Abstract
Pulmonary hypertension (PH) related to old anterior myocardial infarction (OAMI) always accompanies a bad prognosis, and thus, we aimed to screen serum biomarkers related to PH in OAMI patients. According to right ventricular systolic pressure, we divided mice into sham, OAMI, and PH-OAMI groups and evaluated body, heart and lung weight, heart function, pulmonary blood flow velocity, cardiac fibrotic area, and pulmonary arteriole condition. Lung and serum were under the proteomic analysis. Levels of three identified proteins were measured. Compared with sham and OAMI mice, PH-OAMI mice showed heart dysfunction, low pulmonary blood flow, high right ventricular systolic pressure, heavy heart and lung weight, large cardiac fibrotic area, and pathological pulmonary arteriole remodeling (P<0.05 or P<0.01). Haptoglobin, annexin A5, and Ig mu chain C region of lung and serum were changed significantly in PH-OAMI mice (P<0.01). Then, we collected serum and clinical data, measured three serum protein levels, and performed multivariate regression and receiver operating characteristic curve in patients (normal, OAMI, and PH-OAMI groups). Compared with normal and OAMI patients, serum levels of three proteins in PH-OAMI patients were also altered notably (P<0.01). These three proteins can predict PH in OAMI patients (P<0.01). Receiver operating characteristic curve analysis revealed haptoglobin (cut-off value: 78.295, sensitivity: 62.8%, specificity: 94.4%), annexin A5 (cut-off value: 151.925, sensitivity: 41.9%, specificity: 82.4%), and Ig mu chain C region (cut-off value: 168.885, sensitivity: 86.0%, specificity: 79.6%) (P<0.01). Three circulating serum proteins can be useful for the categorization of OAMI patients with and without PH.
Keywords: proteomic analysis, pulmonary hypertension, old anterior myocardial infarction, mice, patients.
Pulmonary hypertension (PH) is one of the most common and serious diseases endangering health in the clinic.1 The progressive increase of pulmonary artery pressure in most patients eventually leads to right heart failure and death.2 The prevalence of patients with PH is about 100 million worldwide.3 At present, PH can be divided into five categories as follows: (1) pulmonary arterial hypertension (PAH); (2) PH secondary to left heart disease (PH-LHD); (3) PH associated with pulmonary disease or hypoxia; (4) chronic thromboembolic PH; (5) PH caused by comprehensive factors.3 PH-LHD is the most common cause of PH, mainly driven by increased left ventricular (LV) or left atrial filling pressure. It belongs to the postcapillary PH.4,5 PH refers to the mean pulmonary artery pressure ≥25 mmHg measured by right cardiac catheterization at rest. Still, the definition of cardiac echography usually refers to the pulmonary arterial systolic pressure (PASP) between 40 and 60 mmHg.5 According to the European Society of Cardiology/European Respiratory Society guidelines in 2015, PASP > 40 mmHg is used to define the PH related to LV systolic dysfunction.5
The previous rheumatic mitral disease is the most common cause of PH-LHD. Epidemiological data show that with the increase of age, heart failure incidence is caused by ischemic cardiomyopathy (ICM) rising worldwide.4–6 Therefore, the more common cause of that is ICM now. ICM or ischemic heart failure refers to hibernating myocardium, diffuse myocardial fibrosis, or multifocal myocardial infarction (MI), which, alone or in combination, results in increased ventricular wall tension, decreased compliance and cardiac cavity expansion, and then a group of clinical syndromes characterized by heart failure.6,7 So far, there is no good therapeutic approach for PH-ICM, mainly including relieving symptoms in heart failure, improving myocardial ischemia, and attenuating pathological ventricular remodeling.5 Furthermore, the prognosis of patients with heart dysfunction who have had PH is poorer, and the mortality rate is more than twice that of the patients without PH.5 Therefore, it is necessary to find better serum biomarkers to predict PH and guide therapy for improving the prognosis in patients with ICM.
Proteomics uses two-dimensional electrophoresis, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, surface-enhanced laser desorption/ionization time-of-flight mass spectrometry, isobaric tags for relative and absolute quantification (iTRAQ), and other proteomic techniques to study the protein composition and their change laws in the cell, tissue, or organism, including the level of protein synthesis, posttranslation modification, protein and protein interaction, etc.8,9 In this way, we can get a comprehensive understanding of the physiological and pathological processes of organisms at the protein level. Old anterior myocardial infarction (OAMI) is an integral part of ICM. However, no research data reported serum biomarkers analysis at the protein level in patients with OAMI to predict PH.
iTRAQ technology has the following advantages: (1) It can detect proteins with low abundance; (2) it has a simple technical route and does not need extra complicated operations; (3) it is a high-throughput research method for the discovery of biomarkers; and (4) it has high qualitative and quantitative accuracy.10,11 Thus, in the present study, we first used iTRAQ technology to analyze proteomic changes of blood serum and lung tissue and screened serum protein biomarkers related to PH in mice with OAMI. The retrospective study was designed to verify these serum biomarkers’ ability to predict PH in patients with OAMI to diagnose and treat this disease better.
Methods
Study animals
Thirty-one two-month-old male mice from C57BL/6 background were housed in groups with 12 h dark–light cycles and free access to food and water. These conditions were under the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH publication no. 85-23, revised in 1996) and in accordance with the regulations on mouse welfare and ethics of our University. The animal protocol was reviewed and approved by the Ethics Committee of our University. The cervical dislocation was a method to provide the mouse with fast and painless death.
MI surgery and sample collection
MI was performed following a method reported previously in mice with slight modifications.12,13 Briefly, mice were anesthetized intraperitoneally with pentobarbital sodium (30–50 mg/kg). A 20-gauge polyethylene catheter was intubated into the trachea. A small animal ventilator (ALC-V8S, Shanghai Orcote Biotech Co., Ltd.) provided positive pressure ventilation at 2–3 mL/cycle and a respiratory rate of 130 cycles/min. After the thoracic cavity at the fourth rib and along the left sternal border was opened, the left anterior descending coronary artery (LADCA) was ligated with a 7-0 silk suture 3 mm from the tip of the left auricle. The chest wall was closed with a continuous 6-0 prolene suture, followed by a 4-0 polyester suture to close the skin. The sham operation procedure was the same as the process mentioned above of MI induction except for LADCA ligation.
After LADCA ligation in mice, ST-segment elevation in the chest leads was the sign of a successful MI surgery checked by small animal electrocardiograph (RM6240E, Shanghai Softmaze Information Technology Co., Ltd.). After surgery in our study, the mortality of these mice was 6% due to cardiac rupture. Four months after MI in mice, samples of blood serum, heart, lung, liver, spleen, and kidney were taken out for testing. Simultaneously, body weight (BW), heart weight (HW), right heart ventricular weight, lung weight (LW), and tibia length (TL) were determined.
Masson’s staining and hematoxylin-eosin staining
We performed protocols for Masson’s staining and hematoxylin-eosin (HE) staining, as reported previously.12,13 Briefly, heart and lung samples were first washed with ice-cold phosphate-buffered saline (PBS) and then fixed in 4% paraformaldehyde at 4°C. The samples were processed successively by (1) a 30-min washing in PBS at 4°C; (2) 15 min each in 30%, 50%, 75%, and 85% ethanol, and then 2 × 10 min of incubation in 95% and 100% ethanol at room temperature (RT); (3) 3 × 10 min of incubation in xylene at RT; (4) 20 min of incubation in paraffin/xylene (1:1) at 65°C; and (5) 3 × 30 min of incubation in fresh paraffin at 65°C. The processed heart and lung samples were embedded in paraffin and sliced into a thickness of 6 µm, and then the sections were stained for Masson and HE.
Heart function and pulmonary velocity-time integral peak velocity assessments by echocardiography in mice
On month 4 following the MI procedure, an echocardiographic examination was performed using a Vevo 770 UBM system (Visual Sonics, Toronto, Canada), equipped with a 30-MHz transducer, which was used for noninvasive transthoracic echocardiography. Two-dimensional guided M-mode tracings were recorded. The LV’s internal diameter in the short-axis plane was measured at end diastole and end systole from M-mode recordings just below mitral valve leaflets’ tips. After the measurement, LV end-diastolic volume (LV Vol;d) as indicative of heart diastolic function was determined. Three indexes of heart systolic function, including LV end-systolic volume (LV Vol;s), LV fractional shortening (LVFS), and LV ejection fraction (LVEF), were calculated. LV mass and LV mass corrected were two indexes of cardiac hypertrophy. Meanwhile, pulmonary velocity-time integral peak velocity was determined to evaluate the condition of pulmonary artery blood flow.12,13
Right ventricular systolic pressure measurement in mice
Before mice were sacrificed, right ventricular systolic pressures (RVSPs) were measured using a high-fidelity pressure sensor catheter inserted directly into the right ventricle. Pressure waveforms were recorded for 2 min for each mouse using the blood pressure recording and analysis system (ALC-MPA, Shanghai Orcote Biotech Co., Ltd.). RVSPs were calculated by averaging ≥20 cardiac cycles for each mouse.14 According to RVSPs of nine mice in the sham operation group were all less than 23.0 mmHg. The OAMI mice were divided into the OAMI group (RVSP < 23.0 mmHg, n = 14) and PH-OAMI group (RVSP ≥23.0 mmHg, n = 8).
Enzyme-linked immunosorbent assay15
Enzyme-linked immunosorbent assay (ELISA) test boxes for N-terminal brain natriuretic peptide (NT-proBNP) (mice), annexin A5 (ANN) (mice and human), haptoglobin (HAP) (mice and human), and total protein (mice and human) were provided by Meimian Biotechnology Co., Ltd. (Wuhan, China). ELISA test boxes (mice and human) of the Ig mu chain C region (IG) and total protein were purchased from SenBeiJia Biological Technology Co., Ltd. (Nanjing, China). Contents of above-mentioned indexes were determined by a microplate reader (XSZ-02, Bio Tek Co., Ltd.). Samples were handled according to manufacturers’ instructions before measured.
Study patients
Patients were enrolled in the study from February 2017 to December 2018 in our hospital. Basic clinical data were recorded during hospitalization. The following were the inclusion criteria: (1) patients without OAMI (ejection fraction (EF) >55% and PASP <40 mmHg) and (2) patients with OAMI (EF <40%). The definition of OAMI was as follows: a history of acute anterior myocardial infarction (AAMI) was identified by an increase of cardiac biomarkers (troponin and/or creatine kinase) and at least one of the following conditions: duration of ischemic chest pain > 20 min, electrocardiogram changes indicating new ischemia and development of abnormal Q wave in the precordial lead, total or subtotal occlusion of the left anterior descending branch shown by coronary angiograms, and cardiac imaging evidence of new viable myocardium loss or new abnormal regional wall motion in the anterior wall of the left ventricle.16,17 OAMI showed a definite medical history of AAMI with a duration of infarction ≥6 months for patients.16,17 In our retrospective study, the time from AAMI to enrollment was 4.2 (2.8, 6.2) years. The following were the exclusion criteria: (1) rheumatic heart disease; (2) valvular heart disease; (3) infectious diseases; (4) severe anemia (SA). SA refers to hemoglobin (Hb) less than 80 g/L; (5) multiple organ dysfunction or failure; (6) rheumatic autoimmune diseases; (7) hyperthyroidism or hypothyroidism; and (8) heart failure due to other causes (dilated cardiomyopathy, hypertrophy cardiomyopathy, viral myocarditis, etc.). According to the EF and PASP, patients are divided into three groups: normal group (EF ≥55% and PASP <40 mmHg, n = 56), OAMI group (EF ≤ 40% and PASP <40 mmHg, n = 52), and PH-OAMI group (EF ≤40% and PASP ≥50 mmHg, n = 43).
Values of serum-related indexes and data measured by cardiac echography among normal, OAMI, and PH-OAMI groups were collected during the hospitalization period. Meanwhile, these patients’ blood sample was extracted from veins, and their blood serum was stored in a refrigerator (–80°C). Baseline characteristics such as history and biochemical blood indicators of all patients were recorded during hospital admission. The data of echocardiography were measured and collected from an experienced echocardiographical doctor blinded to the study. ANN, HAP, and IG of human serum were determined by the ELISA method. The Ethics Committee approved this study of our University.
Statistical analysis
Statistical analyses were performed using SPSS version 20 (SPSS Inc., Chicago, IL, USA). Quantitative data were presented as means ± standard error of the mean (SEM) values. A two-sided t-student test was used between two groups following a P < 0.05 of one-way analysis of variance among the three groups. Discrete data were expressed as absolute values or percentages. Analyses of discrete variables were performed by the chi-square test or Fisher’s exact test where appropriate. Logistic regression analysis was used to explore the best predictor. The area under the receiver operating characteristic (ROC) curve (AUC) was generated to assess the predictive capability of serum-related indexes for PH in patients with OAMI. The optimum cut-off value was determined as the Youden’s index (Sensitivity + Specificity-1) was the highest. A P < 0.05 was considered statistically significant.
Results
Changes of heart function and structure, and pulmonary arteriole condition among sham, OAMI, and PH-OAMI groups of mice
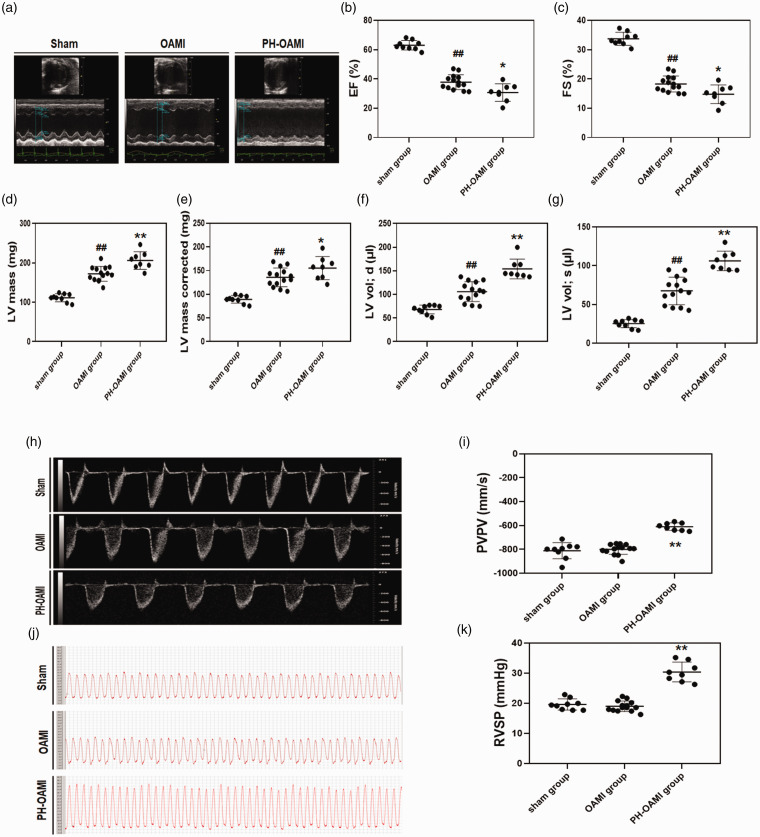
At four months after OAMI, the systolic function of hearts in the OAMI group were considerably impaired compared to the sham group, as indicated by the LVEF, LVFS, and LV Vol;s measurements (P < 0.01). An increase in LV Vol;d is an index for cardiac dilation. LV Vol;d of hearts in the OAMI group were more extensive than those in the sham group (P < 0.01). Cardiac hypertrophy was indicated by LV mass and LV mass corrected. Significant heart hypertrophy was observed in the OAMI group as compared to the sham group. Furthermore, heart systolic and diastolic dysfunction and cardiac hypertrophy were markedly aggravated in the PH-OAMI group compared to the OAMI group (P < 0.05 or P < 0.01) (Fig. 1a–g). The pulmonary valve outflow tract measurement showed a decrease in the velocity-time integral peak velocity in PH-OAMI group, compared with the sham and OAMI groups (P < 0.01) (Fig. 1h, i). RVSP can indirectly reflect the PASP. RVSPs of mice in the PH-OAMI group were higher than those in the sham and OAMI groups (Fig. 1j, k).
Fig. 1.
Changes of heart function, pulmonary blood flow, and RVSP among sham, OAMI, and PH-OAMI groups. (a–g) Echocardiography measurements. sham: n=9; OAMI: n=14; PH-OAMI: n=8. (h, i) PVPV measurement. (j, k) RVSP measurement. Data are given as means ± SEM. ##P < 0.01 versus sham group, *P < 0.05 or **P < 0.01 versus OAMI group.
PH: pulmonary hypertension; OAMI: old anterior myocardial infarction; EF: ejection fraction; FS: fractional shortening; LV: left ventricular; LV vol;d: left ventricular end-diastolic volume; LV vol;s: left ventricular end-systolic volume; PVPV: pulmonary velocity-time integral peak velocity; RVSP: right ventricular systolic pressure.
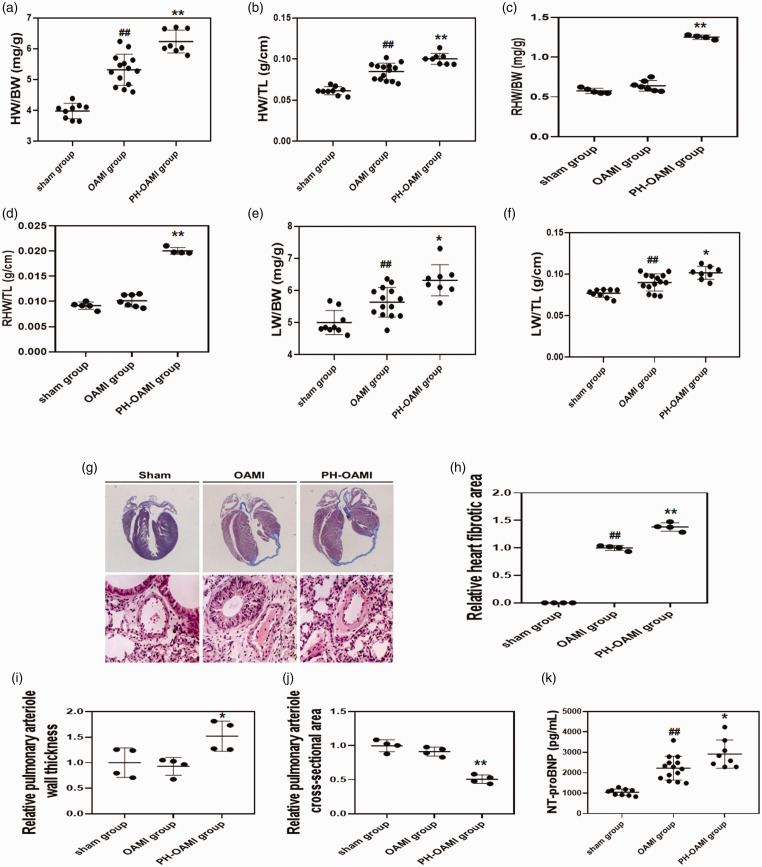
Increased HW and LW indicative of HW/BW (TL) and LW/BW (TL) were observed significantly in the OAMI group compared with the sham group at four months after MI. Besides, the whole and right HW and LW of the PH-OAMI group were more massive than those of the OAMI group (Fig. 2a–f).
Fig. 2.
Changes of HW/BW (TL), RHW/BW (TL), LW/BW (TL), cardiac fibrotic area, pulmonary arteriole condition, and NT-proBNP level among sham, OAMI, and PH-OAMI groups. (a, b) HW/BW (TL) ratio (sham: n = 9; OAMI: n = 14; PH-OAMI: n = 8). (c, d) RHW/BW (TL) ratio (sham: n = 5; OAMI: n = 7; PH-OAMI: n = 4). (e, f) LW/BW (TL) ratio (sham: n = 9; OAMI: n = 14; PH-OAMI: n = 8). (g–j) Determination of cardiac fibrotic area and pulmonary arteriole condition (sham: n = 4; OAMI: n = 4; PH-OAMI: n = 4). (k) Serum NT-proBNP level measurement (sham: n = 9; OAMI: n = 14; PH-OAMI: n = 8). Data are given as means ± SEM. ##P < 0.01 versus sham group, *P < 0.05 or **P < 0.01 versus OAMI group.
OAMI: old anterior myocardial infarction; PH: pulmonary hypertension; HW: heart weight; BW: body weight; TL: tibia length; RHW: right ventricular weight; LW: lung weight; NT-pro BNT: N-terminal brain natriuretic peptide.
Heart fibrotic area and relative pulmonary arteriole wall thickness increased notably in the PH-OAMI group compared to the sham and OAMI groups. Conversely, the relative pulmonary arteriole cross-sectional area of mice in the PH-OAMI group was smaller than those in the sham and OAMI groups (Fig. 2g–j).
NT-proBNP is an index of reflecting cardiac function. In the OAMI group, NT-proBNP levels were significantly high compared to the sham group. Additionally, higher NT-proBNP levels were observed in PH-OAMI group compared with the OAMI group (Fig. 2k).
Mice with PH-OAMI were successfully established, which showed damaged left heart function, extensive cardiac anterior wall fibrosis, high RVSP, pathological pulmonary arteriole remodeling, and right heart ventricular hypertrophy.
Proteomic analysis of lung tissue and blood serum by the iTRAQ method among sham, OAMI, and PH-OAMI groups of mice
The heat map shown in Supplemental Fig. 1a and Supplemental Table 1 showed 905 differential proteins in lung tissues of mice with PH-OAMI as compared to the sham and OAMI groups (P < 0.05 or P < 0.01). Among them, 501 downregulated proteins were found in lung tissue of mice with PH-OAMI compared to the sham and OAMI groups, and 404 proteins of lung tissues were upregulated in the mice of the PH-OAMI group (P < 0.05 or P < 0.01). Next, we analyzed Gene Ontology (GO) information from the biological process (BP), cellular component (CC), and molecular function (MF) categories. In the BP aspect, the oxidation-reduction process, small molecule metabolic process, organonitrogen compound metabolic process, CC assembly, organic acid metabolic process, and protein localization may be the key BPs involving in the development of PH-OAMI (P < 0.01). In the CC aspect, extracellular exosome, extracellular membrane-bounded organelle, vesicle, cytoplasm, and intracellular organelle may play an essential role in the pathology of PH-OAMI (P < 0.01). In the MF aspect, cell adhesion molecule binding, protein complex binding, enzyme binding, coenzyme binding, and transferase activity may play a great role in the development of PH-OAMI (P < 0.01) (Supplemental Fig. 1b–f). Additionally, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway was also considered in our study. We found that carbon metabolism, glutathione metabolism, metabolic pathways, focal adhesion and valine, leucine, and isoleucine degradation may be the significant KEGG pathways participating in the pathology of PH-OAMI (P < 0.01) (Supplemental Fig.1g, h).
Compared with the sham and OAMI groups, the heat map (Supplemental Fig. 2a and Supplemental Table 2) showed that there were 59 differential proteins in blood serum of mice with PH-OAMI, and 41 protein levels were significantly high, and the other 18 protein levels were notably low. Furthermore, we analyzed GO information involving in the pathology of PH-OAMI. BPs of GO analysis mainly included humoral immune response, defense response, adaptive immune response, etc. CCs of GO analysis majorly had blood microparticle, immunoglobulin complex, extracellular exome, and so on. MFs of GO analysis mostly included receptor binding, peptidase regulator activity, enzyme inhibitor activity, and so on (Supplemental Fig. 2b–f). Finally, KEGG pathways (including complement and coagulation cascades, vitamin digestion, and absorption, etc.) may play an essential role in the development of PH-OAMI (Supplemental Fig. 2g, h).
From the analysis of GO and KEGG pathway, we can conclude that multiple BPs, CCs, MFs, and signal pathways of lung tissue and blood serum may participate in the pathogenesis of PH secondary to OAMI.
Levels of identified three proteins in blood serum and various organs among sham, OAMI, and PH-OAMI groups of mice
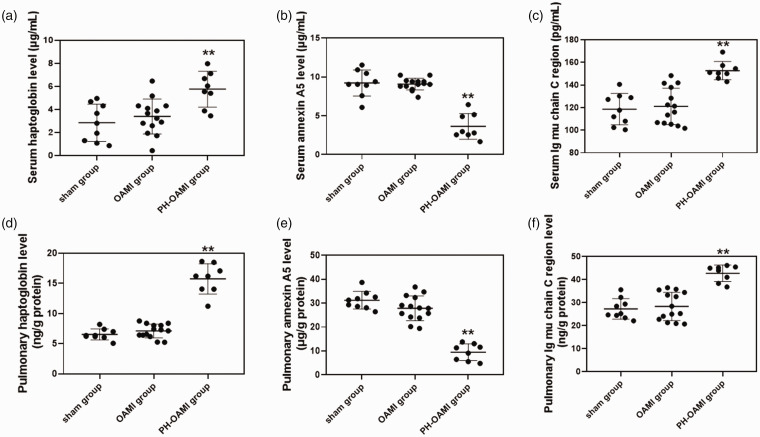
We have screened out three indexes (such as HAP, ANN, and IG) that had significant parallel changes in lung tissue and blood serum from the proteomic analysis results. Levels of HAP and IG (including lung tissue and serum) in the PH-OAMI group were notably higher than those in the sham+OAMI group. However, the level of ANN (including lung tissue and serum) in the PH-OAMI group decreased notably as compared to the sham+OAMI group (P < 0.01) (Supplemental Fig. 3a–f).
Next, these identified proteins were tested in blood serum and lung tissue by ELISA. Levels of HAP and IG in the PH-OAMI group were significantly increased compared to sham and OAMI groups. Still, the level of ANN in the PH-OAMI group was decreased markedly (P < 0.01) (Fig. 3a–f). To further verify the specificity of three identified proteins in serum and lung tissue, we also measured these proteins’ changes in other organs. There were no significant changes in levels of HAP, ANN, and IG in the heart, liver, spleen, and kidney among sham, OAMI, and PH-OAMI groups (Supplemental Figs 4 to 6).
Fig. 3.
Levels of haptoglobin, annexin A5, and Ig mu chain C region in blood serum and lung among sham, OAMI, and PH-OAMI groups of mice. (a) Serum haptoglobin level. (b) Serum annexin A5 level. (c) Serum Ig mu chain C region level. (d) Pulmonary haptoglobin level. (e) Pulmonary annexin A5 level. (f) Pulmonary Ig mu chain C region level. Data are given as means ± SEM. sham: n=9; OAMI: n=14; PH-OAMI: n=8. **P < 0.01 versus sham group or OAMI group.
PH: pulmonary hypertension; OAMI: old anterior myocardial infarction.
In total, these results indicated that levels of HAP, ANN, and IG between blood serum and lung tissue might vary consistently in PH-OAMI mice compared with sham and OAMI mice.
Basic clinical characteristics and blood-related indexes among normal, OAMI, and PH-OAMI groups of patients
Basic clinical characteristics among normal, OAMI, and PH-OAMI groups were shown in Table 1. Not any significant differences were detected about gender, the histories of hypertension, hyperlipidemia, smoking and alcohol, and previous use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers among normal, OAMI, and PH-OAMI groups (P > 0.05). There were significant differences in age, the histories of diabetes and heart function, and previous use of aspirin, clopidogrel/ticagrelor, statin, beta-blocker, calcium channel blocker, antidiabetes drug, furosemide/spironolactone, and digoxin among normal, OAMI, and PH-OAMI groups (P < 0.05 or P < 0.01).
Table 1.
Basic clinical characteristics among normal, OAMI, and PH-OAMI groups.
| Variables | Normal group (n = 56) | OAMI group (n = 52) | PH-OAMI group (n = 43) | P1 value | P2 value | P3 value | P4 value |
|---|---|---|---|---|---|---|---|
| Gender (male/female) | 41/15 | 41/11 | 33/10 | – | 0.510 | 0.810 | 0.816 |
| Age (years) | 63.30±10.17 | 65.73±10.60 | 70.12±13.41 | 0.003 | 0.232 | 0.082 | 0.005 |
| History | |||||||
| Hypertension (%) | 73.21 | 82.69 | 76.74 | – | 0.257 | 0.607 | 0.816 |
| Hyperlipidemia (%) | 7.14 | 7.69 | 9.30 | – | 1.000 | 1.000 | 0.725 |
| Diabetes (%) | 14.29 | 50.00 | 39.53 | – | 0.000 | 0.408 | 0.005 |
| Smoking (%) | 51.79 | 48.08 | 46.51 | – | 0.847 | 1.000 | 0.686 |
| Alcohol (%) | 17.86 | 21.15 | 16.28 | – | 0.808 | 0.607 | 1.000 |
| Heart function (grade) | 1.93±0.26 | 3.02±0.54 | 3.21±0.41 | 0.000 | 0.000 | 0.061 | 0.000 |
| Previous drug medication | |||||||
| Aspirin (%) | 26.78 | 82.69 | 88.37 | – | 0.000 | 0.565 | 0.000 |
| Clopidogrel/ticagrelor (%) | 0 | 51.92 | 46.51 | – | 0.000 | 0.682 | 0.000 |
| Statin (%) | 32.14 | 98.08 | 95.35 | – | 0.000 | 0.588 | 0.000 |
| ACEi/ARB (%) | 53.57 | 67.31 | 53.49 | – | 0.171 | 0.207 | 1.000 |
| Beta-blocker (%) | 37.50 | 63.46 | 65.12 | – | 0.012 | 1.000 | 0.008 |
| CCB (%) | 25.00 | 13.46 | 6.98 | – | 0.151 | 0.504 | 0.029 |
| Antidiabetes drug (%) | 7.14 | 26.92 | 34.88 | – | 0.009 | 0.503 | 0.001 |
| Furosemide/spironolactone (%) | 0 | 67.31 | 79.07 | – | 0.000 | 0.251 | 0.000 |
| Digoxin (%) | 0 | 15.38 | 13.95 | – | 0.002 | 1.000 | 0.005 |
OAMI: old anterior myocardial infarction; PH: pulmonary hypertension; ACEi: angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CCB: calcium channel blocker.
Data were expressed as n (%), mean ± SEM. P1 value was shown a comparison among the three groups. P2 value was shown a comparison between normal and OAMI groups. P3 value was shown a comparison between OAMI and PH-OAMI groups. P4 value was shown a comparison between normal and PH-OAMI groups.
Table 2 showed blood-related indexes among normal, OAMI, and PH-OAMI groups. Blood lipids, glutamic-pyruvic transaminase, active partial thromboplastin time, red blood cell, platelet, and Hb were not significantly changed among normal, OAMI, and PH-OAMI groups (P > 0.05). However, other blood-related indexes including renal function, blood sugar, N-terminal pro-B-type natriuretic peptide (NT-proBNP), glutamic oxaloacetic transaminase (GOT), blood urine acid, high-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, stromelysin-2, markers of myocardial injury, D-dimer, serum fibrinogen, prothrombin time, and white blood cell count were altered obviously among normal, OAMI, and PH-OAMI groups (P < 0.01).
Table 2.
Blood-related indexes, echocardiographical data, and serum levels of identified three proteins among normal, OAMI, and PH-OAMI groups.
| Variables | Normal group (n = 56) | OAMI group (n = 52) | PH-OAMI group (n = 43) | P1 value | P2 value | P3 value | P4 value |
|---|---|---|---|---|---|---|---|
| TCH (mmol/L) | 3.83±0.86 | 4.02±1.41 | 3.74±0.80 | 0.399 | 0.393 | 0.265 | 0.635 |
| LDL-C (mmol/L) | 2.15±0.70 | 2.35±1.18 | 2.27±0.64 | 0.494 | 0.277 | 0.691 | 0.376 |
| HDL-C (mmol/L) | 0.96±0.21 | 0.96±0.26 | 0.95±0.24 | 0.932 | 0.761 | 0.660 | 0.838 |
| TRIG (mmol/L) | 1.89±1.37 | 1.64±0.86 | 1.61±0.48 | 0.308 | 0.270 | 0.863 | 0.217 |
| Scr (μmol/L) | 71.89±12.30 | 93.22±35.85 | 102.67±36.97 | 0.000 | 0.000 | 0.215 | 0.000 |
| BUN (mmol/L) | 5.28±1.34 | 7.39±3.30 | 7.91±4.59 | 0.000 | 0.000 | 0.533 | 0.000 |
| Blood sugar (mmol/L) | 5.42±1.30 | 7.15±2.68 | 6.62±2.60 | 0.001 | 0.000 | 0.332 | 0.004 |
| GHb (%) | 5.74±0.79 | 6.79±1.47 | 6.49±1.15 | 0.000 | 0.000 | 0.292 | 0.000 |
| NT-proBNP (pg/mL) | 141.33±85.05 | 2506.06±3787.17 | 5172.37±5129.62 | 0.000 | 0.000 | 0.005 | 0.000 |
| GPT (U/L) | 34.18±36.68 | 28.37±17.23 | 32.56±35.86 | 0.443 | 0.304 | 0.463 | 0.828 |
| GOT (U/L) | 25.02±15.15 | 34.79±36.97 | 51.00±70.99 | 0.008 | 0.074 | 0.161 | 0.010 |
| Blood urine acid (μmol/L) | 330.39±83.29 | 422.25±137.36 | 449.30±161.30 | 0.000 | 0.000 | 0.385 | 0.000 |
| Hs-CRP (μg/mL) | 3.24±2.40 | 7.08±10.37 | 8.05±7.38 | 0.004 | 0.009 | 0.612 | 0.715 |
| Lp-PLA2 (ng/mL) | 128.53±50.89 | 172.21±111.08 | 180.23±97.12 | 0.008 | 0.010 | 0.000 | 0.001 |
| ST-2 (ng/mL) | 22.43±8.22 | 43.60±21.96 | 64.76±55.91 | 0.000 | 0.000 | 0.015 | 0.000 |
| High serum cTnI level (%) | 0 | 57.69 | 58.14 | – | 0.000 | 1.000 | 0.000 |
| CK (U/L) | 69.18±27.53 | 130.37±97.78 | 101.02±86.21 | 0.001 | 0.000 | 0.132 | 0.012 |
| CK-MB (U/L) | 10.05±5.16 | 17.81±9.61 | 14.23±8.76 | 0.000 | 0.000 | 0.066 | 0.004 |
| D-dimer (μg/mL) | 0.34±0.34 | 0.87±1.14 | 1.18±1.41 | 0.001 | 0.001 | 0.243 | 0.000 |
| serum fibrinogen (g/L) | 2.66±0.73 | 3.35±1.01 | 3.32±0.98 | 0.000 | 0.000 | 0.191 | 0.011 |
| PT (sec) | 10.76±0.92 | 11.04±0.88 | 11.92±1.50 | 0.000 | 0.114 | 0.001 | 0.000 |
| APTT (sec) | 27.09±4.81 | 26.49±4.17 | 27.88±3.32 | 0.194 | 0.495 | 0.082 | 0.362 |
| WBC (109/L) | 6.57±1.54 | 7.79±2.08 | 7.56±2.37 | 0.003 | 0.001 | 0.627 | 0.014 |
| RBC (1012/L) | 4.53±0.54 | 4.58±0.60 | 4.38±0.52 | 0.159 | 0.617 | 0.087 | 0.178 |
| PLT (109/L) | 198.52±57.17 | 215.08±70.46 | 196.33±45.69 | 0.052 | 0.185 | 0.140 | 0.839 |
| Hb (g/L) | 137.72±15.61 | 136.56±18.48 | 131.37±13.74 | 0.069 | 0.728 | 0.135 | 0.039 |
| LAD (mm) | 36.07±2.66 | 46.96±5.50 | 50.16±4.70 | 0.000 | 0.000 | 0.004 | 0.000 |
| LVSd (mm) | 9.75±0.81 | 8.58±2.65 | 8.14±1.88 | 0.000 | 0.002 | 0.370 | 0.000 |
| LVDd (mm) | 47.77±2.97 | 61.29±7.04 | 63.28±7.65 | 0.000 | 0.000 | 0.195 | 0.000 |
| LVPWd (mm) | 9.16±0.84 | 8.75±1.54 | 8.60±1.16 | 0.053 | 0.089 | 0.615 | 0.008 |
| LVDs (mm) | 30.91±2.20 | 50.62±6.51 | 53.67±7.51 | 0.000 | 0.000 | 0.038 | 0.000 |
| FS (mm) | 35.32±2.16 | 17.48±1.96 | 15.67±3.06 | 0.000 | 0.000 | 0.001 | 0.000 |
| EF (%) | 64.64±2.60 | 35.38±3.78 | 32.07±5.99 | 0.000 | 0.000 | 0.002 | 0.000 |
| Annexin A5 (μg/L) | 183.85±28.55 | 178.78±25.84 | 144.47±17.43 | 0.000 | 0.240 | 0.000 | 0.000 |
| Haptoglobin (ng/L) | 61.67±9.75 | 64.75±11.07 | 81.19±10.16 | 0.000 | 0.130 | 0.000 | 0.000 |
| Ig mu chain C region (ng/L) | 153.54±14.24 | 157.74±17.33 | 191.93±22.63 | 0.000 | 0.174 | 0.000 | 0.000 |
PH: pulmonary hypertension; OAMI: old anterior myocardial infarction; TCH: total cholesterol; LDL-C: low density lipoprotein-cholesterol; HDL-C: high density lipoprotein-cholesterol; TRIG: triglyceride; Scr: serum creatinine; BUN: blood urine nitrogen; GHb: glycosylated hemoglobin; NT-proBNP: N-terminal brain natriuretic peptide; GPT: glutamic-pyruvic transaminase; GOT: glutamic oxaloacetic transaminase; Hs-CRP: high-sensitivity C-reactive protein; Lp-PLA2: lipoprotein-associated phospholipase A2; ST-2: stromelysin-2; cTnI: cardiac troponin I; CK: creatine kinase; CK-MB: creatine kinase-MB; PT: prothrombin time; APTT: activated partial thromboplastin time; WBC: white blood cell; RBC: red blood cell; PLT: platelet; Hb: hemoglobin; LAD: left atrial dimension; LVSd: left ventricular septal diameter; LVDd: left ventricular end-diastolic diameter; LVPWd: left ventricular posterior wall depth; LVDs: left ventricular end-systolic diameter; FS: fractional shortening; EF: ejection fraction.
Data were expressed as n (%), mean ± SEM. P1 value was shown a comparison among the three groups. P2 value was shown a comparison between normal and OAMI groups. P3 value was shown a comparison between OAMI and PH-OAMI groups. P4 value was shown a comparison between normal and PH-OAMI groups.
Conclusionally, these results revealed that many basic clinical characteristics and blood-related indexes had changed significantly in patients with PH-OAMI compared with normal and OAMI patients.
Echocardiographic data and serum levels of identified three proteins among normal, OAMI, and PH-OAMI groups of patients
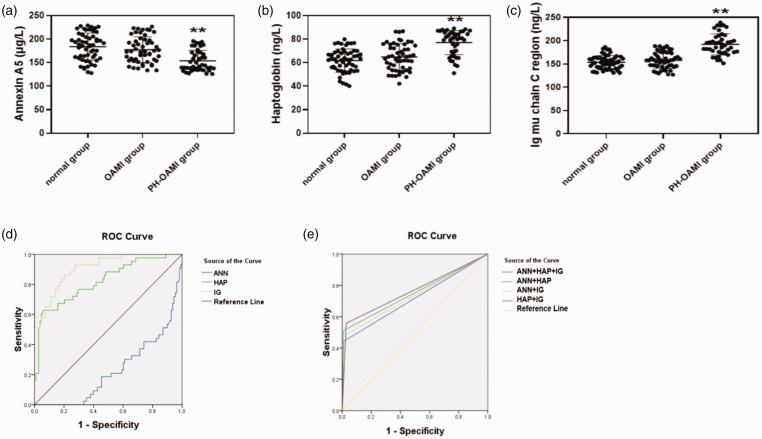
Except for LV posterior wall depth, other echocardiographic data including left atrial dimension (LAD), LV septal diameter, LV end-diastolic diameter, LV end-systolic diameter, fractional shortening (FS), EF, and the incidence of the PASP were dramatically changed among normal, OAMI, and PH-OAMI groups (P < 0.01) (Table 2). Next, we determined serum levels of ANN, HAP, and IG among normal, OAMI, and PH-OAMI groups, and their levels were all altered significantly in the PH-OAMI group compared with normal and OAMI groups (P < 0.01) (Table 2, Fig. 4a–c).
Fig. 4.
Serum levels and the predictive value of identified three proteins among normal, OAMI, and PH-OAMI groups of patients. (a–c) Serum levels of identified three proteins among these patients. Normal: n=56; OAMI: n=52; PH-OAMI: n=43. (d) The predictive value of single serum protein among these patients. (e) The predictive value of two or three serum proteins among these patients. Data are given as means ± SEM. **P < 0.01 versus normal group or OAMI group.
PH: pulmonary hypertension; OAMI: old anterior myocardial infarction; ROC: receiver operating characteristic; HAP: haptoglobin; ANN: annexin 5; IG: Ig mu chain C region.
In a word, these results showed significant heart systolic and diastolic dysfunction and disordered serum levels of ANN, HAP, and IG in PH-OAMI patients as compared to normal and OAMI patients.
Multivariate regression analysis of serum-related factors to predict PH and their predictive value analyzed by ROC curve among normal, OAMI, and PH-OAMI groups
After a pairwise comparison among normal, OAMI, and PH-OAMI groups, the indexes with significant changes were taken into consideration in the multivariate regression analysis, which showed that heart function grade and ANN, HAP, and IG of serum could predict of PH among normal, OAMI, and PH-OAMI groups (Table 3) (P < 0.05 or P < 0.01). The cut-off values of serum levels of ANN, HAP, and IG for predicting PH related to OAMI were 151.925 µg/L (sensitivity: 0.419, specificity: 0.824), 78.295 ng/L (sensitivity: 0.628, specificity: 0.944), and 168.885 ng/L (sensitivity: 0.860, specificity: 0.796), respectively (Table 4, Fig. 4d) (P < 0.01).
Table 3.
Multivariate regression analysis of related factors to predict PH among normal, OAMI, and PH-OAMI groups.
| Variables | OR (95% CI) | P value |
|---|---|---|
| Age (years) | 1.001 (0.997–1.005) | 0.674 |
| Diabetes (%) | 0.880 (0.774–1.001) | 0.052 |
| Heart function (grade) | 1.112 (1.001–1.236) | 0.049 |
| Aspirin (%) | 0.922 (0.793–1.073) | 0.292 |
| Clopidogrel/ticagrelor (%) | 1.026 (0.925–1.139) | 0.627 |
| Statin (%) | 1.007 (0.852–1.190) | 0.936 |
| Beta-blocker (%) | 0.974 (0.889–1.067) | 0.572 |
| CCB (%) | 0.921 (0.820–1.037) | 0.171 |
| Antidiabetes drug (%) | 1.108 (0.955–1.288) | 0.174 |
| Furosemide/spironolactone (%) | 0.994 (0.875–1.129) | 0.924 |
| Digoxin (%) | 1.001 (0.855–1.174) | 0.987 |
| Scr (μmol/L) | 1.000 (0.998–1.002) | 0.858 |
| BUN (mmol/L) | 1.005 (0.983–1.027) | 0.669 |
| Blood sugar (mmol/L) | 1.001 (0.975–1.027) | 0.939 |
| GHb (%) | 0.997 (0.945–1.051) | 0.901 |
| NT-proBNP (pg/mL) | 1.000 (1.000–1.000) | 0.658 |
| GOT (U/L) | 1.001 (1.000–1.002) | 0.135 |
| Blood urine acid (μmol/L) | 1.000 (1.000–1.001) | 0.093 |
| Hs-CRP (μg/mL) | 1.002 (0.994–1.009) | 0.686 |
| Lp-PLA2 (ng/mL) | 1.000 (0.999–1.000) | 0.083 |
| ST-2 (ng/mL) | 1.000 (0.998–1.002) | 0.938 |
| High serum cTnI level (%) | 0.949 (0.845–1.068) | 0.385 |
| CK (U/L) | 1.000 (0.999–1.000) | 0.103 |
| CK-MB (U/L) | 0.997 (0.991–1.004) | 0.456 |
| D-dimer (μg/mL) | 0.991 (0.941–1.043) | 0.716 |
| serum fibrinogen (g/L) | 0.961 (0.914–1.011) | 0.123 |
| PT (sec) | 1.008 (0.963–1.054) | 0.740 |
| WBC (109/L) | 1.007 (0.983–1.031) | 0.562 |
| LAD (mm) | 1.006 (0.996–1.017) | 0.242 |
| LVSd (mm) | 1.002 (0.979–1.025) | 0.882 |
| LVDd (mm) | 0.982 (0.947–1.018) | 0.327 |
| LVDs (mm) | 1.012 (0.969–1.058) | 0.579 |
| FS (mm) | 1.045 (0.980–1.114) | 0.174 |
| EF (%) | 0.973 (0.934–1.014) | 0.191 |
| Annexin A5 (μg/L) | 0.997 (0.995–0.998) | 0.000 |
| Haptoglobin (ng/L) | 1.011 (1.007–1.015) | 0.000 |
| Ig mu chain C region (ng/L) | 1.008 (1.006–1.010) | 0.000 |
OR: odd ratio; CI: confidential interval; CCB: calcium channel blocker; Scr: serum creatinine; BUN: blood urine nitrogen; GHb: glycosylated hemoglobin; NT-proBNP: N-terminal brain natriuretic peptide; GOT: glutamic oxaloacetic transaminase; Hs-CRP: high-sensitivity C-reactive protein; Lp-PLA2: lipoprotein-associated phospholipase A2; ST-2: stromelysin-2; CK: creatine kinase; CK-MB: creatine kinase-MB; PT: prothrombin time; WBC: white blood cell; LAD: left atrial dimension; LVSd: left ventricular septal diameter; LVDd: left ventricular end-diastolic diameter; LVDs: left ventricular end-systolic diameter; FS: fraction shortening; EF: ejection fractions.
Table 4.
The predictive value of identified three proteins analyzed by ROC curve among normal, OAMI, and PH-OAMI groups.
| AUC | Cut-off value | Sensitivity | Specificity | P value | |
|---|---|---|---|---|---|
| Annexin A5 (μg/L) | 0.773 (0.694–0.853) | 151.925 | 41.9% | 82.4% | 0.000 |
| Haptoglobin (ng/L) | 0.823 (0.745–0.901) | 78.295 | 62.8% | 94.4% | 0.000 |
| Ig mu chain C region (ng/L) | 0.909 (0.860–0.957) | 168.885 | 86.0% | 79.6% | 0.000 |
| ANN+HAP+IG | 0.716 (0.613–0.819) | 0.500 | 44.2% | 99.1% | 0.000 |
| ANN+HAP | 0.751 (0.651–0.851) | 0.500 | 51.2% | 99.1% | 0.000 |
| ANN+IG | 0.730 (0.629–0.832) | 0.500 | 48.8% | 97.2% | 0.000 |
| HAP+IG | 0.765 (0.668–0.863) | 0.500 | 55.8% | 97.2% | 0.000 |
AUC: area under the receiver operating characteristic curve; ANN: annexin 5; HAP: haptoglobin; IG: Ig mu chain C region.
Next, we tested the predicted ability of two or three combination about three serum proteins. According to the cut-off values of three serum proteins, if this protein value was more than its cut-off value, it was defined as “1,” or “0” was defined. ANN+HAP+IG (cut-off value: 0.500, sensitivity: 0.442, specificity: 0.991), ANN+HAP (cut-off value: 0.500, sensitivity: 0.512, specificity: 0.991), ANN+IG (cut-off value: 0.500, sensitivity: 0.488, specificity: 0.972), and HAP+IG (cut-off value: 0.500, sensitivity: 0.558, specificity: 0.972) were found (Table 4, Fig. 4e) (P < 0.01).
In brief, these results indicated that serum levels of ANN, HAP, and IG could have an advantage of predicting PH secondary to OAMI, whether it is a single index or a combination of three serum indexes.
Discussion
According to the literature report, there is no basic and clinical study on serum proteomics of PH secondary to OAMI in vivo.18–21 Therefore, in the present study, we first made the PH-OAMI model in mice and then analyzed the proteomics of their lung tissues and serum by iTRAQ technology. After analysis, we screened three proteins (such as ANN, HAP, and IG) to categorize OAMI mice with and without PH. Finally, we found that these three serum proteins can predict PH among normal, OAMI, and PH-OAMI patients.
ANN is widely distributed in various tissues and cells of the organism. It has essential physiological functions (such as antithrombotic, antiapoptotic, and anti-inflammatory properties) through its binding to cell surface-expressed phosphatidylserine (PS).22,23 ANN can bind to PS and form a two-dimensional shielding layer on the membrane, effectively isolating the PS as a coagulation promoting factor from other related coagulation factors, thus playing an anticoagulant role.23 ANN preferentially binds PS with high affinity and inhibits macrophage uptake of apoptotic and necrotic cells, most likely by interfering with PS’s availability for recognition.22 The blood of patients with heart failure is often in the hypercoagulable state, which is easy to form microthrombosis and aggravate PH formation.24 In patients with severe PH, the number of macrophages in lung lesions increases, leading to the release of interleukin (IL)-1 β, IL-6, tumor necrosis factor-alpha, and IL-10. Furthermore, activated macrophages may lead to T cells’ activation and T cell chemokines’ production, thus further promoting PH’s inflammatory process.25 In our study, we found that ANN levels of lung tissues in mice with PH-OAMI were lower than those in mice without OAMI or with OAMI, suggesting that the decrease of ANN level may lead to the aggravation of inflammation and coagulation in lung tissue, which was involved in the development of PH secondary to OAMI.
HAP is a kind of acidic glycoprotein in the serum α2 – globulin component, which widely exists in humans and much mammalian serum and other body fluids. Its principal function is to form HAP–Hb complex by binding with free Hb, transport Hb to the liver for metabolism, avoid the loss of Hb and iron from the kidney, and damage the kidney. Additionally, HAP plays a vital role in repairing damaged tissues, host anti-infection, and internal environment stabilization as an acute phase protein.26,27 Except for the functions mentioned above, HAP also inhibits prostaglandin synthesis, antioxidant activity, promoting immune function, and angiogenesis.27–29 Atsumi et al.30 have reported that serum HAP levels decreased in PH patients and inversely correlated with pulmonary artery pressure in PH related to connective tissue diseases (CTD) patients, suggesting their potential as a surrogate marker for PH-CTD. Buehler et al.31 have found that chronic HAP infusion was a novel therapy for chronic hemolysis-associated PH by preventing Hb-mediated vascular remodeling associated with hypoxia.31 Ivy et al.32 have investigated the serum proteome for a molecular basis of good versus poor outcome to long-term vasodilator therapy in children with IPAH and found that HAP was 1.45-fold lower in that with a good versus poor outcome. However, in our study, HAP levels of lung tissues in mice with PH-OAMI were significantly high compared to sham and OAMI groups, indicating that the compensatory increase of HAP in lung tissue as a protective factor may hinder the progress of PH-OAMI.
Immunoglobulins (Ig), also known as antibodies, are secreted or membrane-bound glycoproteins produced by B lymphocytes. IG is a protein that is encoded by the immunoglobulin heavy constant mu (IGHM) gene in humans. The IGHM gene encodes the C region of Ig mu heavy chain, which defines IgM’s isotype. During an antibody response period, activated B cells can be transformed into the expression of individual downstream heavy chain C region genes via a process of somatic recombination known as isotype switching. Additionally, secreted Ig forms that act as antibodies can be produced by alternative RNA processing of Ig heavy chain C region sequences.33 A variety of autoantibodies can be detected in patients with idiopathic PH, such as antiendothelial cell antibody, antigen fibrin-1 antibody, antiendothelin A receptor antibody, antiangiotensin 1 receptor antibody, antinuclear antibody, etc., suggesting that autoimmunity may participate in the pathogenesis of pH.34,35 Some researchers used CD20 antibody to inhibit the activity of B-lymphocytes in SD rats or used JH-KO rats with B-cell defects to induce pH with monocrotaline. They found that compared with the control group, the RVSP and pulmonary vascular remodeling of rats with B-cell inhibition were significantly improved, suggesting that B-cell activation may be involved in the process of pH.36 In the present study, high expression of the IG in lung tissues of mice with PH-OAMI revealed that overactivation of cellular immunity may play an essential role in developing PH-OAMI.
At the same time, we found that these three proteins (including ANN, HAP, and IG) in serum had parallel changes with those in lung tissue. To show that the changes of these three proteins in serum can expressly reflect their changes in lung tissue, we also measured their changes in other tissues of mice. The results confirmed that the three proteins in serum have specificity to reflect their lung tissue changes. Then, we retrospectively studied the relationship between these three serum proteins and PH condition among normal, OAMI, and PH-OAMI patients and found that they can be useful for categorizing OAMI patients with and without PH.
Study limitations
The limitation of this study was as follows: (1) How these three proteins (including ANN, HAP, and IG) are closely related to the process of PH-OAMI needs further verification in vitro and in vivo; (2) the number of cases in the retrospective study is small, which needs to be further tested by a prospective and multicenter study.
Conclusion
In conclusion, these three serum proteins (including ANN, HAP, and IG) screened from PH-OAMI mice can be applied to evaluate PH condition in OAMI patients, which has not been reported previously. It will supply a good method to guide better diagnosis and treatment in patients with PH-OAMI.
Supplemental Material
Supplemental material, sj-zip-1-pul-10.1177_2045894020969079 for Serum biomarker analysis at the protein level on pulmonary hypertension secondary to old anterior myocardial infarction by Xiangqi Wu, Wei You, Zhiming Wu, Fei Ye and Shaoliang Chen in Pulmonary Circulation
Acknowledgments
The authors thank the physicians, nurses, and patients involved in the study. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository with the dataset identifier PXD019482.37
Author contributions: FY and SC designed the study. XW and WY collected and processed samples. XW, WY, and ZW analyzed samples and data. XW drafted and wrote the manuscript. WY, ZW, FY, and SC revised the manuscript critically for intellectual content. All authors read the final manuscript and agreed to publish it.
Ethical approval: The study was approved by the ethics committee of our institution.
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: This project was supported by the National Natural Science Foundation of China (grant number 81600296), China Postdoctoral Science Foundation (grant number 2019M651886), Jiangsu Postdoctoral Research Foundation (grant number 2018K242C), and Nanjing Municipal Science and Technology Bureau (grant number: 201803008).
Guarantor: SC and FY are the guarantors of the data presented in this paper.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Parikh V, Bhardwaj A, Nair A. Pharmacotherapy for pulmonary arterial hypertension. J Thorac Dis 2019; 11: S1767–S1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai Z, Zhu MM, Peng Y, et al. Therapeutic targeting of vascular remodeling and right heart failure in pulmonary arterial hypertension with a HIF-2alpha inhibitor. Am J Respir Crit Care Med 2018; 198: 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 4.Ramu B, Thenappan T. Evolving concepts of pulmonary hypertension secondary to left heart disease. Curr Heart Fail Rep 2016; 13: 92–102. [DOI] [PubMed] [Google Scholar]
- 5.Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016; 37: 942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association, Chinese Heart Failure Association of Chinese Medical Doctor Association and Editorial Board of Chinese Journal of Cardiology. [Chinese guidelines for the diagnosis and treatment of heart failure 2018]. Zhonghua Xin Xue Guan Bing Za Zhi 2018; 46: 760–789. [DOI] [PubMed] [Google Scholar]
- 7.Panza JA, Ellis AM, Al-Khalidi HR, et al. Myocardial viability and long-term outcomes in ischemic cardiomyopathy. N Engl J Med 2019; 381: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed F, Kumar G, Soliman FM, et al. Proteomics for understanding pathogenesis, immune modulation and host pathogen interactions in aquaculture. Comp Biochem Physiol Part D Genomics Proteomics 2019; 32: 100625. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Yuan Q, Gu Z, et al. Advances of proteomics technologies for multidrug-resistant mechanisms. Future Med Chem 2019; 11: 2573–2593. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Zhu X, Shen J, et al. Quantitative iTRAQ-based proteomic analysis of differentially expressed proteins in aging in human and monkey. BMC Genomics 2019; 20: 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang GM, Yan K, Wang P, et al. ITRAQ-based proteomics analysis reveals the effect of neoliensinine on KCl-induced vascular smooth muscle contraction by inhibiting regulatory light chain phosphorylation. Front Pharmacol 2019; 10: 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, You W, Wu Z, et al. Ivabradine promotes angiogenesis and reduces cardiac hypertrophy in mice with myocardial infarction. Anatol J Cardiol 2018; 20: 266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You W, Wu Z, Ye F, et al. Cardamonin protects against adverse cardiac remodeling through mTORC1 inhibition in mice with myocardial infarction. Pharmazie 2018; 73: 508–512. [DOI] [PubMed] [Google Scholar]
- 14.Novelli EM, Little-Ihrig L, Knupp HE, et al. Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol 2019; 316: L1150–L1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, Cao Z, Wu P, et al. Effect and mechanism of the Bruton tyrosine kinase (Btk) inhibitor ibrutinib on rat model of diabetic foot ulcers. Med Sci Monit 2019; 25: 7951–7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang SF, Zhang XQ, Yang SJ, et al. Intravoxel incoherent motion magnetic resonance imaging with integrated slice-specific shimming for old myocardial infarction: a pilot study. Sci Rep 2019; 9: 19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinozaki K, Tamura A, Kadota J. Associations of positive T wave in lead aVR with hemodynamic, coronary, and left ventricular angiographic findings in anterior wall old myocardial infarction. J Cardiol 2011; 57: 160–164. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Salam VB, Paul GA, Ali JO, et al. Identification of plasma protein biomarkers associated with idiopathic pulmonary arterial hypertension. Proteomics 2006; 6: 2286–2294. [DOI] [PubMed] [Google Scholar]
- 19.Yu M, Wang XX, Zhang FR, et al. Proteomic analysis of the serum in patients with idiopathic pulmonary arterial hypertension. J Zhejiang Univ Sci B 2007; 8: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Zhang Y, Li N, et al. Potential diagnostic biomarkers in serum of idiopathic pulmonary arterial hypertension. Respir Med 2009; 103: 1801–1806. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Hou HT, Wang J, et al. Plasma proteomic study in pulmonary arterial hypertension associated with congenital heart diseases. Sci Rep 2016; 6: 36541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz LE, Frey B, Pausch F, et al. The role of annexin A5 in the modulation of the immune response against dying and dead cells. Curr Med Chem 2007; 14: 271–277. [DOI] [PubMed] [Google Scholar]
- 23.Wahezi DM, Ilowite NT, Wu XX, et al. Atherosclerosis prevention in pediatric lupus erythematosus. Annexin A5 anticoagulant activity in children with systemic lupus erythematosus and the association with antibodies to domain I of beta2-glycoprotein I. Lupus 2013; 22: 702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazan IS, Fares WH. Hypercoagulability in pulmonary hypertension. Clin Chest Med 2018; 39: 595–603. [DOI] [PubMed] [Google Scholar]
- 25.Vergadi E, Chang MS, Lee C, et al. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 2011; 123: 1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem 1996; 42: 1589–1600. [PubMed] [Google Scholar]
- 27.Kato GJ. Haptoglobin halts hemoglobin’s havoc. J Clin Invest 2009; 119: 2140–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Kleijn DP, Smeets MB, Kemmeren PP, et al. Acute-phase protein haptoglobin is a cell migration factor involved in arterial restructuring. FASEB J 2002; 16: 1123–1125. [DOI] [PubMed] [Google Scholar]
- 29.Andersen CBF, Stodkilde K, Saederup KL, et al. Haptoglobin. Antioxid Redox Signal 2017; 26: 814–831. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura H, Kato M, Nakaya T, et al. Decreased haptoglobin levels inversely correlated with pulmonary artery pressure in patients with pulmonary arterial hypertension: a cross-sectional study. Medicine (Baltimore) 2017; 96: e8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwin DC, Baek JH, Hassell K, et al. Hemoglobin-induced lung vascular oxidation, inflammation, and remodeling contribute to the progression of hypoxic pulmonary hypertension and is attenuated in rats with repeated-dose haptoglobin administration. Free Radic Biol Med 2015; 82: 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeager ME, Colvin KL, Everett AD, et al. Plasma proteomics of differential outcome to long-term therapy in children with idiopathic pulmonary arterial hypertension. Proteomics Clin Appl 2012; 6: 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedlander RM, Nussenzweig MC, Leder P. Complete nucleotide sequence of the membrane form of the human IgM heavy chain. Nucleic Acids Res 1990; 18: 4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arends SJ, Damoiseaux J, Duijvestijn A, et al. Prevalence of anti-endothelial cell antibodies in idiopathic pulmonary arterial hypertension. Eur Respir J 2010; 35: 923–925. [DOI] [PubMed] [Google Scholar]
- 35.Yanai-Landau H, Amital H, Bar-Dayan Y, et al. Autoimmune aspects of primary pulmonary hypertension. Pathobiology. 1995; 63: 71–75. [DOI] [PubMed] [Google Scholar]
- 36.Breitling S, Hui Z, Zabini D, et al. The mast cell-B cell axis in lung vascular remodeling and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2017; 312: L710–L721. [DOI] [PubMed] [Google Scholar]
- 37.Ma JT, Chen S, Wu C, et al. iProX: an integrated proteome resource. Nucleic Acids Res 2019; 47: D1211–D1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-zip-1-pul-10.1177_2045894020969079 for Serum biomarker analysis at the protein level on pulmonary hypertension secondary to old anterior myocardial infarction by Xiangqi Wu, Wei You, Zhiming Wu, Fei Ye and Shaoliang Chen in Pulmonary Circulation