experienced lung and lymph nodal progression 4 months after surgery. In another patient with primary SCG to the parotid (case 3) skull base recurrence and brain metastasis occurred 10 months after surgery and adjuvant radiotherapy. A third patient (case 5), who received surgery but refused adjuvant radio/chemotherapy underwent distant disease progression after 30 months. The fourth patient (case 8), who did not receive adjuvant treatment due to clinical deterioration had early recurrence 5 months after surgery, underwent palliative radiotherapy and passed away 1 month later. In consideration of early clinical deterioration, all four patients received only palliative treatment.
Five patients were alive and with no evidence of disease at the last follow up visit, while unfortunately two patients were lost during follow up. Excluding lost patients, in this series the median DFS and OS were 13 months. The only patient with metastasis experienced disease progression after 2 months (PFS = 2 months) (Tables 2 and 3).
Discussion
The remarkable rarity of primary SCG, as well as the need to carry out a differential diagnosis (including squamous metaplasia, benign lesions, malignant squamous component of other tumors such as mucoepidermoid carcinoma, metastatic neck lymph nodes involvement) make this lesion one of the most challenging pathologies in the head and neck region, often requiring strict exclusion criteria in order to perform a definitive diagnosis. Moreover, IHC staining (HER2, androgen receptor, and GATA) is needed as to exclude salivary ductal carcinomas with either squamous differentiation or predominance of spindle-shaped cells (sarcomatoid variant).
In the current literature few data are available about the biological behavior and prognostic and therapeutic indications of squamous SCGs, mainly derived from case reports. The largest cases series was reported by Shemen et al.27 Ying et al.37 reported a retrospective review of 66 cases of squamous cell carcinoma of the parotid gland, but in 41 patients the parotid lesions were considered to be metastases from other primary squamous tumors, while in 16 cases the neoplasia probably originated in the parotid gland. Gaughan et al. reported the clinical behavior and treatment of 18 cases of squamous salivary parotid glands, with an OS of 50% at 5 years.28 Other case series and retrospective reviews described extensive cases of salivary gland tumors including ones with squamous histology, but with little information regarding clinical/pathological features and outcomes.29–37 To our knowledge, this series is one of the most populated and complete in view of the informations provided regarding patient features, treatment, and outcomes (Table 3).
Based on our experience, early onset seems to be an important prognostic factor. SCGs require an accurate initial imaging assessment for staging purposes and to define the multimodal approach. MRI should be considered the imaging method of choice. EUS-FNAB is recommended prior to surgery in order to gain a reliable histological diagnosis.
Shemen et al. reported a very high rate of local recurrences with exclusive surgical management of SCG in both parotid (51%) and submandibular (67%) cases.27 Therefore, SCGs require a complex multimodal treatment involving several specialties based on radical surgical and subsequent adjuvant treatment. Local recurrence in this case series was 3/9 (33%) in parotid and 0/2 in submandibular SCGs. In two patients relapse occurred after integrated treatment (2/8, 25%) while recurrence occurred in 2/3 patients (66%) undergoing surgery only. Moreover, the 2-year disease control rate of the two patients with total parotidectomy confirms the safety of nerve sparing procedures in those cases where there is no direct nerve involvement or where there is a clear cleavage plane between nerve and tumor.
The high rate of cervical lymph node involvement highlights the diagnostic and therapeutic importance of neck dissection with parotidectomy and sialoadenectomy in SCGs.37 lymph node involvement was noted in 6/11 patients. We recommend modified radical neck dissection in all patients with SCG, so that occult lymph node metastases can be detected. Bilateral node dissection should be considered in those rare cases of clinically evident bilateral lymph node metastases in the preoperative setting.
We strongly suggest radio-chemotherapy in association with cisplatin at the standard dose of 100 mg/mq every 3 weeks in young SCG patients with good PS to reduce the high risk of progression after surgical management. Local recurrence has always been associated with systemic metastasis, while systemic spread (without local recurrence) has occurred in only one case confirming the central role of local control in disease management.
Patients with metastatic and/or recurrent SGMTs, when not eligible to loco-regional treatment have a really poor outcome due to the aggressive behavior of SCG. Its profile of chemo-resistance is associated with a rapid decline of clinical condition which quickly occurred in all patients. In addition, data currently available to support clinical decisions comes only from small and heterogeneous series, demonstrating the need for new clinical recommendations to guide clinical practice. To date, single agent or combination chemotherapy are commonly used in the treatment of SCGs not amenable to surgery and/or radiotherapy. Cisplatin, 5-fluororacile, taxanes and anthraciclynes though showed a very limited benefit in terms of overall response and outcome in salivary gland tumors including SCGs.43,44 In this series one patient (case 10) with submandibular squamous cell carcinoma with nodal and lung metastases on diagnosis received two cycles of first line chemotherapy. Due to disease progression and the rapid decline of PS chemotherapy was stopped and the patient received only palliative treatment.
ErbB1 is involved in cell differentiation and proliferation of salivary glands. EGFR is often over expressed in 70% of salivary glands carcinoma and is associated with poor prognosis and aggressive disease, despite the fact that its prognostic and predictive roles have not been completely defined yet, in contrast with other cancers.45–48 Anti-EGFR based therapy could consequently have a strong rationale in metastatic salivary gland management; to date only few clinical trials have investigated its efficacy and safety in metastatic setting and exclusively in monotherapy.49 Moreover, RAS mutation has not been tested yet in clinical trials even though primary resistance to anti-EGFR therapy can be determined by panRAS mutation, which is expressed in 25% of salivary gland carcinomas.50 Due to the uncertain benefit of cetuximab as an addition to chemotherapy, extensive and detailed discussion about the treatment was held with patients, including benefits and potential acute and late sequelae. The other four patients who developed systemic disease progression during follow-up were excluded from treatment due to the rapid deterioration of the general conditions palliative treatment was provided only. At the moment no evidences are available about the role of target therapy in the primary management of SCG, lacking a molecular definition,2,23 due to the extreme rarity of this pathological histotype, the lack of specific studies for this pathology and the aggressive clinical behavior which confirms a drug resistance profile as evidenced in our patients despite treatment. Nonetheless, models of mice with increased Wnt/β-catenin signaling demonstrate a relevant role of this pathway in the carcinogenesis of rapidly growing, aggressive SCGs. The β-catenin pathway drives the SCG tumor proliferation in mice via histone changes at the promoters of stem cell-associated genes.23 It is also known that Wnt/β-catenin signaling is characterized by a stem cell-associated gene signature with a strong increase of tumor cell self-renewal. Loss of membrane β-catenin has been reported to impair cell adhesion enhancing migration, invasion and metastatic potential of cancer cells with associated rapid progression, immunosuppression, and drug resistance.51,52 At the moment no molecular therapeutic targeting the WNT/β catenin pathway has been incorporated into oncological practice. However, some small molecules inhibiting WNT/ β catenin pathway have been evaluated in many preclinical study, since early clinical trials have provided only small evidence of activity in different solid tumors.53,54 Riethdorf et al.55 while analyzing EMM-PRINs (members of the superfamily of receptors inducing expression of matrix metalloproteinases), observed their expression in 100% of SCGs. This shows a possible involvement of the epithelial-to-mesenchymal transition in SCG cancer progression.
In this scenario, immunotherapy in PD-L1 expressing tumors could represents a promising choice for treatment of salivary gland carcinoma. However, the role of the immune system in carcinogenesis and tumor progression of SCG is still uncertain, as well as the role of PD1 positive T lymphocytes in tumor microenvironment and the real expression of PD-L1 in SCG. In order to improve these disappointing data we prospect the evaluation of EGFR, RAS and BRAF mutational status, and PD-L1 to plan a treatment based on molecular features as required in rare metastatic tumors.8,56
Conclusion
High-volume centers and highly dedicated surgical, radiotherapy, and oncological expertises are needed to define the most appropriate treatment for each patient with SCG and to improve patient outcomes. Treatment of resectable lesions should be aggressive and multimodal, with surgery and adjuvant radiotherapy representing the mainstay of treatment aiming to achieve radical loco-regional control. Metastatic disease is often characterized by an aggressive behavior and would require a therapeutic strategy tailored to the molecular profile (evaluation of EGFR, panRAS and BRAF mutational status, and PD-L1 expression) in order to improve the currently disappointing results in the treatment of this rapidly progressive, drug resistant, rare type of malignant salivary gland tumor.
Footnotes
Author contributions: Conception and design: S.M., A.B., P.M.; Acquisition of data: G.P., M.D.M., F.D.F.; Analysis and interpretation of data: G.P., M.D.M., F.D.F., D.M.; Writing: S.M., G.P.; Review: F.D.F. and D.M. (radiotherapy), M.D.M. (surgery), F.V. (imaging), C.D.G., B.C., and R.C. (pathological finding) A.B. (oncology); S.A.; Revision of the manuscript: all the authors; Final approval: all the authors.
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PAOLO MARCHETTI (PM) has/had a consultant/advisory role for BMS, RocheGenentech, MSD, Novartis, Amgen, Merck Serono, Pierre Fabre, Incyte. The other authors declare they have no competing interests.
Ethical approval: Ethical approval was waived by the local Department Scientific Committee of “Sapienza” University of Rome.
Funding: The author(s) received financial support for the publication of this article by FFABR MIUR 49910478(20478).
Informed consent: All the procedures performed were part of the routine care.
ORCID iD: Giulia Pomati  https://orcid.org/0000-0001-6715-5443
https://orcid.org/0000-0001-6715-5443
References
- 1. Mifsud MJ, Burton JN, Trotti AM, et al. Multidisciplinary management of salivary gland cancers. Cancer Control 2016; 23(3): 242–248. [DOI] [PubMed] [Google Scholar]
- 2. Yin LX, Ha PK. Genetic alterations in salivary gland cancers. Cancer 2016; 122(12): 1822–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Terhaard CH, Lubsen H, Van der Tweel I, et al. Salivary gland carcinoma: independent prognostic factors for locoregional control, distant metastases, and overall survival: results of the Dutch head and neck oncology cooperative group. Head Neck 2004; 26(8): 681–692. [DOI] [PubMed] [Google Scholar]
- 4. Sung MW, Kim KH, Kim JW, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 2003; 129(11): 1193–1197. [DOI] [PubMed] [Google Scholar]
- 5. Jayaprakash V, Merzianu M, Warren GW, et al. Survival rates and prognostic factors for infiltrating salivary duct carcinoma: Analysis of 228 cases from the Surveillance, Epidemiology, and End Results database. Head Neck 2014; 36(5): 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Otsuka K, Imanishi Y, Tada Y, et al. Clinical outcomes and prognostic factors for salivary duct carcinoma: a multi-institutional analysis of 141 patients. Ann Surg Oncol 2016; 23(6): 2038–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Felice F, de Vincentiis M, Valentini V, et al. Management of salivary gland malignant tumor: the Policlinico Umberto I, “Sapienza” University of Rome Head and Neck Unit clinical recommendations. Crit Rev Hematol Oncol 2017; 120: 93–97. [DOI] [PubMed] [Google Scholar]
- 8. Kurzrock R, Bowles DW, Kang H, et al. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: results from MyPathway, a phase IIa multiple basket study. Ann Oncol 2020; 31(3): 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nashed M, Casasola RJ. Biological therapy of salivary duct carcinoma. J Laryngol Otol 2009; 123(2): 250–252. [DOI] [PubMed] [Google Scholar]
- 10. Haddad R, Colevas AD, Krane JF, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol 2003; 39(7): 724–727. [DOI] [PubMed] [Google Scholar]
- 11. Nabili V, Tan JW, Bhuta S, et al. Salivary duct carcinoma: a clinical and histologic review with implications for trastuzumab therapy. Head Neck 2007; 29(10): 907–912. [DOI] [PubMed] [Google Scholar]
- 12. Limaye SA, Posner MR, Krane JF, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist 2013; 18(3): 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fan CY, Wang J, Barnes EL. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol 2000; 24: 579–586. [DOI] [PubMed] [Google Scholar]
- 14. Jaspers HC, Verbist BM, Schoffelen R, et al. Androgen receptor-positive salivary duct carcinoma: a disease entity with promising new treatment options. J Clin Oncol 2011; 29(16): e473–e476. [DOI] [PubMed] [Google Scholar]
- 15. Bell D, Hanna EY, Miele L, et al. Expression and significance of notch signaling pathway in salivary adenoid cystic carcinoma. Ann Diagn Pathol 2014; 18(1): 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tonon G, Modi S, Wu L, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet 2003; 33: 208–213. [DOI] [PubMed] [Google Scholar]
- 17. Mukaigawa T, Hayashi R, Hashimoto K, et al. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J Surg Oncol 2016;114(1): 36–43. [DOI] [PubMed] [Google Scholar]
- 18. Cohen RB, Delord JP, Doi T, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the Phase 1b KEYNOTE-028 Study. Am J Clin Oncol 2018; 41(11): 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin HH, Limesand KH, Ann DK. Current State of knowledge on salivary gland cancers. Crit Rev Oncol 2018; 23(3-4): 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boukheris H, Curtis RE, Land CE, et al. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: a population-based study in the United States. Cancer Epidemiol Biomarkers Prev 2009; 18(11): 2899–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Panchbhai AS. Primary squamous cell carcinoma of salivary gland: Report of a rare case. J Can Res Ther 2015; 11(3): 664. [DOI] [PubMed] [Google Scholar]
- 22. Batsakis JG, McClatchey KD, Johns M, et al. Primary squamous cell carcinoma of the parotid gland. Arch Otolaryngol 1976; 102(6): 355–357. [DOI] [PubMed] [Google Scholar]
- 23. Wend P, Fang L, Zhu Q, et al. Wnt/β-catenin signalling induces MLL to create epigenetic changes in salivary gland tumours. EMBO J 2013; 32(14): 1977–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agulnik M, Cohen EW, Cohen RB, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol 2007; 25: 3978–3984. [DOI] [PubMed] [Google Scholar]
- 25. Jakob JA, Kies MS, Glisson BS, et al. A phase II study of gefitinib in patients with advanced salivary gland cancers. Head Neck 2015; 37: 644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Locati LD, Bossi P, Perrone F, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: a phase II study. Oral Oncol 2009; 45: 574–578. [DOI] [PubMed] [Google Scholar]
- 27. Shemen LJ, Huvos AG, Spiro RH. Squamous cell carcinoma of salivary gland origin. Head Neck Surg 1987; 9(4): 235–240. [DOI] [PubMed] [Google Scholar]
- 28. Gaughan RK, Olsen KD, Lewis JE. Primary squamous cell carcinoma of the parotid gland. Arch Otolaryngol Head Neck Surg 1992; 118: 798–801. [DOI] [PubMed] [Google Scholar]
- 29. Spitz MR, Batsakis JG. Major salivary gland carcinoma: descriptive epidemiology and survival of 498 patients. Arch Otolaryngol 1984; 110: 45–49. [DOI] [PubMed] [Google Scholar]
- 30. Kane WJ, McCaffrey TV, Olsen KD, et al. Primary parotid malignancies: a clinical and pathologic review. Arch Otolaryngol Head Neck Surg 1991; 117: 307–315. [DOI] [PubMed] [Google Scholar]
- 31. O’Brien CJ, Soong SJ, Herrera GA, et al. Malignant salivary tumors: analysis of prognostic factors and survival. Head Neck Surg 1986; 9: 82–92. [DOI] [PubMed] [Google Scholar]
- 32. Marks MW, Ryan RF, Litwin MS, et al. Squamous cell carcinoma of the parotid gland. Plast Reconstr Surg 1987; 79: 550–554. [DOI] [PubMed] [Google Scholar]
- 33. Witten J, Hybert F, Hansen HS. Treatment of malignant tumors in the parotid glands. Cancer 1990; 65: 2515–2520. [DOI] [PubMed] [Google Scholar]
- 34. Andersen LJ, Therkildsen MH, Ockelmann HH, et al. Malignant epithelial tumors in the minor salivary glands, the submandibular gland and the sublingual gland: prognostic factors and treatment results. Cancer 1991; 68: 2431–2437. [DOI] [PubMed] [Google Scholar]
- 35. Eneroth CM. Salivary gland tumors in the parotid gland, submandibular gland and the palate region. Cancer 1971; 27: 1415–1418. [DOI] [PubMed] [Google Scholar]
- 36. Eveson JW, Cawson RA. Salivary gland tumours: a review of 2410 cases with particular reference to histological types, site, age and sex distribution. J Pathol 1985; 146: 51–58. [DOI] [PubMed] [Google Scholar]
- 37. Ying YLM, Johnson JT, Myers EN. Squamous cell carcinoma of the parotid gland. Head Neck 2006; 28: 626–632. [DOI] [PubMed] [Google Scholar]
- 38. Seifert G, Donath K. Hybrid tumours of salivary glands. Definition and classification of five rare cases. Eur J Cancer B Oral Oncol 1996; 32B(4): 251–259. [DOI] [PubMed] [Google Scholar]
- 39. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017; 67(2): 122–137. [DOI] [PubMed] [Google Scholar]
- 40. De Felice F, de Vincentiis M, Valentini V, et al. Follow-up program in head and neck cancer. Crit Rev Oncol Hematol 2017; 113: 151–155. [DOI] [PubMed] [Google Scholar]
- 41. Ghi MG, Paccagnella A, Ferrari D, et al. GSTTC (Gruppo di Studio Tumori della Testa e del Collo): Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II–III trial. Ann Oncol 2017; 28(9): 2206–2212. [DOI] [PubMed] [Google Scholar]
- 42. Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008; 359(11): 1116–1127. [DOI] [PubMed] [Google Scholar]
- 43. Laurie SA, Licitra L. Systemic therapy in the palliative management of advanced salivary gland cancers. J Clin Oncol 2006; 24: 2673–2678. [DOI] [PubMed] [Google Scholar]
- 44. Agulnik M, Siu LL. An update on the systemic therapy of malignant salivary gland cancers: role of chemotherapy and molecular targeted agents. Curr Med Chem Anticancer Agents 2004; 4(6): 543–551. [DOI] [PubMed] [Google Scholar]
- 45. Gibbons MD, Manne U, Carroll WR, et al. Molecular differences in mucoepidermoid carcinoma and adenoid cystic carcinoma of the major salivary glands. Laryngoscope 2001; 111(8): 1373–1378. [DOI] [PubMed] [Google Scholar]
- 46. Milano A, Longo F, Basile M, et al. Recent advances in the treatment of salivary gland cancers: emphasis on molecular targeted therapy. Oral Oncol 2007; 43: 729–734. [DOI] [PubMed] [Google Scholar]
- 47. Chiappetta C, Proietti I, Soccodato V, et al. BRAF and NRAS mutations are heterogeneous and not mutually exclusive in nodular melanoma. Appl Immunohistochem Mol Morphol 2015; 23(3): 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Capalbo C, Belardinilli F, Raimondo D, et al. A simplified genomic profiling approach predicts outcome in metastatic colorectal cancer. Cancers (Basel) 2019; 11(2): 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Licitra L, Locati LD, Potepan P, et al. Cetuximab (C225) in recurrent and/or metastatic salivary gland carcinomas (RMSGCs): A monoinstitutional phase II study. Proc Am Soc Clin Oncol 2006; 24: 64. [Google Scholar]
- 50. Grünewald I, Vollbrecht C, Meinrath J, et al. Targeted next generation sequencing of parotid gland cancer uncovers genetic heterogeneity. Oncotarget 2015; 6(20): 18224–18237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2010; 120(5): 1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mezi S, Chiappetta C, Carletti R, et al. Clinical significance of epithelial-to-mesenchymal transition in laryngeal carcinoma: its role in the different subsites. Head Neck 2017; 39(9):1806–1818. [DOI] [PubMed] [Google Scholar]
- 53. Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: Update on effectors and inhibitors. Cancer Treat Rev 2018; 62: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Birkeland AC, Owen JH, Prince ME. Targeting head and neck cancer stem cells: current advances and future challenges. J Dent Res 2015; 94(11): 1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Riethdorf S, Reimers N, Assmann V, et al. High incidence of EMMPRIN expression in human tumors. Int J Cancer 2006; 119: 1800–1810. [DOI] [PubMed] [Google Scholar]
- 56. Chiappetta C, Mancini M, Lessi F, et al. Whole-exome analysis in osteosarcoma to identify a personalized therapy. Oncotarget 2017; 8(46): 80416–80428. [DOI] [PMC free article] [PubMed] [Google Scholar]