Abstract
Microglia are diverse cells that acquire different functional phenotypes in response to microenvironment in which they reside. Several transcriptional regulators have been identified that regulate different microglia phenotypes. They are mainly stimulated into two opposing phenotypes, classically (M1) and alternatively (M2) phenotype. Regulating microglia polarization from M1 to M2 state has been suggested as a potential therapeutic approach in treatment of CNS disorders. Candesartan, an angiotensin II type I receptors antagonist, exerts beneficial effects for antioxidant, anti-inflammation, neurotrophic, and anti-apoptotic function. However, the effect of candesartan on microglia polarization and underlying mechanisms remain unknown. In this study, the resting microglia were stimulated to M1 microglia with lipopolysaccharide (LPS) and interferon-γ (IFN-γ), and then treated with vehicle or candesartan for 24 h. RT-PCR was utilized to detect the mRNA expression of microglia phenotype markers and inflammatory cytokines. Microglia phenotype markers and toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) pathway were determined by western blot. A neuron-microglia co-culture system was used to determine whether candesartan could ameliorate the neurotoxic effect of M1 microglia to oxygen-glucose deprivation (OGD) neuron. Candesartan treatment reduced the expression of M1 markers, and increased M2 markers. Meanwhile, candesartan reduced fluorescence intensity and protein level of M1 marker and enhanced M2 marker. Candesartan also regulated the neuroinflammatory response via reducing the release of pro-inflammatory cytokines and increasing anti-inflammatory cytokines in LPS + IFN-γ stimulated BV2 cells. Candesartan markedly inhibited the protein level of TLR4, the phosphorylation of IKBα and p65, and suppressed nuclear translocation of NF-κB p65. BAY 11-7085, a NF-κB inhibitor, remarkably enlarged the inhibitory effect of candesartan on NF-κB pathway. In addition, M1 phenotype microglia exacerbated post-OGD N2a cells death and LDH release, whereas candesartan reversed such neurotoxic effect. Candesartan treatment may ameliorate stroke-induced neuronal damage through shifting microglia to M2 phenotype in a TLR4/NF-κB-dependent manner.
Keywords: BV2 cells, candesartan, microglia polarization, neuroinflammation, NF-κB
Introduction
Microglia are specialized innate immune macrophages in the central nervous system (CNS) and perform numerous functions required for CNS development, homeostasis, and repair.1,2 Clinical and experimental studies support that microglia play important roles in regulating immune and inflammatory responses for pathogenesis and repairment in CNS injuries, including Alzheimer’s disease, Parkinson’s disease, and stroke.3,4 Many lines of evidence indicate that microglia can exhibit a diverse range of phenotypes with beneficial or deleterious functions in response to their surroundings.5 Microglia phenotypes have the characteristics of spatial, temporal and functional diversity during the progression of CNS disease.6 For instance, the classically activated M1 microglia are stimulated by lipopolysaccharide (LPS) and interferon-γ (IFN-γ), which lead to inflammatory amplification through the secretion of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IFN-γ.7 In contrast, alternatively activated M2 microglia are induced by interleukin-4 (IL-4) or interleukin-13 (IL-13), which exert neuroprotective effects by removing cell debris and producing anti-inflammatory cytokines such as transforming growth factor-β (TGF-β) and interleukin-10 (IL-10).8 Several transcriptional regulators have been identified that regulate different microglia phenotypes, including nuclear factor kappa-B (NF-κB), signal transducer and activator of transcription (STATs), interferon regulatory factors (IRFs), and so on.1 Excessive inflammation responses during the course of CNS injury are detrimental for the recovery of neurological function.2 Not surprisingly, balancing between M1 and M2 microglia polarization play a key role during the CNS disease progression.9
Candesartan is an antagonist blocking angiotensin II type I receptors (AT1R, encoded by Atgr1) stimulation by angiotensin II when administered systemically.10 More observations strongly suggest that angiotensin II signaling may participate in multiple functions for cerebral circulation, stress responses and inflammation through the AT1Rs activation.11,12 But decreasing AT1R activity with the AT1R antagonist candesartan is therapeutic function on hypertension,13 heart failure,14 cerebrovascular accident, or stroke.15 Toll-like receptor 4 (TLR4) can be stimulated by LPS, and further activates downstream NF-κB, which eventually induces the transcription of pro-inflammatory mediators and promotes the polarization of M1 phenotypic microglia.16 In LPS model rats, candesartan was also found to reduce gene expression of pro-inflammatory cytokines, and microglia activation in the brain.17 The anti-inflammatory effects of candesartan may result from the inhibition of Angiotensin II and LPS common signaling cascades. In LPS-stimulated human renal tubular epithelial model, candesartan inhibited inflammatory factors likely via reducing TLR4, but independent on AT1R.18 These studies indicated that candesartan treatment could ameliorate different pathological effect, yet candesartan has not been assessed for regulation on microglia polarization.
The present study was designed to test the underlying mechanism of candesartan in modulating microglia polarization. Firstly, candesartan was demonstrated to inhibit microglia M1 polarization and promote M2 polarization. Furthermore, candesartan treatment also significantly modulated neuroinflammatory response via attenuating pro-inflammatory cytokines and increasing anti-inflammatory factors. The cellular mechanisms of this process may be associated with the inhibition of TLR4/NF-κB signaling pathway. In addition, candesartan inhibited the neurotoxic effect of M1 microglia on OGD neurons in neuron-microglia co-culture system. Therefore, these findings support the view that candesartan alleviates neuronal damage at least partially by modulating the balance between M1 and M2 microglia polarization in a TLR4/NF-κB-dependent manner.
Materials and methods
Microglia cell model and candesartan treatment
Both mouse microglia (BV2 cells) and neuron (N2a cells) were purchased from Cell Center, Institute of Basic Medical Sciences, CAMS and PUMC, Beijing, China. BV2 cells were cultured in DMEM medium (Gibco), which was supplemented with 10% fetal bovine serum (Gibco), and 1% penicillin-streptomycin (Gibco). The cells were then incubated at 37°C in a humidified atmosphere of 5% CO2. For M1 microglia, microglial cells were stimulated with 100 ng/mL LPS + 20 ng/mL IFN-γ (PeproTech),19,20 and then treated with vehicle or candesartan (Sigma). For M2 microglia, microglial cells were induced with 20 ng/mL IL-4 (PeproTech).20 After 24 h treatment, cells were harvested for testing the changes of mRNA and protein level, and the supernatant was collected for measuring the level of inflammatory cytokines.
Cell viability and LDH assay
BV2 cells (3 × 104/well) were seeded into 96-well plates (Gibco), and treated with vehicle or different concentrations of candesartan (0.5, 1, 5, 10, 20, 30, 40, and 50 µM) for 24 h. The cell viability of BV2 microglial cells was evaluated by the cell counting kit-8 assay (CCK-8 assay, Beyotime). Each well was exposed to 10 μL of CCK-8 reagent (Beyotime). During the last 1 h incubation at 37°C, all wells were measured for the absorbance at 450 nm in a microplate reader (Biotek). The cytotoxicity of candesartan on BV2 cells was analyzed by a lactate dehydrogenase (LDH) assay kit (Beyotime) according to the manufacturer’s protocol. The concentrations of LDH were measured for the absorbance at 490 nm using a microplate reader (Biotek). The data were expressed as percentages of the value of vehicle cells.
Nitric oxide assay
BV2 cells (1.5 × 106/well) were cultured in 60 cm culture dish, and treated with LPS (100 ng/mL) + IFN-γ (20 ng/mL) followed by vehicle or optimal dose of candesartan for 24 h. The concentration of nitric oxide (NO) in supernatant was performed using the Griess reaction kit according to the manufacturer’s instruction (Nanjing Jiancheng Bioengineering Institute). The 490 nm absorbance was measured by a microplate reader (Biotek). The NO concentration was calculated using the standard curve of sodium nitrate.
ELISA measurements
BV2 cells (1.5 × 106 cells/well) were cultured in 60 cm culture dish, and treated with LPS (100 ng/mL) + IFN-γ (20 ng/mL), together with vehicle or optimal dose of candesartan for 24 h. The supernatant was collected for detecting the protein level of pro-inflammatory (TNF-α, IL-12p70, and IL-6) and anti-inflammatory (TGF-β and IL-10) according to the instructions of ELISA kits (Nanjing Jiancheng Bioengineering Institute).
Immunofluorescence
BV2 microglial cells (1.5 × 104 cells/well) were seeded in a 24-well plate containing the coverslip. The cells were treated with LPS (100 ng/mL) + IFN-γ (20 ng/mL) followed by vehicle or selected dose of candesartan. After 24 h, cells were fixed with 4% paraformaldehyde followed by blocking with 5% donkey serum. The cells were incubated with primary antibodies, rabbit anti-iNOS (1:100 dilution, Abcam) or rabbit anti-CD206 (1:200 dilution, Abcam) at 4°C overnight. Cells were washed with PBS, followed by incubation with the secondary antibody for 2 h at room temperature, Alexa Fluor 488 donkey anti-rabbit IgG (1:400 dilution, Invitrogen). After being washed, the nucleus was stained with DAPI. All samples were observed with a laser confocal microscope (Nikon).
Quantitative RT-PCR
Total RNA in BV2 cells was extracted using RNA prep Pure cell kit (TIANGEN) according to the manufacturer’s instructions. The cDNA was prepared by using Quantscript RT Kit (TIANGEN). Quantitative PCR was performed on quantitative PCR systems (Applied Biosystems 7500 Real-Time PCR Systems, Thermo Fisher Scientific, Waltham, MA, USA) using the synthetic primers and the SuperReal PreMix Plus (SYBR Green) kit (TIANGEN). Gene specific primers used for cDNA amplification were as listed in Table 1. The data were analyzed by the 2−ΔΔCt method.21
Table 1.
Gene specific primers used for cDNA amplification.
| Genes | Primers(5′-3′) | |
|---|---|---|
| CD11b | Forward | CCAAGACGATCTCAGCATCA |
| Reverse | TTCTGGCTTGCTGAATCCTT | |
| iNOS | Forward | CAAGCACCTTGGAAGAGGAG |
| Reverse | AAGGCCAAACACAGCATACC | |
| YM1/2 | Forward | CAGGGTAATGAGTGGGTTGG |
| Reverse | CACGGCACCTCCTAAATTGT | |
| CCL22 | Forward | CTGATGCAGGTCCCTATGGT |
| Reverse | GCAGGATTTTGAGGTCCAGA | |
| IL-6 | Forward | GAGGATACCCCCAACAGACC |
| Reverse | AAGTGCATCATCGTTGTTCATACA | |
| TNF-α | Forward | GATCTCAAAGACAACCAACTAGTG |
| Reverse | CTCCAGCTGGAAGACTCCTCCCAG | |
| IL-10 | Forward | CCAAGCCTTATCGGAAATGA |
| Reverse | TTTTCACAGGGGAGAAATCG | |
| GAPDH | Forward | AGGTCGGTGTGAACGGATTTG |
| Reverse | GGGGTCGTTGATGGCAACA |
Western blot
Proteins extracted from BV2 cells by RIPA buffer containing the protease and phosphatase inhibitors (Roche) were used for western blotting. In brief, protein samples (30 µg/lane) were separated on 12% SDS-PAGE and transferred to PVDF membrane (Millipore). Membranes were blocked with fat-free milk (5%) in TBST for 2 h at room temperature. Membranes were then respectively incubated with the primary antibodies overnight at 4°C, including iNOS (1:500, Abcam), CD206 (1:1000, Abcam), TLR4 (1:500, Abcam), NF-κB p65 (1:2000, Abcam), phospho-NF-κB p65 (p-p65, 1:2000, Abcam), IKBα (1:1000, Abcam), phospho-IKBα (p- IKBα, 1:2000, Abcam), GAPDH (1:2000, Abcam), LaminB1 (1:2000, Abcam). After washing 3 times with TBST, membranes were respectively incubated with HRP-conjugated secondary antibodies (1:4000) for 2 h at room temperature. GAPDH and LaminB1 were used as the internal control. Membranes were detected by the ECL chemiluminescence detection system (Millipore). The band intensity was measured using Image J.
Neuron-microglia co-culture
BV2 microglia and N2a cells were co-cultured in transwell-24 system (Corning) as our previous study.22 The cell density ratio is 1:10 (BV2 microglia: N2a cells). BV2 cells were seeded on removable culture inserts, and then treated with LPS (100 ng/mL) + IFN-γ (20 ng/mL), together with vehicle or selected dose of candesartan for 24 h. Remove the medium and wash microglia with fresh medium for 3 times. N2a cells growing in 24-well plate were subjected to oxygen glucose deprivation (OGD) for 3 h. Microglia-neuron co-cultures were executed by placing microglia inserts on the top of no-OGD or post-OGD N2a cells. After 24 h co-culture, microglia inserts were removed. Neuronal survival was measured by MTT assay (Roche). Neuronal cytotoxicity was detected by LDH assay (Beyotime). MTT and LDH assay were carried out according to the manufacturer’s protocol.
Statistical analysis
The results are expressed as the means ± SEM. Statistical significance among multiple experimental groups was determined by one-way ANOVA of variance with prism software version 5.0 (GraphPad Software, San Diego, CA, USA). P values <0.05 were considered statistically significant.
Results
Effects of candesartan on cellular viability and cytotoxicity in BV2 cells
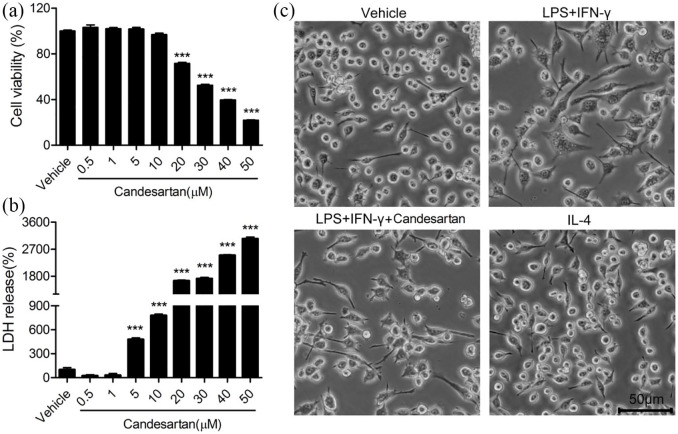
To assess the effects of candesartan on cellular viability and LDH release in BV2 cells, the cells were cultured with vehicle or different concentration of candesartan (0.5, 1, 5, 10, 20, 30, 40, and 50 µM) for 24 h. Compared to vehicle group, the cell viability was markedly reduced by 20, 30, 40, and 50 µM candesartan (Figure 1a). However, 0.5, 1, 5, and 10 µM candesartan did not significantly affect the cell viability. In LDH assay, no cytotoxic effects were observed following culture of BV2 with 0.5, 1 µM candesartan for 24 h. But candesartan (5, 10, 20, 30, 40, and 50 µM) resulted in the significant release of LDH in BV2 cells (Figure 1b). According to two experimental results, the 1 µM concentration was selected for further experiments. As shown in Figure 1c, BV2 microglia were subjected to LPS (100 ng/mL) + IFN-γ (20 ng/mL), or IL-4 (20 ng/mL), and then treated with vehicle or candesartan (1 µM) for 24 h. Compared with the vehicle, LPS + IFN-γ induced microglia polarization toward M1 phenotype, which showed cell swelling, irregular boundary, and less cell number. But candesartan treatment improved morphology and numbers, including smaller cell body and more clear boundaries. IL-4-treated microglia, M2 phenotype, represented as the similar morphology and cell number with the vehicle group.
Figure 1.
Effects of candesartan on cell viability and LDH release of BV2 microglial cells. BV2 cells were treated with vehicle or candesartan (0.5, 1, 5, 10, 20, 30, 40, 50 μM) for 24 h. (a) The cell viability was revealed by CCK-8 assay. (b) The cytotoxicity of candesartan on BV2 cells was detected by LDH assay. (c) Images of BV2 cells were taken after treatment with LPS (100 ng/mL) + IFN-γ (20 ng/mL), or IL-4 (20 ng/mL), together with vehicle, candesartan (1 μM) for 24 h.
Scale bars represent 50 μm (lower). Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate.
***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
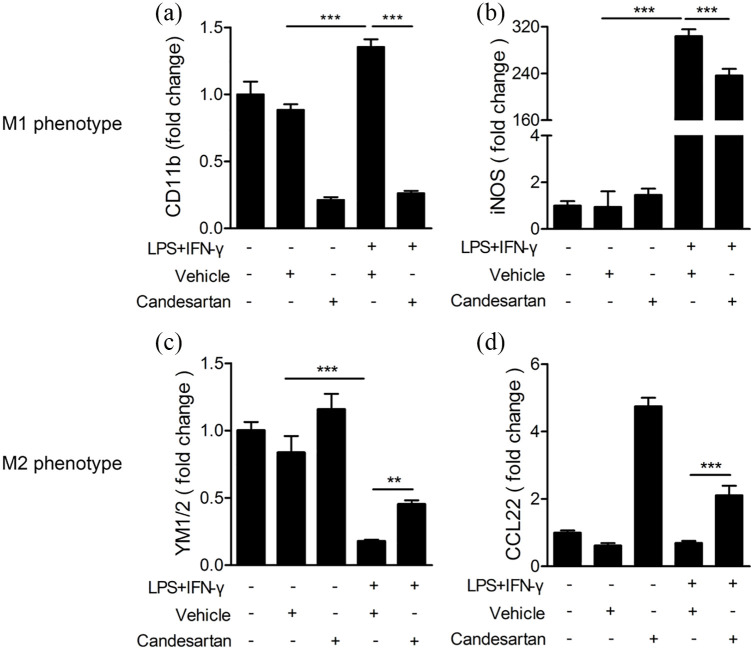
Candesartan treatment inhibits the mRNA level of M1 markers and enhances M2 markers
To determine the effect of candesartan on microglia polarization, we measured the mRNA expression of microglia phenotype markers in BV2 cells using RT-PCR at 24 h after LPS + IFN-γ stimulation. The mRNA expression of M1 markers (CD11b and iNOS) were markedly increased under the stimulation of LPS + IFN-γ, but significantly reduced after treatment with candesartan (1 μM) (Figure 2a and b). However, the mRNA expression of M2 marker YM1/2 was obviously reduced by LPS + IFN-γ. The candesartan treatment reversed the reduction in the mRNA level of YM1/2 and CCL22 in LPS + IFN-γ induced BV2 cells (Figure 2c and d). The results reveal that candesartan modulates microglia polarization toward M2 phenotype.
Figure 2.
Candesartan suppresses the mRNA expression of M1 markers and promotes M2 markers in LPS + IFN-γ stimulated BV2 microglia cells. BV2 cells were stimulated with LPS (100 ng/mL) + IFN-γ (20 ng/mL), and then treated with vehicle or candesartan (1 μM) for 24 h. The mRNA expression for M1 markers CD11b (a) and iNOS (b), and M2 markers YM1/2 (c) and CCL22 (d) were examined by RT-PCR.
Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate.
**P < 0.01, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
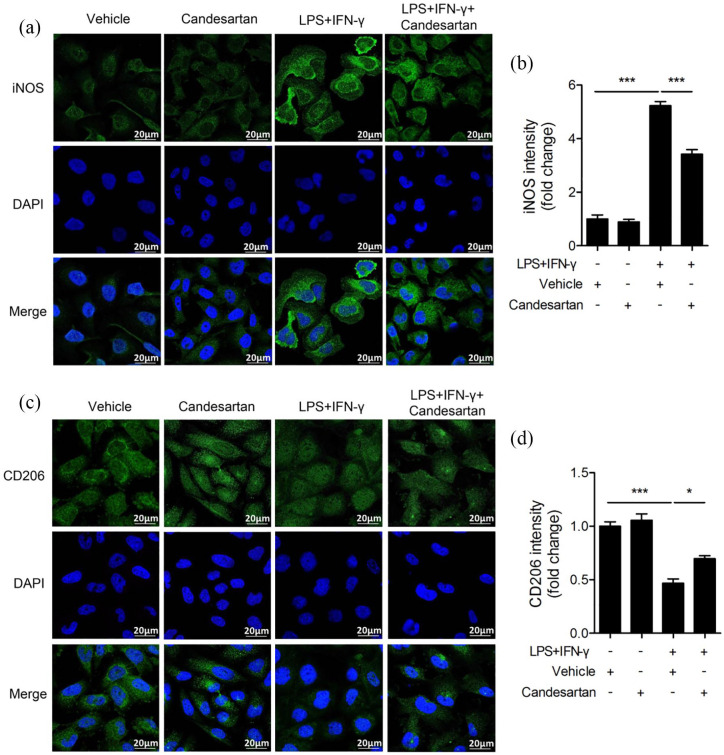
Candesartan treatment inhibits the protein level of M1 marker and enhances M2 marker
To further investigate the effect of candesartan on microglia polarization, we measured the protein expression of microglia phenotypic markers in LPS + IFN-γ stimulated BV2 cells using immunofluorescence staining and western blot at 24 h. As shown in Figure 3, LPS + IFN-γ markedly increased the fluorescence intensity of iNOS (M1 marker), and significantly decreased CD206 (M2 marker). However, candesartan treatment greatly inhibited the fluorescence intensity of iNOS while significantly enhanced CD206 in LPS + IFN-γ treated BV2 cells.
Figure 3.
Candesartan treatment reduces fluorescence intensity of M1 marker and enhances M2 marker in BV2 microglia cells. BV2 cells were stimulated with LPS (100 ng/mL) + IFN-γ (20 ng/mL), and then treated with vehicle or candesartan (1 μM) for 24 h. Representative fluorescence images show M1 marker iNOS (green, a) and M2 marker CD206 (green, c) staining. The nuclei were stained with DAPI (blue) solution. Scale bars = 20 μm. Quantification of the fluorescence intensity of iNOS (b) and CD206 (d) in BV2 cells.
Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate.
*P < 0.05, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
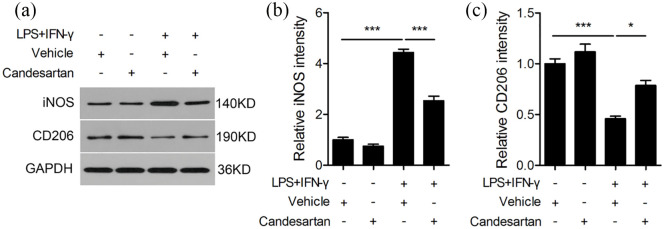
Western blot results showed that the protein level of iNOS was significantly higher, and CD206 was greatly lower in LPS + IFN-γ treated BV2 cells compared to the vehicle group. But candesartan treatment remarkably inhibited the protein level of iNOS and enhanced CD206 (Figure 4). Here, these results demonstrate that candesartan promotes M2 polarization and inhibits M1 polarization, indicating that candesartan shifts microglia from M1 to M2 phenotype.
Figure 4.
Candesartan treatment decreases the protein expression of M1 marker and increases M2 marker in BV2 cells. BV2 microglia cells were stimulated with LPS (100 ng/mL) + IFN-γ (20 ng/mL), and then treated with vehicle or candesartan (1 μM) for 24 h. Representative Western blot (a) and quantitative analysis of M1 marker iNOS (b) and M2 marker CD206 (c). GAPDH was used as a loading control.
Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate.
*P < 0.05, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
Candesartan alleviates the pro-inflammatory responses in LPS + IFN-γ stimulated BV2 cells
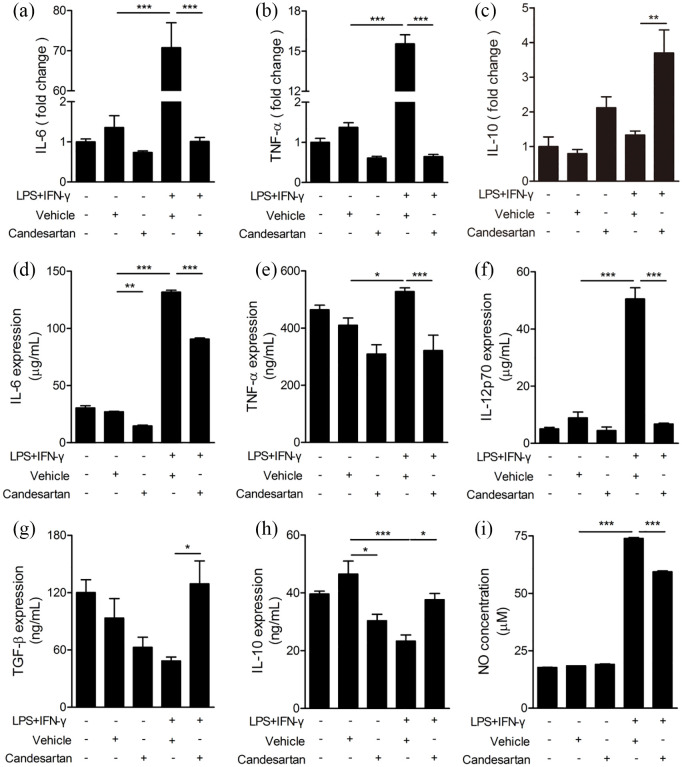
To further determine the effects of candesartan on inflammatory responses, the level of inflammatory factor was detected by RT-PCR and ELISA. RT-PCR results showed that LPS + IFN-γ treatment significantly up-regulated the mRNA level of pro-inflammatory cytokine (IL-6, TNF-α) in BV2 cells compared to the vehicle group. Candesartan significantly reduced the mRNA expression of IL-6 and TNF-α), and increased anti-inflammatory factor IL-10 in LPS + IFN-γ stimulated BV2 cells (Figure 5a–c). Next, the release of inflammatory cytokines in culture supernatant after inflammatory stimulation was measured by ELISA. LPS + IFN-γ significantly enhanced the release of pro-inflammatory (IL-6, TNF-α, IL-12p70) and suppressed anti-inflammatory (IL-10). Candesartan markedly inhibited the release of pro-inflammatory cytokines (IL-6, TNF-α, IL-12p70) (Figure 5d–f), and promoted anti-inflammatory factors (TGF-β, IL-10) in LPS + IFN-γ induced BV2 cells (Figure 5g and h). In addition, LPS + IFN-γ markedly up-regulated the level of NO in culture supernatant compared to the vehicle group. But candesartan obviously down-regulated the NO level in LPS+IFN-γ treated BV2 cells (Figure 5i). Taken together, the data show that candesartan inhibits pro-inflammatory responses after inflammatory stimulation.
Figure 5.
Candesartan inhibits pro-inflammatory cytokines and enhances anti-inflammatory cytokines in LPS + IFN-γ activated BV2 microglial cells. BV2 cells were cultured with LPS (100 ng/mL) + IFN-γ (20 ng/mL), together with vehicle or candesartan (1 μM) for 24 h. (a–c) The mRNA expressions for pro-inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokine (IL-10) were examined by RT-PCR. (d–h) The concentration of pro-inflammatory cytokines (IL-6, TNF-α, and IL-12p70) and anti-inflammatory cytokines (TGF-β and IL-10) were examined by ELISA assay. (i) NO assay.
Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate.
*P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
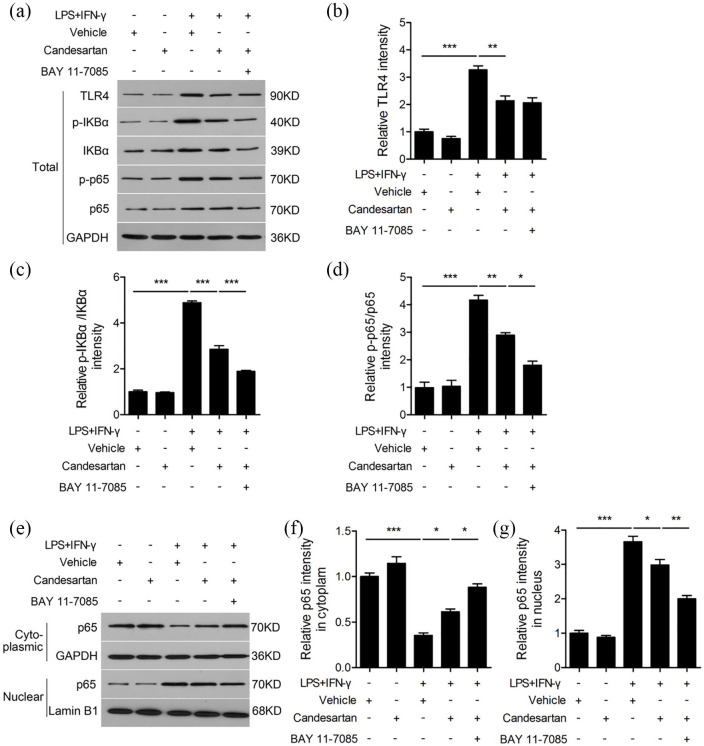
Candesartan regulates microglia polarization toward M2 phenotype through TLR4/NF-кB pathway
TLR4/NF-кB pathway participates in regulation of microglia phenotype polarization.16 In order to reveal the underlying mechanism of candesartan on modulation of microglia polarization, we examined the protein level of TLR4, p-IKBα, IKBα, p-p65, p65 in BV2 cells (Figure 6a). LPS + IFN-γ increased the protein level of TLR4, the ratio of p-IKBα/IKBα and p-p65/p65 compared to vehicle, whereas candesartan dramatically reduced the expression of TLR4, the ratio of p-IKBα/IKBα and p-p65/p65 (Figure 6b–d) in LPS + IFN-γ stimulated BV2 cells. To further clarify the underlying role of NF-кB pathway, BAY 11-7085 was used to inhibit activation of NF-кB. We found that the decrease of p-IKBα/IKBα and p-p65/p65 ratio were further aggravated when BAY 11-7085 was added to LPS + IFN-γ stimulated BV2 cells in the presence of candesartan (Figure 6c and d).
Figure 6.
Candesartan shifts microglia polarization via the inhibition of TLR4/NF-κB signaling pathway. BV2 was treated for 30 min with an NF-κB inhibitor (BAY 11-7085, 1 μg/mL), followed by stimulation with LPS (100 ng/mL) + IFN-γ (20 ng/mL) in the presence of vehicle or candesartan (1 μM) for 24 h. Representative western blot (a) and quantification analysis of TLR4 (b), p-IKBα/IKBα (c), and p-p65/p65 (d). (e–g) The protein expressions of p65 in nuclear and cytoplasm were measured by western blot, respectively.
Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate.
*P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
To determine the effect of candesartan on nuclear translocation of NF-κB, we examined the protein level of p65 in nuclear and cytoplasm (Figure 6e). LPS + IFN-γ decreased p65 protein level in cytoplasm, but increased in nucleus. Candesartan markedly reversed the nuclear translocation of p65 through increasing the protein level of p65 in cytoplasm and decreasing p65 in nucleus after LPS + IFN-γ stimulation. What’s more, the nuclear translocation of p65 was obviously decreased when BAY 11-7085 was added to LPS + IFN-γ-stimulated BV2 cells in the presence of candesartan (Figure 6f and g). These results indicate that candesartan shifts microglia phenotype polarization at least partially in dependent on TLR4/NF-кB pathway.
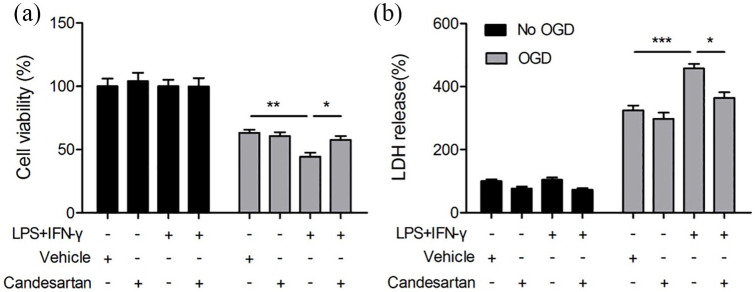
Candesartan reduces the neurotoxic effect of M1 microglia on post-OGD neurons
We have demonstrated that candesartan promoted microgli toward M2 polarization and enhanced the expression of anti-inflammatory cytokine in BV2 cells at 24 h after LPS + IFN-γ (Figures 2–5). To further confirm whether candesartan may protect against neuronal death through the regulation of microglial polarization, the neuron-microglia co-culture system was performed as we showed previously.22 LPS + IFN-γ could stimulate microglia toward M1 polarization. When M1 microglia co-cultured with post-OGD neurons, they significantly exacerbated neuronal death and enhanced LDH release. However, candesartan increased neuronal viability and inhibited LDH release in neuron-microglia co-culture system (Figure 7). These findings suggest that candesartan inhibits the neurotoxic effect of M1 microglia to OGD neurons via suppressing M1 but enhancing M2 microglial polarization.
Figure 7.
Candesartan reduces the neurotoxic effect of M1 microglia in co-cultured neurons. BV2 cells in transwell were exposed to regular microglia media (resting phenotype), or LPS (100 ng/mL) + IFN-γ (20 ng/mL) (M1 phenotype) for 24 h in the absence or presence of candesartan (1 μM). Neuronal N2a cells were subjected to OGD for 3 h. BV2 cells in transwell was applied over the non-OGD or post-OGD N2a cultures for 24 h. (a) N2a viability was quantified by MTT assay. (b) Cytotoxicity was quantified by LDH release.
Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate.
*P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
Discussion
Candesartan (C24H20N6O3) is an oral angiotensin II receptor type I receptors antagonist, which may act against vasoconstriction and reduce peripheral vascular resistance.21 Angiotensin II also binds to AT2R, which is thought to be activated by candesartan as it blocks the actions of AT1R.23 The therapeutic potential of candesartan has been reported in some diseases, such as hypertension,24 renal damage,25 Alzheimer disease.26 More emerging evidence revealed that candesartan could effectively inhibit glial activation,27 reduce inflammatory response,28 and block NF-κB activity.24 However, in the present study, we showed that candesartan inhibited inflammatory response and induced the shift of microglia from pro-inflammatory M1 to anti-inflammatory M2 phenotype, which may be associated with TLR4-mediated NF-κB pathway.
Microglia are the brain-resident macrophages in the central nervous system (CNS), which are the target of inflammatory mediators in the CNS injuries and play a foremost role in the neuroinflammatory response.29 In different environmental stimuli, microglia/macrophage could differentiate into M1 or M2 phenotype. M1-polarized microglia release high level of pro-inflammatory cytokines and upregulate the expression of M1 markers. However, M2-polarized microglia enhance the level of anti-inflammatory factors and up-regulate M2 markers.30 According to the plasticity of microglia/macrophages, the balance of between M1 and M2 microglia polarization is a key player in neuroinflammatory response in CNS injuries.2 Candesartan not only blocks the activation of AT1R, but also promotes angiotensin II-mediated AT2R stimulation.12 Telmisartan, an antagonist blocking AT1R, was shown to prevent the LPS-induced microglia activation by promoting M2 polarization via CaMKKbeta-dependent AMPK activation.31 In this study, our results showed that candesartan can shift microglia polarization from M1 to M2 phenotype. AT1R blockade by candesartan inhibits glial activation and neuroinflammation response induced by LPS.27 Consistent with the previous report, candesartan inhibited the release of pro-inflammatory cytokine (IL-6, TNF-α, and IL-12p70), promoted the level of anti-inflammatory cytokine (IL-10, TGF-β) in LPS + IFN-γ induced BV2 cells. The data suggest that candesartan treatment inhibits inflammation response via regulating the balance of M1/M2 microglia polarization.
Our studies demonstrated that candesartan regulated microglia polarization, which was accompanied by the regulation of inflammatory response. But the mechanisms whereby candesartan promotes microglial polarization to M2 subtype are not yet clear. Several transcriptional regulators have been identified to participate in regulating microglia polarization toward M1 phenotype such as activator protein-1 family (AP-1), NF-κB family, STAT1.1 TLR4/NF-κB signaling pathway is one of the most widely recognized intracellular signaling pathways in inflammatory responses, which is demonstrated to participate in shifting microglial polarization toward M1.16 Curcumin was reported to alleviate neuroinflammation responses through promoting microglia phenotype shift toward M2, which may be associated with the suppression of the TLR4-mediated NF-κB signaling pathway.32 Pinocembrin reduced hemorrhagic brain injury and suppressed M1 phenotype microglia through inhibiting TLR4.33 In agreement with the above observation, candesartan markedly inhibited the protein expression of TLR4 and the phosphorylation of IKBα and p65 in LPS + IFN-γ treated BV-2 cells. Besides, candesartan markedly reversed the nuclear translocation of p65 in LPS + IFN-γ stimulated BV2 cells. The inhibitory effect of candesartan on NF-кB pathway was further aggravated by the NF-кB inhibitor, BAY 11-7085. These results further illustrate that candesartan regulates microglia polarization at least partially through TLR4-mediated NF-κB pathways. Meisoindigo protected against focal cerebral ischemia-reperfusion injury by regulating microglia/macrophage polarization via NF-κB signaling pathway.34 Candesartan was reported to reduce infarct volume and improve behavior after stroke via reducing of the TLR signaling cascade.35 In our co-culture system, candesartan ameliorated the neurotoxic effect of M1 microglia to OGD neurons via regulating microglia polarization toward M2 phenotype. The data indicate that candesartan may alleviate stoke-induced neuronal damage through suppressing inflammatory response and modulating microglia polarization through inhibiting TLR4/NF-κB signaling pathway. However, both the in vivo and primary cell experiments are still required to confirm the underlying mechanism.
Conclusion
This present study demonstrates that candesartan inhibited LPS + IFN-γ induced pro-inflammatory response and increased anti-inflammatory response. Candesartan has a significant regulatory effect on the balance of microglia polarization, shifting microglia to M2 phenotype and inhibiting the polarization of M1 microglia phenotype. The inhibitory effects of candesartan on the neurotoxic effect of M1 microglia to OGD neurons may be associated with modulating inflammatory response and microglia polarization in a TLR4-mediated NF-κB dependent manner. Our findings suggest that candesartan can indirectly reduce neuronal damage via modulating microglial polarization, providing new evidence that candesartan might be a promising therapeutic strategy for stoke.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Natural Science Foundation of China (81671161) to Z Liu.
ORCID iD: Zongjian Liu  https://orcid.org/0000-0003-4685-1057
https://orcid.org/0000-0003-4685-1057
References
- 1. Holtman IR, Skola D, Glass CK. (2017) Transcriptional control of microglia phenotypes in health and disease. The Journal of Clinical Investigation 127(9): 3220–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hu XM, Leak RK, Shi Y, et al. (2015) Microglial and macrophage polarization—new prospects for brain repair. Nature Reviews. Neurology 11(1): 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keren-Shaul H, Spinrad A, Weiner A, et al. (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169(7): 1276–1290. [DOI] [PubMed] [Google Scholar]
- 4. Hu X, Liou AKF, Leak RK, et al. (2014) Neurobiology of microglial action in CNS injuries: Receptor-mediated signaling mechanisms and functional roles. Progress in Neurobiology 119–120: 60–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erny D, Prinz M. (2020) How microbiota shape microglial phenotypes and epigenetics. Glia 68(8): 1655–1672. [DOI] [PubMed] [Google Scholar]
- 6. Masuda T, Sankowski R, Staszewski O, et al. (2020) Microglia heterogeneity in the single-cell era. Cell Reports 30(5): 1271–1281. [DOI] [PubMed] [Google Scholar]
- 7. Orihuela R, McPherson CA, Harry GJ. (2016) Microglial M1/M2 polarization and metabolic states. British Journal of Pharmacology 173(4): 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Subramaniam SR, Federoff HJ. (2017) Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Frontiers in Aging Neuroscience 9: 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang F, Zhong R, Li S, et al. (2017) Acute hypoxia induced an imbalanced M1/M2 activation of microglia through NF-κB signaling in Alzheimer’s disease mice and wild-type littermates. Frontiers in Aging Neuroscience 9: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seltzer A, Bregonzio C, Armando I, et al. (2004) Oral administration of an AT1 receptor antagonist prevents the central effects of angiotensin II in spontaneously hypertensive rats. Brain Research 1028(1): 9–18. [DOI] [PubMed] [Google Scholar]
- 11. Ohshima K, Mogi M, Horiuchi M. (2013) Therapeutic approach for neuronal disease by regulating renin-angiotensin system. Current Hypertension Reviews 9(2): 99–107. [DOI] [PubMed] [Google Scholar]
- 12. Villapol S, Saavedra JM. (2015) Neuroprotective effects of angiotensin receptor blockers. American Journal of Hypertension 28(3): 289–299. [DOI] [PubMed] [Google Scholar]
- 13. Gohlke P, Jürgensen T, von Kügelgen S, et al. (1999) Candesartan cilexetil: development and preclinical studies. Drugs of Today 35(2): 105–115. [DOI] [PubMed] [Google Scholar]
- 14. Lund LH, Claggett B, Liu J, et al. (2018) Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. European Journal of Heart Failure 20(8): 1230–1239. [DOI] [PubMed] [Google Scholar]
- 15. Fu H, Hosomi N, Pelisch N, et al. (2011) Therapeutic effects of postischemic treatment with hypotensive doses of an angiotensin II receptor blocker on transient focal cerebral ischemia. Journal of Hypertension 29(11): 2210–2219. [DOI] [PubMed] [Google Scholar]
- 16. Saijo K, Glass CK. (2011) Microglial cell origin and phenotypes in health and disease. Nature Reviews. Immunology 11(11): 775–787. [DOI] [PubMed] [Google Scholar]
- 17. Benicky J, Sánchez-Lemus E, Pavel J, et al. (2009) Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cellular and Molecular Neurobiology 29(6–7): 781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao L-Q, Huang J-L, Yu Y, et al. (2013) Candesartan inhibits LPS-induced expression increase of toll-like receptor 4 and downstream inflammatory factors likely via angiotensin II type 1 receptor independent pathway in human renal tubular epithelial cells. Sheng Li Xue Bao : [Acta physiologica Sinica] 65(6): 623–630. [PubMed] [Google Scholar]
- 19. Liu Z, Ran Y, Huang S, et al. (2017) Curcumin protects against ischemic stroke by titrating microglia/macrophage polarization. Frontiers in Aging Neuroscience 9: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang G, Shi Y, Jiang X, et al. (2015) HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proceedings of the National Academy of Sciences of the United States of America 112(9): 2853–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsutamoto T, Nishiyama K, Yamaji M, et al. (2010) Comparison of the long‑term effects of candesartan and olmesartan on plasma angiotensin II and left ventricular mass index in patients with hypertension. Hypertension Research: Official Journal of the Japanese Society of Hypertension 33(2): 118–122. [DOI] [PubMed] [Google Scholar]
- 22. Liu Z-J, Ran Y-Y, Qie S-Y, et al. (2019) Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neuroscience & Therapeutics 25(12): 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bessaguet F, Danigo A, Magy L, et al. (2017) Candesartan prevents resiniferatoxin-induced sensory small-fiber neuropathy in mice by promoting angiotensin ii-mediated AT2 receptor stimulation. Neuropharmacology 126: 142–150. [DOI] [PubMed] [Google Scholar]
- 24. Zhao X, Wang X. (2018) Candesartan targeting of angiotensin II type 1 receptor demonstrates benefits for hypertension in pregnancy via the NF-κB signaling pathway. Molecular Medicine Reports 18(1): 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmed HI, Mohamed EA. (2019) Candesartan and epigallocatechin-3-gallate ameliorate gentamicin-induced renal damage in rats through p38-MAPK and NF-κB pathways. Journal of Biochemical and Molecular Toxicology 33(3): e22254. [DOI] [PubMed] [Google Scholar]
- 26. Trigiani LJ, Royea J, Lacalle-Aurioles M, et al. (2018) Pleiotropic benefits of the angiotensin receptor blocker candesartan in a mouse model of Alzheimer disease. Hypertension 72(5): 1217–1226. [DOI] [PubMed] [Google Scholar]
- 27. Bhat SA, Goel R, Shukla R, et al. (2016) Angiotensin receptor blockade modulates NFκB and STAT3 signaling and inhibits glial activation and neuroinflammation better than angiotensin-converting enzyme inhibition. Molecular Neurobiology 53(10): 6950–6967. [DOI] [PubMed] [Google Scholar]
- 28. Larrayoz IM, Pang T, Benicky J, et al. (2009) Candesartan reduces the innate immune response to lipopolysaccharide in human monocytes. Journal of Hypertension 27(12): 2365–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Skaper SD, Giusti P, Facci L. (2012) Microglia and mast cells: Two tracks on the road to neuroinflammation. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 26(8): 3103–3117. [DOI] [PubMed] [Google Scholar]
- 30. Sica A, Mantovani A. (2012) Macrophage plasticity and polarization: in vivo veritas. The Journal of Clinical Investigation 122(3): 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu Y, Xu Y, Wang Y, et al. (2015) Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKβ-dependent AMPK activation. Brain, Behavior, and Immunity 50: 298–313. [DOI] [PubMed] [Google Scholar]
- 32. Gao YY, Zhuang Z, Lu Y, et al. (2019) Curcumin mitigates neuro-inflammation by modulating microglia polarization through inhibiting TLR4 axis signaling pathway following experimental subarachnoid hemorrhage. Frontiers in Neuroscience 13: 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lan X, Han X, Li Q, et al. (2017) Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia. Brain, Behavior, and Immunity 61:326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ye Y, Jin T, Zhang X, et al. (2019) Meisoindigo protects against focal cerebral ischemia-reperfusion injury by inhibiting NLRP3 inflammasome activation and regulating microglia/macrophage polarization via TLR4/NF-κB signaling pathway. Frontiers in Cellular Neuroscience 13: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barakat W, Safwet N, El-Maraghy NN, et al. (2019) Candesartan and glycyrrhizin ameliorate ischemic brain damage through downregulation of the TLR signaling cascade. European Journal of Pharmacology 724: 43–50. [DOI] [PubMed] [Google Scholar]