Abstract
Background
The efficacy of negative pressure wound therapy (NPWT) in the acute management of burns remains unclear. The purpose of this trial was to compare standard Acticoat™ and Mepitel™ dressings with combined Acticoat™, Mepitel™ and continuous NPWT to determine the effect of adjunctive NPWT on re‐epithelialization in paediatric burns.
Methods
This two‐arm, single‐centre RCT recruited children with acute thermal burns covering less than 5 per cent of their total body surface area. The primary outcome was time to re‐epithelialization. Blinded assessments were performed using photographs captured every 3–5 days until discharge. Secondary measures included pain, itch, grafting, perfusion and scar management referrals.
Results
Some 114 patients were randomized. Median time to re‐epithelialization was 8 (i.q.r. 7–11) days in the NPWT group and 10 (8–14) days in the control group. In a multivariable model, NPWT decreased the expected time to wound closure by 22 (95 per cent c.i. 7 to 34) per cent (P = 0·005). The risk of referral to scar management was reduced by 60 (18 to 81) per cent (P = 0·013). Four participants in the control group and one in the NPWT group underwent grafting. There were no statistically significant differences between groups in pain, itch or laser Doppler measures of perfusion. Adverse events were rare and minor, although NPWT carried a moderate treatment burden, with ten patients discontinuing early.
Conclusion
Adjunctive NPWT hastened re‐epithelialization in small‐area burn injuries in children, but had a greater treatment burden than standard dressings alone. Registration number: ACTRN12618000256279 ( http://ANZCTR.org.au).
In this RCT, children with small‐area thermal burns were randomized to standard silver‐impregnated dressings or standard dressings in conjunction with continuous negative pressure wound therapy (NPWT). Adjunctive NPWT decreased time to re‐epithelialization, with attendant improvements in dressing change requirements and scar management referrals. There were no statistically significant differences in pain, itch, or laser Doppler measures of perfusion.
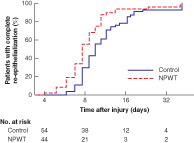
Negative pressure wound therapy hastens epithelialization
Antecedentes
La eficacia del tratamiento de las heridas con presión negativa (negative pressure wound therapy, NPWT) en el tratamiento agudo de las quemaduras sigue sin estar claro. El propósito de este ensayo clínico fue comparar los apósitos estándar del tipo Acticoat™ y Mepitel™ con la combinación de Acticoat™, Mepitel™ y NPWT continua para determinar el efecto de la adición de NPWT en la reepitelización de las quemaduras en pediatría.
Métodos
Ensayo controlado y aleatorizado, con dos brazos y unicéntrico, que reclutó niños con quemaduras térmicas agudas que afectaban < 5% de la superficie corporal total. El resultado primario fue el tiempo hasta la reepitelización. Se realizaron evaluaciones a ciegas utilizando fotografías tomadas cada 3‐5 días hasta el alta hospitalaria. Las medidas secundarias incluían dolor, picor, injerto, perfusión y derivación para el tratamiento de las cicatrices.
Resultados
Se aleatorizaron un total de 114 pacientes. La mediana de tiempo hasta la reepitelización fue 8 días (rango intercuartílico, interquartile range, IQR 7‐11) en el grupo NPWT y 10 días (8‐14) en el grupo control. En el modelo multivariable, el uso de NPWT disminuyó los días previstos hasta el cierre de la herida en un 22% (i.c. del 95% 7‐34%; P = 0,005). El riesgo de ser derivado para el tratamiento de la cicatriz se redujo en un 60% (18‐81%; P = 0,013). Cuatro participantes en el grupo control y uno en el grupo NPWT fueron sometidos a injertos. No hubo diferencias estadísticamente significativas en el dolor, picor, o mediciones de la perfusión con Doppler laser. Los eventos adversos fueron raros y menores, aunque NPWT conllevó una carga de tratamiento moderada con 10 pacientes que lo suspendieron precozmente.
Conclusión
El tratamiento complementario de la herida con presión negativa acelera el tiempo hasta la reepitelización en quemaduras de pequeña extensión en niños, pero implica una mayor carga de tratamiento.
Introduction
Small‐area burns remain a common form of childhood injury, contributing substantially to the non‐fatal burden of disease 1 . Although typically handled on an outpatient basis, these burns nevertheless demand careful treatment to ward off infection and optimize healing 2 . Failure to achieve prompt wound closure significantly increases the risk of hypertrophic scar formation 3 . Affecting between 16 and 35 per cent of children after burn injury 4 , 5 , 6 , 7 , 8 , scarring carries the potential to have a detrimental effect on long‐term physical, cosmetic and psychological outcomes.
Negative pressure wound therapy (NPWT) is a non‐invasive treatment that has shown promise as a facilitator of burn wound healing 9 , 10 , 11 . Nonetheless, the body of rigorous evidence from RCTs has not been commensurate with its growing popularity 12 , 13 , 14 . Recently, three small RCTs 15 , 16 , 17 reported significant improvements with NPWT in re‐epithelialization, histological and biochemical markers of wound healing, skin grafting, bacterial infection and scarring. These trials, however, were constrained by their reliance on suboptimal treatment comparators and unvalidated surrogate outcomes. The aim of this research was to determine the efficacy of NPWT as an adjunct to standard silver‐impregnated dressings, with a focus on patient‐centred outcomes in children with small‐area burns.
Methods
SONATA in C (Study Of Negative pressure wound therapy as an Adjunctive Treatment for Acute burns in Children) is a single‐centre, two‐arm, parallel, randomized, active‐controlled trial. The trial protocol received approval from the Children's Health Queensland Hospital and Health Service and University of Queensland Human Research Ethics Committees (HREC/17/QRCH/279, SSA/17/QRCH/292, 2018000335/HREC/17/QRCH/279). The methodology has been published previously 18 and the trial was registered with the Australian New Zealand Clinical Trials Registry before the start of recruitment (ACTRN12618000256279).
Setting and recruitment
Participants were recruited from a large tertiary‐level metropolitan children's hospital. Children aged less than 17 years were eligible if they presented with a thermal burn covering less than 5 per cent of their total body surface area (TBSA). Patients were excluded if the burn injury occurred more than 7 days before presentation, affected the face, or was expected by clinicians to achieve full re‐epithelialization with standard care before the next dressing change 3–5 days later.
Procedures and interventions
Participants were randomized to receive either standard dressings (control group) or standard dressings in combination with NPWT (NPWT group). The standard dressings consisted of Acticoat™ (Smith & Nephew, Hull, UK), a nanocrystalline silver‐impregnated fibre mesh, and Mepitel™ (Mölnlycke Healthcare, Mikkeli, Finland), a silicone interface. The intervention was delivered via a RENASYS TOUCH™ vacuum pump (Smith & Nephew) set to a continuous subatmospheric pressure of 80 mmHg. For burns involving the extremities in children aged less than 12 months, a pressure of 40 mmHg was employed instead to reduce the risk of ischaemic damage. Participants returned to the burn centre every 3–5 days for removal and reapplication of NPWT and/or standard dressings until wound closure.
Baseline characteristics
Investigators not involved in the care of participants were responsible for trial recruitment, allocation and data collection. They recorded the following baseline characteristics for all eligible patients: age, sex, Fitzpatrick skin type, time of injury, wound aetiology, anatomical location, previous wound care, and clinical assessments of depth and TBSA.
Primary outcome measure
The primary outcome was the time elapsed, in days, from the point of injury to re‐epithelialization of the wound. Photographs of wounds captured during dressing changes underwent blinded review by a panel of three experienced burn clinicians, who indicated for each participant the dressing change at which the wounds were at least 95 per cent re‐epithelialized. The assessments favoured by at least two of the blinded reviewers were used in the analysis. In two instances where there was no majority, members of the panel convened with a fourth burn clinician to reach a consensus.
Secondary outcome measures
Pain and itch
At each dressing change, investigators recorded preprocedural and postprocedural pain and itch, as well as retrospective measures of peak procedural pain. Caregivers were also prompted to document pain and itch at home by means of an electronic survey 24 h after each dressing change.
Observational measures were obtained from clinicians and caregivers. Clinicians evaluated pain and distress in clinic via the Face, Legs, Activity, Cry, Consolability scale 19 . Caregivers used an 11‐point (0–10) numerical rating scale (NRS) to evaluate pain, and the Toronto Paediatric Itch Scale 20 to assess itch in participants under 5 years of age. Self‐reports of pain were obtained using the Revised Faces Pain Scale 21 in children aged 4–7 years, and the NRS in those aged 8 years and older. Participants older than 5 years self‐reported itch by means of the Itch Man Scale 22 .
In accordance with the burn centre's standard practice, pharmacological analgesia on initial presentation and first dressing change consisted of a combination of oxycodone (0·1–0·2 mg/kg), paracetamol (15 mg/kg) and/or ibuprofen (10 mg/kg). Participants with high levels of pain were also provided with nitrous oxide/oxygen (Entonox™; BOC Healthcare, Manchester, UK).
Wound perfusion
Laser Doppler imaging (LDI) was performed to assess mean flux of the defined wound area using a MoorLDI2‐Burn Imager™ (Moor Instruments, Axminster, UK) at the baseline visit and first dressing change 23 .
Skin grafting, adverse events and scar management
Investigators noted all cases of skin grafting, adverse events and referrals to scar management. Patients were referred to scar management if re‐epithelialization took 14 days or more 6 , they received a graft, or were judged by clinicians to be at high risk of functionally or cosmetically impactful scar formation.
Management, movement and satisfaction with treatment
At every visit, clinicians were asked to rate the ease of applying and removing dressings on an 11‐point NRS. Caregivers and children aged 8 years and older assessed the impact on their ease of movement, again using an 11‐point NRS. The original protocol called for a concurrent measure of treatment satisfaction. This was abandoned at an early stage, however, as a result of most families' self‐acknowledged lack of familiarity with burn management. Therefore, the scale was modified to assess the ease of managing the dressings at home.
Scarring
Participants were invited to attend follow‐up appointments 3 and 6 months after injury to evaluate between‐group differences in hypertrophic scarring. Ultrasound imaging and colorimetry were performed at both the burn site and a comparator region of unaffected skin on the contralateral side (or the nearest region of unaffected skin). Images captured using a BT12 Venue 40 MSK ultrasound machine (General Electric, Little Chalfont, UK) were evaluated by a blinded reviewer, who measured the distance between the outer border of the epidermis and the inner border of the dermis. Colorimetry was undertaken using a DSM II ColorMeter® (Cortex Technology, Hadsund, Denmark), with three readings recorded per site of pigmentation (L) and erythema (a). Caregivers and/or children completed the patient component of the Patient and Observer Scar Assessment Scale (POSAS) 24 and Brisbane Burn Scar Impact Profile (BBSIP) 25 to provide subjective assessments of scar severity and burn‐specific health‐related quality of life respectively. Families who declined to attend the appointments were instead sent online POSAS and BBSIP questionnaires.
Randomization
An unpredictable 1 : 1 allocation sequence was generated by a statistician 26 . Randomization was stratified by age (0–3, 4–7, 8–16 years), and a permutated block method with random block sizes of 4 and 6 was used. The sequence was uploaded to the REDCap randomization module, which concealed allocation until the point of randomization.
Blinding
Blinding was not possible for participants and treating clinicians because of obvious differences in physical appearance between the interventions. However, the primary outcome was evaluated by blinded review. Two of the reviewers were involved in the care of at least one of the trial participants. To reduce the possibility of recognition, the images were edited to remove all potentially identifying attributes.
Statistical analysis
The sample size was calculated from the primary outcome using an independent‐samples t test. A previous study 27 reported a mean(s.d.) time to re‐epithelialization of 12·4(5·4) days with Acticoat™ therapy. Given the finding of Deitch and colleagues 3 that hypertrophic scarring was absent in patients whose burn injury healed in less than 10 days, a mean time to re‐epithelialization of 9 days for the NPWT group was viewed as a minimum clinically important improvement. With more than 80 per cent power and a significance level of 0·05, and assuming an attrition rate of 20 per cent, it was determined that a minimum enrolment of 104 participants was required. No interim analyses were performed.
Between‐group differences in demographic and baseline data were assessed using the Student t test and Mann–Whitney U test for continuous variables, and the χ2 test and Fisher's exact test for categorical variables.
Time to re‐epithelialization was analysed by negative binomial regression 28 , 29 . The ratio of means (NPWT versus control group) was reported using the incidence rate ratio (IRR) with 95 per cent confidence interval. The primary analysis sought to incorporate participants who underwent split‐thickness skin grafting. Because the time of grafting is often influenced by factors unrelated to the wound, such as operating room availability, a previously described method 30 was employed to estimate spontaneous time to re‐epithelialization using a dummy value of 1 day greater than the longest duration of wound closure documented for any non‐grafted wound. The wound with the greatest re‐epithelialization time (40 days) had the same depth as the grafted burns. A sensitivity analysis excluding all patients with grafted wounds was also performed. Further sensitivity analyses were undertaken using Kaplan–Meier and Cox proportional hazards models, with estimation of hazard ratios and 95 per cent confidence intervals. A post hoc subgroup analysis was conducted assessing re‐epithelialization in participants treated within a 48‐h time frame versus those who presented later. A subgroup × NPWT interaction term and a main effect for subgroup were added to the Cox model. A modified Poisson regression model was used to assess the need for scar management. Pain and itch were evaluated via multilevel generalized linear mixed‐effects models with a log‐link function and Poisson distribution, with preprocedural pain at first presentation entered as a co‐variable in pain analyses.
Prespecified demographic and injury‐related variables (time to treatment, depth, TBSA, aetiology, anatomical location and skin type) were tested against primary and secondary outcomes. Any variables with P ≤ 0·100 in univariable analyses were included as co‐variables in the multivariable analyses. Significance was set at P < 0·050. Analyses were conducted using SPSS® version 25 (IBM, Armonk, New York, USA) and Stata® version 16 (StataCorp, College Station, Texas, USA).
Results
From May 2018 to January 2019, 114 patients were randomized (Fig. 1 ). Demographic and injury characteristics were balanced across the two groups (Table 1 ).
Fig. 1.
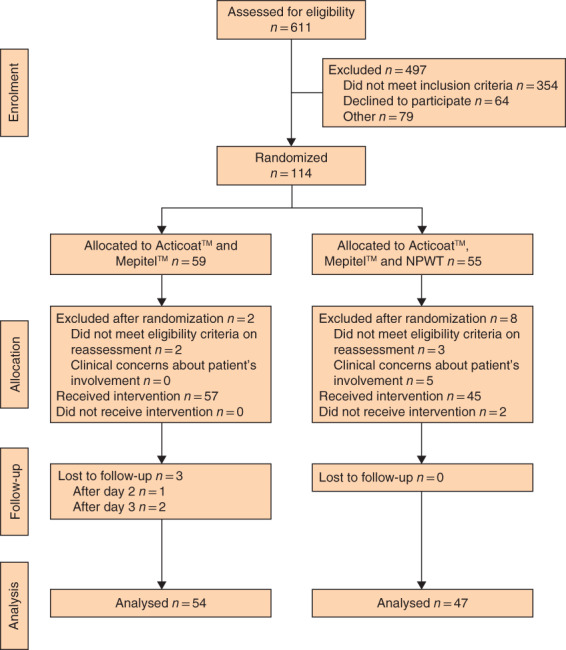
CONSORT diagram for the trial
Table 1.
Demographic and baseline clinical characteristics
| Control (n = 54)‡ | NPWT (n = 47) | |
|---|---|---|
| Age (years) * | 4 (1–9) | 4 (1–8) |
| < 8 | 35 (65) | 31 (66) |
| Sex ratio (M : F) | 31 : 23 | 28 : 19 |
| Total body surface area affected (%) * | 1 (1–2) | 1·5 (1–2) |
| Depth | ||
| Superficial partial thickness | 36 (67) | 32 (68) |
| Deep partial thickness | 18 (33) | 15 (32) |
| Baseline perfusion (PU) †, § | 846(286) | 866(252) |
| Mechanism of burn | ||
| Scald | 35 (65) | 28 (60) |
| Contact | 18 (33) | 17 (36) |
| Flame | 1 (2) | 2 (4) |
| Time to presentation (days) * | 3 (2–3) | 3 (1–4) |
| Presentation within 48 h of injury | 18 (33) | 16 (34) |
| Adequate first aid provided | 43 (80) | 38 (81) |
| Anatomical location | ||
| Upper limb(s) | 17 (31) | 21 (45) |
| Lower limb(s) | 21 (39) | 15 (32) |
| Chest, torso, back | 12 (22) | 8 (17) |
| Genitals, buttocks | 2 (4) | 2 (4) |
| Multiple sites | 2 (4) | 1 (2) |
| Fitzpatrick skin type | ||
| 1 | 3 (6) | 5 (11) |
| 2 | 17 (31) | 18 (38) |
| 3 | 20 (37) | 16 (34) |
| 4 | 11 (20) | 6 (13) |
| 5 | 1 (2) | 1 (2) |
| 6 | 2 (4) | 1 (2) |
Values in parentheses are percentages unless indicated otherwise; values are
median (i.q.r.) and
mean(s.d.).
Excluding three participants who were lost to follow‐up.
Data collected from 40 participants (74 per cent) in control group and 32 (68 per cent) in negative pressure wound therapy (NPWT) group. PU, perfusion units.
Two participants in the control group and three in the NPWT group were excluded after randomization owing to either a modification of their history or a reassessment indicating ineligibility. A further five participants in the NPWT group were excluded because of concerns regarding baseline anxiety levels or social circumstances (such as lack of easy access to assistance in the event of device malfunction). The decision was made by treating clinicians to exclude these participants from the trial altogether. All had superficial partial‐thickness burns with a TBSA of 2 per cent or less.
The intervention was administered for a minimum of 2 days in 45 of 47 children in the NPWT group. Of these, 31 participants received the intervention until wound closure. Among the remaining 14, the median duration of NPWT was 3 (i.q.r. 3–4) days. It was discontinued in four children for clinical reasons. In the other ten, participants or caregivers declined reapplication of NPWT at the first or second dressing change.
Time to re‐epithelialization
A total of 96 of 101 participants (95·0 per cent) achieved full re‐epithelialization spontaneously. There was good agreement among the blinded assessors (intraclass correlation coefficient 0·85). The median time to re‐epithelialization was 10 (i.q.r. 8–14) days in the control group and 8 (7–11) days in the NPWT group. After adjusting for depth, anatomical location and aetiology, NPWT reduced the expected time to re‐epithelialization by 22 per cent (IRR 0·78, 95 per cent c.i. 0·66 to 0·93; P = 0·005). Kaplan–Meier curves for re‐epithelialization time in the NPWT and control groups are shown in Fig. 2 . In a multivariable Cox regression model adjusted for depth and anatomical location, the hazard ratio for the NPWT group was 1·66 (95 per cent c.i. 1·11 to 2·47; P = 0·014).
Fig. 2.
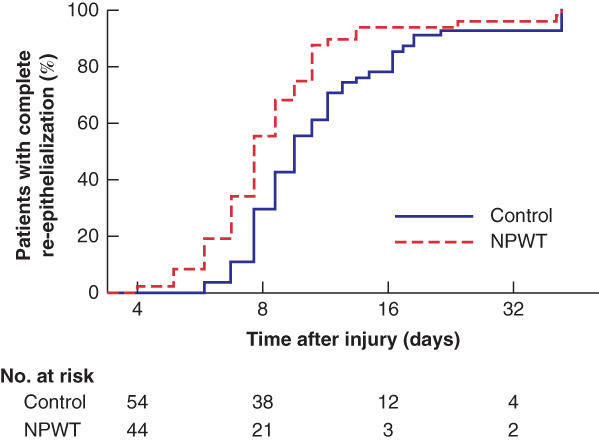
Kaplan–Meier analysis of time to re‐epithelialization by treatment group NPWT, negative pressure wound therapy.
A sensitivity analysis excluding patients who received grafts yielded consistent findings (IRR 0·85, 95 per cent c.i. 0·73 to 0·98; P = 0·027). Median time to wound closure remained unchanged in both groups. A post hoc subgroup analysis investigating whether NPWT exhibited greater efficacy if applied within 48 h of the burn was inconclusive (Table 2 ). The hazard ratio for the subgroup in which the NPWT was applied within 48 h was 1·54 (95 per cent c.i. 0·64 to 3·62) times that for the subgroup with a longer time to treatment (P = 0·330).
Table 2.
Subgroup analysis of time to re‐epithelialization
| Time to re‐epithelialization (days)* | |||||
|---|---|---|---|---|---|
| Time to presentation (h) | Control | NPWT | Hazard ratio†, ‡ | P | P for interaction |
| < 48 | 9 (8–13·75) (n = 18) | 7·5 (5·25–8) (n = 16) | 2·26 (1·11, 4·61) | 0·025 | 0·330 |
| ≥ 48 | 10·5 (9–14·75) (n = 36) | 9 (7–11) (n = 31) | 1·47 (0·90, 2·40) | 0·121 | |
Values are
median (i.q.r.);
values in parentheses are 95 per cent confidence intervals. NPWT, negative pressure wound therapy.
Cox proportional hazards analysis adjusted for depth and anatomical location.
Pain and itch
There were no statistically significant differences between treatment groups in pain or itch (Table 3 ; Table S1 , supporting information).
Table 3.
Mixed‐effects modelling for pain and itch measures
| Ratio of mean score for NPWT/control | P | |
|---|---|---|
| During dressing application/removal | ||
| FLACC scale | 1·09 (0·83, 1·43) | 0·534 |
| Observational NRS | 1·02 (0·68, 1·54) | 0·917 |
| Self‐reported NRS | 1·74 (0·95, 3·19) | 0·072 |
| After dressing application/removal | ||
| Observational NRS | 1·28 (0·64, 2·58) | 0·484 |
| Self‐reported NRS | 1·36 (0·57, 3·25) | 0·485 |
| Toronto Pediatric Itch Scale | 1·19 (0·29, 4·85) | 0·808 |
| Itch Man Scale | 1·21 (0·49, 2·99) | 0·679 |
| At home | ||
| Observational NRS | 0·86 (0·52, 1·41) | 0·554 |
| Self‐reported NRS | 1·05 (0·55, 2·01) | 0·874 |
| Toronto Pediatric Itch Scale | 0·79 (0·40, 1·56) | 0·499 |
| Itch Man Scale | 1·39 (0·95, 2·05) | 0·092 |
Values in parentheses are 95 per cent confidence intervals. Data from the first two clinical visits and home questionnaires were used in the analysis. Only 17 children fell within the 4–7‐year age range, too few to allow a proper analysis of Revised Faces Pain Scale data. Additionally, three participants in the control group and one in the negative pressure wound therapy (NPWT) group had a dressing change under general anaesthesia; it was not possible to collect pain or itch measures for these procedures. FLACC, Face, Legs, Activity, Cry, Consolability; NRS, numerical rating scale.
Wound perfusion
LDI was performed successfully in 75 of 104 participants (72·1 per cent) at the baseline visit, 64 (61·5 per cent) at the first dressing change, and 50 (48·1 per cent) at both time points. At the first change of dressings, mean perfusion was higher in the NPWT group than in the control group (mean(s.d.) 981(296) versus 882(250) perfusion units (PU); P = 0·153). In a separate exploratory analysis, the wound area of children who received NPWT within 48 h of injury exhibited higher mean perfusion at 3–5 days after the burn compared with the remainder of the cohort (1085(294) versus 951(254) PU; P = 0·104).
Grafting
Split‐thickness skin grafting was undertaken in four participants (7 per cent) in the control group and one (2 per cent) in the NPWT group.
Scar management referrals
A statistically higher proportion of children in the control group received referrals to scar management than in the NPWT group: 15 of 54 (28 per cent) versus five of 47 (11 per cent) (P = 0·031). After adjusting for age and depth, the risk of referral to scar management was reduced by 60 per cent in the NPWT group (relative risk 0·40, 95 per cent c.i. 0·19 to 0·82; P = 0·013).
Dressing changes
The median number of dressing changes until wound closure was 2 (i.q.r. 2–3) in the NPWT group and 3 (2–4) in the control group (P = 0·003).
Scarring
Among the 96 participants whose wound re‐epithelialized spontaneously, scar questionnaires were completed by 67 (70 per cent) at 3 months and 58 (60 per cent) at 6 months, with 53 (55 per cent) participating at both time points. Most were submitted online, as rates of attendance at in‐person follow‐up visits were low: 28 children (29 per cent) presented at 3 months, 23 (24 per cent) at 6 months and 14 (15 per cent) at both time points. The questionnaires showed that caregiver perception of scar severity was modestly improved in the NPWT group, although these improvements were not statistically significant (Table S2 , supporting information). No statistically significant differences were detected at either time point in burn‐specific health‐related quality of life (Tables S3 and S4 , supporting information) or colorimetry (Table S5 , supporting information). The median relative difference in scar thickness was statistically lower in the NPWT group at 3 months (P = 0·018), but not at 6 months (P = 0·928) (Table S5 , supporting information).
Movement and management
At the first dressing change, caregivers rated movement and management as more difficult in the NPWT group for participants aged less than 8 years (P < 0·001) (Table 4 ). The intervention was also rated as more difficult to manage by clinicians (P < 0·001), taking longer to apply (P = 0·002) and remove (P < 0·001). Self‐reports by older children, however, revealed no statistically significant between‐group differences in management (P = 0·116) or movement (P = 0·061).
Table 4.
Assessments of ease of management and movement
| Control | NPWT | P * | |
|---|---|---|---|
| Initial visit | |||
| Ease of application | 8 (7–10) (n = 54) | 7 (5–8) (n = 45) | < 0·001 |
| Duration of application (min) | 11 (7–15·75) (n = 56) | 16 (10–19) (n = 47) | 0·002 |
| Second visit | |||
| Ease of removal | 9 (8–10) (n = 50) | 7 (6–8) (n = 41) | < 0·001 |
| Duration of removal (min) | 5 (4–8) (n = 53) | 8 (6–10·5) (n = 45) | < 0·001 |
| Ease of management at home | |||
| Caregiver report (child <8 years) | 10 (9–10) (n = 35) | 7·5 (5–10) (n = 30) | < 0·001 |
| Child self‐report (≥ 8 years) | 10 (8–10) (n = 19) | 8·5 (6·25–9) (n = 16) | 0·116 |
| Ease of movement | |||
| Caregiver report (child <8 years) | 10 (8–10) (n = 35) | 6·5 (4–8) (n = 30) | < 0·001 |
| Child self‐report (≥ 8 years) | 10 (6–10) (n = 19) | 7 (5–8) (n = 16) | 0·061 |
Values are median (i.q.r.); unless indicated otherwise, values are scores on an 11‐point Likert scale (0, extremely difficult to manage/move; 10, extremely easy to manage/move). NPWT, negative pressure wound therapy.
Mann–Whitney U test.
Adverse events
NPWT was discontinued in four patients for clinical reasons: wound maceration (1), periwound blistering (a skin reaction to the adhesive film; 1) and exacerbation of pre‐existing viral illnesses unrelated to burns (2). Blistering was observed in an additional two participants whose burns were fully re‐epithelialized and did not require further care. There were no instances of wound infection. In the control group, there was a single case of exacerbation of a pre‐existing viral disease, but no other adverse events.
Treatment burden
Ten families in the intervention group requested discontinuation of NPWT before the primary endpoint had been reached. Among the reasons provided for declining reapplication, caregivers listed the physical burden of the pump, mechanical issues and difficulties attending school. Three families presented to the emergency department with technical problems (such as air leaks and charging abnormalities) they could not resolve at home.
Discussion
NPWT accelerated re‐epithelialization in children with partial‐thickness thermal burns of less than 5 per cent TBSA. The decrease in expected time to wound closure by 22 per cent corresponded to a reduction in the number of dressing changes required, and a 60 per cent decline in the risk of referral for scar management. Prompt wound healing represents one of the foremost challenges in the management of small‐area thermal injuries 2 , which comprise the majority of cases treated by paediatric burn services 31 , 32 , 33 . Delays in healing lead to extended resource‐ and time‐intensive care, and increase the risk of complications such as infection and hypertrophic scarring. In children for whom the healing process continues beyond 8 days, there is a sigmoidal relationship between time to re‐epithelialization and the incidence of scarring, with the risk of hypertrophic scar formation increasing significantly with each additional day without epithelial closure 5 . Even incremental improvements in re‐epithelialization therefore hold promise for minimizing the physical, psychosocial and financial burdens of scarring 8 , 34 .
The trial results build on past experimental 9 and prospective human 10 , 11 , 35 , 36 , 37 studies that suggested NPWT to be beneficial in the treatment of thermal injury. In a previous RCT 17 involving 64 children with deep dermal partial‐thickness burns, NPWT reduced the duration of wound closure, bacterial colonization, skin grafting and scar severity. However, participants in the control group were treated with silver sulphadiazine cream. The silver fabric dressings employed in the present study are generally preferred by paediatric burn services because of their proven superiority in dressing change frequency, re‐epithelialization and procedural pain 27 , 38 , 39 . Another RCT 15 , which focused on burns affecting a larger TBSA in 50 mainly adult patients, showed that NPWT improved histopathological and biochemical markers of healing by 10 days after injury. These findings were not reported alongside any clinical outcomes. In a similar cohort of 45 adults 16 , NPWT decreased bacterial counts, wound surface area and duration of hospital stay.
Several hypotheses exist regarding the mechanism by which NPWT contributes to wound healing 40 . Its known actions include reduction in tissue oedema, maintenance of a moist environment, and induction, through microdeformational forces and localized hypoxia, of an anti‐inflammatory phenotype that promotes granulation tissue synthesis, neovascularization and remodelling of the extracellular matrix 41 . Owing to its procirculatory effects, NPWT has been considered a promising tool for attenuating burn wound progression 42 if applied within the 48‐h interval after injury in which progressive vascular compromise is known to continue 10 , 43 . The present trial, which set an eligibility window of 7 days, was not able to ascertain definitively whether NPWT was more efficacious if administered within this time frame. It appears likely, based on evidence from this study and others 9 , that patients might benefit from early administration, but further research is required.
The finding that NPWT produced only a moderate increase in perfusion that was not statistically significant must be interpreted with caution as there were missing data. LDI measures of mean perfusion were obtained from less than half of participants at both of the first two visits. The challenges of performing LDI in children have been well documented 28 . In previous trials 10 , 11 , 15 , 35 , NPWT had positive effects on blood flow and histological neovascularization, as well as the more clinically relevant indicator of advanced depth, the need for skin grafting. As only five patients in the present study underwent grafting, the impact of NPWT on surgical requirements could not be determined precisely.
Pain represents a common concern surrounding the use of NPWT in children 44 . Encouragingly, the results showed that procedures involving NPWT were not perceived by clinicians, caregivers or children as significantly more painful than standard dressing changes. Effective pharmacological analgesia could be one plausible explanation for the low levels of pain reported across the two groups. Clinicians also took deliberate action to curb the most distressing aspects of NPWT, such as applying Niltac™ (ConvaTec, Greenlane, New Zealand) to ease extraction of the adhesive film.
The 3‐ and 6‐month follow‐up results demonstrated modest improvements in scar outcomes with NPWT. There was a statistically significant reduction in only one measure, the difference in relative scar thickness at 3 months. Whether these assessments provided an accurate representation of hypertrophic scar formation in the cohort, however, is uncertain given several limiting factors. As with previous paediatric burn studies 45 , the trial recorded low rates of long‐term follow‐up: only 15 per cent of participants attended both appointments. It also bears emphasis that the study predominantly involved superficial partial‐thickness burns with a TBSA of under 2 per cent, which are not in the highest risk category for hypertrophic scarring 6 . Additional studies are needed to determine whether the beneficial effects of NPWT might translate to a more meaningful impact on scarring in children with larger, deeper burns.
The disadvantages of NPWT warrant consideration. Six children experienced minor periwound blistering or maceration, although these incidents did not appear to impair recovery. Of greater concern were the practical challenges. Clinical management of the NPWT apparatus was more laborious than that of standard dressings, with the creation and removal of an airtight seal often requiring the most time and effort. Some caregivers struggled with technical issues that led them to seek out‐of‐hours assistance, including visits to the emergency department. Air leaks in particular were a recurrent problem, especially in burns involving joints. Finally, the size and weight (1·2 kg) of the device rendered it physically onerous to very young patients. It is possible that ultraportable NPWT systems 46 , 47 could provide the same therapeutic benefit with reduced burden.
A major strength of the present trial was its collection of validated patient‐centred endpoints. Many previous RCTs investigating NPWT 15 , 16 , 17 based their findings on surrogate outcomes, which tend to overestimate treatment effects 14 .
In addition to the limitations already described, the 3–5‐day intervals between visits restricted the precision of the re‐epithelialization assessments. The nature of the intervention also precluded blinding of participants and clinicians, although the primary outcome was assessed by blinded review. Finally, despite eligibility criteria designed to be broadly inclusive, 57·9 per cent of patients who presented during the study period were ruled to be ineligible, principally owing to insufficient injury severity. Eligibility assessments may have been influenced by clinicians' perceptions of treatment burden, possibly constraining the generalizability of the trial's findings.
Supporting information
Table S1 Assessments of pain and itch
Table S2 Scar severity*
Table S3 Burn‐specific health‐related quality of life*: Caregivers of children <8 years
Table S4 Burn‐specific health‐related quality of life*: Caregivers of children ≥8 years
Table S5 Objective measures of scar severity (thickness* and colour†)
Acknowledgements
The authors acknowledge the children, families and clinicians who participated in the SONATA in C trial. They thank A. Bairagi, C. McMillan, B. Meikle and R. Walker for their contribution to the blinded reviews, and A. Bernard and F. Zahir for statistical input. Funding for the study was provided by an Australian Government Research Training Scholarship and a research grant from Smith & Nephew to the Queensland University of Technology. L.C. acknowledges Fellowship funding from the Australian Government, National Health and Medical Research Council (APP1130862). Smith & Nephew's involvement in the research was limited to its financial support. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: The authors declare no other conflict of interest.
Presented to the 43rd Annual Scientific Meeting of the Australian and New Zealand Burn Association, Hobart, Tasmania, Australia, October 2019
References
- 1. Peck MD. Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns 2011; 37: 1087–1100. [DOI] [PubMed] [Google Scholar]
- 2. Greenhalgh D. Management of burns. N Engl J Med 2019; 380: 2349–2359. [DOI] [PubMed] [Google Scholar]
- 3. Deitch EA, Wheelahan TM, Rose MP, Clothier J, Cotter J. Hypertrophic burn scars: analysis of variables. J Trauma 1983; 23: 895–898. [PubMed] [Google Scholar]
- 4. Lonie S, Baker P, Teixeira RP. Healing time and incidence of hypertrophic scarring in paediatric scalds. Burns 2016; 43: 509–513. [DOI] [PubMed] [Google Scholar]
- 5. Chipp E, Charles L, Thomas C, Whiting K, Moiemen N, Wilson Y. A prospective study of time to healing and hypertrophic scarring in paediatric burns: every day counts. Burns Trauma 2017; 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallace HJ, Fear MW, Crowe MM, Martin LJ, Wood FM. Identification of factors predicting scar outcome after burn injury in children: a prospective case–control study. Burns Trauma 2017; 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dedovic Z, Koupilova I, Brychta P. Time trends in incidence of hypertrophic scarring in children treated for burns. Acta Chir Plast 2000; 41: 87–90. [PubMed] [Google Scholar]
- 8. Cubison TC, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns 2006; 32: 992–999. [DOI] [PubMed] [Google Scholar]
- 9. Morykwas MJ, David LR, Schneider AM, Whang C, Jennings DA, Canty C et al Use of subatmospheric pressure to prevent progression of partial‐thickness burns in a swine model. J Burn Care Rehabil 1999; 20: 15–21. [DOI] [PubMed] [Google Scholar]
- 10. Kamolz LP, Andel H, Haslik W, Winter W, Meissl G, Frey M. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns 2004; 30: 253–258. [DOI] [PubMed] [Google Scholar]
- 11. Schrank C, Mayr M, Overesch M, Molnar J, Henkel VDG, Muhlbauer W et al [Results of vacuum therapy (V.A.C.) of superficial and deep dermal burns]. Zentralbl Chir 2004; 129: 59–61. [DOI] [PubMed] [Google Scholar]
- 12. Dumville JC, Munson C, Christie J. Negative pressure wound therapy for partial‐thickness burns. Cochrane Database Syst Rev 2014; (12)CD006215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kantak NA, Mistry R, Halvorson EG. A review of negative‐pressure wound therapy in the management of burn wounds. Burns 2016; 42: 1623–1633. [DOI] [PubMed] [Google Scholar]
- 14. Peinemann F, Labeit A. Negative pressure wound therapy: a systematic review of randomized controlled trials from 2000 to 2017. J Evid Based Med 2018; 12: 125–132. [DOI] [PubMed] [Google Scholar]
- 15. Honnegowda TM, Padmanabha Udupa EG, Rao P, Kumar P, Singh R. Superficial burn wound healing with intermittent negative pressure wound therapy under limited access and conventional dressings. World J Plast Surg 2016; 5: 265–273. [PMC free article] [PubMed] [Google Scholar]
- 16. Ibrahim ZM, Waked IS, Ibrahim O. Negative pressure wound therapy versus microcurrent electrical stimulation in wound healing in burns. J Wound Care 2019; 28: 214–219. [DOI] [PubMed] [Google Scholar]
- 17. Zheng XP, Chen J, Chen TS, Jiang YN, Shen T, Xiao SC et al [Preliminary effect observation on the application of micro‐negative pressure in children with small‐area deep partial‐thickness burn]. Zhonghua Shao Shang Za Zhi 2019; 35: 720–725. [DOI] [PubMed] [Google Scholar]
- 18. Frear CC, Griffin B, Cuttle L, McPhail SM, Kimble R. Study of negative pressure wound therapy as an adjunct treatment for acute burns in children (SONATA in C): protocol for a randomised controlled trial. Trials 2019; 20: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Merkel SI, Voepel‐Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs 1997; 23: 293–297. [PubMed] [Google Scholar]
- 20. Everett T, Parker K, Fish J, Pehora C, Budd D, Kelly C et al The construction and implementation of a novel postburn pruritus scale for infants and children aged five years or less: introducing the Toronto Pediatric Itch Scale. J Burn Care Res 2015; 36: 44–49. [DOI] [PubMed] [Google Scholar]
- 21. Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale‐Revised: toward a common metric in pediatric pain measurement. Pain 2001; 93: 173–183. [DOI] [PubMed] [Google Scholar]
- 22. Morris V, Murphy LM, Rosenberg M, Rosenberg L, Holzer CE III, Meyer WJ III. Itch assessment scale for the pediatric burn survivor. J Burn Care Res 2012; 33: 419–424. [DOI] [PubMed] [Google Scholar]
- 23. Hoeksema H, Van de Sijpe K, Tondu T, Hamdi M, Van Landuyt K, Blondeel P et al Accuracy of early burn depth assessment by laser Doppler imaging on different days post burn. Burns 2009; 35: 36–45. [DOI] [PubMed] [Google Scholar]
- 24. Draaijers LJ, Tempelman FR, Botman YA, Tuinebreijer WE, Middelkoop E, Kreis RW et al The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 2004; 113: 1960–1967. [DOI] [PubMed] [Google Scholar]
- 25. Tyack Z, Ziviani J, Kimble R, Plaza A, Jones A, Cuttle L et al Measuring the impact of burn scarring on health‐related quality of life: development and preliminary content validation of the Brisbane Burn Scar Impact Profile (BBSIP) for children and adults. Burns 2015; 41: 1405–1419. [DOI] [PubMed] [Google Scholar]
- 26. Snow G. Blockrand: randomization for block random clinical trials. R package version 1.3. 2013. [Google Scholar]
- 27. Huang Y, Li X, Liao Z, Zhang G, Liu Q, Tang J et al A randomized comparative trial between Acticoat and SD‐Ag in the treatment of residual burn wounds, including safety analysis. Burns 2006; 33: 161–166. [DOI] [PubMed] [Google Scholar]
- 28. Gee Kee EL, Kimble RM, Cuttle L, Khan A, Stockton KA. Randomized controlled trial of three burns dressings for partial thickness burns in children. Burns 2015; 41: 946–955. [DOI] [PubMed] [Google Scholar]
- 29. McBride CA, Kimble RM, Stockton KA. Prospective randomised controlled trial of Algisite™ M, Cuticerin™, and Sorbact® as donor site dressings in paediatric split‐thickness skin grafts. Burns Trauma 2018; 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gee Kee E, Stockton K, Kimble RM, Cuttle L, McPhail SM. Cost‐effectiveness of silver dressings for paediatric partial thickness burns: an economic evaluation from a randomized controlled trial. Burns 2017; 43: 724–732. [DOI] [PubMed] [Google Scholar]
- 31. Stockton KA, Harvey J, Kimble RM. A prospective observational study investigating all children presenting to a specialty paediatric burns centre. Burns 2014; 41: 476–483. [DOI] [PubMed] [Google Scholar]
- 32. Shah A, Suresh S, Thomas R, Smith S. Epidemiology and profile of pediatric burns in a large referral center. Clin Pediatr 2011; 50: 391–395. [DOI] [PubMed] [Google Scholar]
- 33. Warner PM, Coffee TL, Yowler CJ. Outpatient burn management. Surg Clin North Am 2014; 94: 879–892. [DOI] [PubMed] [Google Scholar]
- 34. Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 2016; 388: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Molnar J, Heimbach DM, Tredget EE, Hickerson WL, Still JM, Luterman A. Prospective randomized controlled mulitcenter trial applying subatmospheric pressure to acute hand burns: an interim report. Meeting of 2nd World Union of Wound Healing Societies Paris, 2004. [Google Scholar]
- 36. Haslik W, Kamolz L, Andel H, Meissl G, Frey M. The use of subatmospheric pressure to prevent burn wound progression: first experiences in burn wound treatment. Zentralblatt fur Chirurgie 2004; 129: S62–S63. [DOI] [PubMed] [Google Scholar]
- 37. Chen J, Zhou J, Su G, Shi J, Su S. Evaluation of the clinical curative effect of applying vacuum sealing drainage therapy in treating deep partial‐thickness burn wound at the initial stage. Chinese J Burns 2010; 26: 170–174. [PubMed] [Google Scholar]
- 38. Cuttle L, Naidu S, Mill J, Hoskins W, Das K, Kimble RM. A retrospective cohort study of Acticoat versus Silvazine in a paediatric population. Burns 2007; 33: 701–707. [DOI] [PubMed] [Google Scholar]
- 39. Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev 2013; (3)CD002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang C, Leavitt T, Bayer LR, Orgill DP. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg 2014; 51: 301–331. [DOI] [PubMed] [Google Scholar]
- 41. Glass GE, Murphy GF, Esmaeili A, Lai LM, Nanchahal J. Systematic review of molecular mechanism of action of negative‐pressure wound therapy. Br J Surg 2014; 101: 1627–1636. [DOI] [PubMed] [Google Scholar]
- 42. Jackson DM. The diagnosis of the depth of burning. Br J Surg 1953; 40: 588–596. [DOI] [PubMed] [Google Scholar]
- 43. Krug E, Berg L, Lee C, Hudson D, Birke‐Sorensen H, Depoorter M et al Evidence‐based recommendations for the use of negative pressure wound therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury 2011; 42: S1–S12. [DOI] [PubMed] [Google Scholar]
- 44. Santosa KB, Keller M, Olsen MA, Keane AM, Sears ED, Snyder‐Warwick AK. Negative‐pressure wound therapy in infants and children: a population‐based study. J Surg Res 2019; 235: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McQuaid D, Barton J, Campbell E. Researchers BEWARE! Attrition and nonparticipation at large. J Burn Care Rehabil 2003; 24: 203–207. [DOI] [PubMed] [Google Scholar]
- 46. Hudson DA, Adams KG, Van Huyssteen A, Martin R, Huddleston EM. Simplified negative pressure wound therapy: clinical evaluation of an ultraportable, no‐canister system. Int Wound J 2015; 12: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fong KD, Hu D, Eichstadt SL, Gorell E, Munoz CA, Lorenz HP et al Initial clinical experience using a novel ultraportable negative pressure wound therapy device. Wounds 2010; 22: 230–236. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Assessments of pain and itch
Table S2 Scar severity*
Table S3 Burn‐specific health‐related quality of life*: Caregivers of children <8 years
Table S4 Burn‐specific health‐related quality of life*: Caregivers of children ≥8 years
Table S5 Objective measures of scar severity (thickness* and colour†)


