Abstract
Introduction
Inappropriate antimicrobial use increases the prevalence of antimicrobial-resistant bacteria. Surgeons are reluctant to implement recommendations of guidelines in clinical practice. Antimicrobial stewardship (AMS) is effective in antimicrobial management, but it remains labour intensive. The computerised decision support system (CDSS) has been identified as an effective way to enable key elements of AMS in clinical settings. However, insufficient evidence is available to evaluate the efficacy of computerised AMS in surgical settings.
Methods and analysis
The Evaluate of the Potential Impact of Computerised AMS trial is an open-label, single-centre, two-arm, cluster-randomised, controlled trial, which aims to determine whether a multicomponent CDSS intervention reduces overall antimicrobial use after cardiovascular surgeries compared with usual clinical care in a specialty hospital with a big volume of cardiovascular surgeries. Eighteen cardiovascular surgical teams will be randomised 1:1 to either the intervention or the control arm. The intervention will consist of (1) re-evaluation alerts and decision support for the duration of antimicrobial treatment decision, (2) re-evaluation alerts and decision support for the choice of antimicrobial, (3) quality control audit and feedback. The primary outcome will be the overall systemic antimicrobial use measured in days of therapy (DOT) per admission and DOT per 1000 patient-days over the whole intervention period (6 months). Secondary outcomes include a series of indices to evaluate antimicrobial use, microbial resistance, perioperative infection outcomes, patient safety, resource consumption, and user compliance and satisfaction.
Ethics and dissemination
The Ethics Committee in Fuwai Hospital approved this study (2020-1329). The results of the trial will be submitted for publication in a peer-reviewed journal.
Trial registration number
Keywords: cardiac surgery, adult cardiology, clinical trials
Strengths and limitations of this study.
This adequately powered, cluster-randomised controlled trial addresses many inadequacies in designs of the previous studies.
Different from previous studies in terms of the scope, setting and timing, the Evaluate of the Potential Impact of Computerised antimicrobial trial is among the first to assess the impact of computerised decision support system tools on antimicrobial use in hospital settings.
To the best of our knowledge, this trial will be one of the first trials carried out in surgery settings.
This trial is a single centre study which may increase type II error.
Introduction
Antimicrobial drug resistance among common bacterial pathogens has become a global health crisis.1–3 It is reported that more than two million illnesses and 23 000 deaths are caused by antimicrobial-resistant bacteria in the USA in 2017.4 This crisis is even more serious in low-income to middle-income countries.5
Inappropriate antimicrobial use after surgeries increases the prevalence of antimicrobial-resistant bacteria and subsequently unnecessary risk of adverse drug events to patients as well as loads heavy economic burden on the healthcare system.6 7 Despite many published guidelines of antimicrobial use and decades of efforts to change prescribing patterns, a survey revealed that the practice of antimicrobial use varies substantially among surgeons.8 Furthermore, studies have shown that surgeons are reluctant to implement recommendations of guidelines in their regular clinical practice.9 10 Therefore, interventions to standardise surgeons’ practice of antimicrobial use are highly important.
Antimicrobial stewardship (AMS), the primary goal of which is to optimise antimicrobial use, has been proven to be effective to improve surgical outcomes with increasing evidence.11–13 However, as the idea becomes more widespread, implementing AMS remains a big challenge. Most of the AMS interventions require manual assessment and are best served by the expertise of infectious disease physicians or clinical pharmacists. The labour-intensive nature has impeded AMS implementation on a large and sustainable scale.14 15 Under circumstances where the important personnel are not adequate, computerised decision support system (CDSS) has been identified as one way to enable key elements of AMS in clinical settings.
However, little evidence supports the application of CDSS in the AMS system in surgical settings. The controlled before–after and non-randomised study design in the related studies may lead to bias and reduce the validity of causal inference.16 In addition, previous studies mainly focused on the primary care and little high-quality studies assessed the computer-based intervention for the in-hospital antimicrobial use in both surgical and non-surgical settings.17–19 Therefore, based on the moderate-quality evidence in the literature, the 2016 AMS guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America gave ‘weak recommendation’ on the integration of CDSS into AMS programmes.20
To address this evidence gap, we planned to start a cluster-randomised trial in the largest cardiovascular surgery specialty hospital in China. We chose cardiovascular surgery rather than other surgical procedures because surgical site infections (SSIs) associated with cardiovascular surgeries are particularly severe; moreover, cardiovascular surgery-related SSIs are typically associated with skin flora, and thus, the evidence from this population may have significance for other surgical procedures.21–26
The Evaluate the Potential Impact of Computer AMS (EPIC) trial aims to assess if a multicomponent computer-based system incorporated into the workflow will reduce days of therapy (DOT) per admission and DOT per 1000 patient-day after cardiovascular surgeries in the intervention surgical teams compared with the controlled surgical teams, over a 6-month period.
Methods/design
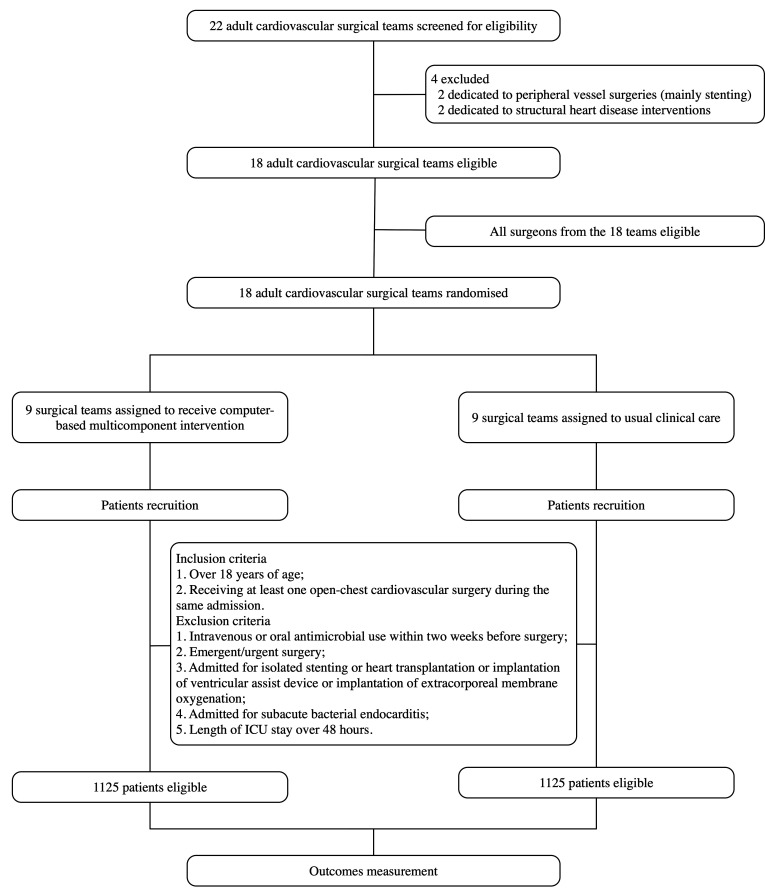
This trial is an open-label, two-arm, cluster-randomised controlled trial with cardiovascular surgical teams as the unit of randomisation (figure 1, flow chart).27 Eligible teams (as defined in the Inclusion/exclusion criteria section) with written consent are randomised to the intervention or control arm by using an interactive web response system. The computer-based, multicomponent intervention targeting the reduction of perioperative antimicrobial use will be delivered to the intervention teams and the control teams will keep the usual clinical care.
Figure 1.
Flow chart of the study design.
A trial steering committee has been set up to monitor the conduct of the trial and the management of the data. Members of the trial steering committee will meet throughout the study period. The committee will include research staff, a clinical pharmacist and two surgeons who are not directly involved in the trial.
Study setting
The study will be launched in Fuwai Hospital, a 1500-bed tertiary care medical centre with an annual cardiovascular surgery volume of approximately 15 000 cases. Twenty-two surgical teams led by paid specialists in Fuwai perform approximately 10 000 various cardiovascular surgeries independently for adult patients (over 18 years old).
Fuwai has deployed an in-house electronic medical record (EMR) system and a computerised physician order entry (CPOE) system since 2009. All surgical teams fulfil the function of medical record management and physician order entry by using the in-house EMR and CPOE systems.
Inclusion/exclusion criteria
At the cluster level, 18 adult cardiovascular surgical teams in Fuwai Hospital will be invited to participate in this trial. Two surgical teams dedicated to peripheral vessel surgeries (mainly stenting) and two dedicated to structural heart disease interventions, which performed operations without opening the chest, are excluded because of their different AMS protocols.
At the physician level, the participants are the surgeons who prescribe antimicrobial to patients in the surgical teams.
At the patient level, the inclusion criteria are: (1) over 18 years of age and (2) receiving at least one open-chest cardiovascular surgery during the same admission. The exclusion criteria are: (1) intravenous or oral antimicrobial use within 2 weeks before surgery; (2) emergent/urgent surgery; (3) admitted for isolated stenting, heart transplantation or implantation of ventricular assist device, or implantation of extracorporeal membrane oxygenation; (4) admitted for subacute bacterial endocarditis and (5) length of ICU stay over 48 hours.
AMS intervention
AMS protocol in Fuwai Hospital
The development of AMS programme in Fuwai Hospital is based on previous guidelines as well as local policies.20 28–31 The programme is multifunctional with the review of all positive blood cultures, regular teaching sessions for physicians and internal/external audit of antimicrobial use and resistance. The programme is regularly updated according to antimicrobial prescribing guidelines.
Briefly, a bundled intervention is implemented in regular workflow and comprises: (1) preoperative screening and decolonisation; (2) an infusion of antimicrobial 30–60 min before incision; (3) intraoperative redosing if the duration of the procedure exceeds 3 hours or two half-lives of the antimicrobial or there is excessive blood loss (mainly aortic surgeries); (4) a duration of antimicrobial prophylaxis less than 48 hours at the postoperative stage and (5) evaluation of microbiological findings, appropriateness of antimicrobial therapy and de-escalation strategies at the postoperative stage.
Computer-based AMS intervention system
The intervention in the EPIC trial targets the control of postoperative antimicrobial use. The development of the computer-based multicomponent intervention is informed by existing medical records, behavioural intervention theory, systematic review evidence, qualitative research with trial and non-trial practices, clinical guidelines and national policies.18–20 28–33
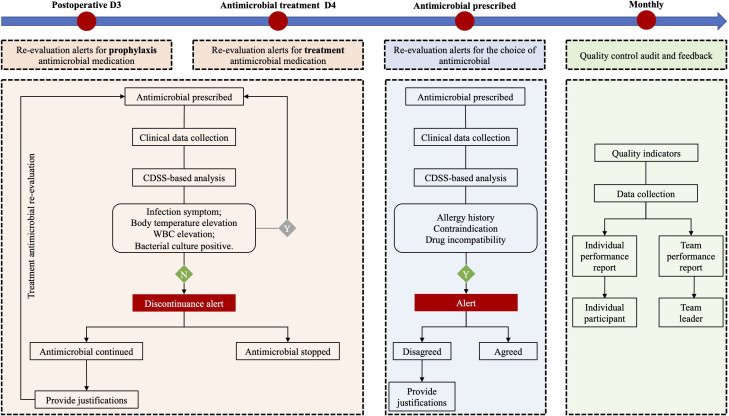
The computer-based AMS intervention system was set up based on the EMR and CPOE systems on the server of Information Centre, which could access all the information from the EMR and CPOE systems in real time. The computer-based evaluation will be activated at the time of the entry of antimicrobial order in the CPOE system. Popup banners, in a man-machine interactive manner, will appear in the centre of the screen to inform the physicians if violation against AMS rules is detected. General information about AMS rules will be provided as information buttons on the lower right corner of the screen. The interventions function in three domains (figure 2):
Figure 2.
The multicomponent, computer-based interventions in the Evaluate the Potential Impact of Computer trial. CDSS, computerised decision support system. WBC, white blood cell.
-
Re-evaluation alerts and decision support for the duration of antimicrobial treatment:
For prophylaxis use:
On postoperative calendar day 3, a visual alert will routinely appear on the CPOE screen to remind the physicians to stop antimicrobial prophylaxis.
To be noted, the system will assess patient-specific data such as clinical manifestations, routine blood tests, chest X-ray, microbiological results and use of other medications within the first two postoperative days. If there are no signs of infection, discontinuance reminder will appear even if the duration of the antimicrobial prophylaxis treatment doesn’t reach in 2 days.
For treatment use:
The same method for postoperative antimicrobial treatment (with signs of postoperative infection) will be applied. Alert will appear on the calendar day 6 of the treatment. Discontinuance alert, on the basis of clinical data, will appear on any day before calendar day 6 if there are no signs of infection.
If the antimicrobial treatment is modified before calendar day 6, the system will assume to set up a re-evaluation and no alert will be displayed on day 6.
If the alerts mentioned above are ignored and the antimicrobial treatment is continued, physicians will be asked to provide accountable justifications. The options for justifications include prophylaxis, empirical and targeted treatment; as for targeted treatment, a predefined list of potential reasons will be provided with the availability to also enter free text, making it possible to assess prescribing quality and to provide specific decision supports.
-
Re-evaluation alerts and decision support for the choice of antimicrobial:
Physicians will be asked to select the treatment type at the time of prescribing (prophylaxis, empirical or targeted treatment). At the same time, the system will evaluate the justifications of the prescription on clinical data and according to the basic AMS rules (history of drug allergy, serum creatinine, drug incompatibility, etc).
If the existing treatment strategy violates the basic AMS rules, the prescriber will be offered the choice to switch to the guideline-recommended treatment. Otherwise, prescribers will be asked to provide a justification for the deviation from the guidelines.
Moreover, treatment with regard to intravenous oral switch, de-escalation or stopping therapy will be recommended by the system if it is appropriate.
-
Quality control audit and feedback:
Quality indicators of antimicrobial prescribing such as concordance with local guidelines (in terms of duration of therapy and antimicrobial selected) will be automatically assessed based on the information collected during the prescribing process.
Team leaders in a given participant team in the intervention arm will receive monthly graphical reports outlining the performance of the team compared with the other participating teams and compared with the guideline recommendation (if applicable). The individual participant surgeons will receive the monthly audit report of their own performance.
Outcomes measures
Table 1 gives detailed information about primary and secondary outcomes, including full names, abbreviations and evaluation purposes. The definitions of the terms were listed in online supplemental table S1.
Table 1.
Outline of primary and secondary outcomes
| Outcomes | Evaluation purposes |
| Primary outcomes | |
| 1. Days of antimicrobial therapy (DOT) per admission | To evaluate the difference in overall systemic antimicrobial use in terms of duration of treatment and combination therapies between the intervention arm and control arm. |
| 2. DOT per 1000 patient-days (PD) | |
| Secondary outcomes | |
| Antimicrobial use indicators | |
| 1. Drug usage per 100 PD and per admission | The same as the evaluation purposes for ‘DOT per admission’. |
| 2. Length of therapy per 100 PD and per admission | |
| 3. Days per treatment period overall and for specific indications | |
| Postoperative microbial resistance indicators | |
| 1. Clostridium difficile colitis | To evaluate the efficacies of the computer-based multicomponent intervention to reduce the incidence of antimicrobial resistance. |
| 2. Incident clinical cultures with multidrug-resistant organisms (MRSA, ESBL-E, CRE, VRE or Pseudomonas aeruginosa) per 1000 PD and admission. | |
| Postoperative infection indicators | |
| 1. In-hospital or 30-day surgical site infections | To evaluate the potential side effects of the computer-based multicomponent intervention to elevate the incidence of antimicrobial resistance. |
| 2. In-hospital bloodstream infections | |
| 3. In-hospital pneumonia | |
| Patient safety indicators | |
| 1. In-hospital or 30-day mortality, postoperative | We do not anticipate any potential serious adverse events that could be directly attributable to the intervention but we could not rule out the indirect association between these outcomes and the intervention. |
| 2. In-hospital or 30-day myocardial infarction, postoperative and newly onset | Therefore, in consideration of patient safety issues, we will compare the surgical-related complications between the two arms. |
| 3. In-hospital or 30-day stroke, postoperative and newly onset | |
| 4. In-hospital or 30-day acute kidney injury, postoperative and newly onset | |
| Resource-consuming indicators | |
| 1. Length of hospital stay | One of the main interest to various parts of the healthcare system.44 These indicators are set to evaluate the efficacies of the computer-based AMS system to reduce the overall resource consumption. |
| 2. Costs of administered antimicrobials (overall and by class) per admission | |
| 3. Total costs of hospitalisation. | |
| User compliance and satisfaction indicators | |
| 1. User satisfaction with the system | These two indices are to evaluate the barriers and facilitators to implementation and the use of the computer-based intervention. |
| 2. User compliance with the system | |
AMS, antimicrobial stewardship; DDD, defined daily dose; ESBL-E, extended spectrum beta-lactamase producing Enterobacteriaceae; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
bmjopen-2020-039717supp001.pdf (77.1KB, pdf)
The primary outcome will be the overall systemic antimicrobial use measured in DOT of systemic antimicrobial use per admission and per 1000 patient-day based on CPOE-derived data.
Secondary outcomes include a series of indices to evaluate antimicrobial use, microbial resistance, perioperative infection outcomes, patient safety, resource consumption and user compliance/satisfaction.
Sample size
The sample size calculation is based on the primary outcome (DOT per admission and DOT per 1000 patient-day) and has been performed taking into account the clustered design of the study according to the approach proposed in the literature.34
The mean annual surgery volume of a team is about 450 cases in Fuwai Hospital, then one team will include 225 patients who are undergoing adult cardiac surgeries over the research period (6 months). Assuming one team will recruit 125 eligible patients and assuming nine teams per arm will have an average size of 1125 admissions, antimicrobial use of 5.0 DOT/admission in the control group with an SD of 2.0 (based on antimicrobial use data of 2019 in Fuwai Hospital) and a two-sided type I error of 0.05, we would have a power of 80% to detect an absolute difference of at least 0.5 in average DOT/admission between the intervention and control arm.
Blinding and randomisation
The trial steering committee is responsible for recruiting surgical teams to the trial and supervising the research process but had no access to the randomisation procedure. The extraction of the outcome measures will be performed primarily by research staff not directly involved in the study. The data analysts will be blinded to the randomisation.
Neither the research staff directly involved in the intervention, nor the participant surgeons, nor the participant patients are blinded to the randomisation due to the nature of the intervention.
Surgical teams will be randomised 1:1 to the intervention or control arm using an interactive web response system. The randomisation plan will be established by research staff not directly involved in the study.
Scheme for statistical analysis
The efficacy of the intervention will be evaluated by analysing EMR and CPOE data that are routinely collected into the Fuwai database. Patients’ data will be collected by their anonymised electronic case report form, including preoperative information (demographics, diagnosis and comorbidities), surgical information and details of prescriptions; anonymised surgeon information will be retrieved from the database of the personnel division of Fuwai. Written consents will be obtained from the participant patients.
Outcome variables will first be summarised across treatment and intervention groups and then explored using descriptive statistics. The DOT/admission at the individual level and DOT/1000 patient-day will be compared between two arms using a random-effects Poisson model. The following confounders will be considered: (1) patient: sex, age, type of comorbidities and type of cardiovascular surgeries and (2) surgeon: age, annual volume, professional title and academic title. All variables that result in a change of >5% in the coefficient for the intervention effect in bivariate regression will be added to the multivariate model, and the most parsimonious model will be selected through the conditional Akaike information criterion (AIC). Collinearity will be checked through a correlation matrix, whereby the most relevant, clinical variable will be selected in case of R2 >0.8. The inverse probability of treatment weighting will be applied, if imbalances exist after randomisation.
The logistic regression analysis for clinical outcomes (indicators of patient safety, infection and antimicrobial resistance) will estimate the difference (95% CI) in the outcome between intervention and control arms, adjusting for variables at patient level as well as surgeon level.
Data for healthcare usage and costs will be analysed at the individual level as reported previously.35 Total cost and antimicrobial cost will be compared between trial arms. A general linear model will be used to estimate the mean costs for the patients.
As a part of process evaluation, users’ compliance and satisfaction with the computer-based intervention protocol will be assessed. As for user compliance, the evaluation will be done by documenting the total number of times the intervention tools fail to change the physicians’ decision on antimicrobial prescription over the intervention period. The number representing compliance will be divided into quartiles and a trend test will be implemented by introducing these into analyses as continuous variables.
As for user satisfaction, a series of questionnaires will be developed to explore participants’ experiences of using the intervention tools and experiences of the study implementation. Inductive thematic analysis will be used to analyse qualitative data.
Data collection and process
The in-hospital information will be retrieved from the hospital’s database which is stored in the form of electronic case report form. Surgically associated adverse events and SSIs events within 30 days will be followed up. The detailed protocol about the follow-up was described elsewhere.36 Briefly, patients discharged alive were followed at regular time intervals including the time point of postoperative 30 days. If the patients reported adverse events, the medical records of the patients in the outpatient clinic of Fuwai Hospital are double-checked. If the patients visit another hospital, they will be required to send paper copies of medical records by mail or photocopies through the internet. De-identified data for research use will be stored in password-protected Microsoft Excel files on secured hospital servers.
For analysis, data will be imported into SAS V.9.4 (SAS Institute). Only investigators directly involved in the trial will have access to the data. The data will be stored on secure servers with backup systems for 5 years after the end of the trial.
Duration of the trial
The intervention period, lasting 9 months, is composed of two parts: an internal pilot period (3 months) and the research period (6 months).
Before the launch of the research, an internal pilot will be conducted to demonstrate the feasibility and acceptability of the intervention. Also, the pilot will allow a period for the participant surgical teams to get familiar with the new computer-based tools for AMS.
In the pilot phase, intermediate outcome measures will include (1) the compatibility of the new operation module with our EMR and EPOE systems; (2) evidence that the intervention tools are accessed and used by prescribing members of staff in surgical teams and (3) successful delivery of regular feedback reports to surgical teams.
Ethics approval
The Ethics Committee in Fuwai Hospital approved this study. Participant surgeons in Fuwai Hospital gave informed consent to the study. Although the intervention is at the surgical team level, patients’ informed consent will be obtained. In addition, an information leaflet will be provided to patients in the participating surgical teams.
Patient and public involvement
Patients and public will not get involved in the development of the research question, study design or any other part of this protocol.
Dissemination and reporting
Several publications in peer-reviewed journals are expected from this trial, and these will include description of the intervention development of the intervention content and main findings of the trial. Also, the findings are planned to be presented at national and international conferences.
Discussion
Enlightened by the evidence in the literature, the EPIC trial is designed to evaluate the efficacy of CDSS support tools to reduce postoperative antimicrobial use. This study has several strengths and limitations.
Strengths: (1) the adequately powered, cluster-randomised controlled trial addresses many inadequacies in designs of the previous studies33 37–39; (2) different from previous studies in terms of the scope, setting and timing17–19 40 the EPIC trial is among the first to assess the impact of CDSS tools on antimicrobial use in hospital settings and (3) also, to the best of our knowledge, this trial will be one of the first trials carried out in surgery settings. On the basis of the increasing incidence of antimicrobial resistance, we are trying to figure out a method to achieve the goal of a more reasonable use of antimicrobial agents.41 42
Limitations: this trial is a single centre study which may increase type II error. However, heterogeneous organisations of AMS programmes are noted among healthcare providers, possibly due to patient-specific considerations, institution-specific factors and local antimicrobial use policies.43 It is a challenge to carry out multicentre trials because the factors above may be hard to be balanced or a huge sample size will be required which is beyond the sample size of the programme recruitment. Therefore, to carry out a single centre trial in a large volume, hospital with adequate surgical teams under the same AMS system is required. The feedback is a part of the computerised tools in management of antimicrobial, which may also influence antimicrobial use outcomes and behaviour patterns that will limit external validity outside of this trial design. Further study will be conducted to investigate the influence of the feedback.
An important output of this research will figure out a way of delivering a set of computer-based multicomponent interventions to reduce antimicrobial use in surgical settings. As a part of the study, rigorous audit mechanisms will examine facilitators and barriers to implementation of the intervention and assess user compliance/satisfaction with the intervention protocol. The process above will expose whether surgeons’ behaviours will be changed by the CDSS during the intervention period. As a result, a similar, low-cost system could be applied for the regular surgical workflow in other hospitals.
Supplementary Material
Acknowledgments
The authors would like to thank all participants of the EPIC trial team for the valuable work in the study, including the cardiovascular surgeons and their patients.
Footnotes
XY and KC contributed equally.
Contributors: SSH conceived the original idea for this study which was further developed with all authors, and secured funding for the study. XY and KC wrote the first draft of this manuscript and designed the CDSS. ShuH provided input regarding the sample size calculations and statistical analysis. WZ, FY and XD programmed CDSS. XC reviewed the regulations of CDSS according to the guidelines and policy. The manuscript was reviewed and edited by all authors.
Funding: This work is supported by the Foundation No.83 of Fuwai Hospital.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Tackling a Crisis for the Future Health and Wealth of Nations Review on antimicrobial resistance website. Available: http://amr-review.org/.December2014 [Accessed 4 Mar 2020].
- 2.Office of the Press Secretary, The White House Executive order—combating antibiotic-resistant bacteria. Whitehouse. govwebsite, 2014. Available: https://www.whitehouse.gov/the-press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria [Accessed 4 Mar 2020].
- 3.Branch-Elliman W, O'Brien W, Strymish J, et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg 2019;154:590–8. 10.1001/jamasurg.2019.0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control Antibiotic/antimicrobial resistance, 2017. Available: https://www.cdc.gov/drugresistance/ [Accessed 4 Mar 2020].
- 5.Leaper DJ, Edmiston CE. World Health Organization: global guidelines for the prevention of surgical site infection. J Hosp Infect 2017;95:135–6. 10.1016/j.jhin.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 6.Al-Mousa HH, Omar AA, Rosenthal VD, et al. Device-associated infection rates, bacterial resistance, length of stay, and mortality in Kuwait: International Nosocomial Infection Consortium findings. Am J Infect Control 2016;44:444–9. 10.1016/j.ajic.2015.10.031 [DOI] [PubMed] [Google Scholar]
- 7.Karanika S, Grigoras C, Flokas ME, et al. The attributable burden of Clostridium difficile infection to long-term care facilities stay: a clinical study. J Am Geriatr Soc 2017;65:1733–40. 10.1111/jgs.14863 [DOI] [PubMed] [Google Scholar]
- 8.Alexiou VG, Ierodiakonou V, Peppas G, et al. Antimicrobial prophylaxis in surgery: an international survey. Surg Infect 2010;11:343–8. 10.1089/sur.2009.023 [DOI] [PubMed] [Google Scholar]
- 9.Tourmousoglou CE, Yiannakopoulou EC, Kalapothaki V, et al. Adherence to guidelines for antibiotic prophylaxis in general surgery: a critical appraisal. J Antimicrob Chemother 2008;61:214–8. 10.1093/jac/dkm406 [DOI] [PubMed] [Google Scholar]
- 10.Gagliotti C, Ravaglia F, Resi D, et al. Quality of local guidelines for surgical antimicrobial prophylaxis. J Hosp Infect 2004;56:67–70. 10.1016/j.jhin.2003.09.007 [DOI] [PubMed] [Google Scholar]
- 11.Manuel-Vázquez A, Palacios-Ortega F, García-Septiem J, et al. Antimicrobial stewardship programs are required in a department of surgery: ‘how’ is the question a quasi-experimental study: results after three years. Surg Infect 2020;21:35–42. 10.1089/sur.2018.311 [DOI] [PubMed] [Google Scholar]
- 12.Ashfaq A, Zhu A, Iyengar A, et al. Impact of an institutional antimicrobial stewardship program on bacteriology of surgical site infections in cardiac surgery. J Card Surg 2016;31:367–72. 10.1111/jocs.12756 [DOI] [PubMed] [Google Scholar]
- 13.Schröder S, Klein M-K, Heising B, et al. Sustainable implementation of antibiotic stewardship on a surgical intensive care unit evaluated over a 10-year period. Infection 2020;48:117–24. 10.1007/s15010-019-01375-6 [DOI] [PubMed] [Google Scholar]
- 14.Hersh AL, Beekmann SE, Polgreen PM, et al. Antimicrobial stewardship programs in pediatrics. Infect Control Hosp Epidemiol 2009;30:1211–7. 10.1086/648088 [DOI] [PubMed] [Google Scholar]
- 15.Johannsson B, Beekmann SE, Srinivasan A, et al. Improving antimicrobial stewardship: the evolution of programmatic strategies and barriers. Infect Control Hosp Epidemiol 2011;32:367–74. 10.1086/658946 [DOI] [PubMed] [Google Scholar]
- 16.de Kraker MEA, Abbas M, Huttner B, et al. Good epidemiological practice: a narrative review of appropriate scientific methods to evaluate the impact of antimicrobial stewardship interventions. Clin Microbiol Infect 2017;23:819–25. 10.1016/j.cmi.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 17.Gonzales R, Anderer T, McCulloch CE, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Intern Med 2013;173:267–73. 10.1001/jamainternmed.2013.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulliford MC, Prevost AT, Charlton J, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: reduce cluster randomised trial. BMJ 2019;364:l236. 10.1136/bmj.l236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis CE, Al Bahar F, Marriott JF. The effectiveness of computerised decision support on antibiotic use in hospitals: a systematic review. PLoS One 2017;12:e0183062. 10.1371/journal.pone.0183062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the infectious diseases Society of America and the Society for healthcare epidemiology of America. Clin Infect Dis 2016;62:e51–77. 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohmen PMCE. Influence of skin flora and preventive measures on surgical site infection during cardiac surgery. Surg Infect 2006;7:s13–17. 10.1089/sur.2006.7.s1-13 [DOI] [PubMed] [Google Scholar]
- 22.Schweizer M, Perencevich E, McDanel J, et al. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram positive surgical site infections after cardiac or orthopedic surgery: systematic review and meta-analysis. BMJ 2013;346:f2743. 10.1136/bmj.f2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garey KW, Dao T, Chen H, et al. Timing of vancomycin prophylaxis for cardiac surgery patients and the risk of surgical site infections. J Antimicrob Chemother 2006;58:645–50. 10.1093/jac/dkl279 [DOI] [PubMed] [Google Scholar]
- 24.Schersten H. Modified prophylaxis for preventing deep sternal wound infection after cardiac surgery. APMIS 2007;115:1025–8. 10.1111/j.1600-0463.2007.00837.x [DOI] [PubMed] [Google Scholar]
- 25.Garey KW, Amrutkar P, Dao-Tran TK, et al. Economic benefit of appropriate timing of vancomycin prophylaxis in patients undergoing cardiovascular surgery. Pharmacotherapy 2008;28:699–706. 10.1592/phco.28.6.699 [DOI] [PubMed] [Google Scholar]
- 26.Garey KW, Lai D, Dao-Tran TK, et al. Interrupted time series analysis of vancomycin compared to cefuroxime for surgical prophylaxis in patients undergoing cardiac surgery. Antimicrob Agents Chemother 2008;52:446–51. 10.1128/AAC.00495-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linnan L, Steckler A. Process evaluation for public health interventions and research. California: Jossey-Bass San Francisco, 2002. [Google Scholar]
- 28.Sousa-Uva M, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg 2018;53:5–33. 10.1093/ejcts/ezx314 [DOI] [PubMed] [Google Scholar]
- 29.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013;70:195–283. 10.2146/ajhp120568 [DOI] [PubMed] [Google Scholar]
- 30.Reinforce the national antimicrobial stewardship regulations enclosed, 2015. Available: http://www.nhc.gov.cn/yzygj/s3593/201508/f0fdf1f52df14b87aa97be53819f1036.shtml [Accessed 6 Mar 2020].
- 31.Continue to implement national antimicrobial stewardship, 2019. Available: http://www.nhc.gov.cn/yzygj/s7659/201903/1d487eb7b7c74abc9fcb104f8b0905f2.shtml [Accessed 6 Mar 2020].
- 32.Linder JA, Meeker D, Fox CR, et al. Effects of behavioral interventions on inappropriate antibiotic prescribing in primary care 12 months after stopping interventions. JAMA 2017;318:1391–2. 10.1001/jama.2017.11152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demonchy E, Dufour J-C, Gaudart J, et al. Impact of a computerized decision support system on compliance with guidelines on antibiotics prescribed for urinary tract infections in emergency departments: a multicentre prospective before-and-after controlled interventional study. J Antimicrob Chemother 2014;69:2857–63. 10.1093/jac/dku191 [DOI] [PubMed] [Google Scholar]
- 34.Leyrat C, Morgan KE, Leurent B, et al. Cluster randomized trials with a small number of clusters: which analyses should be used? Int J Epidemiol 2018;47:321–31. 10.1093/ije/dyx169 [DOI] [PubMed] [Google Scholar]
- 35.Bhattarai N, Charlton J, Rudisill C, et al. Prevalence of depression and utilization of health care in single and multiple morbidity: a population-based cohort study. Psychol Med 2013;43:1423–31. 10.1017/S0033291712002498 [DOI] [PubMed] [Google Scholar]
- 36.Hu S, Zheng Z, Yuan X, et al. Increasing long-term major vascular events and resource consumption in patients receiving off-pump coronary artery bypass: a single-center prospective observational study. Circulation 2010;121:1800–8. 10.1161/CIRCULATIONAHA.109.894543 [DOI] [PubMed] [Google Scholar]
- 37.Baysari MT, Lehnbom EC, Li L, et al. The effectiveness of information technology to improve antimicrobial prescribing in hospitals: a systematic review and meta-analysis. Int J Med Inform 2016;92:15–34. 10.1016/j.ijmedinf.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 38.Nachtigall I, Tafelski S, Deja M, et al. Long-term effect of computer-assisted decision support for antibiotic treatment in critically ill patients: a prospective 'before/after' cohort study. BMJ Open 2014;4:e005370. 10.1136/bmjopen-2014-005370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul M, Andreassen S, Tacconelli E, et al. Improving empirical antibiotic treatment using TREAT, a computerized decision support system: cluster randomized trial. J Antimicrob Chemother 2006;58:1238–45. 10.1093/jac/dkl372 [DOI] [PubMed] [Google Scholar]
- 40.Vaisman A, McCready J, Hicks S, et al. Optimizing preoperative prophylaxis in patients with reported β-lactam allergy: a novel extension of antimicrobial stewardship. J Antimicrob Chemother 2017;72:2657–60. 10.1093/jac/dkx171 [DOI] [PubMed] [Google Scholar]
- 41.Bratzler DW, Houck PM, Surgical Infection Prevention Guidelines Writers Workgroup . Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 2004;38:1706–15. 10.1086/421095 [DOI] [PubMed] [Google Scholar]
- 42.Miller LG, McKinnell JA, Vollmer ME, et al. Impact of methicillin-resistant Staphylococcus aureus prevalence among S. aureus isolates on surgical site infection risk after coronary artery bypass surgery. Infect Control Hosp Epidemiol 2011;32:342–50. 10.1086/658668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sartelli M, Labricciosa FM, Barbadoro P, et al. The Global Alliance for Infections in Surgery: defining a model for antimicrobial stewardship-results from an international cross-sectional survey. World J Emerg Surg 2017;12:34. 10.1186/s13017-017-0145-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibrahim NH, Maruan K, Mohd Khairy HA, et al. Economic evaluations on antimicrobial stewardship programme: a systematic review. J Pharm Pharm Sci 2017;20:397–406. 10.18433/J3NW7G [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-039717supp001.pdf (77.1KB, pdf)