Abstract
In various crops, genetic bottlenecks occurring through domestication can limit crop resilience to biotic and abiotic stresses. In the present study, we investigated nucleotide diversity in tomato chloroplast genome through sequencing seven plastomes of cultivated accessions from the Campania region (Southern Italy) and two wild species among the closest (Solanum pimpinellifolium) and most distantly related (S. neorickii) species to cultivated tomatoes. Comparative analyses among the chloroplast genomes sequenced in this work and those available in GenBank allowed evaluating the variability of plastomes and defining phylogenetic relationships. A dramatic reduction in genetic diversity was detected in cultivated tomatoes, nonetheless, a few de novo mutations, which still differentiated the cultivated tomatoes from the closest wild relative S. pimpinellifolium, were detected and are potentially utilizable as diagnostic markers. Phylogenetic analyses confirmed that S. pimpinellifolium is the closest ancestor of all cultivated tomatoes. Local accessions all clustered together and were strictly related with other cultivated tomatoes (S. lycopersicum group). Noteworthy, S. lycopersicum var. cerasiforme resulted in a mixture of both cultivated and wild tomato genotypes since one of the two analyzed accessions clustered with cultivated tomato, whereas the other with S. pimpinellifolium. Overall, our results revealed a very reduced cytoplasmic variability in cultivated tomatoes and suggest the occurrence of a cytoplasmic bottleneck during their domestication.
Keywords: next-generation sequencing, Solanum, Italian landraces, plastome, molecular markers, phylogenetic analysis
1. Introduction
Domestication of crops was one of the most complex and dynamic processes in plant evolution driven by humans, as it changed the distribution and frequency of plant species on the planet. Crop domestication, through natural or artificial selection, generally results in a reduction of genetic diversity and in the loss of many adaptive traits from wild relatives [1,2]. The analysis of the genetic diversity of wild relatives and cultivated crops provided insight into the geographic and temporal details of domestication, whilst its estimation may provide the basis for developing suitable strategies for crop improvement, conservation and sustainable use [1]. Over past decades, molecular methods have been used to assess genetic diversity and, more recently, high throughput DNA sequencing technologies gave a huge boost to the estimation of genetic and adaptive diversity in crops and model plants [3,4,5,6].
Tomato (Solanum lycopersicum L.) is one of the most consumed vegetables in the world and belongs to the Solanaceae family, which includes species with a considerable economic importance (e.g., potato, pepper, eggplant, tobacco, and petunia) [7]. Within this family, Solanum is the largest and probably the most economically important genus, including both potatoes and tomatoes [8,9]. The original place of tomato domestication is still debated, however it is very likely that it occurred independently in the Peruvianum and Mexican regions [7]. The cultivated tomato, S. lycopersicum is divided into two botanical varieties S. lycopersicum var. cerasiforme (i.e., cherry tomato) and S. lycopersicum var. lycopersicum. Cherry tomato is native to the Andean region, but it also occurs in the subtropical areas and grows either as a true wild or cultivated species. For several years, cherry tomato has been considered an evolutionary intermediate between S. pimpinellifolium, the closest wild ancestor, and the cultivated S. lycopersicum. Recently, genetic studies [10] found cherry tomatoes were a mixture of wild and cultivated forms that likely originated from S. pimpinellifolium.
S. lycopersicum var. lycopersicum derived from cherry tomato through a multiphases process of domestication [11,12]. In particular, Blanca et al. [11] assumed a predomestication in the Andean regions that resulted into a wide morphological diversity of cherry tomatoes; then these genotypes reached Mesoamerica where the true domestication occurred. Here, traditional tomato varieties were developed and spread by Spanish conquistadors in Spain and Italy and, then, in the rest of the World. Since the late 18th century a strong selection activities has taken place in Europe, giving rise to a wide collection of tomato landraces adapted to local cultivation practices and environmental conditions [13,14,15,16]. More recently, these landraces gained increasing attention because of the high quality of fruits, their extended shelf-life and tolerance to environmental stresses [17,18,19]. Accordingly, several studies focused on the genome-wide characterization of the nuclear genetic diversity of various landraces [14,15,16,20,21,22].
Although it has been widely demonstrated the potentiality of cytoplasmic markers to study crop evolution and assess cytoplasmic bottlenecks occurred during the domestication history of several crops (i.e., rice, barley, potato, maize, and wheat) [23,24,25,26,27,28], to date little attention has been given to the analysis of the chloroplast genome in tomato landraces. Furthermore, a deeper knowledge of tomato plastomes would allow a better understanding of nuclear and cytoplasmic genome coevolution, and favor phylogenetic/barcoding studies and novel biotechnological approaches for breeding purposes [29,30,31].
In this work, we reported the complete plastome sequences of seven Italian cultivated tomato accessions grown in the Campania region (Southern Italy) and two wild species, namely S. pimpinellifolium and S. neorickii. Among Italian tomato accessions we selected the “Corbarino” landrace (processed tomato) characterized by obovoid fruits and moderate shelf-life, and six accessions belonging to the “Vesuviano” landrace (long shelf-life) characterized by hearth-shaped fruits with a pronounced pointed apex. Although they have the same place of origin, analysis based on nuclear single nucleotide polymorphisms (SNPs) showed a different clustering for some of these accessions [22]. The selected wild species are among the phylogenetically closest and most distantly related species to cultivated tomatoes and belong to two different phyletic groups characterized by red/orange- or green-fruited species, respectively. In particular, we aim to estimate the nucleotide diversity of tomato plastomes, inferring phylogenetic relationships, shedding lights on de novo mutations likely associated with the domestication and on the potential cytoplasmic bottleneck occurred during such a process.
2. Results
2.1. Chloroplast Genome Size and Organization
Sequencing of the nine tomato genotypes produced from 6.2 M (ves2001) to over 11.5 M (pol) high quality paired-end reads.
Cultivated tomato accessions, namely cor, pds, pol, ves2001, vfr, and vpz, had exactly the same plastome size (155,435 bp), with the exception of pgl (only one bp shorter), whereas plastome size in wild species was slightly larger in S. neorickii 1 (155,515 bp) and smaller in S. pimpinellifolium 1 (155,420 bp; Table 1). All genotypes exhibited the typical quadripartite structure of angiosperms plastome, including a pair of inverted repeats (IRs) separated by a large single copy (LSC) and a small single copy (SSC) regions (Table 1).
Table 1.
Plastome features of the sequenced tomato genotypes.
| Code | Species | Cultivar/Accession | Size (Base Pairs) | |||
|---|---|---|---|---|---|---|
| Total | LSC | SSC | IR | |||
| Cor | S. lycopersicum | Corbarino | 155435 | 85857 | 18364 | 25607 |
| Pds | S. lycopersicum | PDS | 155435 | 85857 | 18364 | 25607 |
| Pgl | S. lycopersicum | Piennolo giallo | 155434 | 85857 | 18363 | 25607 |
| Pol | S. lycopersicum | Pollena | 155435 | 85857 | 18364 | 25607 |
| ves2001 | S. lycopersicum | Vesuvio 2001 | 155435 | 85857 | 18364 | 25607 |
| Vfr | S. lycopersicum | Vesuvio foglia riccia | 155435 | 85857 | 18364 | 25607 |
| Vpz | S. lycopersicum | Vesuviano pizzo | 155435 | 85857 | 18364 | 25607 |
| S. neorickii 1 | S. neorickii | LA2133 | 155515 | 85918 | 18379 | 25609 |
| S. pimpinellifolium 1 | S. pimpinellifolium | LA0722 | 155420 | 85842 | 18362 | 25608 |
2.2. Genetic Variability and Phylogenetic Analyses
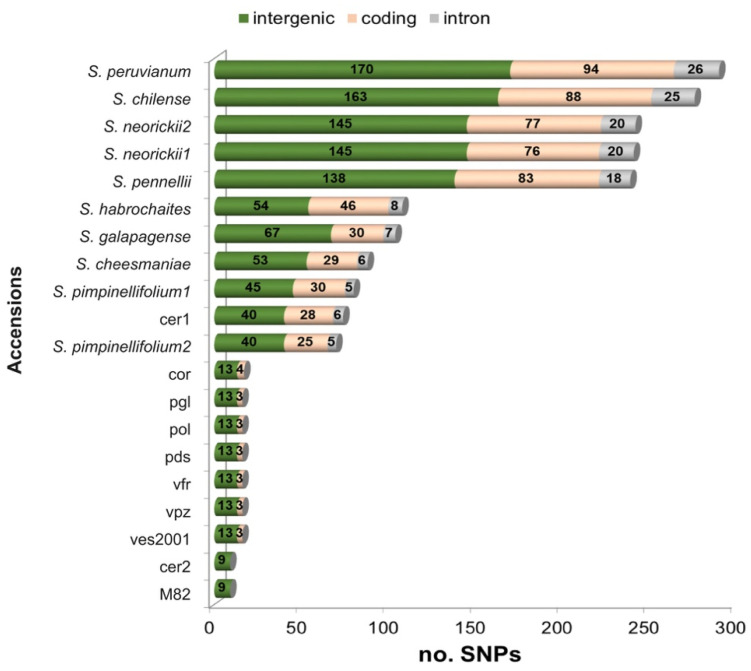
Comparative analyses were performed in order to identify patterns of nucleotide variability among the tomato plastomes (the nine genotypes sequenced in this work and the twelve genotypes retrieved from GenBank). An overview of the nucleotide variability was shown in Figure S1. A variable number of SNPs (from a minimum of 9 to a maximum of 290) was observed when cultivated and wild plastomes were compared with the reference genome IPA-6 (Figure 1). Particularly, in cultivated tomatoes the number of SNPs was markedly low (from 9 to 17 SNPs), with the notable exception of cer1 that differed for 74 SNPs from IPA-6, a difference comparable to that of wild S. pimpinellifolium. All local accessions showed identical plastome sequences, with the exception of cor that differed for one point mutation in the exon 2 of the rpoC1 gene and pgl that was one bp shorter.
Figure 1.
Stacked bar chart showing the distribution of single nucleotide polymorphisms (SNPs) that fall within coding sequences of genes, introns, and intergenic regions of the nine tomato plastomes sequenced in this work and in those of eleven species retrieved from GenBank. The plastome of IPA-6 (AM087200) was used as reference for SNP calling.
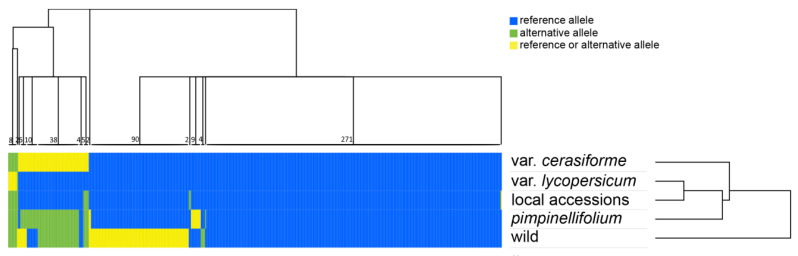
Considering the low variability detected, to verify whether the SNPs identified in cultivated genotypes were ancestral or de novo mutations likely evolved before or after the domestication process, a comparative analysis was performed on the investigated tomato genotypes clustered into five groups: (1) the S. lycopersicum var. lycopersicum tomato commercial varieties IPA-6 and M82; (2) the seven local accessions from Campania region; (3) the S. lycopersicum var. cerasiforme cer1 and cer2; (4) the S. pimpinellifolium, S. pimpinellifolium 1 and S. pimpinellifolium 2, and (5) the wild including S. habrochaites, S. cheesmaniae and S. galapagense, phylogenetically closer to cultivated tomato than other wild species (Figure 2).
Figure 2.
Hierarchical clustered heatmap representing color-coded SNP alleles as scored across 5 different groups of genotypes, i.e., var. cerasiforme; var. lycopersicum; local accessions; pimpinellifolium; wild species (including S. habrochaites, S. cheesmaniae, and S. galapagense). Numbers at the base of the tree indicate the SNP(s) that fall into each group. Blue: reference allele; green: alternative allele; yellow: reference or alternative allele.
Notably, a high number of SNPs (271) was common between all five groups and different from distantly related wild species, thus being ancestral mutations evolved in the phyletic lineages including cultivated tomatoes. Ninety SNPs were common between the cultivated tomatoes (S. lycopersicum var. cerasiforme, S. lycopersicum var. lycopersicum and local accessions) and the S. pimpinellifolium groups, whereas other wild species showed either the reference or the alternative allele. It is very likely that these latter 90 SNPs have been fixed only in the phyletic lineage of wild S. pimpinellifolium and cultivated tomatoes. Only two SNPs distinguished the local accessions from the remaining ones, but 38 SNPs (invariable among the cultivated genotypes) were different between cultivated tomatoes and S. pimpinellifolium. Notably, the S. lycopersicum var. cerasiforme group (cer1 and cer2) showed either the reference or the alternative allele of these 38 SNPs with cer1 sharing the S. pimpinellifolium allele, whilst cer2 the cultivated one. This result suggests that these 38 SNPs evolved as de novo mutations after the separation of cultivated forms from wild S. pimpinellifolium but were already present in the ancestral domesticated gene pool (including the S. lycopersicum var. cerasiforme group) and only subsequently fixed in the cultivated S. lycopersicum var. lycopersicum and local accessions groups. The other five SNPs were common between local accessions, S. pimpinellifolium and wild groups, while the S. lycopersicum var. lycopersicum and the S. lycopersicum var. cerasiforme groups showed, respectively, the reference and either the reference or the alternative allele. By excluding cer1, in cultivated tomatoes seven SNPs (including the latter five and the two exclusive point mutations of local accessions) represent the only differences between plastomes of S. lycopersicum var. lycopersicum and local accessions.
As expected, wild species showed the highest number of SNPs independently from the phylogenetic distance to the reference genome (Figure 1 and Figure 2) with variation detected even between accessions of the same species: ten different SNPs were found between the two accessions of S. pimpinellifolium. By looking at the distribution of SNPs in coding sequences, introns, and intergenic regions, the highest number of SNPs was scored in intergenic regions ranging from 9 to 13 in cultivated tomatoes and cer2, 40 in cer1, and 40-170 in the wild relatives. The same trend was observed for SNP distribution in coding sequences (Figure 1). Particularly, SNPs in wild species ranged from 25 to 94 and were dispersed as 1-2 variations per gene in most genes, whereas among cultivated genotypes up to four SNPs in local accessions were located in matK, exon 2 of the rpoC1, and ycf1 coding sequences, one of these being in charge of an amino acid change. In contrast to all other cultivated landraces, once again, cer1 showed the number and distribution of SNPs similar to that found in the wild S. pimpinellifolium.
The most variable genes, especially among wild species, were ndhH and ycf1 with 9 and 42 SNPs, respectively (Figure S2 and Figure S3). The mutations observed in the ndhH gene were synonymous (i.e., not causing changes in the amino acid sequence), whilst the nucleotide variability observed in ycf1 was also reflected at the amino acid level. Interestingly, a SNP variation produced an amino acid change between the var. lycopersicum and the local accessions (Figure S3).
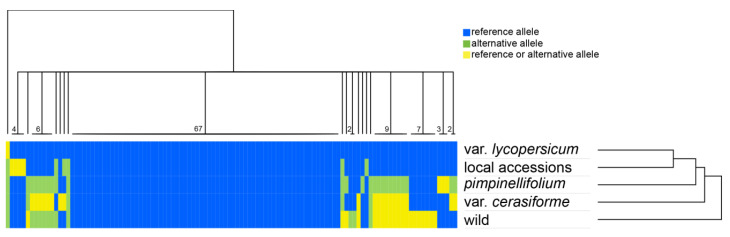
One hundred and fourteen simple sequence repeats (SSRs) were identified. The mononucleotide repeat (adenosine or thymine) was the most common type of microsatellite. Only four wild genotypes showed dinucleotide repeats (S. neorickii 1 and 2, S. peruvianum, and S. chilense). As observed for SNPs, clustered heatmap of SSRs across grouped genotypes revealed a very low level of polymorphism (Figure 3).
Figure 3.
Hierarchical clustered heatmap representing color-coded simple sequence repeat (SSR) alleles as scored across 5 different groups of genotypes, i.e., var. lycopersicum; local accessions; S. pimpinellifolium; var. cerasiforme; wild species (including S. habrochaites, S. cheesmaniae, and S. galapagense). Numbers at the base of the tree indicate the SSR(s) that fall into each group. Blue: reference allele; green: alternative allele; yellow: reference or alternative allele.
Sixty-seven SSRs were ancestral (same number of repeat units), being shared by all the analyzed genotypes as compared with the most distantly related wild species not included in the wild group; six SSRs have the same number of repeat units both in S. lycopersicum var. lycopersicum and local accessions groups, whereas S. pimpinellifolium and wild groups displayed a different number of repeat units, and S. lycopersicum var. cerasiforme both. One SSR displayed 13 repeats shared between S. lycopersicum var. lycopersicum and local accessions groups (i.e., atpB-rbcL intergenic region). An exclusive number of repeat units in the local accessions group was detected in the ndhC-tRNA-Val (UAC) intergenic region, while a number of repeat units exclusive of S. lycopersicum var. lycopersicum group was found in the psbE-petL intergenic region (Figure 3, Table S2). Interestingly, one SSR in the atpH-atpI intergenic region has the same number of repeat units both in all cultivated genotypes and wild group, while S. pimpinellifolium displayed a different number (Table S2). A complete description of SSR variability was shown in Figure S4a. As already observed for SNPs, SSRs were mainly located in intergenic regions (58%) and were mostly included in the LSC (75%; Figure S4b).
Among the in silico identified microsatellites, eight SSR loci with small variation in the number of repeat units were experimentally tested to verify the correct estimation of their length. No variation in the number of repeat units was detected both in silico and in the electrophoresis profiles in a representation of the nine genotypes sequenced in this work and in a large dataset including additional local accessions and processed/fresh market tomatoes (e.g., Acampora, Lucariello, San Marzano, and Sorrento) confirming the absence of SSR variation within and among cultivated tomatoes. A notable exception was the one basis difference found in the microsatellite located in the ndhF-rpl32 intergenic region that allowed distinguishing local accessions group from other tomato landraces and that was also confirmed by the electrophoresis profiles (data not shown).
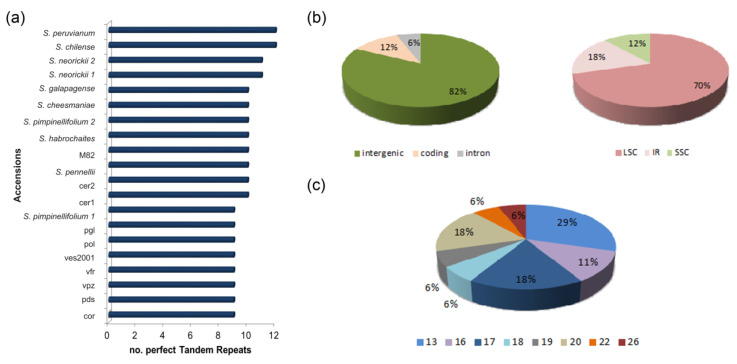
Additionally, 17 perfect tandem repeats (TRs) were found, with cultivated species displaying a lower TR number when compared with wild species (Figure 4a). The identified TRs were mainly located in the LSC and intergenic regions (70 and 82%, respectively); two TRs found in all genotypes were in the coding region of the rps16 and rps4 genes (Figure 4b). The TR period size ranged from 13 to 26 bp (Figure 4c). TRs confirmed the low variability among the analyzed tomato genotypes. No TR was specific to any cultivated tomato; neither de novo TRs could be identified. A TR located in the tRNA-Gln (UUG)-psbK intergenic region was the only one to be found variable among species (Table S3). Particularly, local accessions and S. pimpinellifolium 1 had one copy, S. neorickii 1 and 2 had three copies, while S. lycopersicum var. lycopersicum (IPA-6 and M82), S. lycopersicum var. cerasiforme (cer1 and cer2), S. pimpinellifolium 2, and the remaining wild species had two copies (Table S3). Interestingly, a de novo duplication of four bases motif (ATAA)2, exclusive of the local accessions, was scored by MSA (Figure S5).
Figure 4.
Perfect tandem repeats (TRs) in the nine plastomes sequenced in this work and in the plastome sequence of eleven species available in GenBank. The plastome of IPA-6 (AM087200) was used as reference. (a) Bar chart reporting the total number of TRs in each genotype. (b) Pie charts describing the percentage of TRs located in the coding sequences of genes, introns, and intergenic regions and in the large single copy (LSC), small single copy (SSC), and inverted repeat b (IR) regions. (c) Pie chart describing the percentage of TRs with a specific period size.
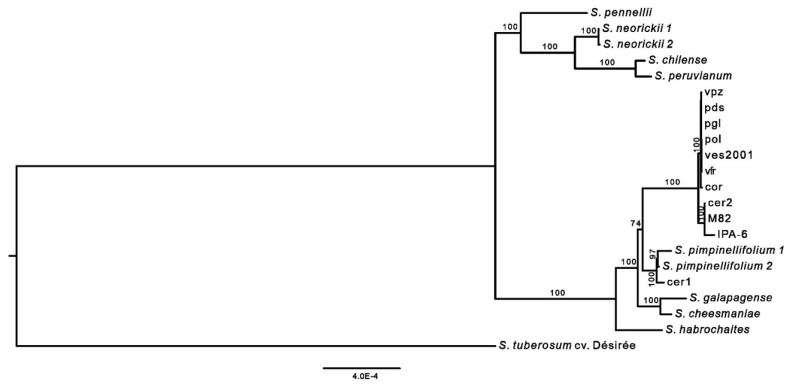
Phylogenetic tree inferred from the complete plastomes of the twenty-one tomato genotypes using the potato chloroplast genome (S. tuberosum cv. Désirée, DQ386163) as an outgroup, showed two main clades with strong bootstrap support (100%; Figure 5). One clade included some wild species (S. pennellii, S. neorickii 1 and 2, S. peruvianum, and S. chilense) with S. pennellii as the basal species. The other clade is further separated into several subclades. In particular, the group that included the seven local accessions from the Campania region was closely related to a cluster populated by other cultivated varieties (IPA-6, M82, and cer2). As expected, all cultivated genotypes were more closely related to the clade comprising the two S. pimpinellifolium accessions and cer1. The remaining wild species (S. galapagense, S. cheesmaniae, and S. habrochaites) were in a separate clade. Finally, the phylogenetic analysis confirmed the admixed nature of S. lycopersicum var. cerasiforme as cer1 was closely related to the wild species (S. pimpinellifolium 1 and 2), while cer2 was part of the cultivated genotypes clade (M82 and IPA-6).
Figure 5.
Phylogenetic tree of cultivated and wild tomato genotypes. Phylogram of the best maximum-likelihood (ML) tree on the complete plastome dataset using Solanum tuberosum cv. Désirée (DQ386163) as the outgroup. Numbers associated with branches are ML bootstrap support values. Bootstraps higher than 70% are reported on the nodes.
3. Discussion
Most crops experienced a reduction in genetic diversity (genetic bottleneck) due to the domestication process [32]. Indeed, the development of high yielding crops for food, feed, and other uses required the desirable phenotypes to be selected at the expense of variability present in their wild ancestors (founder effect) [33,34,35]. However, such “uniformity” often resulted in more vulnerable plants that are not able anymore to cope with biotic and abiotic stresses. As a consequence, wild relative species are often exploited as a reservoir of “exotic” alleles to secondarily increase variability in previously selected traits, thus favoring adaptation to changed conditions [34].
Landraces are locally adapted cultivars that are gaining increasing attention considering their typical traits (e.g., high quality of fruits and yield stability in low input agricultural systems) [17,19,36,37,38]. Although it has been widely demonstrated that the chloroplast genome is a valuable resource to study evolution and phylogenetic relationships among species [39,40], the genetic diversity of tomato landraces was largely based on the genome-wide characterization of their nuclear DNA variability [11,12,14,15,16,20,21,22,41]. Further, due the uniparental mode of inheritance, genetic bottleneck in organellar DNA may not necessarily reflect nuclear variability, thus providing additional/complementary information on the domestication process.
Comparative analyses of the nine plastomes sequenced in this work and of twelve plastomes retrieved from GenBank allowed both to evaluate the extent of the genetic bottleneck on the tomato chloroplast genome and define phylogenetic relationships among wild and cultivated accessions. For these aims, SNPs and SSRs were revealed to be more informative than TRs since no specific TR for cultivated tomato genotypes, or de novo TRs were identified in our survey.
Very low cpDNA variability was detected in tomato varieties with respect to that observed in wild species, thus indicating the occurrence of a very strong cytoplasmic bottleneck during domestication. The number of SNPs in wild species is 24-fold higher than in cultivated tomatoes (389 polymorphic SNPs out of 454 (86%)), while SSRs were slightly lower (49 polymorphic SSRs out of 114 (43%), 4-fold those observed in tomato varieties). The heterogeneous nature of the S. lycopersicum var. cerasiforme group is remarkable, namely, the two analyzed accessions showed a different behavior. Collected data and phylogeny clearly highlighted higher variability in cer1 compared with cer2 and suggest that although cer1 belongs to cerasiforme group, probably it was not subjected to the domestication process and can be considered as “wild” cultivated accession.
Detected levels of plastome variability are consistent with the extensive genetic erosion of cultivated tomato, especially in the light of the large diversity observed across wild relatives [5]. Similarly, pepper wild species displayed a number of SNP and SSR respectively 8-fold and 3-fold greater than that of cultivated genotypes [42].
Only 16 out of 454 SNPs were found polymorphic among cultivated tomato genotypes (3.5%). Comparable results were found in pepper varieties, where only the 4% of the scored SNP loci were polymorphic in cultivated accessions [42].
Similarly, only 12 out of 114 identified SSRs were polymorphic among cultivated tomato genotypes (11%). Comparable results were reported in cultivated barley showing one polymorphism out of seven analyzed SSRs (14%) [27] and pepper varieties, showing 19 polymorphic SSRs out of 92 (21%) [42]. Contrariwise, 16 out of 17 (94%) SSRs were polymorphic among cultivated bean [43].
As previously argued, genetic bottleneck at the nuclear level may not be reflected at the cytoplasmic level. An extreme cytoplasmic bottleneck has been previously hypothesized in cultivated potato by the analysis of SSR markers but no decreased levels of nuclear SSR diversity were recorded [26,39]. On the contrary, the genetic diversity analysis between American and European collections of common bean highlighted the absence of evident cytoplasmic bottleneck (only 2% loss of cpSSR diversity) [44], and a stronger nuclear bottleneck (30% loss of SSR diversity) [45] likely indicating that the founding common bean populations introduced in Europe were still highly variable in their cytoplasmic DNAs [46].
SNP arrays on some tomato cultivars, partially shared with this work (i.e., M82, cor, pgl, vfr, and ves2001), revealed a reduced nuclear genetic diversity [22].
Concordantly, the cpDNA analyses suggest an extreme low cytoplasmic variability of the founding cultivated tomato population. Indeed, cultivated varieties shared 361 out of 454 SNPs (79%) and 74 out of 114 SSRs (65%) with the ancestor S. pimpinellifolium (i.e., same SNP alleles and same SSR haplotypes) and only seven de novo SNPs and two de novo SSRs were different between S. lycopersicum var. lycopersicum and local accessions groups. All analyzed local accessions showed identical cpDNA sequences suggesting that these accessions have a unique domestication origin and that their cytoplasm has evolved monophyletically from the founder tomato gene pool, rather than representing an independent introduction. Still, the local accessions have distinctive sequences from the other commercial tomatoes (i.e., S. lycopersicum the var. lycopersicum group) excluding multiple independent selections of the obovoid fruits (Corbarino) or the hearth-shaped fruits with a pronounced pointed apex (the remaining accessions).
In this work, we also detected plastome variability between wild S. pimpinellifolium 1 and 2. These differences could be due to natural variability among accessions and/or possible errors in the sequencing/assembly procedure. The former hypotheses, however, is supported by differences also observed among other related wild species (S. galapagense, S. cheesmaniae, and S. habrochaites). Thus, the significant reduction in cpDNA variability found in the cultivated tomato gene pool can be directly ascribed as a consequence of the domestication process rather than to an already occurred loss of genetic variation in the closest wild relative, S. pimpinellifolium. Therefore, the present study suggests that a severe ‘cytoplasmic bottleneck’ occurred during the domestication of tomato, as has been reported in other crops: barley [27], lentil [47], onion [48], and potato [26].
A strict relationship between cultivated tomato varieties and the ancestor S. pimpinellifolium was supported by phylogeny. Species belonging to the Lycopersicon group (S. lycopersicum, S. pimpinellifolium, S. cheesmaniae, and S. galapagense) [49] form a well-supported clade in agreement with previous phylogenetic studies [5].
In particular, all local accessions clustered together in a subgroup with S. lycopersicum var. lycopersicum and cer 2. On the contrary, some accessions (i.e., cor, pgl, vfr, and ves2001) were grouped in different clusters based on nuclear SNP genotyping [22]. Noteworthy, cer1 was included in the same group of S. pimpinellifolium accessions, thus plastome diversity analysis confirmed the mixed nature of S. lycopersicum var. cerasiforme as previously observed with the analysis of nuclear variability [10,11,50,51].
The observed low variability of the cultivated tomatoes chloroplast genome can be explained by taking into account both the genetic bottleneck during their domestication and its low mutation rate. Notably, comparison of the plastome sequences of the two modern tomato varieties IPA-6 and Ailsa Craig, the former bred in South America and the latter in Europe, resulted in identical cpDNA sequences, thus demonstrating the stability of plastome in tomato cultivars over a period of at least a few hundred years of separation [52] without the insurgence of any de novo mutation. Although low variation is the rule in tomato cpDNA, few plastid regions have been identified that might be exploited as diagnostic markers: two de novo SNPs, one SSR and a short sequence duplication (ATAA)2 were exclusive of all local accessions, whereas, one SSR was typical of all the var. lycopersicum group.
Variability found in all tomato genotypes mainly affected intergenic regions. However, the most variable genes were ycf1 (showing both synonymous and non-synonymous mutations) and ndhH. Both these genes have been proposed as tools to resolve the phylogenetic relationships among closely related genera and species [53,54,55] and at least ycf1 was found variable even within cultivated plastomes leading to amino acid change (Figure S3).
Overall, our work contributes to the characterization of tomato plastid genomes and their phylogenetic relationships, and especially highlights the severe reduction in variability at plastid DNA as a consequence of the strong genetic bottleneck occurred in the founding population during the domestication process.
4. Materials and Methods
4.1. Plant Material
Seven Italian cultivated tomato accessions grown in the Campania region (Southern Italy), Corbarino (cor) landrace, and six accessions belonging to the “Vesuviano” landrace, Pollena (pol), PDS (pds), Vesuvio 2001 (ves2001), Vesuviano foglia riccia (vfr), Vesuviano pizzo (vpz), Piennolo giallo (pgl), and two wild tomato species, S. pimpinellifolium (LA0722, Peru) and S. neorickii (LA2133, Ecuador) were sampled for chloroplast isolation, DNA extraction and sequencing. Drs. M.S. Grillo and S. Grandillo from the CNR, Institute of Bioscience and BioResources, Portici, kindly provided the seeds.
4.2. Chloroplast DNA Isolation and Extraction
Plants were kept in the dark for 48 h before harvesting to reduce starch accumulation. Fresh leaves (15–25 g) were collected and used for chloroplast isolation with discontinuous sucrose gradient [56]. Purified chloroplasts were lysed with a detergent and proteins eliminated by proteinase K and phenol/chloroform treatments following the procedure described by [57].
4.3. Genome Sequencing, Assembling, and Annotation
DNA samples were sequenced using the GA II Illumina sequencer (2 × 75 paired-end reads with an estimated inset size of 400 bp). Quality check on raw reads was performed using FastQC v.0.11.2 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Then, the fastq_quality_filter utility from the FASTX-toolkit (http://hannonlab.cshl.edu/fastx_toolkit/) was used to remove sequences with a quality score equal or lower than 30 in more than 90% of the read length. Illumina technical sequences were removed by using Trimmomatic v.0.32 [58]. Reference-based assembly was performed using the Columbus module within the Velvet package [59] with a k-mer size of 65. The chloroplast genome sequence of S. lycopersicum cv. IPA-6 (AM087200) was used as reference. Contigs were ordered and oriented by using ABACAS [60] for the final assembly. Finally, high quality reads were aligned back onto the assemblies using Bowtie2 [61] with default settings to validate and manually fix errors in the assemblies. Per base genome coverage was computed using the genomecov utility of bedtools version 2.20.1 (Figure S6) [62]. The annotation of chloroplast genomes was performed using GeSeq (https://chlorobox.mpimp-golm.mpg.de/geseq.html). Gene annotations were manually curated using S. lycopersicum cv. IPA-6 (AM087200) annotations as reference. Chloroplast genome sequences and annotations produced in this study can be found in GenBank under accession numbers MT811790-MT811798.
4.4. Detection and Analysis of Sequence Variations
Single nucleotide variants (SNVs) were identified using the snp-sites tool (https://github.com/sanger-pathogens/snp-sites). Such a tool extracts SNPs from a multiple sequence alignment using the cpDNA of S. lycopersicum cv. IPA-6 as reference sequence. SNP annotation was manually curated.
The microsatellite (MISA) identification tool (http://pgrc.ipk-gatersleben.de/misa/) was run to identify microsatellites (SSR) using the unit_size/min_repeats parameters as follows: 1/8, 2/6, 3/5, 4/5, 5/5, 6/5. The Tandem Repeat Finder web tool accessible at https://tandem.bu.edu/trf/trf.basic.submit.html was used to detect perfect tandem repeats with default settings.
In silico identified SSR loci were experimentally tested for variation in the number of repeat units. For this aim, 8 SSR loci were selected from the MISA output by focusing on those with small variation in the number of repeat units to verify the correct estimation of their repeat length. Primers were designed with Primer3 (http://frodo.wi.mit.edu/primer3/). The primer size was set from 18 to 25 bp, the Tm ranged from 51 to 59 °C and the other parameters were set as default (Table S1). For each microsatellite locus, the forward primers were labeled with the different fluorescent dyes 6-FAM, ATTO550, ATTO565, and HEX (Sigma Aldrich, USA). Beside the sequenced local accessions, we applied these primers to 19 additional local genotypes, namely further seven local accessions and twelve processed/fresh market tomatoes.
All PCR amplifications were performed by a Perkin Elmer 9700 thermocycler according to PCR conditions as reported in [63]. The conditions were maintained constant for all loci in order to maximize standardization. Amplified microsatellite products were then genotyped using an Applied Biosystem 3130 automatic sequencer with LIZ (500) as an internal standard and sized with GENEMAPPER software v. 3.7 (Thermo Fisher Scientific-Applera, USA).
Multiple sequence alignments (MSA) were generated using MAFFT version 7 [64] with default settings. Single-nucleotide variants were identified by the snp-sites software [65] using as input the plastomes MSA and the cpDNA of S. lycopersicum cv. IPA-6 (AM087200) as reference. To highlight differences among nucleotide sequences of plastomes, MSA were visualized using the NCBI Multiple Sequence Alignment Viewer available at https://www.ncbi.nlm.nih.gov/projects/msaviewer/.
RAxML [66] was used to build a maximum-likelihood (ML) tree with 10,000 rapid bootstrap inferences, a generalized time reversible (GTR) substitution matrix and Gamma model of rate heterogeneity. The plastome of S. tuberosum cv. Désirée (DQ386163) was used as the outgroup. The ML tree was visualized with FigTree v.1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
In addition to S. lycopersicum cv. IPA-6 (AM087200), eleven tomato genotypes available in GenBank: S. peruvianum (KP117026), S. chilense (KP117021), S. neorickii (S. neorickii 2, KP117025), S. pennellii (HG975452), S. habrochaites (KP117023), S. galapagense (NC_026878), S. cheesmaniae (NC_026876), S. pimpinellifolium (S. pimpinellifolium 2, KP117027), S. lycopersicum (cv M82, HG975525), and S. lycopersicum var. cerasiforme (cer1, KY887588; and cer2, KY887587) were retrieved for comparative analyses. Heatmaps were generated using Morpheus (https://software.broadinstitute.org/morpheus). Single-linkage hierarchical clustering on both rows and columns was based on the metric “Euclidean distance”.
Acknowledgments
Technical assistance of Mr. G. Guarino and Mr. R. Nocerino (CNR-IBBR, Portici, Italy) with artworks and plant growth is gratefully acknowledged.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/11/1443/s1, Figure S1: Overview of the nucleotide variability in nine plastomes sequenced in this study and in eleven species available in GenBank. The accession number AM087200 (cv. IPA-6) was used as reference. Red lines represent variable regions, Figure S2: Schematic representation of the nucleotide variability observed in the ndhH gene for the plastomes under investigation. Grey bar represents the nucleotide multiple-sequence alignment (MSA) and it is scaled according to the MSA length. Black boxes indicate variable regions in the MSA. Above and below each box, a snapshot of the MSA along with alignment positions is reported, Figure S3: Schematic representation of the amino acid variability observed in the Ycf1 protein for the plastomes under investigation. Grey bar represents the amino acid multiple-sequence alignment (MSA) and it is scaled according to the MSA length. Black boxes indicate variable regions in the MSA. Above and below each box, a snapshot of the MSA along with alignment positions is reported; Figure S4: Simple sequence repeats (SSRs) in nine plastomes sequenced in this study and in eleven species available in GenBank. The plastome of IPA-6 (AM087200) was used as reference a) Heatmap representing differences in SSR size; colors range from red (SSR size larger than the reference) through yellow to blue (SSR size smaller than the reference). Black is for missing SSRs. b) Pie chart describing the percentage of SSRs located in coding sequences of genes, introns, and intergenic regions and in the large single copy (LSC), small single copy (SSC), and inverted repeat b (IR) regions, Figure S5: Multiple-sequence alignment (MSA) of the region harboring the duplicated sequence (ATAA)2 scored only in local landraces, Figure S6: Distribution of per-base sequencing depth for each chloroplast genome sequenced in this work. The average coverage per-base is also reported. Table S1: Tomato cpSSR primers developed in this study, Table S2: Simple sequence repeats (SSRs) in the twenty-one tomato chloroplast genomes using IPA-6 (AM087200) as the reference genome. SSRs size, location, and distribution among different regions, namely coding, intron, and intergenic are reported. The unit_size/min_repeats parameters were as follows: 1/8, 2/6, 3/5, 4/5, 5/5, and 6/5. SSRs located in IRa were not counted. SSRs were identified using MISA—microsatellite identification tool (http://pgrc.ipk-gatersleben.de/misa/), Table S3: Tandem repeats (TRs) in the twenty-one tomato chloroplast genomes using IPA-6 (AM087200) as the reference genome. TRs copy number and location are reported. TRs were identified using the tool available at https://tandem.bu.edu/trf/trf.basic.submit.html.
Author Contributions
T.C., S.C., N.D. and N.S. conceived and designed the research. C.C., N.D. performed bioinformatic analyses. R.T., L.S., D.C. and N.S. carried out wet-lab experiments. L.O. performed Illumina sequencing of plastomes. R.T., D.C., S.C., N.D. and N.S. contributed to data interpretation. R.T. and N.S. wrote the manuscript. T.C., S.C., N.D. and N.S. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by grant from the Italian Ministry of Economy and Finance (MEF)-National Research Council of Italy (CNR), Project “Conoscenze Integrate per la Sostenibilità e l’Innovazione del made in Italy Agroalimentare” (CISIA), Legge n. 191/2009.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meyer R.S., DuVal A.E., Jensen H.R. Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytol. 2012;196:29–48. doi: 10.1111/j.1469-8137.2012.04253.x. [DOI] [PubMed] [Google Scholar]
- 2.Smykal P., Nelson M.N., Berger J.D., von Wettberg E.J.B. The impact of genetic changes during crop domestication. Agronomy. 2018;8:119. doi: 10.3390/agronomy8070119. [DOI] [Google Scholar]
- 3.Weigel D., Mott R. The 1001 genomes project for Arabidopsis thaliana. Genome Biol. 2009;10:107. doi: 10.1186/gb-2009-10-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Tomato Genome Consortium The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 2012;485:635–641. doi: 10.1038/nature11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aflitos S., Schijlen E., de Jong H., de Ridder D., Smit S., Finkers R., Wang J., Zhang G., Li N., Mao L., et al. Exploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant J. 2014;80:136–148. doi: 10.1111/tpj.12616. [DOI] [PubMed] [Google Scholar]
- 6.Acquadro A., Barchi L., Portis E., Nourdine M., Carli C., Monge S., Valentino D., Lanteri S. Whole genome resequencing of four Italian sweet pepper landraces provides insights on sequence variation in genes of agronomic value. Sci. Rep. 2020;10:9189. doi: 10.1038/s41598-020-66053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergougnoux V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014;32:170–189. doi: 10.1016/j.biotechadv.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Peralta I.E., Spooner D.M. Granule-bound starch synthase (GBSSI) gene phylogeny of wild tomatoes (Solanum L. section Lycopersicon [Mill.] Wettst. subsection Lycopersicon) Am. J. Bot. 2001;88:1888–1902. doi: 10.2307/3558365. [DOI] [PubMed] [Google Scholar]
- 9.Peralta I.E., Spooner D.M. In: History, Origin and Early Cultivation of Tomato (Solanaceae) Razdan M.K., Matto A.K., editors. Science Publishers; Plymouth, MA, USA: 2007. pp. 1–27. Volume Tomato: Genetic Improvement of Solanaceous Crops. [Google Scholar]
- 10.Nesbitt T.C., Tanksley S.D. Comparative sequencing in the genus Lycopersicon: Implication for the evolution of fruit size in the domestication of the cultivated tomatoes. Genetics. 2002;162:365–379. doi: 10.1093/genetics/162.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanca J., Canizares J., Cordero L., Pascual L., Diez M.J., Nuez F. Variation revealed by SNP genotyping and morphology provides insight into the origin of the tomato. PLoS ONE. 2012;7:e48198. doi: 10.1371/journal.pone.0048198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanca J., Montero-Pau J., Sauvage C., Bauchet G., Illa E., Diez M.J., Francis D., Causse M., van der Knaap E., Canizares J. Genomic variation in tomato, from wild ancestors to contemporary breeding accessions. BMC Genom. 2015;16:257. doi: 10.1186/s12864-015-1444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai Y., Lindhout P. Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Ann. Bot. 2007;100:1085–1094. doi: 10.1093/aob/mcm150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrado G., Caramante M., Piffanelli P., Rao R. Genetic diversity in Italian tomato landraces: Implications for the development of a core collection. Sci. Hortic. 2014;168:138–144. doi: 10.1016/j.scienta.2014.01.027. [DOI] [Google Scholar]
- 15.Corrado G., Piffanelli P., Caramante M., Coppola M., Rao R. SNP genotyping reveals genetic diversity between cultivated landraces and contemporary varieties of tomato. BMC Genom. 2013;14:835. doi: 10.1186/1471-2164-14-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ercolano M.R., Sacco A., Ferriello F., D’Alessandro R., Tononi P., Traini A., Barone A., Zago E., Chiusano M.L., Buson G., et al. Patchwork sequencing of tomato San Marzano and Vesuviano varieties highlights genome-wide variations. BMC Genom. 2014;15:138. doi: 10.1186/1471-2164-15-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreakis N., Giordano I., Pentangelo A., Fogliano V., Graziani G., Monti L.M., Rao R. DNA fingerprinting and quality traits of Corbarino cherry-like tomato landraces. J. Agric. Food Chem. 2004;52:3366–3371. doi: 10.1021/jf049963y. [DOI] [PubMed] [Google Scholar]
- 18.Digilio M.C., Corrado G., Sasso R., Coppola V., Iodice L., Pasquariello M., Bossi S., Maffei M.E., Coppola M., Pennacchio F., et al. Molecular and chemical mechanisms involved in aphid resistance in cultivated tomato. New Phytol. 2010;187:1089–1101. doi: 10.1111/j.1469-8137.2010.03314.x. [DOI] [PubMed] [Google Scholar]
- 19.Galmés J., Conesa M.À., Ochogavía J.M., Perdomo J.A., Francis D.M., Ribas M., Savé R., Flexas J., Medrano H., Cifre J. Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant Cell Environ. 2011;34:245–260. doi: 10.1111/j.1365-3040.2010.02239.x. [DOI] [PubMed] [Google Scholar]
- 20.Sacco A., Ruggieri V., Parisi M., Festa G., Rigano M.M., Picarella M.E., Mazzucato A., Barone A. Exploring a tomato landraces collection for fruit-related traits by the aid of a high-throughput genomic platform. PLoS ONE. 2015;10:e0137139. doi: 10.1371/journal.pone.0137139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tranchida-Lombardo V., Aiese Cigliano R., Anzar I., Landi S., Palombieri S., Colantuono C., Bostan H., Termolino P., Aversano R., Batelli G., et al. Whole-genome re-sequencing of two Italian tomato landraces reveals sequence variations in genes associated with stress tolerance, fruit quality and long shelf-life traits. DNA Res. 2018;25:149–160. doi: 10.1093/dnares/dsx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tranchida-Lombardo V., Mercati F., Avino M., Punzo P., Fiore M.C., Poma I., Patanè C., Guarracino M.R., Sunseri F., Tucci M., et al. Genetic diversity in a collection of Italian long storage tomato landraces as revealed by SNP markers array. Plant Biosyst. 2019;153:288–297. doi: 10.1080/11263504.2018.1478900. [DOI] [Google Scholar]
- 23.Provan J., Corbett G., McNicol J.W., Powell W. Chloroplast DNA variability in wild and cultivated rice (Oryza spp.) revealed by polymorphic chloroplast simple sequence repeats. Genome. 1997;40:104–110. doi: 10.1139/g97-014. [DOI] [PubMed] [Google Scholar]
- 24.Provan J., Corbett G., Waugh R., McNicol J.W., Morgante M., Powell W. DNA fingerprints of rice (Oryza sativa) obtained from hypervariable chloroplast simple sequence repeats. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1996;263:1275–1281. doi: 10.1098/rspb.1996.0187. [DOI] [PubMed] [Google Scholar]
- 25.Provan J., Lawrenc P., Young G., Wright F., Bird R., Paglia G.P., Cattonaro F., Morgante M., Powell W. Analysis of the genus Zea (Poaceae) using polymorphic chloroplast simple sequence repeats. Plant Syst. Evol. 1999;218:245–256. doi: 10.1007/BF01089230. [DOI] [Google Scholar]
- 26.Provan J., Powell W., Dewar H., Bryan G., Machray G.C., Waugh R. An extreme cytoplasmic bottleneck in the modern European cultivated potato (Solanum tuberosum) is not reflected in decreased levels of nuclear diversity. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1999;266:633–639. doi: 10.1098/rspb.1999.0683. [DOI] [Google Scholar]
- 27.Provan J., Russell J.R., Booth A., Powell W. Polymorphic chloroplast simple sequence repeat primers for systematic and population studies in the genus Hordeum. Mol. Ecol. 1999;8:505–511. doi: 10.1046/j.1365-294X.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 28.Provan J., Wolters P., Caldwell K.H., Powell W. High-resolution organellar genome analysis of Triticum and Aegilops sheds new light on cytoplasm evolution in wheat. Theor. Appl. Genet. 2004;108:1182–1190. doi: 10.1007/s00122-003-1538-z. [DOI] [PubMed] [Google Scholar]
- 29.Rogalski M., do Nascimento Vieira L., Fraga H.P., Guerra M.P. Plastid genomics in horticultural species: Importance and applications for plant population genetics, evolution, and biotechnology. Front. Plant Sci. 2015;6:586. doi: 10.3389/fpls.2015.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniell H., Lin C.S., Yu M., Chang W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tonti-Filippini J., Nevill P.G., Dixon K., Small I. What can we do with 1000 plastid genomes? Plant J. 2017;90:808–818. doi: 10.1111/tpj.13491. [DOI] [PubMed] [Google Scholar]
- 32.Doebley J.F., Gaut B.S., Smith B.D. The molecular genetics of crop domestication. Cell. 2006;127:1309–1321. doi: 10.1016/j.cell.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Ladizinsky G. Founder effect in crop-plant evolution. Econ. Bot. 1985;39:191–199. doi: 10.1007/BF02907844. [DOI] [Google Scholar]
- 34.Tanksley S.D., McCouch S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science. 1997;277:1063–1066. doi: 10.1126/science.277.5329.1063. [DOI] [PubMed] [Google Scholar]
- 35.Saini P., Saini P., Kaur J.J., Francies R.M., Gani M., Rajendra A.A., Negi N., Jagtap A., Kadam A., Singh C., et al. Molecular approaches for harvesting natural diversity for crop improvement. In: Salgotra R., Zargar S., editors. Rediscovery of Genetic and Genomic Resources for Future Food Security. Springer; Singapore: 2020. pp. 67–169. [Google Scholar]
- 36.Fernie A.R., Tadmor Y., Zamir D. Natural genetic variation for improving crop quality. Curr. Opin. Plant Biol. 2006;9:196–202. doi: 10.1016/j.pbi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Berg T. Landraces and folk varieties: A conceptual reappraisal of terminology. Euphytica. 2009;166:423–430. doi: 10.1007/s10681-008-9829-8. [DOI] [Google Scholar]
- 38.Conesa M.A., Fullana-Pericas M., Granell A., Galmes J. Mediterranean long shelf-life landraces: An untapped genetic resource for tomato improvement. Front. Plant Sci. 2020;10:1651. doi: 10.3389/fpls.2019.01651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Provan J., Powell W., Hollingsworth P.M. Chloroplast microsatellites: New tools for studies in plant ecology and evolution. Trends Ecol. Evol. 2001;16:142–147. doi: 10.1016/S0169-5347(00)02097-8. [DOI] [PubMed] [Google Scholar]
- 40.Song Y., Wang S., Ding Y., Xu J., Li M.F., Zhu S., Chen N. Chloroplast genomic resource of Paris for species discrimination. Sci. Rep. 2017;7:3427. doi: 10.1038/s41598-017-02083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sim S.C., Robbins M.D., Van Deynze A., Michel A.P., Francis D.M. Population structure and genetic differentiation associated with breeding history and selection in tomato (Solanum lycopersicum L.) Heredity. 2011;106:927–935. doi: 10.1038/hdy.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Agostino N., Tamburino R., Cantarella C., De Carluccio V., Sannino L., Cozzolino S., Cardi T., Scotti N. The complete plastome sequences of eleven Capsicum genotypes: Insights into DNA variation and molecular evolution. Genes. 2018;9:503. doi: 10.3390/genes9100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desiderio F., Bitocchi E., Bellucci E., Rau D., Rodriguez M., Attene G., Papa R., Nanni L. Chloroplast microsatellite diversity in Phaseolus vulgaris. Front. Plant Sci. 2013;3:312. doi: 10.3389/fpls.2012.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angioi S.A., Rau D., Attene G., Nanni L., Bellucci E., Logozzo G., Negri V., Zeuli P.L.S., Papa R. Beans in Europe: Origin and structure of the European landraces of Phaseolus vulgaris L. Theor. Appl. Genet. 2010;121:829–843. doi: 10.1007/s00122-010-1353-2. [DOI] [PubMed] [Google Scholar]
- 45.Papa R., Nanni L., Sicard D., Rao D., Attene G. The evolution of genetic diversity in Phaseolus vulgaris. In: Motley T.J., Zerega N., Cross H., editors. Darwin’s Harvest: New Approaches to the Origins, Evolution and Conservation of Crops. Columbia University Press; NewYork, NY, USA: 2006. pp. 121–142. [Google Scholar]
- 46.Bellucci E., Bitocchi E., Rau D., Rodriguez M., Biagetti E., Giardini A., Attene G., Nanni L., Papa R. Genomics of origin, domestication and evolution of Phaseolus vulgaris. In: Tuberosa R., Graner A., Frison E., editors. Genomics of Plant Genetic Resources. Volume 1. Springer; Singapore: 2014. pp. 483–507. [Google Scholar]
- 47.Ladizinsky G. Identification of the lentil wild genetic stock. Genet. Resour. Crop Evol. 1999;46:115–118. doi: 10.1023/A:1008626128871. [DOI] [Google Scholar]
- 48.Friesen N., Pollner S., Bachmann K., Blattner F.R. RAPDs and noncoding chloroplast DNA reveal a single origin of the cultivated Allium fistulosum from A. altaicum (Alliaceae) Am. J. Bot. 1999;86:554–562. doi: 10.2307/2656817. [DOI] [PubMed] [Google Scholar]
- 49.Peralta I.E., Spooner D.M., Knapp S. In: Taxonomy of Wild Tomatoes and Their Relatives (Solanum sect. Lycopersicoides, sect Jugandifolia, sect. Lycopersicon; Solanaceae) Anderson C., editor. American Society of Plant Taxonomists; Ann Arbor, MI, USA: 2008. pp. 1–186. [Google Scholar]
- 50.Ranc N., Muños S., Xu J., Le Paslier M.C., Chauveau A., Bounon R., Rolland S., Bouchet J.P., Brunel D., Causse M. Genome-wide association mapping in tomato (Solanum lycopersicum) is possible using genome admixture of Solanum lycopersicum var. cerasiforme. G3. 2012;2:853–864. doi: 10.1534/g3.112.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Causse M., Desplat N., Pascual L., Le Paslier M.-C., Sauvage C., Bauchet G., Bérard A., Bounon R., Tchoumakov M., Brunel D., et al. Whole genome resequencing in tomato reveals variation associated with introgression and breeding events. BMC Genom. 2013;14:791. doi: 10.1186/1471-2164-14-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahlau S., Aspinall S., Gray J.C., Bock R. Sequence of the tomato chloroplast DNA and evolutionary comparison of solanaceous plastid genomes. J. Mol. Evol. 2006;63:194–207. doi: 10.1007/s00239-005-0254-5. [DOI] [PubMed] [Google Scholar]
- 53.Martín M., Sabater B. Plastid ndh genes in plant evolution. Plant Physiol. Biochem. 2010;48:636–645. doi: 10.1016/j.plaphy.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Dong W., Xu C., Li C., Sun J., Zuo Y., Shi S., Cheng T., Guo J., Zhou S. ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015;5:8348. doi: 10.1038/srep08348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strand D.D., D’Andrea L., Bock R. The plastid NAD(P)H dehydrogenase-like complex: Structure, function and evolutionary dynamics. Biochem. J. 2019;476:2743–2756. doi: 10.1042/BCJ20190365. [DOI] [PubMed] [Google Scholar]
- 56.Kemble R.J. A rapid, single leaf, nucleic acid assay for determining the cytoplasmic organelle complement of rapeseed and related Brassica species. Theor. Appl. Genet. 1987;73:364–370. doi: 10.1007/BF00262502. [DOI] [PubMed] [Google Scholar]
- 57.Scotti N., Cardi T., Marechal Drouard L. Mitochondrial DNA and RNA isolation from small amounts of potato tissue. Plant Mol. Biol. Rep. 2001;19:67a–67h. doi: 10.1007/BF02824080. [DOI] [Google Scholar]
- 58.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zerbino D.R., Birney E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Assefa T., Mahama A.A., Brown A.V., Cannon E.K.S., Rubyogo J.C., Rao I.M., Blair M.W., Cannon S.B. A review of breeding objectives, genomic resources, and marker-assisted methods in common bean (Phaseolus vulgaris L.) Mol. Breed. 2019;39:20. doi: 10.1007/s11032-018-0920-0. [DOI] [Google Scholar]
- 61.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quinlan A.R., Hall I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weising K., Gardner R.C. A set of conserved PCR primers for the analysis of simple sequence repeats polymorphisms in chloroplast genomes of dicotyledonous angiosperms. Genome. 1999;42:9–19. doi: 10.1139/g98-104. [DOI] [PubMed] [Google Scholar]
- 64.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Page A.J., Taylor B., Delaney A.J., Soares J., Seemann T., Keane J.A., Harris S.R. Snp-sites: Rapid efficient extraction of SNPs from multi-fasta alignments. Microb. Genom. 2016;2:e000056. doi: 10.1099/mgen.0.000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.