Abstract
Limited data about the long-term prognosis and response to therapy in pulmonary arterial hypertension patients with World Health Organization functional class I/II symptoms are available. PubMed and Embase were searched for publications of observational registries and randomized, controlled trials in pulmonary arterial hypertension patients published between January 2001 and January 2018. Eligible registries enrolled pulmonary arterial hypertension patients ≥18 years, N > 30, and reported survival by functional class. Randomized, controlled trial inclusion criteria were pulmonary arterial hypertension patients ≥18 years, ≥6 months of treatment, and morbidity, mortality, or time to worsening as end points reported by functional class. The primary outcomes were survival for registries and clinical event rates for randomized, controlled trials. Separate random effects models were calculated for registries and randomized, controlled trials. Four randomized, controlled trials (n = 2482) and 10 registries (n = 6580) were included. Registries enrolled 9%–47% functional class I/II patients (the vast majority being functional class II) with various pulmonary arterial hypertension etiologies. Survival rates for functional class I/II patients at one, two, and three years were 93% (95% confidence interval (CI): 91%–95%), 86% (95% CI: 82%–89%), and 78% (95% CI: 73%–83%), respectively. The hazard ratio for the treatment effect in randomized, controlled trials overall was 0.61 (95% CI: 0.51–0.74) and 0.60 (95% CI: 0.44–0.82) for functional class I/II patients and 0.62 (95% CI: 0.49–0.78) for functional class III/IV. The calculated risk of death of 22% within three years for functional class I/II patients underlines the need for careful assessment and optimal treatment of patients with functional class I/II disease. The randomized, controlled trial analysis demonstrates that current medical therapies have a beneficial treatment effect in this population.
Keywords: clinical worsening, functional class, meta-analysis, morbidity, mortality, pulmonary arterial hypertension
Introduction
Pulmonary arterial hypertension (PAH) is a serious and progressive cardiopulmonary disease that is characterized by remodeling of the pulmonary vasculature and elevated pulmonary arterial pressure.1–3 The consequences of PAH, particularly for patients who do not receive timely and appropriate treatment, include right ventricular failure, diminished functional status, and death.1,3–5 In recent years, a number of medical therapies have been approved for the treatment of PAH, including endothelin receptor antagonists, phosphodiesterase type 5 inhibitors, parenteral and nonparenteral prostacyclins, a soluble guanylate cyclase stimulator, and a non-prostanoid prostacyclin receptor agonist.6–13 While these therapies improve symptoms, exercise capacity, hemodynamics, and functional status, as well as reduce hospitalizations and delay disease progression,1,8 PAH remains a chronic, progressive, and incurable disease.14,15
Functional class (FC) is widely used as a clinical end point in studies of cardiovascular diseases, including heart failure,16 coronary artery disease,17 and congenital heart disease.18 In patients with PAH, FC is strongly associated with survival and is considered to be an essential component of risk assessment and treatment planning.19,20 Patients in FC I or FC II generally have lower one- and five-year mortality rates compared with those in FC III or FC IV. The Patient Registry for the Characterization of Primary Pulmonary Hypertension reported a median survival of almost six years for patients in FC I or FC II compared with 2.5 years and six months for those in FC III or FC IV, respectively, in an era when medical treatments for PAH were limited.21 Despite recent advances in medical therapy for patients with PAH, mortality remains high and continues to be associated with FC. The Registry to Evaluate Early and Long-term PAH Disease Management (REVEAL) reported five-year survival rates of 72.2%, 71.7%, 60.0%, and 43.8% for newly diagnosed patients in FC I, II, III, and IV, respectively.4 However, recent guidance on PAH treatment suggests that patient risk should be assessed using a number of variables, not solely FC, and the goal of treatment should be to attain and maintain a low-risk status.22–24 Estimated one-year morality in patients in the low-risk group is <5%.22,23 Nevertheless, it is generally accepted that FC I or II patients are at low risk for clinical worsening or death.
Meta-analysis provides a method to pool data from a number of studies, thus increasing statistical power and providing more precise effect estimates. In this study, we performed two separate meta-analyses of patients with PAH to characterize long-term survival outcomes and treatment effects by FC. In the first meta-analysis, we evaluated survival outcomes reported in observational registries with PAH patients stratified by FC. In the second meta-analysis, we quantified the treatment effects observed in randomized, controlled trials (RCTs) of approved PAH therapies in FC I or II patients. To further understand the prognostic value of FC, we also assessed treatment effects in patients in FC III or IV.
Materials and methods
The protocol for this meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO) and published (CRD42018092820).25 The study adhered to the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis.26 Review and approval by an ethics committee were not required because aggregate data were extracted from published studies.
Data sources and search strategy
We systematically searched the PubMed and Embase databases to identify relevant English-language observational registry studies published between 1 January 2001, and 22 January 2018, that reported survival outcomes for PAH patients in FC I through FC IV, and RCTs that reported treatment effects by FC. Terms for the PubMed search for observational registries were “pulmonary arterial hypertension” AND “registry”. search term used to identify RCTs in PubMed was “pulmonary arterial hypertension”, with the search restricted to phase 3 trials.
The Embase search strategy for registries used the terms “disease registry/exp” OR “disease registry” AND “pulmonary hypertension/exp” OR “pulmonary hypertension”. The search strategy for RCTs in Embase used RCT as a filter and required that the phrase “pulmonary hypertension” appeared in the title of the article and the word “placebo” was included in the title, abstract, or keywords. The broader term of “pulmonary hypertension” was used in the Embase searches because “pulmonary arterial hypertension” was not a search term in Embase. The results of the systematic PubMed and Embase searches were supplemented by data that were available in prescribing information and the annual reports of registries.
Inclusion and exclusion criteria
Studies eligible for inclusion in the meta-analysis of observational registries were restricted to those that enrolled primarily adult (≥18 years) patients with World Health Organization (WHO) Group 1 pulmonary hypertension (ie, PAH),27 although registries that were devoted to specific PAH etiologies (e.g. PAH associated with connective tissue disease (CTD)) were permitted. We included only registries that reported overall survival by WHO or New York Health Association FC and that had survival data for at least one year of follow-up. Registries that mandated medical therapy and those that enrolled fewer than 30 patients were excluded.
Studies included in the meta-analysis of RCTs were limited to those that were randomized, double-blind, placebo-controlled, phase 3 studies that (a) enrolled primarily adults (≥18 years) with PAH, (b) reported data by FC, (c) had an active intervention arm with an average duration of exposure ≥6 months, (d) had a placebo or other intervention comparison group, and (e) reported morbidity, mortality, or time to clinical worsening by WHO or New York Health Association FC as primary or secondary study end points.
Studies that enrolled mostly adolescents or children were excluded to eliminate variability in survival outcomes or treatment response that might be attributable to age. Only peer-reviewed studies were included. Conference abstracts and publications that did not contain the end points of interest were excluded. The remaining inclusion criteria were intentionally kept as broad as possible to reduce potential bias.
Study selection and data extraction
The full citation and source database were recorded for all studies that were identified through the searches of the PubMed and Embase databases and other sources. Two reviewers independently screened each title and abstract to determine if the study was eligible for inclusion in the registry meta-analysis. Full-text reviews were performed if information contained in the abstract and title was not sufficient to determine if the publication met the inclusion criteria. Cases of disagreement were resolved by discussion between the two reviewers, with direction provided by the senior author, as needed. The reason for exclusion was recorded for all studies that were considered ineligible for inclusion. The RCTS were known—there have been only five long-term (i.e. outcomes measured at ≥6 months) event-driven RCTs of PAH medications since 2013—and these were confirmed by the authors.
Data from each eligible study were extracted from the publication into Excel files and verified by an independent reviewer. Data discrepancies were resolved by discussion and in consultation with the senior author and the statistician, when necessary. Patient age, sex, PAH etiology, FC, six-minute walk distance (6MWD), and survival information, as well as first author, study dates, and publication year, were extracted for all registry studies. Data recorded for each RCT included the name of the first author, year of publication, study design, PAH therapy, control intervention, number of patients overall and per treatment group, and baseline patient demographic and clinical characteristics (age, sex, PAH etiology, FC, and 6MWD). Time to the occurrence of clinical events (e.g. death, hospitalization, clinical worsening, or disease progression), including the hazard ratio (HR) and associated confidence interval (CI), was recorded for each RCT. The HR measures the reduction in risk of occurrence of such an event between the treatment arm and the control arm. The definition of clinical event could vary between the RCTs. Secondary end points, such as 6MWD, FC, N-terminal pro hormone B-type natriuretic peptide levels, B-type natriuretic peptide levels, pulmonary vascular resistance, cardiac index, pulmonary artery pressure, and right atrial pressure were recorded for observational registries and RCTs if they were reported.
Quality assessment and publication bias
Patient characteristics were reviewed across studies, and sensitivity analyses removing studies with potential differences in baseline conditions were performed. For RCTs, selections were limited to randomized phase 3 studies to avoid bias. For registries, a sample size >30 was required to avoid bias introduced by small sample sizes.
Statistical analysis
Overall survival rates with 95% CIs were calculated for patients in FC I or II and for those in FC III or IV at study enrollment at years 1, 2, and 3 in each observational registry. The overall observed event rates and CIs were calculated with the Mantel–Haenszel method. Study-specific HRs with 95% CIs were calculated to evaluate the treatment effect for patients in FC I or II and for those in FC III or IV at study enrollment in each RCT. Statistical heterogeneity in survival rates and treatment effects was examined with the I2 statistic,28 with values >50%, indicating substantial heterogeneity.29
Separate meta-analysis models were calculated for the observational registry studies and for the RCTs. The I2 statistic of the fixed effects model for the registry studies indicated substantial heterogeneity (value >50%) among the studies (Supplemental Table 1). Therefore, a random effects meta-analysis model using the inverse variance method of DerSimonian and Laird30 which reduces the impact of heterogeneity was used to combine estimates of survival from the observational registry studies stratified by FC I or II and by FC III or IV. While the I2 statistic of the fixed effects model for the RCTs was not >50%, it was sufficiently close to 50% (Supplemental Table 2) that we elected to use a random effects model to summarize the pooled treatment effect from the RCTs, stratified by FC I or II and by FC III or IV. All statistical analyses were performed with Comprehensive Meta-Analysis Version 3 Software (Biostat, Englewood, NJ).
Sensitivity analyses
Studies with potential outliers in baseline demographic or clinical characteristics and outcome variables were identified. The meta-analyses for the observational registries and RCTs were performed with and without these studies to determine their impact on the overall estimates of survival and treatment effect.
An additional sensitivity analysis of newly diagnosed patients pooling data from all newly diagnosed patients for which data were reported by FC was performed using a random effects model.
Results
Search results for observational registries
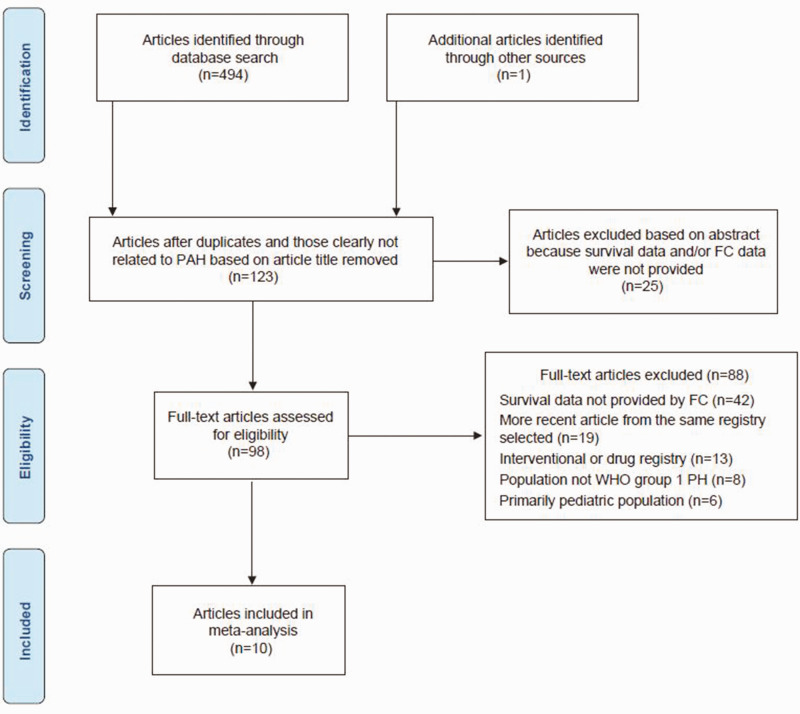
A total of 495 studies were identified from the PubMed, Embase, and annual reports of registry searches. Of these, 397 did not meet the inclusion criteria for observational registries based on the abstract and title review (Fig. 1). A total of 98 full-text articles were reviewed. Following review of the full-text publications, 10 studies were included in the registry meta-analysis.4,31–40
Fig. 1.
Flowchart of studies for the meta-analysis of observational registries. FC, functional class; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; WHO, World Health Organization.
Characteristics of observational registries
A total of 6580 patients were enrolled in the 10 registry studies, with enrollment periods ranging from 1995–200434 to 2013–201439 (Table 1). Overall, the mean patient age was 50.4 years, ranging from 3631,35 to 61 years.36 One registry study included patients ≥14 years with an overall mean age of 36 years.35 The majority of registry patients (70%) were female, ranging from 51% in the United Kingdom National Pulmonary Arterial Hypertension Registry32 to 82% in the Connective Tissue Disease-Pulmonary Arterial Hypertension registry that was also established in the United Kingdom.36 Across the 10 registries, 46% of patients were diagnosed with idiopathic PAH or familial PAH and 20% had PAH associated with CTD or other etiologies.
Table 1.
Baseline patient demographic and disease characteristics from the observational registries.
| Characteristics |
|||||||
|---|---|---|---|---|---|---|---|
| Study | Enrollment period | Number of patients | Age (years), mean (SD) | Female (%) | IPAH + FPAH/CTD/ other (%) | FC I or II (%) | 6MWD (m), mean |
| Condliffe et al.36 | 1/2001–6/2006 | 343 | 61 (12) | 82 | 0/100/0 | 14 | 231 |
| Farber et al.4 | 3/2006–12/2009 | 2749 | 52 (15) | 79 | 50/26/24 | 42 | 359 |
| HSCIC39 | 8/2013–3/2014 | 1116 | 58 (20) | 66 | 100/0/0 | 9 | NR |
| Humbert et al.33,40 | 10/2002–10/2003 | 674 | 50 (15) | 65 | 43/15/42 | 25 | 329 |
| Idrees et al.35 | 12/2009–11/2012 | 107 | 36 (8) | 63 | 55/15/30 | 27 | 298 |
| Jing et al.31 | 1/1999–10/2004 | 72 | 36 (12) | 71 | 100/0/0 | 39 | NR |
| Kane et al.34 | 1/1995–12/2004 | 484 | 52 (15) | 75 | 56/24/20 | 29 | 329 |
| Korsholm et al.37 | 1/2000–3/2012 | 134 | 50 (21) | 58 | 33/23/44 | 26 | 328 |
| McLaughlin et al.38 | 8/2005–7/2007 | 791 | 55 (16) | 77 | 38/29/33 | 47 | NR |
| Sithamparanathan et al.32 | 1/2001–12/2010 | 110 | 53 (12) | 51 | 0/0/100 | 21 | NR |
| Overall | – | 6580 | 50.4 | 70 | 46/20/34 | 26 | 314.9 |
6MWD: six-minute walk distance; CTD: connective tissue disease; FC: functional class; FPAH: familial pulmonary arterial hypertension; HSCIC: Health and Social Care Information Center; IPAH: idiopathic pulmonary arterial hypertension; SD: standard deviation.
Meta-analysis of observational registries
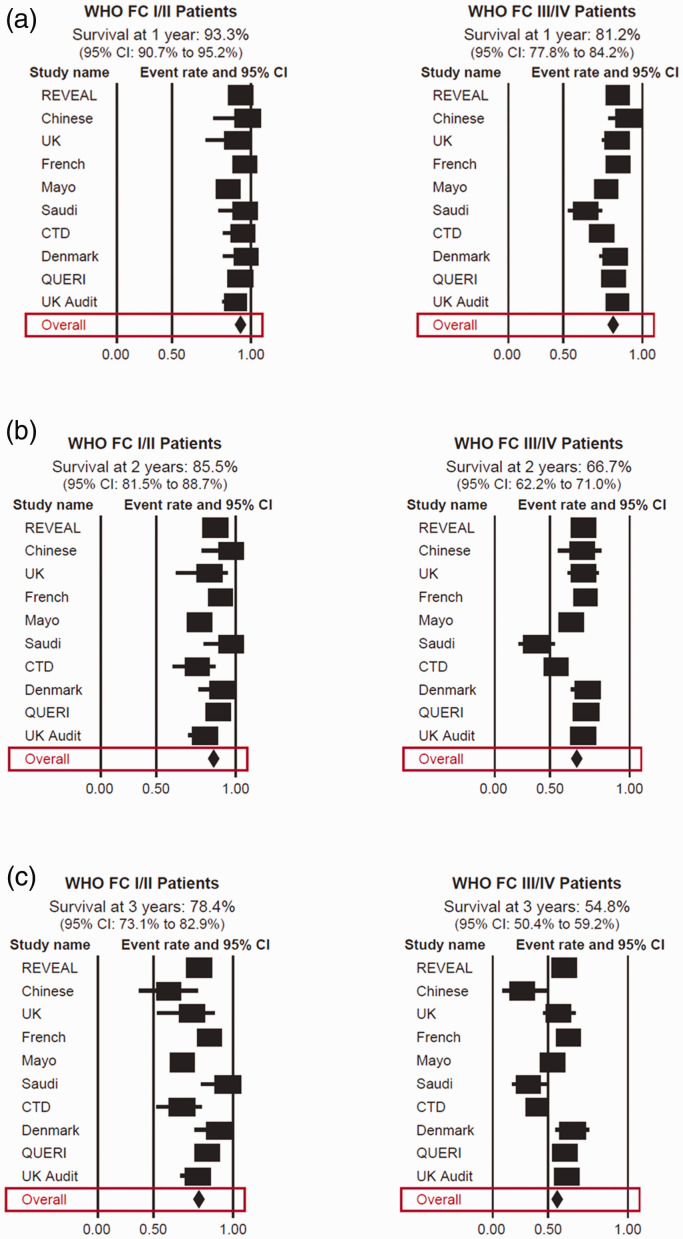
The pooled analysis by FC showed a survival rate at year 1 of 93.3% (95% CI: 90.7–95.2) for WHO FC I or II patients compared with 81.2% (95% CI: 77.8–84.2) for FC III or IV patients (Fig. 2a and Table 2). Survival rates at year 2 declined for both patient groups to 85.5% (95% CI: 81.5–88.7) and 66.7% (95% CI: 62.2–71.0) for those in FC I or II and FC III or IV, respectively (Fig. 2b and Table 2). The pooled survival rate at year 3 for FC I or II patients was 78.4% (95% CI: 73.1–82.9) compared with 54.8% (95% CI: 50.4–59.2) for those in FC III or IV (Fig. 2c and Table 2).
Fig. 2.
Forest plots of survival at one year (a), two years (b), and three years (c) by WHO FC. CI, confidence interval; CTD, connective tissue disease; FC, functional class; QuERI, Quality Enhancement Research Initiative; REVEAL, Registry to Evaluate Early and Long-term PAH Disease Management; SD, standard deviation; UK, United Kingdom; WHO, World Health Organization.
Table 2.
Overall survival in observational registries.
| FC I/II | FC III/IV | |
|---|---|---|
| Survival at one year (95% CI) | 93.3% (90.7%–95.2%) | 81.2% (77.8%–84.2%) |
| Survival at two years (95% CI) | 85.5% (81.5%–88.7%) | 66.7% (62.2%–71.0%) |
| Survival at three years (95% CI) | 78.4% (73.1%–82.9%) | 54.8% (50.4%–59.2%) |
CI: confidence interval; FC: functional class.
Search results for RCTs
Of the five long-term, event-driven phase 3 RCTs of PAH medications published since 2013, four met the inclusion criteria for the RCT meta-analysis.41–44 The fifth trial, Endothelin Antagonist Trial in Mildly Symptomatic PAH Patients (EARLY)45 limited enrollment to only patients in FC II and, therefore, was excluded from the meta-analysis.
Characteristics of RCTs
The patient populations and definitions of clinical events are summarized in Table 3, with all studies reporting death as well as disease progression as primary end points. Other clinical end points included the need for a lung transplant or balloon atrial septostomy, changes in medical therapy, hospitalization for worsening PAH, decreased 6MWD, and worsening FC.
Table 3.
Definitions of clinical events and patient populations and for the randomized controlled trials.
| Study | |||
|---|---|---|---|
| GRIPHON41 | SERAPHIN42 | AMBITION43 | COMPASS-244 |
| Clinical event definitions for morbidity and mortality | |||
| • Death • Hospitalization for worsening PAH • Need for lung transplant or balloon atrial septostomy • Initiation of parenteral prostanoid therapy • Initiation of long-term oxygen therapy • Disease progression indicated by: o Decreased 6MWD and worsening of WHO FC o Need for additional PAH medication | • Death • Need for lung transplant or balloon atrial septostomy • Initiation of intravenous prostanoid therapy • Disease progression indicated by decreased 6MWD and worsening of symptoms (based on change in WHO FC or right heart failure) AND need for additional PAH medication | • Death • Hospitalization for worsening PAH • Disease progression indicated by decreased 6MWD and worsening WHO FC • Unsatisfactory long-term clinical response (based on worsening 6MWD and WHO FC after ≥6 months) | • Death • Hospitalization for worsening PAH • Need for lung transplant or balloon atrial septostomy • Initiation of intravenous prostanoid therapy • Disease progression indicated by: o Increased symptoms on a patient-reported instrument AND need for additional PAH medication o Decreased 6MWD AND need for additional PAH medication |
| Patient population | |||
| • Background therapy with ERA and/or PDE5i medications permitted | • Background therapy with non-ERA medications permitted | • Treatment-naive | • On a stable dose of sildenafil and no other PAH medication in the prior three months |
Italics indicate difference in definition of clinical event compared to the other studies. 6MWD: six-minute walk distance; AMBITION: Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension; COMPASS-2: Effects of the Combination of Bosentan and Sildenafil Versus Sildenafil Monotherapy on Pulmonary Arterial Hypertension; ERA: endothelin receptor antagonist; FC: functional class; GRIPHON: Prostacyclin (PGI2) Receptor Agonist In Pulmonary Arterial Hypertension; PAH: pulmonary arterial hypertension; PDE5i: phosphodiesterase type 5 inhibitor; SERAPHIN: Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve.
The demographic and clinical characteristics of patients enrolled in the four RCTs are presented in Table 4. The studies were published between 2013 and 2017 with a total enrollment of 2482 patients, including 1228 who received study medication and 1254 who were randomized to comparator arms. Overall, the majority of patients were female. The mean age of patients was 50.2 and 51.0 years in the intervention and comparison groups, respectively. PAH etiology was balanced between the intervention and comparator arms. In the intervention and comparator arms, 55% and 57%, respectively, had idiopathic PAH and 32% and 31% had CTD-associated PAH. Patients in FC I or II comprised 43% and 42% of the intervention and control groups, respectively, although one RCT43 included 30% FC I or II patients and 70% FC III or IV. Mean 6MWD was similar between the intervention and comparator groups.
Table 4.
Baseline demographic and disease characteristics for the randomized controlled trials.
| Intervention |
Comparison |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Number of patients | Age (years), mean (SD) | Female (%) | IPAH/CTD/ other (%) | FC I or II/III or IV (%) | 6MWD (m), mean | Number of patients | Age (years), mean (SD) | Female (%) | IPAH/CTD/ other (%) | FC I or II/III or IV (%) | 6MWD (m), mean |
| AMBITION43 | 253 | 55 (14) | 74 | 50/41/9 | 30/70 | 354 | 247 | 54 (15) | 81 | 56/34/10 | 32/68 | 352 |
| COMPASS-244 | 159 | 53 (15) | 79 | 62/27/11 | 45/55 | 363 | 175 | 55 (16) | 73 | 65/26/9 | 39/61 | 358 |
| GRIPHON41 | 574 | 48 (15) | 80 | 54/29/17 | 48/52 | 359 | 582 | 48 (16) | 80 | 58/29/13 | 45/55 | 348 |
| SERAPHIN42 | 242 | 46 (15) | 80 | 56/30/14 | 50/50 | 363 | 250 | 47 (17) | 74 | 51/33/16 | 52/48 | 352 |
| Overall | 1,228 | 50.2 | 78 | 55/32/13 | 43/56 | 359 | 1254 | 51.0 | 77 | 57/31/12 | 42/58 | 351 |
6MWD: six-minute walk distance; AMBITION: Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension; COMPASS-2: Effects of the Combination of Bosentan and Sildenafil Versus Sildenafil Monotherapy on Pulmonary Arterial Hypertension; CTD: connective tissue disease; FC: functional class; GRIPHON: Prostacyclin (PGI2) Receptor Agonist In Pulmonary Arterial Hypertension; IPAH: idiopathic pulmonary arterial hypertension; SD: standard deviation; SERAPHIN: Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome.
Meta-analysis of RCTs
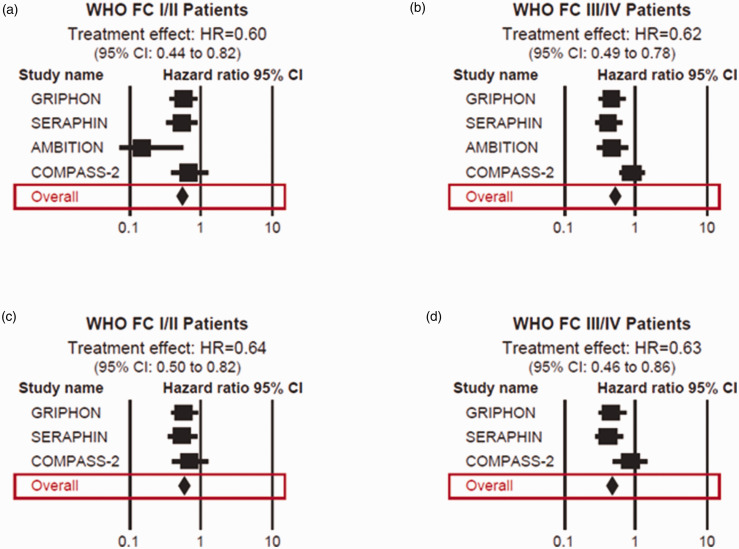
The pooled treatment effect for the entire population, regardless of FC, was HR = 0.61 (95% CI: 0.51–0.74), indicating a 39% reduction in the risk of having a morbidity or mortality event in patients being treated with the specific PAH therapy. The overall observed morbidity/mortality clinical event rate in patients with FC I or FC II disease in the treatment arms was 18.9% (95% CI: 11.5%–29.4%) compared to 30.3% (95% CI: 24.4%–37.0%) in the control arms. The overall observed morbidity/mortality clinical event rate in patients with FC III or FC IV disease in the treatment arms was 36.6% (95% CI: 27.3%–47.1%) compared to 52.5% (95% CI: 41.2%–63.5%) in the control arms. The pooled treatment effect for FC I or II patients was HR = 0.60 (95% CI: 0.44–0.82) (Fig. 3a) and, for FC III or IV patients, it was HR = 0.62 (95% CI: 0.49–0.78) (Fig. 3b), indicating a 40% and a 38% reduction in risk, respectively.
Fig. 3.
Forest plots of treatment effect in PAH RCTs in FC I/II patients (a) and in FC III/IV patients (b). Treatment effect in PAH RCTs excluding AMBITION in FC I/II patients (c) and in FC III/IV patients (d). AMBITION, Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension; CI, confidence interval; FC, functional class; GRIPHON, Prostacyclin (PGI2) Receptor Agonist In Pulmonary Arterial Hypertension; HR, hazard ratio; REVEAL, Registry to Evaluate Early and Long-term PAH Disease Management; SERAPHIN, Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension to Improve Clinical Outcome.
Sensitivity analyses
Review of the clinical characteristics of patients enrolled in the four RCTs revealed that the Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension (AMBITION) trial enrolled substantially fewer patients in FC I (0%) or II (31%)43 than the other three RCTs in which nearly 50% of the patients were FC I or II.41,42,44 In addition, treatment with any PAH medication was an exclusion criterion in AMBITION,43 while in the other three RCTs, 64%–100% of patients were being treated with other PAH therapies at enrollment and throughout the studies.41,42,44 Therefore, a separate meta-analysis was performed in which the AMBITION trial was excluded. The results were similar to the overall analysis. The HR was 0.64 for patients in WHO FC I or II (95% CI: 0.50–0.82) (Fig. 3c) and 0.63 (95% CI: 0.46–0.86) for those in FC III or IV (Fig. 3d).
Discussion
It has generally been assumed that the risk of disease progression or death is low in patients with FC I or II PAH while those in FC III or IV are considered to be at increased risk for poor outcomes. However, reliance on FC as the sole prognostic factor has been challenged with current treatment guidelines recommending multiparameter risk assessments, with the goal of treatment being to attain and/or maintain a low-risk status.22–24 Our results support this recommendation by showing that patients in FC I or II can have a substantial mortality risk.
We performed two meta-analyses resulting in complementary findings. The meta-analysis of observational registry studies was designed to examine survival rates by WHO FC. The meta-analysis of RCTs was designed to quantify the effect of PAH therapy on morbidity and mortality by WHO FC. The observational registry meta-analysis revealed that, despite a lower risk of death compared with patients in FC III or IV, those with FC I or II PAH were not “low risk”—as defined by PAH treatment guidelines22,23—with a 7% one-year morality rate. This mortality risk increased substantially to 22% at three years. Results from the meta-analysis of RCTs indicated that PAH medications reduced the risk of morbidity and mortality by 35%–40% in patients with FC I or II PAH, which was comparable to the effect of medical therapy for patients in FC III or IV. Together, these two analyses establish that some patients with FC I or II PAH are at a higher risk of death or worsening of PAH and medical therapy for PAH may offer considerable clinical benefit in such patients.
In selecting the registry studies, we aimed to gather a widely representative sample. Indeed, the patient populations varied by geographic region, PAH etiology, date of enrollment, and potentially other factors that were not captured. Heterogeneity analysis indicated substantial heterogeneity among the studies; however, the impact of this was reduced by using a random effects model. The CIs of the HRs for death were not large, and we believe this analysis provides a reasonable estimate of mortality over time in this patient population. The three-year mortality of 22% in FC I/II patients determined by our meta-analysis is higher than the rate of 6% (FC I) to 1% (FC II) in previously diagnosed FC I/II patients and similar to the rate of 22% (FC II) to 28% (FC I) in newly diagnosed FC I/II patients reported in the REVEAL registry,4 which enrolled patients in a similar time window as the current study. Our analysis included studies which enrolled newly and previously diagnosed patients; however, only the REVEAL study reported survival data by FC in newly and in previously diagnosed patients, precluding an analysis of the effect of diagnostic status by FC. We performed an exploratory sensitivity analysis to estimate survival in the newly diagnosed patients in our sample (Supplementary Table 3). Mortality rates for FC I/II patients were 29% at three years, a rate comparable to the rate observed in newly diagnosed patients in REVEAL. Greater survival rates in previously diagnosed patients may be attributed, at least in part, to survivor bias.
Differences in survival estimates overall between studies may also be affected by geographic differences in standard of care and availability of therapies. Pooling data using meta-analysis are particularly useful to better understand rare diseases like PAH because it substantially increases the sample size thereby increasing the reliability of the results. Even though it is a pooled estimate, the analysis of registries is limited in that all registries completed enrollment prior to 2015 when the first study using upfront combination therapy was published (AMBITION);43 thus, improved outcomes resulting from more intensive therapeutic regimens are not reflected in the survival estimates reported here. However, given the perception that patients with FC I/II disease are “low risk”, it is not clear that these patients would be treated with more intensive treatment regimens if they were available. An additional limitation of the registry analysis is the lack of data for patients in FC I. In the 10 registries, 31% of patients were FC I/II. In the five registries reporting baseline disease characteristics separately for FC I patients, only 6% were FC I. Thus, these survival estimates are more broadly applicable to patients in FC II.
Our analysis of RCTs was limited in that the definition of a clinical event was not the same in each study (Table 3); however, there was a high degree of similarity across studies. There was also a risk of publication bias in the analysis, because studies with positive results are more likely to be published. Finally, the pooled data were not adjusted or stratified by patient factors other than FC, such as age, sex, comorbid health conditions, whether patients were newly or previously diagnosed, or background PAH therapy. It is likely that these demographic and clinical characteristics also play a role in patient response to PAH treatment. This analysis does not provide information on which therapeutic regimens are most effective, rather it suggests that all regimens reported in the RCTs substantially reduced the risk of a morbidity or mortality event in patients with FC I/II symptoms. Analyzing the relationship between specific regimens and survival in the registry studies is beyond the scope of this analysis.
In summary, this report describes the first comprehensive analysis of the long-term clinical outcomes of PAH patients in FC I or II assessing both observational real-world registries and RCTs. Our meta-analysis of survival stratified by FC establishes that the risk of death for patients with FC I or II PAH is considerable. The observed considerable mortality rate in FC I or II patients and a favorable response to medical therapies support the current treatment guidelines that even FC I or II patients require close surveillance and optimization of PAH therapy.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020935291 for Long-term outcomes in pulmonary arterial hypertension by functional class: a meta-analysis of randomized controlled trials and observational registries by Nick H. Kim, Micah Fisher, David Poch, Carol Zhao, Mehul Shah and Sonja Bartolome in Pulmonary Circulation
Acknowledgments
The authors would like to thank Dr Saling Huang for consulting on the statistical analysis. Medical writing support was provided by Holly Strausbaugh, PhD, of Twist Medical, LLC, and funded by Janssen Pharmaceuticals, Inc.
Footnotes
Contributorship: NHK developed the first draft of the manuscript with assistance from a professional medical writer. CZ performed the data analysis. All authors contributed to data interpretation, manuscript writing and critical analysis of the manuscript and provided final approval for manuscript submission.
Conflict of interest: Nick H. Kim has served as a consultant for Actelion Pharmaceuticals, Arena Pharmaceuticals, Bayer, and Merck and has been a member of a speaker’s bureau for Actelion Pharmaceuticals and Bayer. His institution has received research funding from Bellerophon Therapeutics, Eiger Biopharmaceuticals, Lung Biotechnology, and SoniVie. Micah Fisher reports no disclosures. David Poch has served as a consultant and has been a member of a speaker’s bureau for Bayer. Carol Zhao and Mehul Shah are employees of and own stock in Janssen Pharmaceuticals, Inc. Sonja Bartolome has served as a consultant for Actelion Pharmaceuticals, Arena Pharmaceuticals, and Bayer. Her institution has received research funding from Arena Pharmaceuticals, Bellerophon Therapeutics, Eiger Biopharmaceuticals, Reata, and United Therapeutics.
Funding: Funding for this analysis was provided by Janssen Pharmaceuticals, Inc.
Guarantor: NHK
Supplemental material: Supplemental material for this article is available online.
References
- 1.Hill NS, Cawley MJ, Heggen-Peay CL. New therapeutic paradigms and guidelines in the management of pulmonary arterial hypertension. J Manag Care Spec Pharm 2016; 22(3 Suppl A): S3–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeper MM, Bogaard H, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D42–D50. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation 2006; 114: 1417–1431. [DOI] [PubMed] [Google Scholar]
- 4.Farber HW, Miller DP, Poms AD, et al. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest 2015; 148: 1043–1054. [DOI] [PubMed] [Google Scholar]
- 5.Nickel N, Golpon H, Greer M, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2012; 39: 589–596. [DOI] [PubMed] [Google Scholar]
- 6.Badiani B, Messori A. Targeted treatments for pulmonary arterial hypertension: interpreting outcomes by network meta-analysis. Heart Lung Circ 2016; 25: 46–52. [DOI] [PubMed] [Google Scholar]
- 7.Correale M, Ferraretti A, Monaco I, et al. Endothelin-receptor antagonists in the management of pulmonary arterial hypertension: where do we stand?. Vasc Health Risk Manag 2018; 14: 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galiè N, Manes A, Negro L, et al. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J 2009; 30: 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J 2008; 31: 891–901. [DOI] [PubMed] [Google Scholar]
- 10.Jain S, Khera R, Girotra S, et al. Comparative effectiveness of pharmacologic interventions for pulmonary arterial hypertension: a systematic review and network meta-analysis. Chest 2017; 151: 90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Callaghan DS, Savale L, Montani D, et al. Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol 2011; 8: 526–538. [DOI] [PubMed] [Google Scholar]
- 12.Wilkins MR, Wharton J, Grimminger F, et al. Phosphodiesterase inhibitors for the treatment of pulmonary hypertension. Eur Respir J 2008; 32: 198–209. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Li X, Huang J, et al. Comparative efficacy and safety of prostacyclin analogs for pulmonary arterial hypertension: a network meta-analysis. Medicine (Baltimore) 2016; 95: e2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin V. Managing pulmonary arterial hypertension and optimizing treatment options: prognosis of pulmonary artery hypertension. Am J Cardiol 2013; 111: 10C–5C. [DOI] [PubMed] [Google Scholar]
- 15.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. [DOI] [PubMed] [Google Scholar]
- 16.Packer M, McMurray JJV, Krum H, et al. Long-term effect of endothelin receptor antagonism with bosentan on the morbidity and mortality of patients with severe chronic heart failure: primary results of the ENABLE trials. JACC Heart Fail 2017; 5: 317–326. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Li B, Wang Y, et al. Experience of the management of coronary artery bypass graft only on moderate ischemic mitral regurgitation: a single-center retrospective study. Medicine (Baltimore) 2019; 98: e14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ombelet F, Goossens E, Apers S, et al. Predicting 15-year mortality in adults with congenital heart disease using disease severity and functional indices. Can J Cardiol 2019; 35: 907–913. [DOI] [PubMed] [Google Scholar]
- 19.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 20.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 21.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 34–39. [DOI] [PubMed] [Google Scholar]
- 22.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 23.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest 2019; 155: 565–586. [DOI] [PubMed] [Google Scholar]
- 25.Bartolome S, Fisher M, Poch D, et al. Meta-analysis of long-term outcomes in pulmonary arterial hypertension by disease severity. PROSPERO 2018 CRD42018092820, http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018092820 (2018, accessed 12 December 2019).
- 26.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 27.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schünemann H, Bro ż ek J, Guyatt G, et al. GRADE handbook. Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group Web site, https://gdt.gradepro.org/app/handbook/handbook.html. Updated October 2013 (accessed 4 May 2020).
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 31.Jing ZC, Xu XQ, Han ZY, et al. Registry and survival study in Chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest 2007; 132: 373–379. [DOI] [PubMed] [Google Scholar]
- 32.Sithamparanathan S, Nair A, Thirugnanasothy L, et al. Survival in portopulmonary hypertension: outcomes of the United Kingdom National Pulmonary Arterial Hypertension Registry. J Heart Lung Transplant 2017; 36: 770–779. [DOI] [PubMed] [Google Scholar]
- 33.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 34.Kane GC, Maradit-Kremers H, Slusser JP, et al. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest 2011; 139: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 35.Idrees M, Alnajashi K, Abdulhameed J, et al. Saudi experience in the management of pulmonary arterial hypertension; the outcome of PAH therapy with the exclusion of chronic parenteral prostacyclin. Ann Thorac Med 2015; 10: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Condliffe R, Kiely DG, Peacock AJ, et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am J Respir Crit Care Med 2009; 179: 151–157. [DOI] [PubMed] [Google Scholar]
- 37.Korsholm K, Andersen A, Kirkfeldt RE, et al. Survival in an incident cohort of patients with pulmonary arterial hypertension in Denmark. Pulm Circ 2015; 5: 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaughlin VV, Langer A, Tan M, et al. Contemporary trends in the diagnosis and management of pulmonary arterial hypertension: an initiative to close the care gap. Chest 2013; 143: 324–332. [DOI] [PubMed] [Google Scholar]
- 39.Health and Social Care Information Center. Fifth Annual Report: key findings from the National Audit of Pulmonary Hypertension for the United Kingdom, Channel Islands, Gibraltar and Isle of Man. Report for the audit period April 2013 to March 2014, https://files.digital.nhs.uk/publicationimport/pub17xxx/pub17264/nati-pulm-hype-audi-2014-rep.pdf (2015, accessed 12 December 2019).
- 40.Humbert M, Sitbon O, Yaïci A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010; 36: 549–555. [DOI] [PubMed] [Google Scholar]
- 41.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 42.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 43.Galiè N, Barberà JA, Frost AE, et al. Initial use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin V, Channick RN, Ghofrani HA, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 2015; 46: 405–413. [DOI] [PubMed] [Google Scholar]
- 45.Galiè N, Rubin LJ, Hoeper M, et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 2008; 371: 2093–2100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020935291 for Long-term outcomes in pulmonary arterial hypertension by functional class: a meta-analysis of randomized controlled trials and observational registries by Nick H. Kim, Micah Fisher, David Poch, Carol Zhao, Mehul Shah and Sonja Bartolome in Pulmonary Circulation