Abstract
Crohn’s Disease (CD) is a chronic inflammatory disorder that potentially involves the entire gastrointestinal tract. Perianal fistulizing CD (pCD) is a serious and frequent complication associated with significant morbidities and a heavy negative impact on quality of life. The aim of CD treatment is to induce and maintain disease remission and to promote mucosal repair. Unfortunately, even the best therapeutic regimens in pCD do not have long-term efficacy and cause a significant number of side effects. Therefore, it is mandatory to study new therapeutical options such as the use of mesenchymal stromal cells (MSCs). These cells promote tissue repair via the induction of immunomodulation. The present review aims to analyze the existing updated scientific literature on MSCs adoption in the treatment of pCD to evaluate its efficacy and safety and to compare the use of bone marrow and adipose tissue derived MSCs, type of administration, and dose required for recovery.
Keywords: mesenchymal stromal cells, stem cells, Crohn disease, perianal fistula, fistulizing CD, surgical treatment
1. Introduction
Crohn’s disease (CD) is a chronic inflammatory disorder that may potentially involve any portion of the gastrointestinal tract. Most patients initially complain of significant mucosal inflammation. Unfortunately, over time, disease behavior can change, and many patients progress to penetrating complications, with a full thickness involvement of the gut wall, including sinus, abscess and fistulas [1,2,3,4]. The transmural inflammation predisposes CD patients to fistula formation, but the first step in this process is generally assumed to be tissue destruction. In this context, several studies suggested the role of epithelial-to-mesenchymal-transition (EMT) as a driving force behind the development of fistulizing CD being TGF-β the principal inducer [5,6].
Perianal Fistula in CD (pCD) were first described in 1938 as a typical complication of terminal ileitis and, as demonstrated by several population-based studies, at least one third of CD patients will develop fistulas over the disease’s course [5,7]. Perianal fistula development depends on the localization of CD. Frequently, it is associated with colonic and rectal involvement (92% of patients), while it is rare in patients with isolated ileitis (12% of patients) [8,9].
Perineal fistulizing CD develops in the 20% of patients and typically shows a relapsing and remitting course [5]. It is possible to identify high levels of tumor necrosis factor (TNF), IL-13 and TGF-β all around the fistula. The presence of these factors supports the hypothesis of intestinal microbiota and EMT active role in the pathogenesis of the disease. Fistulization is a negative predictor of long-term disease and its treatment requires a multidisciplinary approach. In this context, the use of mesenchymal stromal cells (MSCs) is attracting the interest of scientific community [10].
2. Mesenchymal Stromal Cells
MSCs are multipotent cells capable of self-renewal and differentiation. In particular, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) identified the three minimal criteria to define MSCs: (1) adherence to plastic; (2) expression of specific surface antigens such as CD105, CD73 and CD90; (3) and the ability to differentiate in standard vitro tissue-culture condition into the main trilineages of mesenchymal derivation or rather osteoblast, adipocytes and chondroblasts [11]. MSCs have been isolated in various tissues such as circulating blood, umbilical cord or placenta, but the main donors are adipose tissue and bone marrow [12,13,14,15]. However, within adipose tissue, it is possible to extract a greater number of MSCs, or adipose-derived stem cells (ASCs), than those isolated in the bone marrow [16].
The methods of extraction of ASCs keep changing over time. In this regard, Cytori Therapeutics Inc. (San Diego, CA, USA) created the CelutionTM system, or rather a closed system capable of extracting ASCs from subcutaneous tissue. The extraction involves several steps including the use of collagenases at a concentration of 0.075% and short centrifugation cycles until a minimum volume of ready-to-use MSCs is extracted, normally within 1 h [17].
Lipogems® technology was first described in 2010, given the need to have a minimally manipulated product with a shorter production time.
In fact, a small amount of fat tissue is harvested from the donor site and it is subsequently processed using the Lipogems® device, being ready for clinical use after less than 20 min. The latter represents a great advantage especially if compared with the time required for enzymatic digestion.
Lastly, it preserves an intact stromal vascular niche due to the use of slight mechanical forces [18]. However, despite the many advantages, in patients with a low BMI, such as those with CD, the lower amount of stem cells extracted with this method could be a problem in term of efficacy.
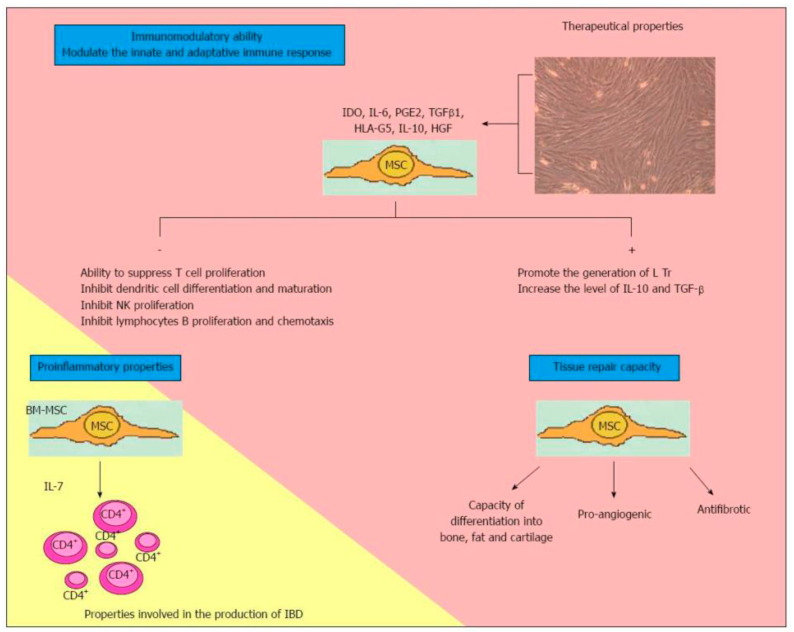
The use of MSCs for the treatment of pCD seems to be justified by their immunomodulatory effect (Figure 1) [19]. This latter lies in the ability of MSCs to upregulate a subset of TCD4 + cells of regulatory T cells (Treg) [20,21]. In fact, a decrease in Treg cells and the coexistence of an altered ratio between Treg cells and T effector cells represent the basis of CD pathogenesis [22,23]. MSCs, therefore, can reduce the immune response both through the upregulation of the Treg, migrating in the inflammation sites and also determining regeneration, and the healing of damaged tissues [24,25]. Nevertheless, their immunomodulatory function can be achieved through the involvement of several other molecules and patterns, such as B-cell proliferation or production of CXCL9, CXCL10 and CCL-2. In fact, they assume a proinflammatory or immunosuppressive phenotype based on the TRL signals received [21].
Figure 1.
Characteristics of multipotent mesenchymal stromal cells. MSCs: Multipotent mesenchymal cells; IDO: Indoleamine 2,3 dioxygenase; IL: Interleukin; PGE2: Prostaglandin E2; TGFβ1: Transforming growth factor beta-1; HLA-G5: Human Leucocyte Antigen G5; HGF: Human growth factor; NK: Natural killer; Tregs: Regulatory T cells; BM-MSC: Bone-marrow-mesenchymal stem cell; CD4+: Activated lymphocytes CD4; IBD: Inflammatory bowel disease. Reproduced with permission from Martínez-Montiel MDP et al. [19].
The present study aims to report the current evidence on the efficacy and safety of MSCs in the treatment of pCD.
3. Surgical Management
The treatment of pCD still represents a clinical challenge; in this context, surgical treatment represents an essential step for both definitive healing and infection control. The choice of the most appropriate surgical technique depends on different factors such as the anatomy of the fistula, the presence of local inflammation and the level of experience of the surgeon [26,27,28,29,30].
Among the most commonly adopted surgical techniques, the use of a draining seton allows a bridge to surgery, whereas the use of a cutting seton on one side may permit fistula healing, but on the other one may cause faecal incontinencein in 5–10% of patients [30,31].
The most used sphincter-saving procedure is represented by the endorectal advancement flaps, but when used in fistulas associated to CD, it shows a lower rate of success in comparison with fistulas of cryptoglandular etiology [32,33,34,35]. Other sphincter-saving approaches such as the use of fibrin glue [36,37], Fistula-Tract Laser Closure (FiLaC) [30,38], Ligation of Intersphincteric Fistula Tract (LIFT) [39] or Video-Assisted Anal Fistula Treatment (VAAFT) [38,40] can be considered, but their success rate remains low, especially in patients with uncontrolled pCD. As far as refractory pCD is concerned, a temporary or definitive faecal diversion can be considered and discussed with the patients [39,41].
4. State of the Art
4.1. Mesenchymal Stromal Cells in Perianal Fistulizing Crohn Disease
The progenitor cells were identified in the bone marrow about a century ago, and the observations of Friedenstein and colleagues, obtained through the isolation of cells adhering to plastic or colony-forming unit fibroblasts, allowed Owen first and Caplan later to define these colonies as “mesenchymal stem cells” in 1980 [42,43,44,45,46]. They consist of only 0.001% to 0.01% of the total nuclear cells, representing a lower percentage if compared with the hemopoietic stem cells [47,48].
Although most studies about MSCs have been carried out on cells derived from bone marrow, cells deriving from muscular tissue, adipose tissue, blood and umbilical cord are also under examination [49].
In addition to the ability to support hemopoiesis in the bone marrow [50], MSCs are able to promote tissue repair and inflammation control in situ via some cellular signals which are still not fully understood—in particular, both the insulin-like growth factor and the angiopoietin-1 recruit macrophages and fibroblasts, which are essential in the production of collagen.
Among the immunomodulatory characteristics of MSCs, three steps were identified:
Their migration into the inflammation site or tissue injury [51];
The secretion of anti-inflammatory molecular factors such as Interleukin-10 (IL-10), HGF, TGFβ1 and Indoleamine 2,3-dioxygenase (IDO) [52,53];
The maintenance of the local anti-inflammatory effect by sending paracrine signals to neighboring cells [54,55].
These immunomodulatory properties, influencing the profiles mentioned and modulating immune cells such as lymphocytes, dendritic cells and macrophages, allowed their therapeutic use in serious diseases such as Graft versus Host Disease (GvHD) [56,57], systemic lupus erythematosus (SLE) [58], myocardial infarction [59], multiple sclerosis [60], COronaVIrus Disease (COVID-19) [61,62], enterocutaneous fistulas [63] and CD [64], even if the exact therapeutic mechanism is not yet known.
4.2. Literature Review
The first Phase I study carried out to demonstrate the efficacy and safety of autologous stem cell treatment for pCD was conducted by Garcia-Olmo et al., 2005. This prospective study involved five patients (three males and two females) but due to gram-positive bacterial contamination of cultured cells, one patient was excluded. A total of nine fistulas (one suprasphincteric, three rectovaginal, five enterocutaneous) were locally inoculated with 3 × 106 ASCs from lipoaspirate, and eight fistulas were able to continue the follow-up at 8 weeks, as the patient with enterocutaneous fistula was excluded due to emergency abdominal surgery. Of the remaining eight fistulas, six achieved complete healing (75%); the other two fistulas achieved partial healing with reduced external drainage. No adverse effects were detected and, to confirm the safety and feasibility of treatment with ASCs, histopathological analisis were carried out and showed that there was no cytological transformation (Table 1) [64].
Table 1.
Trial summary.
| Authors | Year | n Patients (Missing) | Cell Type | Intervention | Time-Point | Healing (%) | Follow-Up | Recurrence % |
|---|---|---|---|---|---|---|---|---|
| Garcia-Olmo et al. [64] | 2005 | 9 fistulas in 4 patients with CD: -1 PF (Suprasphincteric) -3 Rectovaginal -5 Enterocutaneous |
Adipose autologous | Local injection of 3 × 106 stem cells | 8 weeks | 6/8 (75%) -1/1 -2/3 -3/4 (1 NA) |
NA | NA |
| Wainstein et al. [111] | 2008 | 11 fistulas in 8 patients with CD 8 PF (Trans-sphincteric) 1 PF (Inter-sphincteric) 2 Pouch-vaginal |
ASCs + Platelet-rich plasma (PRP) | Local injection of 100–120 million ASCs + PRP | 21–37 months | 10/11 (91%) CH 1/11 (9%) PH |
37 months | 0% |
| Garcia-Olmo et al. [75] | 2009 | 49(1) patients (of which 14 with CD) -24 fistulas of which 7 PF + CD -25 fistulas of which 7 PF + CD |
Adipose autologous | Local injection of 2 × 106 of Stem cells + Fibrin glue Fibrin glue alone |
8 weeks | -17/24 (70.8%) of which 5/7 (71%) with CD -4/25 (16%) of which 1/7 (14%) with CD |
52 weeks | (17.6%) |
| Ciccocioppo et al. [103,104] | 2011 | 10 patients with CD: -9 PF (NS) -1Enterocutaneous |
Bone marrow autologous | Local injection of 1.5–3 × 107 | 52 weeks | 7/10 (70%) -6/9 -1/1 |
52 weeks | 0% |
| de la Portilla et al. [106] | 2012 | 24 (8) patients with PF in CD: 17 PF (Trans-sphincteric) 5 PF (Inter-sphincteric) 1 PF (Extra-sphincteric) 1 PF (Supra-sphincteric) |
Adipose allogeneic | Local injection of 2 × 107 (+4 × 107) | 24 weeks | 8/16 (50%) | 24 weeks | 20.0% |
| Cho et al. [105] | 2013 | 9 (1) patients with CD: -3 PF -3 PF -3 PF of which: 5 (Trans-sphincteric) 4 (Supra-sphincteric) 1 (Extra-sphincteric) |
Adipose autologous | Local injection of 1 × 107 or 2 × 107 or 4 × 107 | 8 weeks | 3/9 (33.3%) -0/3 -2/3 -1/3 |
8 months | 0% |
| Lee et al. [96,108] | 2013 | 33 (17) patients with PF -24 PF (Trans-sphincteric) -4 PF (Inter-sphincteric) -5 PF (Extrasphincteric) |
Adipose autologous | Local injection 3 × 107 or 6 × 107 | 8 weeks | 27/33 (81.8%) | 1 year/2 years After 1 year 23/26 (88.5%) CH After 2 years 20/24 (83.3%) CH |
8 weeks:11.1% 1 year: 11.5% 2 years:16.7% |
| Molendijk et al. [77] | 2015 | 21 patients (with 23 PF): -5 patients with: 3 Trans-sphincteric 1 Inter-sphincteric 1 Supra-sphincteric -5 patients with: 2 Trans-sphincteric 1 Inter-sphincteric 2 Extra-sphincteric -5 patients with: 5 Trans-sphincteric -6 patients with: 5 Trans-sphincteric 1 Inter-sphincteric 1 Superficial 1 Extrasphinteric |
Bone marrow alogeneic | Local injection: -1 × 107 -3 × 107 -9 × 107 -NaCl (Placebo) |
24 weeks | -4/5 (80%) -4/5 (80%) -1/5 (20%) -2/6 (33.3%) |
24 weeks | Only 1 Extrasphinteric fistula in placebo group recurred 1/23 = 0.04% |
| Garcia-Olmo-Guadalajara [110] | 2015 | 10 patients with PF (7 non-CD; 3 CD) Type of PF: NS |
Autologous ASCs or Allogenic ASCs or SVF |
Local injection of 2–3 × 104 cells | 8 weeks | 6/10 (60%) CH 3/10 (30%) PH Of 3 patients with CD: 1 with CD: CH 1 with CD: PH 1 with CD: NO healing |
1 year 60.0% CH |
40.0% |
| Park et al. [107] | 2015 | 6 patients with CD: -3 PF (Suprasphincteric) -3 Fistulas: 1 PF (Suprasphincteric) 1 PF (Trans-sphincteric) 1 Rectovaginal |
Adipose allogeneic | Local injection of 4.33 × 107 17 × 107 |
34 weeks | 3/6 (50%) -2/3 -1/3 |
NA | |
| Panes et al. [68,94] | 2016 | 171(41) patients with PF: -88 (19) PF -83 (22) PF Type of PF: NS |
Adipose allogeneic | Local injection: -12 × 107 -Placebo |
24 weeks | 53/107(49.5%) 36/105(34.3%) |
52 weeks | (25%) (44.1%) |
| Dietz et al. [98] | 2017 | 12 patients with PF in CD: 8 PF (Trans-sphincteric) 3 PF (Inter-sphincteric) 1 PF (Suprasphincteric) |
Adipose autologous | Local injection of 2 × 106 on GoreBioA plug | 26 weeks | 10/12 (83.3%) | NA | |
| Herreros et al. [93] | 2019 | 45 patients (52 fistulas): 18 PF in CD (NS) 24 PF non-CD (NS) 7 Rectovaginal 1 Urethrorectal 1 Sacral 1 Hidradenitis suppurativa |
Autologous ASCs or Allogenic ASCs or SVF |
Local injection of around 48 million cells: SVF (31/52) 60% Allo-ASCs (12/52) 23% Auto-ASCs (9/52) 17% |
26 weeks | 49/52 (94.2%) PH–CH 24 /52 (46.2%) CH Of 18 CD fistulas: 10/18 (55.5%) CH |
1 year | 0% |
| Barnhoorn et al. [109] | 2020 | 21(5) patients with PF: -5(1) patients -5 (1) patients -5 patients -6 (3) patients Type of PF: NS |
Bone marrow alogeneic | Local injection: -1 × 107 -3 × 107 -9 × 107 -Placebo |
4 years | -3/4(75%) -4/4(100%) -1/5(20%) -0/3(0%) |
4 years | Only in placebo-group all fistulas recurred after 4 year. |
NA: not analyzed; CD: Crohn Disease; NS: not specified; SVF: Stromal Vascular Fraction; PH: Partial Healing; CH: Complete Healing; PF: Perianal Fistula; PRP: Platelet-rich plasma.
However, even if no cases of malignant transformation after perianal treatment with MSCs were registered, these cells could have protumorigenic abilities on tumors by promoting the proliferation of neoplastic cells and neoangiogenesis. Therefore, further studies with long-term follow-up are necessary [65,66,67].
Local injection demonstrated a superior therapeutic efficacy respect to systemic administration. In fact, the systemic use does not allow the migration of a sufficient number of MSCs towards the inflammation site [68,69,70,71,72,73,74]. Moreover, local treatment of MSCs combined with fibrin glue resulted in the healing of 71% of pCD cases, compared to the injection of fibrin glue alone, which was efficacious in 16% of pCD cases [75].
Garcia-Olmo [75,76] first, and Molendijk [77,78] later, proposed a standardized method for the local injection of MSCs in pCD. According to the latter, the use of MRI and examination under anesthesia to locate the fistula is mandatory. Following the drainage of the abscess, a loose seton should be placed and patients with proctitis should also start medical therapy. Before local injection, and after the removal of the seton, the fistulous tract is curetted, and the internal orifice closed. MSCs should be injected around the internal orifice, and also through the external one, avoiding direct injection into the lumen in order to not waste therapeutic agents.
Those results were confirmed by an observational study published by Garcia-Olmo and colleagues concerning the treatment of enterocutaneaous fistulas in patients with CD [79]. In particular, a complete re-epithelialization was observed in 75% (3 out of 4) of fistulas treated with expanded ASCs compared to 25% (1 out of 4) of those treated with the not-expanded stromal vascular fraction (SVF) directly from the lipoaspirate sample.
After the extraction and expansion, ASCs are more genetically and morphologically stable and show greater proliferative capacity and angiogenetic properties [80,81,82]. Furthermore, the greatest strength is represented by the superior expression of tissue growth factor, reducing hemocompatibility [83].
There are no trials comparing autologous and allogeneic MSCs. It is evident that the expansion of autologous MSCs in vitro takes several weeks and, before administration, there are several preparation phases with a greater risk of loss of stem cells. Nevertheless, cell quality is also affected by patients’ disease status [84,85,86].
To date, the European Medicines Agency (EMA) has approved Cx601-Darvadstrocel (Alofisel®) for the treatment of refractory pCD in adult patients with inactive/mildly active luminal CD who have shown no response to the first-line approach with conventional or biological therapy [87,88,89]. Darvadstrocel is a preparation of human allogeneic expanded adipose-derived stem cells (eASCs) [90,91].
Darvadstrocel is administered in single-dose by intralesional injection of 4 vials of 120 million in 24 mL after curettage of the fistulous tract and closure of the internal orifice [90]. Up to three fistulous tracts can be treated, but its use is only approved for clinical trials and compassionate use programs regulated by the European Medicines Agency [90,92].
Herreros et al. in 2019 evaluated the efficacy of compassionate use of ASCs (autologous eASCs, allogenic eASCs and SVF) in the treatment of 42 perianal fistulas and a total of 52 cases including 7 rectovaginal, 1 sacral, 1 urethrorectal fistula, and 1 suppurative hidradenitis. In particular, 18 perianal fistulas were related to CD. Partial improvement or complete healing were observed in 49 out of 52 cases (94.2%) in almost 6 weeks. Conversely, considering only CD-associated fistulas, 100% (18/18) experienced improvement or complete recovery in a mean time of 5.3 weeks, and healing was achieved in 10 out of 18 cases (55.5%) [93].
The most important study that has evaluated the efficacy and safety of ASCs in pCD is the Adipose-Derived Mesenchymal Stem Cells for Induction of Remission in Perianal Fistulizing Crohn’s Disease (ADMIRE-CD), a phase III, randomised, double-blind, parallel group, placebo-controlled study [68]. The primary outcome of the ADMIRE-CD study was to assess the closure of external draining openings and the absence of collections >2 cm observed on MRI after 24 weeks [68,94].
The patients selected in the study were aged >18 years, with inactive or slightly active disease, Crohn’s Disease Activity Index (CDAI) of 220 or less, with at least two and a maximum of three external openings and with up to two internal openings.
Patients were randomized (1:1 ratio) in two groups: the active group (n = 107) who received a single injection of Cx601, a 24 mL solution with 120 million of ASCs, two weeks after courettage of the fistula and, possibly, after the placement of a draining seton removed at the time of injection; the placebo group (n = 105 patients), in which the same volume of saline solution was administered. The 24 weeks follow-up was completed by 171 out of 212 patients (81%).
The primary endpoint was achieved in the Intention-to-analysis (ITT) by 53 out of 107 patients (49.5%) in the Cx601 group, and by 36 out of 105 (34.2%) in the placebo group. These results were consistent with those reported in the modified ITT (mITT) analysis, in which 53 out of 103 (51.4%) patients of the active group and 36 out of 101 (35.6%) patients of the placebo group achieved the primary outcome [68]. A total of 131 patients (61.8%) completed the 52-week follow up and the mITT analysis showed a combined remission rate of 56.3% (58/103 patients) in the Cx601 group and 38.6% (39/101 patients) for the placebo group, while a clinical remission was observed in 59.2% of patients in comparison to 41.6% of the control group [94].
The most common treatment-related adverse events were anal abscess (six in the Cx601 group and nine in the placebo group) and proctalgia (five in the Cx601 group and nine in the placebo group) [68]. After 52 weeks, there was a higher rate of adverse events in both groups (76.7% in the Cx601 group and 72.5% in the placebo group). Around 8.7% patients experienced a side effect which led to study withdrawal, while most of the patients referred mild to medium events such as nasopharyngitis, diarrhoea, pyrexia, arthralgia, abdominal pain, flare of Crohn’s disease. According to the authors, one of the most important findings was the maintenance of efficacy for up to one year [94].
Furthermore, in ADMIRE-CD, clinical recovery has been reached in 59.2% patients at 52 weeks, whereas in ACCENT II study (A Crohn’s Disease Clinical Trial Evaluating Infiximab in a New Long-term Treatment Regimen in Patients With Fistulizing Crohn’s Disease), recovery rate was 36% at 54 weeks [95]. In fact, the ACCENT II study is a randomized, double-blind, placebo-controlled study that evaluated the efficacy and safety of infliximab treatment for the maintenance of rectovaginal fistula’s closure in patients with fistulizing CD through intravenous infusions of 5 mg/kg of infliximab, the success rate, and thus the fistula’s closure.
These data highlighted the need for new trials on infliximab treatment in combination with eASC to evaluate the possible superiority of their combination [94] and were consistent with those described by other phase I-II trials [75,96].
In the United States, a similar study, the so called ADMIRE-CD-II (https://clinicaltrials.gov/ct2/show/NCT03279081), is underway, with the aim of demonstrating the efficacy of darvadstrocel in the treatment of complex PF in patients with CD and 600 patients have been enrolled to date [91].
In Europe, there is a postmarketing registry called INSPIRE, an observatIoNal poSt-marketing registry on the effectiveness and safety of darvadstrocel in PatIents with CRohn’s disease and complex pErianal fistulas. This registry aims to establish a framework for acquiring real-world data about the efficacy and safety of the commercially available local MSC therapy. Collected data will soon be available [97].
Recently, Dietz at al. described the effect of autologous ASCs after extraction, cryopreservation and thawing at the time of use. After 6 months, 10 out of 12 (83%) patients showed complete healing and only one patient had abscess formation [98]. Dige et al. [99], however, in a study with 21 patients directly injected the adipose tissue containing the autologous ASCs and demonstrated their effectiveness.
A further study was conducted by Zhou et al. in 2020. Twenty-two patients were enrolled in an open-label, randomized, controlled clinical trial and the safety and efficacy of treatment with ASCs was compared with the incision-thread-drawing procedure. Follow-up was performed at 3 months, 6 months, and 12 months, although the latter was completed by 17 out of 22 patients. The primary endpoint was fistula’s closure and was achieved in both groups with no substantial differences: 10/11 (90.9%) vs. 5/11 (45.5%) at 3 months; 8/11 (72.7%) vs. 6/11 (54.5%) at 6 months; and 7/11 (63.6%) vs. 6/11 (54.5%) at 12 months. Nevertheless, there was a statistically significant superiority of the ASCs group in terms of improvement of the secondary endpoints (simplified Crohn’s Disease Activity Index (CDAI), Perianal Disease Activity Index (PDAI), pain scores with Visual Analog Score (VAS), Inflammatory Bowel Disease Questionnaire (IBDQ), and Wexner score). Neither adverse events nor incontinence problems occurred in both groups, even if sphincter function has been shown to be better in the ASCs group [100].
A recently published meta-analysis reported that the percentage of complete healing of perianal fistulas in CD using MSCs is 64.1% (95% CI 52.3–74.5), which is a higher percentage than the one observed in cryptoglandular fistulas (61.5%; 95% CI 36.8–81.4). There are elements of heterogeneity that limit the execution of a noteworthy meta-analysis, since the numerous studies conducted to date have used different type of MSCs, different origins, different amounts and different injection methods. Despite these limitations, it seems that MSCs can be considered a valid alternative for the treatment of both CD and non-CD fistulas and autologous MSCs are able to achieve higher healing rates than allogenic MSCs. The outcomes were favorable when the amount of MSCs injected is proportionate to the size of the fistula [101].
An interesting application of ASCs concerns the treatment of pCD in pregnant women. In a recent retrospective study, the possible influence of intralesional therapy with ASCs (autologous and/or allogenic) on fertility and fetal development was investigated. Six women, with a mean age of 34.4 years during therapy with ASCs (from a minimum of 17 months to a maximum of 6 years), decided to become pregnant and were included in the study. One patient was lost at follow-up and was excluded from the analysis. One patient had both rectovaginal and perianal fistulas, two patients had only rectovaginal fistulas and two patients had only perianal fistulas. At the end of gestation (mean age 36.6 years), four women underwent a caesarean section to provide perineal protection. No complications occurred.
Only one patient had two first-trimester miscarriages, but ASCs therapy does not appear to be associated with them. According to the authors, therapy with ASCs, from the analyzed data of this small study, does not appear to affect fertility and fetal development. However, larger studies are needed to obtain more reliable information [102].
In Table 1, we summarized the literature reviewed regarding the use of MSCs for pCD [64,68,75,93,94,96,98,103,104,105,106,107,108,109,110,111].
5. Conclusions
There are still many unsolved questions regarding MSCs therapy for pCD. Firstly, it is necessary to evaluate the optimal dosage of MSCs treatment and, secondly, their clinical application in CD patients with active proctitis who have been excluded from many studies is still debated. Lastly, the optimal route and modality of administration should be established and standardized.
Author Contributions
G.G., V.T. and G.S. contributed equally to this work: substantial contributions to the conception and design of the work; the acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved. S.F., G.D.P. and G.V. contributed equally to this work: Substantial contributions to the conception or design of the work; acquisition, analysis, and interpretation of data for the work; final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cosnes J., Gower-Rousseau C., Seksik P., Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Parks A.G., Gordon P.H., Hardcastle J.D. A classification of fistula-in-ano. Br. J. Surg. 1976;63:1–12. doi: 10.1002/bjs.1800630102. [DOI] [PubMed] [Google Scholar]
- 3.Odze R., Goldblum J. Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. Elsevier, Saunders; Philadelphia, PA, USA: 2015. [Google Scholar]
- 4.Bataille F., Klebl F., Rummele P., Schroeder J., Farkas S., Wild P.J., Fürst A., Hofstädter F., Schölmerich J., Herfarth H., et al. Morphological characterisation of Crohn’s disease fistulae. Gut. 2004;53:1314–1321. doi: 10.1136/gut.2003.038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz D.A., Loftus E.V., Jr., Tremaine W.J., Panaccione R., Harmsen W.S., Zinsmeister A.R., Sandborn W.J. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 6.Lightner A.L., Ashburnb J.H., Mantaj S.B., Carvello M., Chandrasinghe P., de Buck van Overstraeten A., Fleshner P.R., Gallo G., Kotze P.G., Holubar S.D., et al. Fistulizing Crohn’s disease. Curr. Probl. Surg. 2020 doi: 10.1016/j.cpsurg.2020.100808. [DOI] [PubMed] [Google Scholar]
- 7.Penner A., Crohn B.B. Perianal fistulae as a complication of regional ileitis. Ann. Surg. 1938;108:867–873. doi: 10.1097/00000658-193811000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellers G., Bergstrand O., Ewerth S., Holmström B. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980;21:525–527. doi: 10.1136/gut.21.6.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasparek M.S., Glatzle J., Temeltcheva T., Mueller M.H., Koenigsrainer A., Kreis M.E. Long-term quality of life in patients with Crohn’s disease and perianal fistulas: Influence of fecal diversion. Dis. Colon Rectum. 2007;50:2067–2074. doi: 10.1007/s10350-007-9006-5. [DOI] [PubMed] [Google Scholar]
- 10.Guadalajara H., García-Arranz M., Herreros M.D., Borycka-Kiciak K., Lightner A.L., García-Olmo D. Mesenchymal stem cells in perianal Crohn’s disease. Tech. Coloproctol. 2020;24:883–889. doi: 10.1007/s10151-020-02250-5. [DOI] [PubMed] [Google Scholar]
- 11.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 12.Bieback K., Kluter H. Mesenchymal Stromal Cells from Umbilical Cord Blood. Curr. Stem Cell Res. 2007;2:310–323. doi: 10.2174/157488807782793763. [DOI] [PubMed] [Google Scholar]
- 13.Miao Z., Jin J., Chen L., Zhu J., Huang W., Zhao J., Qian H., Zhang X. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol. Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 14.In’t Anker P.S., Scherjon S.A., Kleijburg-van der Keur C., de Groot-Swings G.M.J.S., Claas F.H.J., Fibbe W.E., Kanhai H.H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 15.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 16.Helder M.N., Knippenberg M., Klein-Nulend J., Wuisman P.I.J.M. Stem Cells from Adipose Tissue Allow Challenging New Concepts for Regenerative Medicine. Tissue. Eng. 2017;13:1799–1808. doi: 10.1089/ten.2006.0165. [DOI] [PubMed] [Google Scholar]
- 17.Lin K., Matsubara Y., Masuda Y., Togashi K., Ohno T., Tamura T., Toyoshima Y., Sugimachi K., Toyoda M., Marc H., et al. Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy. 2008;10:417–426. doi: 10.1080/14653240801982979. [DOI] [PubMed] [Google Scholar]
- 18.Tremolada C., Colombo V., Ventura C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016;2:304–312. doi: 10.1007/s40778-016-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Montiel, Mdel, P.; Gómez-Gómez, G.J.; Flores, A.I. Therapy with stem cells in inflammatory bowel disease. World J. Gastroenterol. 2014;20:1211–1227. doi: 10.3748/wjg.v20.i5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H.-S., Hung S.-C., Peng S.-T., Huang C.-C., Wei H.-M., Guo Y.-J., Fu Y.-S., Lai M.-C., Chen C.-C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 21.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 22.Mayne C.G., Williams C.B. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 2013;19:1772–1788. doi: 10.1097/MIB.0b013e318281f5a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 24.Ponte A.L., Marais E., Gallay N., Langonné A., Delorme B., Hérault O., Charbord P., Domenech J. The In Vitro Migration Capacity of Human Bone Marrow Mesenchymal Stem Cells: Comparison of Chemokine and Growth Factor Chemotactic Activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 25.Salem H.K., Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold S.L., Cohen-Mekelburg S., Schneider Y., Steinlauf A. Perianal Fistulas in Patients with Crohn’s Disease, Part 2. Gastroenterol. Hepatol. 2018;14:521–528. [PMC free article] [PubMed] [Google Scholar]
- 27.Sandborn W.J., Fazio V.W., Feagan B.G., Hanauer S.B. AGA technical review on perianal Crohn’s disease. Gastroenterology. 2003;125:1508–1530. doi: 10.1016/j.gastro.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Hyder S.A., Travis S.P.L., Jewell D.P., Neil J., George B.D. Fistulating anal Crohn’s disease: Results of combined surgical and infliximab treatment. Dis. Colon Rectum. 2006;49:1837–1841. doi: 10.1007/s10350-006-0656-5. [DOI] [PubMed] [Google Scholar]
- 29.Gaertner W.B., Decanini A., Mellgren A., Lowry A.C., Goldberg S.M., Madoff R.D., Spencer M.P. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis. Colon Rectum. 2007;50:1754–1760. doi: 10.1007/s10350-007-9077-3. [DOI] [PubMed] [Google Scholar]
- 30.Bubbers E.J., Cologne K.G. Management of complex anal fistulas. Clin. Colon Rectal. Surg. 2016;29:43–49. doi: 10.1055/s-0035-1570392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ritchie R.D., Sackier J.M., Hodde J.P. Incontinence rates after cutting seton treatment for anal fistula. Colorectal Dis. 2009;11:564–571. doi: 10.1111/j.1463-1318.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- 32.Mizrahi N., Wexner S.D., Zmora O., Da Silva G., Efron J., Weiss E.G., Vernava A.M., 3rd, Nogueras J.J. Endorectal advancement flap: Are there predictors of failure? Dis. Colon Rectum. 2002;45:1616–1621. doi: 10.1007/s10350-004-7248-z. [DOI] [PubMed] [Google Scholar]
- 33.Sonoda T., Hull T., Piedmonte M.R., Fazio V.W. Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis. Colon Rectum. 2002;45:1622–1628. doi: 10.1007/s10350-004-7249-y. [DOI] [PubMed] [Google Scholar]
- 34.Makowiec F., Jehle E.C., Becker H.D., Starlinger M. Clinical course after transanal advancement flap repair of perianal fistula in patients with Crohn’s disease. Br. J. Surg. 1995;82:603–606. doi: 10.1002/bjs.1800820509. [DOI] [PubMed] [Google Scholar]
- 35.Joo J.S., Weiss E.G., Nogueras J.J., Wexner S.D. Endorectal advancement flap in perianal Crohn’s disease. Am. Surg. 1998;64:147–150. [PubMed] [Google Scholar]
- 36.Grimaud J.C., Munoz-Bongrand N., Siproudhis L., Abramowitz L., Sénéjoux A., Vitton V., Gambiez L., Flourié B., Hébuterne X., Louis E., et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2010;138:2275–2281. doi: 10.1053/j.gastro.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Loungnarath R., Dietz D.W., Mutch M.G., Birnbaum E.H., Kodner I.J., Fleshman J.W. Fibrin glue treatment of complex anal fistulas has low success rate. Dis. Colon Rectum. 2004;47:432–436. doi: 10.1007/s10350-003-0076-8. [DOI] [PubMed] [Google Scholar]
- 38.Limura E., Giordano P. Modern management of anal fistula. World J. Gastroenterol. 2015;21:12–20. doi: 10.3748/wjg.v21.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rojanasakul A. LIFT procedure: A simplified technique for fistula-in-ano. Tech. Coloproctol. 2009;13:237–240. doi: 10.1007/s10151-009-0522-2. [DOI] [PubMed] [Google Scholar]
- 40.Kotze P.G., Shen B., Lightner A., Yamamoto T., Spinelli A., Ghosh S., Panaccione R. Modern management of perianal fistulas in Crohn’s disease: Future directions. Gut. 2018;67:1181–1194. doi: 10.1136/gutjnl-2017-314918. [DOI] [PubMed] [Google Scholar]
- 41.Singh S., Ding N.S., Mathis K.L., Dulai P.S., Farrell A.M., Pemberton J.H., Hart A.L., Sandborn W.J., Loftus E.V., Jr. Systematic review with meta-analysis: Faecal diversion for management of perianal Crohn’s disease. Aliment. Pharm. Ther. 2015;42:783–792. doi: 10.1111/apt.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedenstein A.J., Piatetzky-Shapiro I.I., Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 43.Friedenstein A.J., Petrakova K.V., Kurolesova A.I., Frolova G.P. Heterotopic of bone marrow: Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Owen M. Marrow stromal stem cells. J. Cell Sci. Suppl. 1988;10:63–76. doi: 10.1242/jcs.1988.Supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 45.Sale G.E., Storb R. Bilateral diffuse pulmonary ectopic ossification after marrow allograft in a dog: Evidence for allotransplantation of hemopoietic and mesenchymal stem cells. Exp. Hematol. 1983;11:961–966. [PubMed] [Google Scholar]
- 46.Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 47.Tyndall A., Walker U.A., Cope A., Dazzi F., De Bari C., Fibbe W., Guiducci S., Jones S., Jorgensen C., Feldmann M., et al. Immunomodulatory properties of mesenchymal stem cells: A review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res. 2007;9:301. doi: 10.1186/ar2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pittenger M.F., Martin B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 49.Da Silva Meirelles L., Chagastelles P.C., Nardi N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 50.Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Dai W., Hale S.L., Martin B.J., Kuang J.Q., Dow J.S., Wold L.E., Kloner R.A. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 52.Ryan J.M., Barry F., Murphy J.M., Mahon B.P. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meisel R., Zibert A., Laryea M., Göbel U., Däubener W., Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 54.Horton J.A., Hudak K.E., Chung E.J., White A.O., Scroggins B.T., Burkeen J.F., Citrin D.E. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells. 2013;31:2231–2241. doi: 10.1002/stem.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartholomew A., Sturgeon C., Siatskas M., Ferrer K., McIntosh K., Patil S., Hardy W., Devine S., Ucker D., Deans R., et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002;30:42–48. doi: 10.1016/S0301-472X(01)00769-X. [DOI] [PubMed] [Google Scholar]
- 56.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M.E., Remberger M., et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 57.Ryan J.M., Barry F.P., Murphy J.M., Mahon B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun L., Wang D., Liang J., Zhang H., Feng X., Wang H., Hua B., Liu B., Ye S., Hu X., et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 59.Lee R.H., Pulin A.A., Seo M.J., Kota D.J., Ylostalo J., Larson B.L., Semprun-Prieto L., Delafontaine P., Prockop D.J. Intravenous hMSCs Improve Myocardial Infarction in Mice because Cells Embolized in Lung Are Activated to Secrete the Anti-inflammatory Protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamout B., Hourani R., Salti H., Barada W., El-Hajj T., Al-Kutoubi A., Herlopian A., Baz E.K., Mahfouz R., Khalil-Hamdan R., et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J. Neuroimmunol. 2010;227:185–189. doi: 10.1016/j.jneuroim.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Gallo G., La Torre M., Pietroletti R., Bianco F., Altomare D.F., Pucciarelli S., Gagliardi G., Perinotti R. Italian society of colorectal surgery recommendations for good clinical practice in colorectal surgery during the novel coronavirus pandemic. Tech. Coloproctol. 2020;24:501–505. doi: 10.1007/s10151-020-02209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lightner A.L., García-Olmo D. Mesenchymal Stem Cell Therapy Can Transcend Perianal Crohn’s Disease: How Colorectal Surgeons Can Help in the Coronavirus Disease 2019 Crisis. Dis. Colon Rectum. 2020;63:874–878. doi: 10.1097/DCR.0000000000001700. [DOI] [PubMed] [Google Scholar]
- 63.Mizushima T., Takahashi H., Takeyama H., Naito A., Haraguchi N., Uemura M., Nishimura J., Hata T., Takemasa I., Yamamoto H., et al. A clinical trial of autologous adipose-derived regenerative cell transplantation for a postoperative enterocutaneous fistula. Surg. Today. 2016;46:835–842. doi: 10.1007/s00595-015-1246-8. [DOI] [PubMed] [Google Scholar]
- 64.García-Olmo D., García-Arranz M., Herreros D., Pascual I., Peiro C., Rodríguez-Montes J.A. A phase I clinical trial of the treatment of crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis. Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 65.Qiu Y., Li M.Y., Feng T., Feng R., Mao R., Chen B.L., He Y., Zeng Z.R., Zhang S.H., Chen M.H. Systematic review with meta-analysis: The efficacy and safety of stem cell therapy for Crohn’s disease. Stem Cell Res. 2017;8:136. doi: 10.1186/s13287-017-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang W.H., Chang M.C., Tsai K.S., Hung M.C., Chen H.L., Hung S.C. Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene. 2013;32:4343–4354. doi: 10.1038/onc.2012.458. [DOI] [PubMed] [Google Scholar]
- 67.Tsai K., Yang S., Lei Y., Tsai C., Chen H., Hsu C., Chen L., Wang H., Miller S.A., Chiou S., et al. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology. 2011;141:1046–1056. doi: 10.1053/j.gastro.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 68.Panés J., Garcia-Olmo D., van Assche G., Colombel J.F., Reinisch W., Baumgart D.C., Dignass A., Nachury M., Ferrante M., Danese S., et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- 69.Sasaki M., Abe R., Fujita Y., Ando S., Inokuma D., Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y., Xiang L.X., Shao J.Z., Pan R.L., Wang Y.X., Dong X.J., Zhang G.R. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J. Cell Mol. Med. 2010;14:1494–1508. doi: 10.1111/j.1582-4934.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang L., Dong C., Chen X., Fang Z., Xu J., Liu M., Zhang X., Gu D.S., Wang D., Han Z.C., et al. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transpl. 2011;20:1395–1408. doi: 10.3727/096368910X557245. [DOI] [PubMed] [Google Scholar]
- 72.Kraitchman D.L., Tatsumi M., Gilson W.D., Ishimori T., Kedziorek D., Walczak P., Segars W.P., Chen H.H., Fritzges D., Bulte J.W.M., et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anjos-Afonso F., Siapati E.K., Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J. Cell Sci. 2004;117:5655–5664. doi: 10.1242/jcs.01488. [DOI] [PubMed] [Google Scholar]
- 74.Ruster B., Gottig S., Ludwig R.J., Bistrain R., Muller S., Seifried E., Gille J., Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Olmo D., Herreros D., Pascual I., Pascual J.A., Del-Valle E., Zorrilla J., De-La-Quintana P., Garcia-Arranz M., Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: A phase II clinical trial. Dis. Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Olmo D., Guadalajara-Labajo H. Stem Cell Application in Fistula Disease. In: Abcarian H., editor. Anal Fistula. Springer; New York, NY, USA: 2014. pp. 129–138. [DOI] [Google Scholar]
- 77.Molendijk I., Bonsing B.A., Roelofs H., Peeters K.C.M.J., Wasser M.N.J.M., Dijkstra G., Van Der Woude C.J., Duijvestein M., Veenendaal R.A., Zwaginga J.J. Allogeneic Bone Marrow—Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology. 2015;149:918–927. doi: 10.1053/j.gastro.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 78.Molendijk I., van der Meulen-de Jong A.E., Verspaget H.W., Veenendaal R.A., Hommes D.W., Bonsing B.A., Peeters K.C.M.J. Standardization of mesenchymal stromal cell therapy for perianal fistulizing Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2018;30:1148–1154. doi: 10.1097/MEG.0000000000001208. [DOI] [PubMed] [Google Scholar]
- 79.Garcia-Olmo D., Herreros D., Pascual M., Pascual I., De-La-Quintana P., Trebol J., Garcia-Arranz M. Treatment of enterocutaneous fistula in Crohn’s Disease with adipose-derived stem cells: A comparison of protocols with and without cell expansion. Int. J. Colorectal Dis. 2009;24:27–30. doi: 10.1007/s00384-008-0559-0. [DOI] [PubMed] [Google Scholar]
- 80.Kim Y., Kim H., Cho H., Bae Y., Suh K., Jung J. Direct comparison of human mesench-ymal stem cells derived from adipose tissues and bone marrow inmediating neovascularization in response to vascular ischemia. Cell Physiol. Biochem. 2007;20:867–876. doi: 10.1159/000110447. [DOI] [PubMed] [Google Scholar]
- 81.Kern S., Eichler H., Stoeve J., Klüter H., Bieback K. Comparative analysis of mesench-ymal stem cells from bone marrow, umbilical cord blood, or adi-pose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 82.Izadpanah R., Trygg C., Patel B., Kriedt C., Dufour J., Gimble J.M., Bunnell B.A. Biologic properties of mesench-ymal stem cells derived from bone marrow and adipose tissue. J. Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nikolic M., Stift A., Reinisch W., Vogelsang H., Matic A., Müller C., von Strauss und Torney M., Riss S. Allogeneic expanded-adipose derived stem cells in the treatment of rectovaginal fistulas in Crohn’s disease. Colorectal Dis. 2020 doi: 10.1111/codi.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nie Y., Lau C., Lie A., Chan G., Mok M. Defective phenotype of mesenchymalstem cells in patients with systemic lupus erythematosus. Lupus. 2010;19:850–859. doi: 10.1177/0961203310361482. [DOI] [PubMed] [Google Scholar]
- 85.Choudhery M.S., Khan M., Mahmood R., Mehmood A., Khan S.N., Riazuddin S. Bone marrow derivedmesenchymal stem cells from aged mice have reduced woundhealing, angiogenesis, proliferation and anti-apoptosiscapabilities. Cell Biol. Int. 2012;36:747–753. doi: 10.1042/CBI20110183. [DOI] [PubMed] [Google Scholar]
- 86.Georgiev-Hristov T., Guadalajara H., Herreros M.D., Lightner A.L., Dozois E.J., García-Arranz M., García-Olmo D. A Step-By-Step Surgical Protocol for the Treatment of Perianal Fistula with Adipose-Derived Mesenchymal Stem Cells. J. Gastrointest. Surg. 2018;22:2003–2012. doi: 10.1007/s11605-018-3895-6. [DOI] [PubMed] [Google Scholar]
- 87.Chudy-Onwugaje K.O., Christian K.E., Farraye F.A., Cross R.K. A State-of-the-Art Review of New and Emerging Therapies for the Treatment of IBD. Inflamm. Bowel Dis. 2019;25:820–830. doi: 10.1093/ibd/izy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scott L.J. Darvadstrocel: A Review in Treatment-Refractory Complex Perianal Fistulas in Crohn’s Disease. BioDrugs. 2018;32:627–634. doi: 10.1007/s40259-018-0311-4. [DOI] [PubMed] [Google Scholar]
- 89.Kotze P.G., Spinelli A., Warusavitarne J., Di Candido F., Sahnan K., Adegbola S.O., Danese S. Darvadstrocel for the treatment of patients with perianal fistulas in Crohn’s disease. Drugs Today. 2019;55:95–105. doi: 10.1358/dot.2019.55.2.2914336. [DOI] [PubMed] [Google Scholar]
- 90.CHMP . European Medicines Agency: EMA/CHMP/64055/2018 Committee for Medicinal Products for Human Use (CHMP) Assessment Report Alofisel [Internet] CHMP; London, UK: 2017. [(accessed on 3 December 2019)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/alofisel. [Google Scholar]
- 91.Carvello M., Lightner A., Yamamoto T., Kotze P.G., Spinelli A. Mesenchymal Stem Cells for Perianal Crohn’s Disease. Cells. 2019;8:764. doi: 10.3390/cells8070764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bislenghi G., Wolthuis A., Van Assche G., Vermeire S., Ferrante M., D’Hoore A. Expert Opinion on Biological Therapy Cx601 (darvadstrocel) for the treatment of perianal fistulizing Crohn’s disease. Expert Opin. Biol. 2019;19:607–616. doi: 10.1080/14712598.2019.1623876. [DOI] [PubMed] [Google Scholar]
- 93.Herreros M.D., Garcia-Olmo D., Guadalajara H., Georgiev-Hristov T., Brandariz L., Garcia-Arranz M. Stem Cell Therapy: A Compassionate Use Program in Perianal Fistula. Stem Cells Int. 2019;2019:6132340. doi: 10.1155/2019/6132340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Panés J., García-Olmo D., Van Assche G., Colombel J.F., Reinisch W., Baumgart D.C., Dignass A., Nachury M., Ferrante M., Danese S. Long-term Efficacy and Safety of Stem Cell Therapy (Cx601) for Complex Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology. 2018;154:1334–1342. doi: 10.1053/j.gastro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 95.Sands B.E., Anderson F.H., Bernstein C.N., Chey W.Y., Feagan B.G., Fedorak R.N., Kamm M.A., Korzenik J.R., Lashner B.A., Rutgeerts P. Infliximab Maintenance Therapy for Fistulizing Crohn’s Disease. N. Engl. J. Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 96.Lee W.Y., Park K.J., Cho Y.B., Yoon S.N., Song K.H., Kim D.S., Jung S.H., Kim M., Yoo H.W., Kim I., et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for crohn’s fistula. Stem Cells. 2013;31:2575–2581. doi: 10.1002/stem.1357. [DOI] [PubMed] [Google Scholar]
- 97.Vaegler M., Maerz J., Amend B., Silva L., Mannheim J., Fuchs K., Will S., Sievert K., Stenzl A., Hart M., et al. Labelling and Tracking of Human Mesenchymal Stromal Cells in Preclinical Studies and Large Animal Models of Degenerative Diseases. Curr. Stem Cell Res. 2014;9:444–450. doi: 10.2174/1574888X09666140521144559. [DOI] [PubMed] [Google Scholar]
- 98.Dietz A.B., Dozois E.J., Fletcher J.G., Butler G.W., Radel D., Lightner A.L., Dave M., Friton J., Nair A., Faubion W.A., et al. Autologous Mesenchymal Stem Cells, Applied in a Bioabsorbable Matrix, for Treatment of Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology. 2017;153:59–62. doi: 10.1053/j.gastro.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dige A., Hougaard H.T., Agnholt J., Pedersen B.G., Tencerova M., Kassem M., Krogh K., Lundbyet L. Efficacy of Injection of Freshly Collected Autologous Adipose Tissue into Perianal Fistulas in Patients with Crohn’s Disease. Gastroenterology. 2019;156:2208–2216. doi: 10.1053/j.gastro.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Zhou C., Li M., Zhang Y., Ni M., Wang Y., Xu D., Shi Y., Zhang B., Chen Y., Huang Y., et al. Autologous adipose-derived stem cells for the treatment of Crohn’s fistula-in-ano: An open-label, controlled trial. Stem Cell Res. 2020;11:124. doi: 10.1186/s13287-020-01636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi S., Jeon B.G., Chae G., Lee S.J. The clinical efficacy of stem cell therapy for complex perianal fistulas: A meta-analysis. Tech. Coloproctol. 2019;23:411–427. doi: 10.1007/s10151-019-01994-z. [DOI] [PubMed] [Google Scholar]
- 102.Sanz-Baro R., García-Arranz M., Guadalajara H., de la Quintana P., Herreros M.D., García-Olmo D. First-in-Human Case Study: Pregnancy in Women with Crohn’s Perianal Fistula Treated With Adipose-Derived Stem Cells: A Safety Study. Stem Cells Transl. Med. 2015;4:598–602. doi: 10.5966/sctm.2014-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ciccocioppo R., Bernardo M.E., Sgarella A., Maccario R., Avanzini M.A., Ubezio C., Minelli A., Alvisi C., Vanoli A., Corazza G.R., et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulizing Crohn’s disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 104.Ciccocioppo R., Gallia A., Sgarella A., Kruzliak P., Gobbi P.G., Corazza G.R. Long-term follow-up of Crohn disease fistulas after local injections of bone marrow-derived mesenchymal stem cells. Mayo Clin. Proc. 2015;90:747–755. doi: 10.1016/j.mayocp.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 105.Cho Y.B., Lee W.Y., Park K.J., Kim M., Yoo H.W., Yu C.S. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: A Phase I clinical study. Cell Transpl. 2013;22:279–285. doi: 10.3727/096368912X656045. [DOI] [PubMed] [Google Scholar]
- 106.De La Portilla F., Alba F., Garcia-Olmo D., Herrerias J.M., Gonzalez F.X., Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: Results from a multicenter Phase I/IIa clinical trial. Int. J. Colorectal Dis. 2013;28:313–323. doi: 10.1007/s00384-012-1581-9. [DOI] [PubMed] [Google Scholar]
- 107.Park K.J., Ryoo S.B., Kim J.S., Kim T.I., Baik S.H., Lee K.Y., Kim M., Kim W.H. Allogeneic adipose-derived stem cells for the treatment of perianal fistula in Crohn’s disease: A pilot clinical trial. Colorectal Dis. 2016;18:468–476. doi: 10.1111/codi.13223. [DOI] [PubMed] [Google Scholar]
- 108.Cho Y.B., Park K.J., Yoon S.N., Song K.H., Kim D.S., Jung S.H., Kim M., Jeong H.Y., Yu C.S. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl. Med. 2015;4:532–537. doi: 10.5966/sctm.2014-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barnhoorn M.C., Wasser M.N.J.M., Roelofs H., Maljaars P.W.J., Ilse Molendijk I., Bonsing B.A., Liesbeth E.M., Oosten L.E.M., Dijkstra G., van der Woude C.J., et al. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J. Crohn’s Colitis. 2020;14:64–70. doi: 10.1093/ecco-jcc/jjz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garcia-Olmo D., Guadalajara H., Rubio-Perez I., Herreros M.D., de-la-Quintana P., Garcia-Arranz M. Recurrent anal fistulae: Limited surgery supported by stem cells. World J. Gastroenterol. 2015;21:3330–3336. doi: 10.3748/wjg.v21.i11.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wainstein C., Quera R., Fluxá D., Kronberg U., Conejero A., López-Köstner F., Jofre C., Zarate A.J. Stem Cell Therapy in Refractory Perineal Crohn’s Disease: Long-term Follow-up. Colorectal Dis. 2018 doi: 10.1111/codi.14002. [DOI] [PubMed] [Google Scholar]