Abstract
Simple Summary
Many significant human and animal diseases are spread by blood feeding insects and other arthropod vectors. Arthropod pests and disease vectors rely heavily on chemical cues to identify and locate important resources such as their preferred animal hosts. Although there are abundant studies on the means by which biting insects—especially mosquitoes—are attracted to humans, this focus overlooks the veterinary and medical importance of other host–pest relationships and the chemical signals that underpin them. This review documents the published data on airborne (volatile) chemicals emitted from non-human animals, highlighting the subset of these emissions that play a role in guiding host choice by arthropod pests. The paper exposes some of the complexities associated with existing methods for collecting relevant chemical features from animal subjects, cautions against extrapolating the ecological significance of volatile emissions, and highlights opportunities to explore research gaps. Although the literature is less comprehensive than human studies, understanding the chemical drivers behind host selection creates opportunities to interrupt pest attack and disease transmission, enabling more efficient pest management.
Abstract
Many arthropod pests of humans and other animals select their preferred hosts by recognising volatile odour compounds contained in the hosts’ ‘volatilome’. Although there is prolific literature on chemical emissions from humans, published data on volatiles and vector attraction in other species are more sporadic. Despite several decades since the identification of a small number of critical volatiles underpinning specific host–vector relationships, synthetic chemicals or mixtures still largely fail to reproduce the attractiveness of natural hosts to their disease vectors. This review documents allelochemicals from non-human terrestrial animals and considers where challenges in collection and analysis have left shortfalls in animal volatilome research. A total of 1287 volatile organic compounds were identified from 141 species. Despite comparable diversity of entities in each compound class, no specific chemical is ubiquitous in all species reviewed, and over half are reported as unique to a single species. This review provides a rationale for future enquiries by highlighting research gaps, such as disregard for the contribution of breath volatiles to the whole animal volatilome and evaluating the role of allomones as vector deterrents. New opportunities to improve vector surveillance and disrupt disease transmission may be unveiled by understanding the host-associated stimuli that drive vector-host interactions.
Keywords: volatilome, host–parasite interactions, vector, VOC, allelochemical, allomone, kairomone, non-host volatile
1. Introduction
Many agriculturally and medically significant diseases are vectored by arthropod pests, which locate and identify their preferred host species using a variety of host-associated stimuli. Temperature, humidity, and visual signals contribute to host seeking [1], but odour cues contained in the profile of volatile organic compounds (VOCs) emitted by the host—the ‘volatilome’ [2]—are the most important signals. Although a wide variety of VOCs comprise the volatilome, often only a small subset of these are significant for arthropod host selection. Those compounds emitted by one species that modify the physiology or behaviour of another species are known as ‘allelochemicals’ [3]. Allelochemicals are classified further by the ecological benefit or detriment they infer on the recipient: Those that attract pest species are ‘kairomones’; those that repel pest species are ‘allomones’.
There is much to be gained by studying animal volatilomes and the allelochemicals they contain. There is an abundance of knowledge on allelochemicals in the human volatilome, particularly the identity of kairomones and their role in attracting anthropophilic mosquitoes [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. By contrast, our understanding of the volatilomes of other vertebrates, and their relevance to arthropod–host interactions, is far less mature. Nevertheless, this field is of increasing importance as the relative plasticity or specificity of the feeding habits of arthropod vectors determines the rate of spread of the disease agents they transmit [19,20]. As three-quarters of emerging infectious diseases are zoonoses and/or are vector-borne [21], increasing our understanding of disease transmission has substantial implications for human health. The veterinary impact of ectoparasitic and predatory arthropods is also a significant burden on livestock industries and companion animal husbandry, driving concerns both to animal welfare and the economic productivity of important agricultural industries. Knowledge of volatile allelochemicals creates opportunities to disrupt pest attack and disease transmission by developing repellents or attractants to enhance vector-trapping for surveillance and monitoring, enabling more efficient pest management.
This paper compiles current knowledge on the chemical ecology of mammalian and avian volatilomes, focusing on allelochemicals of relevance to arthropod pests. A comprehensive table is presented for easy reference to volatile chemicals identified from the species considered. A subselection from this total compendium is also included, indicating those VOCs with allelochemical functionality. This latter table is valuable for comparison of known host–arthropod interactions. Although it is possible that the compounds described in this review include some that are erroneously reported in the primary literature, it is nevertheless a valuable catalogue to compare against the human volatilome. These data represent a starting point to explore chemical means of manipulating host seeking by arthropod pests of veterinary and medical significance. Developing our understanding of the volatile profiles from different species may help to define the drivers for host selection by proposing indicators of non-human hosts and non-hosts.
Inclusion of all research that has contributed to our understanding of ecological interactions is beyond the scope of this review and in this sense, the present work does not intend to be exhaustive. Instead, the aim of this paper is to provide an overview of the evidence that pest species use the volatilomes of animals to discriminate host from non-host species and to document the known specific VOCs responsible for mediating these interactions. By identifying and investigating challenges in collecting non-human animal volatilomes, and in identifying bioactive allelochemical VOCs from these odour profiles, this paper cautions inferences and extrapolations about ecological significance of volatile emissions and highlights opportunities to explore knowledge gaps.
2. Challenges in Collecting Volatilomes and Identifying Allelochemicals
As with any field of biochemistry, methodology can strongly influence results. When working with VOCs, techniques for sample collection, VOC discrimination, and elucidation of their biological activity can all significantly influence the findings. In studies of animal volatilomes, many approaches follow procedures developed for investigating the human volatilome. Such investigations are driven by an interest in VOCs for applications such as medical diagnosis and monitoring [22,23,24,25], locating entrapped persons [26,27], or forensic purposes [28], and have documented nearly 2000 compounds from the human volatile odour signature [29]. As these methods have been described and reviewed in other publications [29,30,31] the section below focuses on issues relevant to the collection or testing of non-human animal volatilomes, including identification of VOCs and assessing animal odours to establish allelochemical function.
The transient nature of naturally occurring volatilomes and the chemical properties of VOCs make their accurate capture and identification challenging. VOCs originating from different anatomical or functional compartments have non-uniform representation in each, and the contribution of each compartment to the whole-animal volatile profile varies in time and abundance. Some, such as urinary and faecal emissions, flatulence, and eructation, are transient odour sources. Chemical representations may also be manipulated by microbial community composition and activity, diet, seasonal variation, and environmental conditions. Volatile emissions from animal production, such as broiler facilities [32,33,34], swine fattening operations [35], and cattle feedlots or dairy farms [36,37] can substantially contribute to the environmental odour associated with animals. When investigating arthropod–host interactions, it is important we consider this natural complexity and the methods used to interpret volatilomes.
2.1. Methods for Volatilome Collection
No universally accepted methods exist for volatilome collection and many different techniques have been used to sample various anatomical compartments. Collecting animal volatilomes is more challenging compared to human sample collection, in large part because non-human animals cannot be instructed to provide samples on-demand. This is most notable for animal breath collection [38].
For human breath collection—a burgeoning field driven by an interest in non-invasive disease diagnostics—samples are acquired using a variety of methods, although most are aimed at collecting the alveolar, or end-tidal portion of breath [24,31]. To selectively capture alveolar breath, which contains volatile metabolites from the blood stream as well as those arising in lung tissue, air is first expelled from the upper airways, before the latter fraction of breath is exhaled into a collection receptacle for subsequent analysis [31]. Alternatively, methods that monitor exhaled gases in real time can sample the requisite breath directly as is expelled [39,40]. Although some direct-sampling strategies have been adapted from human breath-collection methods [41,42,43,44], the expiratory resistance in these systems and prolonged sampling duration may interfere with normal breathing patterns in non-human animals. The absence of standardised approaches or commercially available collection devices for animals means the experience can be distressing for conscious subjects [38]. Consequently, animal breath samples are usually captured using bag collection techniques [45,46] or from anaesthetised subjects. With only a few studies, typically of small sample size, it is difficult to be confident about the extent of biological and analytical variation or the reproducibility of findings using different methods. Furthermore, for many animals, the influence of contaminants from the nasal passage (for example, for obligate nasal breathers such as horses), or from saliva is unknown. Thus, there are limited data on the contribution from breath to animal volatilomes, and a preference for obtaining samples from more readily accessible compartments such as integument and body odours, which can be acquired without the need for elaborate apparatus or techniques.
To collect volatile compounds released from integument (skin, hair, feathers, etc.), chemicals may be extracted with a liquid solvent phase or captured from the air when they are naturally emitted. Solvent extraction methods are simpler to perform and do not require specialised equipment, but are unlikely to reflect the natural relative distribution and abundance of compounds in the host volatilome [47]. This is predominantly because different solvents achieve extracts that differ qualitatively and quantitatively in their analyte composition according to their chemical properties [48]. For example, relatively non-polar hexane extracts capture 2-hexanol, 3-hexanol, 2-hexanone, and 3-hexanone from chicken feathers, whereas these analytes are absent in solvent extractions performed with more polar diethyl ether [49]. Extracts produced from these two solvents vary significantly in their appeal to Culex mosquitoes [50]. Moreover, in addition to capturing volatile constituents, solvent extraction also captures non-volatile chemicals, such as long-chain alcohol and acid compounds that dominate in avian preen oils. Solvent extraction techniques frequently involve pH manipulation and chemical derivatisation (e.g., transesterification), which may alter the relative abundance of VOCs and the overall volatile profile of compounds present compared to the natural volatile emissions.
Predictably, chemical sampling methods that directly capture compounds released into the environment immediately surrounding the animal (its ‘headspace’) reveal fewer analytes compared to liquid extraction techniques. By selectively trapping volatile chemicals, systems such as solid phase microextraction (SPME), thermal desorption (TD), or other polymer trapping techniques (e.g., SuperQ or Tenax) deliver a more discerning representation of the natural volatilome profile [51,52,53]. For example, Gabiriot et al. compared traditional solvent extraction with SPME and TD methods to explore the musky odour of the blue petrel (Halobaena caerulea) [51]. The volatile chemicals collected with gas-phase sampling techniques were well represented by low molecular weight compounds including sulfides, furans, and imidazole. These smaller VOCs—compounds with much greater vapour pressures—will disperse substantially further than the large waxy materials obtained in abundance from feathers by solvent extraction. Similarly, solvent-free gas-phase sampling techniques were used to capture the short-chain saturated and mono-unsaturated aldehydes that give rise to the distinctive citrus-like aroma of crested auklets (Aethia cristatella) and whiskered auklets (A. pygmaea) [52,53,54].
Given that methods for collecting volatiles from animal species are less well developed and may be more challenging compared with human research, the existing literature does not yet offer a representative reflection of natural non-human animal volatilomes. In the case of breath volatilomes, even if reproducible methods to collect VOCs from animals are developed, these may not be directly comparable with studies conducted with humans because human data are predominantly obtained from alveolar samples whereas collection from non-human animals principally represents whole-breath samples.
2.2. Methods for Volatilome Analysis
In addition to the issues of sample collection outlined above, major challenges in comparing volatilomes from different species arise due to variability between analytical methods, instrumentation, and misapplication of standards between studies. Gas chromatography with mass spectrometric detection (GC-MS) is the principal analytical technique that has been used for studying animal volatilomes, as it is suitable for analysing mixed chemical entities at very low abundance (e.g., pictogram levels). A range of specialised GC-MS based techniques have been developed to increase sensitivity and discrimination of VOCs, and these have been reviewed elsewhere [55,56]. However, analyte quantification obtained on one mass analyser cannot be compared to data acquired on different mass analysers. Instrument stability over time, as well as sample stability, need to be assessed for each instrument [57]. Furthermore, analytes tentatively identified from biological samples through mass spectral library matches (e.g., NIST) should be unequivocally confirmed by applying the same chromatographic methods to authentic standards so that retention times and mass spectral features of putative and reference compounds can be confidently compared. Unfortunately, in several studies, identification of chromatographic peaks has been based solely on mass spectral library matches without validation with synthetic materials [45,48,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73]. Such studies cannot identify chromatographic features with confidence.
Misidentification based on library searches can occur for many reasons, including co-elution chromatographic peaks, which may result in a mass spectrum reflecting the summation of the peak composition; the compound being absent from the MS library; and MS similarities, particularly for positional isomers of hydrocarbons. Misidentification is a concern when a compound is considered a distinguishing feature of the volatilome. Furthermore, over-identification is problematic. Mass spectral libraries cannot distinguish between optical isomers; a chiral GC-column would be necessary for chromatographic resolution of these. Nevertheless, many studies have reported a specific isomer despite using an achiral column. It may be that physiological or behavioural activity of an acquired optically active reference material has been demonstrated. Rarely have both isomers been compared or the identity of the natural VOC determined. Reducing the dependence on library matches and ensuring verification with chemical standards will improve the confidence of VOC identification within animal volatilomes.
2.3. Methods to Determine Allelochemical Function of Volatiles
Identification of specific putative allelochemicals with ecological relevance is investigated using a suite of field- and/or laboratory-based enquiries. For example, the relative appeal of specific hosts to tsetse flies (Glossina sp.) in the field can be measured from the ‘forage ratio’, which assesses the proportion of blood feeding taken from the host species in question, relative to its abundance in the environment [74,75]. Analysis of blood meals imbibed by tsetse flies reveals that a narrow selection of ungulate species, including ox (Bos indicus) and buffalo (Syncerus caffa) are most favoured, while others such as waterbuck (Kobus defassa), hartebeest (Alcelaphus buselaphus), and impala (Aepyceros melampus), despite being locally abundant, are avoided [76]. Such field-based findings indicate the feeding preference in natural conditions but offer no information about the chemical drivers behind this choice.
At the other end of the spectrum of methods exploring selective feeding behaviours are electrophysiological studies that measure the ability of individual chemicals or volatile mixtures to stimulate neuronal activity in arthropods (electroantennography, EAG). Frequently, electroantennographic detection is coupled with gas chromatography (GC-EAD) to determine which VOC components in a natural mixture stimulate activity, with mass spectral information typically collected in a separate step by matching retention indices of chromatographic features. A shortfall in these studies is that while a neuronal response signifies the presence of appropriate molecular machinery to recognise and perceive a compound, it gives no information about the behavioural role these VOCs may play.
A range of behavioural studies are used to elucidate the ecological chemistry at play, to determine if a host-derived chemical acts as an attractant (kairomone) or repellent (allomone) for a given arthropod. These behavioural tests may take the form of laboratory-based choice assays, using apparatus such as Y-tube olfactometers, or field-based lures and traps. Caution must be used in interpreting results for individual compounds assessed in laboratory studies, which might stimulate attraction of the test species compared to a neutral control. Although this provides preliminary evidence for a VOC’s ecological role—assuming it is a natural component of the host’s volatilome—in isolation from a range of other cues, it is difficult to determine if this behaviour replicates the response to the natural host. On the other hand, a lack of response to an isolated chemical is not sufficient to dismiss it as unimportant in host seeking. Currently, neither electrophysiological nor behavioural studies that assess individual chemicals or synthetic mixtures replicate the complexity of a natural host, which includes olfactory interactions and synergistic non-VOC signals such as temperature, humidity, and visual cues.
When neither electrophyisiological nor behavioural response is tested, the identity of allelochemicals within a volatilome sample has sometimes been based solely on conjecture from differing abundance in host vs. non-host species or extrapolated from the behavioural role that specific animal VOCs play in other host–pest relationships. For instance, Douglas et al. suggest that as octanal and hexanal stint bug secretions are potent repellents against invertebrate threats, their presence in crested auklet feathers also serves to fumigate nest sites from ectoparasites [53]. While possible, this assertion lacks evidence. In other research, when behavioural response to synthetic chemicals is documented without regard to the presence or absence of these compounds in the host volatilome [77], the ecological significance of these findings is questionable.
3. Known Mammalian and Avian Volatiles and Allelochemicals
There is ample evidence that kairomone and allomone VOCs create an olfactory landscape that vectors navigate in locating a suitable blood source [78,79,80]. Across all the reviewed literature, almost 1300 compounds have been reported as volatiles from non-human mammalian and avian species (Supplementary File 1). No chemical has been reported in all species reviewed. Indeed, half (52%) have only been detected from an individual species, and only a quarter (26%) of the compounds from non-human animal volatilomes have also been recorded from the human volatilome [29]. It is likely that the compounds in Supplementary File 1 will contain inaccuracies arising from the challenges described previously, and it is certainly far from complete.
This list of volatiles in Supplementary File 1, grouped by chemical class (acids, esters, aldehydes, ketones, alcohols, phenols, ethers and furans, hydrocarbons, nitrogen-containing, sulfur-containing, halogenated, and miscellaneous) can be navigated by considering the relevant CAS number for a compound of interest. Although much of the primary literature does not contain CAS numbers, their use should be encouraged to avoid ambiguity arising from non-IUPAC naming conventions, and they have been included where available. Another way to consider these data is to search for an analyte by chemical formula or molecular mass, which may be of value for researches pursuing possible identities of chromatographic features of interest. A subset of the total 1287 VOCs is presented here (Table 1), detailing those volatiles from common domestic species for which evidence of allelochemical potential in selected arthropod pests has been demonstrated.
Table 1.
A selection of the total (1287) volatile organic compounds (VOCs) collated from the literature from mammalian and avian emissions. The full table (Supplementary File 1) lists and compares the VOCs identified in 141 species across 17 orders. The compounds displayed in this excerpt, from a selection of domestic species, are those with physiological and/or behavioural evidence of allelochemical function in arthropod species of medical or veterinary significance.
| Chemical Class | CAS Number | Analyte | Chemical Formula | Molecular Mass | Arthropod Pest(s) for which VOC Displays an Allelochemical Role |
Horse (Equus equus) | Cattle (Bos taurus) | Goat (Capra aegagrus hircus) | Sheep (Ovis aries) | Pig (Sus scrofa) | Human (Homo sapiens) | Dog (Canis familiaris) | Guinea pig (Cavia porcellus) | Rabbit (Oryctolagus cuniculus) | Chicken (Gallus gallus) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hydrocarbons | 1120-21-4 | undecane | C11H24 | 156.1878 | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| hydrocarbons | 629-62-9 | pentadecane | C15H32 | 212.2504 | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| hydrocarbons | 544-76-3 | hexadecane | C16H34 | 226.2661 | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| hydrocarbons | 629-78-7 | heptadecane | C17H36 | 240.2817 | 1 | ✓ | |||||||||
| hydrocarbons | 6874-32-4 | 3,7-dimethyl-(Z)-oct-2-ene | C10H20 | 140.1565 | 9 | ✓ | ✓ | ||||||||
| hydrocarbons | 33501-88-1 | 2,3,6-trimethylhepta-1,5-diene | C10H18 | 138.1409 | 9 | ✓ | ✓ | ||||||||
| hydrocarbons | 13828-31-4 | 1-methyl-3-(1-methylethyl) cyclohexene | C10H18 | 138.1409 | 9 | ✓ | ✓ | ||||||||
| hydrocarbons | 637-50-3 | 1-propenylbenzene | C9H10 | 118.0783 | 9 | ✓ | ✓ | ||||||||
| hydrocarbons | 91-20-3 | naphthalene | C10H8 | 128.0626 | 1, 9 | ✓ | ✓ | ✓ | ✓ | ||||||
| hydrocarbons | 138-86-3 | limonene | C10H16 | 136.1252 | 1, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| hydrocarbons | 2436-90-0 | citronellene/dihydromyrcene | C10H18 | 138.1409 | 9 | ✓ | ✓ | ✓ | ✓ | ||||||
| hydrocarbons | 6753-98-6 | α-humulene/ α-caryophyllene |
C15H24 | 204.1878 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| hydrocarbons | 87-44-5 | β-caryophyllene | C15H24 | 204.1878 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| alcohols | 111-70-6 | 1-heptanol | C7H16O | 116.1201 | 9 | ✓ | ✓ | ||||||||
| alcohols | 543-49-7 | 2-heptanol | C7H16O | 116.1201 | 5 | ✓ | ✓ | ||||||||
| alcohols | 111-87-5 | 1-octanol | C8H18O | 130.1358 | 9 | ✓ | ✓ | ✓ | ✓ | ||||||
| alcohols | 123-96-6 | 2-octanol | C8H18O | 130.1358 | 5 | ✓ | ✓ | ||||||||
| alcohols | 589-98-0 | 3-octanol | C8H18O | 130.1358 | 9 | ✓ | ✓ | ✓ | ✓ | ||||||
| alcohols | 143-08-8 | 1-nonanol | C9H20O | 144.1514 | 9 | ✓ | ✓ | ✓ | |||||||
| alcohols | 112-30-1 | 1-decanol | C10H22O | 158.1671 | 3 | ✓ | |||||||||
| alcohols | 1120-06-5 | 2-decanol | C10H22O | 158.1671 | 9 | ✓ | ✓ | ||||||||
| alcohols | 928-96-1 | (Z)-3-hexen-1-ol | C6H12O | 100.0888 | 9 | ✓ | ✓ | ||||||||
| alcohols | 928-92-7 | 4-hexen-1-ol | C6H12O | 100.0888 | 1 | ✓ | |||||||||
| alcohols | 4938-52-7 | 1-hepten-3-ol | C7H14O | 114.1045 | 1 | ✓ | |||||||||
| alcohols | 3391-86-4 | 1-octen-3-ol | C8H16O | 128.1201 | 2, 4, 5, 7, 8, 9 | ✓ | ✓ | ✓ | ✓ | ||||||
| alcohols | 123-51-3 | 3-methyl-1-butanol/isopentanol | C5H12O | 88.0888 | 2 | ✓ | ✓ | ||||||||
| alcohols | 104-76-7 | 2-ethylhexanol | C8H18O | 130.1358 | 1, 7, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| alcohols | 107-21-1 | ethan-1,2-diol/ethylene glycol | C2H6O2 | 62.0368 | 3 | ✓ | |||||||||
| alcohols | 56-81-5 | 1,2,3-propanetriol/glycerol | C3H8O3 | 92.0473 | 1, 3 | ✓ | |||||||||
| alcohols | 18479-58-8 | dihydromyrcenol | C10H20O | 156.1514 | 1 | ✓ | ✓ | ||||||||
| alcohols | 78-70-6 | linalool | C10H18O | 154.2530 | 1, 9 | ✓ | ✓ | ✓ | ✓ | ||||||
| alcohols | 89-78-1 | menthol | C10H20O | 156.1514 | 1 | ✓ | ✓ | ||||||||
| alcohols | 106-22-9 | dihydrogeraniol/citronellol | C10H20O | 156.1514 | 9 | ✓ | |||||||||
| alcohols | 57-88-5 | cholesterol | C27H46O | 386.3549 | 3 | ✓ | |||||||||
| alcohols | 100-51-6 | benzyl alcohol | C7H8O | 108.0575 | 3 | ✓ | ✓ | ✓ | ✓ | ||||||
| phenols | 108-95-2 | phenol | C6H6O | 94.0419 | 6, 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| phenols | 108-39-4 | 3-methylphenol/ m-cresol |
C7H8O | 108.0575 | 4, 6, 7, 9 | ✓ | ✓ | ✓ | ✓ | ||||||
| phenols | 106-44-5 | 4-methylphenol/ p-cresol |
C7H8O | 108.0575 | 2, 4, 5, 6, 7, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| phenols | 620-17-7 | 3-ethylphenol | C8H10O | 122.0732 | 4, 5, 7 | ✓ | |||||||||
| phenols | 123-07-9 | 4-ethylphenol | C8H10O | 122.0732 | 2, 6, 7 | ✓ | ✓ | ||||||||
| phenols | 621-27-2 | 3-propylphenol | C9H12O | 136.0888 | 4, 5, 6, 7 | ✓ | ✓ | ✓ | |||||||
| phenols | 645-56-7 | 4-propylphenol | C9H12O | 136.0888 | 7 | ✓ | ✓ | ||||||||
| phenols | 499-75-2 | 3-isopropyl-6-methylphenol | C10H14O | 150.1045 | 4 | ✓ | ✓ | ||||||||
| phenols | 90-05-1 | 2-methoxyphenol | C7H8O2 | 124.0524 | 4, 5, 7, 9 | ✓ | ✓ | ✓ | ✓ | ||||||
| phenols | 119-33-5 | 4-methyl-2-nitrophenol | C7H7NO3 | 124.0524 | 9 | ✓ | |||||||||
| acids | 64-18-6 | methanoic acid/formic acid | CH2O2 | 46.0055 | 1 | ✓ | |||||||||
| acids | 64-19-7 | ethanoic acid/ acetic acid |
C2H4O2 | 60.0211 | 1, 3, 5, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| acids | 79-09-4 | propanoic acid | C3H6O2 | 74.0368 | 1, 2, 5, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| acids | 107-92-6 | butanoic acid | C4H8O2 | 88.0524 | 1, 2, 5, 8, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| acids | 109-52-4 | pentanoic acid/valeric acid | C5H10O2 | 102.0681 | 1, 2, 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| acids | 142-62-1 | hexanoic acid | C6H12O2 | 116.0837 | 1, 2, 3, 8, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| acids | 111-14-8 | heptanoic acid | C7H14O2 | 130.0994 | 1, 2, 3, 8 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| acids | 124-07-2 | octanoic acid | C8H16O2 | 144.115 | 1, 2, 3 | ✓ | ✓ | ✓ | ✓ | ||||||
| acids | 112-05-0 | nonanoic acid | C9H18O2 | 158.1307 | 1, 2, 3 | ✓ | ✓ | ||||||||
| acids | 334-48-5 | decanoic acid | C10H20O2 | 172.1463 | 1, 2, 3 | ✓ | ✓ | ✓ | |||||||
| acids | 112-37-8 | undecanoic acid | C11H22O2 | 186.162 | 1, 2, 3 | ✓ | |||||||||
| acids | 143-07-7 | dodecanoic acid | C12H24O2 | 200.1776 | 1, 2, 3 | ✓ | ✓ | ✓ | ✓ | ||||||
| acids | 638-53-9 | tridecanoic acid | C13H26O2 | 214.1933 | 1, 2, 3 | ✓ | ✓ | ||||||||
| acids | 544-63-8 | tetradecanoic acid/myristic acid | C14H28O2 | 228.2089 | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| acids | 1002-84-2 | pentadecanoic acid | C15H30O2 | 242.2246 | 1, 3 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| acids | 57-10-3 | hexadecanoic acid/palmitic acid | C16H32O2 | 256.2402 | 1, 2, 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| acids | 506-12-7 | heptadecanoic acid | C17H34O2 | 270.2559 | 1, 3 | ✓ | ✓ | ✓ | ✓ | ||||||
| acids | 57-11-4 | octadecanoic acid/stearic acid | C18H36O2 | 284.2715 | 1, 2, 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| acids | 79-31-2 | 2-methylpropanoic acid/isobutyric acid | C4H8O2 | 88.0524 | 5, 8, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| acids | 503-74-2 | 3-methylbutanoic acid/isovaleric acid | C5H10O2 | 102.0681 | 1, 2, 5, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| acids | 27960-21-0 | (E)-3-methyl-2-hexenoic acid | C7H12O2 | 128.0837 | 2 | ✓ | |||||||||
| acids | 18719-24-9 | 7-octenoic acid (+CO2) | C8H14O2 | 142.0994 | 2 | ✓ | |||||||||
| acids | 50-21-5 | 2-hydroxypropanoic acid/lactic acid | C3H6O3 | 90.0317 | 1, 2, 3, 6, 7 | ✓ | |||||||||
| acids | 98-89-5 | cyclohexanecarboxylic acid | C7H12O2 | 128.0837 | 5 | ✓ | ✓ | ||||||||
| esters | 698-76-0 | δ-octalactone | C8H14O2 | 142.0994 | 4, 5 | ||||||||||
| esters | 105-66-8 | propyl butanoate | C7H14O2 | 130.0994 | 9 | ✓ | ✓ | ✓ | |||||||
| acids | 65-85-0 | benzoic acid | C7H6O2 | 122.0368 | 1, 3, 8 | ✓ | ✓ | ✓ | ✓ | ||||||
| aldehydes | 123-38-6 | propanal | C3H6O | 58.0419 | 3 | ✓ | ✓ | ||||||||
| aldehydes | 66-25-1 | hexanal | C6H12O | 100.0888 | 1, 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| aldehydes | 111-71-7 | heptanal | C7H14O | 114.1045 | 1, 3, 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| aldehydes | 124-13-0 | octanal | C8H16O | 128.1201 | 1, 4, 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| aldehydes | 124-19-6 | nonanal | C9H18O | 142.1358 | 1, 3, 4, 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| aldehydes | 112-31-2 | decanal | C10H20O | 156.1514 | 1, 4, 7, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| aldehydes | 112-44-7 | undecanal | C11H22O | 170.1671 | 4 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| aldehydes | 112-54-9 | dodecanal | C12H24O | 184.1827 | 1, 4 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| aldehydes | 18829-55-5 | (E)-2-heptenal | C7H12O | 112.0888 | 5, 4 | ✓ | ✓ | ✓ | |||||||
| aldehydes | 18829-56-6 | (E)-2-nonenal | C9H16O | 140.1201 | 1, 5, 7 | ✓ | ✓ | ✓ | ✓ | ||||||
| aldehydes | 53448-07-0 | (E)-2-undecenal | C11H20O | 168.1514 | 4 | ✓ | ✓ | ||||||||
| aldehydes | 432-25-7 | β-cyclocitral | C10H16O | 152.1201 | 9 | ✓ | |||||||||
| aldehydes | 100-52-7 | benzaldehyde | C7H6O | 106.0419 | 1, 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| aldehydes | 122-78-1 | phenylacetaldehyde | C8H8O | 120.0575 | 3, 7 | ✓ | ✓ | ✓ | ✓ | ||||||
| ketones | 67-64-1 | acetone | C3H6O | 58.0419 | 1, 2, 6, 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| ketones | 78-93-3 | butanone | C4H8O | 72.0575 | 1, 6, 7 | ✓ | ✓ | ✓ | ✓ | ||||||
| ketones | 107-87-9 | 2-pentanone | C5H10O | 86.0732 | 1 | ✓ | ✓ | ✓ | ✓ | ||||||
| ketones | 96-22-0 | 3-pentanone | C5H10O | 86.0732 | 1 | ✓ | ✓ | ||||||||
| ketones | 110-43-0 | 2-heptanone | C7H14O | 114.1045 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| ketones | 111-13-7 | 2-octanone | C8H16O | 128.1201 | 4, 5 | ✓ | ✓ | ||||||||
| ketones | 106-68-3 | 3-octanone | C8H16O | 128.1201 | 9 | ✓ | ✓ | ✓ | ✓ | ||||||
| ketones | 821-55-6 | 2-nonanone | C9H18O | 142.1358 | 5, 4 | ✓ | ✓ | ||||||||
| ketones | 693-54-9 | 2-decanone | C10H20O | 156.1514 | 1, 4 | ✓ | |||||||||
| ketones | 112-12-9 | 2-undecanone | C11H22O | 170.1671 | 9, 4 | ✓ | ✓ | ||||||||
| ketones | 6175-49-1 | 2-dodecanone | C12H24O | 184.1827 | 4 | ✓ | ✓ | ||||||||
| ketones | 110-93-0 | 6-methyl-5-hepten-2-one | C8H12O | 124.0888 | 1, 2, 7, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| ketones | 3796-70-1 | (E)-geranylacetone | C13H22O | 194.1671 | 1, 2, 4, 5, 9 | ✓ | ✓ | ✓ | |||||||
| ketones | 7764-50-3 | dihydrocarvone | C10H16O | 152.1201 | 9 | ✓ | |||||||||
| ketone | 76-22-2 | camphor | C10H16O | 152.1201 | 5 | ✓ | ✓ | ✓ | |||||||
| ketones | 98-86-2 | acetophenone | C8H8O | 120.0575 | 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| amines | 7664-41-7 | ammonia | NH3 | 17.0265 | 1, 2 | ✓ | ✓ | ✓ | |||||||
| amines | 120-72-9 | indole | C8H7N | 117.0578 | 1, 2, 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| amines | 83-34-1 | 3-methylindole/skatole | C9H9N | 131.0735 | 5, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| halogenated | 75-09-2 | dichloromethane | CH2Cl2 | 83.9534 | 1 | ✓ | ✓ | ||||||||
| halogenated | 106-46-7 | 1,4-dichlorobenzene | C6H4Cl2 | 145.969 | 1 | ✓ | ✓ | ||||||||
| sulfides | 75-18-3 | dimethyl sulfide | C2H6S | 62.019 | 1 | ✓ | ✓ | ✓ | ✓ | ||||||
| sulfides | 624-92-0 | dimethyl disulfide | C2H6S2 | 93.9911 | 1 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| sulfides | 110-81-6 | ethyl disulfide | C4H10S2 | 122.0224 | 1 | ✓ | |||||||||
| sulfides | 2179-60-4 | methyl propyl disulfide | C4H10S2 | 122.0224 | 1, 3 | ✓ | |||||||||
| sulfides | 3658-80-8 | dimethyl trisulfide | C2H6S3 | 125.9632 | 1, 3, 5, 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| sulfides | 75-15-0 | carbon disulfide | CS2 | 75.9441 | 1 | ✓ | ✓ | ✓ | ✓ | ||||||
| thiazole | 95-16-9 | benzothiazole | C7H5NS | 135.0143 | 2 | ✓ | ✓ | ✓ |
1Aedesaegypti. 2Anophelesgambiae. 3Culexquinquefasciatus. 4Glossinamorsitans. 5Glossinapallipides. 6Culicoidesimpunctatus. 7Culicoidesnubeculosus. 8Rhipicephalusmicroplus. 9Stomoxyscalcitrans.
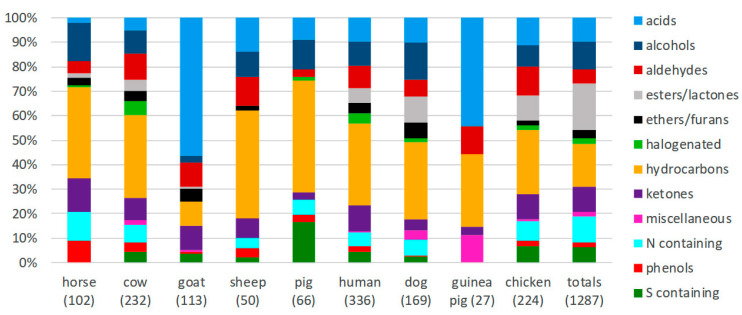
While the specific identity of volatile compounds varies, the diversity of VOCs in different chemical classes is similar between species, with hydrocarbons making up a large proportion (Figure 1). The exceptions to this trend are for those species from which data are scarce, where the reported distribution of VOCs may be heavily biased by a small number of studies. For example, records of caprine volatile emissions are swayed significantly by a small number of studies exploring fleece extracts, which are dominated by organic acids, so their emissions may be disproportionately represented by these VOCs [81,82].
Figure 1.
Relative proportion of volatile organic compounds (VOCs) in different chemical classes that have been documented from domestic animal emissions, and across all species reviewed. Numbers in parentheses are the total number of unique compounds reported for the species. Percentages indicate the relative abundance of chemical entities described in each compound class, not their relative concentrations.
Similarly, the data are predisposed to reflect the chemical collection and analysis techniques used. Research with a specific focus or methodology, may over—or under—represent the VOC diversity and chemical class distribution, rather than reflecting the total volatilome composition. For instance, information on avian VOC profiles is based largely on samples collected using solvent extraction of waxy uropygial gland contents and feather materials. The uropygial, or preen gland, is a key source of avian oils, which are applied to feathers to aid water-repellency, thermoregulation, hygiene, and in some instances controlling fungal and bacterial flora [83,84,85,86]. The chemical profiles of these oils are species specific [87] and are predominantly complex mixtures of high-molecular weight esters with characteristic branching patterns and alcohol-acid combinations. Typical esters are composed of C20–C40 (and up to C50) carbon chains [49,50,73,88]. Conversely, almost all odorant chemicals, regardless of their structure or proportions, are compounds with a molecular weight below 300–400Da [89] Although abundant, the physical properties of uropygial and feather waxes means they are unlikely to feature prominently in birds’ volatile odour profiles at ambient temperature. Some authors even suggest that the low volatility of uropygial waxes reduce olfactory conspicuousness to predators [90].
Many studies into host chemistry and vector perception have been performed with different agenda, so that either the ecological significance of volatilome components is unknown, or host attraction has not been pursued with rigorous chemical analysis. Research with a specific focus or methodology may misrepresent the compound diversity and chemical class distribution, rather than reflecting the total volatilome composition. The presence of unique compounds in a given volatilome has often been interpreted as implicit evidence for the relevance of these as allelochemicals in vector–host interaction. Without at least electrophysiological data, and ultimately behavioural studies to reinforce these hypotheses, these inferences remain unproven.
Collecting VOCs from animals is significantly more challenging than from human sources. Given this challenge, it is possible that some compounds reported in Supplementary File 1 are not of endogenous origin but arise through sample contamination during collection or storage, through degradation, or changes brought about by measurement/analysis activities, e.g., oxidation or rearrangement with heating. There are significant gaps in our understanding of the origin of many of the VOCs reported, including absences from otherwise continuous homologous series. Are these absences real (and significant), or do they reflect a failure of detection? The occurrence in several studies of compounds known to be plasticisers or material stabilisers needs to be viewed with suspicion as potential contaminants from collection apparatus.
4. Animal Volatilomes Influence Host–Pest Interaction
It is well established that odours derived from humans attract insect vectors [91,92]. Similarly, in non-human animals, there is ample evidence for the existence of volatile allelochemical odour clouds that influence interspecies interactions. This section describes examples in which the presence of allelochemicals within host emissions is indicated, but where the identity of the inherent chemicals has not been determined. The following section describes identified allelochemicals within host emissions.
Despite their limited environmental mobility, several tick species are recognised to exhibit marked host preference. For instance, breeds of domestic cattle that belong to Bos taurus taurus subspecies (e.g., Holstein) suffer significantly greater prevalence and severity of tick-fever compared with B. taurus indicus breeds (e.g., Nelore) and their crosses [93,94]. B. taurus indicus breeds have a high degree of innate resistance to Babesia parasites and are marginally more resistant to Anaplasma parasites [95,96,97]—both of which can cause tick-fever—transmitted by cattle ticks Rhipicephalus (Boophilus) microplus sensu lato [93,98,99]. While both tick-susceptible and tick-resistant cattle breeds attract tick larvae, significantly more are attracted to olfactory cues in skin rubbings from Holstein calves than those from Nelore calves [100]. Genes that encode enzymes for volatile compound production are expressed at significantly higher levels in susceptible compared with tick-resistant breeds, which may contribute to their increased attractiveness to ticks. In response to biting ticks, an inflammatory response is also exhibited earlier in tick-resistant hosts, correlating with decreased production of tick salivary proteins. Not only do B. taurus taurus cattle develop more debilitating disease when exposed to tick-fever organisms, but they are also at increased risk of exposure as they are more attractive hosts to the tick vectors [100,101,102]. The identity of the attractive or repellent volatiles involved has not been determined. Similarly, brown dog ticks (Rhipicephalus sanguineus) have markedly different reproductive and development success after feeding on different dog breeds [103,104]. Beagles are unusual in that they are tick-resistant, compared to English cocker spaniels, which are particularly susceptible. Ticks are significantly more attracted to extracts of hair or skin rubbings from tick-susceptible compared to tick-resistant canines [105].
Volatiles cues that provoke differential attraction to specific mosquitoes are well explored, largely in the context of understanding anthropophagic feeding habits amongst a mosaic of alternative host options. Zoophilic and ornithophilic mosquito species also respond selectively to host-derived VOCs. Emissions from avian and bovine blood provoke different attraction and landing responses for female Aedes aegypti and Culex quinquefasciatus mosquitoes [4]. These differences mirror the host preferences of zoophilic or ornithophilic species, respectively. Volatile emissions from bacteria originating from human skin attract a significantly higher proportion of Anopheles gambiae (a highly anthrophophilic species) compared to An. arabiensis (a generalist species), whereas the inverse is seen for volatiles emitted by chicken skin flora. Equally, while An. gambiae demonstrates a preference for bacterial species that are strongly associated with humans, An. arabiensis is uniformly attracted to emissions produced by four diverse bacterial species evaluated, perhaps reflecting the broad range of hosts with their associated microbiota on which it feeds [78].
Host-associated microbial volatiles influence other pest arthropods, such as several species of predatory fly. Odours released from mixed-species bacterial communities colonising wounds on warm-blooded animals act as kairomones for screwworm fly species (Cochliomyia sp.), which are attracted to and lay their eggs in these lesions [106]. Volatiles released from necrotic tissues are important for advertising oviposition substrates to gravid female screwworm flies [107], which are particularly attracted to tissues already infested with screwworm larvae and wound-associated bacteria [108,109].
As well as odours from live animals and their resident microbial flora, urine and faecal odours and decaying animal remains can also act as arthropod attractants. Tsetse flies are attracted to urine, in particular aged urine, from domestic ox (Bos indicus), African buffalo (Syncerus caffer), and bush pig (Potomochoerus porcus) [110,111]. Faecal waste accumulation increases the attraction of Aedes and Culex mosquitoes towards live hamsters [112]. Presumably aged waste residues are important to pest species as they indicate reliable host habitat [106].
These studies provide abundant evidence that VOCs play as important a role in animal–pest interactions, as they do in human–pest interactions. In these examples, a behavioural response of an arthropod to volatile emissions from a range of animal compartments or animal-associated materials, has been demonstrated. In the following section, evidence that specific chemical analytes act as allelochemicals within these emissions is reviewed.
5. Allelochemical VOCs Identified from the Volatilomes of Animals
Carbon dioxide (CO2), a ubiquitous respiratory compound, plays important roles in the behaviours of a wide range of arthropods, especially in insect–vertebrate interactions [113]. It is well-established as a kairomone for foraging blood-seeking insects and is particularly effective in luring generalist mosquito species [114,115]. Data from a range of mosquito species and their mammalian hosts indicate that it dictates at least two types of interactions: It activates host searching, and, in the presence of other host factors, facilitates attraction with orientation and movement towards the source [116]. It also demonstrates a synergistic effect when combined with other host odours [117,118].
Octenol (1-octen-3-ol) is another widespread VOC that can be either a kairomone or allomone when released by vertebrates [119]. First identified as an attractant of tsetse flies (Glossina sp.)—vectors of African trypanosomiasis—to cattle [120], it is also very effective at attracting other haematophagous insects to hosts that emit it. In combination with CO2 and other olfactory cues, it is an important kairomone for zoophilic and anthropophilic mosquitoes such as Aedes and Anopheles [121,122,123], and female biting midges, Culicoides impunctatus, and C. nubeculosus [124], which feed mainly on large mammals such as equids and bovids. Conversely, a lower response or allomonal effect is observed for Culex species, which are generally regarded as having ornithophilic feeding preferences [125].
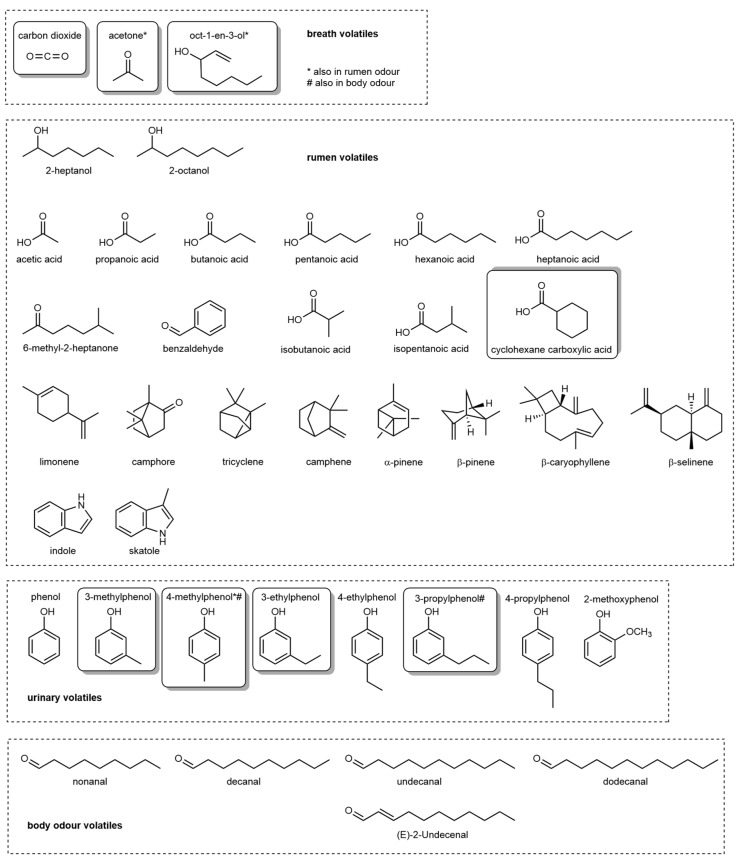
In addition to the compounds described above, which are common to a range of host–pest interactions, specific VOCs discussed below, have been shown to be important for certain taxa. Aside from human VOCs, bovine volatiles are probably among the most thoroughly investigated odours in the context of attracting arthropod pests. The area developed from the 1970–1980s’ research on tsetse flies, which formed the groundwork for laboratory and field studies into host finding by chemosensory cues [126]. A 1984 study capturing whole animal emissions from oxen held in tents for up to 80 h collected compounds holistically from skin, breath, rumen eructations, urine, and faeces [120]. Screening total volatile emissions led to the identification of a number of potent tsetse allelochemicals, including CO2 [127], acetone [77], and octenol [120,128] in the bovine odour cloud (Figure 2).
Figure 2.
Olfactory attractants of tsetse flies (Glossina) from bovine volatile emissions. Broken outlines delineate the different odour compartments from which the analytes are emitted. Solid boxes identify chemical structures that have been shown to demonstrate both electroantennographic and behavioural activity.
Ruminant breath is regularly and frequently contaminated with rumen gases during eructation [129,130]. Metabolic end products that are eructed, such as methane and volatile fatty acids (VFAs), mix with exhaled air when it is expelled via the upper airways. Metabolites that are systemically absorbed from the digestive tract can also be measured in breath as they are eliminated into the airways by blood-gas exchange in the alveoli. Consequently, emanations from breath and rumen gases are often considered together. Despite limited efforts exploring the bovine breath volatilome as an avenue to develop diagnostic tools as indicators of animal health status [45,61,62,63,129,131,132], which follow a parallel path to the flourishing field of human breath analysis [22,23,24,31,133,134,135], few studies have explored animal respiratory emissions as a source of kairomones in arthropod interactions.
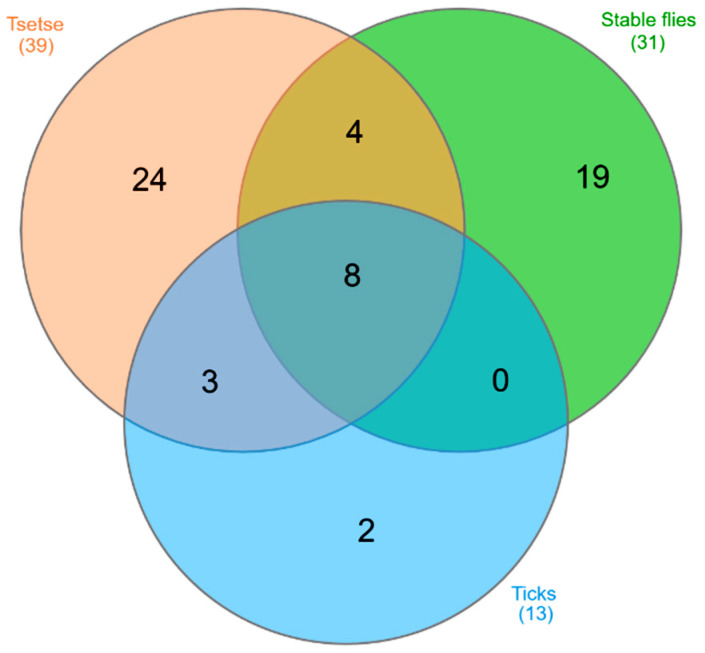
Those studies exploring odours associated with rumen fluid show that at least 60 rumen volatiles are attractive to various arthropod pests, including hard tick species [136], tsetse flies [130], and stable flies [137]. Some of these allelochemicals appear to be universally attractive across a range of species, signifying the relevance of rumen eructations to host-seeking arthropods, but others have been noted as attractants in only one or two pest groups (Figure 3). Harraca et al. [130] suggest that the profile of VOCs regularly eructed by ruminants provides chemostimuli that enable tsetse flies to locate their preferred hosts. At least 30 compounds in emissions from bovine rumen contents have been shown to elicit electrophysiological and/or behavioural activity in the stable fly (Stomoxys calcitrans), a serious pest of large ungulates such as cattle (Table 1). Of these, 4-methylphenol, dimethyl trisulfide, and butanoic acid were noted for their role as kairomones [137].
Figure 3.
Number of bioactive volatiles from bovine rumen odour that have been shown to stimulate electrophysiological and/or behavioural activity in tsetse fly (39), stable fly (31), and tick (13) species. Numbers in parentheses indicate the total number of allelochemicals in rumen emissions for each pest type; numbers in compartments of the figure show the chemicals with allelochemical function that are shared by different arthropod taxa [138].
Unsurprisingly, in their passage through the digestive tract, many of the VOCs that are detectable in rumen fluid also ultimately reach faecal emissions. Odours common to both sources (dimethyl trisulphide, butanoic, acid and p-cresol) may act as kairomones to attract tabanids to both oviposition sites (faeces) and nutrition sources (living ruminant host) [137]. Though the two behaviours (feeding and oviposition) rely on attraction to the resource in question (host body and faeces, respectively), it is difficult to establish in laboratory and field studies whether the role of the common chemicals as attractants is relevant to both life history scenarios.
Urine of several ungulate species contains kairomones for many arthropod pests. Tsetse flies are particularly attracted to urinary residues from domestic ox (B. indicus), African buffalo (S. caffer), or bush pig (P. porcus) [110,111], in particular when aged. Unbranched alkyl phenols in these emissions were confirmed through laboratory [139] and field bioassays [139,140] as the attractive components (Figure 2). These kairomones have also been assessed against tabanids flies, including horseflies and deerflies (Tabanus bromis and Atylotus quadrifarius), which are large, diurnal, somewhat opportunistic haematophagous insects that are significant livestock pests and vectors of animal pathogens [141,142]. The urinary phenols elicited electroantennographic responses and increased trap catches [141] but in isolation, the synthetic VOCs were less attractive to tabanids than natural sources of ungulate urine [143]. Addition of ammonia synergistically enhanced their attractive properties [142].
Ammonia accumulates in urine over time with aerobic fermentation of urea, accounting for the increased attractiveness of aged urine. Aging of urine also increases the levels of the phenolic attractants themselves [144]. While trace quantities of the free phenols exist in fresh buffalo urine, the majority are present as glucuronate and sulfate conjugates [111]. Specific microorganisms liberate the attractive phenols by hydrolytic activity over time. Sterile urine samples, or those lacking the appropriate microbes, do not yield appreciable quantities of free phenols [144]. This interplay between host kairomones, microbial activity, and insect pests mirrors the changes seen in attractiveness of fresh and aged human sweat samples to Anopheles gambiae [6].
Studies of semiochemical emissions that contribute to territory marking pheromones are well represented from various carnivores [145,146,147,148,149,150,151], but less attention has been given to the existence of allelochemicals in their volatilomes and their role in influencing host location by arthropod pests. Like other mammalian hosts, domestic dogs (Canis lupus familiaris) are susceptible to blood-feeding arthropods and the pathogens they transmit. Extracts of hair and skin rubbings from beagles contained an abundance of analytes that were absent from cocker spaniel extracts, suggesting the differential attraction of brown dog ticks, R. sanguineus, to different canine races might be due to these differences. Synthetic preparations of the beagle-specific volatiles 2-hexanone and benzaldehyde, and to a lesser extent undecane, acted as non-host allomones to orchestrate avoidance by ticks [152].
Relative to what is known of mammalian volatiles, the chemosensory information contained in the volatilomes of birds has been comparatively underexplored. Campagna and colleagues’ review of avian chemicals highlights a bias for analysis of solvent extractable compounds from the uropygial gland contents and feathers [88], focusing on pheromone-based intraspecific olfactory communication. Fewer studies have investigated allelochemicals driving host–parasite interactions (kairomones or allomones). No doubt practical challenges to collecting volatile odours from avian subjects plays into this, but it is also likely that until relatively recently the significance of avian allelochemicals has been overlooked.
Despite the abundance of low volatility uropygial waxes in oily feather coatings, volatile constituents that can act as long-range allelochemicals are also present. Supporting this are studies demonstrating that chicken feathers or their extracts attract Ae. aegypti, Cx. quinquefasciatus, and Cx. nigripalpus (but not Cx. tarsalis) in dual-port olfactometer experiments [50]. Although dominated by high molecular weight, non-volatile compounds—the uropygiols [153]—these extracts also contain compounds that are candidates for air-borne mosquito-attractants. These volatile attractants include ketones, alcohols, aldehydes, C6–C9 carboxylic acids and various saturated and unsaturated hydrocarbons [49,50,73].
On the other hand, the response rate for several mosquito species was substantially greater when presented with a whole live chicken than with isolated feathers, CO2, or a combination of feathers and CO2 [50]. This suggests a component of avian kairomones must originate from unexplored components of integument and breath, or other compartments. The allelochemical contribution of VOCs from skin of live birds has been largely unexplored. Unlike mammalian hair follicles, feather follicles do not contain glands. Instead, the entire avian skin acts as a sebum-producing holocrine unit, with the potential for odour production and secretion [47].
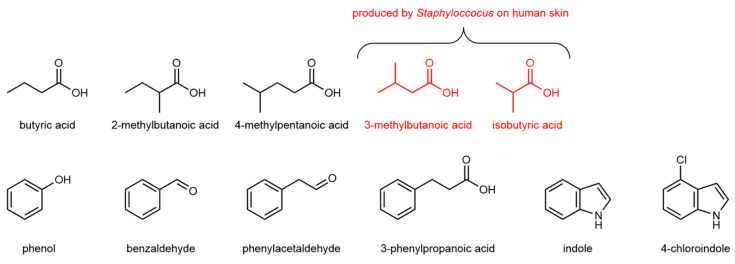
Odour production in avian species may also involve microbial action on non-volatile integument precursors to produce smaller, more-volatile metabolites [49,88]. The malodorous uropygial secretions of hoopoes (Upupidae) and woodhoopoes (Phoeniculidae) appear to depend on the symbiosis of Enterococcus bacteria [154,155]. The volatile emissions include phenolic and indolic compounds, and branched short-chain organic acids similar to those produced by Staphylococcus metabolism of human sweat [156] (Figure 4). If administered antibiotics eliminate the birds’ uropygial flora, many of the volatiles contributing to the pungent odours are lost [86]. Although it has not been established whether these modifications influence host-finding behaviour by arthropod pests, the significance of microbial participation in vector attraction has been recognised for the human skin microbiome: The compounds produced by microbial degradation of human sweat elicit physiological and behavioural responses in anthropophilic mosquitoes [6,157,158,159,160].
Figure 4.
Volatile organic compounds derived from microbial metabolism of hoopoe uropygial secretions [86]. Generation of malodorous fatty acid shows similarities to the compounds produced by cutaneous bacteria on human skin [156].
Crested (Aethia cristatella) and whiskered auklets (A. pygmaea) both produce hexanal, hexanoic acid, octanal, and (Z)-4-decenal in their volatilomes [54]. Synthetic mixtures of these compounds act as allomones to Ixodes uriae and Amblyomma americanum ticks [54], and inhibit landing of the yellow fever mosquito (Aedes aegypti) [161]. Chewing lice in the genera Austromenopon and Quadraceps also showed adverse responses when exposed to high concentrations of octanal and (Z)-4-decenal. The relevance of these anti-parasitic responses is still uncertain; it has been suggested that the volatile emissions of crested auklets may act as broad-spectrum deterrents against various arthropods [162].
Faecal odour is a conspicuous source of volatile compounds from birds [47]. Cooperband et al. (2008) showed in dual-choice experiments that chicken faeces attracted Culex quinquefasciatus [163]. Mosquito attractant assays and electroantennograms indicated that faecal fractions contained physiologically active compounds including (E)-2-decenal, nonanal, undecanal, dodecanal, tetradecanal, pentadecanal, hexadecanal, heptadecanal, and octadecanol. In birds, faecal contamination of nest materials can contribute significantly to the odours emitted from nests, which may be relevant to blood-feeding vectors by way of advertising host habitat [164]. Parent birds frequently perform nest sanitation by removing nestling excrement, which is hypothesised to reduce the intensity of predation, bacterial contamination, arthropod parasitism, and ultimately increase fledgling success [165,166]. Given that avian faeces are recognised as an odour source, it is surprising that few studies have explored the molecular nature of this odour from the perspective of inadvertent signalling to ornithophagic pests.
6. How Allelochemicals Influence Host Selection
The above highlights that VOCs in animal volatilomes act as allelochemicals. In this section, the different ways that allelochemicals influence host selection are examined. Allelochemicals can act as kairomones or allomones allowing pest species to fine-tune their feeding choice. The interplay between host-derived chemicals that allows vectors to make specific foraging decisions regarding their preferred host species also permits them to select the individual, and even the body area on which to feed. There is evidence to suggest that some arbovectors are attracted to hosts that are already infected with the agent that they transmit.
6.1. Allelochemicals Used to Distinguish Host and Non-Host Species
Allelochemicals are an important factor in an arthropods’ ability to distinguish and choose a host from a non-host animal. Selection could be driven by the presence of kairomones or the absence of allomones, or the varying levels of both in the volatilome of a potential host.
A good example is from tsetse flies where electrophysiological studies suggest that the pests may use both attractive aldehydes and a range of deterrents to distinguish between preferred (ox and buffalo) and non-preferred (waterbuck) hosts [76]. Preferred host volatilomes contain medium-chain (C7-C11) saturated and unsaturated aldehydes, and phenolic components identical to those found in fermented host urine. Although the volatilome of non-preferred waterbuck also contains some of these compounds (4-methylphenol and 3-n-propylphenol), it includes fewer of the tsetse-attractive aldehydes. The non-host volatilome includes compounds that act as allomones: 2-methoxyphenol (guaiacol), 3-isopropyl-6-methylphenol, δ-octalactone, an array of methyl-ketones (C8–C13), and straight-chain fatty acids (C5–C9). The difference in body odour between these bovids—creating a kairomonal lure in the case of oxen and buffalos, and an allomonal defense for waterbuck—provides a chemical rationale for host discrimination by tsetse flies.
Certain chemicals can act as either kairomone or allomone for different arthropod species. L-lactic acid is a kairomone for vectors that are anthropophagic, such as the human malaria vector An. gambiae [10], and Ae. aegypti [91], but is an allomone for zoophilic and ornithophilic mosquitoes [91] and for tsetse flies, which avoid feeding on humans [167]. The effect of acetone and dimethyl disulfide also depends on the species under investigation: Mosquito response to these human-associated volatiles mirrors their preference for human, other mammalian or avian hosts [91].
6.2. Culex Host Switching
Several mosquito species in the Culex genus can divert their normally ornithophagic preference to different hosts, based on the availability of the hosts. For instance, when avian hosts migrate away in late summer, Cx. pipiens turns its feeding bias towards mammals, including humans [168]. Similarly, the feeding preference of Cx. annulirostris in Australia, which acts as a vector for arboviruses with zoonotic potential (e.g., Ross River, Kunjin and Murray Valley encephalitis viruses), differs depending on regional availability of mammals or birds [73]. It has been suggested that nonanal, a major constituent in the headspace volatiles from human skin, whole chickens, and domestic pigeons (Columba livia), and a demonstrated kairomone to Cx. pipiens quinquefasciatus, allows this host-switching behaviour to occur [15].
6.3. Allelochemicals That are Used to Isolate Individual Hosts within a Population
The tendency for certain people to be more heavily preyed upon by mosquitos than others is anecdotally well-recognised. Similar intraspecific preferences for individual animals within a population have been identified among other arthropods. Differences in the attractiveness of individual cattle towards haematophagic pests is likely attributable to their VOC phenotype. Holstein-Friesian heifers (B. taurus) exhibit variable susceptibility towards horn flies (Haematobia irritans) [169]. Fly-resistant or fly-vulnerable individuals display differences in volatile chemical emissions, with several of these chemicals reported in other studies as allelochemicals governing interactions of haemotophagous pests [170,171].
Some vectors can discriminate the pathogen infection status of individual hosts and apparently choose accordingly. Domestic canaries (Serinus canaria) that are chronically infected with the avian malaria parasite (Plasmodium relictum) are more attractive to Cx. pipiens mosquitoes than uninfected controls. Conversely, birds suffering from acute infection are less attractive [172]. Whether infection-induced changes to the host volatilome profiles have adaptive significance to the malaria parasite in terms of attracting blood-seeking vectors, is uncertain. A previous study of birds exposed to natural malaria infections, in which the duration of the disease for each individual was unknown, reported greater attractiveness of uninfected hosts [173].
Dogs play an important role as reservoirs for Leishmania infantum, which has significant zoonotic potential, primarily through transmission by sand fly vectors (Lutzomayia longipalpis). Hair from dogs with leishmaniasis contains a variety of VOCs that are up- or down-regulated compared to uninfected individuals, making them putative biomarkers [174]. Pathogen-induced modification of the host volatilome to facilitate vector transmission has been suggested for golden hamsters (Mesocricetus auratus), which are more appealing to female sand flies when infected with L. infantum [175]. Although laboratory assays with synthetic compounds demonstrated activation and attraction of sand flies to the canine VOCs [176], as some of these biomarkers are down-regulated with infection status, the ecological significance of these changes for vector attraction is dubious. Ultimately, evaluating volatilome components out of context from their natural presentation risks misrepresenting their significance as allelochemicals.
Gravid female screwworm flies (Cochliomyia sp.) are particularly attracted to tissues that are already infested with screwworm larvae. In this case, it is well established that the change to the volatilome brought about by microbial activity plays an important part in attracting adult females to an appropriate oviposition site [109,177].
6.4. Allelochemicals That are Used to Identify Preferred On-Host Feeding-Sites
Some haematophageous pests exhibit on-host feeding-site preferences. The cues that guide the feeding-site orientation are likely to be due to short-range chemicals or changes to the behavioural function of a chemical with local concentration. The highly anthropophilic An. gambiae selectively bites human feet and ankles, while the opportunistic An. atroparvus has a propensity to bite around the head and shoulders [9]. Short-chain fatty acids produced by coryneform bacteria found on the skin between human toes acts as a kairomone for An. gambiae [178] and washing feet with antibacterial soap markedly diverts the biting-site preference of this pest to other body regions. Equally, by excluding exhaled breath, and so removing the main source of CO2, the preference of An. atroparvus for feeding around the head region is significantly diminished [9].
Similarly, tick species with discernible differences in their preferred on-host feeding sites are guided to these regions by attractive odours and repelled from other areas by compounds emanating locally from them. The behaviours of the brown ear tick (Rhipicephalus appendiculatus) and the red-legged tick (R. evertsi), which share a geographic range and a common host, are differentiated by their respective feeding sites once on-host. R. appendiculatus has a predilection for feeding inside the ears of bovine hosts, whereas R. evertsi is generally located around the anal regions [179]. The ability of adult ticks of these two species to successfully orient and navigate from an alternative place to their usual feeding location on a bovine host, suggests they follow gradients of volatile attractants to the source. Furthermore, when released on-host at the preferred feeding site of the other tick, both species commenced movement to find their favoured destination more rapidly than if released at an otherwise ‘neutral’ location. This indicates that as well as being attracted towards site-specific kairomones, local allomones also act to repel ticks from non-preferred areas. Volatiles collected from the inside of cattle ears and the anal region attracted and repelled R. appendiculatus, respectively. The opposite effect was observed for R. evertsi.
There is evidence that VOCs dictate arthropod selection of their host at the level of species, individual, and even feeding site. The interplay between host-derived chemicals that allows vectors to make specific, highly tuned decisions regarding their preferred host species, and also permits them to select the individual and even the body area on which to feed.
6.5. Manipulation of Host Parasitism through Application of Synthetic Allelochemicals
Recognising that specific volatile chemical cues drive host selection by arthropod pests suggests opportunities for their artificial application to interfere with host attack. Specifically, the application of allomone compounds identified from non-host odours, can be used to provide an olfactory camouflage to protect otherwise appealing and susceptible hosts. This principal has been most effectively demonstrated in development of a tsetse fly repellent based on VOCs from the non-preferred waterbuck volatilome. A synthetic mixture of geranylacetone, pentanoic acid, guaiacol, and δ-octalactone released from a collar-mounted dispensing system, provided long-term and significant protection to cattle (preferred-hosts) against African trypanosomiasis, vectored by tsetse flies. The large-scale field trial demonstrated significant reduction in disease, and improved bovine health characteristics, which correlated with marked improvement in socioeconomic measures and food security [180]. A similar approach was taken for managing biting black flies (Diptera: Simuliidae) on equine hosts. In this study, a slow-release synthetic preparation of two saturated compounds, C8 and C9 (chemical identities were not provided), mimicking VOC emissions from the European badger, Meles meles, offered protection to horse ears from black fly attack [181].
7. Conclusions
Almost 1300 analytes have been documented in the volatilomes of non-human animals (Supplementary File 1). Studies of volatilome components as they pertain to arthropod–host interactions are limited: Most studies on mammalian chemical profiles have been limited to herbivores and many studies on birds have focused primarily on waxy uropygial secretions, despite evidence that VOCs from other sources may play a greater role as allelochemicals. These gaps in knowledge arise due to challenges in obtaining the samples that contribute to the volatilome, which are exacerbated in non-human subjects. A lack of consistency in volatile-collection methods makes comparisons between studies difficult. In some work, when analysis has relied on chemical libraries without validation using authentic standards, misidentification or overidentification of VOCs is possible, so conclusions need to be interpreted with caution.
It is apparent from the studies reported to date that VOCs originating from different anatomical or functional compartments have non-uniform representation in each. Furthermore, the contribution of each compartment to the whole-animal volatile profile varies. Some, such as urinary and faecal emissions, are transient sources. Chemical representations may also be manipulated by microbial activity, diet, seasonal variation and environmental conditions, and these changes may influence the allelochemical representation and therefore the host appeal.
To date, most studies of the interaction between arthropod pests and vertebrates focus on the kairomones that actively lure ectoparasites and predatory insects to their preferred hosts, with limited research into allomones. There is a need for greater understanding overall, and especially in the identification of allomones. The possible use of allomones to protect animals and control disease transmission warrants further research in this area.
Several things are clear from the available data. Not all VOCs are physiologically active, and not all physiologically active VOCs are ecologically relevant. By and large we have failed to replicate the attractiveness of natural hosts with any chemical or synthetic mixture of chemicals. The implications of this is that allelochemicals are interaction-specific and complex. These chemicals do not work in isolation in nature and it is most likely that the chemical fingerprint alone, although critical, is not the only stimulus. Other cues such as temperature, humidity, and visual signs contribute to host seeking and selection. It is unlikely that there is a single ‘rule’ governing what constitutes an effective vector-host interaction. We will not identify a single panacea to minimise the impact of arthropod pests on human or animal health and comfort. However, by filling in the gaps for the most significant species—whether from an economic, welfare, or One Health perspective—we can determine where best to focus limited resources.
Acknowledgments
J.P. would like to thank Tara Sutherland for her comments on the manuscript.
Supplementary Materials
The following is available online at https://www.mdpi.com/2076-2615/10/11/1984/s1, Supplementary File 1: All 1287 volatile organic compounds reported in the literature from 141 non-human animal species. References [182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332] are cited in the supplementary materials.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Crooks E., Randolph S.E. Walking by Ixodes ricinus ticks: Intrinsic and extrinsic factors determine the attraction of moisture or host odour. J. Exp. Biol. 2006;209:2138–2142. doi: 10.1242/jeb.02238. [DOI] [PubMed] [Google Scholar]
- 2.Zwiebel L.J., Takken W. Olfactory regulation of mosquito-host interactions. Insect Biochem. Mol. Biol. 2004;34:645–652. doi: 10.1016/j.ibmb.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson B., Wicher D. Chapter 2—Chemical Ecology in Insects. In: Zufall F., Munger S.D., editors. Chemosensory Transduction. Academic Press; Cambridge, MA, USA: 2016. pp. 29–45. [Google Scholar]
- 4.Allan S.A., Bernier U.R., Kline D.L. Attraction of mosquitoes to volatiles associated with blood. J. Vector Ecol. 2006;31:71–78. doi: 10.3376/1081-1710(2006)31[71:AOMTVA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Brady J., Costantini C., Sagnon N., Gibson G., Coluzzi M. The role of body odours in the relative attractiveness of different men to malarial vectors in Burkina Faso. Ann. Trop. Med. Parasitol. 1997;91:S121–S122. doi: 10.1080/00034983.1997.11813252. [DOI] [Google Scholar]
- 6.Braks M., Meijerink J., Takken W. The response of the malaria mosquito, Anopheles gambiae, to two components of human sweat, ammonia and L-lactic acid, in an olfactometer. Physiol. Entomol. 2001;26:142–148. doi: 10.1046/j.1365-3032.2001.00227.x. [DOI] [Google Scholar]
- 7.De Boer J.G., Robinson A., Powers S.J., Burgers S.L.G.E., Caulfield J.C., Birkett M.A., Smallegange R.C., van Genderen P.J.J., Bousema T., Sauerwein R.W., et al. Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions. Sci. Rep. 2017;7:9283. doi: 10.1038/s41598-017-08978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jong R., Knols B.G.J. Olfactory responses of host-seeking Anopheles gambiae s.s. Giles (Diptera: Culicidae) Acta Trop. 1995;59:333–335. doi: 10.1016/0001-706X(95)00090-2. [DOI] [PubMed] [Google Scholar]
- 9.De Jong R., Knols B.G.J. Selection of biting sites on man by two malaria mosquito species. Experientia. 1995;51:80–84. doi: 10.1007/BF01964925. [DOI] [PubMed] [Google Scholar]
- 10.Dekker T., Steib B., Cardé R.T., Geier M. L-lactic acid: A human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med. Vet. Entomol. 2002;16:91–98. doi: 10.1046/j.0269-283x.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- 11.Harraca V., Ryne C., Birgersson G., Ignell R. Smelling your way to food: Can bed bugs use our odour? J. Exp. Biol. 2012;215:623–629. doi: 10.1242/jeb.065748. [DOI] [PubMed] [Google Scholar]
- 12.Logan J.G., Birkett M.A., Clark S.J., Powers S., Seal N.J., Wadhams L.J., Mordue Luntz A.J., Pickett J.A. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J. Chem. Ecol. 2008;34:308–322. doi: 10.1007/s10886-008-9436-0. [DOI] [PubMed] [Google Scholar]
- 13.Mukabana W.R., Takken W., Killeen G.F., Knols B.G.J. Allomonal effect of breath contributes to differential attractiveness of humans to the African malaria vector Anopheles gambiae. Malar. J. 2004;3:1. doi: 10.1186/1475-2875-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smallegange R.C., Qiu Y.T., van Loon J.J., Takken W. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae) Chem. Senses. 2005;30:145–152. doi: 10.1093/chemse/bji010. [DOI] [PubMed] [Google Scholar]
- 15.Syed Z., Leal W.S. Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc. Natl. Acad. Sci. USA. 2009;106:18803–18808. doi: 10.1073/pnas.0906932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takken W. The role of olfaction in host-seeking of mosquitoes: A review. Int. J. Trop. Insect Sci. 1991;12:287–295. doi: 10.1017/S1742758400020816. [DOI] [Google Scholar]
- 17.Takken W., Verhulst N.O. Chemical signaling in mosquito–host interactions: The role of human skin microbiota. Curr. Opin. Insect Sci. 2017;20:68–74. doi: 10.1016/j.cois.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Young R.M., Burkett-Cadena N.D., McGaha T.W., Jr., Rodriguez-Perez M.A., Toé L.D., Adeleke M.A., Sanfo M., Soungalo T., Katholi C.R., Noblet R., et al. Identification of human semiochemicals attractive to the major vectors of onchocerciasis. PLoS Negl. Trop. Dis. 2015;9:e3450. doi: 10.1371/journal.pntd.0003450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keesing F., Holt R.D., Ostfeld R.S. Effects of species diversity on disease risk. Ecol. Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 20.Simpson J.E., Hurtado P.J., Medlock J., Molaei G., Andreadis T.G., Galvani A.P., Diuk-Wasser M.A. Vector host-feeding preferences drive transmission of multi-host pathogens: West Nile virus as a model system. Proc. Biol. Sci. 2012;279:925–933. doi: 10.1098/rspb.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L.-F., Crameri G. Emerging zoonotic viral diseases. Rev. Sci. Tech. 2014;33:569–581. doi: 10.20506/rst.33.2.2311. [DOI] [PubMed] [Google Scholar]
- 22.Boots A.W., van Berkel J.J., Dallinga J.W., Smolinska A., Wouters E.F., van Schooten F.J. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath Res. 2012;6:027108. doi: 10.1088/1752-7155/6/2/027108. [DOI] [PubMed] [Google Scholar]
- 23.Mazzatenta A., Di Giulio C., Pokorski M. Pathologies currently identified by exhaled biomarkers. Resp. Physiol. Neurobi. 2013;187:128–134. doi: 10.1016/j.resp.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Sun X., Shao K., Wang T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal. Bioanal. Chem. 2016;408:2759–2780. doi: 10.1007/s00216-015-9200-6. [DOI] [PubMed] [Google Scholar]
- 25.Thomas A.N., Riazanskaia S., Cheung W., Xu Y., Goodacre R., Thomas C.L., Baguneid M.S., Bayat A. Novel noninvasive identification of biomarkers by analytical profiling of chronic wounds using volatile organic compounds. Wound Repair Regen. 2010;18:391–400. doi: 10.1111/j.1524-475X.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 26.Agapiou A., Amann A., Mochalski P., Statheropoulos M., Thomas C.L.P. Trace detection of endogenous human volatile organic compounds for search, rescue and emergency applications. Trends Analyt. Chem. 2015;66:158–175. [Google Scholar]
- 27.Mochalski P., Unterkofler K., Teschl G., Amann A. Potential of volatile organic compounds as markers of entrapped humans for use in urban search-and-rescue operations. Trends Analyt. Chem. 2015;68:88–106. [Google Scholar]
- 28.Kusano M., Mendez E., Furton K.G. Comparison of the volatile organic compounds from different biological specimens for profiling potential. J. Forensic Sci. 2013;58:29–39. doi: 10.1111/j.1556-4029.2012.02215.x. [DOI] [PubMed] [Google Scholar]
- 29.De Lacy Costello B., Amann A., Al-Kateb H., Flynn C., Filipiak W., Khalid T., Osborne D., Ratcliffe N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014;8:014001. doi: 10.1088/1752-7155/8/1/014001. [DOI] [PubMed] [Google Scholar]
- 30.Amann A., de Lacy Costello B., Miekisch W., Schubert J., Buszewski B., Pleil J., Ratcliffe N., Risby T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014;8:034001. doi: 10.1088/1752-7155/8/3/034001. [DOI] [PubMed] [Google Scholar]
- 31.Beale D.J., Jones O.A., Karpe A.V., Dayalan S., Oh D.Y., Kouremenos K.A., Ahmed W., Palombo E.A. A review of analytical techniques and their application in disease diagnosis in breathomics and salivaomics research. Int. J. Mol. Sci. 2017;18:24. doi: 10.3390/ijms18010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunlop M.W., Blackall P.J., Stuetz R.M. Odour emissions from poultry litter—A review litter properties, odour formation and odorant emissions from porous materials. J. Environ. Manage. 2016;177:306–319. doi: 10.1016/j.jenvman.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Murphy K.R., Parcsi G., Stuetz R.M. Non-methane volatile organic compounds predict odor emitted from five tunnel ventilated broiler sheds. Chemosphere. 2014;95:423–432. doi: 10.1016/j.chemosphere.2013.09.076. [DOI] [PubMed] [Google Scholar]
- 34.Trabue S., Scoggin K., Li H., Burns R., Xin H., Hatfield J. Speciation of volatile organic compounds from poultry production. Atmos. Environ. 2010;44:3538–3546. [Google Scholar]
- 35.Chakroborty N.K., Bienefeld K., Menzel R. Odor learning and odor discrimination of bees selected for enhanced hygienic behavior. Apidologie. 2015;46:499–514. [Google Scholar]
- 36.Filipy J., Rumburg B., Mount G., Westberg H., Lamb B. Identification and quantification of volatile organic compounds from a dairy. Atmos. Environ. 2006;40:1480–1494. [Google Scholar]
- 37.Trabue S., Scoggin K., McConnell L., Maghirang R., Razote E., Hatfield J. Identifying and tracking key odorants from cattle feedlots. Atmos. Environ. 2011;45:4243–4251. [Google Scholar]
- 38.Cathcart M.P., Love S., Hughes K.J. The application of exhaled breath gas and exhaled breath condensate analysis in the investigation of the lower respiratory tract in veterinary medicine: A review. Vet. J. 2012;191:282–291. doi: 10.1016/j.tvjl.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Kleeblatt J., Schubert J.K., Zimmermann R. Detection of gaseous compounds by needle trap sampling and direct thermal-desorption photoionization mass spectrometry: Concept and demonstrative application to breath gas analysis. Anal. Chem. 2015;87:1773–1781. doi: 10.1021/ac5039829. [DOI] [PubMed] [Google Scholar]
- 40.Trefz P., Rösner L., Hein D., Schubert J.K., Miekisch W. Evaluation of needle trap micro-extraction and automatic alveolar sampling for point-of-care breath analysis. Anal. Bioanal. Chem. 2013;405:3105–3115. doi: 10.1007/s00216-013-6781-9. [DOI] [PubMed] [Google Scholar]
- 41.Fischer S., Bergmann A., Steffens M., Trefz P., Ziller M., Miekisch W., Schubert J.S., Kohler H., Reinhold P. Impact of food intake on in vivo VOC concentrations in exhaled breath assessed in a caprine animal model. J. Breath Res. 2015;9:047113. doi: 10.1088/1752-7155/9/4/047113. [DOI] [PubMed] [Google Scholar]
- 42.Fischer S., Trefz P., Bergmann A., Steffens M., Ziller M., Miekisch W., Schubert J.S., Kohler H., Reinhold P. Physiological variability in volatile organic compounds (VOCs) in exhaled breath and released from faeces due to nutrition and somatic growth in a standardized caprine animal model. J. Breath Res. 2015;9:027108. doi: 10.1088/1752-7155/9/2/027108. [DOI] [PubMed] [Google Scholar]
- 43.Purkhart R., Köhler H., Liebler-Tenorio E., Meyer M., Becher G., Kikowatz A., Reinhold P. Chronic intestinal Mycobacteria infection: Discrimination via VOC analysis in exhaled breath and headspace of feces using differential ion mobility spectrometry. J. Breath Res. 2011;5:027103. doi: 10.1088/1752-7155/5/2/027103. [DOI] [PubMed] [Google Scholar]
- 44.Traxler S., Bischoff A.-C., Saß R., Trefz P., Gierschner P., Brock B., Schwaiger T., Karte C., Blohm U., Schröder C. VOC breath profile in spontaneously breathing awake swine during Influenza A infection. Sci. Rep. 2018;8:14857. doi: 10.1038/s41598-018-33061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peled N., Ionescu R., Nol P., Barash O., McCollum M., VerCauteren K., Koslow M., Stahl R., Rhyan J., Haick H. Detection of volatile organic compounds in cattle naturally infected with Mycobacterium bovis. Sens. Actuators B Chem. 2012;171:588–594. [Google Scholar]
- 46.Turner C., Knobloch H., Richards J., Richards P., Mottram T.T.F., Marlin D., Chambers M.A. Development of a device for sampling cattle breath. Biosys. Eng. 2012;112:75–81. [Google Scholar]
- 47.Nevitt G.A., Prada P.A. The chemistry of avian odors: An introduction to best practices. In: Doty R.L., editor. Handbook of Olfaction and Gustation. John Wiley & Sons; Hoboken, NJ, USA: 2015. pp. 565–578. [Google Scholar]
- 48.Barman P., Yadav M.C., Kumar H., Meur S.K., Ghosh S.K. Gas chromatographic-mass spectrometric analysis of chemical volatiles in buffalo (Bubalus bubalis) urine. Theriogenology. 2013;80:654–658. doi: 10.1016/j.theriogenology.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Bernier U.R., Allan S.A., Quinn B.P., Kline D.L., Barnard D.R., Clark G.G. Volatile compounds from the integument of white leghorn chickens (Gallus gallus domesticus L.): Candidate attractants of ornithophilic mosquito species. J. Sep. Sci. 2008;31:1092–1099. doi: 10.1002/jssc.200700434. [DOI] [PubMed] [Google Scholar]
- 50.Allan S.A., Bernier U.R., Kline D.L. Laboratory evaluation of avian odors for mosquito (Diptera: Culicidae) attraction. J. Med. Entomol. 2006;43:225–231. doi: 10.1603/0022-2585(2006)043[0225:leoaof]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 51.Gabirot M., Mardon J., Campagna S., West N., Bonadonna F., Saunders S.M. Guidelines for collecting and extracting avian odors in a remote field: Case study of a subantarctic seabird. In: Schulte B.A., editor. Chemical Signals in Vertebrates 13. Springer International Publishing; Cham, Switzerland: 2016. pp. 435–460. [Google Scholar]
- 52.Douglas H.D. Measurement of chemical emissions in crested auklets (Aethia cristatella) J. Chem. Ecol. 2006;32:2559–2567. doi: 10.1007/s10886-006-9164-2. [DOI] [PubMed] [Google Scholar]
- 53.Douglas H., Co J., Jones T., Conner W. Heteropteran chemical repellents identified in the citrus odor of a seabird (crested auklet: Aethia cristatella): Evolutionary convergence in chemical ecology. Naturwissenschaften. 2001;88:330–332. doi: 10.1007/s001140100236. [DOI] [PubMed] [Google Scholar]
- 54.Douglas H.D., Co J.E., Jones T.H., Conner W.E. Interspecific differences in Aethia spp. Auklet odorants and evidence for chemical defense against ectoparasites. J. Chem. Ecol. 2004;30:1921–1935. doi: 10.1023/b:joec.0000045586.59468.de. [DOI] [PubMed] [Google Scholar]
- 55.Kuehnbaum N.L., Britz-McKibbin P. New advances in separation science for metabolomics: Resolving chemical diversity in a post-genomic era. Chem. Rev. 2013;113:2437–2468. doi: 10.1021/cr300484s. [DOI] [PubMed] [Google Scholar]
- 56.Schymanski E.L., Jeon J., Gulde R., Fenner K., Ruff M., Singer H.P., Hollender J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014;48:2097–2098. doi: 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- 57.Wang X.R., Cassells J., Berna A.Z. Stability control for breath analysis using GC-MS. J. Chromatogr. B. 2018;1097:27–34. doi: 10.1016/j.jchromb.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersen K., Vulpius T. Urinary volatile constituents of the lion, Panthera leo. Chem. Senses. 1999;24:179–189. doi: 10.1093/chemse/24.2.179. [DOI] [PubMed] [Google Scholar]
- 59.Archunan G., Rajagopal T. Detection of estrus in Indian blackbuck: Behavioural, hormonal and urinary volatiles evaluation. Gen. Comp. Endocrinol. 2013;181:156–166. doi: 10.1016/j.ygcen.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 60.Deshpande K., Furton K.G., De Etta K. The equine volatilome: Volatile organic compounds as discriminatory markers. J. Equine Vet. Sci. 2018;62:47–53. doi: 10.1016/j.jevs.2017.05.013. [DOI] [Google Scholar]
- 61.Elliott-Martin R.J., Mottram T.T., Gardner J.W., Hobbs P.J., Bartlett P.N. Preliminary investigation of breath sampling as a monitor of health in dairy cattle. J. Agr. Eng. Res. 1997;67:267–275. [Google Scholar]
- 62.Ellis C.K., Rice S., Maurer D., Stahl R., Waters W.R., Palmer M.V., Nol P., Rhyan J.C., VerCauteren K.C., Koziel J.A. Use of fecal volatile organic compound analysis to discriminate between non-vaccinated and BCG—Vaccinated cattle prior to and after Mycobacterium bovis challenge. PLoS ONE. 2017;12:e0179914. doi: 10.1371/journal.pone.0179914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ellis C.K., Stahl R.S., Nol P., Waters W.R., Palmer M.V., Rhyan J.C., VerCauteren K.C., McCollum M., Salman M.D. A pilot study exploring the use of breath analysis to differentiate healthy cattle from cattle experimentally infected with Mycobacterium bovis. PLoS ONE. 2014;9:e89280. doi: 10.1371/journal.pone.0089280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayes R.A., Morelli T., Wright P. Anogenital gland secretions of Lemur catta and Propithecus verreauxi coquereli: A preliminary chemical examination. Am. J. Primatol. 2004;63:49–62. doi: 10.1002/ajp.20038. [DOI] [PubMed] [Google Scholar]
- 65.Holderman C.J., Kaufman P.E., Booth M.M., Bernier U.R. Novel collection method for volatile organic compounds (VOCs) from dogs. J. Chromatogr. B. 2017;1061:1–4. doi: 10.1016/j.jchromb.2017.06.044. [DOI] [PubMed] [Google Scholar]
- 66.Iwata E., Kikusui T., Takeuchi Y., Mori Y. Substances derived from 4-ethyl octanoic acid account for primer pheromone activity for the “male effect” in goats. J. Vet. Med. Sci. 2003;65:1019–1021. doi: 10.1292/jvms.65.1019. [DOI] [PubMed] [Google Scholar]
- 67.Muñoz-Romo M., Nielsen L.T., Nassar J.M., Kunz T.H. Chemical composition of the substances from dorsal patches of males of the Curaçaoan long-nosed bat, Leptonycteris curasoae (Phyllostomidae: Glossophaginae) Acta Chiropt. 2012;14:213–224. doi: 10.3161/150811012X654411. [DOI] [Google Scholar]
- 68.Nielsen L.T., Eaton D.K., Wright D.W., Schmidt-French B. Characteristic odors of Tadarida brasiliensis mexicana Chiroptera: Molossidae. J. Cave Karst Stud. 2006;68:27–31. [Google Scholar]
- 69.Scordato E.S., Dubay G., Drea C.M. Chemical composition of scent marks in the ringtailed lemur (Lemur catta): Glandular differences, seasonal variation, and individual signatures. Chem. Senses. 2007;32:493–504. doi: 10.1093/chemse/bjm018. [DOI] [PubMed] [Google Scholar]
- 70.Selvaraj R., Archunan G. Chemical identification and bioactivity of rat (Rattus rattus) urinary compounds. Zool. Stud. 2002;41:127–135. [Google Scholar]
- 71.Setchell J.M., Vaglio S., Moggi-Cecchi J., Boscaro F., Calamai L., Knapp L.A. Chemical composition of scent-gland secretions in an Old World monkey (Mandrillus sphinx): Influence of sex, male status, and individual identity. Chem. Senses. 2010;35:205–220. doi: 10.1093/chemse/bjp105. [DOI] [PubMed] [Google Scholar]
- 72.Vogt K., Boos S., Breitenmoser U., Kölliker M. Chemical composition of Eurasian lynx urine conveys information on reproductive state, individual identity, and urine age. Chemoecology. 2016;26:205–217. doi: 10.1007/s00049-016-0220-2. [DOI] [Google Scholar]
- 73.Williams C.R., Kokkin M.J., Smith B.P. Intraspecific variation in odor-mediated host preference of the mosquito Culex annulirostris. J. Chem. Ecol. 2003;29:1889–1903. doi: 10.1023/A:1024806429366. [DOI] [PubMed] [Google Scholar]
- 74.Baldacchino F., Gardès L., De Stordeur E., Jay-Robert P., Garros C. Blood-feeding patterns of horse flies in the French Pyrenees. Vet. Parasitol. 2014;199:283–288. doi: 10.1016/j.vetpar.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 75.Jaleta K.T., Sharon Rose H., Birgersson G., Habte T., Ignell R. Chicken volatiles repel host-seeking malaria mosquitoes. Malar. J. 2016;15:354. doi: 10.1186/s12936-016-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gikonyo N.K., Hassanali A., Njagi P.G.N., Gitu P.M., Midiwo J.O. Odour composition of preferred (buffalo and ox) and non-preferred (waterbuck) hosts of some savanna tsetse flies. J. Chem. Ecol. 2002;28:969–981. doi: 10.1023/A:1015205716921. [DOI] [PubMed] [Google Scholar]
- 77.Vale G.A. Field studies of the responses of tsetse flies (Glossinidae) and other Diptera to carbon dioxide, acetone and other chemicals. Bull. Entomol. Res. 1980;70:563–570. doi: 10.1017/S0007485300007860. [DOI] [Google Scholar]
- 78.Busula A.O., Takken W., de Boer J.G., Mukabana W.R., Verhulst N.O. Variation in host preferences of malaria mosquitoes is mediated by skin bacterial volatiles. Med. Vet. Entomol. 2017;31:320–326. doi: 10.1111/mve.12242. [DOI] [PubMed] [Google Scholar]
- 79.Dekker T., Takken W. Differential responses of mosquito sibling species Anopheles arabiensis and An. quadriannulatus to carbon dioxide, a man or a calf. Med. Vet. Entomol. 1998;12:136–140. doi: 10.1046/j.1365-2915.1998.00073.x. [DOI] [PubMed] [Google Scholar]
- 80.Dekker T., Takken W., Braks M.A.H. Innate preference for host-odor blends modulates degree of anthropophagy of Anopheles gambiae sensu lato (Diptera: Culicidae) J. Med. Entomol. 2001;38:868–871. doi: 10.1603/0022-2585-38.6.868. [DOI] [PubMed] [Google Scholar]
- 81.Hillbrick G., Tucker D., Smith G. The lipid composition of cashmere goat fleece. Aust. J. Agric. Res. 1995;46:1259–1271. doi: 10.1071/AR9951259. [DOI] [Google Scholar]
- 82.Sugiyama T., Sasada H., Masaki J., Yamashita K. Unusual fatty acids with specific odor from mature male goat. Agric. Biol. Chem. 1981;45:2655–2658. [Google Scholar]
- 83.Rajchard J. Biologically active substances of bird skin: A review. Vet. Med. 2010;55:413–421. doi: 10.17221/2981-VETMED. [DOI] [Google Scholar]
- 84.Moyer B.R., Rock A.N., Clayton D.H., Prum R. Experimental test of the importance of preen oil in rock doves (Columba livia) Auk. 2003;120:490–496. doi: 10.1093/auk/120.2.490. [DOI] [Google Scholar]
- 85.Moreno-Rueda G. Preen oil and bird fitness: A critical review of the evidence. Biol. Rev. 2017;92:2131–2143. doi: 10.1111/brv.12324. [DOI] [PubMed] [Google Scholar]
- 86.Martín-Vivaldi M., Peña A., Peralta-Sánchez J.M., Sánchez L., Ananou S., Ruiz-Rodríguez M., Soler J.J. Antimicrobial chemicals in hoopoe preen secretions are produced by symbiotic bacteria. Proc. Biol. Sci. 2010;277:123–130. doi: 10.1098/rspb.2009.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacob J., Grimmer G. Composition of uropygial gland waxes in relation to the classification of some passerine birds. Biochem. Syst. Ecol. 1975;3:267–271. doi: 10.1016/0305-1978(75)90013-7. [DOI] [Google Scholar]
- 88.Campagna S., Mardon J., Celerier A., Bonadonna F. Potential semiochemical molecules from birds: A practical and comprehensive compilation of the last 20 years studies. Chem. Senses. 2012;37:3–25. doi: 10.1093/chemse/bjr067. [DOI] [PubMed] [Google Scholar]
- 89.Tegoni M., Pelosi P., Vincent F., Spinelli S., Campanacci V., Grolli S., Ramoni R., Cambillau C. Mammalian odorant binding proteins. Biochim. Biophys. Acta. 2000;1482:229–240. doi: 10.1016/S0167-4838(00)00167-9. [DOI] [PubMed] [Google Scholar]
- 90.Reneerkens J., Piersma T., Damsté J.S.S. Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002;269:2135–2139. doi: 10.1098/rspb.2002.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Allan S.A., Bernier U.R., Kline D.L. Laboratory evaluation of lactic acid on attraction of Culex spp. (Diptera: Culicidae) J. Vector Ecol. 2010;35:318–324. doi: 10.1111/j.1948-7134.2010.00089.x. [DOI] [PubMed] [Google Scholar]
- 92.Smallegange R.C., Takken W. Host-seeking behaviour of mosquitoes: Responses to olfactory stimuli in the laboratory. In: Takken W., Knols B.G.J., editors. Olfaction in Vector-Host Interactions, Ecology and Control of Vector Borne Diseases. Volume 2. Wageningen Academic Publishers; Wageningen, The Netherlands: 2010. pp. 143–180. [Google Scholar]
- 93.Jonsson N.N., Bock R.E., Jorgensen W.K. Productivity and health effects of anaplasmosis and babesiosis on Bos indicus cattle and their crosses, and the effects of differing intensity of tick control in Australia. Vet. Parasitol. 2008;155:1–9. doi: 10.1016/j.vetpar.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 94.Bock R., Jackson L., De Vos A., Jorgensen W. Babesiosis of cattle. Parasitology. 2004;129:S247–S269. doi: 10.1017/S0031182004005190. [DOI] [PubMed] [Google Scholar]
- 95.Bock R., Kingston T., De Vos A. Effect of breed of cattle on transmission rate and innate resistance to infection with Babesia bovis and B bigemina transmitted by Boophilus microplus. Aust. Vet. J. 1999;77:461–464. doi: 10.1111/j.1751-0813.1999.tb12093.x. [DOI] [PubMed] [Google Scholar]
- 96.Bock R., De Vos A., Kingston T., McLellan D. Effect of breed of cattle on innate resistance to infection with Babesia bovis, B bigemina and Anaplasma marginale. Aust. Vet. J. 1997;75:337–340. doi: 10.1111/j.1751-0813.1997.tb15706.x. [DOI] [PubMed] [Google Scholar]
- 97.Bock R., Kingston T., De Vos A. Effect of breed of cattle on innate resistance to infection with Anaplasma marginale transmitted by Boophilus microplus. Aust. Vet. J. 1999;77:748–751. doi: 10.1111/j.1751-0813.1999.tb12920.x. [DOI] [PubMed] [Google Scholar]
- 98.Ali A., Parizi L.F., Ferreira B.R., da Silva Vaz I., Jr. A revision of two distinct species of Rhipicephalus: R. microplus and R. australis. Ciênc. Rural. 2016;46:1240–1248. doi: 10.1590/0103-8478cr20151416. [DOI] [Google Scholar]
- 99.Jonsson N.N. The productivity effects of cattle tick (Boophilus microplus) infestation on cattle, with particular reference to Bos indicus cattle and their crosses. Vet. Parasitol. 2006;137:1–10. doi: 10.1016/j.vetpar.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Franzin A.M., Maruyama S.R., Garcia G.R., Oliveira R.P., Ribeiro J.M., Bishop R., Maia A.A., More D.D., Ferreira B.R., Santos I.K. Immune and biochemical responses in skin differ between bovine hosts genetically susceptible and resistant to the cattle tick Rhipicephalus microplus. Parasites Vectors. 2017;10:51. doi: 10.1186/s13071-016-1945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Borges L.M.F., Duarte S.C., Louly C.C.B. Cattle tick differentiates between the odors of Holstein Friesian and Nelore cattle. Ciênc. Rural. 2015;45:2023–2025. doi: 10.1590/0103-8478cr20150010. [DOI] [Google Scholar]
- 102.Wambura P.N., Gwakisa P.S., Silayo R.S., Rugaimukamu E.A. Breed-associated resistance to tick infestation in Bos indicus and their crosses with Bos taurus. Vet. Parasitol. 1998;77:63–70. doi: 10.1016/S0304-4017(97)00229-X. [DOI] [PubMed] [Google Scholar]
- 103.Louly C.C.B., Soares S.F., Silveira D.N., Neto O.J.S., Silva A.C., Borges L.M.F. Differences in the susceptibility of two breeds of dogs, English cocker spaniel and beagle, to Rhipicephalus sanguineus (Acari: Ixodidae) Int. J. Acarol. 2009;35:25–32. doi: 10.1080/01647950802655251. [DOI] [Google Scholar]
- 104.Louly C.C.B., Fonseca I.N., Oliveira V.F.D., Linhares G.F.C., Menezes L.B.D., Borges L.M.F. Seasonal dynamics of Rhipicephalus sanguineus (Acari: Ixodidae) in dogs from a police unit in Goiânia, Goiás, Brazil. Ciênc. Rural. 2007;37:464–469. doi: 10.1590/S0103-84782007000200026. [DOI] [Google Scholar]
- 105.Louly C.C., Soares S.F., da Nobrega Silveira D., Guimaraes M.S., Borges L.M. Differences in the behavior of Rhipicephalus sanguineus tested against resistant and susceptible dogs. Exp. Appl. Acarol. 2010;51:353–362. doi: 10.1007/s10493-009-9334-3. [DOI] [PubMed] [Google Scholar]
- 106.Hall M. Trapping the flies that cause myiasis: Their responses to host-stimuli. Ann. Trop. Med. Parasitol. 1995;89:333–357. doi: 10.1080/00034983.1995.11812964. [DOI] [PubMed] [Google Scholar]
- 107.Zhu J., Chaudhury M., Tangtrakulwanich K., Skoda S. Identification of oviposition attractants of the secondary screwworm, Cochliomyia macellaria (F.) released from rotten chicken liver. J. Chem. Ecol. 2013;39:1407–1414. doi: 10.1007/s10886-013-0359-z. [DOI] [PubMed] [Google Scholar]
- 108.Chaudhury M.F., Skoda S.R., Sagel A., Welch J.B. Volatiles emitted from eight wound-isolated bacteria differentially attract gravid screwworms (Diptera: Calliphoridae) to oviposit. J. Med. Entomol. 2010;47:349–354. doi: 10.1093/jmedent/47.3.349. [DOI] [PubMed] [Google Scholar]
- 109.Chaudhury M.F., Zhu J.J., Skoda S.R. Bacterial volatiles attract gravid secondary screwworms (Diptera: Calliphoridae) J. Econ. Entomol. 2016;109:947–951. doi: 10.1093/jee/tov390. [DOI] [PubMed] [Google Scholar]
- 110.Vale G.A., Flint S., Hall D.R. The field responses of tsetse flies, Glossina spp. (Diptera: Glossinidae), to odours of host residues. Bull. Entomol. Res. 1986;76:685–693. doi: 10.1017/S0007485300015170. [DOI] [Google Scholar]
- 111.Hassanali A., McDowell P.G., Owaga M.L.A., Saini R.K. Identification of tsetse attractants from excretory products of a wild host animal, Syncerus caffer. Insect Sci. Appl. 1986;7:5–9. doi: 10.1017/S1742758400003027. [DOI] [Google Scholar]
- 112.Becker N., Zgomba M., Petric D., Ludwig M. Comparison of carbon dioxide, octenol and a host-odour as mosquito attractants in the Upper Rhine Valley, Germany. Med. Vet. Entomol. 1995;9:377–380. doi: 10.1111/j.1365-2915.1995.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 113.Guerenstein P.G., Hildebrand J.G. Roles and effects of environmental carbon dioxide in insect life. Annu. Rev. Entomol. 2008;53:161–178. doi: 10.1146/annurev.ento.53.103106.093402. [DOI] [PubMed] [Google Scholar]
- 114.Reeves W.C. Quantitative field studies on a carbon dioxide chemotropism of mosquitoes. Am. J. Trop. Med. Hyg. 1953;2:325–331. doi: 10.4269/ajtmh.1953.2.325. [DOI] [PubMed] [Google Scholar]
- 115.Costantini C., Gibson G., Sagnon N.f., della Torre A., Brady J., Coluzzi M. Mosquito responses to carbon dioxide in a West African Sudan savanna village. Med. Vet. Entomol. 1996;10:220–227. doi: 10.1111/j.1365-2915.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 116.Gillies M.T. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): A review. Bull. Entomol. Res. 1980;70:525–532. doi: 10.1017/S0007485300007811. [DOI] [Google Scholar]
- 117.Laarman J. The host-seeking behaviour of anopheline mosquitoes. Trop. Geogr. Med. 1958;10:293–305. [PubMed] [Google Scholar]
- 118.Bos H., Laarman J. Guinea pig, lysine, cadaverine and estradiol as attractants for the malaria mosquito Anopheles stephensi. Entomol. Exp. Appl. 1975;18:161–172. doi: 10.1111/j.1570-7458.1975.tb02366.x. [DOI] [Google Scholar]
- 119.Dekel A., Pitts R.J., Yakir E., Bohbot J.D. Evolutionarily conserved odorant receptor function questions ecological context of octenol role in mosquitoes. Sci. Rep. 2016;6:37330. doi: 10.1038/srep37330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hall D.R., Beevor P.S., Cork A., Nesbitt B.F., Vale G.A. 1-Octen-3-ol. A potent olfactory stimulant and attractant for tsetse isolated from cattle odours. Int. J. Trop. Insect Sci. 1984;5:335–339. doi: 10.1017/S1742758400008626. [DOI] [Google Scholar]
- 121.Takken W., Kline D.L. Carbon dioxide and 1-octen-3-ol as mosquito attractants. J. Am. Mosq. Control Assoc. 1989;5:311–316. [PubMed] [Google Scholar]
- 122.Kline D.L. Olfactory attractants for mosquito surveillance and control: 1-octen-3-ol. J. Am. Mosq. Control Assoc. 1994;10:280–287. [PubMed] [Google Scholar]
- 123.Cork A. Olfactory basis of host location by mosquitoes and other haematophagous Diptera. In: Bock G.R., Cardew G., editors. Symposium on Olfaction in Mosquito-Host Interactions. John Wiley and Sons; Chichester, UK: 1996. pp. 71–88. [DOI] [PubMed] [Google Scholar]
- 124.Bhasin A., Mordue W. Electrophysiological and behavioural identification of host kairomones as olfactory cues for Culicoides impunctatus and C. nubeculosus. Physiol. Entomol. 2000;25:6–16. doi: 10.1046/j.1365-3032.2000.00157.x. [DOI] [Google Scholar]
- 125.Kline D.L., Takken W., Wood J.R., Carlson D.A. Field studies on the potential of butanone, carbon dioxide, honey extract, 1-octen-3-ol, L-lactic acid and phenols as attractants for mosquitoes. Med. Vet. Entomol. 1990;4:383–391. doi: 10.1111/j.1365-2915.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 126.Muirhead-Thomson R.C. Trap Responses of Flying Insects. Academic Press; London, UK: 1991. Chapter 8—Animal-baited traps and animal odours; pp. 225–246. [Google Scholar]
- 127.Frézil J.-L., Carnevale P. Utilisation de la carboglace pour la capture de Glossina fuscipes quanzensis Pires, 1948, avec le piège Challier-Laveissière. Conséquences épidémiologiques. Cah. ORSTOM Séries Ent. Med. Parasitol. 1976;14:225–233. [Google Scholar]
- 128.Vale G.A., Hall D.R. The role of 1-octen-3-ol, acetone and carbon dioxide in the attraction of tsetse flies, Glossina spp. (Diptera: Glossinidae), to ox odour. Bull. Entomol. Res. 1985;75:209–218. doi: 10.1017/S0007485300014292. [DOI] [Google Scholar]
- 129.Spinhirne J.P., Koziel J.A., Chirase N.K. Sampling and analysis of volatile organic compounds in bovine breath by solid-phase microextraction and gas chromatography–mass spectrometry. J. Chromatogr. 2004;1025:63–69. doi: 10.1016/j.chroma.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 130.Harraca V., Syed Z., Guerin P.M. Olfactory and behavioural responses of tsetse flies, Glossina spp., to rumen metabolites. J. Comp. Physiol. A. 2009;195:815–824. doi: 10.1007/s00359-009-0459-y. [DOI] [PubMed] [Google Scholar]
- 131.Bayn A., Nol P., Tisch U., Rhyan J., Ellis C.K., Haick H. Detection of volatile organic compounds in Brucella abortus-seropositive bison. Anal. Chem. 2013;85:11146–11152. doi: 10.1021/ac403134f. [DOI] [PubMed] [Google Scholar]
- 132.Spinhirne J.P., Koziel J.A., Chirase N.K. A device for non-invasive on-site sampling of cattle breath with solid-phase microextraction. Biosys. Eng. 2003;84:239–246. doi: 10.1016/S1537-5110(02)00240-4. [DOI] [Google Scholar]
- 133.Beauchamp J. Inhaled today, not gone tomorrow: Pharmacokinetics and environmental exposure of volatiles in exhaled breath. J. Breath Res. 2011;5:037103. doi: 10.1088/1752-7155/5/3/037103. [DOI] [PubMed] [Google Scholar]
- 134.Berna A.Z., McCarthy J.S., Wang R.X., Saliba K.J., Bravo F.G., Cassells J., Padovan B., Trowell S.C. Analysis of breath specimens for biomarkers of Plasmodium falciparum infection. J. Infect. Dis. 2015;212:1120–1128. doi: 10.1093/infdis/jiv176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mochalski P., King J., Klieber M., Unterkofler K., Hinterhuber H., Baumann M., Amann A. Blood and breath levels of selected volatile organic compounds in healthy volunteers. Analyst. 2013;138:2134–2145. doi: 10.1039/c3an36756h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Donzé G., McMahon C., Guerin P.M. Rumen metabolites serve ticks to exploit large mammals. J. Exp. Biol. 2004;207:4283–4289. doi: 10.1242/jeb.01241. [DOI] [PubMed] [Google Scholar]
- 137.Jeanbourquin P., Guerin P.M. Sensory and behavioural responses of the stable fly Stomoxys calcitrans to rumen volatiles. Med. Vet. Entomol. 2007;21:217–224. doi: 10.1111/j.1365-2915.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- 138.Heberle H., Meirelles G.V., da Silva F.R., Telles G.P., Minghim R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015;16:169. doi: 10.1186/s12859-015-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bursell E., Gough A.J.E., Beevor P.S., Cork A., Hall D.R., Vale G.A. Identification of components of cattle urine attractive to tsetse flies, Glossina spp. (Diptera: Glossinidae) Bull. Entomol. Res. 1988;78:281–291. doi: 10.1017/S0007485300013043. [DOI] [Google Scholar]
- 140.Owaga M.L.A., Hassanali A., McDowell P.G. The role of 4-cresol and 3-n-propylphenol in the attraction of tsetse flies to buffalo urine. Insect Sci. Appl. 1988;9:95–100. doi: 10.1017/S1742758400010110. [DOI] [Google Scholar]
- 141.Baldacchino F., Carier J., Porciani A., Buatois B., Dormont L., Jay-Robert P. Behavioural and electrophysiological responses of females of two species of tabanid to volatiles in urine of different mammals. Med. Vet. Entomol. 2013;27:77–85. doi: 10.1111/j.1365-2915.2012.01022.x. [DOI] [PubMed] [Google Scholar]
- 142.Mihok S., Lange K. Synergism between ammonia and phenols for Hybomitra tabanids in northern and temperate Canada. Med. Vet. Entomol. 2012;26:282–290. doi: 10.1111/j.1365-2915.2011.00999.x. [DOI] [PubMed] [Google Scholar]
- 143.Baldacchino F., Manon S., Puech L., Buatois B., Dormont L., Jay-Robert P. Olfactory and behavioural responses of tabanid horseflies to octenol, phenols and aged horse urine. Med. Vet. Entomol. 2014;28:201–209. doi: 10.1111/mve.12038. [DOI] [PubMed] [Google Scholar]
- 144.Okech M., Hassanali A. The origin of phenolic tsetse attractants from host urine: Studies on the pro-attractants and microbes involved. Int. J. Trop. Insect Sci. 1990;11:363–368. doi: 10.1017/S1742758400012789. [DOI] [Google Scholar]
- 145.Apps P., Mmualefe L., McNutt J.W. Identification of volatiles from the secretions and excretions of African wild dogs (Lycaon pictus) J. Chem. Ecol. 2012;38:1450–1461. doi: 10.1007/s10886-012-0206-7. [DOI] [PubMed] [Google Scholar]
- 146.Raymer J., Wiesler D., Novotny M., Asa C., Seal U., Mech L. Volatile constituents of wolf (Canis lupus) urine as related to gender and season. Experientia. 1984;40:707–709. doi: 10.1007/BF01949734. [DOI] [PubMed] [Google Scholar]
- 147.Raymer J., Wiesler D., Novotny M., Asa C., Seal U., Mech L. Chemical investigations of wolf (Canis lupus) anal-sac secretion in relation to breeding season. J. Chem. Ecol. 1985;11:593–608. doi: 10.1007/BF00988570. [DOI] [PubMed] [Google Scholar]
- 148.Schultz T.H., Flath R.A., Stern D.J., Mon T.R., Teranishi R., Kruse S.M., Butler B., Howard W.E. Coyote estrous urine volatiles. J. Chem. Ecol. 1988;14:701–712. doi: 10.1007/BF01013917. [DOI] [PubMed] [Google Scholar]
- 149.Schultz T.H., Kruse S.M., Flath R.A. Some volatile constituents of female dog urine. J. Chem. Ecol. 1985;11:169–175. doi: 10.1007/BF00988199. [DOI] [PubMed] [Google Scholar]
- 150.Preti G., Muetterties E.L., Furman J.M., Kennelly J.J., Johns B.E. Volatile constituents of dog (Canis familiaris) and coyote (Canis latrans) anal sacs. J. Chem. Ecol. 1976;2:177–186. doi: 10.1007/BF00987740. [DOI] [Google Scholar]
- 151.Burger B., Viviers M., Bekker J., Le Roux M., Fish N., Fourie W., Weibchen G. Chemical characterization of territorial marking fluid of male Bengal tiger, Panthera tigris. J. Chem. Ecol. 2008;34:659. doi: 10.1007/s10886-008-9462-y. [DOI] [PubMed] [Google Scholar]
- 152.Borges L.M.F., de Oliveira Filho J.G., Ferreira L.L., Louly C.C.B., Pickett J.A., Birkett M.A. Identification of non-host semiochemicals for the brown dog tick, Rhipicephalus sanguineus sensu lato (Acari: Ixodidae), from tick-resistant beagles, Canis lupus familiaris. Ticks Tick Borne Dis. 2015;6:676–682. doi: 10.1016/j.ttbdis.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 153.Haahti E.O.A., Fales H.M. The uropygiols: Identification of the unsaponifiable constituent of a diester wax from chicken preen glands. J. Lipid Res. 1967;8:131–137. [PubMed] [Google Scholar]
- 154.Burger B.V., Reiter B., Borzyk O., du Plessis M.A. Avian exocrine secretions. I. Chemical characterization of the volatile fraction of the uropygial secretion of the green woodhoopoe, Phoeniculus purpureus. J. Chem. Ecol. 2004;30:1603–1611. doi: 10.1023/B:JOEC.0000042071.65335.f3. [DOI] [PubMed] [Google Scholar]
- 155.Soler J.J., Martín-Vivaldi M., Ruiz-Rodríguez M., Valdivia E., Martín-Platero A.M., Martínez-Bueno M., Peralta-Sánchez J.M., Méndez M. Symbiotic association between hoopoes and antibiotic-producing bacteria that live in their uropygial gland. Funct. Ecol. 2008;22:864–871. doi: 10.1111/j.1365-2435.2008.01448.x. [DOI] [Google Scholar]
- 156.James A.G., Hyliands D., Johnston H. Generation of volatile fatty acids by axillary bacteria. Int. J. Cosmetic Sci. 2004;26:149–156. doi: 10.1111/j.1467-2494.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 157.Braks M., Takken W. Incubated human sweat but not fresh sweat attracts the malaria mosquito Anopheles gambiae sensu stricto. J. Chem. Ecol. 1999;25:663–672. doi: 10.1023/A:1020970307748. [DOI] [Google Scholar]
- 158.Smallegange R.C., Verhulst N.O., Takken W. Sweaty skin: An invitation to bite? Trends Parasitol. 2011;27:143–148. doi: 10.1016/j.pt.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 159.Bernier U.R., Kline D.L., Posey K.H. Human emanations and related natural compounds that inhibit mosquito host-finding abilities. In: Debboun M., Frances S.P., Strickman D., editors. Insect Repellents: Principles, Methods, and Uses. CRC Press; Boca Raton, FL, USA: 2006. pp. 77–100. [Google Scholar]
- 160.Verhulst N.O., Boulanger N., Spitzen J. Chapter 3—Impact of skin microbiome on attractiveness to arthropod vectors and pathogen transmission. In: Boulanger N., editor. Skin and Arthropod Vectors. Academic Press; Cambridge, MA, USA: 2018. pp. 55–81. [Google Scholar]
- 161.Douglas H.D., Co J.E., Jones T.H., Conner W.E., Day J.F. Chemical odorant of colonial seabird repels mosquitoes. J. Med. Entomol. 2005;42:647–651. doi: 10.1093/jmedent/42.4.647. [DOI] [PubMed] [Google Scholar]
- 162.Clayton D.H., Koop J.A., Harbison C.W., Moyer B.R., Bush S.E. How birds combat ectoparasites. Open Ornithol. J. 2010;3:41–71. doi: 10.2174/1874453201003010041. [DOI] [Google Scholar]
- 163.Cooperband M.F., McElfresh J.S., Millar J.G., Cardé R.T. Attraction of female Culex quinquefasciatus Say (Diptera: Culicidae) to odors from chicken feces. J. Insect Physiol. 2008;54:1184–1192. doi: 10.1016/j.jinsphys.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 164.Shutler D. Some important overlooked aspects of odors in avian nesting ecology. J. Avian Biol. 2019;50:e02003. doi: 10.1111/jav.02003. [DOI] [Google Scholar]
- 165.Ibáñez-Álamo J.D., Ruiz-Raya F., Rodríguez L., Soler M. Fecal sacs attract insects to the nest and provoke an activation of the immune system of nestlings. Front. Zool. 2016;13:3. doi: 10.1186/s12983-016-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Azcárate-García M., Ruiz-Rodríguez M., Díaz-Lora S., Ruiz-Castellano C., Soler J.J. Experimentally broken faecal sacs affect nest bacterial environment, development and survival of spotless starling nestlings. J. Avian Biol. 2019;50:e02044. doi: 10.1111/jav.02044. [DOI] [Google Scholar]
- 167.Vale G.A. Field responses of tsetse flies (Diptera: Glossinidae) to odours of men, lactic acid and carbon dioxide. Bull. Entomol. Res. 1979;69:459–467. doi: 10.1017/S0007485300018964. [DOI] [Google Scholar]
- 168.Kilpatrick A., Kramer L.J., Jones M., Marra P., Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jensen K.M.V., Jespersen J.B., Birkett M.A., Pickett J.A., Thomas G., Wadhams L.J., Woodcock C.M. Variation in the load of the horn fly, Haematobia irritans, in cattle herds is determined by the presence or absence of individual heifers. Med. Vet. Entomol. 2004;18:275–280. doi: 10.1111/j.0269-283X.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 170.Oyarzún M.P., Palma R., Alberti E., Hormazabal E., Pardo F., Birkett M.A., Quiroz A. Olfactory response of Haematobia irritans (Diptera: Muscidae) to cattle-derived volatile compounds. J. Med. Entomol. 2009;46:1320–1326. doi: 10.1603/033.046.0610. [DOI] [PubMed] [Google Scholar]
- 171.Birkett M.A., Agelopoulos N., Jensen K.-M.V., Jespersen J.B., Pickett J.A., Prijs H.J., Thomas G., Trapman J.J., Wadhams L.J., Woodcock C.M. The role of volatile semiochemicals in mediating host location and selection by nuisance and disease-transmitting cattle flies Med. Vet. Entomol. 2004;18:313–322. doi: 10.1111/j.0269-283X.2004.00528.x. [DOI] [PubMed] [Google Scholar]
- 172.Cornet S., Nicot A., Rivero A., Gandon S. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol. Lett. 2013;16:323–329. doi: 10.1111/ele.12041. [DOI] [PubMed] [Google Scholar]
- 173.Lalubin F., Bize P., van Rooyen J., Christe P., Glaizot O. Potential evidence of parasite avoidance in an avian malarial vector. Anim. Behav. 2012;84:539–545. doi: 10.1016/j.anbehav.2012.06.004. [DOI] [Google Scholar]
- 174.Magalhães J.T., Jr., Mesquita P.R.R., Oliveira W.F.d.S., Oliveira F.S., Franke C.R., Rodrigues F.d.M., de Andrade J.B., Barrouin-Melo S.M. Identification of biomarkers in the hair of dogs: New diagnostic possibilities in the study and control of visceral leishmaniasis. Anal. Bioanal. Chem. 2014;406:6691–6700. doi: 10.1007/s00216-014-8103-2. [DOI] [PubMed] [Google Scholar]
- 175.Nevatte T., Ward R., Sedda L., Hamilton J. After infection with Leishmania infantum, Golden Hamsters (Mesocricetus auratus) become more attractive to female sand flies (Lutzomyia longipalpis) Sci. Rep. 2017;7:6104. doi: 10.1038/s41598-017-06313-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Magalhães J., Jr., Oliva-Filho A.D.A., Novais H., Mesquita P.M., Rodrigues F., Pinto M., Barrouin-Melo S. Attraction of the sandfly Lutzomyia longipalpis to possible biomarker compounds from dogs infected with Leishmania infantum. Med. Vet. Entomol. 2019;33:322–325. doi: 10.1111/mve.12357. [DOI] [PubMed] [Google Scholar]
- 177.Zhu J.J., Chaudhury M.F., Durso L.M., Sagel A., Skoda S.R., Jelvez-Serra N.S., Goulart Santanab E.G. Semiochemicals released from five bacteria identified from animal wounds infested by primary screwworms and their effects on fly behavioral activity. PLoS ONE. 2017;12:e0179090. doi: 10.1371/journal.pone.0179090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Knols B.G.J., van Loon J.J.A., Cork A., Robinson R.D., Adam W., Meijerink J., Jong R.D., Takken W. Behavioural and electrophysiological responses of the female malaria mosquito Anopheles gambiae (Diptera: Culicidae) to Limburger cheese volatiles. Bull. Entomol. Res. 1997;87:151–159. doi: 10.1017/S0007485300027292. [DOI] [Google Scholar]
- 179.Wanzala W., Sika N.F.K., Gule S., Hassanali A. Attractive and repellent host odours guide ticks to their respective feeding sites. Chemoecology. 2004;14:229–232. doi: 10.1007/s00049-004-0280-6. [DOI] [Google Scholar]
- 180.Saini R.K., Orindi B.O., Mbahin N., Andoke J.A., Muasa P.N., Mbuvi D.M., Muya C.M., Pickett J.A., Borgemeister C.W. Protecting cows in small holder farms in East Africa from tsetse flies by mimicking the odor profile of a non-host bovid. PLoS Negl. Trop. Dis. 2017;11:e0005977. doi: 10.1371/journal.pntd.0005977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Creton B., Pageat P., Robejean M., Lafont-Lecuelle C., Cozzi A. Protection of horse ears against Simulid parasitism: Efficacy of a mammal semiochemical solution over 10 hours. Vet. Parasitol. 2016;227:15–19. doi: 10.1016/j.vetpar.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 182.Dehnhard M., Heistermann M., Goritz F., Hermes R., Hildebrandt T., Haber H. Demonstration of 2-unsaturated C19-steroids in the urine of female Asian elephants, Elephas maximus, and their dependence on ovarian activity. Reproduction. 2001;121:475–484. doi: 10.1530/rep.0.1210475. [DOI] [PubMed] [Google Scholar]
- 183.Greenwood D.R., Comeskey D., Hunt M.B., Rasmussen L.E.L. Chirality in elephant pheromones. Nature. 2005;438:1097–1098. doi: 10.1038/4381097a. [DOI] [PubMed] [Google Scholar]
- 184.Perrin T.E., Rasmussen L. Chemosensory responses of female Asian elephants (Elephas maximus) to cyclohexanone. J. Chem. Ecol. 1994;20:2857–2866. doi: 10.1007/BF02098394. [DOI] [PubMed] [Google Scholar]
- 185.Perrin T.E., Rasmussen L., Gunawardena R., Rasmussen R. A method for collection, long-term storage, and bioassay of labile volatile chemosignals. J. Chem. Ecol. 1996;22:207–221. doi: 10.1007/BF02055093. [DOI] [PubMed] [Google Scholar]
- 186.Rasmussen L. Source and cyclic release pattern of (Z)-7-dodecenyl acetate, the pre-ovulatory pheromone of the female Asian elephant. Chem. Senses. 2001;26:611–623. doi: 10.1093/chemse/26.6.611. [DOI] [PubMed] [Google Scholar]
- 187.Rasmussen L., Lee T.D., Daves G.D., Schmidt M.J. Female-to-male sex pheromones of low volatility in the Asian elephant, Elephas maximus. J. Chem. Ecol. 1993;19:2115–2128. doi: 10.1007/BF00979651. [DOI] [PubMed] [Google Scholar]
- 188.Rasmussen L., Lee T.D., Roelofs W.L., Zhang A., Daves G.D. Insect pheromone in elephants. Nature. 1996;379:684-684. doi: 10.1038/379684a0. [DOI] [PubMed] [Google Scholar]
- 189.Adams J., Garcia A., Foote C.S. Some chemical constituents of the secretion from the temporal gland of the African elephant (Loxodonta africana) J. Chem. Ecol. 1978;4:17–25. doi: 10.1007/BF00988256. [DOI] [Google Scholar]
- 190.Goodwin T.E., Brown F.D., Counts R.W., Dowdy N.C., Fraley P.L., Hughes R.A., Liu D.Z., Mashburn C.D., Rankin J.D., Roberson R.S. African elephant sesquiterpenes. II. Identification and synthesis of new derivatives of 2, 3-dihydrofarnesol. J. Nat. Prod. 2002;65:1319–1322. doi: 10.1021/np010647c. [DOI] [PubMed] [Google Scholar]
- 191.Goodwin T.E., Eggert M.S., House S.J., Weddell M.E., Schulte B.A., Rasmussen L. Insect pheromones and precursors in female African elephant urine. J. Chem. Ecol. 2006;32:1849–1853. doi: 10.1007/s10886-006-9094-z. [DOI] [PubMed] [Google Scholar]
- 192.Goodwin T.E., Rasmussen E.L., Guinn A.C., McKelvey S.S., Gunawardena R., Riddle S.W., Riddle H.S. African elephant sesquiterpenes. J. Nat. Prod. 1999;62:1570–1572. doi: 10.1021/np990191n. [DOI] [PubMed] [Google Scholar]
- 193.Wheeler J., Rasmussen L., Ayorinde F., Buss I.O., Smuts G. Constituents of temporal gland secretion of the African elephant, Loxodonta africana. J. Chem. Ecol. 1982;8:821–835. doi: 10.1007/BF00994782. [DOI] [PubMed] [Google Scholar]
- 194.Būda V., Mozūraitis R., Kutra J., Borg-Karlson A.-K. p-Cresol: A sex pheromone component identified from the estrous urine of mares. J. Chem. Ecol. 2012;38:811–813. doi: 10.1007/s10886-012-0138-2. [DOI] [PubMed] [Google Scholar]
- 195.Dormont L., Jay-Robert P., Bessiere J.-M., Rapior S., Lumaret J.-P. Innate olfactory preferences in dung beetles. J. Exp. Biol. 2010;213:3177–3186. doi: 10.1242/jeb.040964. [DOI] [PubMed] [Google Scholar]
- 196.Mozūraitis R., Būda V., Kutra J., Borg-Karlson A.-K. p-and m-Cresols emitted from estrous urine are reliable volatile chemical markers of ovulation in mares. Anim. Reprod. Sci. 2012;130:51–56. doi: 10.1016/j.anireprosci.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 197.Datta S., Harris H. Urinary amino-acid patterns of some mammals. Ann. Eugen. 1953;18:107–116. doi: 10.1111/j.1469-1809.1952.tb02503.x. [DOI] [PubMed] [Google Scholar]
- 198.Kimura R. Volatile substances in feces, urine and urine-marked feces of feral horses. Can. J. Anim. Sci. 2001;81:411–420. doi: 10.4141/A00-068. [DOI] [Google Scholar]
- 199.Tchouassi D.P., Sang R., Sole C.L., Bastos A.D., Teal P.E., Borgemeister C., Torto B. Common host-derived chemicals increase catches of disease-transmitting mosquitoes and can improve early warning systems for Rift Valley fever virus. PLoS Negl. Trop. Dis. 2013;7:e2007. doi: 10.1371/journal.pntd.0002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ferreira L.L., Sarria A.L.F., de Oliveira Filho J.G., de Silva F.d.O., Powers S.J., Caulfield J.C., Pickett J.A., Birkett M.A., Borges L.M.F. Identification of a non-host semiochemical from tick-resistant donkeys (Equus asinus) against Amblyomma sculptum ticks. Ticks Tick Borne Dis. 2019;10:621–627. doi: 10.1016/j.ttbdis.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Reiter B., Burger B., Dry J. Mammalian exocrine secretions. XVIII: Chemical characterization of interdigital secretion of red hartebeest, Alcelaphus buselaphus caama. J. Chem. Ecol. 2003;29:2235–2252. doi: 10.1023/A:1026218213151. [DOI] [PubMed] [Google Scholar]
- 202.Burger B., Le Roux M., Spies H., Truter V. Mammalian pheromone studies. III: (E, E)-7,11,15-trimethyl-3-methylenehexadeca-1,6,10,14-tetraene, a new diterpene analogue of beta-farnesene from the dorsal gland of the springbok, Antidorcas marsupialis. Tetrahedron Lett. 1978;52:5221–5224. doi: 10.1016/S0040-4039(01)85855-X. [DOI] [Google Scholar]
- 203.Burger B., le Roux M., Spies H., Truter V., Bigalke R. Mammalian pheromone studies, IV. Terpenoid compounds and hydroxy esters from the dorsal gland of the springbok, Antidorcas marsupialis. Z. Naturforsch. C. 1981;36:340–343. doi: 10.1515/znc-1981-3-426. [DOI] [Google Scholar]
- 204.Fischer-Tenhagen C., Johnen D., Le Danvic C., Gatien J., Salvetti P., Tenhagen B.A., Heuwieser W. Validation of bovine oestrous-specific synthetic molecules with trained scent dogs; similarities between natural and synthetic oestrous smell. Reprod. Domest. Anim. 2015;50:7–12. doi: 10.1111/rda.12440. [DOI] [PubMed] [Google Scholar]
- 205.Hales K., Parker D.B., Cole N.A. Volatile organic compound flux from manure of cattle fed diets differing in grain processing method and co-product inclusion. Atmos. Environ. 2015;100:20–24. doi: 10.1016/j.atmosenv.2014.10.037. [DOI] [Google Scholar]
- 206.Hales K.E., Parker D.B., Cole N.A. Potential odorous volatile organic compound emissions from feces and urine from cattle fed corn-based diets with wet distillers grains and solubles. Atmos. Environ. 2012;60:292–297. doi: 10.1016/j.atmosenv.2012.06.080. [DOI] [Google Scholar]
- 207.Isberg E., Bray D.P., Birgersson G., Hillbur Y., Ignell R. Identification of cattle-derived volatiles that modulate the behavioral response of the biting midge Culicoides nubeculosus. J. Chem. Ecol. 2016;42:24–32. doi: 10.1007/s10886-015-0663-x. [DOI] [PubMed] [Google Scholar]
- 208.Kumar K.R., Archunan G., Jeyaraman R., Narasimhan S. Chemical characterization of bovine urine with special reference to oestrus. Vet. Res. Commun. 2000;24:445–454. doi: 10.1023/A:1006495404407. [DOI] [PubMed] [Google Scholar]
- 209.Preti G. Method for detecting bovine estrus by determining methyl heptanol concentrations in vaginal secretions. 4,467,814. U.S. Patent. 1984 Aug 28
- 210.Sankar R., Archunan G., Habara Y. Detection of oestrous-related odour in bovine (Bos taurus) saliva: Bioassay of identified compounds. Animal. 2007;1:1321–1327. doi: 10.1017/S1751731107000614. [DOI] [PubMed] [Google Scholar]
- 211.Vale G.A., Hall D.R., Gough A.J.E. The olfactory responses of tsetse flies, Glossina spp. (Diptera: Glossinidae), to phenols and urine in the field. Bull. Entomol. Res. 1988;78:293–300. doi: 10.1017/S0007485300013055. [DOI] [Google Scholar]
- 212.Rajanarayanan S., Archunan G. Identification of urinary sex pheromones in female buffaloes and their influence on bull reproductive behaviour. Res. Vet. Sci. 2011;91:301–305. doi: 10.1016/j.rvsc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 213.Burger B., Pretorius P. Mammalian pheromone studies, VI. Compounds from the preorbital gland of the blue duiker, Cephalophus monticola. Z. Naturforsch. C. 1987;42:1355–1357. doi: 10.1515/znc-1987-11-1238. [DOI] [Google Scholar]
- 214.Burger B., Pretorius P., Stander J., Grierson G. Mammalian pheromone studies, VII. Identification of thiazole derivatives in the preorbital gland secretions of the grey duiker, Sylvicapra grimmia, and the red duiker, Cephalophus natalensis. Z. Naturforsch. C. 1988;43:731–736. doi: 10.1515/znc-1988-9-1016. [DOI] [PubMed] [Google Scholar]
- 215.Burger B., le Roux M., Garbers C., Bigalke R., Pachler K., Wessels P., Christ V., Maurer K. Studies on mammalian pheromones, II. Further compounds from the pedal gland of the bontebok (Damaliscus dorcas dorcas) Z. Naturforsch. C. 1977;32:49–56. doi: 10.1515/znc-1977-1-207. [DOI] [PubMed] [Google Scholar]
- 216.Burger B., Le Roux M., Garbers C., Spies H., Bigalke R., Pachler K., Wessels P., Christ V., Maurer K. Studies on mammalian pheromones, I. Ketones from the pedal gland of the bontebok (Damaliscus dorcas dorcas) Z. Naturforsch. C. 1976;31:21–28. doi: 10.1515/znc-1976-1-208. [DOI] [PubMed] [Google Scholar]
- 217.Burger B., Nell A., Spies H., Le Roux M., Bigalke R. Mammalian exocrine secretions. XIII: Constituents of preorbital secretions of bontebok, Damaliscus dorcas dorcas, and blesbok, D. d. phillipsi. J. Chem. Ecol. 1999;25:2085–2097. doi: 10.1023/A:1021088807149. [DOI] [Google Scholar]
- 218.Burger B., Nell A., Spies H., Le Roux M., Bigalke R., Brand P. Mammalian exocrine secretions. XII: Constituents of interdigital secretions of bontebok, Damaliscus dorcas dorcas, and blesbok, D. d. phillipsi. J. Chem. Ecol. 1999;25:2057–2084. doi: 10.1023/A:1021036823079. [DOI] [Google Scholar]
- 219.Stander M., Burger B., Le Roux M. Mammalian exocrine secretions. XVII: Chemical characterization of preorbital secretion of male suni, Neotragus moschatus. J. Chem. Ecol. 2002;28:89–101. doi: 10.1023/A:1013562818965. [DOI] [PubMed] [Google Scholar]
- 220.Burger B., Yang T.-P., Le Roux M., Brandt W., Cox A., Hart P. Mammalian exocrine secretions XI. Constituents of the preorbital secretion of klipspringer, Oreotragus oreotragus. J. Chem. Ecol. 1997;23:2383–2400. doi: 10.1023/B:JOEC.0000006681.33646.f4. [DOI] [Google Scholar]
- 221.Flood P.F., Abrams S.R., Muir G.D., Rowell J.E. Odor of the muskox. J. Chem. Ecol. 1989;15:2207–2217. doi: 10.1007/BF01014110. [DOI] [PubMed] [Google Scholar]
- 222.Mo W.P., Burger B.V., LeRoux M., Spies H.S.C. Mammalian exocrine secretions: IX. Constituents of preorbital secretion of oribi, Ourebia, ourebi. J. Chem. Ecol. 1995;21:1191–1215. doi: 10.1007/BF02228320. [DOI] [PubMed] [Google Scholar]
- 223.Burger B.V., Tien F.C., Le Roux M., Mo W.P. Mammalian exocrine secretions: X. constituents of preorbital secretion of grysbok, Raphicerus melanotis. J. Chem. Ecol. 1996;22:739–764. doi: 10.1007/BF02033583. [DOI] [PubMed] [Google Scholar]
- 224.Burger B., Pretorius P., Spies H., Bigalke R., Grierson G. Mammalian pheromones VIII Chemical characterization of preorbital gland secretion of grey duiker, Sylvicapra grimmia (Artiodactyla: Bovidae) J. Chem. Ecol. 1990;16:397–416. doi: 10.1007/BF01021773. [DOI] [PubMed] [Google Scholar]
- 225.Madubunyi L.C., Hassanali A., Ouma W., Nyarango D., Kabii J. Chemoecological role of mammalian urine in host location by tsetse, Glossina spp. (Diptera: Glossinidae) J. Chem. Ecol. 1996;22:1187–1199. doi: 10.1007/BF02027954. [DOI] [PubMed] [Google Scholar]
- 226.Ishii H., Krane S., Itagaki Y., Berova N., Nakanishi K., Weldon P.J. Absolute configuration of a hydroxyfuranoid acid from the pelage of the genus Bos, 18-(6S,9R,10R)-bovidic acid. J. Nat. Prod. 2004;67:1426–1430. doi: 10.1021/np049937u. [DOI] [PubMed] [Google Scholar]
- 227.Cohen-Tannoudji J., Einhorn J., Signoret J. Ram sexual pheromone: First approach of chemical identification. Physiol. Behav. 1994;56:955–961. doi: 10.1016/0031-9384(94)90329-8. [DOI] [PubMed] [Google Scholar]
- 228.Ryan J.P. Determination of volatile fatty acids and some related compounds in ovine rumen fluid, urine, and blood plasma, by gas-liquid chromatography. Anal. Biochem. 1980;108:374–384. doi: 10.1016/0003-2697(80)90602-8. [DOI] [PubMed] [Google Scholar]
- 229.Chmielowiec-Korzeniowska A. The concentration of volatile organic compounds [VOCs] in pig farm air. Ann. Agric. Environ. Med. 2009;16:249–256. [PubMed] [Google Scholar]
- 230.Pageat P. Pig appeasing pheromones to decrease stress, anxiety and aggressiveness. 6,169,113. U.S. Patent. 2001 Jan 2
- 231.Sinclair P.A., Squires E.J., Raeside J.I., Renaud R. Synthesis of free and sulphoconjugated 16-androstene steroids by the Leydig cells of the mature domestic boar. J. Steroid Biochem. Mol. Biol. 2005;96:217–228. doi: 10.1016/j.jsbmb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 232.Müller-Schwarze D., Müller-Schwarze C., Singer A.G., Silverstein R.M. Mammalian pheromone: Identification of active component in the subauricular scent of the male pronghorn. Science. 1974;183:860–862. doi: 10.1126/science.183.4127.860. [DOI] [PubMed] [Google Scholar]
- 233.Wood W. 2-Ethyl-3-methylpyrazine in the subauricular and median glands of pronghorn, Antilocapra americana. Biochem. Syst. Ecol. 2011;39:159–160. doi: 10.1016/j.bse.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 234.Ayorinde F., Wheeler J., Wemmer C., Murtaugh J. Volatile components of the occipital gland secretion of the bactrian camel (Camelus bactrianus) J. Chem. Ecol. 1982;8:177–183. doi: 10.1007/BF00984014. [DOI] [PubMed] [Google Scholar]
- 235.Whittle C.L., Bowyer R.T., Clausen T.P., Duffy L.K. Putative pheromones in urine of rutting male moose (Alces alces): Evolution of honest advertisement? J. Chem. Ecol. 2000;26:2747–2762. doi: 10.1023/A:1026485725805. [DOI] [Google Scholar]
- 236.Bakke J.M., Figenschou E. Volatile compounds from the red deer (Cervus elaphus) secretion from the tail gland. J. Chem. Ecol. 1983;9:513–520. doi: 10.1007/BF00990223. [DOI] [PubMed] [Google Scholar]
- 237.Wood W.F. Volatile components in metatarsal glands of sika deer, Cervus nippon. J. Chem. Ecol. 2003;29:2729–2733. doi: 10.1023/B:JOEC.0000008016.44795.8a. [DOI] [PubMed] [Google Scholar]
- 238.Wood W.F. Straight and branched-chain fatty acids in preorbital glands of sika deer, Cervus nippon. J. Chem. Ecol. 2004;30:479–482. doi: 10.1023/B:JOEC.0000017996.65270.d7. [DOI] [PubMed] [Google Scholar]
- 239.Ruzicka L. Zur Kenntnis des Kohlenstoffringes VII. Über die Konstitution des Muscons. Helv. Chim. Acta. 1926;9:715–729. doi: 10.1002/hlca.19260090197. [DOI] [Google Scholar]
- 240.Brownlee R.G., Silverstein R.M., Müller-Schwarze D., Singer A.G. Isolation, identification and function of the chief component of the male tarsal scent in black-tailed deer. Nature. 1969;221:284–285. doi: 10.1038/221284a0. [DOI] [Google Scholar]
- 241.Gassett J., Wiesler D., Baker A., Osborn D., Miller K., Marchinton R., Novotny M. Volatile compounds from interdigital gland of male white-tailed deer (Odocoileus virginianus) J. Chem. Ecol. 1996;22:1689–1696. doi: 10.1007/BF02272407. [DOI] [PubMed] [Google Scholar]
- 242.Andersson G. Volatile ketones from the preorbital gland of reindeer (Rangifer t. tarandus L.) J. Chem. Ecol. 1979;5:629–634. doi: 10.1007/BF00987847. [DOI] [Google Scholar]
- 243.Andersson G., Andersson K., Brundin A., Rappe C. Volatile compounds from the tarsal scent gland of reindeer (Rangifer tarandus) J. Chem. Ecol. 1975;1:275–281. doi: 10.1007/BF00987876. [DOI] [Google Scholar]
- 244.Andersson G., Brundin A., Andersson K. Volatile compounds from the interdigital gland of reindeer (Rangifer t. tarandus L.) J. Chem. Ecol. 1979;5:321–333. doi: 10.1007/BF00987918. [DOI] [Google Scholar]
- 245.Brundin A., Andersson G. Seasonal variation of three ketones in the interdigital gland secretion of reindeer (Rangifer tarandus L.) J. Chem. Ecol. 1979;5:881–889. doi: 10.1007/BF00990210. [DOI] [Google Scholar]
- 246.Brundin A., Andersson G., Andersson K., Mossing T., Källquist L. Short-chain aliphatic acids in the interdigital gland secretion of reindeer (Rangifer tarandus L.), and their discrimination by reindeer. J. Chem. Ecol. 1978;4:613–622. doi: 10.1007/BF00988924. [DOI] [Google Scholar]
- 247.Müller-Schwarze D., Quay W., Brundin A. The caudal gland in reindeer (Rangifer tarandus L.): Its behavioral role, histology, and chemistry. J. Chem. Ecol. 1977;3:591–601. doi: 10.1007/BF00989079. [DOI] [Google Scholar]
- 248.Wood W.F., Weldon P.J. The scent of the reticulated giraffe (Giraffa camelopardalis reticulata) Biochem. Syst. Ecol. 2002;30:913–917. doi: 10.1016/S0305-1978(02)00037-6. [DOI] [Google Scholar]
- 249.Waterhouse J.S., Hudson M., Pickett J.A., Weldon P.J. Volatile components in dorsal gland secretions of the white-lipped peccary, Tayassu pecari, from Bolivia. J. Chem. Ecol. 2001;27:2459–2469. doi: 10.1023/A:1013675431162. [DOI] [PubMed] [Google Scholar]
- 250.Waterhouse J.S., Ke J., Pickett J.A., Weldon P.J. Volatile components in dorsal gland secretions of the collared peccary, Tayassu tajacu (Tayassuidae, Mammalia) J. Chem. Ecol. 1996;22:1307–1314. doi: 10.1007/BF02266967. [DOI] [PubMed] [Google Scholar]
- 251.Michael R.P., Keverne E., Bonsall R. Pheromones: Isolation of male sex attractants from a female primate. Science. 1971;172:964–966. doi: 10.1126/science.172.3986.964. [DOI] [PubMed] [Google Scholar]
- 252.MacDonald E.A., Fernandez-duque E., Evans S., Hagey L.R. Sex, age, and family differences in the chemical composition of owl monkey (Aotus nancymaae) subcaudal scent secretions. Am. J. Primatol. 2008;70:12–18. doi: 10.1002/ajp.20450. [DOI] [PubMed] [Google Scholar]
- 253.Smith A.B., Belcher A.M., Epple G., Jurs P.C., Lavine B. Computerized pattern recognition: A new technique for the analysis of chemical communication. Science. 1985;228:175–177. doi: 10.1126/science.3975636. [DOI] [PubMed] [Google Scholar]
- 254.Belcher A., Epple G., Küderling I., Smith A. Volatile components of scent material from Cotton-top Tamarin (Saguinus o. oedipus) J. Chem. Ecol. 1988;14:1367–1384. doi: 10.1007/BF01020141. [DOI] [PubMed] [Google Scholar]
- 255.Crewe R., Burger B., Le Roux M., Katsir Z. Chemical constituents of the chest gland secretion of the thick-tailed galago (Galago crassicaudatus) J. Chem. Ecol. 1979;5:861–868. doi: 10.1007/BF00986569. [DOI] [Google Scholar]
- 256.Wheeler J., Blum M., Clark A. ß-(p-hydroxyphenyl) ethanol in the chest gland secretion of a galago (Galago crassicaudatus) Experientia. 1977;33:988–989. doi: 10.1007/BF01945921. [DOI] [PubMed] [Google Scholar]
- 257.Burger B.V., Visser R., Moses A., Le Roux M. Elemental sulfur identified in urine of cheetah, Acinonyx jubatus. J. Chem. Ecol. 2006;32:1347–1352. doi: 10.1007/s10886-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 258.Poddar-Sarkar M., Brahmachary R. Putative semiochernicals in the African cheetah (Acinonyx jubatus) J. Lipid Mediat. Cell. 1997;15:285–287. doi: 10.1016/S0929-7855(96)00561-5. [DOI] [PubMed] [Google Scholar]
- 259.Apps P., Mmualefe L., Jordan N.R., Golabek K.A., McNutt J.W. The “tomcat compound” 3-mercapto-3-methylbutanol occurs in the urine of free-ranging leopards but not in African lions or cheetahs. Biochem. Syst. Ecol. 2014;53:17–19. doi: 10.1016/j.bse.2013.12.013. [DOI] [Google Scholar]
- 260.Mattina M., Pignatello J., Swihart R. Identification of volatile components of bobcat (Lynx rufus) urine. J. Chem. Ecol. 1991;17:451–462. doi: 10.1007/BF00994344. [DOI] [PubMed] [Google Scholar]
- 261.Soini H.A., Linville S.U., Wiesler D., Posto A.L., Williams D.R., Novotny M.V. Investigation of scents on cheeks and foreheads of large felines in connection to the facial marking behavior. J. Chem. Ecol. 2012;38:145–156. doi: 10.1007/s10886-012-0075-0. [DOI] [PubMed] [Google Scholar]
- 262.Albone E.S., Eglinton G., Walker J.M., Ware G.C. The anal sac secretion of the red fox (Vulpes vulpes); its chemistry and microbiology. A comparison with the anal sac secretion of the lion (Panthera leo) Life Sci. 1974;14:387–400. doi: 10.1016/0024-3205(74)90069-1. [DOI] [PubMed] [Google Scholar]
- 263.Soso S.B., Koziel J.A. Characterizing the scent and chemical composition of Panthera leo marking fluid using solid-phase microextraction and multidimensional gas chromatography–mass spectrometry-olfactometry. Sci. Rep. 2017;7:1–15. doi: 10.1038/s41598-017-04973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Poddar-Sarkar M. Putative chemical signals of leopard. Anim. Biol. 2004;54:255–259. [Google Scholar]
- 265.Brahmachary R. The expanding world of 2-acetyl-1-pyrroline. Curr. Sci. 1996;71:257–258. [Google Scholar]
- 266.Brahmachary R., Dutta J. Phenylethylamine as a biochemical marker of tiger. Z. Naturforsch. C. 1979;34:632–633. doi: 10.1515/znc-1979-7-824. [DOI] [PubMed] [Google Scholar]
- 267.Soso S.B., Koziel J.A. Analysis of odorants in marking fluid of Siberian tiger (Panthera tigris altaica) using simultaneous sensory and chemical analysis with headspace solid-phase microextraction and multidimensional gas chromatography-mass spectrometry-olfactometry. Molecules. 2016;21:834. doi: 10.3390/molecules21070834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Banks G.R., Buglass A.J., Waterhouse J.S. Amines in the marking fluid and anal sac secretion of the tiger, Panthera tigris. Z. Naturforsch. C. 1992;47:618–620. doi: 10.1515/znc-1992-7-821. [DOI] [PubMed] [Google Scholar]
- 269.Goodwin M., Gooding K., Regnier F. Sex pheromone in the dog. Science. 1979;203:559–561. doi: 10.1126/science.569903. [DOI] [PubMed] [Google Scholar]
- 270.Martín J., Barja I., López P. Chemical scent constituents in feces of wild Iberian wolves (Canis lupus signatus) Biochem. Syst. Ecol. 2010;38:1096–1102. doi: 10.1016/j.bse.2010.10.014. [DOI] [Google Scholar]
- 271.Goodwin T.E., Songsasen N., Broederdorf L.J., Burkert B.A., Chen C.J., Jackson S.R., Keplinger K.B., Rountree M.E., Waldrip Z.J., Weddell M.E. Hemiterpenoids and pyrazines in the odoriferous urine of the maned wolf (Chrysocyon brachyurus) In: East M.L., Dehnhard M., editors. Chemical Signals in Vertebrates 12. Springer; New York, NY, USA: 2013. pp. 171–184. [Google Scholar]
- 272.Albone E., Perry G. Anal sac secretion of the red fox, Vulpes vulpes; volatile fatty acids and diamines: Implications for a fermentation hypothesis of chemical recognition. J. Chem. Ecol. 1975;2:101–111. doi: 10.1007/BF00988029. [DOI] [Google Scholar]
- 273.Albone E., Robins S., Patel D. 5-Aminovaleric acid, a major free amino acid component of the anal sac secretion of the red fox, Vulpes vulpes. Comp. Biochem. Physiol., B. 1976;55:483–486. doi: 10.1016/0305-0491(76)90004-3. [DOI] [PubMed] [Google Scholar]
- 274.Jorgenson J., Novotny M., Carmack M., Copland G., Wilson S., Katona S., Whitten W. Chemical scent constituents in the urine of the red fox (Vulpes vulpes L.) during the winter season. Science. 1978;199:796–798. doi: 10.1126/science.199.4330.796. [DOI] [PubMed] [Google Scholar]
- 275.Apps P., Viljoen H., Taylor P. Volatile components from the anal glands of the yellow mongoose Cynictis penicillata. Afr. Zool. 1989;24:361–362. [Google Scholar]
- 276.Gorman M.L. A mechanism for individual recognition by odour in Herpestes auropunctatus (Carnivora: Viverridae) Anim. Behav. 1976;24:141–145. doi: 10.1016/S0003-3472(76)80107-8. [DOI] [Google Scholar]
- 277.Hefetz A., Ben-Yaacov R., Yom-Tov Y. Sex specificity in the anal gland secretion of the Egyptian mongoose Herpestes ichneumon. J. Zool. 1984;203:205–209. doi: 10.1111/j.1469-7998.1984.tb02327.x. [DOI] [Google Scholar]
- 278.Wood W.F., Fisher C.O., Graham G.A. Volatile components in defensive spray of the hog-nosed skunk, Conepatus mesoleucus. J. Chem. Ecol. 1993;19:837–841. doi: 10.1007/BF00985013. [DOI] [PubMed] [Google Scholar]
- 279.Wood W.F., Sollers B.G., Dragoo G.A., Dragoo J.W. Volatile components in defensive spray of the hooded skunk, Mephitis macroura. J. Chem. Ecol. 2002;28:1865–1870. doi: 10.1023/A:1020573404341. [DOI] [PubMed] [Google Scholar]
- 280.Andersen K.K., Bernstein D.T. Some chemical constituents of the scent of the striped skunk (Mephitis mephitis) J. Chem. Ecol. 1975;1:493–499. doi: 10.1007/BF00988589. [DOI] [Google Scholar]
- 281.Wood W.F. New components in defensive secretion of the striped skunk, Mephitis mephitis. J. Chem. Ecol. 1990;16:2057–2065. doi: 10.1007/BF01020516. [DOI] [PubMed] [Google Scholar]
- 282.Wood W.F., Morgan C.G., Miller A. Volatile components in defensive spray of the spotted skunk, Spilogale putorius. J. Chem. Ecol. 1991;17:1415–1420. doi: 10.1007/BF00983773. [DOI] [PubMed] [Google Scholar]
- 283.Bradshaw A., Beckmann M., Stevens R., Slater F. Anal scent gland secretion of the European otter (Lutra Lutra) In: Marchlewska-Koj A., Lepri J.J., Müller-Schwarze D., editors. Chemical Signals in Vertebrates 9. Springer; Boston, MA, USA: 2001. pp. 313–319. [Google Scholar]
- 284.Davies M.J. Ph.D. Thesis. The University of Hull; Hull, UK: 2008. Chemical communication in the European otter, Lutra lutra. [Google Scholar]
- 285.Wood W.F., Copeland J.P., Yates R.E., Horsey I.K., McGreevy L.R. Potential semiochemicals in urine from free ranging wolverines (Gulo gulo Pallas, 1780) Biochem. Syst. Ecol. 2009;37:574–578. doi: 10.1016/j.bse.2009.09.007. [DOI] [Google Scholar]
- 286.Wood W.F., Terwilliger M.N., Copeland J.P. Volatile compounds from anal glands of the wolverine, Gulo gulo. J. Chem. Ecol. 2005;31:2111–2117. doi: 10.1007/s10886-005-6080-9. [DOI] [PubMed] [Google Scholar]
- 287.Apps P.J., Viljoen H.W., Pretorius V., Rohwer E.R. Volatile components of the anal gland secretion of the striped polecat Ictonyx striatus. S. Afr. J. Zool. 1988;23:136–137. [Google Scholar]
- 288.Wheeler J.W., Nyalley L., Davis D.M., Weldon P.J. Additional sulfur compounds from the anal glands of the striped polecat, Ictonyx striatus (Mustelidae, Mammalia) Z. Naturforsch. C. 1997;52:283–285. doi: 10.1515/znc-1997-3-425. [DOI] [PubMed] [Google Scholar]
- 289.Brinck C., Erlinge S., Sandell M. Anal sac secretion in mustelids a comparison. J. Chem. Ecol. 1983;9:727–745. doi: 10.1007/BF00988779. [DOI] [PubMed] [Google Scholar]
- 290.Crump D.R. 2-Propylithietane, the major malodorous substance from the anal gland of the stoat (Mustela erminea) Tetrahedron Lett. 1978;19:5233–5234. doi: 10.1016/S0040-4039(01)85858-5. [DOI] [Google Scholar]
- 291.Crump D.R. Thietanes and dithiolanes from the anal gland of the stoat (Mustela erminea) J. Chem. Ecol. 1980;6:341–347. doi: 10.1007/BF01402912. [DOI] [Google Scholar]
- 292.Zhang J.-X., Sun L., Zhang Z.-B., Wang Z.-W., Chen Y., Wang R. Volatile compounds in anal gland of Siberian weasels (Mustela sibirica) and steppe polecats (M. eversmanni) J. Chem. Ecol. 2002;28:1287–1297. doi: 10.1023/A:1016246120479. [DOI] [PubMed] [Google Scholar]
- 293.Schildknecht H., Wilz I., Enzmann F., Grund N., Ziegler M. Mustelan, the malodorous substance from the anal gland of the mink (Mustela vison) and the polecat (Mustela putorius) Angew. Chem. Int. Ed. 1976;15:242–243. doi: 10.1002/anie.197602421. [DOI] [PubMed] [Google Scholar]
- 294.Crump D.R. Anal gland secretion of the ferret (Mustela putorius forma furo) J. Chem. Ecol. 1980;6:837–844. doi: 10.1007/BF00990407. [DOI] [PubMed] [Google Scholar]
- 295.Zhang J.-X., Soini H.A., Bruce K.E., Wiesler D., Woodley S.K., Baum M.J., Novotny M.V. Putative chemosignals of the ferret (Mustela furo) associated with individual and gender recognition. Chem. Senses. 2005;30:727–737. doi: 10.1093/chemse/bji065. [DOI] [PubMed] [Google Scholar]
- 296.Brinck C., Gerell R., Odham G. Anal pouch secretion in mink Mustela vison. Chemical communication in mustelidae. Oikos. 1978;30:68–75. doi: 10.2307/3543523. [DOI] [Google Scholar]
- 297.Buesching C.D., Waterhouse J.S., Macdonald D.W. Gas-chromatographic analyses of the subcaudal gland secretion of the European badger (Meles meles) part I: Chemical differences related to individual parameters. J. Chem. Ecol. 2002;28:41–56. doi: 10.1023/A:1013558718057. [DOI] [PubMed] [Google Scholar]
- 298.Davies J.M., Lachno D.R., Roper T.J. The anal gland secretion of the European badger (Meles meles) and its role in social communication. J. Zool. 1988;216:455–463. doi: 10.1111/j.1469-7998.1988.tb02441.x. [DOI] [Google Scholar]
- 299.Peet D.J., Wettenhall R.E., Rivett D.E., Allen A.K. A comparative study of covalently-bound fatty acids in keratinized tissues. Comp. Biochem. Physiol., B. 1992;102:363–366. doi: 10.1016/0305-0491(92)90135-E. [DOI] [PubMed] [Google Scholar]
- 300.Hagey L., MacDonald E. Chemical cues identify gender and individuality in giant pandas (Ailuropoda melanoleuca) J. Chem. Ecol. 2003;29:1479–1488. doi: 10.1023/A:1024225806263. [DOI] [PubMed] [Google Scholar]
- 301.Zhang J.-X., Liu D., Sun L., Wei R., Zhang G., Wu H., Zhang H., Zhao C. Potential chemosignals in the anogenital gland secretion of giant pandas, Ailuropoda melanoleuca, associated with sex and individual identity. J. Chem. Ecol. 2008;34:398–407. doi: 10.1007/s10886-008-9441-3. [DOI] [PubMed] [Google Scholar]
- 302.Apps P., Claase M., Yexley B., McNutt J.W. Interspecific responses of wild African carnivores to odour of 3-mercapto-3-methylbutanol, a component of wildcat and leopard urine. J. Ethol. 2017;35:153–159. doi: 10.1007/s10164-016-0503-7. [DOI] [Google Scholar]
- 303.van Dorp D.A., Klok R., Nugteren D.H. New macrocyclic compounds from the secretions of the civet cat and the musk rat. Recl. Trav. Chim. Pay-B. 1973;92:915–928. doi: 10.1002/recl.19730920813. [DOI] [Google Scholar]
- 304.O’Connell R.J., Singer A.G., Macrides F., Pfaffmann C., Agosta W.C. Responses of the male golden hamster to mixtures of odorants identified from vaginal discharge. Behav. Biol. 1978;24:244–255. doi: 10.1016/S0091-6773(78)93110-3. [DOI] [PubMed] [Google Scholar]
- 305.Boyer M.L., Jemiolo B., Andreolini F., Wiesler D., Novotny M. Urinary volatile profiles of pine vole, Microtus pinetorum, and their endocrine dependency. J. Chem. Ecol. 1989;15:649–662. doi: 10.1007/BF01014708. [DOI] [PubMed] [Google Scholar]
- 306.Ma W., Wiesler D., Novotny M.V. Urinary volatile profiles of the deermouse (Peromyscus maniculatus) pertaining to gender and age. J. Chem. Ecol. 1999;25:417–431. doi: 10.1023/A:1020937400480. [DOI] [Google Scholar]
- 307.Soini H.A., Wiesler D., Apfelbach R., König P., Vasilieva N.Y., Novotny M.V. Comparative investigation of the volatile urinary profiles in different Phodopus hamster species. J. Chem. Ecol. 2005;31:1125–1143. doi: 10.1007/s10886-005-4252-2. [DOI] [PubMed] [Google Scholar]
- 308.Burger B., Smit D., Spies H., Schmidt C., Schmidt U., Telitsina A. Mammalian exocrine secretions XVI. Constituents of secretion of supplementary sacculi of dwarf hamster, Phodopus sungorus sungorus. J. Chem. Ecol. 2001;27:1277–1288. doi: 10.1023/A:1010380315961. [DOI] [PubMed] [Google Scholar]
- 309.Burger B., Smit D., Spies H., Schmidt C., Schmidt U., Telitsina A., Grierson G. Mammalian exocrine secretions XV. Constituents of secretion of ventral gland of male dwarf hamster, Phodopus sungorus sungorus. J. Chem. Ecol. 2001;27:1259–1276. doi: 10.1023/A:1010328331891. [DOI] [PubMed] [Google Scholar]
- 310.Maurer B.v., Ohloff G. Zur kenntnis der stickstoffhaltigen inhaltsstoffe von castoreum. Helv. Chim. Acta. 1976;59:1169–1185. doi: 10.1002/hlca.19760590420. [DOI] [Google Scholar]
- 311.Li G., Roze U., Locke D.C. Warning odor of the North American porcupine (Erethizon dorsatum) J. Chem. Ecol. 1997;23:2737–2754. doi: 10.1023/A:1022511026529. [DOI] [Google Scholar]
- 312.Massolo A., Dani F.R., Bella N. Sexual and individual cues in the peri-anal gland secretum of crested porcupines (Hystrix cristata) Mamm. Biol. 2009;74:488–496. doi: 10.1016/j.mambio.2009.07.004. [DOI] [Google Scholar]
- 313.Poddar-Sarkar M., Raha P., Bhar R., Chakraborty A., Brahmachary R.L. Ultrastructure and lipid chemistry of specialized epidermal structure of Indian porcupines and hedgehog. Acta Zool. 2011;92:134–140. doi: 10.1111/j.1463-6395.2010.00452.x. [DOI] [Google Scholar]
- 314.Apps P., Gordon D., Viljoen H., Pretorius V. Chromatographic analysis of species specific odor profiles in Mastomys natalensis and M. coucha (Rodentia: Muridae) J. Chem. Ecol. 1990;16:2667–2676. doi: 10.1007/BF00988077. [DOI] [PubMed] [Google Scholar]
- 315.Otsuka H., Otsuru O., Kasama T., Kawaguchi A., Yamasaki K., Seyama Y. Stereochemistry of 2, 3-alkanediols obtained from the Harderian gland of Mongolian gerbil (Meriones unguiculatus) J. Biochem. 1986;99:1339–1344. doi: 10.1093/oxfordjournals.jbchem.a135601. [DOI] [PubMed] [Google Scholar]
- 316.Achiraman S., Archunan G. Characterization of urinary volatiles in Swiss male mice (Mus musculus): Bioassay of identified compounds. J. Biosci. (Bangalore) 2002;27:679–686. doi: 10.1007/BF02708376. [DOI] [PubMed] [Google Scholar]
- 317.Achiraman S., Archunan G. 3-Ethyl-2, 7-dimethyl octane, a testosterone dependent unique urinary sex pheromone in male mouse (Mus musculus) Anim. Reprod. Sci. 2005;87:151–161. doi: 10.1016/j.anireprosci.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 318.Achiraman S., Archunan G., Ponmanickam P., Rameshkumar K., Kannan S., John G. 1–Iodo-2 methylundecane [1I2MU]: An estrogen-dependent urinary sex pheromone of female mice. Theriogenology. 2010;74:345–353. doi: 10.1016/j.theriogenology.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 319.Cavaggioni A., Mucignat-Caretta C., Zagotto G. Absolute configuration of 2-sec-butyl-4,5-dihydrothiazole in male mouse urine. Chem. Senses. 2003;28:791–797. doi: 10.1093/chemse/bjg073. [DOI] [PubMed] [Google Scholar]
- 320.Novotny M., Harvey S., Jemiolo B., Alberts J. Synthetic pheromones that promote inter-male aggression in mice. Proc. Natl. Acad. Sci. USA. 1985;82:2059–2061. doi: 10.1073/pnas.82.7.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Novotny M., Xie T.-M., Harvey S., Wiesler D., Jemiolo B., Carmack M. Stereoselectivity in mammalian chemical communication: Male mouse pheromones. Experientia. 1995;51:738–743. doi: 10.1007/BF01941272. [DOI] [PubMed] [Google Scholar]
- 322.Takács S., Gries R., Zhai H., Gries G. The sex attractant pheromone of male brown rats: Identification and field experiment. Angew. Chem. Int. Ed. 2016;55:6062–6066. doi: 10.1002/anie.201511864. [DOI] [PubMed] [Google Scholar]
- 323.Zhang J.-X., Sun L., Zhang J.-H., Feng Z.-Y. Sex-and gonad-affecting scent compounds and 3 male pheromones in the rat. Chem. Senses. 2008;33:611–621. doi: 10.1093/chemse/bjn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lee H., Finckbeiner S., Jose S.Y., Wiemer D.F., Eisner T., Attygalle A.B. Characterization of (E,E)-farnesol and its fatty acid esters from anal scent glands of nutria (Myocastor coypus) by gas chromatography–mass spectrometry and gas chromatography–infrared spectrometry. J. Chromatogr. 2007;1165:136–143. doi: 10.1016/j.chroma.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 325.Toftegaards C.L., Moore C., Bradley A.J. Chemical characterization of urinary pheromones in brown antechinus, Antechinus stuartii. J. Chem. Ecol. 1999;25:527–535. doi: 10.1023/A:1020901820044. [DOI] [Google Scholar]
- 326.Church J., Evans D., Woodhead A. The characterization of Tasmanian devil Sarcophilius harrisii pelage fibres and their associated lipids. Acta Zool. 2009;90:380–389. doi: 10.1111/j.1463-6395.2008.00383.x. [DOI] [Google Scholar]
- 327.Goodrich B., Hesterman E., Murray K., Mykytowycz R., Stanley G., Sugowdz G. Identification of behaviorally significant volatile compounds in the anal gland of the rabbit, Oryctolagus cuniculus. J. Chem. Ecol. 1978;4:581–594. doi: 10.1007/BF00988922. [DOI] [Google Scholar]
- 328.Hayes R.A., Richardson B.J., Claus S.C., Wyllie S.G. Semiochemicals and social signaling in the wild European rabbit in Australia: II. Variations in chemical composition of chin gland secretion across sampling sites. J. Chem. Ecol. 2002;28:2613–2625. doi: 10.1023/A:1021452623055. [DOI] [PubMed] [Google Scholar]
- 329.Schaal B., Coureaud G., Langlois D., Ginies C., Sémon E., Perrier G. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature. 2003;424:68–72. doi: 10.1038/nature01739. [DOI] [PubMed] [Google Scholar]
- 330.McLean S., Brandon S., Davies N.W., Boyle R., Foley W.J., Moore B., Pass G.J. Glucuronuria in the koala. J. Chem. Ecol. 2003;29:1465–1477. doi: 10.1023/A:1024273722192. [DOI] [PubMed] [Google Scholar]
- 331.Stralendorff F.v. A behaviorally relevant component of the scent signals of male Tupaia belangeri: 2, 5-dimethylpyrazine. Behav. Ecol. Sociobiol. 1982:101–107. doi: 10.1007/BF00300098. [DOI] [Google Scholar]
- 332.Garner C.E., Smith S., Elviss N.C., Humphrey T.J., White P., Ratcliffe N.M., Probert C.S. Identification of Campylobacter infection in chickens from volatile faecal emissions. Biomarkers. 2008;13:413–421. doi: 10.1080/13547500801966443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.