Abstract
Food contact materials (FCM) can contain chemicals that could migrate from the material itself to the foodstuff posing health concerns if ingested in non‐safe quantities by the consumer. FCM include containers, packaging, machinery or kitchenware and can be made from different materials like plastics, paper and board, metal or glass. Printing inks are also an important part of FCM. FCM have an important role in preventing damage or spoilage of the foodstuff and are essential along the food chain. Therefore, their safety needs to be carefully assessed in order to reduce the exposure to potentially hazardous substances and protect the health of the consumer. At the EU level, the legislation on FCM establishes general safety requirements for FCM. In addition, for certain materials, specific measures concerning usage and release of substances have been set. For materials or articles not specifically regulated in this harmonised framework, safety must be proven on a case‐by‐case basis. National legislations and lists of substances evaluated by competent authorities are important data sources in this context. One of the most important databases are the ‘BfR Recommendations on Food Contact Materials’ and the soon to come German national regulation on printing inks. BfR Unit 74, besides dealing with chemical risk assessment of FCM, is responsible for the evaluation of application dossiers for including substances into the BfR recommendations on FCM or the substance list of the printing inks regulation. Through the proposed work programme the fellow has been involved in risk assessment of substances that migrate from FCM into foodstuff gaining experience in the methodologies used to perform the scientific data evaluation as well as to support the BfR Unit 74s work.
Keywords: chemical risk assessment, food contact materials, in silico toxicology
1. Introduction
Food contact materials (FCM) are materials and articles intended to come into contact with food at any level of the food chain including processing, preparation, storage, serving, etc. Chemical substances used in the production of FCM are several thousand considering both intentionally added as well as known and unknown non‐intentionally added substances (NIAS) (Geueke et al., 2014). Migration of these substances or their breakdown or reaction products into foods can occur. In order to protect the consumer health an appropriate chemical risk assessment needs to be undertaken.
The framework regulation for FCM is the regulation (EC) No 1935/2004 (European Commission, 2004) which lays down the general safety principles for all FCM – first of all, that FCM must not endanger human health. Another EU regulation, which FCM have to comply with, is regulation (EC) No 2023/2006 on ‘Good manufacturing practice’ (GMP) (European Commission, 2006a) that applies to all stages in their manufacturing chain. Besides the general legislation, specific European Union (EU) measures exist for some FCM such as plastic materials (also recycled), ceramics, regenerated cellulose films, active and intelligent materials as well as for some substances including bisphenol A, epoxy derivatives and nitrosamines. However, there are many materials and substances not specifically regulated in a harmonised way and in these cases risk assessment has to be conducted on a case by case basis.
BfR Recommendations on Food Contact Materials (https://bfr.ble.de/kse/faces/DBEmpfehlung_en.jsp) represent a standard for the production of materials not subjected to any specific legislation and are well accepted by other European Commission member states according to the mutual recognition principle. BfR Recommendations are not legal norms but ‘reflect the current state of science and technology for the conditions under which consumer products made of high polymer materials meet the requirements of § 31.1 of the German Foods, Consumer Articles and Feed Act (LFGB) and Article 3.1 of Regulation (EC) 1935/2004 on Materials and Articles Intended to Come into Contact with Food as to their safety for human health’.
The BfR unit 74 ‘Safety of Food Contact Materials’, where the fellow has been placed in, deals with risk assessment of chemical substances that migrate from FCM into food or food simulant. The unit is part of the Department 7 ‘Chemicals and Product Safety’ of the BfR that assesses substances in context of the REACH regulation and is involved in the assessment of the health risks relative to chemicals, cosmetics, FCM, toys and other consumer products.
One important task of the BfR Unit 74 is to evaluate dossiers for including substances into the BfR Recommendations. The dossier evaluation process includes checking compliance with the requirements set by the EFSA ‘Note for Guidance For the Preparation of an Application for the Safety Assessment of a Substance to be used in Plastic Food Contact Materials’ (https://www.efsa.europa.eu/en/efsajournal/pub/rn-21) as well as assessing the scientific information provided by the applicant. The evaluated dossiers are then further discussed with external experts in the Panels ‘Toxicology’ and ‘Petitions’ of the BfR Committee for Consumer Products (BeKo). The unit elaborates also scientific opinions about health risk of substances who need a review or an up‐to‐date.
2. Description of the work programme
2.1. Aims
The overall aim of the programme was to learn how the risk assessment workflow of substances from FCM works. To achieve this purpose, the fellow has been involved in the ongoing activities and projects of the BfR unit 74. Specifically, the training objectives to be addressed included the dossier evaluation process, insight into the analytical and toxicological data, the use of in silico tools such as QSAR Toolbox, Toxtree, Derek and Sarah Nexus and practical experience in the German National Reference Laboratory for Materials in contact with food (NRL‐FCM). Moreover, the training purpose of the work programme has been a suitable opportunity for the fellow to take part actively to the projects and to contribute to current assessment issues of the host unit.
2.2. Activities/methods
In order to accomplish the training objectives the following activities, concerning relevant risk assessment issue of the unit, were carried out:
scientific evaluation of two dossiers (confidential) from current issues. The evaluation consisted in reviewing analytical and toxicological data provided by the applicant in accordance to the EFSA note for guidance. The dossiers are submitted for substances to be included in the BfR recommendations on food contact materials.
review and update of the risk assessment for certain substances, used for printing inks in FCM (presented at the 5th German Pharma‐Tox Summit in Leipzig, 2–5 March 2020, see Annex A)
review and update of the risk assessment of styrene oligomers with special focus on in silico genotoxicity prediction
determination of migration of aluminium and boron from paper and board FCM by means of cold‐water extract in combination with inductively coupled plasma mass spectrometry (ICP‐MS).
Because of data confidentiality concerning the dossiers, only the projects (ii)–(iv) will be further elaborated.
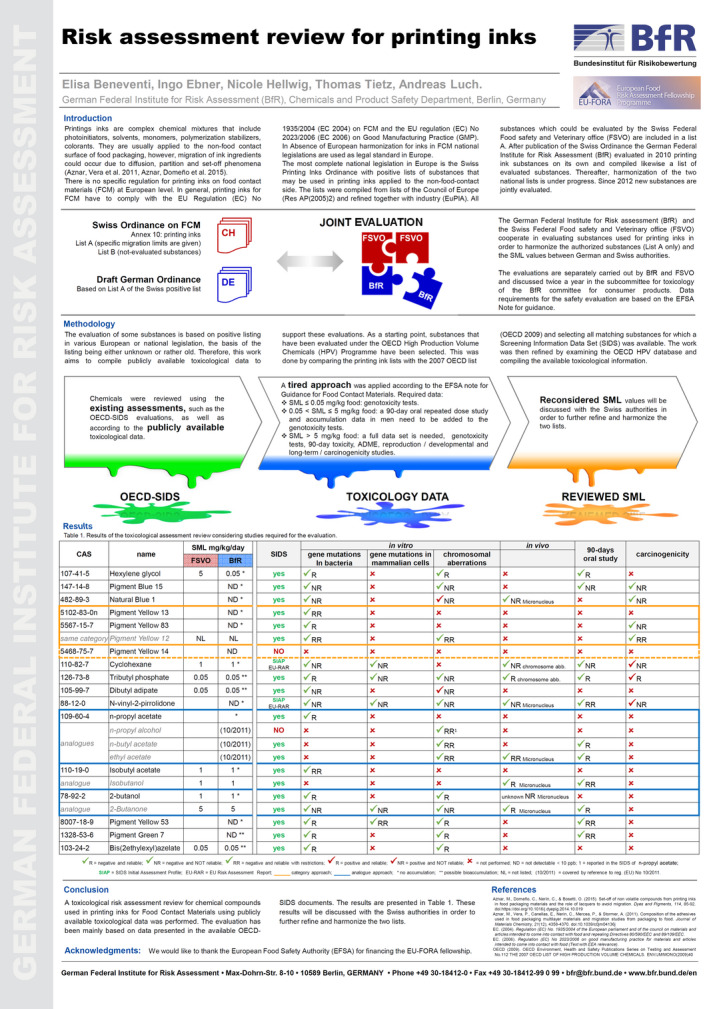
2.2.1. Risk Assessment review for printing inks in FCM (see Annex A)
Printing inks are complex chemical mixtures that can include pigments, solvents, monomers, photoinitiators and others. They are mainly applied to the non‐food contact surface of the food packaging but, although the printed surface is not in direct contact with the foodstuff, migration can still occur through diffusion, partition or set‐off phenomena (Aznar et al., 2015; Lago et al., 2015).
At the European level, a specific regulation for printing inks in FCM is missing. As a final component of a packaging material, they have to comply with the EU Regulation (EC) No 1935/2004 (European Commission, 2004) on FCM and the EU regulation (EC) No 2023/2006 (European Commission, 2006a) on GMP. In absence of European specific regulation for printing inks in FCM, evaluations of national authorities and national legislations are used to prove the safety of the inks according to regulation (EC) 1935/2004.
The most complete national legislation in Europe is the Swiss Printing Inks Ordinance (https://www.blv.admin.ch/blv/en/home/gebrauchsgegenstaende/materialien-in-kontakt-mit-lebensmitteln.html). Specifically, Annex 10 of the Swiss Ordinance deals with printing inks included in a positive list which is further divided into List A (evaluated substances for which a specific migration limit is given) and List B (non‐evaluated substances).
The BfR started its own evaluation on printing inks in 2010 with a positive list based on the Swiss list A. Since 2012 BfR and Swiss Federal Food Safety and Veterinary Office (FSVO) started a joint evaluation for printing inks in FCM in order to harmonise the two national positive lists. The German and the Swiss authorities independently carry out evaluations for the new substances and twice a year the submitted dossiers are discussed and approved in a joint meeting.
For some of the already evaluated substances, the basis for the positive listing is old or unknown. In order to fill the possible gaps and to better harmonise the two lists, a risk assessment review for these substances has been performed.
As a starting point, substances that have been already evaluated under the OECD high production volume (HPV) chemicals Programme with an existing assessment (OECD‐SIDS), have been selected. Then, a tiered approach for toxicological data was applied based on the EFSA note for guidance. According to that, in case of low migration of the substance into food (< 0.05 mg/kg food) only absence of genotoxicity has to be proven. For migration values between 0.05 and 5 mg/kg food, an in vivo subchronic study and data on accumulation in men have to be provided in addition to the genotoxicity tests, while for high migration values (> 5 mg/kg per food) a full toxicological data set (including information on absorption, distribution, metabolism and excretion (ADME), studies on reproductive toxicity, teratogenicity, chronic toxicity/carcinogenicity studies) is needed.
For each substance, a careful revision of the toxicological data provided in the OECD‐SIDS was performed by the fellow. In addition, a literature search for new data was conducted with a focus on in vitro and in vivo genotoxicity tests, 90‐day repeated oral toxicity and carcinogenicity studies. The reliability of each test was a key point of the review. Overall, 16 substances were re‐evaluated (Table 1).
Table 1.
Results of the toxicological assessment review considering studies required for the evaluation
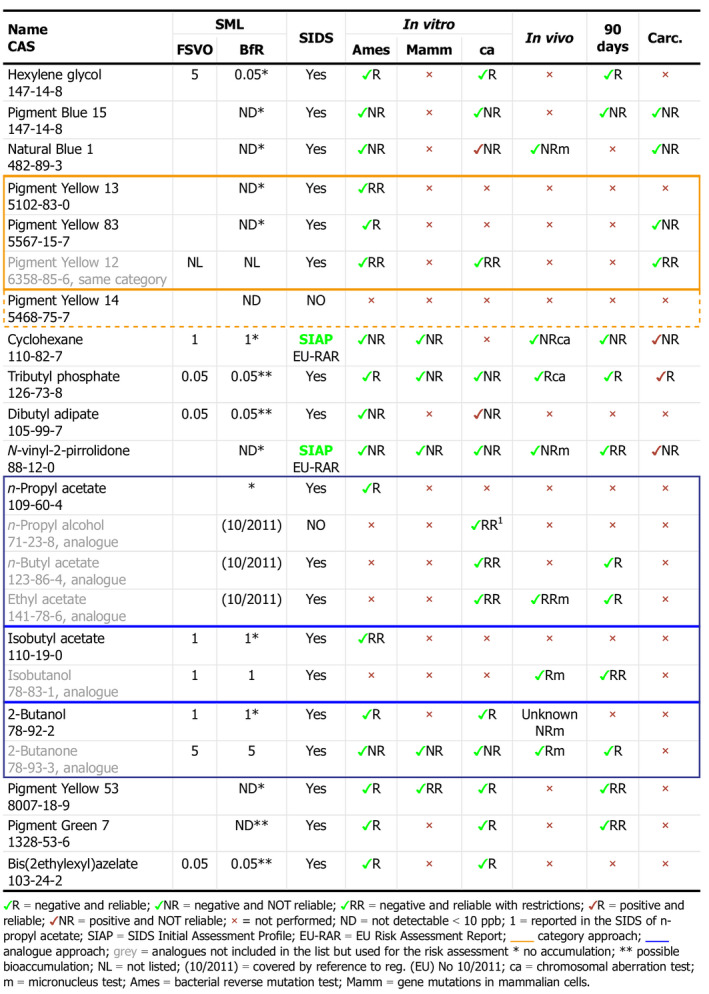
Two pigments have been assessed considering the category approach and three solvents using the analogue approach meaning that data on chemicals belonging to the same category or to metabolites or analogues were considered for the evaluation. Particular attention has been given to the genotoxicity endpoint, which needs to be ruled out. As already mentioned, reliability has been a crucial point; it reflects data quality and can deeply influence the overall outcome. The reviewed specific migration limit (SML) values have been reported and compared with the ones already published in the Annex 10 of the Swiss Ordinance. These preliminary results will be discussed with the FSVO in order to further refine and harmonise the two lists.
2.2.2. In silico genotoxicity prediction of styrene oligomers
Styrene oligomers are NIAS produced during the manufacturing process of polystyrene. The latter can be used for FCM, e.g. packaging or take‐away tableware. The Commission Regulation (EU) No 10/2011 on food contact plastics requires the risk assessment of NIAS by the business operator (Article 19 (European Commission, 2011)).
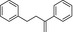
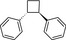
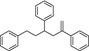
Recently, an official German laboratory for food surveillance (CVUA‐MEL) performed migration tests on 7 different styrene (Table 2) from 12 commercially available polystyrene FCM. A summed migration of up to 51 μg/kg food simulant (Ethanol 50%, 2 hours at 70°C) was measured. Considering that, in 2016 BfR performed the risk assessment of these investigated styrene oligomers (BfR, 2016). Based on the level of migration and on the available toxicological data, there was no evidence for health risk.
Table 2.
Chemical structure of styrene dimers and trimers
| Dimers |

|
|
|
||
| Trimers |
|
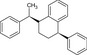
|
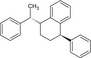
|
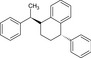
|
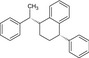
|
In 2019, a new risk assessment on styrene oligomers from polystyrene containers was published (Gelbke et al., 2019). A detailed literature search was conducted updating the information on migration and toxicological studies. Margin of safety (MoS) were calculated considering different approaches (no observed adverse effect level (NOAEL) or threshold of toxicological concern, TTC) and different tiers of exposure were applied (migration from food simulants, concentration in foodstuff, FACET methodology). Overall, the performed risk assessment showed a low risk for consumers.
Only two genotoxicity studies on styrene dimers and trimers are available so far. In 1990, Grifoll and co‐workers performed Ames tests using the Salmonella Typhimurium strain TA98 with metabolic activation on chromatographic fractions isolated from river and marine sediments (Grifoll et al., 1990). In Nakai et al. (2014), investigated styrene oligomers extracted from polystyrene intended for FCM use in an Ames test and in an in vitro chromosomal aberration test according to the relative OECD guidelines. In both studies, no evidence for genotoxicity was observed but considering each specific assay, many limitations apply.
When data on genetic toxicity of NIAS are scarce, computational (in silico) methods can be used in combination with existing data in order to improve the confidence of the outcome. In recent years, these in silico methodologies have been promoted by regulatory authorities (European Commission, 2006b; EFSA, 2017; EMA, 2018) and have been used in many regulatory fields (impurities in pharmaceuticals, NIAS).
The main goal of this project was to review of the literature on styrene oligomers focusing on migration, (geno)toxicity and endocrine disruptor activity data as well as to use in silico tools for genotoxicity prediction in order to strengthen the risk assessment. For genotoxicity prediction four independent software programs were used, the knowledge‐based OECD (Q)SAR (quantitative/qualitative structure activity relationships) Toolbox v. 4.4 and DEREK Nexus v. 6.0.1 (Lhasa Limited 2.2.2) and the statistically based Sarah Nexus v. 3.0.0 (Lhasa Limited 2.2.2) and DTU (Q)SAR database (http://qsar.food.dtu.dk). The results are intended to be presented in a publication later on.
2.2.3. Content determination of aluminium and boron from cold‐water extracts in paper and board
Paper and board are commonly used as FCM, and they can contain certain elements or their salts as residues derived from the production process. If they migrate into food, some of them could rise toxicological concerns because of their potential for accumulation in the cells and organs and for causing many diseases and disorders even at low concentration.
In the BfR recommendation XXXVI on Paper and Paperboard, guidance values for the maximum content or migration are given for many substances, including aluminium‐ and boron‐compounds (BfR‐Recommendations). In this context, the aim of this study was to determine the aluminium and boron release from paper samples. The work has been performed at the NRL‐FCM, which develops and validates analytical methods for the determination of the release of chemicals from FCM, conducts comparative laboratory tests or proficiency tests and establishes a national network within Germany for laboratories responsible for FCM.
Cold‐water extraction was performed according to CEN standard EN 645 (Gelbke et al., 2019).
ICP‐MS (iCAP™ TQ Thermo Scientific™) was used for the determination of aluminium and boron in the extracts according to a standard operating procedure. For each paper sample 12 replicate extractions were carried out, and measurements were performed in triplicate.
3. Conclusions
Overall, the work programme allowed the fellow to get a deep insight into chemical risk assessment applied to food contact materials. Specifically, it has provided the fellow to experience the dossier evaluation procedure and the decision‐making process for the inclusion of substances into the ‘BfR recommendations on Food Contact Materials’. Then, other projects were performed: the risk assessment review for printing inks, that implied to retrieve relevant data from existing assessments with a specific attention given to their reliability, and the in silico genotoxicity prediction for styrene oligomers, that allowed the fellow to learn using in silico tools for the toxicological assessment of NIAS. Furthermore, some practical work in the NRL‐FCM has been performed to complete the training objectives.
Beside the scientific training and achievements, the programme has been a unique opportunity for the fellow to build a strong network with the experts gaining a worthwhile experience and a growth mind‐set that will be valuable beyond the fellowship itself.
4. Disclaimer
The results of the in silico genotoxicity prediction of styrene oligomers are intended to be published in a peer‐review journal. In order to avoid copyright claims they were not included in the technical report.
Abbreviations
- ADME
absorption, distribution, metabolism and excretion
- BeKo
BfR Committee for Consumer Products
- BfR
German Federal Institute for Risk Assessment
- CEN
European Committee for Standardization
- CVUA‐MEL
Chemical and Veterinary Investigation Office Münsterland‐Emscher‐Lippe
- DTU
Technical University of Denmark
- FACET
Flavours, Additives, and food Contact materials Exposure Task
- FCM
food contact materials
- FSVO
Federal Food Safety and Veterinary Office
- GMP
Good Manufacturing Practice
- HPV
high production volume
- ICP‐MS
inductively coupled plasma‐mass spectrometry
- MoS
margin of safety
- NIAS
non‐intentionally added substances
- NOAEL
no observed adverse effect level
- NRL‐FCM
German National Reference Laboratory for Materials in contact with food
- OECD
Organisation for Economic Co‐operation and Development
- QSAR
quantitative/qualitative structure activity relationships
- SIDS
Screening Information Datasheet
- SML
specific migration limit
- TTC
threshold of toxicological concern
Appendix A – Training activities
1.
| What | Title | Contribution | Where | Date |
|---|---|---|---|---|
| Seminar |
Big data and high‐throughput‐driven modeling of health effects of environmental agents Prof. Roland Grafström, Karolinska Institut Stockholm |
Attendance | BfR | 6.11.2019 |
| Meeting |
Toxicological Subcommittee meeting BfR unit 74 |
Oral presentation | BfR | 12.11.2019 |
| Webinar | Testing the study appraisal methodology for the re‐evaluation of BPA safety EFSA | Attendance | On‐line | 14.11.2019 |
| Seminar |
An introduction to the BfR library and its services Benedikt Hummel, Head of BfR Library |
Attendance | BfR | 15.11.2019 |
| Workshop |
Harmonized exchange of food safety models using web‐based services from RAKIP and the AGINFRA+ project BfR |
Attendance | BfR | 9.12.2019 |
| Seminar/Workshop |
Creating characters for the BfR as a new line of communication” plus Workshop Claudio Canales Rios, BfR |
Attendance | BfR | 10.1.2020 |
| Seminar |
Trust – how we understand, measure and build it Michelle Patel (Food Standards Agency, UK) |
Attendance | BfR | 29.1.2020 |
| Workshop |
10th Berlin Workshop on Developmental Toxicology BfR |
Attendance | BfR | 19–20.2.2020 |
| Conference | 5th German Pharm‐Tox Summit 2020 – Leipzig | Poster presentation | University of Leipzig | 2–5.2.2020 |
| Webinar |
Introductory GastroPlus® Simulation and Modeling Maxime Le Merdy |
Attendance | Online | 13.4.2020 |
| Workshop |
Risk Assessment‐ Food contamination by plasticisers Dr. Zellmer, Dr. Pirow |
Attendance | BfR | 5.5.2020 |
| Conference |
One Health EJP Annual Scientific Meeting 2020 |
Attendance | Online | 27–29.5.2020 |
| Workshop |
Risk Assessment and Risk Management of Genetically Modified Organisms (GMO) Hermann Broll |
Attendance | BfR | 9.6.2020 |
Annex A – Poster presented at the 5th German Pharma‐Tox Summit in Leipzig, 2‐5 March 2020
1.
Suggested citation: German Federal Institute for Risk Assessment (BfR), Department of Chemicals and Product Safety, Berlin, Germany , Beneventi E, Tietz T and Merkel S, 2020. Risk Assessment of Food Contact Materials. EFSA Journal 2020;18(S1):e181109, 11 pp. 10.2903/j.efsa.2020.181109
Acknowledgements: This report is funded by EFSA as part of the EU‐FORA programme.
Approved: 7 September 2020
References
- Aznar M, Domeño C, Nerín C and Bosetti O, 2015. Set‐off of non volatile compounds from printing inks in food packaging materials and the role of lacquers to avoid migration. Dyes and Pigments, 114, 85–92. 10.1016/j.dyepig.2014.10.019 [DOI] [Google Scholar]
- BfR , 2016. Levels of styrene oligomers measured in food simulants show that health risks are unlikely. BfR Opinion 023/2016.
- BfR‐Recommendations . Database BfR Recommendations on Food Contact Materials. Available online: https://bfr.ble.de/kse/faces/DBEmpfehlung_en.jsp
- EFSA (European Food Safety and Authority), 2017. Guidance on the use of the weight of evidence approach in scientific assessments. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed]
- EMA (European Medicines Agency), 2018. ICH guideline M7(R1) on assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. Available online: https://www.ema.europa.eu/en/ich-m7-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit-potential#current-effective-version–section
- European Commission , 2004. Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX:32004R1935
- European Commission , 2006a. Commission Regulation (EC) No 2023/2006 of 22 December 2006 on good manufacturing practice for materials and articles intended to come into contact with food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02006R2023-20080417
- European Commission , 2006b. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC (Text with EEA relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20140410
- European Commission , 2011. Commision Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02011R0010-20190829
- Gelbke H‐P, Banton M, Block C, Dawkins G, Eisert R, Leibold E, Pemberton M, Puijk IM, Sakoda A and Yasukawa A, 2019. Risk assessment for migration of styrene oligomers into food from polystyrene food containers. Food and Chemical Toxicology, 124, 151–167. 10.1016/j.fct.2018.11.017 [DOI] [PubMed] [Google Scholar]
- Geueke B, Wagner CC and Muncke J, 2014. Food contact substances and chemicals of concern: a comparison of inventories. Food Additives & Contaminants: Part A, 31, 1438–1450. 10.1080/19440049.2014.931600 [DOI] [PubMed] [Google Scholar]
- Grifoll M, Solanas AM and Bayona JM, 1990. Characterization of genotoxic components in sediments by mass spectrometric techniques combined with Salmonella/microsome test. Archives of Environmental Contamination and Toxicology, 19, 175–184. 10.1007/BF01056084 [DOI] [PubMed] [Google Scholar]
- Lago MA, Rodríguez‐Bernaldo de Quirós A, Sendón R, Bustos J, Nieto MT and Paseiro P, 2015. Photoinitiators: a food safety review. Food Additives & Contaminants: Part A, 32, 779–798. 10.1080/19440049.2015.1014866 [DOI] [PubMed] [Google Scholar]
- Nakai M, Tsubokura M, Suzuki M, Fujishima S, Watanabe Y, Hasegawa Y, Oyama K and Ogura S, 2014. Genotoxicity of styrene oligomers extracted from polystyrene intended for use in contact with food. Toxicology Reports, 1, 1175–1180. 10.1016/j.toxrep.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]


