Abstract
Aim
To perform a systematic review summarizing the knowledge of genetic variants, gene, and protein expression changes in humans and animals associated with urgency urinary incontinence (UUI) and to provide an overview of the known molecular mechanisms related to UUI.
Methods
A systematic search was performed on March 2, 2020, in PubMed, Embase, Web of Science, and the Cochrane library. Retrieved studies were screened for eligibility. The risk of bias was assessed using the ROBINS‐I (human) and SYRCLE (animal) tool. Data were presented in a structured manner and in the case of greater than five studies on a homogeneous outcome, a meta‐analysis was performed.
Results
Altogether, a total of 10,785 records were screened of which 37 studies met the inclusion criteria. Notably, 24/37 studies scored medium‐high to high on risk of bias, affecting the value of the included studies. The analysis of 70 unique genes and proteins and three genome‐wide association studies showed that specific signal transduction pathways and inflammation are associated with UUI. A meta‐analysis on the predictive value of urinary nerve growth factor (NGF) levels showed that increased urinary NGF levels correlate with UUI.
Conclusion
The collective evidence showed the involvement of two molecular mechanisms (signal transduction and inflammation) and NGF in UUI, enhancing our understanding of the pathophysiology of UUI. Unfortunately, the risk of bias was medium‐high to high for most studies and the value of many observations remains unclear. Future studies should focus on elucidating how deficits in the two identified molecular mechanisms contribute to UUI and should avoid bias.
Keywords: gene expression changes, genetic variants, protein expression changes, urgency urinary incontinence
Abbreviations
- ATP
adenosine triphosphate
- BDNF
brain‐derived neurotrophic factor
- BMI
body mass index
- CI
confidence interval
- Cr
creatinine
- CRP
C‐reactive protein
- ECM
extracellular matrix
- GWAS
genome‐wide association study
- MCP
monocyte chemoattractant protein
- M‐Ras
muscarinic‐Ras
- NGF
nerve growth factor
- OAB
overactive bladder
- ROBINS‐I
risk of bias in non‐randomized studies of interventions
- SD
standard deviation
- SE
standard error
- SMD
standardized mean difference
- SYRCLE
SYstematic Review Centre for Laboratory animal Experimentation
- TRPV1
transient receptor potential cation channel subfamily V member 1
- UUI
urgency urinary incontinence
1. INTRODUCTION
Urgency urinary incontinence (UUI) is a prevalent symptom that negatively impacts the quality of life. 1 , 2 Patients with UUI sense a sudden, compelling desire to pass urine that is difficult to defer combined with the involuntary loss of urine. 2 , 3 The reported prevalence of UUI ranges between 1.8% and 30.5%, and differs substantially due to different definitions in studies. 4 Clear risk factors for UUI are age, obesity, and postmenopausal status in women. 5 , 6 , 7 , 8
The pathophysiology of UUI is considered to be multifactorial: both intrinsic and environmental factors are involved.
The underlying processes that contribute to the development of UUI are still unresolved. Although the cellular and/or molecular mechanisms related to UUI have been studied, most studies focus on the overarching overactive bladder (OAB) syndrome. The current systematic review focusses on one clinically well‐defined and objectively measurable symptom (UUI) to be able to study the relation between a clear phenotype and/or population and cellular/molecular mechanisms. Because several symptoms (urgency, urinary frequency, nocturia, and/or UUI) may indicate OAB, a systematic review of all these symptoms or OAB as a whole may lead to inaccuracy or cluttering in the results due to ill‐defined (mixed) populations or a combination of phenotypes. This was one of the drawbacks noticed in a recent systematic review of biomarkers of several lower urinary tract symptoms (LUTS), including OAB. 9 A systematic review summarizing and critically evaluating all available evidence of cellular and/or molecular mechanisms underlying UUI is lacking. Such overview is critical to understand the mechanisms involved in the pathophysiology that leads to UUI.
1.1. Objective
This systematic review combines and summarizes studies—both human and animal–on genetic variants, gene, and protein expression changes in relation to UUI.
2. MATERIALS AND METHODS
We investigated human and animal studies (domain) on genetic variants, gene and/or protein expression changes (outcome) in relation to UUI (determinant). A prospectively registered protocol in Prospero was used concerning genetic variants, gene, and protein expression changes in relation to urinary incontinence (UI) in general (Supporting Information 1), ultimately narrowed to UUI only instead of UI in general to examine a more homogeneous population.
2.1. Information sources and search strategy
On March 2, 2020, a systematic search was performed in PubMed, Embase, Web of Science, and the Cochrane library to identify available studies using the search strategy described in Supporting Information 2. The terms used were related to UI in general and a broad spectrum of genetic and protein expression terms and assays. References of reviews and included studies were cross‐checked for studies not retrieved by the database search.
Studies were screened by two independent reviewers in two phases (title/abstract and full‐text phase). Studies judged as eligible for full‐text screening by one of the reviewers were screened for full text by both. The inclusion criteria were: studies with primary research data of affected cases (UUI) and controls of both humans and animals (all species and genders/sexes), examining genetic variants, gene expression, or protein expression differences, with sufficient information to determine the risk of bias. Nocturnal enuresis in children was beyond the scope of this review and was excluded. We included studies on OAB when the criterium of incontinence was met in greater than 50% of the patients. For animal studies, a clear UUI model must be defined.
2.2. Data extraction
The extraction of study characteristics was performed by one reviewer and verified by a second reviewer. Characteristics extracted for human studies were: first author and year of publication, gender, number of participants, definition and diagnosis‐method of UUI, assessed material, assay method, investigated gene/protein/genetic variant, and results of the (value) differences between the groups (UUI and controls). For animal studies, first author and year of publication, type of animal, sex, number of subjects, UUI induction method and confirmation of diagnosis, assessed material, assay method, and investigated gene/protein/genetic variant were extracted.
The risk of bias assessment was performed by one and checked by a second reviewer. All discrepancies were discussed until agreement was reached and with the help of a third reviewer when necessary. The tools used were the Cochrane risk of bias in non‐randomized studies of interventions (ROBINS‐I) 10 for human studies, and the SYRCLE risk of bias tool 11 for animal studies. Differences between cases and controls in age, body mass index (BMI), and menopausal status were recorded and taken into account in the risk of bias assessment. The basic signaling questions of the ROBINS‐I tool do not cover in vitro aspects of studies, that is, the derivation and preparation of cell material for outcome assessment. Therefore, seven signaling questions addressing the risk of bias for in vitro aspects of studies were used if applicable (Supporting Information 3), based on a tool developed in 2016 for in vitro studies by the National Toxicology Program. 12
The retrieved data per outcome measure were presented in a structured manner. Outcome measures were grouped in themes according to the knowledge of their functions. Since it was expected that the outcomes of the included studies were too diverse for an overall meta‐analysis, we decided that when more than five homogeneous studies explored one outcome measure, a meta‐analysis of that outcome measure would be performed. When a standard error (SE) was provided instead of the standard deviation (SD) in the studies included in the meta‐analysis, SDs were calculated using the following formula: SD = SE × . Standardized mean difference (SMD) was used as an effect size measure. Subsequently, a random effect meta‐analysis was performed using STATA version 15, and forest plots were created. For quantifying heterogeneity, I 2 was used.
3. RESULTS
3.1. Study selection
Figure 1 shows the flow chart of the review. Out of 10,785 retrieved articles, only 37 studies met the inclusion criteria and were included in the final analysis.
Figure 1.
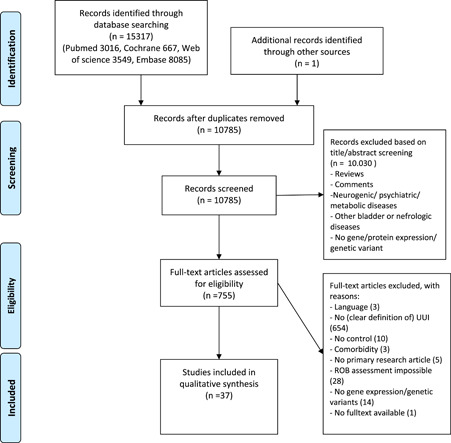
Systematic selection of articles and main reasons for exclusion based on Prisma 2009. ROB, risk of bias;
UUI, urgency urinary incontinence 13
3.2. Study characteristics
Tables 1 and 2 show an overview of the characteristics of the human (N = 33) and animal (N = 4) studies, respectively. Nine studies investigated genetic variants, 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 4 studies gene expression changes, 23 , 24 , 25 , 26 and 26 studies protein expression changes 24 , 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 including 17 studies on urinary or serum biomarkers. 27 , 28 , 31 , 32 , 33 , 34 , 35 , 36 , 38 , 39 , 41 , 43 , 44 , 45 , 47 , 49 , 50 Four studies investigated possible associations in a nonhypothesis‐driven manner: three genome‐wide association studies (GWASs) 15 , 17 , 18 and one whole‐genome expression microarray. 23 Seventy unique genes/proteins/protein‐related products were analyzed in hypothesis‐driven studies, and the majority of the genes/proteins/protein‐related products (83%) were examined in only one study. The four animal studies were all (conditional) gene knockout mouse models. 19 , 20 , 21 , 22 A more extensive description of study characteristics is presented in Tables S1 and S2.
Table 1.
Study characteristics of human studies
| First author (year of publication) | Assay/method | n, UUI | n, controls | Definition trait | Assessed material | Analyzed genes/proteins | ||
|---|---|---|---|---|---|---|---|---|
| Severity | Assessment of clinical symptoms | UDT | ||||||
| Alkis et al. (2017) 27 | ELISA | 16 | 45 | NI | By 3‐day VD | Y | Urine | BDNF, GAG, MCP‐1, and NGF |
| Antunes‐Lopes et al. (2013) 28 | ELISA | 37 | 20 | Naïve to any form of treatment, symptoms ≥ 6 months in duration | By 7‐day VD and USS | NI | Urine | BDNF, GDNF, and NGF |
| Birder et al. (2013) 29 | Western blot | 8 | 7 | Nonneurogenic UUI refractory to antimuscarinics: frequency > 10/day, ≥1 UUI/day | NI | NI | Primary HBUC of biopsies of posterior wall | M3R and TRPV1 |
| Carey et al. (2000) 30 | EM/IHC | 13/7 | 11/5 | Severe idiopathic detrusor instability not specified | NI | Y | UBSM biopsies above the trigone and midline | (complementary) Dense plaques, membrane caveolae, and vinculin |
| Cartwright et al. (2010) 23 | Whole‐genome expression microarray | 5 | 5 | Detrusor overactivity, with symptomatic urinary urgency and UUI, >10 voids/day | By 3‐day VD and ICIQ‐FLUTS | Y | Urothelium, lamina propria, and UBSM from biopsies of the posterior bladder wall | Whole genome |
| Cornu et al. (2011) 14 | DNA sequencing | 30 | 66 | UUI frequency not specified, >3 months | NI | NI | Blood | Androgen receptor CYP‐17, CYP‐19, and estrogen receptor‐1 |
| Christiaansen et al. (2011) 24 | RT‐PCR, FACS, and ELISA | 3 | 3 | Urinary frequency > 10/day, ≥1 UUI/day | NI | NI | HBUC of random biopsies | HIF‐1a, HIF‐2a, and VEGF |
| Chuang et al. (2010) 31 | High‐sensitivity CRP assay | 18 | 20 | UUI ≥ 1/day | NI | NI | Serum and urine | CRP |
| Farhan et al. (2019) 32 | ELISA | 18 | 10 | UUI ≥ 1/day | By 3‐day VD | NI | Urine | MCP‐1 |
| Funada et al. (2018) 15 | GWAS | 187 | 4096 | UUI (OABSS ≥ 3, urgency score ≥ 2, UUI score > 2 in OABSS) | By OABSS | NI | Blood | GWAS with 99,059 SNPs, and additionally three genes previously associated with UUI (ADAMTS16, CIT, and ZNF521) |
| Honda et al. (2014) 16 | PCR‐based | 61 | 100 | UUI ≥ 1/day | By 3‐day VD | NI | Hair | 1 Variant in B3‐AR |
| Hsiao et al. (2012) 33 | Particle‐enhanced turbidimetric assay | 39 | 18 | UUI ≥ 1/3 days | By 3‐day VD, OABSS, and modified IUSS | Y | Serum | CRP |
| Keske et al. (2019) 34 | Colorimetric assays | 38 | 29 | NI | NI | Y | Serum | TAC, TOS, PON, arylesterase, AOPP, and IMA |
| Kim et al. (2015) 35 | ELISA | 39 | 62 | ≥3 UUI/3 days, no history of diagnosis/treatment for OAB | By 3‐day VD | Y | Urine | HB‐EGF and NGF |
| Kubota et al. (2018) 36 | ELISA | 612 | 147 | NI | By OABSS | NI | Urine | Stem cell factor |
| Kumar et al. (2010) 37 | Luminometry | 8 | 9 | Refractory symptoms of UUI, frequency, and urgency, not further specified | NI | Y | Urothelium. Patients: bladder dome, Controls: site distant from tumor (nonirradiated bladder), normal looking bladder area | ATP |
| Kuo et al. (2010) 38 | ELISA | 25 | 28 | ≥1 UUI/3 days | By 3‐day VD | Y | Urine | NGF |
| Kuo et al. (2010) 39 | ELISA | 22 | 49 | ≥1 UUI/3 days | By 3‐day VD | Y | Urine | NGF |
| Li et al. (2011) 25 | Immunofluorescence, PCR, and Western blot | 2 | 2 | Nonneurogenic UUI refractory to antimuscarinics: frequency > 10/day, ≥1 UUI/day | NI | NI | HBUC | TRPV1 |
| Li et al. (2013) 40 | Immunohisto‐fluorescence and HPLC | 4 | 6 | ≥1 UUI/day, frequency > 10/day | NI | NI | HBUC | Polyamines |
| Liu et al. (2007) 26 | Quantitative competitive RT‐PCR | 12 | 42 | Refractory UUI, frequency, urgency, and nocturia, despite ≥ 2 anticholinergics and bladder training >1 year | NI | Y | Urothelium, lamina propria, and UBSM biopsies from body 2 cm from the left ureteric orifice and central trigone | TRPV1 |
| Liu et al. (2008)41 | ELISA | 80 | 40 | ≥1UUI/day, urgency and frequency | By 3‐day VD | Y | Urine | NGF |
| Liu et al. (2010) 42 | IHC and ELISA | 18 | 14 | UUI patients who underwent botulinum toxin A injection | NI | Y | Urothelium (location unknown) and urine | NGF |
| Liu et al. (2011) 43 | ELISA | 17 | 31 | ≥3 UUI/3 days, refractory to 3 months of treatment | By 3‐day VD | Y | Serum and urine | NGF |
| Liu et al. (2011) 44 | ELISA | 106 | 84 | ≥1 UUI/3 days | By 3‐day VD | NI | Urine | NGF |
| Liu et al. (2013) 45 | Bead‐based human serum adipokine panel B kit and particle‐enhanced turbidimetric assay | 14 | 26 | ≥3 UUI/3 days, refractory to previous antimuscarinic therapy | By 3‐day VD | NI | Serum | CRP, IL‐1b, IL‐6, IL‐8, insulin, leptin, MCP‐1, NGF, and TNF‐ a |
| Moore et al. (2001) 46 | IHC | 18 | 22 | UUI refractory to antimuscarinic drugs for > 12 months | NI | Y | Bladder biopsy tissue including UBSM cells | P2X(1–7) |
| Penney et al. (2019) 17 | GWAS | 1942 | 4811 | UUI weekly | Biennial questionnaire | NI | Blood and/or cheek cell sample | GWAS of 1,410,640 variants |
| Richter et al. (2015) 18 | GWAS and replication in a second cohort | 1102 | 405 | Anamnestic symptoms of UUI, >1/month who leaked sufficiently to wet or soak their underpants or clothes | NI | NI | Blood | GWAS of 975,508 variants, after imputation 9,077,347 |
| Richter et al. (2017) 47 | ELISA and magnetic polystyrene bead‐based immunoassay | 260 | 54 | Refractory UUI of ≥ 6/3 days, despite ≥ 1 supervised behavioral/physical therapy and ≥ 2 anticholinergic drugs | By 3‐day VD | Y | Urine | BDNF, CGRP, collagenase activity, GMC‐SF, IL‐Ib, IL‐6, IL‐8, MMP‐1, MMP‐2, MMP‐9, NGF, NTx, TNF‐a, tropoelastin, and substance P |
| Schofield et al. (2005) 48 | IHC | 18 | 23 | Refractory UUI, persistent disabling urgency of ≥ 8 voids/24 h, despite ≥2 anticholinergic drugs > 12 months | By 3‐day VD | Y | Subepithelial and UBSM nerve fibers | GAP‐43 |
| Silva‐Ramos et al. (2013) 49 | Luciferin–luciferase bioluminescence assay and ELISA | 34 | 36 | UUI ≥ 1/day, urgency, frequency ≥ 8 voids/day | NI | Y | Urine | ATP and NGF |
| Ustundag et al. (2019) 50 | Commercially available kits, immunoassay, and nephelometry | 42 | 34 | OAB‐questionnaire score > 11 | By OAB‐questionnaire | NI | Serum | Calcium, triglyceride, HDL, LDL, total cholesterol, Hba1c, parathormone, vitamin D CRP, ferric reducing power of plasma, albumin, IMA, native thiol, total thiol, and disulfide |
Abbreviations: ATP, adenosine triphosphate; B1 integrin, β1 integrin; B3‐AR, Beta‐3 adrenergic receptor; BDNF, brain‐derived neurotrophic factor; CGRP, calcitonin gene‐related peptide; CRP, C‐reactive protein; ELISA, enzyme‐linked immuno sorbent assay; FACS, fluorescence‐activated cell sorting; GAG, glycosaminoglycans; GAP‐43, growth association protein 43; GDNF, glial cell line‐derived neurotrophic factor; GMC‐SF, granulocyte‐macrophage colony‐stimulating factor; GWAS, genome‐wide association study; HB‐EGF, heparin‐binding epidermal growth factor‐like growth factor; HBUC, human bladder urothelium cells; HDL, high‐density lipoprotein; HIF, hypoxia‐inducible factor; HPLC, high‐performance liquid chromatography; ICIQ‐FLUTS, International Consultation on Incontinence Questionnaire‐Female Lower Urinary Tract Symptoms; IHC, immunohistochemistry; IL, interleukin; IUSS, Indevus Urgency Severity Score; LDL, low‐density lipoprotein; M2/3R, muscarinic 2/3 receptor; MCP‐1, monocyte chemoattractant protein‐1; MMP, matrix metalloproteinase; M‐Ras, muscarinic‐Ras; NGF, nerve growth factor; NI, no information; NTx, N‐terminal telopeptide type 1 collagen; OAB, overactive bladder; OABSS overactive bladder symptom score; SNP, single‐nucleotide polymorphism; TNF‐α, tumor necrosis factor α; TRPV1, transient receptor potential cation channel subfamily V member 1; UBSM, urinary bladder smooth muscle; UDT, urodynamic test; UUI, urgency urinary incontinence; VD, voiding diary; VEGF, vascular endothelial growth factor; Y, yes.
Table 2.
Study characteristics of animal studies ([conditional] gene deletion mouse models)
| First author (year of publication) | Animal characteristics | n, UUI | n, controls | UUI diagnosis | (c)KO | Analyzed tissue | Analyzed genes/proteins |
|---|---|---|---|---|---|---|---|
| Ehrhardt et al. (2015) 19 | Gene deletion: KO mice backcrossed with C57Bl/6 till F10 | PAM | PAM | Increased amplitudes of spontaneous bladder contractions, increased number of urine spots | KO | Whole animal, bladder special focus on UBSM | M‐Ras, M2R, and M3R |
| WT: C57Bl/6 mice | |||||||
| Kanasaki et al. (2013) 20 | 16 Gene deletion: B1‐integrin floxed/floxed | PAM | PAM | Dramatic loss of voiding control, that is, inability to restrict voiding location and distribution of spot sizes | cKO | Whole bladder, special focus on urothelium | B1‐integrin |
| B6; 129‐Itgb1tm1EfuJ mice expressing Cre | |||||||
| WT: B1‐integrin floxed/floxed | |||||||
| B6; 129‐Itgb1tm1EfuJ not expressing Cre | |||||||
| Meredith et al. (2004) 21 | Gene deletion: KO C57Bl/6 mice | PAM | PAM | Many small urination spots, yellow perineal staining | KO | Whole bladder, special focus on UBSM | BK channel and Slo1 |
| WT: C57Bl/6 mice | |||||||
| Thorneloe et al. (2005) 22 | Gene deletion: Slo−/− mice not further specified | 8 | 8 | Increased bladder pressures, increased frequency of pressure oscillations, and urine leakage | KO | UBSM stripes | BK channel and Slo1 |
| WT: Slo+/+ mice not further specified |
Abbreviations: cKO, conditional knockout; KO, knockout; PAM, per assay mentioned; UBSM, urinary bladder smooth muscle; UUI, urgency urinary incontinence; WT, wildtype.
3.3. Risk of bias of included studies
In the overall risk of bias judgment with the ROBINS‐I tool, 6 studies scored high, 29 , 31 , 34 , 37 , 39 , 42 18 medium‐high, 15 , 23 , 24 , 25 , 27 , 28 , 33 , 35 , 36 , 38 , 40 , 41 , 43 , 44 , 45 , 46 , 48 , 50 and 9 medium‐low 14 , 16 , 17 , 18 , 26 , 30 , 32 , 47 , 49 (Figure 2). Human studies scored poor on the topics of confounding, bias in the measurement of outcomes, and reporting of deviations between study groups. In the SYRCLE tool, all four animal studies scored unclear on risk of bias for most items (Figure 3). 19 , 20 , 21 , 22 The topics blinding (performance and detection), random outcome assessment, and incomplete outcome data were scored most often as contributing to unclear the risk of bias. Tables S3 and S4 show the risk of bias assessment per study.
Figure 2.
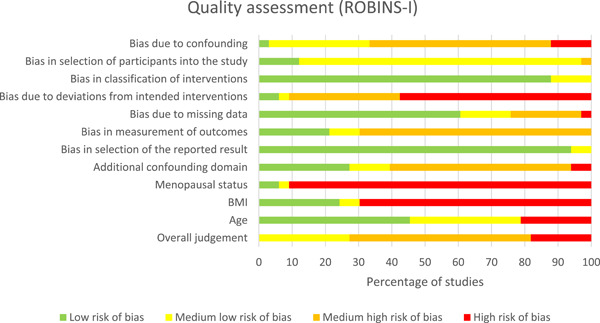
Risk of bias graph of each item from the Cochrane ROBINS‐I tool that was applied to all included human studies and scored by two investigators. For each item, several questions were scored with answers ranging from yes/probably yes/probably no/no/no information/not applicable. Finally, all items were scored as low risk of bias, medium‐low risk of bias, medium‐high risk of bias, and high risk of bias. BMI, body mass index; ROBINS‐I, risk of bias in non‐randomized studies of interventions
Figure 3.
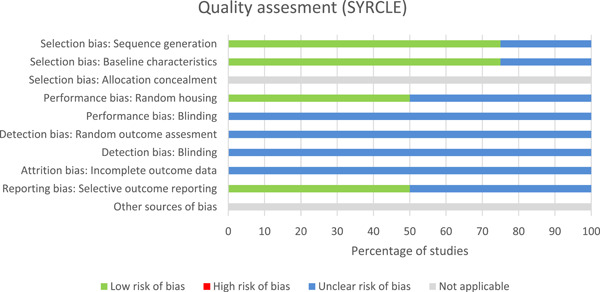
Risk of bias graph of each item from the SYRCLE tool that was applied to all included animal studies and scored by two independent investigators. For each item, “yes” correlates to low risk of bias and scores 1, while “no” correlates to high risk of bias and scores 0. SYRCLE, SYstematic Review Centre for Laboratory animal Experimentation
3.4. Synthesis of the results
The overview of the extracted results of the included studies are displayed in Tables 3 (nonhypothesis‐driven) and 4 (examining specific gene/protein/protein‐related product). Concerning genetic variants, the three GWASs did not find any replicable association (Table 3). 15 , 17 , 18 Another human study, investigating specific predefined polymorphisms, indicated an association between a polymorphism in the androgen receptor and UUI. 14 The (conditional) knockout of muscarinic‐Ras (M‐Ras), 19 β‐1 integrin, 20 and Slo1 21 , 22 genes led to UUI‐like symptoms in animals, demonstrating a functional correlation between these genes and the occurrence of UUI.
Table 3.
Results of nonhypothesis‐driven studies
| First author (year of publication) | Type of study | Population studied | Results | Extra | |
|---|---|---|---|---|---|
| Cartwright et al. (2010) 23 | Transcriptome analysis of bladder biopsies (Affymetrix array) | Women with and without UUI | ≥Twofold change, p < .005: FAM69C, MYOM2, SLC5A9, C3orf16, RUNX1, GAN, PWRN1, PDE5A, NCAM1, LOC100126784, MYLK4, GFRA3, SPTBN1, S100B, PTGS1, RGS11, MYOM1, PLN, NRP2, FXYD7, RYR2, MAEL, DCAF12L1, and CHRM3 | Pathway analysis: cytoskeleton remodeling, cell adhesion, smooth muscle contraction, cholinergic, G‐protein coupled, and calcium‐dependent signaling | |
| p < .01: 1115 Differentially expressed genes | |||||
| Richter et al. (2015) 18 | Two‐stage GWAS | Postmenopausal women with or without UUI | Discovery cohort: | Replication cohort: replication failed | Meta‐analysis of both cohorts: 17 genetic variants: (15 new) 5p15 (ADAMTS16), 10p12 (LINC01516), 11q14 (intergenic), 12p11 (intergenic), 12q24 (CIT gene), and 18q11 (ZNF521; p = 1.91−9.47 × 10−7) |
| 17 genetic variants: CIT‐gene, SLC16A7, and intergenic | |||||
| (p = 4.57−9.32 × 10−7) | |||||
| Funada et al. (2018) 15 | Two‐stage GWAS | General population with or without UUI | Discovery cohort: rs4467538 (p = 8.47 × 10−8) | Replication cohort: replication failed | Checked for associations between UUI and ADAMTS16, CIT, and ZNF521, replication failed |
| Penney et al. (2019) 17 | GWAS | Nurse participants with or without UUI | No genome‐wide significant associations | NA | |
Abbreviations: GWAS, genome‐wide association study; NA, not applicable; UUI, urgency urinary incontinence.
Table 4.
Results of studies researching specific genes/proteins/product
| Analyzed gene/protein/product | Study | Tissuea | Resultb UUI | Control | p | Unit |
|---|---|---|---|---|---|---|
| AIP | Ustundag et al. (2019) 50 | Serum | 0.048 ± 0.31 | 0.046 ± 0.26 | .982 | NI |
| Albumin | Ustundag et al. (2019) 50 | Serum | 46 ± 10 | 55 ± 17 | .151 | g/L |
| Androgen receptor | Cornu et al. (2011) 14 | Blood | AR polymorphism (combination of two alleles containing more than 21 CAG repeats) is significantly associated with UUI | .02 | NA | |
| AOPP | Keske et al. (2019) 34 | Serum | 134.4 ± 32.6 | 138.9 ± 46.0 | .641 | NI |
| Arylesterase | Keske et al. (2019) 34 | Serum | 184.6 ± 39.2 | 189.7 ± 55.7 | .662 | NI |
| ATP | Kumar et al. (2010) 37 | Urothelium | 1064.2 ± 238.9 | 45.7 ± 4.9 | NI | pmol/g |
| Silva‐Ramos et al. (2013) 49 | Urine | 27.5 ± 8.3 | 7.2 ± 1.7 | .022 | pM | |
| B1‐integrin | Kanasaki et al. (2013) 20 | Mutated mice | B1‐KO mice exhibited UUI phenotype compared with controls. Urine spot number and spot area as a percentage of the filter paper area were significantly greater in the B1‐cKO mice; frequency distribution of urine spot volumes showed B1‐cKO mice had a greater proportion of urine deposits that were moderately large | |||
| B3‐AR | Honda et al. (2014) 16 | Hair | Significantly higher frequency of variant Trp64Arg/Arg64Arg I in B3‐AR n OAB group versus controls. Within OAB‐group no significant difference in UUI pts with and without variant | |||
| BDNF | Alkis et al. (2017) 27 | Urine | 844.3 ± 286.3 | 340.2 ± 199.0 | NI | pg/mg |
| Antunes‐Lopes et al. (2013) 28 | Urine | 628.1 ± 590.5 | 110.4 ± 159.5 | NI | pg/mg | |
| Richter et al. (2017)c , 47 | Urine | 62.0 ± 1.4 | 46.3 ± 1.2 | NS difference | pg/mg | |
| Calcium | Ustundag et al. (2019) 50 | Serum | Median [IQR]: 2.37 [0.12] | 2.39 [0.07] | .724 | mmol/L |
| CGRP | Richter et al. (2017)c , 47 | Urine | 595.5 ± 1.3 | 527.4 ± 1.5 | NS difference | pg/mg |
| Cholesterol, total | Ustundag et al. (2019) 50 | Serum | 5.58 ± 1.08 | 6.05 ± 1.13 | .071 | mmol/L |
| Collagenase I activity | Richter et al. (2017)c , 47 | Urine | 279.2 ± 449.0 | 138.9 ± 321.2 | NS difference | µg/min/mg |
| CRP | Chuang et al. (2010) 31 | Serum | 2.96 ± 0.47 | 0.93 ± 0.27 | .0002 | mg/L |
| Urine | All samples below assay sensitivity | |||||
| Hsiao et al. (2012) 33 | Serum | Median [IQR]: 0.12 [0.03–0.26] | 0.055 [0.04–0.08] | .032 | mg/dl | |
| Liu et al. (2013) 45 | Serum | 0.33 ± 0.37 | 0.06 ± 0 0.04 | .011 | pg/ml | |
| Ustundag et al. (2019) 50 | Serum | Median [IQR]: 29.5 [0.9] | 29.5 [0.9] | .994 | nmol/L | |
| (complementary) Dense plaques | Carey et al. (2000) 30 | Detrusor muscle | No apparent differences between the groups | |||
| CYP‐17 and CYP‐19 | Cornu et al. (2011) 14 | Blood | No significant difference in prevalence of polymorphisms between the groups | |||
| Estrogen receptor‐1 | Cornu et al. (2011) 14 | Blood | No significant difference in prevalence of polymorphisms between the groups | |||
| Disulfide | Ustundag et al. (2019) 50 | Serum | 17.0 ± 4.2 | 19.0 ± 6.2 | .118 | µmol/L |
| FRAP | Ustundag et al. (2019) 50 | Serum | 1135 ± 283 | 1120 ± 264 | .842 | µmol/L |
| GAG | Alkis et al. (2017) 27 | Urine | 126.2 ± 45.1 | 90.9 ± 60.3 | NI | pg/mg |
| GAP‐43 | Schofield et al. (2005) 48 | PNT | NS difference | Area | ||
| GDNF | Antunes‐Lopes et al. (2013) 28 | Urine | 958.1 ± 826.2 | 1.220.5 ± 513.5 | .128 | pg/mg |
| GMC‐SF | Richter et al. (2017)c , 47 | Urine | All samples below assay sensitivity | |||
| Hba1c | Ustundag et al. (2019) 50 | Serum | Median [IQR]: 5.8 [0.5] | 5.9 [0.7] | .363 | % |
| HB‐EGF | Kim et al. (2015) 35 | Urine | 9.4 ± 7.73 | 4.45 ± 2.93 | NI | pg/mg |
| HDL | Ustundag et al. (2019) 50 | Serum | 1.42 ± 0.43 | 1.42 ± 0.38 | .835 | mmol/L |
| HIF‐(1a/2a) | Christiaansen et al. (2011) 24 | HBUC | HIF‐1a: 20.06 ± 11.74% | HIF‐1a: 21.58 ± 12.39% | NS difference | % |
| HIF2‐a: 13.7 ± 1.75% | HIF‐2a: 16.3 ± 3.15% | |||||
| IMA | Keske et al. (2019) 34 | Serum | 0.614 ± 0.106 | 0.530 ± 0.117 | .003 | NI |
| Ustundag et al. (2019) 50 | Serum | 0.629 ± 0.257 | 0.569 ± 0.219 | .335 | Absorbance unit | |
| Insulin | Liu et al. (2013) 45 | Serum | 771.58 ± 502.54 | 759.8 ± 471.7 | .922 | pg/ml |
| Interleukins (IL‐1B, IL‐6, IL‐8) | Liu et al. (2013) 45 | Serum | IL‐Iβ: 4.68 ± 3.10 | IL‐Iβ: 1.64 ± 2.37 | .045 | pg/ml |
| IL‐6: 5.78 ± 9.97 | IL‐6: 0.79 ± 1.05 | .000 | ||||
| IL‐8: 4.12 ± 3.81 | IL‐8: 1.45 ± 1.06 | .000 | ||||
| Richter et al. (2017)c , 47 | Urine | IL‐6: 2.5 ± 1.5 | IL‐6: 3.0 ± 1.1 | NS difference | pg/mg | |
| IL‐8: 38.4 ± 1.1 | IL‐8: 37.2 ± 1.3 | NS difference | ||||
| IL‐1B below assay sensitivity | ||||||
| LDL | Ustundag et al. (2019) 50 | Serum | 3.36 ± 1.00 | 3.7 ± 0.98 | .089 | mmol/L |
| Leptin | Liu et al. (2013) 45 | Serum | 10,942 ± 14,338 | 6242 ± 4038 | .922 | pg/ml |
| MCP‐1 | Alkis et al. (2017) 27 | Urine | 635.7 ± 284.2 | 155.8 ± 79.4 | NI | pg/mg |
| Farhan et al. (2019) 32 | Urine | Mean: 209.25 ± (SEM) 30.5 | 48.02 ± 9 | .001 (ANOVA control‐wet‐dry) | pg/mg | |
| Liu et al. (2013) 45 | Serum | 132.46 ± 18.00 | 104.81 ± 37.39 | .067 | pg/ml | |
| Membrane caveolae | Carey et al. (2000) 30 | Detrusor muscle | No apparent differences between the groups | |||
| MMP(‐1/2/9) | Richter et al. (2017)c , 47 | Urine | MMP‐2: 251.8 ± 1.3 | MMP‐2: 183.8 ± 1.5 | NS difference | pg/mg |
| MMP‐9: 32.8 ± 1.9 | MMP‐9: 28.2 ± 2.1 | NS difference | ng/mg | |||
| MMP‐1 below assay sensitivity | ||||||
| Muscarinic 2/3 receptor/M‐Ras | Birder et al. (2013) 29 | HBUC | Nonsignificant decrease of M3R‐expression in UUI group (shown in figure, exact data not shown) | |||
| Ehrhardt et al. (2015) 19 | Mutated mice | M‐Ras−/− male mice exhibited UUI phenotype. Dysregulation of M2R and M3R in M‐Ras−/− mice; male mice had a higher expression of M2R, female mice lower expression M3R. Significantly more urine spots produced by M‐Ras−/− males compared with WT males (p = .0124), while M‐Ras−/− and WT females produced similar numbers of spots | ||||
| NGF | Alkis et al. (2017) 27 | Urine | 1107 ± 602.5 | 202.9 ± 48.4 | NI | pg/mg |
| Antunes‐Lopes et al. (2013) 28 | Urine | 488.5 ± 591.8 | 188.3 ± 290.2 | .005 | pg/mg | |
| Kim et al. (2015) 35 | Urine | 1.26 ± 1.07 | 0.5 ± 0.29 | <.001 | pg/mg | |
| Kuo et al. (2010) 38 | Urine | 1.66 ± 3.30 | 0.09 ± 0.22 | .015 | pg/mg | |
| Kuo et al. (2010) 39 | Urine | 1.83 ± 0.74 | 0.05 ± 0.02 | .012 | pg/mg | |
| Liu et al. (2008) 41 | Urine | 1.7 ± 0.26 | 0.041 ± 0.026 | .000 | pg/mg | |
| Liu et al. (2010) 42 | Urine | 0.78 ± 1.26 | 0.01 ± 0.02 | .02 | pg/mg | |
| Urothelium | 125.87 ± 21.79 | 135.60 ± 13.50 | .142 | pg/mg | ||
| Liu et al. (2011) 43 | Serum | Median [IQR]: 0.0 [0–33.6] | 0.0728 [0–0.234] | NI | pg/ml | |
| Urine | Median [IQR]: 0.82 [0.13–1.84] | 0.005 [0–0.028] | NI | pg/mg | ||
| Liu et al. (2011) 44 | Urine | 2.13 ± 3.87 | 0.07 ± 0.21 | .000 (ANOVA (control‐dry‐wet) | pg/mg | |
| Liu et al. (2013) 45 | Serum | 3.66 ± 2.45 | 2.57 ± 0.88 | .045 | pg/ml | |
| Richter et al. (2017)c , 47 | Urine | 6.4 ± 1.5 | 5.0 ± 1.5 | NS difference | pg/mg | |
| Silva‐Ramos et al. (2013) 49 | Urine | 109.5 ± 29.0 | 64.0 ± 13.6 | .162 | pg/mg | |
| NTx | Richter et al. (2017)c , 47 | Urine | 31.4 ± 1.3 | 15.6 ± 2.1 | <.001 | nM/mM |
| P2X(1–7) | Moore et al. (2001) 46 | PNT | P2X3 and P2X5: 0% and 0% | P2x3 and P2X5 94 and 91% | NI | % |
| P2X4, P2X6, and P2X7: 36%, 33%, and 67% | P2X4, P2X6, P2X7: 16%, 18%, and 6% | <.0001 in all | ||||
| P2X1 and P2X2: 96% and 99% | P2X1 and P2X2: 97% and 99% | 0.32 and 0.16 | ||||
| Parathormone | Ustundag et al. (2019) 50 | Serum | Median [IQR]: 6.68 [4.0] | 6.15 [3.9] | 0.715 | pmol/L |
| Polyamines | Li et al. (2013) 40 | Urothelium | Putrescine: 0.50 ± 0.15 | Putrescine: 0.16 ± 0.03 | <.05 | nmol/mg |
| Spermidine: 2.4 ± 0.21 | Spermidine: 1.0 ± 0.13 | <.01 | nmol/mg | |||
| Spermine: 1.9 ± 0.27 | Spermine: 0.86 ± 0.26 | <.05 | nmol/mg | |||
| PON | Keske et al. (2019) 34 | Serum | Median [IQR]: 144.1 [91.6–249.5] | 158.6 [91.1–280.8] | .934 | NI |
| Slo | Meredith et al. (2004) 21 | Mutated mice | Slo−/− mice exhibited UUI phenotype compared with controls | |||
| Thorneloe et al. (2005) 22 | Mutated mice | Slo−/− mice exhibited UUI phenotype compared with controls | ||||
| Stem cell factor | Kubota et al. (2018) 36 | Urine | Median [IQR]: 1.30 [0.56–2.71] | 0.26 [0.13–0.43] | <.0001 | pg/mg |
| Substance P | Richter et al. (2017)c , 47 | Urine | 257.5 ± 0.9 | 271.5 ± 1.1 | NS difference | pg/mg |
| TAC | Keske et al. (2019) 34 | Serum | 1.8 ± 0.199 | 2.1 ± 0.216 | <.001 | NI |
| Thiol, native | Ustundag et al. (2019) 50 | Serum | 331 ± 64 | 356 ± 73 | .156 | µmol/L |
| Thiol, total | Ustundag et al. (2019) 50 | Serum | 365 ± 65 | 394 ± 70 | .095 | µmol/L |
| TNF‐α | Liu et al. (2013) 45 | Serum | 3.30 ± 2.60 | 0.91 ± 0.84 | .000 | pg/ml |
| Richter et al. (2017)c , 47 | Urine | All below assay sensitivity | pg/ml | |||
| TOS | Keske et al. (2019) 34 | Serum | 4.7 ± 1.77 | 4.1 ± 1.46 | .109 | NI |
| Triglyceride | Ustundag et al. (2019) 50 | Serum | 1.84 ± 1.08 | 1.86 ± 1.12 | .927 | mmol/L |
| Tropoelastin | Richter et al. (2017)c , 47 | Urine | 17.1 ± 0.9 | 9.6 ± 1.2 | .001 | mg/mg |
| TRPV1 | Birder et al. (2013) 29 | HBUC | Statistically significant higher receptor expression in UUI versus controls (shown in figure, exact data not shown) | |||
| Li et al. (2011) 25 | Urothelium | 0.25 ± 0.005 | 0.125 ± 0.01 | <.05 | Mean density ratio | |
| Liu et al. (2007) 26 | Urothelium | Bladder body median [IQR]: 11.4 [6.7–16.1] | 14.2 [8.2–20.7] | NS difference | 105 Copies/µg | |
| Trigonum median [IQR]: 10.9 [8.5–15.7] | 4.1 [0.77–26.2] | Total RNA | ||||
| VEGF | Christiaansen et al. (2011) 24 | HBUC | 23.51 ± 9.88% | 24.52 ± 4.68% | NS difference | % |
| Vinculin | Carey et al. (2000) 30 | Detrusor muscle | No apparent differences between the groups | |||
| Vitamin D | Ustundag et al. (2019) 50 | Serum | Median [IQR]: 27.0 [27.5] | 33.7 [30.7] | .081 | nmol/L |
All urinary values are adjusted to urinary creatinine levels.
Data are presented as means ± SD unless otherwise described.
Data log‐transformed before analysis.
Abbreviations: AIP, atherogenic index of plasma; ANOVA, analysis of variance; AOPP, advanced oxidation protein products; ATP, adenosine triphosphate; B1 integrin, β1 integrin; B3‐AR, β‐3 adrenergic receptor; BDNF, brain‐derived neurotrophic factor; CGRP, calcitonin gene‐related peptide; CRP, C‐reactive protein; FRAP, ferric reducing power of plasma; GAG, glycosaminoglycans; GAP‐43, growth association protein 43; GDNF, glial cell line‐derived neurotrophic factor; GMC‐SF, granulocyte‐macrophage colony‐stimulating factor; HB‐EGF, heparin‐binding epidermal growth factor‐like growth factor; HBUC, human bladder urothelium cells; HDL, high‐density lipoprotein; HIF, hypoxia‐inducible factor; IL, interleukin; IMA, ischemia modified albumin; IQR, interquartile ratio; LDL, low‐density lipoprotein; M2/3R, muscarinic 2/3 receptor; MCP‐1, monocyte chemoattractant protein‐1; MMP, matrix metalloproteinase; M‐Ras, muscarinic‐Ras; NA, not applicable; NGF, nerve growth factor; NI, no information; NTx, N‐terminal telopeptide type 1 collagen; PNT, parasympathic nerve tissue; PON, paraoxonase; TAC, total antioxidant capacity; TNF‐α, tumor necrosis factor α; TOS, total oxidant status; TRPV1, transient receptor potential cation channel subfamily V member 1; VEGF, vascular endothelial growth fac.
Concerning gene expression differences, a transcriptome analysis of bladder biopsies showed several associated genes per p‐value threshold, suggesting the involvement of multiple molecular pathways 23 (Table 3). In other studies examining gene expression, only one to three genes were examined and the results were conflicting or differences were not significant. 24 , 25 , 26
The vast majority of the included studies examined protein expression(‐related) differences that involved urinary or serum biomarkers (Table 4). These potential biomarkers were often tested in individual studies only and results were not independently validated. In view of the former, these single‐study results are not discussed separately and can be found in Table 4.
Nerve growth factor (NGF) was the most represented biomarker (12 studies). Figure 4 shows the meta‐analysis of urinary NGF/creatinine (Cr) values of patients with UUI versus controls. Two studies were not included in the meta‐analysis because log‐transformed data before analysis were reported 47 or only median and interquartile values were reported 43 instead of means and SD or SE. Five studies included in the meta‐analysis were from one research group. 38 , 39 , 41 , 42 , 44 Communication with the corresponding author of the studies by email confirmed that there was no overlap of included subjects in these studies. Two studies 38 , 44 did not report which measure (SD or SE) was used. Therefore, we employed a conservative approach and assumed that the studies used SE and these were recalculated into SD. This random effect meta‐analysis showed a pooled SMD of 1.01 (confidence interval (CI) = 0.49–1.52, I 2 = 90.0%; Figure 4). A sensitivity analysis, assuming the unknown measurement units were SDs, resulted in a similar pooled effect (Table S5).
Figure 4.
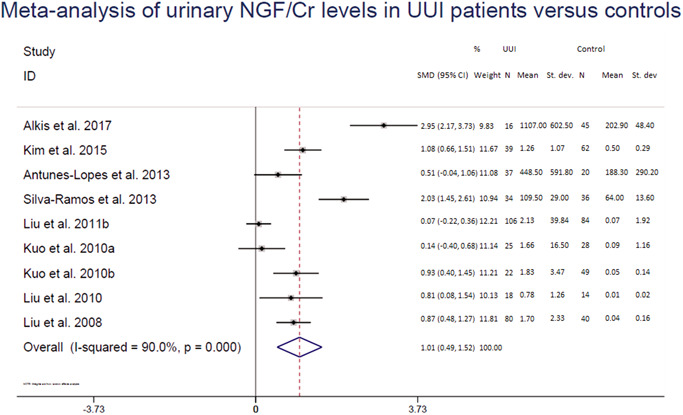
Meta‐analysis of urinary NGF/Cr levels in patients with urgency urinary incontinence versus controls.
CI; confidence interval; Cr, creatinine; NGF, nerve growth factor; SMD, standardized mean difference; st. dev., standard deviation; UUI, urgency urinary incontinence
Two studies examined adenosine triphosphate (ATP) release in relation to UUI. Tissue ATP levels were substantially pronounced and urinary UUI levels were significantly increased in UUI patients. 37 , 49 In three studies, urinary brain‐derived neurotrophic factor (BDNF)/Cr levels were investigated. A trend toward higher BDNF/Cr levels was observed but a clear association between elevated urine BDNF/Cr levels and UUI could not be established. 27 , 28 , 47 For serum C‐reactive protein (CRP), three out of four studies showed increased levels in UUI patients compared with controls, one study failed to demonstrate this association. 31 , 33 , 45 , 50 In two studies examining ischemia modified albumin (IMA) significantly elevated IMA levels were found in UUI patients compared with controls in one study, 32 but this was not confirmed in the second study. 36 Two studies examining urinary 47 or serum 45 levels of interleukin‐1B, ‐6, and ‐8 showed increased levels in the serum of UUI patients but no significant differences in the urine. Finally, in three studies urinary 27 , 32 and serum 45 levels of monocyte chemoattractant protein‐1 (MCP‐1) were investigated, showing a trend toward elevated levels in UUI patients compared with controls but a statistically significant difference was achieved in only one study. 32
4. DISCUSSION
In search of molecular pathways involved in UUI, we performed a systematic review of literature concerning genetic variants, and differences in gene and protein expression in UUI subjects when compared with controls. The symptom of UUI was selected to examine a clear and clinically well‐defined phenotype, with the aim to avoid cluttering of results. After the extended search, only 0.03% of initial studies were ultimately included in the analysis. In general, the risk of bias was judged as medium‐high or high (unclear in animal studies), and the majority of the outcomes were only examined by single studies. Despite the heterogeneity between the studies—which made it a challenge to find common denominators—two major molecular themes were distinguished as being associated with UUI: signal transduction and inflammation.
4.1. Signal transduction
Several studies suggested an association of genetic polymorphisms with UUI. 14 , 18 Polymorphisms in the genes encoding CIT (associated with cytokinesis), the transcription factor ZNF5521, and the androgen receptor were described, but unfortunately, the association could not be validated in independent cohorts. Although this suggests that these genes are not linked to UUI, replication in larger cohorts is necessary to draw firm conclusions. The four animal gene knockout studies all showed an association of specific gene expression with a UUI phenotype. Slo1, investigated in two studies, encodes for the pore‐forming subunit of the calcium‐activated BK potassium channel in bladder smooth muscle cells. 21 , 22 It contributes to the control and regulation of spontaneous bladder contractions by regulating its membrane potential and repolarizing the action potentials and deletion of this gene resulted in a UUI phenotype. 51 , 52 The studies firmly demonstrated a direct relation between Slo1 expression and regulation of bladder contractions and UUI. Whether Slo1 expression or expression levels are involved in human UUI is, however, still unsolved. Male M‐Ras−/− knockout mice developed an UUI phenotype but the female M‐Ras−/− mice did not. 19 The sex‐dependent variation of this phenotype and the expression of both M3R and M2R has not been explained. M‐Ras is expressed predominantly in fibroblasts and skeletal muscle cells and activates a wide variety of proteins. The results suggest that phenotypic changes in these cells contribute to UUI. Finally, the association of B1‐integrin (encoded by the ITGB1 gene), a receptor for collagen, with UUI suggests that an aberrant extracellular matrix (ECM) composition may play a role in UUI. 20
Concerning gene expression studies, the transcriptome analysis comparing normal versus UUI‐derived bladder tissue showed the differential expression of a large number of genes linked to multiple pathways. 23 Interestingly, smooth muscle contraction, cholinergic, G‐protein coupled, and calcium‐dependent signaling were major pathways in which genes that are differentially expressed in UUI are involved.
Collectively, these studies show that the occurrence of aberrant signaling, abnormal responses of contractile cells, and aberrant ECM composition are intimately associated with UUI.
Finally, protein expression studies support the association of signal transduction pathways and UUI. Multiple studies demonstrated a correlation between UUI and an increased urinary NGF/Cr ratio (pooled SMD: 1.01, CI: 0.49–1.52; I 2: 90.0%). An SMD of zero denotes no effect. Nevertheless, this result should be interpreted with caution because the heterogeneity between studies was high in the participants enrolled (difference in the severity of UUI, medication use, and/or age), in the method of collection of the urinary sample, and in the method of NGF/Cr determination. It is intriguing that NGF is associated with neurological effects 53 and the collective evidence suggests that NGF also affects the bladder. 54 , 55 Kashyap et al. 56 showed that, when inducing bladder overactivity with acetic acid in female Sprague‐Dawley rats, NGF overexpression and chemokine upregulation occurred. How this relates to UUI and whether this leads to NGF signaling remains undetermined and deserves further investigation. Importantly, elevated NGF/Cr values are not solely restricted to UUI, as these were also found to be elevated in OAB in general 57 , 58 , 59 and bladder pains syndrome/interstitial cystitis, 60 a conclusion recently confirmed by Siddiqui et al. 9 who reviewed biomarkers related to LUTS. In line with our findings, they judged the included studies as being of poor quality. The studies investigating urinary BDNF/Cr levels in UUI patients were inconclusive. However, transgenic animals overexpressing BDNF in the bladder showed changes in the bladder neurons leading to detrusor overactivity, a common finding in UUI. 61 This does suggest a role for BDNF in UUI, albeit that apparently this may not be related to BDNF levels but to downstream signaling. Combined with the findings on NGF, it could conceivably be hypothesized that UUI is associated with neuronal changes and/or aberrant signaling.
Multiple studies investigated the possible involvement of the purinergic signaling pathway in UUI, showing changes in purinergic receptor (subtypes 3–7) expression, 46 ATP release, 37 , 49 and involvement of transient receptor potential cation channel subfamily V member 1 (TRPV1). 62 Nevertheless, the evidence is currently insufficient to conclude that TRPV1 and purinergic receptors (subtypes 3–7) play a role in the etiology of UUI.
Finally, combining the results of gene expression and protein expression studies, we were also able to identify similarities of association in protein expression from the following pathways indicated by the transcriptome study of Cartwright et al. 23 : calcium‐dependent signaling, 25 , 29 smooth muscle contraction, 19 , 29 , 46 G‐protein coupled, 16 , 19 , 29 and cholinergic signaling. 19 , 29
4.2. Inflammation
Inflammatory responses and UUI appeared to be associated: serum CRP levels were significantly elevated in UUI patients in all three studies included, 31 , 33 , 45 and also levels of other inflammatory markers (interleukins, tumor necrosis factor‐alpha, and MCP‐1) 27 , 32 , 45 , 47 were elevated. The finding that urinary CRP was not elevated in UUI patients 31 implies that the elevated serum CRP levels do not originate from the bladder epithelium, but are possibly a reflection of submucosal inflammatory responses. How this relates to (the development of) UUI is unknown. Possibly, the different urinary microbiome of UUI patients may play a role. 63 Clearly, these aspects deserve attention to understand their role in UUI.
4.3. Considerations and limitations
Despite the high number of studies retrieved by our search, only 37 studies were included. Due to an extensive search and the strict inclusion criteria, with the intention to restrict ourselves to a rather homogeneous and clear study population, many articles were excluded. We believe this method of reviewing associations in a well‐defined population prevented the introduction of significant bias, which would make it difficult to link associations to specific symptoms, a problem Siddiqui et al. 9 described in their review of LUTS. However, despite this precaution, the populations (UUI vs. control) included in the various studies differed substantially, for instance in reporting of age, gender/sex, use of medications, BMI, and controls with different diseases, such as bladder cancer or a combination of these aspects. Therefore, some of the reported outcomes may still not be UUI‐related, since these parameters could affect expression differences or influence genetic variant association. We decided to present these studies but marked them as (medium) high risk of bias.
With the use of different risk of bias tools for animal and human studies, we were able to assess study‐specific determinants in an effort to reduce over/underestimation of the results. Generally, the risk of bias assessment showed a relatively high or unclear risk of bias which is a risk factor for an overestimation of reported associations. The unclear risk of bias was mainly due to the general lack of reporting standards and transparency in animal studies.
Most of the analyzed genes/proteins(‐related products) were studied in isolation and involved individual studies, emphasizing the fragmented nature of the UUI research. Moreover, relatively low numbers of subjects were analyzed, possibly because of ethical considerations. This was also true for the gene knockout animal models. Nevertheless, these are very informative to demonstrate a direct cause‐effect relationship between a certain gene and the UUI phenotype. The animal studies allowed researchers to study individual animals before they acquired UUI, something that is not possible in patients. Thus, the underlying molecular drivers can be studied in more detail. The value of these observations and the relation with clinical UUI remains to be firmly established, due to possible biological differences between animals and humans. The contribution of individual genetic variants to the etiology may also be limited. This may explain the lack of replicated results from the three GWASs. 15 , 17 , 18 In addition, these studies may have been underpowered. For a multifactorial symptom such as UUI, mostly occurring later in life, it is expected that the effect sizes of the variants will be low and therefore, a large group of participants is needed to find a statistically significant and replicable association.
5. CONCLUSIONS
Signal transduction pathways and inflammation emerged as important biological processes potentially associated with UUI. A meta‐analysis suggests a relation between an increased urinary level of NGF and UUI. This suggests aberrant signaling in both smooth muscle and nerve cells with the involvement of inflammation. Studies combining this information might lead to better insights in the development and occurrence of UUI. Despite the high prevalence of UUI, only 37 studies met our inclusion criteria, implying the need for more focused and less fragmented future research with clearly defined populations. This systematic review provides an overview of genetic variants, gene, and protein expression changes in relation to UUI and therefore helps to formulate the actual knowledge gaps and research questions that need to be solved.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENT
The authors acknowledge Onying Chan, librarian at the Medical Library of Radboud University, Nijmegen, for her help in developing the search strategy used for this review. This study was funded by the More Knowledge with Fewer Animals project of ZonMw under Project No. 114024125. Co‐authors W. M. P, A. M. R. Z, G. P, M. J. H. C, E. O, and K. B. K were supported by the European Regional Development Fund (https://ec.europa.eu/regional_policy/en/funding/erdf/) under the Grant Agreement No. PROJ00787 (DIABIP).
Post WM, Ruiz‐Zapata AM, Grens H, et al. Genetic variants and expression changes in urgency urinary incontinence: a systematic review. Neurourology and Urodynamics. 2020;39:2089‐2110. 10.1002/nau.24512
Egbert Oosterwijk and Kirsten B. Kluivers contributed equally to this study.
REFERENCES
- 1. Kupelian V, Wei JT, O'Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) survey. Arch Intern Med. 2006;166(21):2381‐2387. [DOI] [PubMed] [Google Scholar]
- 2. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5‐26. [DOI] [PubMed] [Google Scholar]
- 3. D'Ancona C, Haylen B, Oelke M, et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn. 2019;38(2):433‐477. [DOI] [PubMed] [Google Scholar]
- 4. Milsom I, Coyne KS, Nicholson S, Kvasz M, Chen CI, Wein AJ. Global prevalence and economic burden of urgency urinary incontinence: a systematic review. Eur Urol. 2014;65(1):79‐95. [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Yu W, Yang Y, et al. Association between overactive bladder and peri‐menopause syndrome: a cross‐sectional study of female physicians in China. Int Urol Nephrol. 2015;47(5):743‐749. [DOI] [PubMed] [Google Scholar]
- 6. Varella LR, Bezerra da Silva R, Eugenia de Oliveira MC, Melo PH, Maranhao TM, Micussi MT. Assessment of lower urinary tract symptoms in different stages of menopause. J Phys Ther Sci. 2016;28(11):3116‐3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu JM, Vaughan CP, Goode PS, et al. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014;123(1):141‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aniuliene R, Aniulis P, Steibliene V. Risk factors and types of urinary incontinence among middle‐aged and older male and female primary care patients in Kaunas region of Lithuania: cross sectional study. Urol J. 2016;13(1):2552‐2561. [PubMed] [Google Scholar]
- 9. Siddiqui NY, Helfand BT, Andreev VP, et al. Biomarkers implicated in lower urinary tract symptoms: systematic review and pathway analyses. J Urol. 2019;202(5):880‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes‐Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Toxicology Program Monograph on Immunotoxicity Associated with Exposure to Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). National Toxicology Program; 2016. https://ntp.niehs.nih.gov/ntp/ohat/pfoa_pfos/pfoa_pfosmonograph_508.pdf [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Med. 2019;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cornu JN, Merlet B, Cussenot O, et al. Genetic susceptibility to urinary incontinence: implication of polymorphisms of androgen and oestrogen pathways. World J Urol. 2011;29(2):239‐242. [DOI] [PubMed] [Google Scholar]
- 15. Funada S, Kawaguchi T, Terada N, et al. Cross‐sectional epidemiological analysis of the Nagahama Study for correlates of overactive bladder: genetic and environmental considerations. J Urol. 2018;199(3):774‐778. [DOI] [PubMed] [Google Scholar]
- 16. Honda K, Yamaguchi O, Nomiya M, et al. Association between polymorphism of beta3‐adrenoceptor gene and overactive bladder. Neurourol Urodyn. 2014;33(4):400‐402. [DOI] [PubMed] [Google Scholar]
- 17. Penney KL, Townsend MK, Turman C, et al. Genome‐wide association study for urinary and fecal incontinence in women. J Urol. 2019;203(5):978‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Richter HE, Whitehead N, Arya L, et al. Genetic contributions to urgency urinary incontinence in women. J Urol. 2015;193(6):2020‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehrhardt A, Wang B, Yung AC, et al. Urinary retention, incontinence, and dysregulation of muscarinic receptors in male mice lacking M‐Ras. PLOS One. 2015;10(10):e0141493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanasaki K, Yu W, Bodungen M, et al. Loss of beta1‐integrin from urothelium results in overactive bladder and incontinence in mice: a mechanosensory rather than structural phenotype. J Off Publ Fed Am Soc Exp Biol. 2013;27(5):1950‐1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+‐activated K+channel. J Biol Chem. 2004;279(35):36746‐36752. [DOI] [PubMed] [Google Scholar]
- 22. Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol. 2005;289(3):F604‐F610. [DOI] [PubMed] [Google Scholar]
- 23. Cartwright R, Khullar V, Gopalan V, Lee Y, Fernando R, Bennett P. Whole genome gene expression in bladder tissue from women with detrusor overactivity. Int Urogynecol J Pelvic Floor Dysfunct. 2010;21:S177‐S179. [Google Scholar]
- 24. Christiaansen CE, Sun Y, Hsu YC, Chai TC. Alterations in expression of HIF‐1alpha, HIF‐2alpha, and VEGF by idiopathic overactive bladder urothelial cells during stretch suggest role for hypoxia. Urology. 2011;77(5):1266.e7‐1266.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li M, Sun Y, Simard JM, Chai TC. Increased transient receptor potential vanilloid type 1 (TRPV1) signaling in idiopathic overactive bladder urothelial cells. Neurourol Urodyn. 2011;30(4):606‐611. [DOI] [PubMed] [Google Scholar]
- 26. Liu L, Mansfield KJ, Kristiana I, Vaux KJ, Millard RJ, Burcher E. The molecular basis of urgency: regional difference of vanilloid receptor expression in the human urinary bladder. Neurourol Urodyn. 2007;26(3):433‐438. discussion 439; discussion 451‐433. [DOI] [PubMed] [Google Scholar]
- 27. Alkis O, Zumrutbas AE, Toktas C, Aybek H, Aybek Z. The use of biomarkers in the diagnosis and treatment of overactive bladder: Can we predict the patients who will be resistant to treatment? Neurourol Urodyn. 2017;36(2):390‐393. [DOI] [PubMed] [Google Scholar]
- 28. Antunes‐Lopes T, Pinto R, Barros SC, et al. Urinary neurotrophic factors in healthy individuals and patients with overactive bladder. J Urol. 2013;189(1):359‐365. [DOI] [PubMed] [Google Scholar]
- 29. Birder LA, Wolf‐Johnston AS, Sun Y, Chai TC. Alteration in TRPV1 and Muscarinic (M3) receptor expression and function in idiopathic overactive bladder urothelial cells. Acta Physiol. 2013;207(1):123‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carey MP, De Jong S, Friedhuber A, Moran PA, Dwyer PL, Scurry J. A prospective evaluation of the pathogenesis of detrusor instability in women, using electron microscopy and immunohistochemistry. BJU Int. 2000;86(9):970‐976. [DOI] [PubMed] [Google Scholar]
- 31. Chuang YC, Tyagi V, Liu RT, Chancellor MB, Tyagi P. Urine and serum C‐Reactive protein levels as potential biomarkers of lower urinary tract symptoms. Urol Sci. 2010;21(3):132‐136. [Google Scholar]
- 32. Farhan B, Chang H, Ahmed A, Zaldivair F, Ghoniem G. Characterisation of urinary monocyte chemoattractant protein 1: potential biomarker for patients with overactive bladder. Arab J Urol. 2019;17(1):58‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsiao SM, Lin HH, Kuo HC. The role of serum C‐reactive protein in women with lower urinary tract symptoms. Int Urogynecol J. 2012;23(7):935‐940. [DOI] [PubMed] [Google Scholar]
- 34. Keske M, Gok B, Ener K, et al. Relationship between oxidative stress and detrussor overactivity: a case control study. Urol J. 2019;16(4):371‐374. [DOI] [PubMed] [Google Scholar]
- 35. Kim SR, Moon YJ, Kim SK, Bai SW. NGF and HB‐EGF: potential biomarkers that reflect the effects of fesoterodine in patients with overactive bladder syndrome. Yonsei Med J. 2015;56(1):204‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kubota Y, Hamakawa T, Osaga S, et al. A kit ligand, stem cell factor as a possible mediator inducing overactive bladder. Neurourol Urodyn. 2018;37(4):1258‐1265. [DOI] [PubMed] [Google Scholar]
- 37. Kumar V, Chapple CR, Rosario D, Tophill PR, Chess‐Williams R. In vitro release of adenosine triphosphate from the urothelium of human bladders with detrusor overactivity, both neurogenic and idiopathic. Eur Urol. 2010;57(6):1087‐1092. [DOI] [PubMed] [Google Scholar]
- 38. Kuo HC, Liu HT, Chancellor MB. Urinary nerve growth factor is a better biomarker than detrusor wall thickness for the assessment of overactive bladder with incontinence. Neurourol Urodyn. 2010;29(3):482‐487. [DOI] [PubMed] [Google Scholar]
- 39. Kuo HC, Liu HT, Tyagi P, Chancellor MB. Urinary nerve growth factor levels in urinary tract diseases with or without frequency urgency symptoms. Low Urin Tract Symptoms. 2010;2(2):88‐94. [DOI] [PubMed] [Google Scholar]
- 40. Li MK, Sun Y, Tomiya N, Hsu YC, Chai TC. Elevated polyamines in urothelial cells from OAB subjects mediate oxotremorine‐evoked rapid intracellular calcium rise and delayed acetylcholine release. Am J Physiol Ren Physiol. 2013;305(4):F445‐F450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu HT, Kuo HC. Urinary nerve growth factor level could be a potential biomarker for diagnosis of overactive bladder. J Urol. 2008;179(6):2270‐2274. [DOI] [PubMed] [Google Scholar]
- 42. Liu HT, Wang YS, Kuo HC. Nerve growth factor levels are increased in urine but not urothelium in patients with detrusor overactivity. Tzu Chi Med J. 2010;22(4):165‐170. [Google Scholar]
- 43. Liu HT, Lin H, Kuo HC. Increased serum nerve growth factor levels in patients with overactive bladder syndrome refractory to antimuscarinic therapy. Neurourol Urodyn. 2011;30(8):1525‐1529. [DOI] [PubMed] [Google Scholar]
- 44. Liu HT, Chen CY, Kuo HC. Urinary nerve growth factor in women with overactive bladder syndrome. BJU Int. 2011;107(5):799‐803. [DOI] [PubMed] [Google Scholar]
- 45. Liu HT, Jiang YH, Kuo HC. Increased serum adipokines implicate chronic inflammation in the pathogenesis of overactive bladder syndrome refractory to antimuscarinic therapy. PLOS One. 2013;8(10):e76706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moore KH, Ray FR, Barden JA. Loss of purinergic P2X(3) and P2X(5) receptor innervation in human detrusor from adults with urge incontinence. J Neurosci Off J Soc Neurosci. 2001;21(18):RC166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Richter HE, Moalli P, Amundsen CL, et al. Urinary biomarkers in women with refractory urgency urinary incontinence randomized to sacral neuromodulation versus onabotulinumtoxina compared to controls. J Urol. 2017;197(6):1487‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schofield EC, Clausen JA, Burcher E, Moore KH. GAP‐43 immunoreactivity of subepithelial and detrusor muscle nerve fibres in patients with refractory idiopathic detrusor overactivity. Neurourol Urodyn. 2005;24(4):325‐333. [DOI] [PubMed] [Google Scholar]
- 49. Silva‐Ramos M, Silva I, Oliveira O, et al. Urinary ATP may be a dynamic biomarker of detrusor overactivity in women with overactive bladder syndrome. PLOS One. 2013;8(5):e64696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ustundag Y, Karlıbel A., Sambel M, et al. Vitamin D and thiol‐disulfide homeostasis levels in postmenopausal women with overactive bladder syndrome. J Med Biochem. 2019;39(1):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heppner TJ, Bonev AD, Nelson MT. Ca(2+)‐activated K+channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol. 1997;273(Pt 1):C110‐C117. [DOI] [PubMed] [Google Scholar]
- 52. Steers WD, Tuttle JB. Role of ion channels in bladder function and voiding disorders. Curr Bladder Dysfunct Rep. 2009;4(3):125‐131. [Google Scholar]
- 53. Bradshaw RA, Mobley W, Rush RA. Nerve growth factor and related substances: a brief history and an introduction to the international NGF meeting series. Int J Mol Sci. 2017;18(6):1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Antunes‐Lopes T, Cruz F. Urinary biomarkers in overactive bladder: revisiting the evidence in 2019. Eur Urol Focus. 2019;5(3):329‐336. [DOI] [PubMed] [Google Scholar]
- 55. Chen W, Ye DY, Han DJ, et al. Elevated level of nerve growth factor in the bladder pain syndrome/interstitial cystitis: a meta‐analysis. SpringerPlus 2016;5(1):1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kashyap M, Kawamorita N, Tyagi V, et al. Down‐regulation of nerve growth factor expression in the bladder by antisense oligonucleotides as new treatment for overactive bladder. J Urol. 2013;190(2):757‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Qu HC, Yan S, Zhang XL, Zhu XW, Liu YL, Wang P. Urinary nerve growth factor levels could be a biomarker for overactive bladder symptom: a meta‐analysis. Genet Mol Res. 2014;13(4):8609‐8619. [DOI] [PubMed] [Google Scholar]
- 58. Seth JH, Sahai A, Khan MS, et al. Nerve growth factor (NGF): a potential urinary biomarker for overactive bladder syndrome (OAB)? BJU Int. 2013;111(3):372‐380. [DOI] [PubMed] [Google Scholar]
- 59. Sheng W, Zhang H, Ruth KH. Could urinary nerve growth factor be a biomarker for overactive bladder? A meta‐analysis. Neurourol Urodyn. 2017;36(7):1703‐1710. [DOI] [PubMed] [Google Scholar]
- 60. Lai HH, Pickersgill NA, Vetter JM. Hunner lesion phenotype in interstitial cystitis/bladder pain syndrome: a systematic review and meta‐analysis. J Urol. 2020;204(3):518‐523. [DOI] [PubMed] [Google Scholar]
- 61. Kashyap MP, Pore SK, de Groat WC, Chermansky CJ, Yoshimura N, Tyagi P. BDNF overexpression in the bladder induces neuronal changes to mediate bladder overactivity. Am J Physiol Renal Physiol. 2018;315(1):F45‐F56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP‐evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98(12):6951‐6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Govender Y, Gabriel I, Minassian V, Fichorova R. The current evidence on the association between the urinary microbiome and urinary incontinence in women. Front Cell Infect Microbiol. 2019;9:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information


