Abstract
This study investigated whether a lifestyle modification program that encouraged a ketogenic diet (KD) for participants with lymphedema and obesity would reduce weight and limb volume and improve quality of life. A total of 12 participants with lymphedema and obesity (mean body mass index = 38.38; SD = 7.02) were enrolled in a lifestyle modification group. The timespan from baseline data collection to 30-day follow-up was 18 weeks. Retention rate was 83.3%. Data were analyzed with repeated-measures ANOVA and Pearson correlation. Participants demonstrated statistically significant improvement in most outcome measures. Mean weight loss was 5.18 kg—F(4, 36) = 11.17; P < .001—or 4.8% of mean baseline weight. The average limb volume reduction was 698.9 ml—F(4, 36) = 9.4; P < .001—and was positively correlated with weight loss (r = 0.8; P = .005). There appeared to be a tendency for participants who used a KD (n = 6) to demonstrate superior results in most outcome measures compared with those who did not use the diet (n = 4), although the sample size of the 2 groups was too small to report definitive results. This lifestyle modification program provided insight into the possible value of a KD for obesity and lymphedema management.
Keywords: obesity, ketogenic diet, lifestyle modification, lymphedema
‘Adults with obesity exhibit structural lymphatic abnormality, and in some cases, the lymphatic damage may be irreversible.’
Lymphedema is a chronic condition arising from a compromised lymphatic system and results in a swollen body part. It is most commonly a result of parasitic infection or treatment for cancer1 but is increasingly being attributed to obesity.2,3 The most recent statistics for the United States show the trend toward obesity in adults continuing, with more than 34% considered obese.4 Individuals with obesity and lymphedema commonly return for repeated treatment, often with progressively increasing weight and further exacerbation of swelling. Several authors have reported increased incidence in obesity-related lower-extremity lymphedema.3,5 Therefore, weight management must be included in the effective treatment of lymphedema when obesity is a comorbidity.
Lymphatic function is reciprocally related to adipose expansion.6-9 Adults with obesity exhibit structural lymphatic abnormality, and in some cases, the lymphatic damage may be irreversible.10 Venous insufficiency,11 cellulitis,12 and chronic wounds13 as well as other comorbidities common with obesity create additional challenges for lymphedema management.
If significant weight reduction is to be achieved and sustained, lasting changes to lifestyle must be accomplished.14 Lifestyle modification groups with long-term contact may support sustained weight loss for the greatest number of people at the lowest cost.15 A program using a behavioral group model with multiple modes of support might be the most successful formula.16,17
Part of lifestyle modification is altering dietary intake. A growing body of evidence links obesity with excessive dietary carbohydrate intake coupled with decreased dietary fat consumption.18-21 A ketogenic diet (KD) (carbohydrate restriction to less than 20 g/d, moderate protein intake of 50 to 75 g/d, and fat intake to satiation) may be a favorable method to facilitate weight loss.22,23 Recent studies demonstrate healthy outcomes with a KD even in the presence of obesity-related comorbidities, such as metabolic syndrome, hyperlipidemia, cardiovascular disease, and type 2 diabetes.19,21,24
In summary, better lymphedema management in those with obesity must include weight managment.10,13 A community-based lifestyle modification program for individuals with lymphedema and obesity, utilizing evidence-based intervention strategies and a KD, may be a potent approach to address the urgent need for promoting and sustaining healthy lifestyle behavior changes. The purpose of this pilot study was to investigate the short-term effects of such a program on weight and lymphedema reduction as well as on quality of life.
Methods
This study utilized a single group within-subjects design over a period of 3 months, between March 31 and July 1, 2015. Data were collected at (1) 3 preintervention baselines, (2) after completion of the intervention, and (3) 1 month later. Because scores on all assessment measures vary somewhat from measurement session to measurement session, we took the mean of 3 baseline tests for each outcome measure to improve the accuracy of baseline representation. The University of Utah Institutional Review Board approved the study.
Participants
We recruited a convenience sample of individuals from the primary investigator’s private practice lymphedema clinic. Inclusion criteria were the following: 25 to 75 years old, English speaking, body mass index (BMI) ≥30 kg/m2, and a diagnosis of limb lymphedema. Exclusion criteria were the following: diagnosis of type 1 or 2 diabetes treated with an injectable medication, active untreated cancer, end-stage renal or hepatic disease, dementia or other cognitive impairment, comprehensive metabolic panel results that fell outside of normal range, or blood glucose level ≥150 mg/dL. Participants were not paid and did not have any fees to participate in the study beyond paying for their own meal if they chose to attend the group session held at a local restaurant.
Outcome Measures
The primary outcomes were change in body weight and lymphedema severity. Secondary outcomes included, among others, weight- and lymphedema-related quality of life.
Instruments used to measure weight-related quality of life included the Obesity and Weight-Loss Quality-of-Life (OWLQOL) and Weight-Related Symptoms Measure (WRSM). Both scales have been tested extensively for reliability and validity.25 The Lymphedema Life Impact Scale (LLIS) assessed lymphedema-related quality of life and has been tested for reliability and validity.26
Procedures
After providing written informed consent, participants completed baseline assessments at 3 different sessions, each 2 weeks apart. They then began a 12-week, 1.5-h/wk lifestyle modification group intervention that was modeled after University of Southern California’s Lifestyle Redesign program27 and adjusted for lymphedema and weight management. A schedule of topics for the group meetings is included in Appendix 1. The primary investigator led all sessions. In addition to group sessions, each participant was given the opportunity for individual visits with the primary investigator every 2 weeks, for a maximum of 6 individual visits. Immediately after the last group session, participants repeated the assessment measures, followed by a final assessment session 1 month later.
Data Analysis
All data were analyzed using Statistics Solutions Pro (version v1.15.05.04). Mean scores and SDs were computed for each outcome measure for the group, which were then entered into repeated-measures ANOVAs, using Tukey post hoc testing to assess the pairwise comparisons. The mean of the 3 baseline assessments was used as the baseline score in each analysis. Pearson correlations were used to assess the strength of the relationship between each outcome measure and program attendance as well as between all outcome measures themselves. Only some of the participants implemented the KD. Because of the small number of participants in the adherent and nonadherent groups, descriptive statistics were used to compare differences in the outcome variables between these groups. Statisticians argue that such small samples cannot accurately estimate the variance of the population; thus, the effect size would not be very meaningful in these subgroups. Instead, for outcomes where it is known, the size of the change in relation to the minimal detectable change (MDC) and minimal clinically important difference (MCID) is discussed.
Results
A total of 20 people signed consent forms and underwent a screening process; 8 people failed to meet entry criteria and were excluded from the study. We enrolled the remaining 12 participants (11 women and 1 man) into the study. Data on participant ethnicity and socioeconomic status were not obtained. Two participants were diagnosed with cancer-related lymphedema, whereas the remaining participants were diagnosed with obesity-induced lymphedema. Baseline characteristics of participants are displayed in Table 1. Two people dropped out of the study during the intervention, resulting in a retention rate of 83.3%.
Table 1.
Descriptive Statistics for Baseline Characteristics of Participants.
| Variable | n | Minimum | Maximum | M | SD |
|---|---|---|---|---|---|
| Age | 10 | 57 | 70 | 66.70 | 3.74 |
| Weight (kg) | 10 | 87.21 | 176.25 | 107.72 | 26.69 |
| Waist (cm) | 10 | 96.17 | 157.17 | 113.25 | 19.94 |
| Body fat (%) | 10 | 33.90 | 44.53 | 40.81 | 3.83 |
| BMI | 10 | 31.33 | 54.20 | 38.38 | 7.02 |
| LLIS | 10 | 25.33 | 66.67 | 50.50 | 14.69 |
| OWLQOL | 10 | 8.17 | 81.37 | 44.22 | 25.61 |
| WRSM | 10 | 2.08 | 61.11 | 37.29 | 17.55 |
| COPM-P | 10 | 1.27 | 6.80 | 3.85 | 1.60 |
| COPM-S | 10 | 1.00 | 6.60 | 2.75 | 1.71 |
| Demographic | n | Percentage | |||
| Gender | |||||
| Male | 1 | 10 | |||
| Female | 9 | 90 | |||
| Affected limb | |||||
| Bilateral legs | 8 | 80 | |||
| Bilateral arms | 1 | 10 | |||
| Unilateral arm | 1 | 10 |
Abbreviations: M, mean; BMI, body mass index; LLIS, Lymphedema Life Impact Scale; OWLQOL, Obesity and Weight Loss Quality of Life; WRSM, Weight-Related Symptom Measure; COPM-P, Canadian Occupational Performance Measure–Performance Score; COPM-S, Canadian Occupational Performance Measure–Satisfaction Score.
Postintervention Outcomes
Of 10 participants, 9 demonstrated weight loss after the intervention, with 5 participants each losing more than 6.5 kg. Postintervention mean weight reduced by 4.8% of mean baseline weight. At follow-up 30 days postintervention, participants were able to achieve further improvement. See Table 2 for results of all outcome measures. Only 1 adverse event was reported during the course of the study (retinal artery occlusion). This participant reported that she was not implementing the recommended KD.
Table 2.
Results for All Outcome Measures Over Testing Sessions.
| Outcome Measure | Baselinea | First Posttest | Second Posttest | F(4, 36) | P |
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |||
| Weight (kg) | 107.72 (26.69) | 103.29 (27.81) | 102.54 (28.59) | 11.17 | <.001 |
| Waist (cm) | 113.25 (19.94) | 108.7 (18.65) | 109.5 (19.34) | 3.16 | .025 |
| Body fat (%) | 40.81 (3.83) | 39.79 (3.93) | 39.58 (4.25) | 1.9 | .133 |
| BMI | 38.38 (7.02) | 36.76 (7.32) | 36.27 (7.53) | 11.92 | <.001 |
| Limb volume (mL) | 9690.03 (4339.56) | 9205.76 (4113.77) | 8991.14 (3986.61) | 9.4 | <.001 |
| LLIS | 50.44 (14.63) | 37.9 (8.6) | 38 (18.38) | 5.26 | .002 |
| OWLQOL | 41.51 (22.48) | 63.43 (22.03) | 65.49 (21.52) | 6.12 | <.001 |
| WRSM | 36.97 (18.01) | 21.17 (11.81) | 23.83 (17.58) | 9.99 | <.001 |
| COPM-P | 3.85 (1.6) | 6.12 (1.86) | 6.07 (1.97) | 9.78 | <.001 |
| COPM-S | 2.75 (1.71) | 5.07 (2.25) | 5.41 (2.55) | 9.01 | <.001 |
Abbreviations: M, mean; BMI, body mass index; LLIS, Lymphedema Life Impact Scale; OWLQOL, Obesity and Weight Loss Quality of Life; WRSM, Weight-Related Symptom Measure; COPM-P, Canadian Occupational Performance Measure–Performance Score; COPM-S, Canadian Occupational Performance Measure–Satisfaction Score.
Mean of 3 baseline measures.
Decrease in weight was positively correlated with both limb volume reduction (r = 0.8; P = .005) and decreased impact of lymphedema in the LLIS (r = 0.76; P = .01). No relationship was found between weight loss and OWLQOL or WRSM scores. Limb volume reduction was positively correlated with LLIS scores (r = 0.65; P < .05), suggesting a lessened impact of lymphedema on the individual’s life as limb volume reduced. Furthermore, the mean LLIS score decreased by 12.44, which is greater than both the MDC (11.53) and MCID (7.31).26
Participants were introduced to a KD during the second group session. An 8-page handout adapted from instructional materials from the Duke Lifestyle Medicine Clinic was used to teach principles of a KD. An abbreviated version of the handout can be found in Appendix 2. Diet adherence was not measured, although each participant was asked privately to confirm or deny if they were using a KD. Food logs were provided for each participant’s use, but they were not required to share log contents with the investigator. By self-report, 6 participants used a KD (KD group) over the course of the study, whereas 4 did not (NKD group), for an implementation rate of 60%. Although part of the intent of this study was to test the efficacy of a ketogenic diet, the sizes of the 2 self-selected diet groups were too small to make a definitive statement. Additionally, the 2 groups were not homogeneous at baseline.
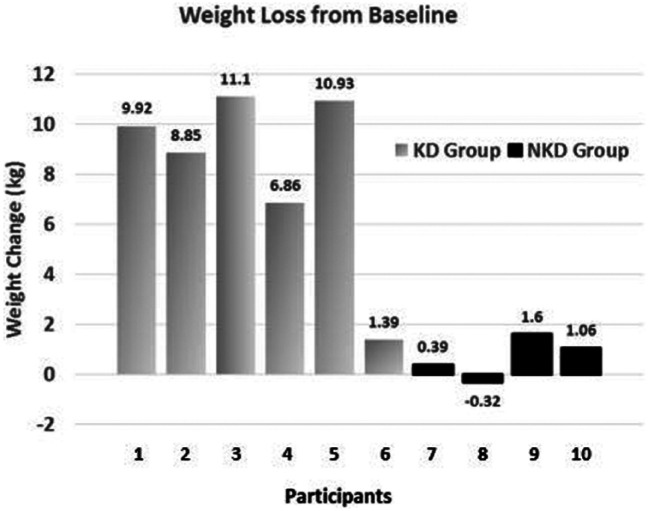
The apparent differences in the outcomes of the 2 groups, however, are noteworthy. The KD group appeared to achieve better improvement for all outcome measures compared with the NKD group. Furthermore, favorable changes in limb volume in the KD group were of clinical significance for edema reduction. See a comparison of the self-selected diet groups in Table 3. The KD group lost more weight and had a larger reduction in BMI than the NKD group. Of 6 KD participants, 5 lost more than 4.5 kg. In contrast, only 1 NKD participant lost more than 2.3 kg (see Figure 1).
Table 3.
Comparison of Outcome Measures Between Participants Who Used and Did Not Use a Ketogenic Diet.
| Outcome Measure | Used Ketogenic Diet (n = 6)a | Percentage Change | Did Not Use Ketogenic Diet (n = 4)a | |||||
|---|---|---|---|---|---|---|---|---|
| Baselineb | First Postmeasure | Second Postmeasure | Baselineb | First Posmeasure | Second Postmeasure | Percentage Change | ||
| Weight (kg) | 102.24 (14.84) | 95.45 (15.87) | 94.07 (16.54) | −7.99 | 115.93 (40.27) | 115.04 (39.93) | 115.25 (40.46) | −0.59 |
| Waist (cm) | 110.25 (16.35) | 102.67 (12.32) | 103 (14.23) | −6.57 | 117.75 (26.5) | 117.75 (24.67) | 119.25 (23.94) | +1.27 |
| Body fat (%) | 41.26 (3.6) | 39.4 (4.27) | 39.12 (4.84) | −5.18 | 40.13 (4.61) | 40.38 (3.91) | 40.28 (3.76) | +0.37 |
| BMI | 37.58 (5.26) | 35.1 (5.46) | 34.45 (5.48) | −8.32 | 39.58 (9.93) | 39.25 (9.85) | 39.25 (10.08) | −0.83 |
| Limb volume | 10 773.64 (2693.77) | 10 063.68 (2691.04) | 9773.06 (2593.1) | −9.28 | 8064.63 (6207.29) | 7918.88 (5917.74) | 7818.25 (5780.59) | −3.05 |
| LLIS | 51.78 (14.22) | 35.17 (1.47) | 29.17 (10.13) | −43.67 | 48.42 (17.2) | 42 (13.44) | 51.25 (21.28) | +5.84 |
| OWLQOL | 41.78 (28.18) | 72.88 (14.08) | 75.32 (12.61) | +80.28 | 41.1 (13.84) | 49.27 (26.08) | 50.74 (25.3) | +23.45 |
| WRSM | 37.13 (15.46) | 18.19 (6.74) | 17.22 (6.4) | −53.62 | 36.74 (23.98) | 25.63 (17.27) | 33.75 (25.3) | −8.14 |
| COPM-P | 3.35 (1.28) | 6.32 (2.23) | 6.52 (1.72) | +94.63 | 4.59 (1.93) | 5.81 (1.39) | 5.39 (2.39) | +17.43 |
| COPM-S | 2.29 (1.25) | 5.25 (2.18) | 5.88 (2.42) | +156.77 | 3.45 (2.27) | 4.79 (2.67) | 4.71 (2.95) | +6.52 |
Abbreviations: BMI, body mass index; LLIS, Lymphedema Life Impact Scale; OWLQOL, Obesity and Weight Loss Quality of Life; WRSM, Weight-Related Symptom Measure; COPM-P, Canadian Occupational Performance Measure–Performance Score; COPM-S, Canadian Occupational Performance Measure–Satisfaction Score.
Data expressed as mean (SD).
Mean of 3 baseline measures.
Figure 1.
Individual data for weight loss from baseline in the 2 self-selected groups: ketogenic diet (KD) group and nonketogenic diet (NKD) group.
Weight-related symptoms appeared to decrease more in the KD group. Of the 6 KD participants, 5 reported fewer symptoms, with 4 of these reporting more than a 13-point improvement on the WRSM. All the participants in the NKD group reported fewer symptoms, but only 2 of them reported a >13-point change. Changes in weight-related quality of life also differed between the KD and NKD groups. The 2 groups had different amounts of change in mean OWLQOL scores. All KD participants improved QOL after the intervention, with 4 of 6 improving more than 25 points on this measure. Although 3 out of the 4 NKD participants reported some improvement in QOL after intervention, only 1 NKD participant improved by more than 25 points.
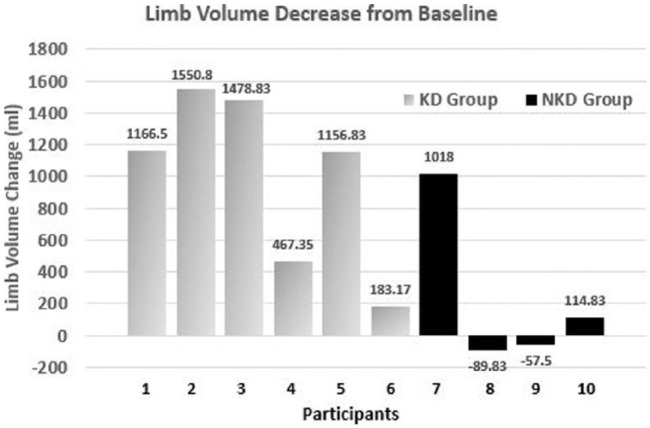
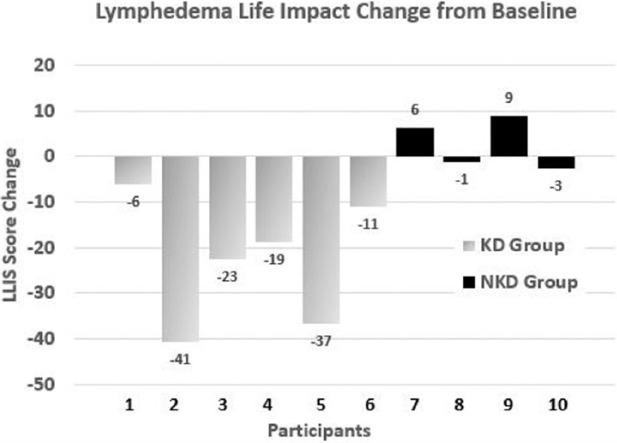
The limb reduction data were less clear. Although all 6 KD participants lost limb volume, the range was quite large (154-2209 mL). Only 2 of the 4 NKD participants lost limb volume (see Figure 2). However, the KD group displayed improved LLIS score reduction, indicating a greater decrease in impact of lymphedema on life over the NKD group. Of the 6 KD participants, 5 experienced a score reduction, with 4 of them reducing by more than 18 points and exceeding the threshold for both the MDC and MCID. Although 3 of the NKD participants had reduced LLIS scores, only 1 participant demonstrated a change that exceeded the threshold for MDC and MCID (see Figure 3).
Figure 2.
Individual data for limb volume decrease in the 2 self-selected groups: ketogenic diet (KD) group and nonketogenic diet (NKD) group.
Figure 3.
Individual data for impact of lymphedema on life by change in Lymphedema Life Impact Scale (LLIS) score in the 2 self-selected groups: ketogenic diet (KD) group and nonketogenic diet (NKD) group. Decrease in score denotes decreased impact of lymphedema on life.
Differences were also evident between the diet groups at final follow-up. The KD group appeared better able to sustain results for 30 days. All 6 participants in the KD group continued losing weight (.09 to 4.13 kg), whereas 3 out of 4 participants in the NKD group gained weight (.09 to 1.0 kg). Five KD participants had decreased limb volume, ranging from 84.6 to 568.5 mL, whereas 3 NKD participants reduced limb volume to a lesser extent (91 to 254 mL). Four of 6 KD participants reported increase in quality of life on the OWLQOL, ranging from 0.98 to 14.7, whereas 2 of 4 NKD participants did so (range 6.86 to 12.07). There was no clear trend in LLIS scores for either group at final follow-up.
Discussion
This study examined the effect of a lifestyle modification program that recommended a well-formulated ketogenic diet on weight loss and limb volume for individuals with lymphedema and obesity. The intervention was realistic and practical, resulting in a high retention rate (83.3%). Whereas, as a group, the participants improved in all study outcomes, it was primarily the participants following the ketogenic diet who drove these changes. More than half of the participants implemented the ketogenic diet, with the majority realizing reductions in weight and limb volume and perceived improvement in quality of life.
People with obesity need only a modest weight loss of 5% to 10% of starting weight to have a significant impact on health.28 Most participants who implemented the ketogenic diet lost at least 5% of their original body weight, with 3 losing 11% or more of baseline values. These results are consistent with other studies that have found a ketogenic diet to be effective in promoting weight loss.23,29
The findings that it was primarily the KD group that appeared to benefit from the intervention raises questions about the need for the lifestyle modification program part of the intervention. This small pilot study is unable to answer that question because there was no group that implemented the ketogenic diet without also receiving the program. Thus, it is not clear whether being in the program helped the participants in the KD group adhere to the ketogenic diet.
The impact of lymphedema and obesity on quality of life can be significant for an individual with these conditions.30-32 In our study, although all participants demonstrated improved quality of life, the KD group appeared to show the greatest increase. Several previous studies found strong correlations between improvements in OWLQOL and WRSM scores and weight loss.33,34 However, although OWLQOL and WRSM scores were statistically significantly correlated in our study, no such correlation was found between OWLQOL and WRSM scores and weight loss. Additionally, in contradiction to a recent LLIS validation study,26 we found a statistically significant correlation in our study between LLIS score improvement and limb volume decrease. It is unclear why our results differed from those of previous studies. Unfortunately, our small sample size precludes definitive conclusions.
Limitations
Study limitations included the following: lack of control group, small sample size, limited participation of men, potential for participant bias, and short follow-up. Additionally, more information on participant baseline characteristics, such as ethnicity and socioeconomic status, and procedures for assessing diet adherence might have provided valuable insight.
Conclusion
The lifestyle modification program used in this study encouraged a well-formulated ketogenic diet as well as other healthy lifestyle changes such as increased physical activity and improved stress management. Participants in the program experienced significant weight loss and limb volume reduction that gave rise to improved quality of life. Participants who implemented the ketogenic diet reduced their weight by 8.0% of baseline weight, with a corresponding decrease in limb volume. These results offer preliminary evidence that this intervention may be a viable adjunct to improve clinical outcomes for individuals with lymphedema and obesity. The apparent differences in results between the 2 diet groups suggest an exciting possibility for improved lymphedema and weight management using a ketogenic diet. However, further research using controlled designs are needed to confirm efficacy and to ascertain which elements of the program are key for facilitating positive results. Additionally, it is important to discover whether the positive results obtained from this study are sustained in the long term.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the assistance of the following individuals in the design and process of this original research: Eric Westman, MD, MHS, and Joanne Wright, PhD.
Appendix 1.
Group Session Schedule of Topics.
| Session | Topic |
|---|---|
| 1 | Introduction to Lifestyle Change |
| 2 | Eating for Health and Weight Loss |
| 3 | Eating Routines |
| 4 | Prevention/Management of Chronic Medical Conditions plus guest lecture (Thyroid and Weight Management) |
| 5 | Barriers to Change and Coping Strategies |
| 6 | Eating Out and Social Eating |
| 7 | Field Trip: Meet at Restaurant |
| 8 | Physical Activity and Exercise |
| 9 | Stress Management plus guest lecture (Meditation) |
| 10 | The Importance of Sleep |
| 11 | Life Balance and Time Management |
| 12 | Wrap-up and review: Planning for Sustained Change |
Appendix 2
“No Sugar, No Starch” Diet
Dr. Eric WestmanChief Medical Officer, HEAL Diabetes and Medical Weight Loss Clinics (healclinics.com)
List of Permitted Foods
This diet is focused on providing your body with the nutrition it needs (protein and fat), while minimizing foods that your body does not need (carbohydrates). To be most effective, you will need to keep the dietary carbohydrate to less than 20 grams per day.
When hungry, EAT AS MUCH AS YOU WANT OF THESE FOODS.
Meat: Beef (hamburger, etc), pork, ham, bacon, lamb, veal, sausage, pepperoni, hot dogs, or other meats.
Poultry: Chicken, turkey, duck, or other fowl.
Fish & Shellfish: Any fish including tuna, salmon, catfish, bass, trout, shrimp, scallops, crab, and lobster.
Eggs: Whole eggs are permitted without restrictions.
Don’t avoid the fat. Oils and butter have no carbs. (Do avoid vegetable oils like canola, corn and soy oil.)
Salad greens and nonstarchy vegetables MUST BE EATEN EVERY DAY:
Leafy greens: 2 cups a day. Examples: all varieties of cabbage, greens and lettuce.
Nonstarchy vegetables: 1 cup (measured uncooked) a day. Examples: broccoli, cauliflower, mushrooms, Brussel sprouts and eggplant.
FOODS THAT ARE ALLOWED IN LIMITED QUANTITIES:
(Check the labels to be sure there is not added carbohydrates.)
Cheese: up to 4 ounces a day.
Examples: Swiss, cheddar, mozzarella, and cream cheese
Cream: up to 2 tablespoonfuls a day.
Olives: up to 6 a day.
Avocado: up to 1 whole fruit a day.
Lemon/lime juice: up to 2 teaspoonfuls a day.
Pickles, dill or sugar-free: up to 2 servings a day.
Zero Carb Snacks: Examples: pork rinds; pepperoni slices; ham, turkey, beef jerky, deviled eggs
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: The authors confirm that this research was conducted in an ethical and responsible manner and complies with all relevant legislation. The results were reported clearly, honestly, and without fabrication, falsification or inappropriate data manipulation.
Informed Consent: Not applicable, because this article does not contain any studies with human or animal subjects.
Trial Registration: Not applicable, because this article does not contain any clinical trials.
Supplemental Material: Supplementary material is available for this article online.
References
- 1. Brayton KM, Hirsch AT, O’Brien PJ, Cheville A, Karaca-Mandic P, Rockson SG. Lymphedema prevalence and treatment in cancer: impact of a therapeutic intervention on health outcomes and costs. PLoS One. 2014;9:e114597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Todd M. Managing chronic oedema in the morbidly obese patient. Br J Nurs. 2009;18:1120-1124. [DOI] [PubMed] [Google Scholar]
- 3. O’Malley E, Ahern T, Dunlevy C, Lehane C, Kirby B, O’Shea D. Obesity-related chronic lymphoedema-like swelling and physical function. QJM. 2015;108:183-187. [DOI] [PubMed] [Google Scholar]
- 4. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fife CE, Benavides S, Otto G. Morbid obesity and lymphedema management. LymphLink. 2007;19:1-3. [Google Scholar]
- 6. Chakraborty S, Zawieja S, Wang W, Zawieja DC, Muthuchamy M. Lymphatic system: a vital link between metabolic syndrome and inflammation.Ann N Y Acad Sci. 2010;1207(suppl 1):E94-E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harvey NL. The link between lymphatic function and adipose biology. Ann N Y Acad Sci. 2008;1131:82-88. [DOI] [PubMed] [Google Scholar]
- 8. Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Resconstr Surg. 2014;134:154e-160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro- and microcirculation of adipose tissue. FEBS J. 2009;276:5738-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greene AK, Grant FD, Slavin SA, Maclellan RA. Obesity-induced lymphedema: clinical and lymphoscintigraphic features. Plast Reconstr Surg. 2015;135:1715-1719. [DOI] [PubMed] [Google Scholar]
- 11. Arngrim N, Simonsen L, Holst JJ, Bülow J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int J Obes (Lond). 2013;37:748-750. [DOI] [PubMed] [Google Scholar]
- 12. Hirschmann JV, Raugi GJ. Lower limb cellulitis and its mimics: part 1. Lower limb cellulitis. J Am Acad Dermatol. 2012;67:163.e1-163.e12. [DOI] [PubMed] [Google Scholar]
- 13. Fife CE, Carter MJ. Lymphedema in the morbidly obese patient: unique challenges in a unique population. Ostomy Wound Manage. 2008;54:44-56. [PubMed] [Google Scholar]
- 14. Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: a randomized trial. Ann Intern Med. 2009;150:255-262. [DOI] [PubMed] [Google Scholar]
- 15. Sniehotta FF, Dombrowski SU, Avenell A, et al. Randomised controlled feasibility trial of an evidence-informed behavioural intervention for obese adults with additional risk factors. PLoS One. 2011;6:e23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34:841-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gellert KS, Aubert RE, Mikami JS. Ke ‘Ano Ola: Moloka‘i’s community-based healthy lifestyle modification program. Am J Public Health. 2010;100:779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31:1-13. [DOI] [PubMed] [Google Scholar]
- 19. Volek JS, Phinney SD, Forsythe CE, et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. 2008;44:297-309. [DOI] [PubMed] [Google Scholar]
- 20. Westman EC, Feinman RD, Mavropoulos JC, et al. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86:276-284. [DOI] [PubMed] [Google Scholar]
- 21. Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769-777. [DOI] [PubMed] [Google Scholar]
- 22. Bazzano LA, Hu T, Reynolds K, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161:309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bueno NB, de Melo IS, de Oliveira SL, da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. 2013;110:1178-1187. [DOI] [PubMed] [Google Scholar]
- 24. Dashti HM, Al-Zaid NS, Mathew TC, et al. Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol Cell Biochem. 2006;286:1-9. [DOI] [PubMed] [Google Scholar]
- 25. Patrick DL, Bushnell DM, Rothman M. Performance of two self-report measures for evaluating obesity and weight loss. Obes Res. 2004;12:48-57. [DOI] [PubMed] [Google Scholar]
- 26. Weiss J, Daniel T. Validation of the Lymphedema Life Impact Scale (LLIS): a condition-specific measurement tool for persons with lymphedema. Lymphology. 2015;48:128-138. [PubMed] [Google Scholar]
- 27. Clark F, Jackson J, Carlson M, et al. Effectiveness of a lifestyle intervention in promoting well-being of independently living older people: results of the Well Elderly 2 Randomised Controlled Trial. J Epidemiol Community Health. 2012;66:782-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pi-Sunyer FX. A review of long-term studies evaluating the efficacy of weight loss in ameliorating disorders associated with obesity. Clin Ther. 1996;18:1006-1035. [DOI] [PubMed] [Google Scholar]
- 29. Moreno B, Bellido D, Sajoux I, et al. Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine. 2014;47:793-805. [DOI] [PubMed] [Google Scholar]
- 30. Yancy WS, Jr, Almirall D, Maciejewski ML, Kolotkin RL, McDuffie JR, Westman EC. Effects of two weight-loss diets on health-related quality of life. Qual Life Res. 2009;18:281-289. [DOI] [PubMed] [Google Scholar]
- 31. Brinkworth GD, Luscombe-Marsh ND, Thompson CH, et al. Long-term effects of very low-carbohydrate and high-carbohydrate weight-loss diets on psychological health in obese adults with type 2 diabetes: randomized controlled trial. J Intern Med. 2016;280:388-397. doi: 10.1111/joim.12501 [DOI] [PubMed] [Google Scholar]
- 32. Stolldorf DP, Dietrich MS, Ridner SH. A comparison of the quality of life in patients with primary and secondary lower limb lymphedema: a mixed-methods study. West J Nurs Res. 2016;38:1313-1334. doi: 10.1177/0193945916647961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cash SW, Beresford SA, Henderson JA, et al. Dietary and physical activity behaviours related to obesity-specific quality of life and work productivity: baseline results from a worksite trial. Br J Nutr. 2012;108:1134-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Billy HT, Sarwer DB, Ponce J, et al. Quality of life after laparoscopic adjustable gastric banding (LAP-BAND): APEX interim 3-year analysis. Postgrad Med. 2014;126:131-140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.