Abstract
Antimicrobial resistance (AMR) is a global public health threat. The main purpose of this study was to evaluate AMR in generic Escherichia coli and Salmonella recovered from broiler chickens in Canada. To do this, an analysis of the antimicrobial susceptibility results was performed on a sample of generic E. coli and Salmonella isolates from the 2012 to 2013 national microbiological baseline study in broiler chicken. Of the 1135 generic E. coli isolates tested, 940 (82.8%) were resistant to at least one antimicrobial, with a large number of unique AMR profiles observed. Of the 1495 Salmonella isolates tested, 879 (58.8%) were resistant to at least one antimicrobial. Resistance was most common to aminoglycosides, β-lactams, and tetracyclines and, for generic E. coli isolates only, folate inhibitors. Differences in AMR patterns were observed across regions for both E. coli and Salmonella. For Salmonella, the levels of resistance were similar across the different sectors sampled along the food chain (e.g., slaughterhouse and retail) and the types of product sampled. There were also considerable differences in the levels and patterns of resistance among different Salmonella serovars, with most Salmonella Enteritidis isolates being susceptible to all antimicrobials tested.
Keywords: antimicrobial resistance, Salmonella, E. coli, broiler chicken, Canada
Introduction
Antimicrobial resistance (AMR) is a global public health threat (WHO, 2019). Governments and international health organizations are increasingly focused on this threat, which reduces our ability to prevent and treat infectious diseases in both humans and animals.
According to the Centers for Disease Control and Prevention (CDC, 2013), at least 2 million individuals contract serious resistant infections in the United States every year and at least 23,000 people die as a result of such infections (CDC, 2013). In Canada, it is estimated that 5400 lives were lost in 2018 as a result of resistant infections (CCA, 2019).
Escherichia coli is a bacterium commonly found in the gastro-intestinal tract of warm-blooded animals. Poultry, in particular, can be a reservoir of antimicrobial-resistant E. coli strains that are capable of being pathogenic to humans (Johnson et al., 2017). Generic E. coli is also commonly used as an indicator organism for AMR.
Poultry is a well-known reservoir of Salmonella serovars of public health significance, particularly Salmonella Enteritidis (FAO-WHO, 2002; Pires et al., 2011; Painter et al., 2013). Gastroenteritis caused by non-typhoidal Salmonella ranks fourth among notifiable enteric bacterial diseases in Canada, with an estimated incidence of 269 cases per 100,000 individuals annually (Thomas et al., 2013). In the United States, more than 1 million non-typhoidal Salmonella cases are estimated to occur per year, including 378 deaths (Scallan et al., 2011). Of these, 100,000 illnesses and 38 deaths were associated with resistant strains (CDC, 2013).
The purpose of this study is to describe antimicrobial susceptibility patterns in E. coli and Salmonella isolates from Canadian poultry and to assess the influence of selected factors, including seasonality, type of sample, and geographical origin, on resistance. An equivalent study for Campylobacter is available (Dramé et al., 2020).
Materials and Methods
A national microbiological baseline study (MBS) was conducted in Canada in collaboration with industry and federal and provincial government partners, to estimate the prevalence and concentrations of Campylobacter and Salmonella in broiler chickens and raw chicken products processed in federally inspected slaughterhouses and sold at retail. Raw chicken products sampled at slaughterhouses were also tested for generic E. coli. Isolates collected during the MBS were randomly selected for antimicrobial susceptibility testing to estimate levels of AMR in different geographical areas and across the poultry supply chain. The study report describes the epidemiological design and sampling and testing methodologies used to collect and analyze the samples (CFIA, 2016). Samples were collected between December 2012 and October 2013 (for E. coli) and between December 2012 and December 2013 (for Salmonella), from 38 broiler slaughterhouses. Retail samples were collected over the same periods from supermarket chains and independent grocers at a 4:1 ratio in five regions (British Columbia [BC], Prairies, Ontario, Quebec, and Atlantic) across Canada.
A total of 1135 generic E. coli isolates from carcasses collected in slaughter establishments and 1495 Salmonella isolates were tested for antimicrobial susceptibility. The majority (72%) of the Salmonella isolates analyzed belonged to serovars Salmonella Kentucky with 662 (44%) isolates, Salmonella Heidelberg (317, 21%), and Salmonella Enteritidis (103, 7%). Supplementary Table S1 provides additional information on serovar distribution. Generic E. coli isolates originated exclusively from individual broiler carcasses collected in slaughterhouses. Salmonella isolates originated from composite cecal samples from 20 broiler chickens, individual broiler carcasses, or parts after chilling in slaughterhouses, and from carcasses or parts collected from retail outlets. These isolates represented three sectors of the food chain: (1) Slaughter: cecal samples, considered a surrogate of the state of contamination on farms; (2) Processing: carcasses or parts, representing the steps after carcasses are chilled and parts are packaged; and (3) Retail: representing chicken products available to consumers. Additional information on the distribution of isolates by sample type and stage in the processing chain is available in Supplementary Table S2.
Isolates were tested for susceptibility against 15 antimicrobials (Table 1) according to a broth microdilution method detailed in the 2013 Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) report (PHAC, 2015). Isolates with intermediate minimum inhibitory concentration values were grouped as susceptible to the antimicrobial being tested (PHAC, 2015).
Table 1.
Percentage of Generic Escherichia coli and Salmonella Isolates Resistant to 15 Antimicrobials
| Antimicrobial | No. of isolates resistant (% of isolates) |
|
|---|---|---|
| Escherichia coli (n = 1135) | Salmonella (n = 1495) | |
| Aminoglycosides | ||
| Gentamicin | 343 (30.2) | 31 (2.1) |
| Kanamycin | 153 (13.5) | 3 (0.2) |
| Streptomycin | 586 (51.6) | 621 (41.5) |
| β-Lactams | ||
| Amoxicillin-clavulanic acid | 406 (35.8) | 466 (31.2) |
| Ampicillin | 581 (51.2) | 484 (32.4) |
| Cefoxitin | 408 (35.9) | 463 (31) |
| Ceftiofur | 336 (29.6) | 469 (31.4) |
| Ceftriaxone | 411 (36.2) | 470 (31.4) |
| Folate pathway inhibitors | ||
| Sulfisoxazole | 650 (57.3) | 93 (6.2) |
| Trimethoprim-sulfamethoxazole | 182 (16) | 18 (1.2) |
| Macrolides | ||
| Azithromycin | 1 (0.1) | 0 (0) |
| Phenicols | ||
| Chloramphenicol | 60 (5.3) | 11 (0.7) |
| Quinolones | ||
| Ciprofloxacin | 2 (0.2) | 0 (0) |
| Nalidixic acid | 78 (6.9) | 7 (0.5) |
| Tetracyclines | ||
| Tetracycline | 523 (46.1) | 642 (42.9) |
Data were analyzed by using software from R Development Core Team, version 3.3.3 (Vienna, Austria), and Stata 15 (StataCorp LLC. 2017. Stata Statistical Software: Release 15; College Station, TX). Logistic regression was used to evaluate associations between resistance and industry sectors, region of sample origin (i.e., BC, Prairies, Ontario, Quebec and Atlantic), and season. Season was defined as: December to May—winter–spring season; June to November—summer–fall season. The significance level was set at p < 0.05 to identify important differences between variables. For Salmonella, the effect of serovar was also included in the model.
Results
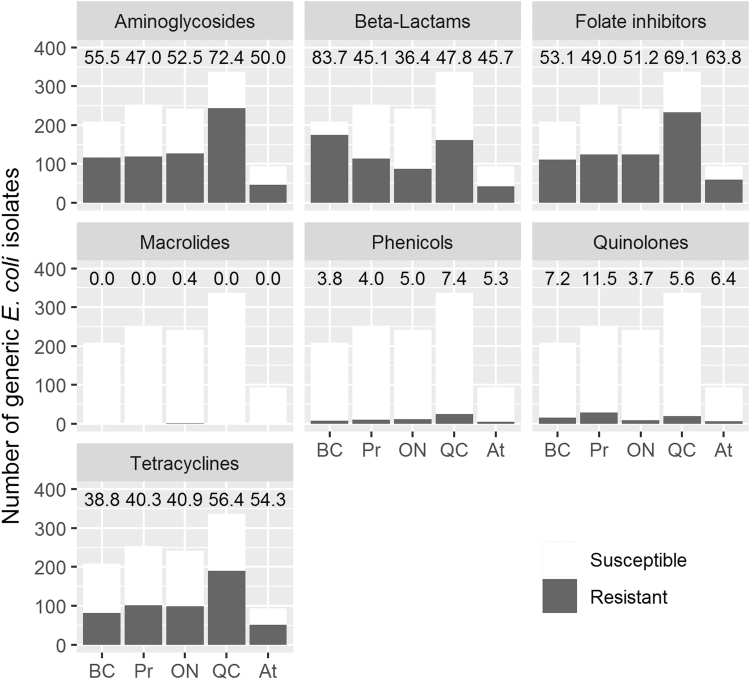
The prevalence of resistant generic E. coli isolates was high, with 940 (83%) isolates being resistant to at least one antimicrobial. Resistance was most common against folate inhibitors, aminoglycosides, β-lactams, and tetracyclines, and 420 (37%) isolates were resistant to at least one Category I* antimicrobial. Resistance against phenicols and quinolones was low across all regions, and only one isolate was resistant against macrolides (Fig. 1).
FIG. 1.
Resistance of generic Escherichia coli isolates to seven different antimicrobial classes by region. Numbers above bars are percentages of resistant isolates. BC, British Columbia; Pr, Prairies Region; ON, Ontario; QC, Quebec; At, Atlantic Region.
Of the 1135 E. coli isolates, 549 (48%) were resistant to two or three classes of antimicrobials and 223 (20%) were resistant to four or five classes. No isolates were resistant to more than five classes. Resistance was highest against sulfisoxazole (57%), streptomycin (52%), and ampicillin (51%). Only one isolate showed resistance to azithromycin and none against ciprofloxacin. The most common patterns were joint resistance against amoxicillin-clavulanic acid, ampicillin, cefoxitin, ceftiofur, and ceftriaxone (63 isolates, 6%) and against gentamicin, streptomycin, and sulfisoxazole (56 isolates, 5%).
We observed statistically significant differences in resistance between regions. There was no effect of season on resistance to any of the antimicrobials tested. Generic E. coli isolates from Ontario were less likely to be resistant to most antimicrobials than in other regions, especially Quebec, BC, and the Prairies, except for chloramphenicol and kanamycin, for which there was no difference. For trimethoprim-sulfamethoxazole, isolates from the Atlantic region, Ontario, and Quebec were more likely to be resistant than those from BC and the Prairies. Azithromycin and ciprofloxacin-resistant isolates were not subjected to logistic regression, because no or very low resistance to these antimicrobials was observed in this study.
Of the 1495 Salmonella isolates, 879 (59%) were resistant to at least one antimicrobial. High levels of resistance in Salmonella isolates were found against aminoglycosides, β-lactams, and tetracyclines, but there were significant differences in resistance across serovars. Resistance was most common to tetracycline and streptomycin, with more than 40% of isolates from cecal samples and chicken products at processing being resistant. No resistance was observed to azithromycin and ciprofloxacin, and resistance to chloramphenicol, kanamycin, and nalidixic acid was present in less than 1% of the isolates.
Although resistance levels were generally higher in generic E. coli isolates compared with Salmonella, the percentage of resistant isolates was fairly similar for β-lactams, macrolides, quinolones, and tetracyclines, as well as for streptomycin (Table 1).
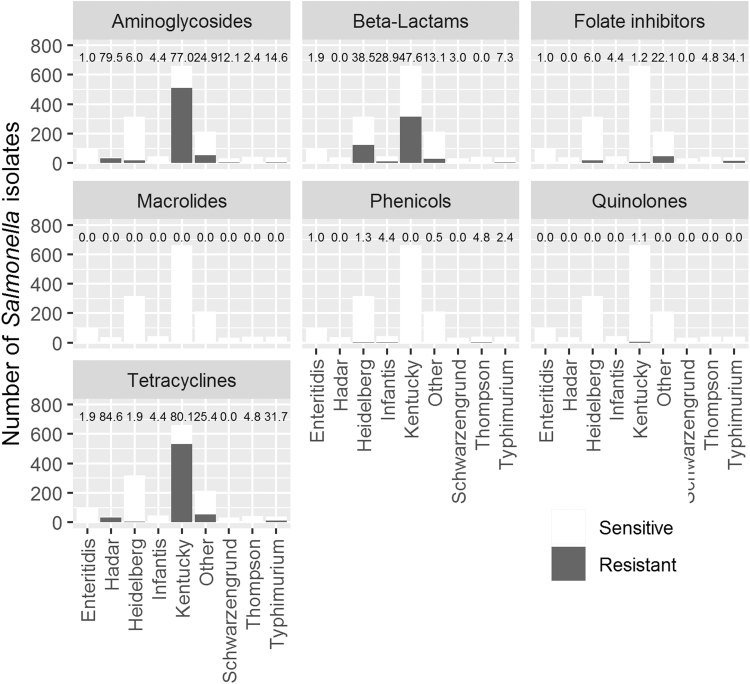
Figure 2 shows differences in resistance between serovars. Salmonella Kentucky was the most resistant serovar to β-lactams, aminoglycosides, and tetracyclines and it was the only serovar that showed quinolone resistance. Salmonella Heidelberg presented the second highest percentage of resistance against β-lactams (38%). Almost all Salmonella Enteritidis isolates were susceptible to all antimicrobial classes. Two Salmonella Enteritidis isolates out of 103 were resistant to β-lactams and tetracyclines and one was resistant to aminoglycosides, folate inhibitors, and phenicols. Salmonella Typhimurium isolates were moderately resistant, especially against tetracyclines (32% of the 41 isolates were resistant). Supplementary Table S3 provides additional information about the distribution of resistant isolates by region.
FIG. 2.
Resistance of Salmonella isolates to seven different antimicrobial classes, by serovar. Numbers above bars are percentages of resistant isolates.
Of the 1495 Salmonella isolates, 238 (16%) were resistant to one antimicrobial class, 626 (42%) to two or three classes, and only 15 (1%) were resistant to four or five classes. No isolate was resistant to more than five antimicrobial classes. The distribution of resistant isolates was very similar for isolates across stages in the production chain and from cecal samples, compared with those obtained from carcasses or parts (Supplementary Tables S4 and S5). The three most common patterns were joint resistance against streptomycin and tetracycline (276 isolates, 18%), amoxicillin-clavulanic acid, ampicillin, cefoxitin, ceftiofur, ceftriaxone, streptomycin and tetracycline (261 isolates, 18%), and amoxicillin-clavulanic acid, ampicillin, cefoxitin, ceftiofur, and ceftriaxone (177 isolates, 12%). The remaining 43 unique profiles were observed in less than 2% of isolates each (ranging from one to 28 isolates).
Results of the logistic regression models showed no industry sector, season, or sample type effects when comparing susceptible isolates with those resistant to at least one antimicrobial. The same was observed when the outcome was resistance against a given antimicrobial class. Assessing resistance by antimicrobial class was not possible for macrolides, as all isolates were susceptible, or for phenicols and quinolones due to lack of statistical power related to the low number of resistant isolates.
The logistic models showed regional differences in resistance, depending on the antimicrobial or antimicrobial class studied. Controlling for the effect of other variables, including serovar, Salmonella isolates from BC and the Prairies were more likely to be resistant against aminoglycosides, β-lactams, and tetracyclines than those from Ontario. Isolates from Quebec were also more likely to be resistant than those from Ontario against aminoglycosides and tetracyclines. Isolates from the Atlantic region had similar odds of resistance to those from Ontario for aminoglycosides and β-lactams, but they showed higher odds of resistance against tetracyclines. Odds of resistance against folate inhibitors were higher in Ontario compared with other regions.
Controlling for region, Salmonella Kentucky isolates were more likely to show resistance to at least one antimicrobial than the other serovars and to show individual resistance to aminoglycosides and tetracyclines. Salmonella Heidelberg resistance to β-lactams and Salmonella Enteritidis resistance to folate inhibitors were not different from Salmonella Kentucky.
Discussion
The high levels of AMR in generic E. coli isolates recovered from abattoir chicken carcasses in this study were similar to those in generic E. coli isolates in samples from poultry meat in CIPARS data from Canadian retail outlets the same year (PHAC, 2015). Higher levels of resistance against β-lactams found in BC were also observed in CIPARS data for the year 2013 (PHAC, 2015). These regional variations could be due to several factors, such as differences in prescribing and usage of antimicrobials, disease pressures, or use of vaccines. For example, regional differences in antimicrobial use in feed and for hatchery-level administration were observed in 2013 (PHAC, 2015).
Generic E. coli isolates in this study showed higher levels of resistance than equivalent isolates from the United States Department of Agriculture Hazard Analysis and Critical Control Points (HACCP) monitoring system for the National Antimicrobial Resistance Monitoring System (NARMS) in 2013 for 13 out of 15 antimicrobials, whereas NARMS isolates had slightly higher levels of resistance for ciprofloxacin and gentamicin (FDA, 2019). The antimicrobial susceptibility testing methodology used for our study is the same as for NARMS; however, there may be variations in sample collection or in primary isolation methods, which could explain these differences.
We compared levels of AMR in generic E. coli isolates with those in broiler meat in Denmark, Germany, Hungary, and Slovenia (EFSA and ECDC, 2015). Among these, Denmark had the lowest level of resistance and also had less resistance than isolates from our study, except for ciprofloxacin. Canadian E. coli isolates had lower levels of resistance for ampicillin, chloramphenicol, ciprofloxacin, and nalidixic acid compared with the other three EU countries. Resistance against gentamicin and streptomycin was higher in Canadian isolates compared with these three countries, and it was similar for tetracyclines. It seems that resistance to older drugs in lower categories of importance in human medicine is higher in Canada compared with the EU, but resistance to drugs in the higher categories of importance is lower in Canada, probably reflecting different antimicrobial use practices in these jurisdictions.
There was no resistance against macrolides and very low resistance against phenicols and quinolones in Salmonella isolates. The levels of resistance in our study were similar irrespective of where along the chain samples were collected, season, or type of product, and they were similar to those found in Salmonella isolates from CIPARS (PHAC, 2015). Data from NARMS (FDA, 2019) also showed that the levels of resistance for individual antimicrobials along the chain are somewhat stable, although the consistency in the levels of resistance across stages was greater in our study.
The levels of AMR in Salmonella isolates resistant to at least one antimicrobial were similar to those found by NARMS in 2013 in retail chicken and in the samples collected in poultry plants as part as their HACCP system (FDA, 2015), although resistance levels varied depending on the specific antimicrobial (FDA, 2019).
A comparison of resistance levels in Canadian Salmonella isolates from cecal samples with those found in broiler flocks in the European Union in 2013 (EFSA and ECDC, 2015) was carried out. Ranking the level of resistance detected in Canada among EU countries, the level of resistance in Canadian isolates was the fourth highest for ampicillin. For ciprofloxacin, Canadian isolates showed no resistance, as did isolates from Ireland, whereas the remaining countries ranged from 3% to 88%. Similarly, Canadian isolates showed the second lowest level of resistance for nalidixic acid, whereas the remaining countries ranged from 3% to 88%. Resistance against gentamicin was low in European countries (less than 5%), except for Romania (20%) and Spain (27%), compared with 2% in Canadian isolates.
Levels of resistance in Salmonella isolates in broiler meat in our study (combining the processing and retail stages) were also compared with those found in EU countries (EFSA and ECDC, 2015). Resistance against ampicillin was only higher in the Netherlands compared with Canadian isolates. Resistance against gentamicin was comparable, although Canadian isolates had lower levels of resistance than the average in the EU. Resistance against ciprofloxacin and nalidixic acid was lower in Canadian isolates compared with EU countries, with levels in our study equal or close to zero, compared with averages higher than 65% in the EU.
There are methodological differences between the sample collection, primary isolation, and AMR testing methods used in the EU compared with this study. Nevertheless, it is reassuring to see that the levels of resistance in Canada are on the lower end of the spectrum compared with those in EU countries, except for tetracyclines in cecal samples and ampicillin in broiler meat, for which resistance in Canadian Salmonella isolates is higher than in most EU countries. As with generic E. coli, resistance to older, less important drugs for human medicine was higher in Canada, but resistance to antimicrobials of medical importance was higher in the EU.
Differences in the levels of resistance between Salmonella serovars have been widely reported previously (Lee et al., 2016; Duc et al., 2019; FDA, 2019). More than 99% of Salmonella Enteriditis isolates from our study were fully susceptible, a result that was also found in CIPARS data from retail for 2013 (PHAC, 2015) and in data from NARMS for the same year (FDA, 2019), but not in the EU, where low to moderate resistance levels were observed (EFSA and ECDC, 2015). The proportion of fully susceptible Salmonella Enteriditis isolates from human cases was lower according to CIPARS data (84% and 83% in 2013 and 2014, respectively) (PHAC, 2015, 2016). This overrepresentation of resistant Salmonella Enteritidis in human infections compared with their prevalence in chicken may be a reflection of the relative importance of travel-associated Salmonella Enteritidis cases (Nesbitt et al., 2012).
Salmonella Heidelberg was the second most common serovar in our study and is one of the most common found in human salmonellosis cases in Canada (Carson et al., 2019). This serovar presented the second highest levels of resistance against β-lactams after Salmonella Kentucky, and higher levels than were observed by NARMS (FDA, 2019). The results presented here are similar to those reported by CIPARS. The CIPARS data have been used to demonstrate that the presence of ceftiofur-resistant Salmonella Heidelberg in poultry meat is associated with both the use of ceftiofur in chicken hatcheries and its incidence in humans in Canada (Dutil et al., 2010; Carson et al., 2019). In 2014, the poultry industry in Canada banned preventive use of ceftiofur. Since that time, ceftiofur resistance in Salmonella Heidelberg from chicken and humans has decreased (Carson et al., 2019).
Salmonella Kentucky was the most common serovar in this study and presented higher levels of resistance to aminoglycosides and tetracyclines than other serovars after controlling for other variables. High resistance in Salmonella Kentucky was also reported by NARMS (FDA, 2019) and the EU (EFSA and ECDC, 2015). The levels of resistance in this serovar have increased compared with when CIPARS first started collecting data in 2002 (Parmley et al., 2013). This is worthy of consideration given that this serovar is an uncommon cause of human disease, possibly due to the lack of SPI2-associated genes (Dhanani et al., 2015). In addition, the primary concern in Salmonella Kentucky isolates from clinical cases in Canada is increasing levels of resistance to quinolones that appears to be associated with travel (Mulvey et al., 2013). These findings stress the importance of conducting further research on the role of poultry meat as a contributor to the AMR burden in human health.
Conclusions
This study provides a comprehensive snapshot of AMR in the poultry production chain in Canada. Levels of resistance were higher in E. coli than in Salmonella isolates, and they did not change significantly across the different stages of the chain or according to season. There was variability in the levels of resistance in both E. coli and Salmonella from different regions. Compared with other jurisdictions, resistance in Canada was higher to older antimicrobials and lower to newer ones. The vast majority of Salmonella Enteritidis isolates were fully susceptible to the antimicrobials tested.
Supplementary Material
Acknowledgments
The authors thank all CFIA staff and industry personnel from federally registered establishments involved in sample collection as well as all the provincial inspectors from the Ministries of Health and Agriculture who collected raw chicken meat products from retail stores across the country. They also acknowledge the contribution of Linda Cole, Laboratory Supervisor, and Andrea Desruisseau, Microbiology Technologist, from the Laboratory for Foodborne Zoonoses in Guelph (now the National Microbiology Laboratory at Guelph) for performing susceptibility testing, serotyping and phage typing of the bacterial isolates. In addition, they would like to thank J.T. McClure, Carol McClure, Matthew Saab, Cynthia Mitchell, and Anne Muckle from the University of Prince Edward Island for primary isolation and AMR testing of CIPARS retail isolates from the Atlantic region.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by Agriculture and Agri-Food Canada via the Agricultural Flexibility Fund (AGRI0008).
Supplementary Material
Health Canada has categorized antimicrobials into four categories depending on their importance in human medicine, with Category I antimicrobials considered the most important. See https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html for details.
References
- Canadian Food Inspection Agency (CFIA). National Microbiological Baseline Study in Broiler Chicken: December 2012–December 2013. 2016. Available at: http://www.inspection.gc.ca/food/chemical-residues-microbiology/food-safety-testing-reports/2016-08-17/december-2012-december-2013/eng/1471358115567/1471358175297, accessed May7, 2020
- Carson C, Li XZ, Agunos A, Loest D, Chapman B, Finley R, Mehrotra M, Sherk LM, Gaumond R, Irwin R. Ceftiofur-resistant Salmonella enterica serovar Heidelberg of poultry origin—A risk profile using the Codex framework. Epidemiol Infect 2019;147:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States. 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf, accessed May7, 2020
- Council of Canadian Academies (CCA). 2019. When Antibiotics Fail. Ottawa, ON, Canada: The Expert Panel on the Potential Socio-Economic Impacts of Antimicrobial Resistance in Canada, Council of Canadian Academies [Google Scholar]
- Dhanani AS, Block G, Dewar K, Forgetta V, Topp E, Beiko RG, Diarra MS Genomic comparison of non-typhoidal Salmonella enterica serovars Typhimurium, Enteritidis, Heidelberg, Hadar and Kentucky isolates from broiler chickens. PLoS One 2015;10:e012; 8773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dramé O, Leclair D, Parmley EJ, Deckert A, Ouattara B, Daignault D, Ravel A. Antimicrobial resistance of Campylobacter in broiler chicken along the food chain in Canada. Foodborne Pathog Dis 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc VM, Nakamoto Y, Fujiwara A, Toyofuku H, Obi T, Chuma T. Prevalence of Salmonella in broiler chickens in Kagoshima, Japan in 2009 to 2012 and the relationship between serovars changing and antimicrobial resistance. BMC Vet Res 2019;15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutil L, Irwin R, Finley R, Ng LK, Avery B, Boerlin P, Bourgault AM, Cole L, Daignault D, Desruisseau A, Demczuk W. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg Infect Dis 2010;16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control). EU Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2013. EFSA J 2015;13:4036., 178 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization (FAO) and World Health Organization (WHO). Risk assessments of Salmonella in eggs and broiler chickens (Vol. 2). 2002. Available at: http://www.fao.org/docrep/005/Y4392E/y4392e00.htm, accessed May7, 2020
- Food and Drug Administration (FDA). NARMS Executive report 2012–2013. 2015. Available at: https://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM453398.pdf, accessed May7, 2020
- Food and Drug Administration (FDA). NARMS Now. Rockville MD: U.S. Department of Health and Human Services. 2019. Availableat: https://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm416741.htm, accessed December20, 2019
- Johnson JR, Porter SB, Johnston B, Thuras P, Clock S, Crupain M, Rangan U. Extraintestinal pathogenic and antimicrobial-resistant Escherichia coli, including sequence type 131 (ST131), from retail chicken breasts in the United States in 2013. Appl Environ Microbiol 2017;83:e02956-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SK, Choi D, Kim HS, Kim DH, Seo KH. Prevalence, seasonal occurrence, and antimicrobial resistance of Salmonella spp. isolates recovered from chicken carcasses sampled at major poultry processing plants of South Korea. Foodborne Pathog Dis 2016;13:544–550 [DOI] [PubMed] [Google Scholar]
- Mulvey MR, Boyd DA, Finley R, Fakharuddin K, Langner S, Allen V, Ang L, Bekal S, El Bailey S, Haldane D, Hoang L. Ciprofloxacin-resistant Salmonella enterica serovar Kentucky in Canada. Emerg Infect Dis 2013;19:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt A, Ravel A, Murray R, McCormick R, Savelli C, Finley R, Parmley J, Agunos A, Majowicz SE, Gilmour M, Canadian Integrated Program for Antimicrobial Resistance Surveillance Public Health Partnership. Integrated surveillance and potential sources of Salmonella Enteritidis in human cases in Canada from 2003 to 2009. Epidemiol Infect 2012;140:1757–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter JA, Hoekstra RM, Ayers T, Tauxe RV, Braden CR, Angulo FJ, Griffin PM. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg Infect Dis 2013;19:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley EJ, Pintar K, Majowicz S, Avery B, Cook A, Jokinen C, Gannon V, Lapen DR, Topp E, Edge TA, Gilmour M. A Canadian application of one health: Integration of Salmonella data from various Canadian surveillance programs (2005–2010). Foodborne Pathog Dis 2013;10:747–756 [DOI] [PubMed] [Google Scholar]
- Pires SM, Knegt L, Hald T. Estimation of the relative contribution of different food and animal sources to human Salmonella infections in the European Union. EFSA Supporting Publications 2011;8 [Google Scholar]
- Public Health Agency of Canada (PHAC). Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2013 Annual Report—Chapter I. Design and Methods. 2015;20–21. Available at: www.phac-aspc.gc.ca/cipars-picra/2012/assets/pdf/HP2-4-2012-1-eng.pdf, accessed May7, 2020 [Google Scholar]
- Public Health Agency of Canada (PHAC). 2016. Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2014 Annual Report. Guelph, Ontario, Public Health Agency of Canada, 2016 [Google Scholar]
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 2011;17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MK, Murray R, Flockhart L, Pintar K, Pollari F, Fazil A, Nesbitt A, Marshall B. Estimates of the burden of foodborne illness in Canada for 30 specified pathogens and unspecified agents, circa 2006. Foodborne Pathog Dis 2013;10:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). No Time to Wait: Securing the future from drug-resistant infections. Report to the Secretary-General of the United Nations, Geneva. Interagency Coordination Group on Antimicrobial Resistance WHO. 2019. Available at: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1, accessed May7, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.