Abstract
Background:
Direct injection of corticosteroids into the joint is a standard treatment for knee osteoarthritis (OA). However, the treatment is somewhat controversial with regard to the benefit of both single and repeated injections; evidence that they are beneficial comes from small studies that show only modest improvements. The aim of this study was to estimate the short- and long-term clinical efficacy and safety of hylan G-F 20 versus intra-articular corticosteroids (IACS) for the treatment of pain in knee OA using Bayesian network meta-analysis.
Methods:
Based on a pre-specified protocol, MEDLINE, Embase, and CENTRAL were searched from inception to June 2018 to identify randomized controlled trials. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials was used to assess the included studies. Hylan G-F 20 and IACS were compared using Bayesian network meta-analysis. Efficacy was evaluated at 1, 3, and 6 months, and at the final follow-up for safety outcomes. A pain hierarchy was used to select 1 pain outcome per study.
Results:
Forty-two trials were included for analysis. The network meta-analysis of pain showed that hylan G-F 20 may be equivalent to IACS in the short-term, but by 6 months the benefit relative to IACS was statistically significant, standardized mean difference (95% credible interval): –0.13 (–0.26, –0.01). There were no statistical differences in adverse events.
Conclusions:
Hylan G-F 20 may perform better in relieving pain at 6 months post-injection compared to IACS. Both agents were relatively well tolerated, with no clear differences in safety.
Keywords: Osteoarthritis, knee, hyaluronic acid, injections, intra-articular, molecular weight, network meta-analysis
Background
Osteoarthritis (OA) is a leading cause of disability and the most common form of arthritis affecting around 250 million people worldwide,1 and more than 27 million people in the United States.2 Multiple factors play a role in development of OA, with elderly females, people with obesity, and African Americans being at greater risk of developing OA.3 Treatment for people with symptomatic OA of the knee starts with participation in self-management programs, neuromuscular education, and engagement in physical activity such as strengthening or low-impact aerobic exercises.4 In addition, pharmacological treatments are conditionally recommended in people who fail to obtain adequate pain relief with over-the-counter acetaminophen, non-steroidal anti-inflammatory drugs, or nutritional supplements.5
In the absence of effective disease-modifying medical interventions for knee OA, treatments are primarily symptomatic in nature, often including intra-articular (IA) injections of a corticosteroid (CS) for pain relief.6 IACS is a standard treatment for knee OA; however, clinical evidence for the effectiveness of this intervention is not robust.7-9 IACS injections have been linked with cartilage loss,7 radiographic worsening,8 and only short-term pain relief.9 In a systematic review of published literature on IACS injections (27 studies with 1767 participants), the quality of the evidence across outcomes was graded low due to inconsistent treatment effect estimates, great variation across trials, imprecise pooled estimates, and high or unclear risk of bias across most included trials.10 For these reasons, in the current study we took particular interest in change in efficacy estimates relative to time from injection.
Hylan G-F 20 is an HA preparation consisting of hylan A, a 6000 kDa HA, and hylan B, a cross-linked derivative of natural HA.11 There are 2 hylan G-F 20 formulations: a single-shot (wherein 6 mL is administered) and the once weekly × 3 approach (wherein 2 mL is administered across multiple injections).12,13 An early Cochrane review found that hylan G-F 20 significantly improved pain and movement relative to placebo, significantly improved pain but not function relative to non-steroidal anti-inflammatory drugs, and significantly improved pain as well as function when added to standard of care.14 Despite mixed results from head-to-head trials comparing different HA formulations,15-17 many of the more recent meta-analyses have taken a broader focus by combining multiple HA formulations, and subsequently found lower efficacy estimates18 and higher rates of adverse events.19 Relative to agents that are low molecular weight and non-crosslinked, high molecular weight crosslinked agents are more effective.20 Hylan G-F 20 is both crosslinked and has a high molecular weight, suggesting it may be more efficacious than other types of IAHA injections. For this reason, it is important to compare the efficacy of hylan G-F 20 to IACS injections in the treatment of knee OA, instead of comparing all IAHA agents as a group to IACS injections. Consequently, one reason for the imprecision and variation in findings from prior studies may be that they have not distinguished the intrinsic properties of HA injections, but have included all types of HA injections regardless of molecular weight or whether they are crosslinked.21
Consistent with these discrepancies in the published reviews, recommendations from multiple international guideline committees22-25 regarding the widespread use of HA to treat pain in knee OA are sometimes discordant.26 A return to more focused meta-analyses will likely benefit this field. Therefore, the objective of this study was to evaluate the clinical efficacy and safety of hylan G-F 20 and IACS in people living with knee OA, 6 months post-treatment. A systematic literature review was conducted to identify relevant published literature, followed by a Bayesian network meta-analysis (NMA).
Methods
Eligibility criteria
Standard methods for conducting systematic reviews as per guidelines provided by the Cochrane Handbook for Systematic Reviews of Interventions were followed.27 Eligibility criteria were developed using the Population, Intervention, Comparator, and Outcome (PICO) framework. RCTs evaluating efficacy and safety of treatments for adults with knee OA who were treated with IACS and HA were included.
Search methods
Relevant studies were identified by conducting searches in the following databases, from inception until 12 June 2018: MEDLINE (via PubMed), Embase (via Ovid), and the Cochrane Central Register of Controlled Trials (via Wiley) (Supplemental Tables 1–3). As well, conference abstracts for the years 2016 to 2018 from the American College of Rheumatology (ACR), European League Against Rheumatism (EULAR), The Asian Pacific League of Associations for Rheumatology (APLAR), and Osteo-Arthritis Research Society International (OARSI) were searched. Hand-searching was also performed on the reference lists of previously published systematic literature reviews on the same topic and eligible articles screened through main database search to capture additional eligible studies that were missed during the main database search.
Study selection
Two investigators reviewed all abstracts identified in the systematic literature review. PICO criteria were applied, and abstracts deemed eligible for inclusion were advanced to full-text screening. Full-text articles were screened by 2 investigators. Articles deemed eligible after full-text screening were included in the systematic literature review. At each stage of the screening process, disagreements due to differences in interpretation between investigators were resolved by a third investigator in order to reach a consensus.
Data extraction
Data was extracted independently by 2 investigators, and if disagreements due to differences in interpretation could not be resolved, a third investigator was consulted to reach consensus. The Digital Outcome Conversion (DOC) Data version 2.0 software platform (Doctor Evidence, LLC, Santa Monica, CA, USA) was used to store and manage data. Extraction included trial characteristics, interventions, participant characteristics, as well as efficacy and safety outcomes. Characteristics of interest were age, gender, race/ethnicity, body mass index (BMI), and whether ACR criteria were used for the diagnosis of knee OA.
Pain scores at 1, 2, 3, and 6 months were the primary efficacy outcomes of interest for this review and analysis. A pain hierarchy was used to select 1 pain outcome from each study.31 Safety outcomes included overall adverse events, treatment-related adverse events, and serious adverse events.
Cochrane risk of bias tool
The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials was used to assess the randomized trials.30 This instrument is used to evaluate 7 domains of bias: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other sources of bias.
Statistical analysis
The primary outcome of interest was pain measured using a pain hierarchy.28 Adverse events (overall, serious, treatment related) were also analyzed. Preliminary pairwise meta-analyses were performed using the DerSimonian-Laird method.29 This was done to assess heterogeneity between studies and assess inconsistency across studies for potential exclusion from the network. For continuous variables, only change from baseline scores were analyzed using a random-effects model. If different studies reported different units for the same outcome, a standardized mean difference (SMD) was calculated. Comparative estimates for the continuous outcomes measured on the same scale were represented as mean difference (MD) with associated 95% confidence intervals (CI). We used these models to assess statistical heterogeneity by inspecting the forest plots, and the calculated I2 using the R software package “metafor.”30 Estimates based on direct and indirect evidence were compared to assess potential inconsistency.
The primary NMA was conducted using standard practice models described by the National Institute for Health and Care Excellence Decision Support Unit, Technical Support Documents (NICE DSU TSD) series.31 All analyses were performed in a Bayesian framework and involved a model with parameters, data, a likelihood distribution, and prior distributions. The NMA was performed for efficacy outcomes at 1, 3, and 6 (± 0.5) months; and the final follow-up time point for safety outcomes. The following characteristics were included in the baseline heterogeneity analysis: age, gender, BMI, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain, VAS pain, WOMAC overall score. Inconsistency between direct and indirect evidence is more prevalent in the medical literature than previously supposed,32 and violates a basic assumption of NMA.33 Consequently, consistency between direct and indirect evidence was evaluated for each path in our model.
For the NMA of continuous data, SMD and credible intervals (CrI) were used because the pain scales across the included studies were different. The SMD was calculated using a normal/identity link-likelihood model. For binary (eg, adverse) events, only intervention groups with at least 1 event were included to avoid divide by 0 errors for odds ratio (OR) and relative risk comparisons. This was done as an alternative to adding pseudo counts to the data. The binary data was analyzed as “OR” using the binomial/logit link-likelihood model. The R software package “gemtc” which utilizes jags was used to perform the calculations within a Bayesian framework using a random-effects model.34 The model fit was evaluated via the deviance information criterion, which supported the use of a random-effects model over a fixed-effects model. A burn in of 5000 for 2 chains and 20 000 iterations were run with a thinning parameter of 10.
Results
Literature search findings
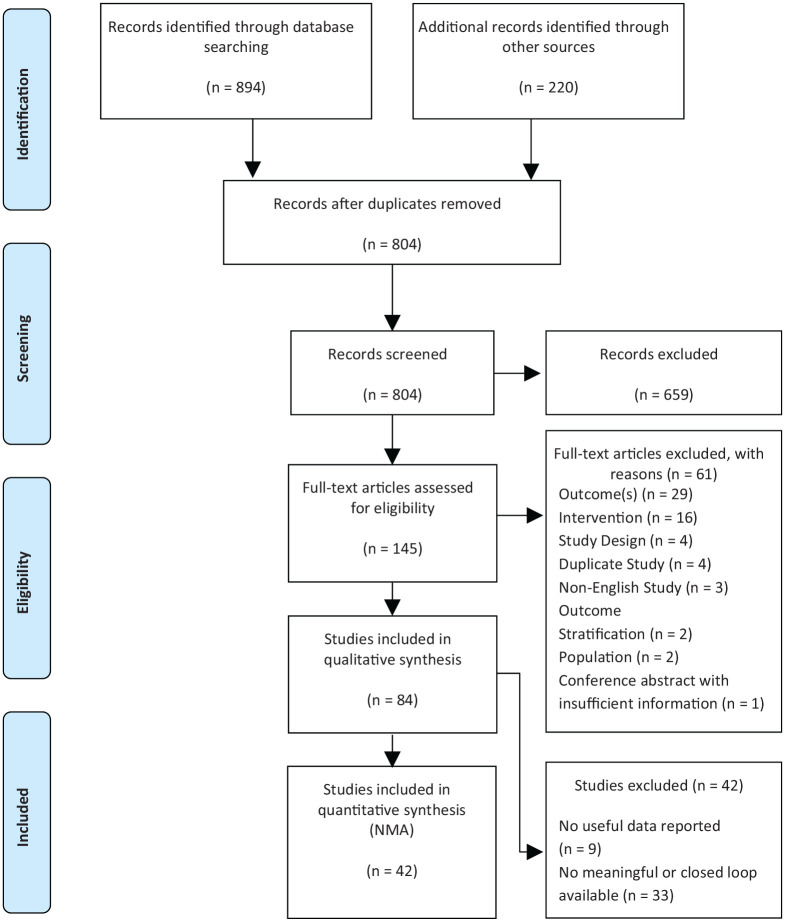
A total of 1114 publications were identified by the systematic review. After removing duplications, 804 unique publications were screened, and 659 were excluded (frequently for wrong study design, or not being an empirical study), leading to inclusion of 145 publications after title and abstract screening. After full-text screening and additional 61 studies were excluded (frequently for having the wrong intervention or lacking an outcome of interest) leaving 84 publications. Out of those 84 included publications, 42 were excluded from the statistical analysis (due to lack of actionable data). In the final analysis, 42 publications reporting on 42 distinct trials representing a total of 8047 adults were included in the NMA.7,16,35-75 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the number of studies at each stage is presented in Figure 1.
Figure 1.
PRISMA flow diagram.
Cochrane risk of bias tool
The studies tended towards low risk of bias, except for the random sequence generation and allocation concealment (selection bias) categories. Of the 41 studies, twenty had low risk of bias associated with random sequence generation (selection bias)7,36,38,41-43,45,46,48,51,55,58,60-62,64,68-71 and 21 had unclear risk due to the fact that although described as randomized, no description of the randomization procedure was presented.16,35,37,39,44,47,49,50,52,54,56,57,59,63,65-67,72-74 The second component of selection bias (allocation concealment) had 20 studies which described concealment methods and therefore had low risk7,16,36,41-43,45,46,48,49,51,54,55,58,60,61,64,65,70,71 and 21 studies with unclear risk as the concealment process was not described.35,37-40,44,47,50,52,56,57,59,62,63,66-69,72-74 In terms of performance bias, 26 studies blinded both participants and personnel and were judged to have low risk.7,16,35,36,40-43,45,46,48-51,54,55,58,60-62,65-67,69-71 Eight studies had unclear risk of performance bias due to either lack of information44,52,63,68,74 or participants but not investigators being blinded.37,47,56 Seven studies were considered at high risk of performance bias due to open-label study design.38,39,57,59,64,72,73 Studies generally had low risk of outcome assessment bias (n = 31).7,16,35,36,38-43,45-51,54,55,58,60-62,64-67,69-72 Six had an unclear risk of bias as there was no specification on the blinding methodology,44,52,63,68,73,74 and 4 studies had high risk of outcome assessment bias due to being unblinded,57 or single blinded.37,56,59 Most studies (n = 28) were assessed to have low risk of attrition bias,7,16,35-38,41-44,46,48,49,51,54-56,58,59,61,62,64,66,68-72 though 7 studies were deemed “unclear” due to unspecified follow-up sample size (n = 6),52,57,63,67,73,74 and 5 were deemed high risk due to attrition rates greater than 20%.39,40,50,65 Almost all studies (n = 39) were assessed to have low risk of reporting bias,7,16,35-37,39-52,54-61,63,64,66-74 though 2 studies had a high risk of bias due to failing to report the use of paracetamol62 or the presence of knee effusion,65 despite specifying that these would be investigated in the methods. Most studies had low risk of other sources of bias (n = 33).16,36,37,39,41,42,45,47-52,54-58,60-74 However, 8 studies were deemed to have unclear risk of additional bias7,35,38,40,43,44,46,59 (eg, baseline differences, power concerns, possible randomization failure, and different amounts of the active intervention and the saline-solution to be injected).
Network meta-analysis
The main network consisted of 4 nodes corresponding to 4 interventions: hylan G-F 20, IACS, HA other than hylan G-F 20, and placebo (administration route was intraarticular in 14 studies and oral in 1 study). Sensitivity analyses showed no significant difference between Synvisc® (hylan G-F 20, 2 ml [16 mg], 3 weekly injections) and Synvisc-One® (hylan G-F 20, 6 ml [48 mg], 1 weekly injection), so they are combined in the primary models.
Trureba et al (2015) and Wobig et al (1998) were excluded from the NMA at 6 months because the magnitude of effects (4 and 1.4, respectively) were larger than all the other studies (effect size range: –0.81 to 0.63), which led to inconsistency in the network by skewing the direct effect point estimate. Karlsson et al (2002) was excluded due to being an outlier in the heterogeneity analysis of age. Iannitti et al was excluded due to being an outlier in the heterogeneity analysis of age and gender. After removal of these outliers, the evidence was consistent with no inconsistency between direct and indirect evidence.49
Pain
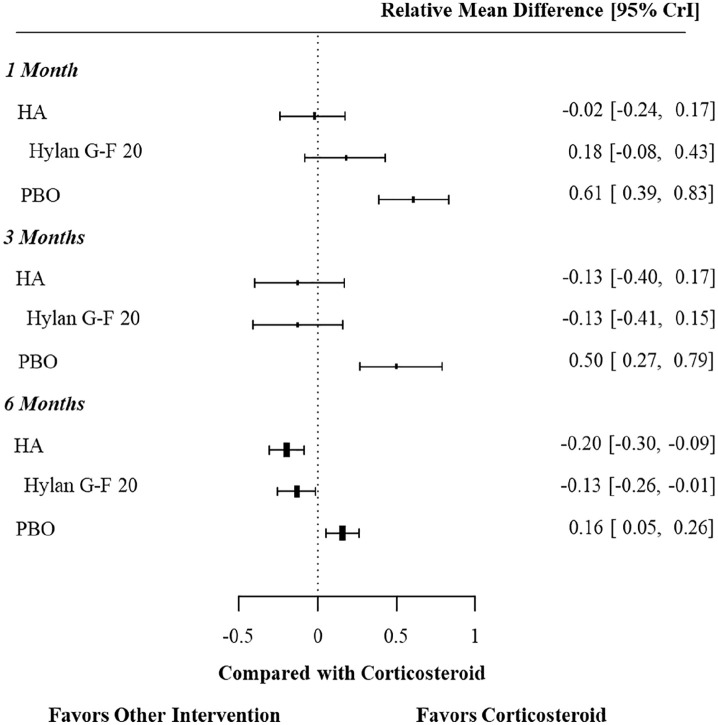
Twenty-four studies reported pain score at 1 month, followed by 21 studies at 3 months, and 21 studies at 6 months (Figure 2 illustrates this network). In analyzing the results for pain reduction at 1 month, all of the 3 treatments (hylan G-F 20, other HA formulations, IACS) were statistically superior to placebo, but not significantly different from each other (Figure 3). However, at 6 months the pain reduction effect of hylan G-F 20 was significantly greater than IACS (SMD: –0.13, 95% Crl: –0.26, –0.01), as was the pain reduction of other HA formulations (SMD: –0.20, 95% Crl: –0.30, –0.09). Hylan G-F 20 and other HA formulations were statistically indistinguishable from one another (Supplemental Table 4). The pain reduction of IACS had attenuated but remained significantly greater than placebo (SMD: –0.16, 95% Crl: –0.26, –0.05).
Figure 2.
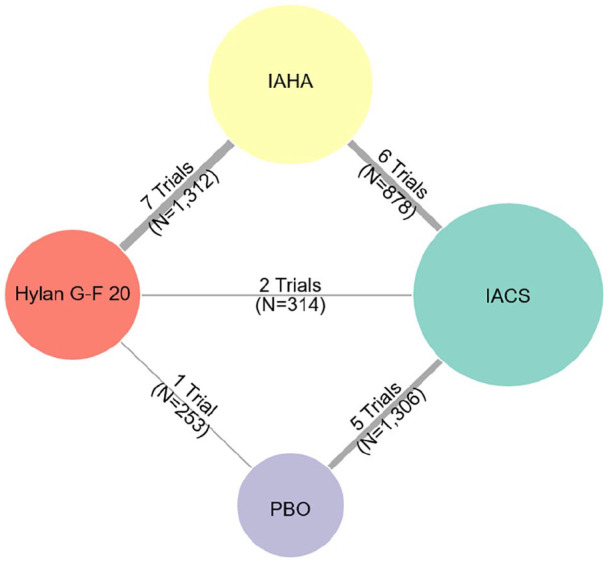
Network for change from baseline to 6 months in pain scores.
The numbers by the vertices indicate how many publications reported the respective comparison between the connected nodes (interventions). IACS, intra-articular corticosteroid; IAHA, intra-articular hyaluronic acid; PBO, placebo.
Figure 3.
Pain score, results from the network meta-analysis.
CrI, credible intervals; HA, intra-articular hyaluronic acid; PBO, placebo.
Safety outcomes
Compared to participants treated with IACS, participants treated with hylan G-F 20 did not experience significantly different odds of adverse events (OR [95% Crl]: 1.32 [0.94, 1.93]), treatment-related adverse events (OR [95% Crl]: 2.72 [0.83, 9.75]), or serious adverse events (treatment-related or unrelated to treatment) (OR [95% Crl]: 0.53 [0.05, 2.53]) (Table 1).
Table 1.
Safety results of the network meta-analysis.
| Comparison | Adverse event | Adverse event, serious | Adverse event, treatment-related |
|---|---|---|---|
| Hylan G-F 20 vs IACS OR (95% Crl) | 1.32 (0.94, 1.93) | 0.53 (0.05, 2.53) | 2.72 (0.83, 9.75) |
CrI, credible interval; IACS, intra-articular corticosteroid; OR, odds ratio.
Discussion
Using a Bayesian NMA approach, we found that a single or 3-injection course of hylan G-F 20 is likely to be superior to IACS at 6 months in terms of pain relief. This result is robust, and it is based on data from 21 published studies of generally low risk across the domains of bias in the Cochrane tool. Prior meta-analytic work has shown the SMD between IACS and placebo for the treatment of knee OA is 0.40 (calculated from a relative risk of 2.09, 95% CI: 1.20, 3.65).75 Our findings show an SMD of 0.13 between hylan G-F 20 and IACS, suggesting a 33% improvement in absolute pain score with hylan G-F 20 over IACS for the treatment of knee OA. Additionally, based on this SMD, we can calculate the number needed to treat (NNT) as 14, using methods described by Furukawa et al 2011.76 Hence, our meta-analysis suggests that when compared with IACS treatment, 14 adults would need to be treated with hylan G-F 20 in order to have 1 experience better pain relief.
Our results also show a time-dependent dynamic for the treatment effects of both IACS and HA. At early stages post-injection (1 month) IACS treatment seems to have a slight advantage. However, at 3 months the effect flips, and HA becomes more effective, with the difference reaching statistical significance at 6 months post-treatment. Our findings are similar to a previous smaller literature review and meta-analysis of 7 trials which found that IACS is more effective than HA in the short term (up to 4 weeks), whereas HA is more effective in the long term (4-26 weeks).6 This delayed effect of hylan G-F 20 has been commented on directly by authors in several of the included studies.36,39,57 It is consistent with the argument that the advantage of a high molecular weight agent, such as hylan G-F 20, may be due to a long-term increase in viscoelasticity of synovial fluid, but indirect actions of HA may be linked also to decreasing extra cellular matrix degradation and inflammation.77-79 The current findings cannot speak to the mechanism by which hylan G-F 20 and other HA formulations show improved efficacy over time, but they do demonstrate that the pattern of improvement continues through 6 months post-treatment.
The safety profiles of these interventions are similar. IACS showed, numerically, less overall adverse events and treatment-related adverse events, whereas hylan G-F 20 showed a smaller incidence of serious adverse events (treatment-related or unrelated to treatment) and injection side flare-ups. Although the overall adverse events were higher with hylan G-F 20, most of them were mild in nature. None of the safety outcome comparisons mentioned above reached statistical significance. However, there still remain safety concerns with IACS.80 A recent review highlighted 4 main adverse joint events following IACS injections including accelerated OA progression, subchondral insufficiency fracture, and complications of osteonecrosis and rapid joint destruction (including bone loss). These findings suggest careful considerations of patient characteristics and needs before administering IACS.80
A final consideration is the less obvious influence of sampling bias on the real-world overall benefit/risk ratio of these 2 treatments. Adults with uncontrolled hypertension, diabetes, immunocompromised status, and drepanocytes are more exposed to complications with IACS and are often excluded from these trials. This means that in addition to the support for the relative benefit of hylan G-F 20 quantified here, there is also an unquantifiable benefit due to these additional IACS-related adverse events which do not manifest in the trial data as those participants are often screened out.
Limitations
A primary limitation was the gap between the number of studies that officially met our PICO criteria and the number of those studies which provided sufficient data to be included in the network. Many studies did not report sufficient data to be included in the meta-analysis (eg, reporting baseline and endpoint data but not variance), and adverse events that had zero reported events in the arms of interest were not included in the analyses, which would have the effect of biasing upward the model-based estimates of adverse events. We attempted to calculate change from baseline scores (if not reported by study authors) using the baseline and endpoint scores. However, some studies did not report the associated variance and, therefore, we could not include these studies. In order to create a more robust network, this analysis used the assumption that, regardless of type, the effectiveness of all individual IAHA (other than hylan G-F 20) are the same. Similarly, all individual IACS were assumed to be the same. However, research indicates that there is some variation between individual IAHA and IACS, therefore a degree of imprecision was introduced into the network.20 This is a limitation to the analysis that was conducted in this study. We removed from the network several studies that were outliers in the preliminary analysis, which, although allowing us to satisfy the consistency assumption necessary for NMA, also had the effect of omitting data.
Conclusion
Overall, the results of the NMA suggest that in people with knee OA, hylan G-F 20 is similar to IACS in improving symptoms in the short term but likely to be better in relieving pain at 6 months post-injection. This effect was also observed in other HA formulations. Both therapies were relatively well tolerated with no clear differences in safety.
Supplemental Material
Supplemental material, sj-pdf-1-amd-10.1177_1179544120967370 for Efficacy and Safety of Hylan G-F 20 Versus Intra-Articular Corticosteroids in People with Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis by Xavier Chevalier, Brendan Sheehan, Craig Whittington, Mir-Masoud Pourrahmat, Lionel Duarte, Wilson Ngai and Gustavo Constantino de Campos in Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders
Acknowledgments
We thank Thomas Schofield from Evidinno Outcomes Research Inc. for editorial support in preparation of this manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Sanofi. The study sponsor was involved in the conception and design of the study, revision of the article for content, and final approval of the article.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: XC reports speaker/honoraria for Sanofi and IBSA Institut Biochimique, and consulting for Pfizer, IBSA Institut Biochimique, and LAB Pharma. BS reports speaker/honoraria and consulting for Sanofi. CW is currently employed by Sanofi, and is a former employee of Doctor Evidence, LLC. MP is currently employed by Evidinno Outcomes Research Inc., which reports contract by Doctor Evidence, LLC. LD is currently employed by Doctor Evidence, LLC, who were contracted by Sanofi to conduct this study. WN is currently employed by Sanofi. GCC reports speaker/honoraria and consulting for Sanofi.
Authors’ contributions: All authors contributed to the conception and design of the study, analysis and interpretation of the data, the drafting of the article, revision of the article for content, and final approval of the article.
Availability of data and materials: Not applicable. The data used for analysis was retrieved from openly published studies listed in our manuscript.
Ethics approval and consent to participate: Since our study is a systematic literature review and network meta-analysis, an Ethical Review Committee Statement is not applicable.
ORCID iD: Craig Whittington  https://orcid.org/0000-0002-1950-0334
https://orcid.org/0000-0002-1950-0334
Supplemental material: Supplemental material for this article is available online.
References
- 1. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162:46-54. [DOI] [PubMed] [Google Scholar]
- 2. Felson DT. Intra-articular corticosteroids and knee osteoarthritis: interpreting different meta-analyses. JAMA. 2016;316:2607-2608. [DOI] [PubMed] [Google Scholar]
- 3. Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J Pain Res. 2018;11:2189-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Academy of Orthopaedic Surgeons. Treatment of osteoarthritis of the knee: evidence-based guideline. 2nd ed https://www.aaos.org/globalassets/quality-and-practice-resources/osteoarthritis-of-the-knee/osteoarthritis-of-the-knee-2nd-editiion-clinical-practice-guideline.pdf [DOI] [PubMed]
- 5. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64:465-474. [DOI] [PubMed] [Google Scholar]
- 6. Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61:1704-1711. [DOI] [PubMed] [Google Scholar]
- 7. McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317:1967-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeng C, Lane NE, Hunter DJ, et al. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: results from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2019;27:855-862. [DOI] [PubMed] [Google Scholar]
- 9. Orchard JW. Is there a place for intra-articular corticosteroid injections in the treatment of knee osteoarthritis? BMJ. 2020;368:l6923. [DOI] [PubMed] [Google Scholar]
- 10. Juni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015;(2):CD005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell J, Bellamy N, Gee T. Differences between systematic reviews/meta-analyses of hyaluronic acid/hyaluronan/hylan in osteoarthritis of the knee. Osteoarthritis Cartilage. 2007;15:1424-1436. [DOI] [PubMed] [Google Scholar]
- 12. SYNVISC (hylan G-F 20) [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014. [Google Scholar]
- 13. Synvisc-One (hylan G-F 20) [prescribing information]. Cambridge, MA: Genzyme Corporation; 2014. [Google Scholar]
- 14. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pritchard CH, Sripada P, Bankes PF, Smith DG, Schneider D. A retrospective comparison of the efficacy and tolerability of sodium hyaluronate and hylan g-f 20 in the treatment of osteoarthritis of the knee. J Musculoskelet Res. 2002;6:197-205. [Google Scholar]
- 16. Wobig M, Bach G, Beks P, et al. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: a comparison of hylan G-F 20 and a lower-molecular-weight hyaluronan. Clin Ther. 1999;21:1549-1562. [DOI] [PubMed] [Google Scholar]
- 17. Zhang H, Zhang K, Zhang X, et al. Comparison of two hyaluronic acid formulations for safety and efficacy (CHASE) study in knee osteoarthritis: a multicenter, randomized, double-blind, 26-week non-inferiority trial comparing Durolane to Artz. Arthritis Res Ther. 2015;17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am. 2015;97:2047-2060. [DOI] [PubMed] [Google Scholar]
- 19. Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157:180-191. [DOI] [PubMed] [Google Scholar]
- 20. Bhandari M, Bannuru RR, Babins EM, et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a Canadian evidence-based perspective. Ther Adv Musculoskelet Dis. 2017;9:231-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gregori D, Giacovelli G, Minto C, et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordan KM, Arden NK, Doherty M, et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62:1145-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Institute for Health and Clinical Excellence: Guidance. Osteoarthritis: Care and Management in Adults. London: National Institute for Health and Care Excellence (UK); 2014. [PubMed] [Google Scholar]
- 24. Brown GA. AAOS clinical practice guideline: treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21:577-579. [DOI] [PubMed] [Google Scholar]
- 25. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363-388. [DOI] [PubMed] [Google Scholar]
- 26. Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: Results of an extensive critical literature review. Semin Arthritis Rheum. 2019;48:563-572. [DOI] [PubMed] [Google Scholar]
- 27. Higgins J, Green S. Handbook for systematic reviews of interventions 2011 (Version 5.1.0). http://handbook.cochrane.org.
- 28. Juhl C, Lund H, Roos EM, Zhang W, Christensen R. A hierarchy of patient-reported outcomes for meta-analysis of knee osteoarthritis trials: empirical evidence from a survey of high impact journals. Arthritis. 2012;2012:136245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [DOI] [PubMed] [Google Scholar]
- 30. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:48. [Google Scholar]
- 31. Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials [Internet]. London: National Institute for Health and Care Excellence (NICE); 2014. https://www.ncbi.nlm.nih.gov/books/NBK310366/. [PubMed] [Google Scholar]
- 32. Song F, Xiong T, Parekh-Bhurke S, et al. Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ. 2011;343:d4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313-2324. [DOI] [PubMed] [Google Scholar]
- 34. van Valkenhoef G, Kuiper J. gemtc: network meta-analysis. R package version 0.8-2. http://CRAN.R-project.org/package=gemtc. Updated 2016.
- 35. Al-Omran A, Azam Q. Efficacy of viscosupplementation in knee osteoarthritis: a clinical trial of three agents. Bahrain Med Bull. 2014;36:150-153. [Google Scholar]
- 36. Askari A, Gholami T, NaghiZadeh MM, Farjam M, Kouhpayeh SA, Shahabfard Z. Hyaluronic acid compared with corticosteroid injections for the treatment of osteoarthritis of the knee: a randomized control trail. SpringerPlus. 2016;5:442-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atamaz F, Kirazli Y, Akkoc Y. A comparison of two different intra-articular hyaluronan drugs and physical therapy in the management of knee osteoarthritis. Rheumatol Int. 2006;26:873-878. [DOI] [PubMed] [Google Scholar]
- 38. Bisicchia S, Bernardi G, Tudisco C. HYADD 4 versus methylprednisolone acetate in symptomatic knee osteoarthritis: a single-centre single blind prospective randomised controlled clinical study with 1-year follow-up. Clin Exp Rheumatol. 2016;34:857-863. [PubMed] [Google Scholar]
- 39. Caborn D, Rush J, Lanzer W, Parenti D, Murray C. A randomized, single-blind comparison of the efficacy and tolerability of hylan G-F 20 and triamcinolone hexacetonide in patients with osteoarthritis of the knee. J Rheumatol. 2004;31:333-343. [PubMed] [Google Scholar]
- 40. Chao J, Wu C, Sun B, et al. Inflammatory characteristics on ultrasound predict poorer longterm response to intraarticular corticosteroid injections in knee osteoarthritis. J Rheumatol. 2010;37:650-655. [DOI] [PubMed] [Google Scholar]
- 41. Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Conaghan PG, Cohen SB, Berenbaum F, Lufkin J, Johnson JR, Bodick N. Phase 2b trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2017;70:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Joint Surg Am. 2018;100:666-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cubukcu D, Ardic F, Karabulut N, Topuz O. Hylan G-F 20 efficacy on articular cartilage quality in patients with knee osteoarthritis: clinical and MRI assessment. Clin Rheumatol. 2005;24:336-341. [DOI] [PubMed] [Google Scholar]
- 45. Dickson DJ, Hosie G, English JR. A double-blind, placebo-controlled comparison of hylan G-F 20 against diclofenac in knee osteoarthritis. J Clin Res. 2001;4:41-52. [Google Scholar]
- 46. Diracoglu D, Vural M, Baskent A, Dikici F, Aksoy C. The effect of viscosupplementation on neuromuscular control of the knee in patients with osteoarthritis. J Back Musculoskelet Rehabil. 2009;22:1-9. [DOI] [PubMed] [Google Scholar]
- 47. Gaffney K, Ledingham J, Perry JD. Intra-articular triamcinolone hexacetonide in knee osteoarthritis: factors influencing the clinical response. Ann Rheum Dis. 1995;54:379-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Housman L, Arden N, Schnitzer TJ, et al. Intra-articular hylastan versus steroid for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2014;22:1684-1692. [DOI] [PubMed] [Google Scholar]
- 49. Iannitti T, Rottigni V, Palmieri B. A pilot study to compare two different hyaluronic acid compounds for treatment of knee osteoarthritis. Int J Immunopathol Pharmacol. 2012;25:1093-1098. [DOI] [PubMed] [Google Scholar]
- 50. Jones AC, Pattrick M, Doherty S, Doherty M. Intra-articular hyaluronic acid compared to intra-articular triamcinolone hexacetonide in inflammatory knee osteoarthritis. Osteoarthritis Cartilage. 1995;3:269-273. [DOI] [PubMed] [Google Scholar]
- 51. Juni P, Reichenbach S, Trelle S, et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum. 2007;56:3610-3619. [DOI] [PubMed] [Google Scholar]
- 52. Karatay S, Kiziltunc A, Yildirim K, Karanfil RC, Senel K. Effects of different hyaluronic acid products on synovial fluid levels of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in knee osteoarthritis. Ann Clin Lab Sci. 2004;34:330-335. [PubMed] [Google Scholar]
- 53. Kelley SD, Johnson JR, Thornton D, Skaar JR, Varsos GV, Peyerl FW. An intra-articular, extended-release formulation of triamcinolone acetonide as a cost-effective therapy for treating osteoarthritis of the knee. Value Health. 2017;20:A145. [Google Scholar]
- 54. Khanasuk Y, Dechmaneenin T, Tanavalee A. Prospective randomized trial comparing the efficacy of single 6-ml injection of hylan G-F 20 and hyaluronic acid for primary knee arthritis: a preliminary study. J Med Assoc Thai. 2012;95:S92-S97. [PubMed] [Google Scholar]
- 55. Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154-162. [DOI] [PubMed] [Google Scholar]
- 56. Leardini G, Franceschini M, Mattara L, Bruno R, Pebrellini A. Intra-articular sodium hyaluronate (Hyalgan) in gonarthrosis: a controlled study comparing methylprednisolone acetate. Clin Trial J. 1987;24:341-350. [Google Scholar]
- 57. Leardini G, Mattara L, Franceschini M, Perbellini A. Intra-articular treatment of knee osteoarthritis. A comparative study between hyaluronic acid and 6-methyl prednisolone acetate. Clin Exp Rheumatol. 1991;9:375-381. [PubMed] [Google Scholar]
- 58. Leighton R, Akermark C, Therrien R, et al. NASHA hyaluronic acid vs. methylprednisolone for knee osteoarthritis: a prospective, multi-centre, randomized, non-inferiority trial. Osteoarthritis Cartilage. 2014;22:17-25. [DOI] [PubMed] [Google Scholar]
- 59. Lyons C, Majeed A, Banarsee R. Effectiveness of high volume intra-articular injection of cortisone and lignocaine in osteoarthritis of the knee: pilot study. North West London J Gen Pract. 2005;11:23-26. [Google Scholar]
- 60. Maheu E, Zaim M, Appelboom T, et al. Comparative efficacy and safety of two different molecular weight (MW) hyaluronans F60027 and Hylan G-F20 in symptomatic osteoarthritis of the knee (KOA). Results of a non inferiority, prospective, randomized, controlled trial. Clin Exp Rheumatol. 2011;29:527-535. [PubMed] [Google Scholar]
- 61. Pavelka K, Uebelhart D. Efficacy evaluation of highly purified intra-articular hyaluronic acid (Sinovial(®)) vs hylan G-F20 (Synvisc(®)) in the treatment of symptomatic knee osteoarthritis. A double-blind, controlled, randomized, parallel-group non-inferiority study. Osteoarthritis Cartilage. 2011;19:1294-1300. [DOI] [PubMed] [Google Scholar]
- 62. Petrella RJ, Emans PJ, Alleyne J, Dellaert F, Gill DP, Maroney M. Safety and performance of hydros and hydros-TA for knee osteoarthritis: a prospective, multicenter, randomized, double-blind feasibility trial. BMC Musculoskelet Disord. 2015;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pietrogrande V, Melanotte PL, D’Agnolo B, et al. Hyaluronic acid versus methylprednisolone intraarticularly injected for treatment of osteoarthritis of the knee. Curr Ther Res Clin Exp. 1991;50:691-701. [Google Scholar]
- 64. Raman R, Dutta A, Day N, Sharma HK, Shaw CJ, Johnson GV. Efficacy of hylan G-F 20 and sodium hyaluronate in the treatment of osteoarthritis of the knee – a prospective randomized clinical trial. Knee. 2008;15:318-324. [DOI] [PubMed] [Google Scholar]
- 65. Ravaud P, Moulinier L, Giraudeau B, et al. Effects of joint lavage and steroid injection in patients with osteoarthritis of the knee: results of a multicenter, randomized, controlled trial. Arthritis Rheum. 1999;42:475-482. [DOI] [PubMed] [Google Scholar]
- 66. Rolf CG, Engstrom B, Ohrvik J, Valentin A, Lilja B, Levine DW. A comparative study of the efficacy and safety of hyaluronan viscosupplements and placebo in patients with symptomatic and arthroscopy-verified cartilage pathology. J Drug Assess. 2005;8:183-200. [Google Scholar]
- 67. Scale D, Wobig M, Wolpert W. Viscosupplementation of osteoarthritic knees with hylan: a treatment schedule study. Curr Ther Res. 1994;55:220-232. [Google Scholar]
- 68. Shimizu M, Higuchi H, Takagishi K, Shinozaki T, Kobayashi T. Clinical and biochemical characteristics after intra-articular injection for the treatment of osteoarthritis of the knee: prospective randomized study of sodium hyaluronate and corticosteroid. J Orthop Sci. 2010;15:51-56. [DOI] [PubMed] [Google Scholar]
- 69. Skwara A, Ponelis R, Tibesku CO, Rosenbaum D, Fuchs-Winkelmann S. Gait patterns after intraarticular treatment of patients with osteoarthritis of the knee–hyaluronan versus triamcinolone: a prospective, randomized, doubleblind, monocentric study. Eur J Med Res. 2009;14:157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sun SF, Hsu CW, Lin HS, Liou IH, Chen YH, Hung CL. Comparison of single intra-articular injection of novel hyaluronan (HYA-JOINT Plus) with synvisc-one for knee osteoarthritis: a randomized, controlled, double-blind trial of efficacy and safety. J Bone Joint Surg Am. 2017;99:462-471. [DOI] [PubMed] [Google Scholar]
- 71. Tammachote N, Kanitnate S, Yakumpor T, Panichkul P. Intra-articular, single-shot hylan G-F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: a double-blind, randomized controlled trial. J Bone Joint Surg Am. 2016;98:885-892. [DOI] [PubMed] [Google Scholar]
- 72. Tasciotaoglu F, Oner C. Efficacy of intra-articular sodium hyaluronate in the treatment of knee osteoarthritis. Clin Rheumatol. 2003;22:112-117. [DOI] [PubMed] [Google Scholar]
- 73. Tekeoglu I, Adak B, Goksoy T, Tosun N. Effects of intra-articular injections of sodium hyaluronate (orthovisc) and betamethasone on osteoarthritis of the knee. J Rheum Med Rehab. 1998;9:220-224. [Google Scholar]
- 74. Yavuz U, Sökücü S, Albayrak A, Oztürk K. Efficacy comparisons of the intraarticular steroidal agents in the patients with knee osteoarthritis. Rheumatol Int. 2012;32:3391-3396. [DOI] [PubMed] [Google Scholar]
- 75. Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Furukawa TA, Leucht S. How to obtain NNT from Cohen’s d: comparison of two methods. PLoS One. 2011;6:e19070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Webb D, Naidoo P. Viscosupplementation for knee osteoarthritis: a focus on Hylan G-F 20. Orthop Res Rev. 2018;10:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fernández-Cuadros ME, Pérez Moro O, Florin MJ, Rabasa S, Tobar-Izquierdo M, Rodriguez-De-Cía J. A new paradigm for the management of knee osteoarthritis: the role of hyaluronic acid, platelet-rich plasma (PRP) and ozone in the modulation of inflammation: a review [published online ahead of print February 2020]. J Surg Rehabil. doi: 10.31487/j.JSR.2020.01.01. [DOI] [Google Scholar]
- 79. Nicholls MA, Fierlinger A, Niazi F, Bhandari M. The disease-modifying effects of hyaluronan in the osteoarthritic disease state. Clin Med Insights Arthritis Musculoskelet Disord. 2017;10. doi: 10.1177/1179544117723611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kompel AJ, Roemer FW, Murakami AM, Diaz LE, Crema MD, Guermazi A. Intra-articular corticosteroid injections in the hip and knee: perhaps not as safe as we thought? Radiology. 2019;293:656-663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-amd-10.1177_1179544120967370 for Efficacy and Safety of Hylan G-F 20 Versus Intra-Articular Corticosteroids in People with Knee Osteoarthritis: A Systematic Review and Network Meta-Analysis by Xavier Chevalier, Brendan Sheehan, Craig Whittington, Mir-Masoud Pourrahmat, Lionel Duarte, Wilson Ngai and Gustavo Constantino de Campos in Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders