Abstract
Simple Summary
The carabid beetles are well known for the consumption of weed seeds in arable land, but how they choose the seeds is poorly known. In this work, we try to explain the patterns in preferences of 37 species of carabids based on eight seed properties of 28 species of seeds. Surprisingly, chemical properties of the seeds did not affect the preferences. Instead, preferences were driven mainly by seed structural properties. The importance of particular seed properties was also affected by the degree of predator specialization.
Abstract
Ground beetles are important invertebrate seed predators in temperate agro-ecosystems. However, there is a lack of information regarding which seed properties are important to carabids when they select seeds for consumption. Therefore, seed properties, such as size, shape, morphological defence, and chemical composition, were measured, and in addition to seed taxonomy and ecology, these data were used to explain carabid preferences. Carabid preferences were assessed using a multi-choice experiment with 28 species of weed seeds presented to 37 species of Carabidae. Multiple regression on distance matrices (MRM) was used to determine the importance of particular sets of seed properties for carabids. The analysis was conducted for the full set of carabids (37 species) as well as for subsets of species belonging to the tribes of Harpalini or Zabrini. For the complete set of species, seed dimensions, seed mass, taxonomy, plant strategy, and seed coat properties significantly explained carabid preferences (proportion of explained variance, R2 = 0.465). The model for Harpalini fit the data comparably well (R2 = 0.477), and seed dimensions, seed mass and seed coat properties were significant. In comparison to that for Harpalini, the model for Zabrini had much lower explanatory power (R2 = 0.248), and the properties that significantly affected the preferences were seed dimensions, seed mass, taxonomy, plant strategy, and seed coat properties. This result suggests that the seed traits that carabids respond to may be specific to taxonomic and likely relate to the degree of specialisation for seeds. This study contributes to understanding the mechanisms that determine the preferences of carabid beetles for seeds.
Keywords: preference, ground beetles, weed seeds, seed properties
1. Introduction
Ground beetles (Coleoptera: Carabidae) are among the most important groups of weed seed predators in temperate agro-ecosystems where they help to reduce weed seeds. These granivorous species of arable land mainly belong to the tribes Harpalini (e.g., genera Harpalus, Ophonus, Acupalpus, Stenolophus, or Anisodactylus) and Zabrini (genera Amara and Zabrus) [1,2], but species from other groups consume seeds as well. Recent findings suggest that granivory is more widespread within this family than previously thought [3,4]. Species that are specialised seed feeders often show distinct seed preferences [5]. Species of Zabrini prefer seeds of Asteraceae, Brassicaceae, or Caryophyllaceae, and they seem to be more selective than species of the tribe Harpalini, which prefer seeds of Violaceae or Asteraceae [6,7]. Many species of these families are considered to be problematic weeds. However, the knowledge on what drives the preferences is not fully understood. Predator identity, taxonomy, body size, size of mandibles [8,9], seed size, and other seed properties may affect carabid preferences for seeds. Understanding the driving factors of the preferences would potentially improve our ability to predict which seeds are the most vulnerable to which predators.
Seeds are usually unevenly scattered on the ground or aggregated in patches near the mother plant; therefore, insect seed predators have to locate the seeds or patches of seeds. However, the seeds try to resist predators. The defence of seeds against predators is divided into two main groups, morphological and chemical traits, which inevitably interact with each other and influence seed dormancy and persistence in soil [10,11], and in this way influence predation in the long term [12].
Although the information on the cues seed predators use is scarce and we hypothesized that the process that ultimately leads to seed predation is similar to the one described for other types of predators [13,14,15]. The typical process of prey location by an insect predator usually includes several steps, each having typical sets of cues involved. Visual or olfactory cues may be important when searching for seeds [16,17,18,19]. Utilising (semio-)chemicals is a common means of communication within food webs [20,21,22]. How important it is for seed predation is poorly understood. Few studies have shown that ground beetles detect volatiles from other animals, such as aphids, springtails, or slugs [23,24], as well as from plants [25,26] and probably seeds [16,17,24]. The chemical properties of seeds may change the behaviour of seed predators (serve as attractants or as repellents). The detection rate may be affected by the properties of seed coats because some are impermeable to gases, chemical compounds, or water [27]. This rate can also be affected by the level of imbibition [16] because the imbibed seeds release different amounts of volatile compounds, including carbon dioxide, alcohols, aldehydes, alkane, ketones, volatile acids [28], or ethylene [29], which can potentially attract or repel beetles.
Seeds vary in their morphological properties, such as mass, size and shape; as well as defensive structural traits. These properties affect seed interception and handling by a predator. Seed mass [30,31,32] and size [6,33,34,35] are major drivers in seed selection by ground beetles and there is a relationships between carabid body size and seed size or mass [6,7]. Larger seeds might be more apparent to predators [36], and they also stay on the soil surface for longer than smaller ones [37,38]. Seed shapes can also affect predation but has never been studied. The smaller, round seeds are able to escape seed predation more than flattened ones [31,39,40]. Round seeds fit better in cracks in the soil where they can escape predation [41]. In comparison to flat seeds, round ones can also be harder to handle because they pop out of mandibles (e.g., seeds of Amaranthaceae) [6].
Once seeds are found, a predator is expected to evaluate seed attractiveness. The chemical profile of the seed surface is often important in this process [17,22]. Waxes or fatty acids present on the seed surface [42] may drive a predator’s decision to feed on it or not [43,44]. Other surface compounds could also affect seed predation. Other surface compounds contain mostly long-chain alkanes or their branched counterparts, which are common constituents of plant waxes [45]. These compounds protect seeds from physical, temperature-related, or water damage to ensure that the plant seed remains in a state of dormancy [46]. Once a predator attempts in feeding, crushing and opening a seed is further affected by physical traits, such as thickness [5] or the strength of the seed coat [5,47]. These are seemingly related, but this is not necessarily the case (for example, seed coats can be relatively thin but hard, e.g., Silene latifolia alba (Mill.) Greut. et Burdet, or thick but soft, e.g., Fumaria officinalis L.). This type of physical defence is potentially more effective, and in comparison to other types of defences, less costly for the plant [48]. A higher investment in a seed coat may increase post-dispersal survival [10,11,48]. That seed coat thickness can be an adaptive defensive trait is supported by the finding of Benkman [49], who documented stronger seed coats in environments with predators rather than without predators. There is also a positive relationship between seed mass and seed coat thickness [50] as well as the interaction among seed size, mass, and strength of seed coat [5]. There may also be an interaction between seed coat hardness and shape, which may explain seed preference [9].
After successfully opening, a predator further evaluates the nutritional composition of a seed (amount of starches, proteins, oils, secondary metabolites, fatty acids, etc.) [22,42,51,52,53,54,55], which stimulates or deters the predator from additionally feeding on conspecific seeds. Some of the chemical compounds can be distasteful or poisonous for their predator (e.g., opium and L-dopa) [56], but insects have evolved systems to detoxify these compounds. In fact, we know only very little about this hypothetical sequence of events leading to the destruction of a seed by the mandibles of insect seed predators.
In addition to seed chemical and morphological properties, plant taxonomy [6] and the life cycle strategy of plants [57] are important determinants of predator preferences. The sister taxa of plants may be more attractive for seed predators than taxa unrelated ones [6], likely because related seeds have similar properties.
The aim of this study is to explore which weed seed properties are decisive for preferences of carabid beetles. We focus on properties related to seed size, shape, mass, and morphological defence; seed chemical properties (volatile compounds, fatty acids, and other surface compounds); and seed ecology and taxonomy.
2. Materials and Methods
2.1. Seed Material
A set of 28 species of weed seeds was used (Table 1). Each year, the seeds were hand-collected de novo from the parental plant at full maturity using laboratory gloves. The seeds were cleaned from dust and admixtures of non-seed plant particles by blowing, dried at room temperature (25 °C for 30 days) and then stored in the freezer (−21 °C) until the experiments.
Table 1.
List of the model organisms that were used in the preference experiment. The plant taxonomy was based on Kubát et al. [58] while that of carabids on Hůrka [2].
| Plants | Carabids | ||
|---|---|---|---|
| Species | Family | Species | Tribe |
| Amaranthus retroflexus L. | Amaranthaceae | Acupalpus meridianus (Linnaeus) | Harpalini |
| Arctium lappa L. | Asteraceae | Amara aenea (DeGeer) | Zabrini |
| Arenaria serpyllifolia agg. | Caryophyllaceae | Amara anthobia (A. Villa et G.B. Villa) | Zabrini |
| Bidens tripartita L. | Asteraceae | Amara apricaria (Paykull) | Zabrini |
| Campanula trachelium L. | Campanulaceae | Amara aulica (Panzer) | Zabrini |
| Capsella bursa-pastoris (L.) Med. | Brassicaceae | Amara bifrons (Gyllenhal) | Zabrini |
| Chenopodium album L. | Amaranthaceae | Amara consularis (Duftschmid) | Zabrini |
| Cichorium intybus L. | Asteraceae | Amara convexior (Stephens) | Zabrini |
| Cirsium arvense (L.) Scop. | Asteraceae | Amara convexiuscula (Marsham) | Zabrini |
| Consolida regalis S.F. Gray | Ranunculaceae | Amara eurynota (Panzer) | Zabrini |
| Crepis biennis L. | Asteraceae | Amara familiaris (Duftschmid) | Zabrini |
| Descurainia sophia (L.) Prantl | Brassicaceae | Amara ingenua (Duftschmid) | Zabrini |
| Fumaria officinalis L. | Papaveraceae | Amara litorea (C.G.Thomson) | Zabrini |
| Galinsoga parviflora Cav. | Asteraceae | Amara montivaga (Sturm) | Zabrini |
| Galium aparine L. | Rubiaceae | Amara ovata (Fabricius) | Zabrini |
| Lapsana communis L. | Asteraceae | Amara sabulosa (Audient-Serville) | Zabrini |
| Leonurus cardiaca L. | Lamiaceae | Amara similata (Gyllenhal) | Zabrini |
| Lepidium ruderale L. | Brassicaceae | Amara spreta (Dejean) | Zabrini |
| Melilotus albus Med. | Fabaceae | Anisodactylus signatus (Panzer) | Harpalini |
| Potentilla argentea L. | Rosaceae | Calathus ambiguus (Paykull) | Sphodrini |
| Silene latifolia alba (Mill.) Greut. et Burdet | Caryophyllaceae | Calathus fuscipes (Goeze) | Sphodrini |
| Sisymbrium loeselii L. | Brassicaceae | Harpalus affinis (Schrank) | Harpalini |
| Stellaria media (L.) Vill. | Caryophyllaceae | Harpalus atratus (Latreille) | Harpalini |
| Taraxacum officinale agg. | Asteraceae | Harpalus distinguendus (Duftschmid) | Harpalini |
| Thlaspi arvense L. | Brassicaceae | Harpalus honestus (Duftschmid) | Harpalini |
| Tripleurospermum inodorum (L.) Schultz-Bip. | Asteraceae | Harpalus luteicornis (Duftschmid) | Harpalini |
| Urtica dioica L. | Urticaceae | Harpalus rubripes (Duftschmid) | Harpalini |
| Viola arvensis Murray | Violaceae | Harpalus signaticornis (Duftschmid) | Harpalini |
| Harpalus subcylindricus (Dejean) | Harpalini | ||
| Ophonus azureus (Fabricius) | Harpalini | ||
| Parophonus maculicornis (Duftschmid) | Harpalini | ||
| Pseudoophonus griseus (Panzer) | Harpalini | ||
| Pseudoophonus rufipes (DeGeer) | Harpalini | ||
| Pterostichus melanarius (Illiger) | Pterostichini | ||
| Stenolophus teutonus (Schrank) | Harpalini | ||
| Trechus quadristriatus (Schrank) | Trechini | ||
| Zabrus tenebrioides (Goeze) | Zabrini | ||
2.2. Preference Experiments
The preferences of 37 species of carabids were evaluated (Table 1). The preferences were determined based on a cafeteria experiment described in Honěk et al. [6] and Saska et al. [7]. The seeds of 28 species of weeds (Table 1) were presented on tin trays filled with white modelling clay (JOVI, Barcelona, Spain). The seed trays were arranged in two concentric rings in Petri dishes (20 cm in diameter) with 10 beetles for five days. The seed consumption was counted daily, after which it was summed and standardised to remove the effect of carabid body size on the total consumption and be able to compare data across the species [6]. Standardisation was performed by converting the actual consumption of seed to the proportion of the most consumed seed.
2.3. Measurement of Seed Morphological Traits
Seed mass was measured by weighing 100 seeds on a balance to a precision of 10−5 g (Sartorius, Göttingen, Germany). Seed dimensions were measured following Bekker et al. [38], using a digital calliper and five seeds per species: A—the longest dimension, B—the longest dimension perpendicular to A within the same plane, and C—the longest dimension perpendicular to the plane of A and B.
These dimensions were used to calculate indices that describe seed shape, flatness, eccentricity, and volume. The shape of the seed was calculated as in Bekker et al. [38], , where x represents a division of either A, B, or C through A and as their mean, and n is 3. vs. ranges from 0 for perfectly spherical seeds to 0.2 for seeds shaped like a thin disc or spindles. The flatness of the seeds [59] was calculated as . FI ranges from 1 for a complete sphere to higher values for plane-like or spindle-like seeds. The eccentricity of the seeds [59] was calculated as . EI ranges from 1 for round seeds to values > 2 for spindle-like seeds. The volume of the seeds was calculated as [59].
Seed coat thickness was measured using a light microscope on sections of seeds. Dry seeds were infiltrated with a 2% sucrose solution for six hours at room temperature, mounted onto cryo-gel on the alum chuck, and sectioned using a cryotome (Shandon SME, Astmoor, UK). Sections were observed using an Olympus BX51 microscope (Olympus Corp., Tokyo, Japan) and documented with an Apogee U4000 digital camera (Apogee Imaging Systems, Inc., Roseville, CA, USA). Five seeds of each species were measured 10 times. The strength of the seed coat was measured on an MTS 02 (Aviko Praha, Praha, Czech Republic), which measures the force developed by the instrument to crack the seed coat [N]. For each species, 10 seeds were measured.
2.4. Chemical Analysis of Seeds
Seeds were subjected to detailed chemical analysis, which differed in the targeted groups of compounds and methods used to detect them. The targeted groups of compounds were considered to be perceived by carabids either from a distance or during handling seeds and included surface waxes, amino acids, and volatile compounds.
Fatty acids from the ground seeds (total fatty acids) as well as from seed surfaces were isolated and derivatized into corresponding volatile methyl esters and then quantified via gas chromatography–mass spectrometry (GC–MS) [60,61,62]. The isolation protocol was optimised for a small-scale experiment using ~50 mg of seeds for surface fatty acids and ~25 mg of seeds for total fatty acids. After isolation with a chloroform:methanol (2:1) mixture, the fatty acids were trans-esterified with a sodium methoxide solution into corresponding methyl esters and then extracted into n-hexane. The solvent was then removed under reduced pressure. Dry samples were dissolved in n-hexane containing 0.1% n-undecane as an internal standard for normalization of chromatographic conditions. All samples were analysed in triplicates. Identification and quantification of fatty acid methyl esters (FAMEs) in seed samples was accomplished via an internal standard calibration curve for 35 FAMEs (Supelco, Darmstadt, Germany). The single ion monitoring (SIM) mode was used for identification and quantification of each particular analyte.
Other surface compounds (waxes, alkanes, phytosterols, etc.) were isolated by dipping 50 mg of intact seeds into chloroform for 30 s [45]. After filtration, chloroform was evaporated under reduced pressure, and isolated compounds were dissolved into n-hexane containing 0.1% n-undecane as the internal standard for normalization of chromatographic conditions. Each seed sample was analysed in triplicate, and the results are presented as the percentage content of chloroform soluble surface compounds.
The volatile compounds from plant seeds were isolated and detected by the static headspace technique. For the analysis, sets of dry and imbibed seeds were used. Imbibed seeds produce other chemical compounds because of the start of the chemical processes during germination. The dry seeds were stored in the freezer. The imbibed seeds (0.5 g of each species) were incubated for 24 h at 25 °C before measurement. Volatile compounds released by seeds were pre-concentrated during incubation into headspace vials, and, therefore, we were able to detect them via a common GC–MS platform.
2.5. Ecology and Taxonomy of Plants
Information on plant ecology (annual, biennial, annual-biennial and perennial) and taxonomic placement were determined from the literature [58,63].
2.6. Data Analyses
The multiple regression on distance matrices (MRM) approach (ecodist package [64] for R version 3.4.1 [65]) was used for data analysis. MRM was preferred over other methods because it allows the regression of a response matrix on multiple explanatory matrices [66]. Raw matrices were created according to the nature of the data and possible correlation between the variables, presumed mechanisms behind the expected influence on the preferences, and methods used to generate them. Most of the available data were formed as two-dimensional matrices with seed species as rows and measured quantities as columns. We considered the following matrices for the initial exploration: carabid preferences (response matrix), seed mass (mass of 100 seeds in grams), seed dimensions (dimensions of the seed on axes A, B, and C), seed shape (indices of seed shape, flatness, eccentricity and volume), seed coat (seed coat thickness and strength), plant taxonomy (family of plants), plant strategy, volatile compounds from dry seeds, volatile compounds from imbibed seeds, fatty acids from seed surface, total fatty acids, and other surface compounds. Before the MRM approach can be applied, raw data matrices must be converted into distance matrices using the vegan package [67]. Bray–Curtis dissimilarities were used to convert the seed preference matrix because consumption was standardised on a scale of 1 to 0. The Mahalanobis distance was used for matrices of seed dimensions, seed coat, surface fatty acids, total fatty acids, volatile compounds from dry seed, volatile compounds from imbibed seeds and other surface compounds because these factors contain continuous numerical variables. The data matrices of seed mass, taxonomy and plant ecology were transformed to distance matrices by using the specified distance measurement. Prior to the analysis, the correlation between the dissimilarity matrices was explored by using Mantel’s permutation test for similarity of two matrices (999 permutations). The following matrices were excluded since they showed correlation with other matrices: seed shape (with seed dimensions; p > 0.001), volatile compounds from dry seeds (with volatile compounds from imbibed seeds; p > 0.009), and fatty acids from seed surface (with other surface compounds and taxonomy; p > 0.011). The distance matrix for carabid preferences was used as a response, and the following distance matrices were used as explanatory terms: seed dimensions, seed mass, seed coat, taxonomy, plant strategy, total fatty acids, other surface compounds and volatile compounds from imbibed seeds. Three different models were fitted that differed according to the carabid preference distance matrix: (i) one model was based on the full set of 37 carabid species, (ii) another model was calculated only for the species of Harpalini (15 species), and (iii) the final model was calculated only for the species of Zabrini (18 species). The variances with associated p-values from the multiple regressions were obtained using Legendre et al. (1994)’s permutation test with 9999 permutations [68]. The level of significance to reject the null hypothesis was set to α < 0.1.
3. Results
3.1. Preferences of Carabids
Seed consumption varied among the species of carabids [7]. The highest preferences by Harpalini were on seeds of Cirsium arvense, Viola arvensis, and Cichorium intybus, while tribe Zabrini preferred seeds of Taraxacum officinale, Tripleurospermum inodorum, or Crepis biennis. The small seeds of Brassicaceae were preferred by small carabids of both tribes. The standardized consumption of all species is in Table S1.
3.2. Morphological Analysis of Seeds
The seed mass of 100 seeds ranged from 0.08 g (Potentilla argentea L.) to 8.72 g (Arctium lappa L.) (Table S2). The seed dimensions were diverse and ranged from 9.076 ± 1.264 mm (dimension A of Bidens tripartita L.) to 0.272 ± 0.372 mm (dimension C of Arenaria serpyllifolia agg.). The shape index ranged from 0.171 ± 0.002 (B. tripartita) to 0.005 ± 0.003 (Fumaria officinalis L.). The flatness index ranged from 13.496 ± 0.73 (B. tripartita L.) to 1.144 ± 0.052 (F. officinalis L.) (Table S2). Eccentricity ranged from 6.319 ± 0.322 (Crepis biennis L.) to 1.03 ± 0.012 (Stellaria media (L.) Vill.). The volume ranged from 16.932 ± 0.926 mm3 (A. lappa) to 0.058 ± 0.006 mm3 (A. serpyllifolia) (Table S2). The strength of the seed coat varied among the species and families as well. The seeds of Galium aparine L required the greatest amount of power (99.47 ± 16.818 N) to crush the seed coat, and Urtica dioica L. required the least amount of power to crush the seed coat (1.14 ± 0.533 N). The seed coat thickness ranged from 0.138 ± 0.043 mm (A. serpyllifolia) to 0.017 ± 0.006 mm (G. aparine) (Table S2).
3.3. Chemical Analyses of Seeds
The majority of the 35 fatty acids from the FAME standard mixture was detected in the analysed seeds (Tables S3 and S4). The greatest concentration of all fatty acids was found in the seeds of Galinsoga parviflora Cav. (467.75 ± 8.40 mg/g dry weight), while the lowest concentration was extracted from G. aparine (33.39 ± 1.26 mg/g DW). The major fatty acid in all seeds analysed was unsaturated linoleic acid, which accounted for ~50% of the total fatty acids quantified. The composition of the surface fatty acids varied between the species more than the composition of total fatty acids (Tables S3 and S4). The highest content of the sum of all surface fatty acids was found in the seeds of Cirsium arvense (114.12 ± 3.21 mg/g DW). Other species had a lower content of surface fatty acids. The lowest amount of all surface fatty acids (6.07 ± 0.32 mg/g DW) was found in Amaranthus retroflexus L. (Table S4). Some of the fatty acids were found just in one species (e.g., cis-5, 8, 11, 14, 17-Eicosapentanoic acid in Lapsana communis L.).
Thirteen volatile compounds were detected in seeds (Tables S5 and S6). The amounts of volatile compounds varied between dry and imbibed seeds. The highest amount of volatile compounds was found in the seeds of Sisymbrium loeselii L. (4.8% of determined volatiles); while no volatile compounds were detected in A. retroflexus (Table S5). The highest amounts of volatile compounds were found in seeds of S. loeselii (4.83% of determined volatiles), and the lowest amount was found in seeds of A. retroflexus, where 0.03% of volatiles were detected (Table S6).
Nineteen other surface compounds (Table S7) were detected in the seeds including long-chain alkanes or their branched counterparts, with significant amounts of phytosterols, such as β-sitosterol, were detected. The composition of the other surface compounds also varied between the species.
3.4. Relationships among Carabid Preferences and Seed Properties
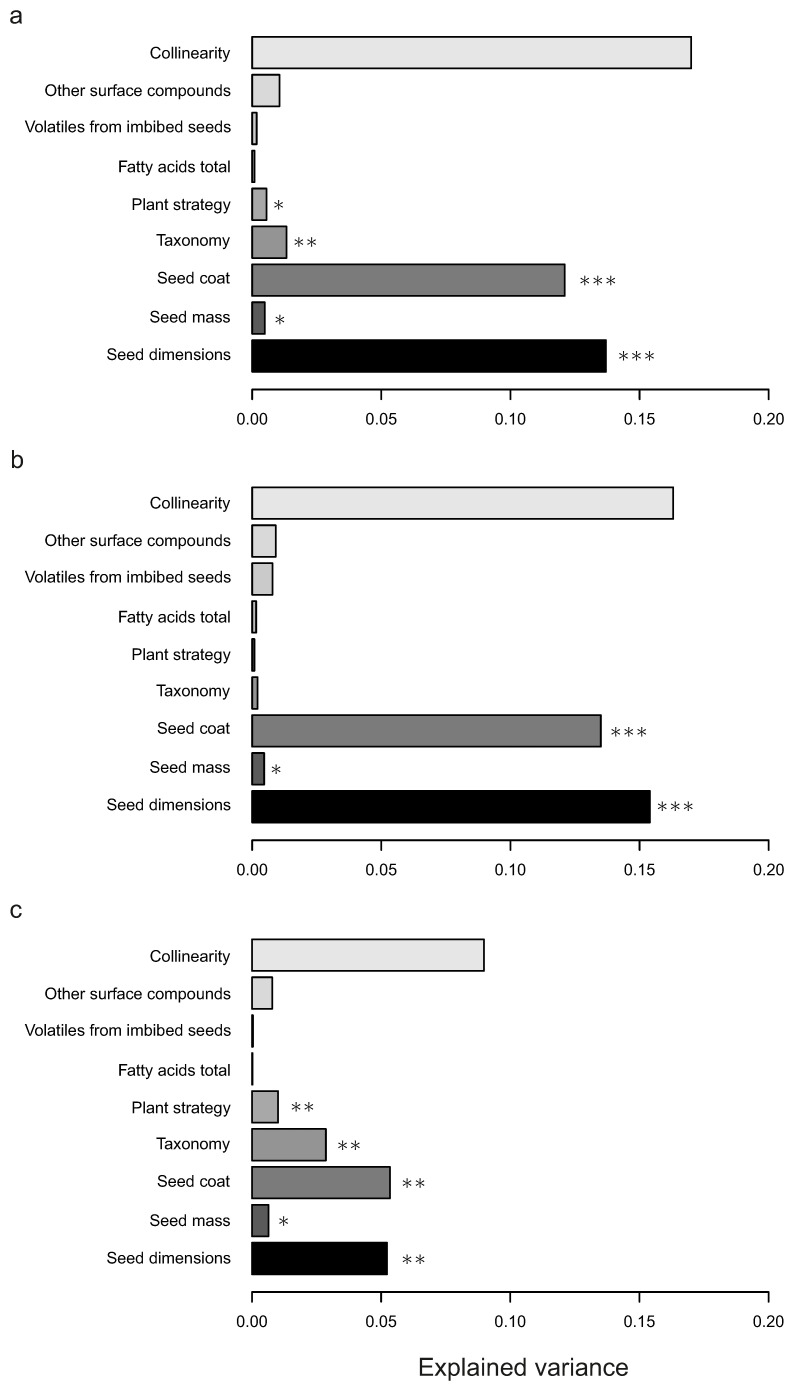
The full model on standardized consumption included matrices on seed mass, seed dimensions, seed coat, seed taxonomy, plant strategy, other surface compounds, total fatty acids, and volatiles released from imbibed seeds. The model explained the variation in consumption across the range of carabid species (R2 = 0.465, p = 0.001; Figure 1a), with the following matrices contributing significantly (at the level of α = 0.1) to the explained variance: seed dimensions (p < 0.001), seed coat (p < 0.001), taxonomy (p = 0.035), seed mass (p = 0.054), and plant strategy (p = 0.058).
Figure 1.
Contribution of the matrices of seed traits to seed preferences of carabid beetles based on a multiple regression on distance matrices (MRM) approach. The horizontal bars indicate the proportion of explained variance by a particular variable in the data. The collinearity shows part of the variation explained by the model, but which cannot be attributed solely to any of the single factors. (a) Full set of the 37 species of carabids (proportion of explained variance, R2 = 0.465, p = 0.01). (b) Species of the tribe Harpalini (R2 = 0.477, p = 0.001). (c) Species of the tribe Zabrini (R2 = 0.248, p = 0.001); * p < 0.1, ** p < 0.05, *** p < 0.001.
However, by re-running the analysis separately for the two major taxonomic groups of carabids, Zabrini and Harpalini, we found specific responses. The model for Harpalini fit the data comparably well to the global one (R2 = 0.477, p = 0.001; Figure 1b), with the following matrices contributing significantly: seed dimensions (p < 0.001), seed coat (p < 0.001) and seed mass (p = 0.062). In no model did seed phytochemistry significantly influence the seed preferences of the carabid beetles included in this study. The model for Zabrini had much lower explanatory power (R2 = 0.248, p = 0.001; Figure 1c), with the following matrices contributing significantly: seed coat (p = 0.002), seed dimensions (p = 0.005), taxonomy (p = 0.005), plant strategy (p = 0.036), and seed mass (p = 0.075).
4. Discussion
Seed properties, such as seed dimensions, mass, taxonomy, plant strategy, and physical seed coat traits were the most important properties affecting the preferences of carabid beetles in this study. The seed dimensions explaining over 13% of the preferences was the main factor affecting seed selection [6,33,34], even when other properties are considered. The interaction between seed size and the mass of carabids should not, however, be overlooked [6,7,53]. The size of the seeds also affects their chemical properties, such as the oil content or stored energy [55], which may affect seed predation.
The properties of the seed coat were also important because the seed coat protects seeds against predators. To open seeds, many species of carabids have evolved broad mandibles with large adductors [69] and bases that are generally triangular. The shape of mandibles varies among the tribes. Species of Harpalini have more asymmetrical mandibles than those of Zabrini. Quadrate mandibles with broadly rounded incisors and a basal face suggest an omnivorous diet in most Harpalini [70]. Species of the tribe Zabrini have short, square-shaped mandibles that are blunt at the tips, and are more adapted for crushing hard seeds [8]. This can explain why the seed coat properties were important properties for seed preference by Harpalini (13% of explained variance) but less so for Zabrini (5% of explained variance).
Seed preferences by species of the studied Zabrini species were related to seed taxonomy, which probably drove the significant response for the entire species set because for the tribe Harpalini, seed properties related to their taxonomy did not appear to be a significant determinant of preferences. This result is in line with the previous findings that Harpalini are less specialised than Zabrini [6]. Our results suggest that the seed traits to which the carabid seed predators respond may be species- or higher taxon-specific and perhaps dependent on the degree of carabid specialisation for seeds. Since Zabrini species are more specialised [6,71] to a narrower range of seeds, often with the same ecology or from the same family, the variables that appeared to be the most influential for preference determination were unexpected. On the other hand, Harpalini species (Figure 1b) are more generalists; therefore, it is ecologically sound that seed mass and dimensions would be the major variable explaining the variation among the matrices of traits for this tribe.
Seed chemistry did not seem to play a crucial role in seed selection by carabid beetles. Although other studies [16,24] have determined that volatiles originating from seeds can attract seed predators, our data do support these observations. There may be several reasons for this lack of support. The seeds used in the multi-choice experiment were dried and mounted on modelling clay [6], which could have limited the amount of volatiles released from the seeds [28,29] compared to those present on the soil surface. The other reason for this difference could be due the cold storage of seeds prior to seed preference assays. Although cold storage does not affect seed viability [72,73], defrosting could have potentially changed the qualitative and quantitative aspects of seed chemical ecology. This needs to be studied. The seeds could have been contaminated by fungi or bacteria [74], which also release their own suite of chemicals. In fact, ethanethiol that was found in the headspace of the tested seeds in our work suggests that some seeds were contaminated, most likely with methanogenic bacteria [75,76]. However, this occurrence should not be considered a problem because in the field, seeds are also colonised by microorganisms [77,78], so the interaction among seeds, microorganisms, and seed predators should be considered a natural component of seed predation and represents an interesting direction for future research.
5. Conclusions
Our data suggest that seed morphological properties are more important than chemical properties in determining the preferences of granivorous carabid beetles. Seed dimensions and seed coat properties were among the most important seed properties affecting carabid preferences. The preferences varied between the taxonomical groups of predators that differ in the degree of specialisation. This paper expands the knowledge on how seed defences influence seed preferences of carabid beetles.
Acknowledgments
We are thankful Jana Kohoutová, Jiří Namyslov, Michaela Broženská, and Alena Dudková for their help with seed processing and measuring. We are also very thankful for Edita Tylová for her supervision and methodological guidance of the microscopic sections of seeds and for Andreas Makiola for his advice on MRM analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/11/11/757/s1, Table S1: Preferences of carabids, Table S2: Morphological properties of the seeds, Table S3: Surface fatty acids from seeds, Table S4: Total fatty acids from seeds, Table S5: Volatiles from imbibed seeds, Table S6: Volatiles from dry seeds, Table S7: Other surface compounds.
Author Contributions
Conceptualization, P.S., A.H., Z.M., and P.T.; methodology, P.S., A.H., Z.M., S.Ć.Z., P.T., and H.F.; validation, P.S., A.H., and P.T.; formal analysis, H.F.; investigation, P.S., A.H., H.F., and S.Ć.Z.; resources, P.S., A.H., H.F., and S.Ć.Z.; data collection, P.S., H.F., and S.Ć.Z.; writing—original draft preparation, H.F. and P.S.; writing—review and editing, P.S., S.Ć.Z., P.T., A.H., and Z.M.; visualization, H.F.; supervision, P.S.; project administration, P.S.; and funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Czech Science Foundation grant #17-00043S and MZE #RO0418.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thiele H.U. Carabid Beetles in Their Environments. Springer Science & Business Media; Berlin, Germany: 1977. [Google Scholar]
- 2.Hůrka K. Carabidae of the Czech and Slovak Republics. Carabidae České a Slovenské Republiky. Kabourek; Zlín, Czech Republic: 1996. p. 565. [Google Scholar]
- 3.Frei B., Guenay Y., Bohan D.A., Traugott M., Wallinger C. Molecular analysis indicates high levels of carabid weed seed consumption in cereal fields across Central Europe. J. Pest Sci. 2019;92:935–942. doi: 10.1007/s10340-019-01109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundgren J.G., Saska P., Honek A. Molecular approach to describing a seed-based food web: The post-dispersal granivore community of an invasive plant. Ecol. Evol. 2013;3:1642–1652. doi: 10.1002/ece3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundgren J.G., Rosentrater K.A. The strength of seeds and their destruction by granivorous insects. Arthropod-Plant Interact. 2007;1:93–99. doi: 10.1007/s11829-007-9008-1. [DOI] [Google Scholar]
- 6.Honek A., Martinkova Z., Saska P., Pekar S. Size and taxonomic constraints determine the seed preferences of Carabidae (Coleoptera) Basic Appl. Ecol. 2007;8:343–353. doi: 10.1016/j.baae.2006.07.002. [DOI] [Google Scholar]
- 7.Saska P., Honek A., Martinkova Z. Preferences of carabid beetles (Coleoptera: Carabidae) for herbaceous seeds. Acta Zool. Acad. Sci. Hung. 2019;65:57–76. doi: 10.17109/AZH.65.Suppl.57.2019. [DOI] [Google Scholar]
- 8.Forsythe T.G. Feeding Mechanisms of Certain Ground Beetles (Coleoptera: Carabidae) Coleopt. Bull. 1982;36:26–73. [Google Scholar]
- 9.Honek A., Saska P., Martinkova Z. Seasonal variation in seed predation by adult carabid beetles. Entomol. Exp. Appl. 2006;118:157–162. doi: 10.1111/j.1570-7458.2006.00376.x. [DOI] [Google Scholar]
- 10.Dalling J.W., Davis A.S., Schutte B.J., Arnold A.E. Seed survival in soil: Interacting effects of predation, dormancy and the soil microbial community. J. Ecol. 2011;99:89–95. doi: 10.1111/j.1365-2745.2010.01739.x. [DOI] [Google Scholar]
- 11.Mazer S.J. Rainforest plants protect their investments. Trends Ecol. Evol. 1998;13:471–473. doi: 10.1016/s0169-5347(98)01487-6. [DOI] [PubMed] [Google Scholar]
- 12.Saska P., Foffová H., Martinková Z., Honěk A. Persistence and Changes in Morphological Traits of Herbaceous Seeds Due to Burial in Soil. Agronomy. 2020;10:448. [Google Scholar]
- 13.Maureaud A., Andersen K.H., Zhang L., Lindegren M. Trait-based food web model reveals the underlying mechanisms of biodiversity-ecosystem functioning relationships. J. Anim. Ecol. 2020;89:1497–1510. doi: 10.1111/1365-2656.13207. [DOI] [PubMed] [Google Scholar]
- 14.Zaguri M., Hawlena D. Odours of non-predatory species help prey moderate their risk assessment. Funct. Ecol. 2020;34:830–839. doi: 10.1111/1365-2435.13509. [DOI] [Google Scholar]
- 15.Antiqueira P.A.P., de Omena P.M., Goncalves-Souza T., Vieira C., Migliorini G.H., Kersch-Becker M.N.F., Bernabe T.N., Recalde F.C., Benavides-Gordillo S., Romero G.Q. Precipitation and predation risk alter the diversity and behavior of pollinators and reduce plant fitness. Oecologia. 2020;192:745–753. doi: 10.1007/s00442-020-04612-0. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni S.S., Dosdall L.M., Spence J.R., Willenborg C.J. Seed Detection and Discrimination by Ground Beetles (Coleoptera: Carabidae) Are Associated with Olfactory Cues. PLoS ONE. 2017;12:11. doi: 10.1371/journal.pone.0170593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law J.J., Gallagher R.S. The role of imbibition on seed selection by Harpalus pensylvanicus. Appl. Soil Ecol. 2015;87:118–124. doi: 10.1016/j.apsoil.2014.11.015. [DOI] [Google Scholar]
- 18.Niu H.Y., Chu W., Yi X.F., Zhang H.M. Visual and auditory cues facilitate cache pilferage of Siberian chipmunks (Tamias sibiricus) under indoor conditions. Integr. Zool. 2019;14:354–365. doi: 10.1111/1749-4877.12373. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro J.F., Vieira E.M. Microhabitat selection for caching and use of potential landmarks for seed recovery by a neotropical rodent. J. Zool. 2016;300:274–280. doi: 10.1111/jzo.12380. [DOI] [Google Scholar]
- 20.Griffin C.A.M., Thaler J.S. Insect predators affect plant resistance via density- and trait-mediated indirect interactions. Ecol. Lett. 2006;9:335–343. doi: 10.1111/j.1461-0248.2005.00880.x. [DOI] [PubMed] [Google Scholar]
- 21.DeAngelis K.M. Chemical communication connects soil food webs. Soil Biol. Biochem. 2016;102:48–51. doi: 10.1016/j.soilbio.2016.06.024. [DOI] [Google Scholar]
- 22.Paulsen T.R., Colville L., Kranner I., Daws M.I., Hogstedt G., Vandvik V., Thompson K. Physical dormancy in seeds: A game of hide and seek? New Phytol. 2013;198:496–503. doi: 10.1111/nph.12191. [DOI] [PubMed] [Google Scholar]
- 23.Kielty J.P., AllenWilliams L.J., Underwood N., Eastwood E.A. Behavioral responses of three species of ground beetle (Coleoptera: Carabidae) to olfactory cues associated with prey and habitat. J. Insect Behav. 1996;9:237–250. doi: 10.1007/BF02213868. [DOI] [Google Scholar]
- 24.Thomas R.S., Glen D.M., Symondson W.O.C. Prey detection through olfaction by the soil-dwelling larvae of the carabid predator Pterostichus melanarius. Soil Biol. Biochem. 2008;40:207–216. doi: 10.1016/j.soilbio.2007.08.002. [DOI] [Google Scholar]
- 25.Thoming G., Solhaug K.A., Norli H.R. Kairomone-assisted trap cropping for protecting spring oilseed rape (Brassica napus) from pollen beetles (Coleoptera: Nitidulidae) Pest Manag. Sci. 2020;11 doi: 10.1002/ps.5882. [DOI] [PubMed] [Google Scholar]
- 26.Ma C., Cui S.W., Bai Q., Tian Z.Y., Zhang Y., Chen G.M., Gao X.Y., Tian Z.Q., Chen H.S., Guo J.Y., et al. Olfactory co-receptor is involved in host recognition and oviposition in Ophraella communa (Coleoptera: Chrysomelidae) Insect Mol. Biol. 2020;10 doi: 10.1111/imb.12643. [DOI] [PubMed] [Google Scholar]
- 27.Baskin C.C., Baskin J.M. Seeds, Ecology, Biogeography, and Evolution of Dormancy and Germination. Elsevier; San Diego, CA, USA: 1998. [Google Scholar]
- 28.Linton C.J., Wright S.J.L. Volatile organic-compounds—Mictrobiologicak aspects and some technological implications. J. Appl. Bacteriol. 1993;75:1–12. doi: 10.1111/j.1365-2672.1993.tb03400.x. [DOI] [Google Scholar]
- 29.Mattoo A.K., Suttle J.C. Plant Hormone Ethylene. CRC Press; Boca Raton, FL, USA: 1991. p. 352. [Google Scholar]
- 30.Honek A., Martinkova Z., Saska P. Effect of size, taxonomic affiliation and geographic origin of dandelion (Taraxacum agg.) seeds on predation by ground beetles (Carabidae, Coleoptera) Basic Appl. Ecol. 2011;12:89–96. doi: 10.1016/j.baae.2010.11.003. [DOI] [Google Scholar]
- 31.Moles A.T., Warton D.I., Westoby M. Do small-seeded species have higher survival through seed predation than large-seeded species? Ecology. 2003;84:3148–3161. doi: 10.1890/02-0662. [DOI] [Google Scholar]
- 32.Honek A., Martinkova Z., Jarosik V. Ground beetles (Carabidae) as seed predators. Eur. J. Entomol. 2003;100:531–544. doi: 10.14411/eje.2003.081. [DOI] [Google Scholar]
- 33.Forsythe T.G. Locomotion in ground beetles (Coleptera: Carabidae)—An interpretation of leg structure in functional terms. J. Zool. 1983;200:493–507. [Google Scholar]
- 34.Acorn J.H., Ball G.E. The mandibles of some adult ground beetles—Structure, function, and the evolution of herbivory (Coleptera: Carabidae) Can. J. Zool. Rev. Can. Zool. 1991;69:638–650. doi: 10.1139/z91-094. [DOI] [Google Scholar]
- 35.Brown J.S., Venable D.L. Evolutionary ecology of seed-bank annuals in temporally varying environments. Am. Nat. 1986;127:31–47. doi: 10.1086/284465. [DOI] [Google Scholar]
- 36.Feeny P.P. Plant apparency and chemical defense. Recent Adv. Phytochem. 1976;10:1–40. [Google Scholar]
- 37.Thompson K., Band S.R., Hodgson J.G. Seed size and shape predict persistence in soil. Funct. Ecol. 1993;7:236–241. doi: 10.2307/2389893. [DOI] [Google Scholar]
- 38.Bekker R.M., Bakker J.P., Grandin U., Kalamees R., Milberg P., Poschlod P., Thompson K., Willems J.H. Seed size, shape and vertical distribution in the soil: Indicators of seed longevity. Funct. Ecol. 1998;12:834–842. doi: 10.1046/j.1365-2435.1998.00252.x. [DOI] [Google Scholar]
- 39.Thompson K., Bakker J., Bekker R. The Soil Seed Banks of North West Europe: Methodology, Destiny and Longevity. Cambridge University Press; Cambridge, UK: 1997. p. 276. [Google Scholar]
- 40.Azcarate F.M., Arqueros L., Sanchez A.M., Peco B. Seed and fruit selection by harvester ants, Messor barbarus, in Mediterranean grassland and scrubland. Funct. Ecol. 2005;19:273–283. doi: 10.1111/j.0269-8463.2005.00956.x. [DOI] [Google Scholar]
- 41.Benvenuti S. Natural weed seed burial: Effect of soil texture, rain and seed characteristics. Seed Sci. Res. 2007;17:211–219. doi: 10.1017/S0960258507782752. [DOI] [Google Scholar]
- 42.Bewley J.D., Black M.J.B. Physiology and Biochemistry of Seeds in Relation to Germination. Springer; Berlin, Germany: 1982. p. 306. [Google Scholar]
- 43.Lanza J., Schmitt M.A., Awad A.B. Comparative chemistry of elaiosomes of 3 species of Trillium. J. Chem. Ecol. 1992;18:209–221. doi: 10.1007/BF00993754. [DOI] [PubMed] [Google Scholar]
- 44.Eigenbrode S.D., Jetter R. Attachment to plant surface waxes by an insect predator. Integr. Comp. Biol. 2002;42:1091–1099. doi: 10.1093/icb/42.6.1091. [DOI] [PubMed] [Google Scholar]
- 45.Shao S.Q., Meyer C.J., Ma F.S., Peterson C.A., Bernards M.A. The outermost cuticle of soybean seeds: Chemical composition and function during imbibition. J. Exp. Bot. 2007;58:1071–1082. doi: 10.1093/jxb/erl268. [DOI] [PubMed] [Google Scholar]
- 46.Mohamedyasseen Y., Barringer S.A., Splittstoesser W.E., Costanza S. The role of seed coats in seed viability. Bot. Rev. 1994;60:426–439. doi: 10.1007/BF02857926. [DOI] [Google Scholar]
- 47.van der Meij M.A.A., Bout R.G. Seed selection in the Java Sparrow (Padda oryzivora): Preference and mechanical constraint. Can. J. Zool. Rev. Can. Zool. 2000;78:1668–1673. doi: 10.1139/z00-114. [DOI] [Google Scholar]
- 48.Rodgerson L. Mechanical defense in seeds adapted for ant dispersal. Ecology. 1998;79:1669–1677. doi: 10.1890/0012-9658(1998)079[1669:MDISAF]2.0.CO;2. [DOI] [Google Scholar]
- 49.Benkman C.W. The impact of tree squirrels (Tamiasciurus) on Limber pine seed dispersal adaptations. Evolution. 1995;49:585–592. doi: 10.2307/2410312. [DOI] [PubMed] [Google Scholar]
- 50.Davis A.S., Schutte B.J., Iannuzzi J., Renner K.A. Chemical and physical defense of weed seeds in relation to soil seedbank persistence. Weed Sci. 2008;56:676–684. doi: 10.1614/WS-07-196.1. [DOI] [Google Scholar]
- 51.Janzen D.H. Seed predation by animals. Curr. Contents/Agric. Biol. Environ. Sci. 1982:465–492. doi: 10.1146/annurev.es.02.110171.002341. [DOI] [Google Scholar]
- 52.Hulme P.E. Herbivory, plant regeneration, and species coexistence. J. Ecol. 1996;84:609–615. doi: 10.2307/2261482. [DOI] [Google Scholar]
- 53.Gaba S., Deroulers P., Bretagnolle F., Bretagnolle V. Lipid content drives weed seed consumption by ground beetles (Coleopterea, Carabidae) within the smallest seeds. Weed Res. 2019;59:170–179. doi: 10.1111/wre.12354. [DOI] [Google Scholar]
- 54.Grubb P.J., Metcalfe D.J., Grubb E.A.A., Jones G.D. Nitrogen-richness and protection of seeds in Australian tropical rainforest: A test of plant defence theory. Oikos. 1998;82:467–482. doi: 10.2307/3546368. [DOI] [Google Scholar]
- 55.Bretagnolle F., Matejicek A., Gregoire S., Reboud X., Gaba S. Determination of fatty acids content, global antioxidant activity and energy value of weed seeds from agricultural fields in France. Weed Res. 2016;56:78–95. doi: 10.1111/wre.12188. [DOI] [Google Scholar]
- 56.Pond C.M. Ecology of Storage. In: Levin S.M., editor. Encyclopedia of Biodiversity. Academic Press; New York, NY, USA: 2013. p. 5504. [Google Scholar]
- 57.Moles A.T., Westoby M. Seed size and plant strategy across the whole life cycle. Oikos. 2006;113:91–105. doi: 10.1111/j.0030-1299.2006.14194.x. [DOI] [Google Scholar]
- 58.Kubát K., Hrouda L., Chrtek J., Kaplan Z., Kirschner, Štěpánek J. Klíč ke Květeně České Republiky. Academia; Praha, Czech Republic: 2002. [Google Scholar]
- 59.Cerda A., Garcia-Fayos P. The influence of seed size and shape on their removal by water erosion. Catena. 2002;48:293–301. doi: 10.1016/S0341-8162(02)00027-9. [DOI] [Google Scholar]
- 60.Carvalho A.P., Malcata F.X. Preparation of fatty acid methyl esters for gas-chromatographic analysis of marine lipids: Insight studies. J. Agric. Food Chem. 2005;53:5049–5059. doi: 10.1021/jf048788i. [DOI] [PubMed] [Google Scholar]
- 61.Stroescu M., Stoica-Guzun A., Ghergu S., Chira N., Jipa I. Optimization of fatty acids extraction from Portulaca oleracea seed using response surface methodology. Ind. Crops Prod. 2013;43:405–411. doi: 10.1016/j.indcrop.2012.07.051. [DOI] [Google Scholar]
- 62.Mira S., Hill L.M., Gonzalez-Benito M.E., Ibanez M.A., Walters C. Volatile emission in dry seeds as a way to probe chemical reactions during initial asymptomatic deterioration. J. Exp. Bot. 2016;67:1783–1793. doi: 10.1093/jxb/erv568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bojnanský V., Fargašová A. Atlas of Seeds and Fruits of Central and East-European Flora. Springer; Dordrecht, The Netherlands: 2007. p. 1046. [Google Scholar]
- 64.Goslee S.C., Urban D.L. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 2007;22:1–19. doi: 10.18637/jss.v022.i07. [DOI] [Google Scholar]
- 65.R Core Team R: A Language and Environment for Statistical Computing. [(accessed on 3 November 2020)]; Available online: https://www.R-project.org/
- 66.Lichstein J.W. Multiple regression on distance matrices: A multivariate spatial analysis tool. Plant Ecol. 2007;188:117–131. doi: 10.1007/s11258-006-9126-3. [DOI] [Google Scholar]
- 67.Oksanen J., Blanchet F.G., Kindt R., Legendre P., O’Hara R.B., Simpson G.L., Solymos P., Stevens M.H.H., Szoecs E., Wagner H. Vegan: Community Ecology Package. R Package Version 1.17–10. [(accessed on 4 November 2020)];2017 Available online: https://CRAN.R-project.org/package=vegan.
- 68.Legendre P., Lapointe F.J., Casgrain P. Modeling brain evolution from behavior—A permutational regretion approach. Evolution. 1994;48:1487–1499. doi: 10.1111/j.1558-5646.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 69.Paarmann W., Faust N., Arndt E., Luchtrath I., Rohe W. Constant seed size and mandible growth—A fundamental problem for granivorous ground beetle larvae (Coleoptera: Carabidae) Entomol. Fenn. 2006;17:334–339. doi: 10.33338/ef.84353. [DOI] [Google Scholar]
- 70.Larochelle A. The Food of Carabid Beetles: (Coleoptera: Carabidae, Including Cicindelinae) Association des Entomologistes du Québec; Varennes, QC, Canada: 1990. p. 132. [Google Scholar]
- 71.Zetto-Brandmayr T. Spermophilus (Seed-Eating) Ground Beetles: First Comparison of the Diet and Ecology of the Harpaline General Harpalus and Ophonus (Col., Carabidae) In: Stork N., editor. The Role of Ground Beetles in Ecological and Environmental Studies. Intercept; Andover, UK: 1990. pp. 307–316. [Google Scholar]
- 72.Jordan L.S., Jordan J.L. Effects of prechilling on Convolvulus arvensis L. seed coat and germination. Ann. Bot. 1982;49:421–423. doi: 10.1093/oxfordjournals.aob.a086266. [DOI] [Google Scholar]
- 73.Eynard I. Herbage Abstracts. Volume 28. CAB International; Wallingford, UK: 1958. The Effect of Very Low Temperatures on Germination of Hard Seeds; p. 1027. [Google Scholar]
- 74.Raza W., Ling N., Liu D.Y., Wei Z., Huang Q.W., Shen Q.R. Volatile Organic compounds produced by Pseudomonas fluorescens WR-1 restrict the growth and virulence traits of Ralstonia solanacearum. Microbiol. Res. 2016;192:103–113. doi: 10.1016/j.micres.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 75.Rajagopal B.S., Daniels L. Invertigation of Mercaptans, organic sulfides, and inorganic sulfur compounds as sulfur sources for the growth of methanogenic bacteria. Curr. Microbiol. 1986;14:137–144. doi: 10.1007/BF01568365. [DOI] [Google Scholar]
- 76.Schulz S., Dickschat J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007;24:814–842. doi: 10.1039/b507392h. [DOI] [PubMed] [Google Scholar]
- 77.Nelson E.B. The seed microbiome: Origins, interactions, and impacts. Plant Soil. 2018;422:7–34. doi: 10.1007/s11104-017-3289-7. [DOI] [Google Scholar]
- 78.Truyens S., Weyens N., Cuypers A., Vangronsveld J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015;7:40–50. doi: 10.1111/1758-2229.12181. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.