Abstract
Pulmonary hypertension is commonly associated with heart failure with preserved ejection fraction. In heart failure with preserved ejection fraction, the elevated left-sided filling pressures result in isolated post-capillary pulmonary hypertension or combined pre- and post-capillary pulmonary hypertension. Although right heart catheterization is the gold standard for diagnosis, it is an invasive test with associated risks. The ability of sub-maximum cardiopulmonary exercise test as an adjunct diagnostic tool in pulmonary hypertension-associated heart failure with preserved ejection fraction is not known. Forty-six patients with heart failure with preserved ejection fraction and pulmonary hypertension (27 patients with combined pre- and post-capillary pulmonary hypertension and 19 patients with isolated post-capillary pulmonary hypertension) underwent sub-maximum cardiopulmonary exercise test followed by right heart catheterization. The study also included 18 age- and gender-matched control subjects. Several sub-maximum gas exchange parameters were examined to determine the ability of sub-maximum cardiopulmonary exercise test to distinguish between isolated post-capillary pulmonary hypertension and combined pre- and post-capillary pulmonary hypertension. Conventional echocardiogram measures did not distinguish between isolated post-capillary pulmonary hypertension and combined pre- and post-capillary pulmonary hypertension. Compared to isolated post-capillary pulmonary hypertension, combined pre- and post-capillary pulmonary hypertension had greater ventilatory equivalent for carbon dioxide (VE/VCO2) slope, reduced delta end-tidal CO2 change during exercise, reduced oxygen uptake efficiency slope, and reduced gas exchange determined pulmonary vascular capacitance. The latter was significantly associated with right heart catheterization determined pulmonary artery compliance (r = 0.5; p = 0.0004). On univariate analysis, sub-maximum VE/VCO2, delta end-tidal carbon dioxide, and gas exchange determined pulmonary vascular capacitance emerged as independent predictors of the extrapolated maximum oxygen uptake (%predicted) (β-coefficient values of –7.32, 95% CI: –13.3 – (–1.32), p = 0.01; 8.01, 95% CI: 1.96–14.05, p = 0.01; 8.78, 95% CI: 2.26–15.29, p = 0.01, respectively). Sub-maximum gas exchange parameters obtained during cardiopulmonary exercise test in an ambulatory setting allows for discrimination between isolated post-capillary pulmonary hypertension and combined pre- and post-capillary pulmonary hypertension. Additionally, sub-maximum cardiopulmonary exercise test derived VE/VCO2, delta end-tidal carbon dioxide, and gas exchange determined pulmonary vascular capacitance influences aerobic capacity in heart failure with preserved ejection fraction.
Keywords: pulmonary hypertension, heart failure with preserved ejection fraction (HFpEF), exercise capacity, cardiopulmonary exercise testing (CPET)
Introduction
Pulmonary hypertension (PH) is a progressive disease that results from various different processes. Regardless of origin, the diagnosis of PH requires the presence of a mean pulmonary artery pressure (mPAP) > 20 mmHg on resting right heart catheterization (RHC).1 In the western world, PH associated with left heart disease (PH-LHD) represents the most common cause of PH and its presence portends a poorer prognosis.2 PH-LHD is sub-divided into two groups according to RHC hemodynamic values as isolated post-capillary PH (Ipc-PH) and combined pre- and post-capillary PH (Cpc-PH). Both sub-groups are defined by elevated pulmonary artery wedge pressure (PAWP) > 15 mmHg but are further dichotomized based on the presence or absence of an abnormal pulmonary vascular resistance (PVR) with Cpc-PH having a PVR ≥3 Woods Unit (WU) and Ipc-PH having a PVR < 3 WU.1 PH-LHD is constituted primarily by left-sided valvular heart disease and those with left heart failure. Left-sided heart failure with preserved ejection fraction (HFpEF), defined as symptomatic heart failure occurring in the setting of a left ventricular ejection fraction (EF) ≥50%, accounts for approximately 50% of patients admitted with heart failure.3
Resting RHC remains the gold standard for diagnosing PH. However, it remains an invasive test with accompanying risks and is used infrequently to monitor disease progression. While imperative for the diagnosis of PH, resting RHC has little utility in examining the dynamic effects of hemodynamic perturbation that accounts for the exertional and functional limitation seen in PH patients during exercise. Trans-thoracic echocardiography (TTE) is often referred to as the first-line test in the initial work-up in patients with suspected PH.4 TTE is a safe, non-invasive imaging test that allows for estimation of pulmonary artery systolic pressure (PASP) from the maximum trans-tricuspid valvular regurgitant jet velocity. However, marked discordance between non-invasive pressure and invasive pressure measurements does occur.5–8 The causes of inaccurate PASP estimation by TTE include poor acoustic windows and Doppler misalignment with the tricuspid regurgitant jet.9 Importantly, while certain echocardiographic features have been shown to differentiate between Ipc-PH and Cpc-PH,10,11 these were either studied in a mixed cohort of left heart disease patients10 or were reliant on estimation of the highly variable PASP.11
In addition to standard diagnostic evaluation, cardiopulmonary exercise testing (CPET) is frequently utilized as an adjunct in the diagnosis and management of patients with PH.12 More recently, sub-maximum CPET has garnered increasing attention in the diagnostic evaluation of patients with PH.13–16 Unlike conventional CPET, a maximum exercise effort is not required, making it an attractive option for patients with cardio-pulmonary or musculoskeletal disorders and elderly patients who often are unable to undergo maximum exercise testing. While previous research have examined the diagnostic utility of sub-maximum CPET in PH, these initial studies were limited to patients with pulmonary arterial hypertension (PAH) only.14–16 Recently, Klaassen et al., showed that an abnormal ventilatory efficiency (VE/VCO2) in HFpEF patients during sub-maximum CPET is associated with greater PVR and increased mortality.13 However, the diagnostic utility of sub-maximum gas exchange parameters to help distinguish Ipc-PH and Cpc-PH remains unknown. Early identification of Ipc-PH can help prevent unnecessary invasive RHC and allow for immediate management directed toward HFpEF symptomatology and its associated conditions.17 The latter intervention could potentially help prevent progression toward Cpc-PH18 which portends a worse prognosis.11
Accordingly, in this study, we sought to examine whether VE/VCO2, oxygen uptake efficiency slope (OUES), gas exchange derived estimate of pulmonary vascular (PV) capacitance (GXCAP), resting end-tidal carbon dioxide (ETCO2), and delta ETCO2 measured during sub-maximum CPET differ between HFpEF patients with Ipc-PH and Cpc-PH in an ambulatory setting. We hypothesize that Cpc-PH would exhibit more significant derangement of the aforementioned variables thus providing a useful adjunct investigative tool in the management of HFpEF.
Methods
Study population and design
We enrolled patients from our Pulmonary Vascular Disease (PVD) clinic between January 2019 and August 2020 who underwent clinically indicated sub-maximum CPET (step test) followed by diagnostic RHC study. The study protocol was approved by our Institutional Review Board (IRB 2000024783). Included patients consented to have their clinical and investigative data used for research purposes.
The study included 46 consecutive patients with HFpEF who were seen at our PVD clinic. All HFpEF patients had left ventricular EF ≥50% with no significant valvular abnormalities19 along with resting supine mPAP > 20 mmHg and PAWP > 15 mmHg on RHC study. This new mPAP threshold replaced the prior mPAP threshold of ≥25 mmHg following the most recent recommendation by the 6th World Symposium on PH Task Force.1 The HFpEF group was further classified as Ipc-PH if the PVR was < 3 WU or as Cpc-PH if the PVR ≥3 WU.1 Once we identified our HFpEF cohort, we then selected 18 age- and gender-matched controls. The control subjects consisted of patients referred for unexplained dyspnea with unremarkable investigative testing at the time of presentation. The diagnostic association of control subjects is described in Table S1. Patients with physical or musculoskeletal limitation (including contracture deformity of the limbs) that precluded satisfactory sub-maximum CPET and those with left ventricular EF < 50% and significant valvular abnormalities were excluded from this study.19
Sub-maximum exercise step protocol
It is our standard of practice to have all patients who are capable to undergo sub-maximum exercise step testing (Shape Medical Systems, Inc., St Paul, MN, USA) during their ambulatory PVD clinic visit. This non-invasive sub-maximum test has been previously shown to differentiate PAH patients from healthy controls16 and help in the diagnosis of systemic sclerosis-associated PH.14,15 It consists of a portable unit with a 14 cm high step that patients step up and down for three minutes. The unit is equipped with a portable metabolic cart and a mouthpiece that is connected to a continuous gas exchange analyzer. The entire duration of the test is six minutes: two minutes of rest for baseline monitoring, three minutes of step exercise followed by one minute of recovery. The test measures sub-maximum and extrapolated maximum exercise oxygen consumption (VO2) (% predicted), ventilatory efficiency expressed as VE/VCO2, OUES, GXCAP, ETCO2 at rest and during exercise, heart rate and rhythm, and peripheral oxygen saturation. After the two minutes of baseline measurements, patients are instructed to “begin exercise” by stepping on and off of a platform at the speed indicated by a metronome set by the test administrator. After each minute of exercise, the test administrator increases the metronome speed. After three minutes of exercise, the patient is instructed to stop and stand idle for an additional minute for data collection. Gas exchange parameters, heart rate, and peripheral O2 saturation are collected throughout the entire two minutes of rest, three minutes of step exercise, and one minute of recovery.
Right heart hemodynamic assessment
RHC was performed in the supine position with a 7.5F Swan-Ganz catheter (Edwards LifeSciences, Irvine, CA, USA) inserted percutaneously under fluoroscopic and ultrasound guidance into the internal jugular vein. Right atrial, right ventricular, pulmonary arterial, and pulmonary arterial wedge pressures along with superior vena cava, right atrial, and pulmonary arterial oxygen saturations were measured. All pressures were recorded at the end of passive exhalation. When significant respirophasic changes to the hemodynamic tracings persisted, an electronic average was used.20 A zero reference was obtained at the mid-thoracic level.21,22 Cardiac output (CO) was determined using the thermodilution method. PVR was calculated by (mPAP – PAWP)/CO and expressed in Wood units (WU). Stroke volume (SV) was calculated as CO divided by the heart rate. CO and SV were indexed to body surface area to obtain both cardiac index and SV index. Pulmonary artery (PA) compliance was calculated as the ratio of SV to PA pulse pressure (the difference between systolic PA pressure and diastolic PA pressure) and expressed as mL/mmHg. Diastolic pressure gradient (DPG) was calculated as the difference between diastolic PA pressure and PAWP.23,24
Statistical analysis
Unless otherwise stated, values are presented as mean and standard deviation. Comparisons of baseline characteristics, echocardiogram data, RHC data, and sub-maximum CPET variables between controls, Ipc-PH, and Cpc-PH were performed using one-way ANOVA with Bonferroni post hoc correction. The comparison of sub-maximum CPET variables between Ipc-PH and Cpc-PH was performed using t-test. Receiver-operating characteristic curve analysis was used to assess the ability of the different sub-maximum CPET variables to distinguish between Ipc-PH and Cpc-PH. Pearson correlation was performed to determine the correlation between sub-maximum gas exchange derived GXCAP and RHC-determined PA compliance. Univariate analysis was performed to determine predictors of extrapolated maximum VO2 on sub-maximum step test. Only non-colinear variables (i.e. Pearson correlation r < 0.6) were incorporate in the univariate model. A probability value of < 0.05 was considered significant. Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, LLC, La Jolla, CA, USA) and SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic and clinical characteristics
There was no statistical difference in the age and gender between the three groups. HFpEF patients had greater body mass index (BMI) compared to controls as well as greater echocardiogram-determined right ventricle (RV) systolic pressure, left atrium indexed volume, and ratio of early mitral inflow velocity and mitral annular early diastolic velocity. There were no differences in any of the echocardiographic parameters between Ipc-PH and Cpc-PH. The baseline characteristics, co-morbidities, and echocardiogram parameters are summarized in Table 1.
Table 1.
Characteristics of patients with HFpEF.
| Control (n = 18) | Ipc-PH (n = 19) | Cpc-PH (n = 27) | |
|---|---|---|---|
| Characteristics | |||
| Age, years | 61 ± 8 | 62 ± 14 | 69 ± 10 |
| BMI (kg/m2) | 25 ± 4 | 41 ± 14a | 32 ± 8a |
| Female gender, n (%) | 13 (72) | 17 (89) | 23 (85) |
| NT-proBNP, pg/mL | n/a | 1215 ± 1203 | 2111 ± 2288 |
| NYHA functional class (n (%)) | |||
| I | 6 (33) | 2 (10) | 2 (7) |
| II | 12 (67) | 12 (63) | 12 (44) |
| III | n/a | 5 (26) | 12 (44) |
| Co-morbidities (n (%)) | |||
| Systemic hypertension | 7 (38) | 15 (79)a | 18 (67) |
| Diabetes | 1 (5) | 7 (37)a | 5 (20) |
| Coronary artery disease | 0 | 3 (15) | 3 (11) |
| Atrial fibrillation | 0 | 6 (32)a | 12 (44)a |
| Obstructive sleep apnea | 1 (7) | 8 (42)a | 6 (22) |
| Interstitial lung disease | 0 | 2 (10) | 3 (11) |
| COPD | 0 | 4 (21) | 3 (12) |
| Medications (n (%)) | |||
| Beta blocker | 1 (6) | 8 (47)a | 15 (55)a |
| Calcium channel blocker | 2 (11) | 7 (41)a | 4 (14) |
| ACE inhibitor or ARB | 1 (6) | 7 (41)a | 8 (30)a |
| Diuretics | 3 (16) | 11 (65)a | 19 (70)a |
| Echocardiogram | |||
| LV ejection fraction (%) | 65 ± 7 | 65 ± 7 | 66 ± 6 |
| RV systolic pressure (mmHg) | 30 ± 12 | 55 ± 14a | 65 ± 19a |
| TAPSE (cm) | 2.4 ± 0.4 | 2.1 ± 0.7 | 2.3 ± 0.4 |
| TAPSE/RVSP (mm/mmHg) | 0.92 ± 0.4 | 0.43 ± 0.2a | 0.33 ± 0.1a |
| LA volume index (cm/m2) | 29 ± 13 | 44 ± 16a | 46 ± 17a |
| E/e’ | 8 ± 1 | 12 ± 4a | 13 ± 6a |
| Right heart catheterization | |||
| Right atrial pressure (mmHg) | 3 ± 3 | 12 ± 3a | 13 ± 5a |
| Cardiac index (L/min/m2) | 3.4 ± 0.8 | 3.1 ± 0.9 | 2.1 ± 0.5a,b |
| SV index (mL/min/m2) | 48.6 ± 12.1 | 42.8 ± 12.1 | 32.0 ± 9.8a,b |
| mPAP (mmHg) | 15 ± 5 | 33 ± 5a | 47 ± 11a,b |
| PAWP (mmHg) | 6 ± 4 | 21 ± 5a | 19 ± 4a |
| DPG (mmHg) | 3 ± 1 | 3 ± 3 | 14 ± 7a,b |
| PA compliance (mL/mmHg) | 7.3 ± 3.4 | 3.7 ± 1.5a | 1.6 ± 0.5a,b |
| PVR (WU) | 1.5 ± 0.6 | 1.9 ± 0.7 | 6.9 ± 2.2a,b |
Note: Data presented as no. (%) or mean ± SD.
Ipc-PH: isolated post-capillary pulmonary hypertension; Cpc-PH: combined pre-and post-capillary pulmonary hypertension; BMI: body mass index; NT-proBNP: N-terminal prohormone brain natriuretic peptide; NYHA: New York Heart Association; COPD: chronic obstructive pulmonary disease; ACE: angiotensin converting enzyme; LV: left ventricle; RV: right ventricle; TAPSE: tricuspid annular systolic plane excursion; RVSP: right ventricular systolic pressure; LA: left atrium; E/e’: ratio of early mitral inflow velocity and mitral annular early diastolic velocity; mPAP: mean pulmonary artery pressure; SV: stroke volume; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; WU: Woods unit; DPG: diastolic pulmonary gradient; PA: pulmonary artery.
ap-value < 0.05 vs. controls.
bp-value < 0.05 vs. Ipc-PH.
Resting RHC data
HFpEF patients had greater right atrial pressure compared to controls. Cpc-PH had greater mPAP (47 ± 11 vs. 33 ± 5 mmHg, p < 0.05) with lower cardiac (2.1 ± 0.1 vs. 3.1 ± 0.9 L/min, p < 0.05) and SV (32.0 ± 9.8 vs. 42.8 ± 12.1 mL/min/m2, p < 0.05) indices compared to Ipc-PH. By study design, Cpc-PH had greater PVR and DPG compared to Ipc-PH. Cpc-PH had the lowest PA compliance between the groups. The resting RHC data are summarized in Table 1.
Sub-maximum CPET data
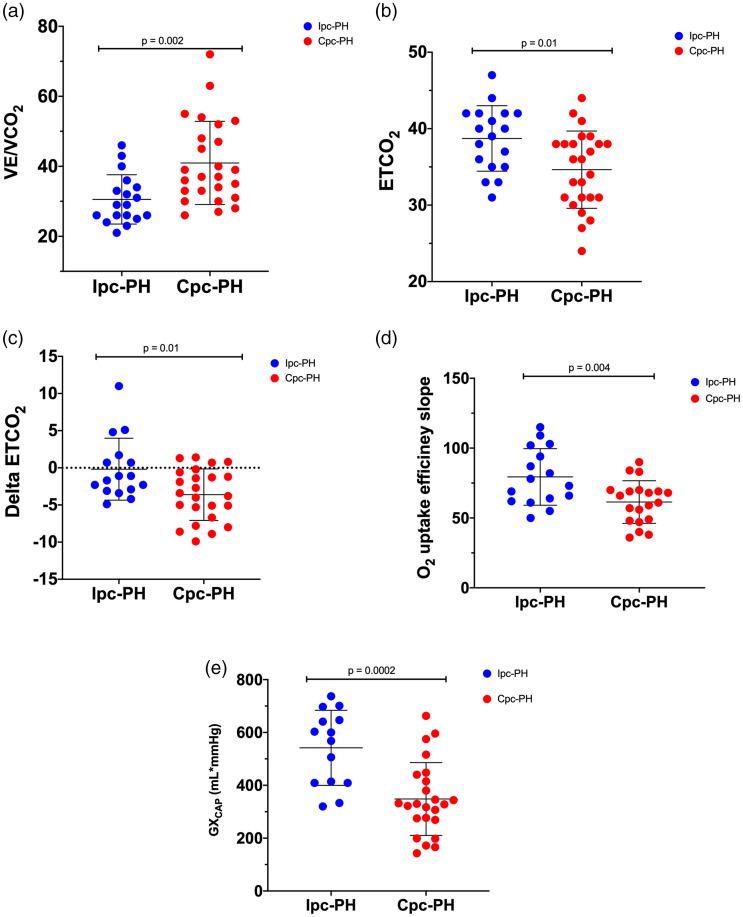
Cpc-PH exhibited greatest VE/VCO2 between the three groups. Delta ETCO2, GXCAP, and OUES were lowest in Cpc-PH group. There was no difference between resting ETCO2, VE/VCO2, delta ETCO2, and GXCAP between Ipc-PH and controls. Peak exercise oxygen saturation (SpO2) was lower in Cpc-PH compared to controls but not different compared to Ipc-PH. Both HFpEF group exhibited similarly depressed sub-maximum VO2 (% predicted) and O2 pulse compared to controls. The sub-maximum CPET parameters between the three groups and between Ipc-PH and Cpc-PH are summarized in Table 2 and Fig. 1, respectively.
Table 2.
Sub-maximum CPET parameters.
| Control (n = 18) | Ipc-PH (n = 19) | Cpc-PH (n = 27) | |
|---|---|---|---|
| Time between RHC and sub-maximum CPET, median (IQR), days | 51 (10–107) | 19 (8–34) | 15 (8–23) |
| ETCO2 (mmHg) | 35 ± 5 | 38 ± 4 | 34 ± 4b |
| Rest SpO2 (%) | 97 ± 1 | 95 ± 2a | 95 ± 2a |
| Peak SpO2 (%) | 95 ± 2 | 90 ± 3a | 90 ± 4a |
| RER | 1.03 ± 0.1 | 1.03 ± 0.1 | 1.04 ± 0.1 |
| VE/VCO2 | 31 ± 10 | 31 ± 7 | 41 ± 11a,b |
| Delta ETCO2 (mmHg) | 1.9 ± 5.0 | −0.2 ± 4.0 | −3.6 ± 3.4a,b |
| GXCAP (mL*mmHg) | 611 ± 247 | 541 ± 141 | 348 ± 137a,b |
| OUES (% predicted) | 102 ± 22 | 79 ± 20a | 61 ± 15a,b |
| O2 pulse (mL/O2/beat) | 180 ± 102 | 101 ± 22a | 98 ± 36a |
| Sub-maximum VO2 (% predicted) | 98 ± 18 | 64 ± 11a | 60 ± 18a |
| Extrapolated maximum VO2(% predicted) | 121 ± 44 | 69 ± 18a | 62 ± 21a |
Note: Data presented as no. (%) or mean ± SD unless otherwise stated.
CPET: cardiopulmonary; Ipc-PH: isolated post-capillary pulmonary hypertension; Cpc-PH: combined pre-and post-capillary pulmonary hypertension; RHC: right heart catheterization; ETCO2: end tidal carbon dioxide; SpO2: peripheral oxygen saturation; RER: respiratory exchange ratio; VE/VCO2: ventilatory efficiency; GXCAP: gas exchange derived pulmonary vascular capacitance; OUES: oxygen uptake efficiency slope; VO2: oxygen consumption.
ap-value < 0.05 vs. controls.
bp-value < 0.05 vs. Ipc-PH.
Fig. 1.
Sub-maximum CPET parameters between patients with isolated post-capillary pulmonary hypertension (Ipc-PH) and combined pre- and post-capillary pulmonary hypertension (Cpc-PH). Data presented as mean ± SD.
VE/VCO2: ventilatory efficiency; ETCO2: end tidal carbon dioxide; delta ETCO2: change in ETCO2 from rest to end exercise; O2: oxygen; GXCAP: gas exchange derived measure of pulmonary vascular capacitance.
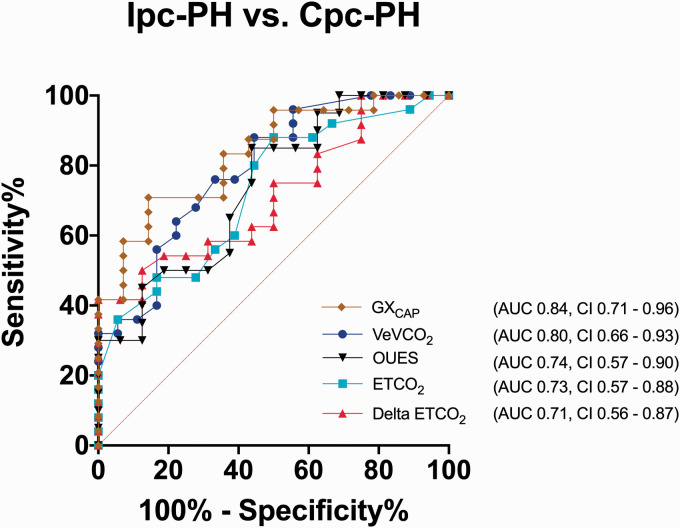
Using the different sub-maximum CPET parameters to distinguish between Cpc-PH from Ipc-PH, GXCAP and VE/VCO2 emerged as the best predictors. The area under the curve (AUC) for GXCAP was 0.84 ± 0.06 (p = 0.001) with a sensitivity of 73% and a specificity of 85% at an optimal cut-off point of 394 mL*mmHg. For VE/VCO2, the AUC was 0.79 ± 0.07 (p = 0.001) with a sensitivity of 76% and a specificity of 67% at an optimal cut-off point of 33. Other sub-maximum CPET parameters including resting ETCO2 (AUC: 0.73 ± 0.08; p = 0.01), OUES (AUC: 0.74 ± 0.08; p = 0.02), and delta ETCO2 (AUC: 0.71 ± 0.08; p = 0.02) provided less discrimination between Ipc-PH and Cpc-PH (Fig. 2). There was a significant correlation between sub-maximum GXCAP from CPET and resting RHC-determined PA compliance (r = 0.5; p = 0.0004) (Figure S1). On univariate analysis, the sub-maximum CPET variables of VE/VCO2, delta ETCO2, and GXCAP were significantly associated with the extrapolated maximum VO2 (%predicted) (Table S2).
Fig. 2.
Receiver operating characteristic curve for discriminating between patients with isolated post-capillary pulmonary hypertension (Ipc-PH) and combined pre- and post-capillary pulmonary hypertension (Cpc-PH). GXCAP and VE/VCO2 provided the best discriminating parameters between Ipc-PH and Cpc-PH.
VE/VCO2: ventilatory efficiency; ETCO2: end tidal carbon dioxide; delta ETCO2: change in ETCO2 from rest to end exercise; O2: oxygen; GXCAP: gas exchange derived measure of pulmonary vascular capacitance; OUES: oxygen uptake efficiency slope; AUC: area under the curve; Ipc-PH: isolated post-capillary pulmonary hypertension; Cpc-PH: combined pre- and post-capillary pulmonary hypertension.
Discussion
In the current study, we demonstrate that an easily executed sub-maximum exercise test incorporating ventilatory gas analysis provides a clear distinction between Ipc-PH and Cpc-PH. This simplified exercise test also provides physiological reasoning for the differential exertional limitation experienced by Ipc-PH and Cpc-PH patients. Additionally, we also show that reduced delta ETCO2 and GXCAP during sub-maximum exercise is associated with reduced exercise capacity in HFpEF.
Why is the distinction between Ipc-PH and Cpc-PH in HFpEF important? Patients with Cpc-PH have worse outcomes compared with those with Ipc-PH owing possibly to worse RV function.11 The sub-maximum exercise testing can serve as an adjunctive diagnostic tool, while also offering pathophysiological reasoning for patients’ exercise limitation and prognostic information in HFpEF. The constellation of a clinical and echocardiographic phenotype consistent with HFpEF, along with abnormal gas-exchange parameters on sub-maximum exercise testing, can help obviate the immediate need for an invasive RHC. Maximum VO2 is an important prognostic indicator in patients with heart failure.25,26 In the present study, similar to prior reports, HFpEF was associated with reduced sub-maximum27 and maximum VO2 (%predicted).28,29 Extrapolated maximum VO2 has been shown to closely correlate with actual maximum VO2 attained during exercise and is independent of exercise duration.30,31 It can be obtained by mathematically extrapolating the relation between VO2 and carbon dioxide production (VCO2) to the point at which respiratory exchange has risen to double the value at the onset of exercise30 or using a logarithmic curve fitting model which incorporates the OUES.31 An important finding of the present study is that the sub-maximum CPET variables VE/VCO2, delta ETCO2, and GXCAP were significantly associated with the extrapolated maximum VO2 (%predicted) (Table S2).
Sub-maximum VE/VCO2 has been previously shown to confer prognostic significance in HFpEF.13 The current study adds further significance to sub-maximally derived CPET parameters as we also demonstrate that reduce delta ETCO2 and GXCAP during sub-maximum exercise is associated with reduced exercise capacity in HFpEF. Notably, unlike VE/VCO2, both of these parameters are not routinely reported on conventional non-invasive CPET and therefore provide a novel prognostic indicator in HFpEF.
In the present study, the two parameters which offered the best discrimination between Ipc-PH and Cpc-PH were GXCAP and VE/VCO2 (Fig. 2). Other gas exchange parameters including resting ETCO2, delta ETCO2, and OUES were also significantly different but provided less discrimination between Ipc-PH and Cpc-PH (Table 2 and Fig. 1). While conventional echocardiographic measures of LV diastolic impairment clearly discriminated HFpEF from controls, they did not distinguish Cpc-PH from Ipc-PH (Table 2). We showed a significant correlation between GXCAP obtained during sub-maximum CPET and PA compliance derived from resting RHC (Figure S1). The lower GXCAP seen in Cpc-PH is likely attributed to the compounding effect of increased resistive RV afterload to an increased pulsatile afterload driven by elevated PAWP. The increase in RV afterload seen with Cpc-PH is similar to PAH in that both are driven by dynamic augmentation of resistive and pulsatile afterload during exercise.28,29 During invasive cardio-pulmonary exercise testing (iCPET), PA compliance (with which GXcap has a significant correlation with) is similarly reduced in PAH and HFpEF patients with abnormal PVR response during exercise.29 However, the mechanism by which PA compliance worsens during exercise and the subsequent increase in RV pulsatile afterload differs between PAH and Cpc-PH. Approximately 20% of PA compliance is stored in the main PA and proximal left and right PAs, with the remaining 80% resides within the distal pulmonary vasculature.32 The pathologic hallmark of PAH implicates a vasculopathy of the distal pre-capillary pulmonary vasculature, in particular the small resistive vessel.29 Hence, the distal pre-capillary PV remodeling seen in PAH reduces PA compliance and this increases RV pulsatile afterload. Additionally, pre-capillary PV remodeling contributes to increases in PVR and impairment of PV distensibility.29 Consequently, in PAH, the inability of the distal pulmonary vasculature to first recruit and later distend culminates in dynamic worsening of RV pulsatile and resistive afterload during exercise.29
The greater RV afterload encountered by Cpc-PH compared to Ipc-PH prevents the expectant increase in pulmonary blood flow during exercise leading to under-perfusion of well-ventilated lung units. Ventilation–perfusion (V/Q) mismatch ensues contributing to the greater VE/VCO2 slope and failure of expectant increase in ETCO2 from rest to peak exercise (i.e. negative delta ETCO2) in Cpc-PH. We recently showed that in HFpEF, RV function and its SV deteriorate by 50% of the peak VO2.27 Consequently, the CO is insufficient to meet the increasing metabolic demands of exercising muscle. This reduction in O2 delivery contributes to the earlier attainment of the anaerobic threshold (AT) and subsequent lactic acidosis. The resulting proton (H+) and CO2 generation stimulates chemoreceptors and increases the ventilatory response.33–36 Patients with Cpc-PH have intrinsic pre-capillary PVD from chronic pulmonary venous hypertension.18 This pre-capillary PV remodeling process likely contributes to the greater degree of hypoxemia seen in Cpc-PH from VQ mismatch and reduced O2 diffusion capacity. The resulting hypoxemia is a potent stimulator of ventilation.16 Additionally, juxta-capillary receptors which terminate in the lung parenchyma adjacent to the capillaries are stimulated by development of interstitial edema.37 Altogether, this hyper-ventilatory response along with reduced pulmonary blood flow results in the lower ETCO2 and greater VE/VCO2 seen in Cpc-PH during exercise.
In response to left atrial hypertension, there is a disproportionate increase in mPAP with resulting reduced PA compliance.23 Consequently, the trans-pulmonary gradient (TPG) and therefore the PVR can be elevated even in the absence of pulmonary vasoconstriction or remodeling. Unlike the TPG, DPG, which is less dependent on volume load and SV, has been shown to be a useful marker to help distinguish between Ipc-PH and Cpc-PH.11,23,38 In the current study, Cpc-PH and Ipc-PH were defined by a PVR ≥3 WU and PVR < 3 WU, respectively. Five out of 27 in Cpc-PH group had DPG < 7 mmHg and 1 out of 19 in Ipc-PH group had DPG ≥7 mmHg. During our RHC study, if the systemic blood pressure permits, we routinely perform intravenous nitroprusside challenge followed by repeat hemodynamic measurement in patients with left heart disease. Three out of five of the Cpc-PH patients who underwent intravenous nitroprusside challenge normalized their PAWP (i.e. < 15 mmHg) with persistent abnormality of their PVR ≥3 WU, indicating the presence of fixed pre-capillary PVD (data not shown). It is therefore unlikely that the incorporation of PVR and DPG would have changed the current interpretation of our results given (a) the severity of our Cpc-PH population (mean DPG in the Cpc-PH was 14 mmHg with mean PVR of 6.9 WU), (b) the similar PAWP between the HFpEF groups, and (c) the majority of Cpc-PH patients exhibited hemodynamic evidence of pre-capillary PVD while only one Ipc-PH patient had a DPG ≥7 mmHg.
The current finding of a greater VE/VCO2 seen in Cpc-PH population is in keeping with prior reports.13,38 VE/VCO2 shares a close relationship with DPG and PVR and can therefore be used a marker of PVD burden.13,38 Using invasive CPET (iCPET), we previously showed that PAH patients exhibited greater degree of ventilatory inefficiency (i.e. abnormal VE/VCO2 slope) compared to HFpEF patients who develop an abnormal PVR response during maximum cycle ergometer testing in the upright position.29 There was no significant difference in other non-invasive CPET variables, including delta ETCO2 and peak VO2 (%predicted). However, the HFpEF population studied was distinct from the current study in that they were defined by their peak exercise hemodynamic values during upright cycle ergometer testing. Caravita and colleagues compared various maximum CPET variables between PAH and Cpc-PH (from underlying HFpEF, systolic heart failure, and valvular heart disease). They found that PAH patients exhibited greater VE/VCO2 with lower prevalence of exercise oscillatory breathing (EOB) compared to Cpc-PH.38 To our knowledge, no prior study has examined whether CPET parameters obtained during sub-maximum or maximum exercise helps differentiate between HFpEF-associated Cpc-PH from PAH.
The OUES reflects the global (pulmonary, cardiovascular, and skeletal muscle) functional impairment that combines VO2 and VE/VCO2 slope. It is calculated as the logarithmic transformation of minute ventilation (VE) with VO2, creating a liner relationship between VE and VO2.12 OUES has been shown to significantly correlate with peak VO2 and remains stable throughout exercise, making it ideal for the evaluation of sub-maximum exercise capacity.39 A reduction in VO2 is the result of depressed peak exercise CO and/or impaired systemic oxygen extraction. We and other groups showed that during maximum invasive CPET, reduced peak VO2 in HFpEF patients is predominantly attributed to CO limitation.28,40 Accordingly, in HFpEF, the reduced blood flow/CO and hyperventilation contributes to reduced OUES. Compared to controls subjects, HFpEF patients are therefore unable to consume as much oxygen (i.e. reduced VO2) for a given increase in VE during exercise leading to a reduced OUES (Table 2).
Several studies that have demonstrated improvement in various non-invasive CPET parameters in patients with chronic thromboembolic pulmonary hypertension41 and heart failure with reduced ejection fraction (HFrEF),42,43 owing in large part to the known pharmacotherapeutic benefits seen with PAH-targeted and HFrEF therapies. In contrast, CPET outcomes in response to pharmacotherapy in HFpEF have not been as favorable.44–46 Because of the limited therapeutic success seen with HFpEF, at the current juncture, there is limited utility in longitudinal testing to assess treatment response in HFpEF. However, the current study supports the prognostic role for longitudinal sub-maximum CPET in HFpEF as it would help identify progression toward Cpc-PH,18 a phenotype that is known to have a worse prognosis compared to Ipc-PH.11
Some of our control subjects are symptomatic patients who have an eventual diagnosis of dysautonomia on maximum iCPET. They represent a cohort of patients with normal resting RHC hemodynamics and are considered to have a “normal” physiological response to exercise as determined by their peak exercise CO and VO2 ≥80% predicted on iCPET. The other groups of patients that make up the majority of the control cohort are patients with connective tissue disease (i.e. approximately 78% of the control subjects). Dysautonomia and connective tissue disease patients commonly exhibit a hyper-ventilatory response during exercise.47 This contributes to excessive ventilation relative to carbon dioxide (CO2) output and likely accounts for the similarly elevated VE/VCO2 and end-exercise ETCO2 seen when compared to Ipc-PH.47
Unlike the findings of the current study, other studies have shown that certain echocardiographic features are able to distinguish between Ipc-PH and Cpc-PH.10,11 There are several potential reasons why there was no significant difference in echocardiographic parameters between Ipc-PH and Cpc-PH in the current study. The study by D’Alto et al. incorporated a different and more specific echocardiographic measures to help distinguish between Ipc-PH and Cpc-PH.10 Additionally, the study population from D’Alto et al., unlike our studied cohort, were not exclusively HFpEF patients. The study from Gerges M et al. demonstrated that echocardiography derived ratio of tricuspid annular plane systolic excursion to estimated PA systolic pressure (TAPSE/PASP) was significantly reduced in Cpc-PH compared to Ipc-PH. In the current study, however, we did not find a significant difference in TAPSE/PASP between Ipc-PH and Cpc-PH. The determination of TAPSE/PASP relies on accurate determination of PASP and therefore on good quality acoustic windows and Doppler alignment. One of the primary reasons for poor acoustic windows relates to a patients’ body habitus. The mean BMI in our Ipc-PH and Cpc-PH groups were 41 ± 14 kg/m2 and 32 ± 8 kg/m2, respectively, which likely limits the acquisition of good quality acoustic windows. This is in contrast to the BMI of the HFpEF group in Gerges M et al.’s study who had a mean BMI of 27 ± 2 kg/m2.
Limitations
VE/VCO2 describes the ventilation needed to eliminate one liter of metabolic CO2 at the level of ventilation that is least variable. Because of this, VE/VCO2 is measured at the level of the AT or the respiratory compensation point. We did not obtain serial serum lactate measurements during the sub-maximal test to determine if subjects reached their lactic acidosis or AT. However, the respiratory exchange ratio (RER), which represents the ratio between the amount of CO2 produced (VCO2) and oxygen uptake (VO2) during exercise, can be used as a gas exchange determinant of the AT. The transition from aerobic metabolism, where VCO2 increases linearly with VO2 yielding a RER of < 1, to anaerobic metabolism where the RER is slightly > 1 defines the lactic acidosis threshold or gas exchange AT.48 In the current study, the mean RER across the three groups was > 1 suggesting that step exercise during sub-maximum CPET was performed at or near the gas exchange AT (Table 2).
There is a time delay between the sub-maximum CPET and RHC study, particularly for the control patients who underwent further investigative work-up with an invasive CPET. However, no treatment was instituted or augmented in any of the HFpEF patients in the interim period between their sub-maximum CPET and RHC. Caravita and colleagues demonstrated that EOB distinguished between Ipc-PH and Cpc-PH during maximum CPET.38 EOB is defined as ≥3 cyclical fluctuations of VE during exercise lasting ≥60% of the exercise duration with an amplitude of either 15% or 25% of the average VE.38,49 However, in the current study, the duration of exercise was not sufficient to allow for adequate observation of EOB.
Conclusions
This study shows that a non-invasive, sub-maximum stress test can be readily performed in an ambulatory setting and that the gas exchange parameters obtained during testing, in particular VE/VCO2 and GXCAP, allows for a clear distinction between HFpEF phenotypes. This study also demonstrates that owing to the additive effects of resistive RV afterload, Cpc-PH encounter greater degree of ventilatory inefficiency and reduced GXCAP compared to Ipc-PH during exercise. Additionally, we show that non-conventional CPET parameters (i.e. reduced delta ETCO2 and GXCAP) during sub-maximum exercise are associated with reduced exercise capacity in HFpEF. Larger studies are warranted to study its utility in monitoring response to therapeutic interventions and as an end-point measure for clinical trials.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020972273 for Diagnostic utility of sub-maximum cardiopulmonary exercise testing in the ambulatory setting for heart failure with preserved ejection fraction by Hannah T. Oakland, Phillip Joseph, Ahmed Elassal, Marjorie Cullinan, Paul M. Heerdt and Inderjit Singh in Pulmonary Circulation
Footnotes
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PMH declares consulting arrangements with Baxter International, Baudax Bio, and is co-founder RVMetrics, LLC.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Hannah T. Oakland https://orcid.org/0000-0003-4678-1554
Phillip Joseph https://orcid.org/0000-0001-9299-8629
Paul M. Heerdt https://orcid.org/0000-0002-9765-9885
Supplemental material: Supplemental material for this article is available online.
References
- 1.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1501913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012; 126: 975–990. [DOI] [PubMed] [Google Scholar]
- 3.Dhingra A, Garg A, Kaur S, et al. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2014; 11: 354–365. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 5.Bossone E, Duong-Wagner TH, Paciocco G, et al. Echocardiographic features of primary pulmonary hypertension. J Am Soc Echocardiogr 1999; 12: 655–662. [DOI] [PubMed] [Google Scholar]
- 6.Brecker SJ, Gibbs JS, Fox KM, et al. Comparison of Doppler derived haemodynamic variables and simultaneous high fidelity pressure measurements in severe pulmonary hypertension. Br Heart J 1994; 72: 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139: 988–993. [DOI] [PubMed] [Google Scholar]
- 8.Murata I, Takenaka K, Yoshinoya S, et al. Clinical evaluation of pulmonary hypertension in systemic sclerosis and related disorders. A Doppler echocardiographic study of 135 Japanese patients. Chest 1997; 111: 36–43. [DOI] [PubMed] [Google Scholar]
- 9.Hur DJ, Sugeng L. Non-invasive multimodality cardiovascular imaging of the right heart and pulmonary circulation in pulmonary hypertension. Front Cardiovasc Med 2019; 6: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Alto M, Romeo E, Argiento P, et al. A simple echocardiographic score for the diagnosis of pulmonary vascular disease in heart failure. J Cardiovasc Med (Hagerstown) 2017; 18: 237–243. [DOI] [PubMed] [Google Scholar]
- 11.Gerges M, Gerges C, Pistritto AM, et al. Pulmonary hypertension in heart failure. Epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med 2015; 192: 1234–1246. [DOI] [PubMed] [Google Scholar]
- 12.Guazzi M, Bandera F, Ozemek C, et al. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol 2017; 70: 1618–1636. [DOI] [PubMed] [Google Scholar]
- 13.Klaassen SHC, Liu LCY, Hummel YM, et al. Clinical and hemodynamic correlates and prognostic value of VE/VCO2 slope in patients with heart failure with preserved ejection fraction and pulmonary hypertension. J Card Fail 2017; 23: 777–782. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein EJ, Gordon JK, Spiera RF, et al. Comparison of change in end tidal carbon dioxide after three minutes of step exercise between systemic sclerosis patients with and without pulmonary hypertension. Rheumatology (Oxford) 2017; 56: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein EJ, Mandl LA, Gordon JK, et al. Submaximal heart and pulmonary evaluation: a novel noninvasive test to identify pulmonary hypertension in patients with systemic sclerosis. Arthritis Care Res (Hoboken) 2013; 65: 1713–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods PR, Frantz RP, Taylor BJ, et al. The usefulness of submaximal exercise gas exchange to define pulmonary arterial hypertension. J Heart Lung Transplant 2011; 30: 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2014; 64: 2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fayyaz AU, Edwards WD, Maleszewski JJ, et al. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 2018; 137: 1796–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pieske B, Tschope C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297–3317. [DOI] [PubMed] [Google Scholar]
- 20.Boerrigter BG, Waxman AB, Westerhof N, et al. Measuring central pulmonary pressures during exercise in COPD: how to cope with respiratory effects. Eur Respir J 2014; 43: 1316–1325. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs G, Avian A, Olschewski A, et al. Zero reference level for right heart catheterisation. Eur Respir J 2013; 42: 1586–1594. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs G, Avian A, Pienn M, et al. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med 2014; 190: 252–257. [DOI] [PubMed] [Google Scholar]
- 23.Naeije R, Vachiery JL, Yerly P, et al. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013; 41: 217–223. [DOI] [PubMed] [Google Scholar]
- 24.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013; 143: 758–766. [DOI] [PubMed] [Google Scholar]
- 25.Mehra MR, Canter CE, Hannan MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016; 35: 1–23. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill JO, Young JB, Pothier CE, et al. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 2005; 111: 2313–2318. [DOI] [PubMed] [Google Scholar]
- 27.Singh I, Oliveira RKF, Heerdt P, et al. Dynamic right ventricular function response to incremental exercise in pulmonary hypertension. Pulm Circ. Epub ahead of print 2020. DOI: 10.1177/2045894020950187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh I, Rahaghi FN, Naeije R, et al. Right ventricular-arterial uncoupling during exercise in heart failure with preserved ejection fraction: role of pulmonary vascular dysfunction. Chest 2019; 156: 933–943. [DOI] [PubMed] [Google Scholar]
- 29.Singh I, Oliveira RKF, Naeije R, et al. Pulmonary vascular distensibility and early pulmonary vascular remodeling in pulmonary hypertension. Chest 2019; 156: 724–732. [DOI] [PubMed] [Google Scholar]
- 30.Buller NP, Poole-Wilson PA. Extrapolated maximal oxygen consumption: a new method for the objective analysis of respiratory gas exchange during exercise. Br Heart J 1988; 59: 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba R, Nagashima M, Goto M, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol 1996; 28: 1567–1572. [DOI] [PubMed] [Google Scholar]
- 32.Saouti N, Westerhof N, Helderman F, et al. RC time constant of single lung equals that of both lungs together: a study in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol 2009; 297: H2154–H2160. [DOI] [PubMed] [Google Scholar]
- 33.Olson TP, Joyner MJ, Johnson BD. Influence of locomotor muscle metaboreceptor stimulation on the ventilatory response to exercise in heart failure. Circ Heart Fail 2010; 3: 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kee K, Stuart-Andrews C, Ellis MJ, et al. Increased dead space ventilation mediates reduced exercise capacity in systolic heart failure. Am J Respir Crit Care Med 2016; 193: 1292–1300. [DOI] [PubMed] [Google Scholar]
- 35.Guazzi M, Reina G, Tumminello G, et al. Exercise ventilation inefficiency and cardiovascular mortality in heart failure: the critical independent prognostic value of the arterial CO2 partial pressure. Eur Heart J 2005; 26: 472–480. [DOI] [PubMed] [Google Scholar]
- 36.Hachamovitch R, Brown HV, Rubin SA. Respiratory and circulatory analysis of CO2 output during exercise in chronic heart failure. Circulation 1991; 84: 605–612. [DOI] [PubMed] [Google Scholar]
- 37.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 1984; 99: 1–110. [DOI] [PubMed] [Google Scholar]
- 38.Caravita S, Faini A, Deboeck G, et al. Pulmonary hypertension and ventilation during exercise: role of the pre-capillary component. J Heart Lung Transplant 2017; 36: 754–762. [DOI] [PubMed] [Google Scholar]
- 39.Van Laethem C, Bartunek J, Goethals M, et al. Oxygen uptake efficiency slope, a new submaximal parameter in evaluating exercise capacity in chronic heart failure patients. Am Heart J 2005; 149: 175–180. [DOI] [PubMed] [Google Scholar]
- 40.Abudiab MM, Redfield MM, Melenovsky V, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 2013; 15: 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charalampopoulos A, Gibbs JS, Davies RJ, et al. Exercise physiological responses to drug treatments in chronic thromboembolic pulmonary hypertension. J Appl Physiol (1985) 2016; 121: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatia V, Bhatia R, Mathew B. Angiotensin receptor blockers in congestive heart failure: evidence, concerns, and controversies. Cardiol Rev 2005; 13: 297–303. [DOI] [PubMed] [Google Scholar]
- 43.Guazzi M, Myers J, Peberdy MA, et al. Ventilatory efficiency and dyspnea on exertion improvements are related to reduced pulmonary pressure in heart failure patients receiving Sildenafil. Int J Cardiol 2010; 144: 410–412. [DOI] [PubMed] [Google Scholar]
- 44.Parthasarathy HK, Pieske B, Weisskopf M, et al. A randomized, double-blind, placebo-controlled study to determine the effects of valsartan on exercise time in patients with symptomatic heart failure with preserved ejection fraction. Eur J Heart Fail 2009; 11: 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal N, Sivaswamy N, Mahmod M, et al. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation 2015; 132: 1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitzman DW, Hundley WG, Brubaker PH, et al. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail 2010; 3: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melamed KH, Santos M, Oliveira RKF, et al. Unexplained exertional intolerance associated with impaired systemic oxygen extraction. Eur J Appl Physiol 2019; 119: 2375–2389. [DOI] [PubMed] [Google Scholar]
- 48.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986; 60: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 49.Murphy RM, Shah RV, Malhotra R, et al. Exercise oscillatory ventilation in systolic heart failure: an indicator of impaired hemodynamic response to exercise. Circulation 2011; 124: 1442–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020972273 for Diagnostic utility of sub-maximum cardiopulmonary exercise testing in the ambulatory setting for heart failure with preserved ejection fraction by Hannah T. Oakland, Phillip Joseph, Ahmed Elassal, Marjorie Cullinan, Paul M. Heerdt and Inderjit Singh in Pulmonary Circulation