Abstract
Background
Belgium was among the first countries in Europe with confirmed coronavirus disease 2019 (COVID-19) cases. Since the first diagnosis on February 3rd, the epidemic has quickly evolved, with Belgium at the crossroads of Europe, being one of the hardest hit countries. Although risk factors for severe disease in COVID-19 patients have been described in Chinese and United States (US) cohorts, good quality studies reporting on clinical characteristics, risk factors and outcome of European COVID-19 patients are still scarce.
Methods
This study describes the clinical characteristics, complications and outcomes of 319 hospitalized COVID-19 patients, admitted to a tertiary care center at the start of the pandemic in Belgium, and aims to identify the main risk factors for in-hospital mortality in a European context using univariate and multivariate logistic regression analysis.
Results
Most patients were male (60%), the median age was 74 (IQR 61–83) and 20% of patients were admitted to the intensive care unit, of whom 63% needed invasive mechanical ventilation. The overall case fatality rate was 25%. The best predictors of in-hospital mortality in multivariate analysis were older age, and renal insufficiency, higher lactate dehydrogenase and thrombocytopenia. Patients admitted early in the epidemic had a higher mortality compared to patients admitted later in the epidemic. In univariate analysis, patients with obesity did have an overall increased risk of death, while overweight on the other hand showed a trend towards lower mortality.
Conclusions
Most patients hospitalized with COVID-19 during the first weeks of the epidemic in Belgium were admitted with severe disease and the overall case fatality rate was high. The identified risk factors for mortality are not easily amenable at short term, underscoring the lasting need of effective therapeutic and preventative measures.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-020-05605-3.
Keywords: COVID-19, Coronavirus, Clinical characteristics, Mortality
Background
A major outbreak of a respiratory illness caused by the novel beta-coronavirus SARS-CoV-2 started in Wuhan, China late 2019 and has since spread rapidly throughout the world [1]. The disease, termed Coronavirus Disease 2019 (COVID-19), has been declared a pandemic by the World Health Organization (WHO), with over 37 million confirmed cases and one million deaths to date [2, 3].
Several publications addressed clinical characteristics of hospitalized COVID-19 patients during the early weeks of the pandemic and determined risk factors for severe disease in Chinese and US cohorts [4, 5]. However, there are differences between Chinese, US and European populations that could have an impact on the generalizability of these results and the translatability to European patients. Thus far comparable good-quality studies in a European context are scarce [6, 7].
Belgium was among the first countries in Europe with confirmed COVID-19 cases [8]. Since the first diagnosis on February 3rd, the epidemic has quickly evolved with Belgium, at the crossroads of Europe, being one of the hardest hit countries [9, 10]. The aim of our study was to describe the clinical characteristics of COVID-19 patients in a tertiary care centre early in the Belgian epidemic and identify independent risk factors for hospital mortality.
Methods
This retrospective cohort study was conducted at the Jessa Hospital in Hasselt, a 981 bed non-academic tertiary care centre located in the centre of the outbreak in Belgium [11]. All patients aged 16 years or older, admitted to hospital for at least 24 h with confirmed COVID-19 until April 15, were included in the study.
Confirmed COVID-19 was defined as a positive real-time reverse transcriptase–polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a respiratory sample at any time during or before admission. Repeated testing was performed when there was a high clinical suspicion, but negative initial testing. Patients were treated in accordance with the Belgian guidelines in force at that time, which included hydroxychloroquine (400 mg twice daily for one day, followed by 200 mg twice daily up to day 5) when presenting with severe disease (defined as need for supplemental oxygen and chest X-ray abnormalities) [12]. The national guidelines recommended lopinavir / ritonavir as a second choice option, but this was not used in our center. Remdesivir was available only through a compassionate use program for patients admitted to the intensive care unit (ICU). Steroids as a systemic adjunctive treatment were not recommended and no specific advice was formulated yet regarding thromboprophylaxis or use of antibiotics. On March 31 we implemented intensified thromboprophylaxis in all patients admitted to the intensive care unit (ICU) [13]. The study was approved by the Ethics Committee of Jessa Hospital (ethical approval number 20.38-infect20.06). The requirement for informed consent was waived because of the retrospective nature of the study.
From the electronic medical records, we collected data on patient characteristics, comorbidities, and clinical symptoms, laboratory and radiology exams at hospital admission. Parameters were selected based on clinical relevance and previously published studies. Univariate logistic regression was used to assess significance of independent risk factors for in-hospital mortality. To reduce estimate’s bias because of small numbers of events per variable, a logistic regression model using Firth’s bias reduction method was used whenever cross table cell counts were below 5 [14]. To address that not all covariates were registered for all patients, missing values were imputed using multiple imputation (five imputations), using the fully conditional specification (FCS) method. This method of multiple imputation does not rely on the assumption of multivariate normality, instead using conditional distributions (regression models) specified for each variable with missing values. Each variable is therefore imputed conditional on the distribution of the remaining variables [15]. After imputation of missing values, a final multivariate logistic regression model was constructed through stepwise model building using pooled p-values (alpha 0.01) [14, 16]. Furthermore, we assessed how the comorbidity, age, lymphocyte count and lactate dehydrogenase score (CALL score), a previously published score for the prediction of progression to severe disease, performed for prediction of in-hospital mortality in our cohort, using the area under the receiver operating characteristic curve (AUC) [17]. Characteristics of patients admitted early in the epidemic (from first hospitalization until March 30), were compared with characteristics of patients admitted later (as from March 31) in the epidemic using univariate logistic regression. All analyses were conducted using SAS v9.4.
Results
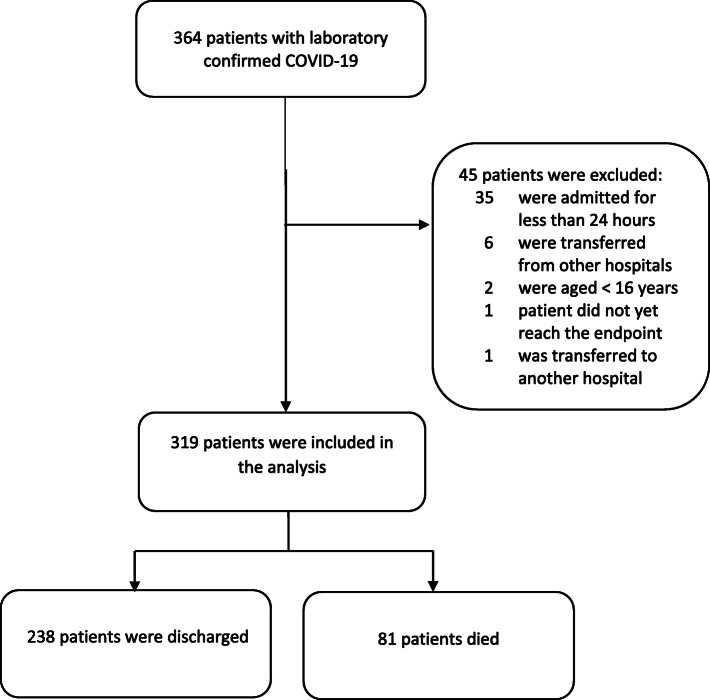
From when the first patient with COVID-19 was admitted on March 11 to April 15, 364 laboratory-confirmed COVID-19 patients had been admitted to our hospital, of which 319 patients met the inclusion criteria (Fig. 1).
Fig. 1.
Study flow diagram
Most patients were male (60%), the median age was 74 (IQR 61–83) years, with 70 patients (22%) being < 60 years of age. The most common comorbidities were hypertension (51%), coronary artery disease (23%), diabetes (20%), and chronic renal disease (20%) (Table 1). A significant proportion of patients (40%) was overweight (body mass index (BMI) 25–30) and 23% classified as obese (BMI > 30). Only 8% had known exposure to a confirmed COVID-19 case, in 7% nosocomial transmission was suspected and 3% of patients were healthcare personnel.
Table 1.
Characteristics of patients at admission
| All patients (n = 319) |
Discharged (n = 238) |
Deceased (n = 81) |
p-value | |
|---|---|---|---|---|
| Baseline and demographic parameters | ||||
| Median age (IQR) — yr | 74 (61–83) | 71 (59–79) | 82 (76–86) | < 0.0001 |
| Male — no. (%) | 188 (58.93) | 138 (57.98) | 50 (61.73) | 0.5530 |
| Nursing home resident — no. (%) | 30/318 (9.43) | 17/137 (7.17) | 13/81 (16.05) | 0.0249 |
| Smoking history | ||||
| - Current smoker — no. (%) | 20/181 (11.05) | 18/131 (13.74) | 2/50 (4.00) | 0.0619c |
| - Former smoker — no. (%) | 63/181 (34.81) | 42/131 (32.06) | 21/50 (42.00) | 0.2131 |
| - Never smoker— no. (%) | 98/181 (54.14) | 71/131 (54.20) | 27/50 (54.00) | 0.9809 |
| BMI (IQR) | 26.75 (23.79–29.74) | 26.35 (23.72–29.19) | 28.17 (23.83–30.97) | 0.0472 |
| - Underweighta - no. (%) | 4/230 (1.74) | 3/174 (1.72) | 1/56 (1.79) | |
| - Normal weighta - no. (%) | 80/230 (34.78) | 63/174 (36.21) | 17/56 (30.36) | 0.4206 |
| - Overweighta - no. (%) | 92/230 (40.00) | 73/174 (41.95) | 19/56 (33.93) | 0.2830 |
| - Obesitya — no. (%) | 54/230 (23.48) | 35/174 (20.11) | 19/56 (33.93) | 0.0390 |
| - Not known | 89 | 64 | 25 | |
| Diabetes — no. (%) | 65 (20.38) | 38 (15.97) | 27 (33.33) | 0.0012 |
| Hypertension — no. (%) | 162 (50.78) | 101 (42.44) | 61 (75.31) | < 0.0001 |
| - Use of ACE inhibitor | 60/251 (23.90) | 39/178 (21.91) | 21/73 (28.77) | 0.2530 |
| - Use of ATII antagonist | 30/250 (12.00) | 18/177 (10.17) | 12/73 (16.44) | 0.1764 |
| COPD — no. (%) | 40 (12.54) | 27 (11.34) | 13 (16.05) | 0.2805 |
| Asthma — no. (%) | 16 (5.02) | 12 (5.04) | 4 (4.94) | 0.9286c |
| Coronary artery disease — no. (%) | 72 (22.57) | 47 (19.75) | 25 (30.86) | 0.0434 |
| Chronic renal disease — no. (%) | 62/318 (19.50) | 34/237 (14.35) | 28/81 (34.57) | 0.0001 |
| - eGFR 30–60 | 36/314 (11.46) | 26/233 (11.16) | 10/81 (12.35) | 0.7743 |
| - eGFR 15–30 | 17/314 (5.41) | 5/233 (2.15) | 12/81 (14.81) | < 0.0001 |
| - eGFR < 15 | 3/314 (0.96) | 0/233 (0.00) | 3/81 (3.70) | 0.0090c |
| - HD/PD | 7 (2.19) | 4 (1.68) | 3 (3.70) | 0.2582c |
| Cerebrovascular disease — no. (%) | 48 (15.05) | 30 (12.61) | 18 (22.22) | 0.0433 |
| Immunocompromised — no. (%) | 42 (13.17) | 32 (13.45) | 10 (12.35) | 0.7992 |
| - Immunosuppressive drugs | 31 (9.72) | 23 (9.66) | 8 (9.88) | 0.9556 |
| - Active chemotherapy | 16 (5.02) | 10 (4.20) | 6 (7.41) | 0.2727 |
| Clinical symptoms | ||||
| Mean duration of symptoms (IQR) – days | 7 (3–10) | 7 (4–10) | 4 (1–7) | 0.0007 |
| - Not known | 30 | 21 | 9 | |
| History of feverb — no. (%) | 191 (59.87) | 148 (62.18) | 43 (53.09) | 0.1510 |
| Cough — no. (%) | 211 (66.14) | 161 (67.65) | 50 (61.73) | 0.3339 |
| - purulent | 47 (14.73) | 36 (15.13) | 11 (13.58) | 0.7327 |
| Dyspnea — no. (%) | 208 (65.20) | 152 (63.87) | 56 (69.14) | 0.3867 |
| Thoracic pain — no. (%) | 29 (9.09) | 26 (10.92) | 3 (3.70) | 0.0488c |
| Myalgia — no. (%) | 61 (19.12) | 57 (23.95) | 4 (4.94) | < 0.0001c |
| Diarrhea — no. (%) | 49 (15.36) | 38 (15.97) | 11 (13.58) | 0.6029 |
| Abdominal pain— no. (%) | 25 (7.84) | 20 (8.40) | 6 (6.17) | 0.5088 |
| Anorexia — no. (%) | 93 (29.15) | 76 (31.93) | 17 (20.99) | 0.0556 |
| Nausea — no. (%) | 55 (17.24) | 45 (18.91) | 10 (12.35) | 0.1644 |
| Vomiting — no. (%) | 45 (14.11) | 37 (15.55) | 8 (9.88) | 0.1908 |
| Syncope — no. (%) | 12 (3.76) | 8 (3.36) | 4 (4.94) | 0.4583c |
| Headache — no. (%) | 35 (10.97) | 32 (13.45) | 3 (3.70) | 0.0117c |
| Confusion — no. (%) | 29 (9.09) | 20 (8.40) | 9 (11.11) | 0.4728 |
| History of falling — no. (%) | 19 (5.96) | 8 (3.36) | 11 (13.58) | 0.0019 |
| Anosmia — no. (%) | 20 (6.27) | 18 (7.56) | 2 (2.47) | 0.1103c |
| Parameters | ||||
| Temperature > 38 °C – no. (%) | 52 (16.30) | 41 (17.23) | 11 (13.58) | 0.4354 |
| Respiratory rate (IQR) – breaths per min | 16 (14–20) | 16 (15–20) | 16 (14–18) | 0.5534 |
| - Not measured | 25 | 16 | 9 | |
| Mean arterial blood pressure (IQR) - mmHg | 94 (84–102) | 93 (84–102) | 97 (83–104) | 0.6908 |
| - Not measured | 7 | 3 | 4 | |
aBody mass index (the weight in kilograms divided by the square of the height in meters) was categorized as: < 18.5, underweight; 18.5–25, normal weight; 25–30, overweight; > 30 obese
bFever was defined as a measured body temperature > 38.0 °C
cUnivariate logistic regression used Firth correction when small cell counts (< 5) occurred
The mean duration of symptoms at the time of presentation to the hospital was 7 days. Most patients had an important degree of hypoxemia, with 88% of patients having a partial pressure of oxygen (PaO2) below 80 mmHg without supplemental oxygen (Table 2). Twenty percent of all patients were admitted to the ICU, of whom 63% needed invasive mechanical ventilation. The most common complications were acute kidney injury, hyponatremia and acute respiratory distress syndrome (ARDS) (Table 3). The overall case fatality rate was 25%. More patients died in the wards (72%) compared to the ICU (28%).
Table 2.
Laboratory and radiology findings at admission
| All patients (n = 319) |
Discharged (n = 238) |
Deceased (n = 81) |
p-value | |
|---|---|---|---|---|
| Laboratory findings | ||||
| White blood cell count (×10*9/L) | 6.33 (4.89–8.50) | 6.27 (4.80–8.41) | 6.63 (5.16–8.97) | 0.2258 |
| - > 10 | 45/315 (14.29) | 31/236 (13.14) | 14/79 (17.72) | 0.3505 |
| - < 4 | 37/315 (11.75) | 25/236 (10.59) | 12/79 (15.19) | 0.1795 |
| - Not measured | 4 | 2 | 2 | |
| Lymphocyte count (×10*9/L) | 0.75 (0.49–1.12) | 0.81 (0.53–1.21) | 0.63 (0.32–0.92) | 0.0720 |
| - < 1 | 211/314 (67.20) | 150/236 (63.56) | 61/78 (78.21) | 0.0097 |
| - Not measured | 5 | 2 | 3 | |
| Platelet count (×10*9/L) | 191 (157–254) | 196 (162–255) | 171 (126–243) | 0.0089 |
| - < 150 | 71/316 (22.47) | 42/237 (17.72) | 29/79 (36.71) | 0.0003 |
| - Not measured | 3 | 1 | 2 | |
| C-reactive protein (mg/L) | 76 (31–130) | 68 (28–110) | 96 (46–160) | 0.0120 |
| - Not measured | 7 | 4 | 3 | |
| Lactate dehydrogenase (U/L) | 320 (250–420) | 300 (230–400) | 390 (280–475) | < 0.0001 |
| - Not measured | 42 | 33 | 9 | |
| Ferritin (ug/L) | 840 (390–1600) | 735 (370–1500) | 1200 (490–2100) | 0.0073 |
| - Not measured | 90 | 62 | 28 | |
| D-dimer (mg/L) | 0.83 (0.53–1.41) | 0.78 (0.51–1.28) | 1.06 (0.62–1.67) | 0.3039 |
| - > 0.5 mg/L | 185/237 (78.06) | 133/177 (75.14) | 52/60 (86.67) | 0.1878 |
| - Not measured | 82 | 61 | 21 | |
| Aspartate aminotransferase (U/L) | 39 (28–56) | 39 (26–54) | 41 (31–60) | 0.4091 |
| - Not measured | 12 | 7 | 5 | |
| Alanine aminotransferase (U/L) | 28 (20–43) | 28 (20–43) | 28 (20–43) | 0.4939 |
| - Not measured | 13 | 8 | 5 | |
| Gamma-glutamyl transferase (U/L) | 45 (26–87) | 46 (25–86) | 42 (27–87) | 0.5703 |
| - Not measured | 22 | 14 | 8 | |
| Alkaline phosphatase (U/L) | 68 (57–93) | 67 (57–93) | 70 (58–90) | 0.4856 |
| - Not measured | 28 | 19 | 9 | |
| Total bilirubin (mg/dl) | 0.49 (0.38–0.68) | 0.49 (0.38–0.67) | 0.56 (0.38–0.72) | 0.9666 |
| - Not measured | 24 | 15 | 9 | |
| Creatinine (mg/dl) | 0.99 (0.79–1.31) | 0.94 (0.76–1.17) | 1.24 (0.92–2.21) | < 0.0001 |
| - Not measured | 9 | 7 | 2 | |
| eGFR (mL/min/1.73 m*2)b− | 70 (48–87) | 74 (58–91) | 48 (25–71) | < 0.0001 |
| - Not measured | 10 | 8 | 2 | |
| Sodium (mmol/L) | 137 (135–140) | 137 (134–139) | 138 (135–141) | 0.7515 |
| - Not measured | 7 | 5 | 2 | |
| Potassium (mmol/L) | 3.94 (3.60–4.26) | 3.91 (3.60–4.26) | 3.97 (3.58–4.26) | 0.4519 |
| - Not measured | 7 | 5 | 2 | |
| Arterial blood gas | ||||
| pH | 7.48 (7.45–7.51) | 7.48 (7.45–7.52) | 7.47 (7.43–7.48) | 0.0091 |
| - Not measured | 69 | 50 | 19 | |
| pO2 without suppl oxygen (mmHg) | 63 (55–71) | 64 (56–73) | 58 (50–67) | 0.0190 |
| - Hypoxemiaa — no. (%) | 212/241 (87.97) | 158/183 (86.34) | 54/58 (93.10) | 0.1105 |
| - Not measured | 78 | 55 | 23 | |
| pCO2 (mmHg) | 31 (28–34) | 31 (28–35) | 31 (28–34) | 0.8152 |
| - Not measured | 78 | 55 | 23 | |
| Lactate | 1.50 (1.10–1.90) | 1.40 (1.10–1.80) | 1.60 (1.30–2.30) | 0.0020 |
| - Not measured | 119 | 95 | 24 | |
| Radiology findings | ||||
| Infiltrates on chest X-ray | 205/292 (70.21) | 154/220 (70.00) | 51/72 (70.83) | 0.8931 |
| - Unilateral | 64/292 (21.92) | 49/220 (22.27) | 15/72 (20.83) | 0.7165 |
| - Bilateral | 140/292 (47.95) | 104/220 (47.27) | 36/72 (50.0) | 0.6846 |
aHypoxemia was defined as a PaO2 < 80 mmHg
bestimated glomerular filtration rate (eGFR) estimated using the CKD-EPI (Chronic Kidney Disease
Epidemiology Collaboration) equation
Table 3.
Complications, Treatment, Outcome
| All patients (n = 319) |
Discharged (n = 238) |
Deceased (n = 81) |
p-value | |
|---|---|---|---|---|
| Treatment | ||||
| Hydroxychloroquine — no. (%) | 164 (51.41) | 130 (54.62) | 34 (41.98) | 0.0489 |
| Antibiotics — no. (%) | 227/318 (71.38) | 161/237 (67.93) | 66/81 (81.48) | 0.0164 |
| Systemic glucocorticoids — no. (%) | 36/317 (11.36) | 25/237 (10.55) | 11/80 (13.75) | 0.4436 |
| Mechanical ventilation — no. (%) | 71 (22.26) | 46 (19.33) | 25 (30.86) | 0.0354 |
| - Invasive ventilation | 40 (12.54) | 23 (9.66) | 17 (20.99) | 0.0112 |
| - Non-invasive ventilation | 22 (6.90) | 15 (6.30) | 7 (8.64) | 0.4830 |
| - High flow oxygen device | 9 (2.82) | 8 (3.36) | 1 (1.23) | 0.2779 |
| Complications | ||||
| Acute respiratory distress syndrome — no. (%) | 45 (14.11) | 24 (10.08) | 21 (25.93) | 0.0008 |
| Acute kidney injuryc — no. (%) | 57 (17.87) | 20 (8.40) | 37 (45.68) | < 0.0001 |
| - Need for renal replacement therapy | 13 (4.08) | 5 (2.10) | 8 (9.88) | 0.0049 |
| Venous thrombo-embolism — no. (%) | 25 (7.84) | 20 (8.40) | 5 (6.17) | 0.5088 |
| - Deep venous thrombosis | 22 (6.90) | 17 (7.14) | 5 (6.17) | 0.7634 |
| - Pulmonary embolism | 5 (1.57) | 3 (1.26) | 2 (2.47) | 0.3853† |
| Hypoglycemiaa — no. (%) | 16 (5.02) | 9 (3.78) | 7 (8.64) | 0.1013 |
| Hyperglycemia b — no. (%) | 22 (6.90) | 12 (5.04) | 10 (12.35) | 0.0343 |
| Stroke — no. (%) | 4 (1.25) | 0 (0.00) | 4 (4.94) | 0.0021 |
| Hyponatraemia < 135 mmol/L — no. (%) | 157 (49.22) | 120 (50.42) | 37 (45.68) | 0.4607 |
| Hyponatraemia < 130 mmol/L — no. (%) | 74 (23.20) | 63 (26.47) | 11 (13.58) | 0.0133 |
| Outcome | ||||
| Admission to the ICU — no. (%) | 63 (19.75) | 39 (16.39) | 24 (29.36) | 0.0123 |
| Median length of stay in hospital — no. (%) | 8 (5–14) | 8 (5–14) | 8 (5–12) | 0.0273 |
| - Not known | 1 | 1 | 0 | |
aHypoglycemia was defined as a serum glucose level of < 70 mg/dl
bHyperglycemia was defined as a serum glucose level of > 250 mg/dl
cAcute kidney injury was defined according to the KDIGO guidelines as an increase in serum creatinine by 0.3 mg/dl within 48 h, or an increase in serum creatinin 1.5 times baseline creatinin, which is known or presumed to have occurred within the prior 7 days
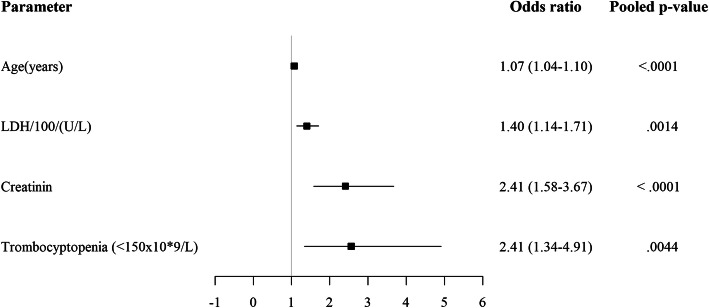
Univariate logistic regression showed that older age, residence in a nursing home, diabetes, pre-existing hypertension, chronic renal disease (eGFR < 30 ml/min), coronary artery disease and cerebrovascular disease were associated with a higher risk of death (Table 1). Smoking, either current or former, was not associated with a higher risk of death. Patients with obesity (BMI > 30) had an overall increased risk of death, but overweight on the other hand showed a trend towards lower mortality. Also, patients that died had reported a significantly shorter duration of symptoms at admission compared to patients that survived (4 vs 7 days, p = 0.0007). Lymphocytopenia, thrombocytopenia, high C-reactive protein (CRP), high lactate dehydrogenase (LDH) and high ferritin levels were significantly more present in patients who died of COVID-19 in hospital (Table 2), possibly reflecting a higher inflammatory state. Using multivariate logistic regression analysis we found the best predictors of in-hospital mortality to be older age, renal insufficiency, higher LDH levels and thrombocytopenia at admission (Fig. 2, Table S1 in supplementary materials).
Fig. 2.
Admission early in the epidemic versus later in the epidemic
We assessed the performance of the CALL score for prediction of in-hospital mortality using the area under the receiver operating characteristic curve (AUC) [17]. Over five imputed datasets, this score had an average AUC of 0.76 (min 0.75, max 0.76) which is considered excellent.
In patients admitted early in the epidemic (from first hospitalization until March 30), the mortality was significantly higher compared to patients admitted later (as from March 31) in the epidemic (Table 4). Mean age was not significantly different between these groups, but more patients admitted in the first weeks had hypertension. The proportion of patients admitted to the intensive care unit was not significantly different between groups.
Table 4.
Admission early in the epidemic versus later in the epidemic
| Early a (n = 206) |
Late b (n = 113) |
p-value | |
|---|---|---|---|
| Age — median (IQR) | 74 (62–83) | 73 (59–83) | 0.3138 |
| Hypertension — no. (%) | 116 (56.31) | 46 (40.71) | 0.008 |
| Diabetes — no. (%) | 45 (21.84) | 20 (17.70) | 0.3754 |
| Coronary heart disease — no. (%) | 45 (21.84) | 27 (23.89) | 0.677 |
| Chronic renal disease — no. (%) | 42 (20.49) | 20 (17.70) | 0.5457 |
| Number of days ill before admission – days (IQR) | 6.5 (2–10) | 7.8 (4–10) | 0.0384 |
| - Not measured | 8 | 22 | |
| Admission to ICU — no. (%) | 44 (21.36) | 19 (16.81) | 0.3248 |
| Death — no. (%) | 62 (30.10) | 19 (16.81) | 0008 |
aPatients admitted between March 11 and March 30
bPatients admitted between March 31 and April 15
Discussion
Our study describes the clinical characteristics, complications and outcomes of a large cohort of hospitalized COVID-19 patients in the early weeks of the pandemic in Belgium. In accordance with other reports, most patients were male [5, 7, 18]. The median age in our cohort was 74 (IGR 61–83), which is higher compared to recent studies in hospitalized patients from Italy (median 61, IQR 50–72), the US (median 63, IQR, 52–75) and China (median 41 years in 43 studies) [4, 5, 7]. The large majority of patients in our cohort presented with severe disease as defined by WHO [19], and we identified older age, renal insufficiency and higher LDH and thrombocytopenia as the most important risk factors for in-hospital mortality, which is in line with other reports [4, 7, 17, 20]. Because not all patient profiles were complete, these findings are based on the assumption that the missing data are missing at random, which means that conditional on the observed values included in the imputation model, the missing values are missing completely at random.
Comparing our results with previously published studies, some findings stand out. In our multivariate analysis, we did not find obesity to be amongst the most important risk factors for mortality. In univariate analysis, patients with obesity (BMI > 30) did have an overall increased risk of death, but overweight on the other hand showed a trend towards lower mortality. Several studies have described the increased need for mechanical ventilation and higher mortality in patients with obesity [20–23]. Several mechanisms are supposedly involved with this increased risk, mainly factors compromising respiratory physiology such as higher airway resistance, impaired gas exchange and lower lung volumes, but also increased risk of venous thromboembolism, a complication that is found to be very prevalent in patients with COVID-19 [24–26].
The association of overweight with lower mortality in both hospitalized and intensive care patients has previously been described and termed the obesity paradox [26, 27]. Despite the previously mentioned risk factors in obese patients, this was also found in a meta-analysis of observational studies in patients with pneumonia [27]. Whether this paradox represents a real protective effect of adipose tissue is uncertain and the topic of ongoing debate. In the setting of acute lung injury, it has been shown that patients with obesity have lower levels of proinflammatory cytokines, which could hypothetically be an advantage in COVID-19 [28]. Also, increased metabolic reserve is hypothesized to potentially be beneficial [27]. Because the obesity paradox thus far is based on observational studies only, it should be interpreted with caution and further data is needed to see whether this paradox truly exists and if so, applies to COVID-19 patients as well.
The incidence of venous thromboembolism in our cohort was low, which is in contrast with recent studies [24, 25]. This difference is most likely caused by detection bias, with a higher threshold for performing computed tomography (CT) scans due to a lower index of suspicion in the very beginning of the epidemic. No recommendations on thrombotic prophylaxis were included in the Belgian guidelines at the time. Currently, the increased risk of thromboembolic disease in COVID-19 is widely recognized, leading to better prophylactic strategies and aiding faster recognition and treatment, likely resulting in better outcome.
A previously published Chinese report found that in multivariate analysis comorbidity, older age, lymphocytopenia and high LDH at presentation were independent risk factors for COVID-19 progression to severe disease. From this, the “CALL” prediction score was developed to predict progression to severe disease [17]. However, most patients in our cohort already classified as having severe disease at admission. When assessing this score for prediction of hospital mortality, it also proved to perform well for the prediction of in-hospital mortality in our cohort.
There was no statistically significant difference in the administration of hydroxychloroquine between the deceased and discharged. In the beginning of the epidemic, off label chloroquine and/or hydroxychloroquine have both been widely advised in national guidelines as a possible treatment option for COVID-19, based mainly on in vitro data. Meanwhile several studies have shown no benefit of hydroxychloroquine on mortality [29–31]. Dexamethason and remdesivir, the only two therapies that have since shown to have an impact on outcomes in patients with severe COVID-19, were not advised at the time of our study [32, 33].
The overall case fatality rate in our cohort was high, similar to previously reported data from the UK and New York [5, 7], but higher compared to previous reports from China and Italy [4, 18]. This difference in reported case fatality rates in hospitalized patients is likely caused by a higher threshold for hospitalization compared to China and Italy, evident from the larger amount of patients being admitted with severe disease as defined by Wu et al. [34]. The fact that most patients died in the wards compared to the ICU reflects the attention for advanced care planning in our hospital consisting of a case-by-case assessment of the potential added value versus harm done by an intensive care admission, taking into careful consideration comorbidities, pre-admission performance status and patient wishes.
We found mortality to be higher in patients admitted in the first few weeks of the epidemic. Except for the prevalence of hypertension, the patient characteristics didn’t change however. We postulate that better understanding of the disease, its management and prevention of complications such as venous thromboembolism led to better treatment and overall outcome of patients admitted later in the epidemic.
Our study has certain limitations. The retrospective cohort analysis of a single centre may hamper generalization of the results in a broader geographical context, however our data are in line with other reported analyses. Furthermore, because not all patient profiles were complete, findings are based on the assumption that the missing data are missing at random, which means that conditional on the observed values included in the imputation model, the missing values are missing completely at random. However, whether missing data were truly missing at random or certain categories of patients were more frequently missing data is unknown. Also, we did not correct for multiple comparisons, making predictors with p-values close to 0.05 less likely to be true associations.
Conclusion
Patients hospitalized with COVID-19 during the first weeks of the epidemic in Belgium were admitted with severe disease, with an overall case fatality rate of 25%. In multivariate analysis we identified older age, and renal insufficiency, higher lactate dehydrogenase and thrombocytopenia but not obesity as the most important risk factors for in-hospital mortality. Mortality was higher for patients admitted in the first few weeks of the epidemic, compared to patients admitted later in the epidemic, most likely reflecting a learning curve in case management. The previously described CALL score was validated as a useful clinical tool for the prediction of COVID-19 related mortality. The identified risk factors for mortality are not easily amenable at short term, underscoring the lasting need of effective therapeutic and preventative measures.
Supplementary Information
Additional file 1: Table S1. Multivariate analysis of risk factors for death versus hospital discharge.
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
Coronavirus disease 2019
- US
United States
- IQR
Interquartile range
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- WHO
World health organisation
- RT-PCR
real-time reverse transcriptase-polymerase chain reaction
- ICU
Intensive care unit
- FCS
Fully conditional specification
- CALL score
Comorbidity, age, lymphocyte count and lactate hydrogenase score
- AUC
Area under the curve
- BMI
Body mass index
- PaO2
Partial pressure of oxygen
- ARDS
Acute respiratory distress syndrome
- eGFR
Estimated glomerular filtration rate
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- KDIGO
Kidney Disease: Improving Global Outcomes
- CRP
C-reactive protein
- LDH
Lactate dehydrogenase
- CT
Computed tomography
Authors’ contributions
KH, JH, PM and RB designed the study, analysed and interpreted the data in addition to writing the manuscript. KH, PD, MO and ES were involved in collecting the data. KH, JH, PM, JC, BS and JD were involved in writing the manuscript and revising it. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Jessa Hospital, Hasselt, Belgium (ethical approval number 20.38-infect20.06). The requirement for informed consent was waived because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Pneumonia of unknown cause 2020, January 5 [Available from: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/. Accessed 8 June 2020.
- 2.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 2020, March 11 [Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19%2D%2D-11-march-2020. Accessed 8 June 2020.
- 3.World Health Organization. Weekly Operational Update on COVID-19. 23 October 2020 Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 27 Oct 2020.
- 4.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, Li P, Zhou Y, Lin YF, Duan Q, Luo G, Fan S, Lu Y, Feng A, Zhan Y, Liang B, Cai W, Zhang L, Du X, Li L, Shu Y, Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80(6):656–65. 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed]
- 5.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–59. 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed]
- 6.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A; COVID-19 Lombardy ICU Network. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–81. 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed]
- 7.Tomlins J, Hamilton F, Gunning S, Sheehy C, Moran E, MacGowan A. Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID-19), the first UK cohort. J Infect. 2020;81(2):e59–e61. 10.1016/j.jinf.2020.04.020. [DOI] [PMC free article] [PubMed]
- 8.Spiteri G, Fielding J, Diercke M, Campese C, Enouf V, Gaymard A, Bella A, Sognamiglio P, Sierra Moros MJ, Riutort AN, Demina YV, Mahieu R, Broas M, Bengnér M, Buda S, Schilling J, Filleul L, Lepoutre A, Saura C, Mailles A, Levy-Bruhl D, Coignard B, Bernard-Stoecklin S, Behillil S, van der Werf S, Valette M, Lina B, Riccardo F, Nicastri E, Casas I, Larrauri A, Salom Castell M, Pozo F, Maksyutov RA, Martin C, Van Ranst M, Bossuyt N,Siira L, Sane J, Tegmark-Wisell K, Palmérus M, Broberg EK, Beauté J, Jorgensen P, Bundle N, Pereyaslov D, Adlhoch C, Pukkila J, Pebody R, Olsen S,Ciancio BC. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9):2000178. 10.2807/1560-7917.ES.2020.25.9.2000178.
- 9.European Centre for Disease Prevention and Control. Rapid risk assessment: coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK– ninth update 2020. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-rapid-risk-assessment-coronavirus-disease-2019-ninth-update-23-april-2020.pdf. Accessed 8 June 2020.
- 10.Sciensano. COVID 19 epidemiologisch bulletin. 2020, April 30. Available from: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_Weekly%20report_20200430%20-%20NL.pdf.
- 11.van Halem K, Cox J, Messiaen P, Pat K, Declercq C, Meersman A, et al. Care for adult non-ICU Covid-19 patients: early experiences from a Belgian tertiary care Centre. Neth J Med. 2020;78(3):111–115. [PubMed] [Google Scholar]
- 12.Sciensano. Interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium. 2020. https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_InterimGuidelines_Treatment_ENG.pdf.
- 13.Stessel B, Vanvuchelen C, Bruckers L, Geebelen L, Callebaut I, Vandenbrande J, et al. Impact of implementation of an individualised thromboprophylaxis protocol in critically ill ICU patients with COVID-19: a longitudinal controlled before-after study. Thromb Res. 2020;194:209–215. doi: 10.1016/j.thromres.2020.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38. doi: 10.1093/biomet/80.1.27. [DOI] [Google Scholar]
- 15.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 16.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons Inc.; 1987. [Google Scholar]
- 17.Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, Chen G, Cheng G, Wang Y, Bi J, Tan L, Lau G, Qin E. Prediction for Progression Risk in Patients With COVID-19 Pneumonia: The CALL Score. Clin Infect Dis. 2020;71(6):1393–99. 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed]
- 18.Giacomelli A, Ridolfo AL, Milazzo L, Oreni L, Bernacchia D, Siano M, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931. doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Management of COVID-19 Interim guidance, 27 may 2020 WHO/2019-nCoV/clinical/2020.5. 2020, May 27 [Available from: Available at https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed 8 June 2020.
- 20.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. Bmj. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16(7):341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, Stachel A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin Infect Dis. 2020;71(15):896–7. 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed]
- 24.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. 10.1111/jth.14888. [DOI] [PMC free article] [PubMed]
- 26.Schetz M, De Jong A, Deane AM, Druml W, Hemelaar P, Pelosi P, et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 27.Nie W, Zhang Y, Jee SH, Jung KJ, Li B, Xiu Q. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med. 2014;12:61. doi: 10.1186/1741-7015-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stapleton RD, Dixon AE, Parsons PE, Ware LB, Suratt BT. The association between BMI and plasma cytokine levels in patients with acute lung injury. Chest. 2010;138(3):568–577. doi: 10.1378/chest.10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, et al. Observational study of Hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM,de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O. Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020:NEJMoa2019014. 10.1056/NEJMoa2019014.
- 31.Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, et al. Association of Treatment with Hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. Jama. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E,Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020;17:NEJMoa2021436. 10.1056/NEJMoa2021436. Epub ahead of print.
- 33.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;8:NEJMoa2007764. 10.1056/NEJMoa2007764. Epub ahead of print.
- 34.Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020;71(15):706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Multivariate analysis of risk factors for death versus hospital discharge.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.