Abstract
Background:
Treatment with gemcitabine/nab-paclitaxel confers a survival benefit over gemcitabine monotherapy in patients with advanced pancreatic cancer (APC). However, such treatment can be associated with significant toxicities especially in older patients and carries practical disadvantages related to a weekly schedule along with financial cost. We retrospectively analyzed patients >65 years of age with APC who received a modified biweekly regimen of gemcitabine/nab-paclitaxel to evaluate efficacy and toxicity.
Methods:
Patients aged >65 years with chemo-naïve APC with Eastern Cooperative Oncology Group performance status ⩽2 were studied. Patients were treated with a modified regimen of gemcitabine 1000 mg/m2 and nab-paclitaxel 125 mg/m2 every 2 weeks on days 1 and 15 of a 28-day cycle. Patients were evaluated for progression-free survival (PFS) and overall survival (OS) with analyses performed using the Kaplan–Meier method. Adverse events were recorded on the day of chemotherapy. Cancer antigen 19.9 was measured in every cycle and restaging scans were performed every two cycles.
Results:
A total of 73 patients (median age: 73 years; range: 66–93) were treated with biweekly gemcitabine/nab-paclitaxel as first-line treatment. The median OS and PFS were 9.1 months and 4.8 months, respectively. Around 66% of patients received growth-factor support based on American Society of Clinical Oncology guidelines and no patient developed neutropenic fever. The incidences of grade ⩾3 toxicity for neutropenia, anemia, thrombocytopenia, and neurotoxicity were 2%, 7%, 3%, and 5%, respectively. Dose reductions of gemcitabine/nab-paclitaxel were required in 10% and 4% patients, respectively.
Conclusion:
In patients older than >65 years of age with APC, a modified regimen of biweekly gemcitabine/nab-paclitaxel was found to be effective when compared with the historical control from the MPACT study. This regimen allowed for fewer dose reductions, reduced healthcare costs from additional appointments, travel-related cost, as well as a favorable side-effect profile while maintaining efficacy. Though retrospective in nature, this study underlines the need for further investigation, particularly in elderly patients with poor performance status, such as those with pancreatic cancer, and in order to combine with a third agent, such as a targeted treatment or immunotherapy.
Keywords: advanced pancreatic cancer, attenuated regimen, biweekly, chemotherapy, elderly, improved toxicity
Introduction
Despite various revolutions in the field of oncology, advanced pancreatic cancer (APC) has a grim prognosis and is now the third leading cause of cancer-related deaths in the USA.1 It is estimated that pancreatic cancer will become the second leading cause of cancer-related deaths in the USA by 2030.2 Approximately 56,770 Americans will be diagnosed with pancreatic cancer and more than 45,750 will die of the disease yearly, highlighting how lethal this disease has become.1 Most patients present with advanced disease leading to the dismal 5-year survival rate of less than 10%. Furthermore, it is estimated that the number of elderly patients with pancreatic cancer will continue to rise due to the aging population.3 Management of these patients is quite limited and further hindered by underrepresentation of this population in clinical trials.4
Gemcitabine historically was considered the standard of care for metastatic pancreatic cancer as it provided a survival benefit as well as alleviation of symptoms compared with fluorouracil (5-FU).5 Many trials after this yielded disappointing results as they failed to show improvement on this landmark trial including combination treatments with novel agents and other chemotherapy agents in addition to gemcitabine monotherapy.6–12
More recently, two chemotherapy combination regimens have shown superiority in patients with metastatic disease in randomized phase III trials. In the PRODIGE/ACCORD trial, a combination of 5-FU with oxaliplatin and irinotecan showed an overall survival benefit with a median overall survival (OS) of 11.1 months compared with 6.8 months in the gemcitabine arm.13 Of note, patients older than 75 years of age were excluded in this trial. In the MPACT trial, the combination of gemcitabine/nab-paclitaxel showed superiority over gemcitabine with a median OS of 8.5 months in the combination arm versus 6.7 months in the monotherapy arm. Treatment was administered with gemcitabine (1000 mg/m2) and nab-paclitaxel (125 mg/m2) given on days 1, 8, and 15 of a 4-week cycle.14 Although these results were promising and effective, they came at a cost of increased toxicity as well as practical disadvantages for the patients. Prior studies have suggested a modified regimen of biweekly gemcitabine/nab-paclitaxel could lessen adverse effects of the regimen while maintaining efficacy.15,16 Although promising, this regimen has not been readily studied in the elderly population and it is unclear how to best manage these patients in the setting of advanced disease. Therefore, we adopted an attenuated regimen of biweekly gemcitabine/nab-paclitaxel for patients older than 65 years of age as treatment for APC and present the efficacy, side-effect profile, and cost-analysis data of our study.
Methods
Patients and study design
This study was approved with written consent by our institution’s Institutional Review Board. Ethics approval was not sought as this was a retrospective review. We performed a retrospective analysis of patients older than 65 years of age with chemo-naïve APC from 2015 to 2017. All patients were treated at our institution and monitored for efficacy as well as toxicity. All patients who were treated with this modified regimen were included in the analysis during this time period.
Eligibility
Patients older than 65 years of age with locally advanced/unresectable or metastatic pancreatic adenocarcinoma were eligible. Diagnosis through tissue biopsy was required. All patients had to have Eastern Cooperative Oncology Group performance status of 0 or 1. Patients were treated with this regimen based on the discretion of the treating physician. Decisions were based upon open discussions with the patients and mostly related to patient preference for not wanting to come to the treatment room for weekly treatments. There were no limitations based on comorbidities as long as performance status was adequate. Patients were chemo-naïve as no previous treatment for pancreatic cancer was allowed.
Treatment schedule
Chemotherapy was administered on days 1 and 15 of a 28-day cycle. Patients received 125 mg/m2 of nab-paclitaxel followed by 1000 mg/m2 of gemcitabine on both days. Pre-medications and anti-emetics were ordered according to American Society of Clinical Oncology (ASCO) guidelines. Granulocyte-colony stimulating factor was also administered according to ASCO guidelines. Treatment was continued until evidence of disease progression or unacceptable toxicity.
Assessments
Comprehensive physician office visits were conducted every 2 weeks prior to initiation of treatment and between each cycle of treatment. Detailed history and physical examination were performed at each clinic visit. Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5. Computed tomography imaging of the chest, abdomen, and pelvis was used to assess disease status every two cycles or as clinically indicated. Baseline blood work was obtained prior to initiating treatment as well as prior to each subsequent treatment. Laboratory values included a complete blood count, comprehensive metabolic panel, and cancer antigen 19.9 levels. Baseline information to assess disease status was also evaluated including demographic information, metastatic sites of disease, number of metastatic sites, biliary stent placement, and subsequent lines of treatment. All information was recorded in each patient’s secure online medical electronic record.
Statistical analyses
Study endpoints that were evaluated included progression-free survival (PFS), OS, and toxicity profile. Descriptive statistics were used to evaluate demographic information, subsequent lines of treatment, as well as toxicity profile. The Kaplan–Meier method was used for the calculation of PFS and OS. PFS was calculated from the start of chemotherapy until disease progression or death, whichever came first. OS was obtained from the initiation of chemotherapy until death from any cause.
Results
A total of 73 patients who received this attenuated regimen of gemcitabine/nab-paclitaxel for APC as first-line treatment were evaluated. Demographic data are presented in Table 1. All patients were older than 65 years of age with a median of age 73 years. The median OS was 9.1 months and the median PFS was 4.8 months. All patients were able to receive second-line treatments once progression of disease was noted. Standard-of-care subsequent treatments were utilized including capecitabine monotherapy or other 5-FU-based regimens as outlined in Figure 1.
Table 1.
Demographics of patients.
| Characteristic | First-line treatment – metastatic disease n = |
|---|---|
| Age (years) | |
| Median | 73 |
| Range | 66–93 |
| Sex | |
| Female | 35 |
| Male | 38 |
| Location of primary tumor | |
| Head | 39 |
| Body | 20 |
| Tail | 14 |
| Unknown/other | |
| Number of metastatic sites | |
| 1 | 14 |
| 2 | 22 |
| 3 | 26 |
| >3 | 11 |
| Sites of metastatic disease | |
| Liver | 41 |
| Lung | 23 |
| Peritoneum | 7 |
| Cancer antigen 19-9 level at start of therapy | |
| Normal (⩽37 U/ml) | 10 |
| Elevated (>37 U/ml) | 63 |
Figure 1.
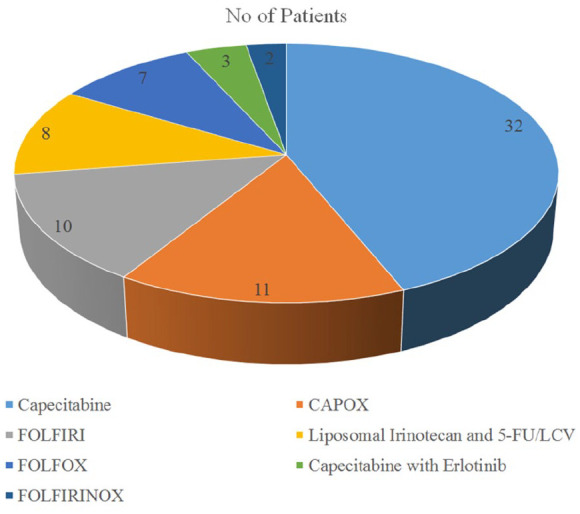
Second-line treatments after progression of disease.
5-FU, fluorouracil; CAPOX, capecitabine and oxaliplatin; FOLFIRI, folinic acid, 5-FU, and irinotecan; FOLFIRINOX, 5-FU, irinotecan, oxaliplatin, and folinic acid; FOLFOX, folinic acid, 5-FU, and oxaliplatin; LCV, leucovorin.
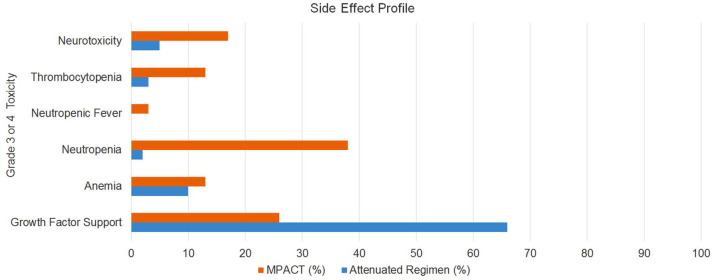
Most toxicities were rated as grade 2 or less and related to fatigue, neuropathy, skin rash, or nausea. Grade 3 or higher toxicities were rare in our cohort. The incidences of grade 3 or 4 hematologic toxicities for neutropenia, anemia, and thrombocytopenia were minimal, resulting in 2%, 10%, and 3%, respectively. Around 66% of patients received growth-factor support based on the ASCO guidelines. Although the standard to receive primary prophylaxis includes regimens where the risk of neutropenia is >20%, many patients in our analysis received growth-factor support largely due to older age and comorbidities. It has been shown that older patients aged greater than 65 years may be more vulnerable to chemotherapy-related febrile neutropenia.17 Furthermore, having more advanced cancer and comorbidities carries a higher risk of complications from chemotherapy. In our analysis, no patients developed neutropenic fever. Furthermore, rates of grade 3 or 4 nonhematologic toxicity were also low. For example, grade 3 or 4 neurotoxicity was found in 5% of patients. Dose reductions of gemcitabine/nab-paclitaxel were required in only 10% and 3% of patients, respectively. The side-effect profile is outlined in Figure 2 and is compared with the historical MPACT trial in Table 2.
Figure 2.
Side-effect profile.
Table 2.
Cross comparison with historical control.
| Our study: n (%) | MPACT trial: n (%) | |
|---|---|---|
| Survival data | ||
| Progression-free survival, median (months) | 4.8 | 5.5 |
| Overall survival, median (months) | 9.1 | 8.5 |
| Grade 3 or 4 toxicities | ||
| Growth-factor support | 48 (66%) | 110 (26%) |
| Anemia | 7 (10%) | 53 (13%) |
| Neutropenia | 2 (2%) | 153 (38%) |
| Neutropenic fever | 0 (0%) | 14 (3%) |
| Thrombocytopenia | 3 (3%) | 52 (13%) |
| Neurotoxicity | 5 (5%) | 70 (17%) |
| Dose reduction in patients | ||
| Nab-paclitaxel | 3 (3%) | 41% |
| Gemcitabine | 7 (10%) | 47% |
Discussion
Our study showed that an alternative biweekly treatment regimen with gemcitabine/nab-paclitaxel appears to have similar efficacy when compared with the historical control from the MPACT trial. Although this trial showed statistically significant results and improved upon the standard of gemcitabine monotherapy, it came at a cost of increased toxicity, dose reductions, and delays in treatment. In this trial, nab-paclitaxel dosing was administered at 125 mg/m2 on days 1, 8, and 15 of a 28-day cycle. Gemcitabine was also administered on days 1, 8, and 15 at 1000 mg/m2. Dose reduction was required with the use of nab-paclitaxel and gemcitabine in 41% and 47% of patients, respectively.14 This compares with a rate of 3% and 10% in our cohort. A total of 22% of patients permanently discontinued treatment in the MPACT study.14 Furthermore, rates of hematologic and nonhematologic toxicities were much lower with the biweekly attenuated regimen.
Previous trials have shown that biweekly gemcitabine monotherapy or combination therapy may improve the side-effect profile while maintaining efficacy in various settings compared with weekly dosing. Previous retrospective studies have also shown that a modified schedule of treatment with gemcitabine/nab-paclitaxel on days 1 and 15 may maintain efficacy while improving upon the toxicity profile of these agents in this setting of APC.15,16
However, these studies did not focus on primarily elderly patients. Pancreatic cancer is known to be a disease of older adults, with the median age of 71 years at diagnosis in the USA.18 In the MPACT study, only 41% of patients were aged 65 years or older. A subgroup analysis showed that there was a benefit of PFS in this population with combination therapy; however, there was no difference in OS with a hazard ratio of 0.81 (0.63–1.03).14 The older population remains a heterogeneous group and difficult to treat based on unclear tolerability of treatments as well as exclusion from some clinical trials.19
The findings from our study suggested that the attenuated regimen maintains relative efficacy with considerable improvement in side-effect profile. Continuing treatments on schedule without dose reductions and interruptions may also help explain why survival was unchanged. Very few patients required dose modifications, and no patients had to discontinue treatment altogether. There were also no instances of neutropenic fever with only 2% of patients experiencing grade 3 or 4 neutropenia. This may explain why survival data are slightly improved in our cohort. However, definite conclusions cannot be made in this retrospective analysis and would require randomized data. It should be noted, however, most patients received growth-factor support due to advanced age in line with current recommendations.
In addition to an improved toxicity profile, the modified biweekly regimen of gemcitabine/nab-paclitaxel also has a significant impact on healthcare costs. By eliminating day 8 of treatment, financial toxicity can be greatly reduced factoring in costs of chemotherapeutic agents, infusion related costs, and personnel need at the cancer center. Furthermore, there is increasing awareness of quality-of-life measures in patients with cancer. By reducing treatment room visits, patients may have considerable improvement in these measures related to convenience factors as well as transportation needs. This may also encompass more quality time with loved ones at home, which is of paramount importance for most patients with APC.20 We did not include a quality-of-life assessment in our study, but this could present an interesting area of research in this population.
Lastly, improvement in these toxicities while producing similar results may allow for additional treatments. By preserving toxic side effects from treatment, further investigation can be performed with the addition of a third agent to this combination. Management of advanced disease is evolving with ongoing research into various targeted treatments and immunotherapies in APC, but mortality rate remains high. With the continued advancements in oncology, there is a new focus on precision medicine and the identification of novel biomarkers.21 With the emergence of immunotherapy and these targeted treatments, there is a research need to explore further these associations. With our attenuated regimen of gemcitabine/nab-paclitaxel, we present an opportunity to further improve outcomes while minimizing toxicities in this historically lethal disease.
We acknowledge the limitations that accompany our study. This was carried out in a retrospective nature which included inherent biases such as the possibility of selection bias. Cross- over comparison from different studies also lends to bias as different populations were evaluated. There is no direct comparison of demographic data and lack of a control arm could limit generalizability of results. A relatively small sample size is also a limitation as this was conducted solely at our institution as a single-center retrospective analysis. However, keeping these points in mind, we believe our patients represented a reasonably similar profile to the general population in this advanced disease setting. All patients had a good performance status at the discretion of the treating physician and were given standard treatment doses. Patients were monitored as they would be in any clinical setting. In the setting of an elderly population, our results showed that a biweekly regimen of gemcitabine/nab-paclitaxel allowed for a favorable side-effect profile while maintaining efficacy in APC.
Conclusion
An attenuated regimen of biweekly gemcitabine/nab-paclitaxel for the treatment of APC in elderly patients showed similar results to historical precedents. Using this regimen minimized toxicities associated with these agents, reduced healthcare costs, and perhaps improved quality-of-life measures in these patients. Better tolerability of these agents may allow for combination with a third agent, such as a targeted treatment or immunotherapy, especially as we enter the era of precision medicine in oncology. Though retrospective in nature, this study underlines the need for further investigation, particularly in elderly patients with APC to optimize outcomes while minimizing toxicities and preserving quality of life.
Footnotes
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Saif had grant funding from Celegene. The rest of the authors have no conflicts of interest to declare.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Muhammad Wasif Saif  https://orcid.org/0000-0003-0599-4991
https://orcid.org/0000-0003-0599-4991
Contributor Information
Hasan Rehman, Northwell Health Cancer Institute, and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, NY, USA.
Jeffrey Chi, Northwell Health Cancer Institute, and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, NY, USA.
Nausheen Hakim, Northwell Health Cancer Institute, and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, NY, USA.
Shreya Prasad Goyal, Northwell Health Cancer Institute, and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, NY, USA.
Coral Olazagasti, Northwell Health Cancer Institute, and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, NY, USA.
Jyothi Jose, Northwell Health Cancer Institute, and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, NY, USA.
Linda Moriarty, Northwell Health Cancer Institute, and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, NY, USA.
Muhammad Wasif Saif, Northwell Health Cancer Institute, Medical Oncology, and Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, 1111 Marcus Avenue, Lake Success, NY 11042, USA.
References
- 1. Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 2019; 10(1): 10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 3. Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009; 27: 2758–2765. [DOI] [PubMed] [Google Scholar]
- 4. Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 2004; 22: 4626–4631. [DOI] [PubMed] [Google Scholar]
- 5. Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–2413. [DOI] [PubMed] [Google Scholar]
- 6. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25: 1960–1966. [DOI] [PubMed] [Google Scholar]
- 7. Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010; 28: 3605–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the cancer and leukemia group B (CALGB 80303). J Clin Oncol 2010; 28: 3617–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005; 23: 3509–3516. [DOI] [PubMed] [Google Scholar]
- 10. Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002; 20: 3270–3275. [DOI] [PubMed] [Google Scholar]
- 11. Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007; 25: 2212–2217. [DOI] [PubMed] [Google Scholar]
- 12. Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol 2004; 22: 3776–3783. [DOI] [PubMed] [Google Scholar]
- 13. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 14. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahn DH, Krishna K, Blazer M, et al. A modified regimen of biweekly gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer is both tolerable and effective: a retrospective analysis. Ther Adv Med Oncol 2017; 9: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kokkali S, Tripodaki ES, Drizou M, et al. Biweekly gemcitabine/nab-paclitaxel as first-line treatment for advanced pancreatic cancer. In Vivo 2018; 32: 653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aapro M, Schwenkglenks M, Lyman GH, et al. Pegfilgrastim primary prophylaxis vs. current practice neutropenia management in elderly breast cancer patients receiving chemotherapy. Crit Rev Oncol Hematol 2010; 74: 203–210. [DOI] [PubMed] [Google Scholar]
- 18. Noonan AM, Howlader N, Krapcho M. SEER cancer statistics review, 1975–2015. Bethesda, MD: National Cancer Institute, 2015. [Google Scholar]
- 19. Higuera O, Ghanem I, Nasimi R, et al. Management of pancreatic cancer in the elderly. World J Gastroenterol 2016; 22: 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bauer MR, Bright EE, MacDonald JJ, et al. Quality of life in patients with pancreatic cancer and their caregivers: a systematic review. Pancreas 2018; 47: 368–375. [DOI] [PubMed] [Google Scholar]
- 21. Torres C, Grippo PJ. Pancreatic cancer subtypes: a roadmap for precision medicine. Ann Med 2018; 50: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]