Abstract
Background and purpose
Unexpected stressful life events may alter immune function and affect susceptibility to autoimmune diseases including multiple sclerosis (MS). Current results from epidemiological investigations examining the role of stress in MS remain inconsistent. The aim was to conduct the hitherto largest population‐based case–control study on this topic.
Methods
Extensive questionnaire information collected on lifestyle environmental factors available for 2930 incident MS cases and 6170 controls were used to assess the association of 10 major life events that had occurred before disease onset with the risk of MS by unconditional logistic regressions, adjusting for potential confounders. Stratified analyses were also performed by sex and time.
Results
Compelling evidence was found for a link between major life events and risk of MS – most events significantly increased disease risk by 17%–30%. It was further observed that women were affected to a greater extent than men under certain stressful scenarios, and that most events that happened recently (≤5 years prior to MS onset) had significant effects on MS, indicating a critical window in disease development.
Conclusion
Stressful life events may have an adverse effect on the risk of MS. Research into the mechanisms of this observation may give important clues to triggering pathogenetic events in MS.
Keywords: association study, epidemiology, major life changes, multiple sclerosis, negative events, risk, sex disparity, stress
Introduction
Multiple sclerosis (MS) is an autoimmune neurodegenerative disorder, characterized by the loss of myelin and damage of axons [1]. Despite the many advances in genome‐wide association studies identifying a significant genetic component [2] and epidemiological studies in support of a low but steadily increasing number of environmental risk factors [3], the disease etiology remains to be understood.
Most humans, across their lifespan, are at some point exposed to trauma or unexpected stressful life events. These events may lead to the development of psychiatric reactions which could affect multiple body systems including immune function, and thus susceptibility to disease [4]. Despite a few lines of epidemiological evidence linking stress‐related disorders to a heightened risk of autoimmune disease overall [5], results remain inconclusive for MS. Several earlier small case–control studies have reported a significant association between emotional stress, negative life events, mental health symptoms and an increased risk of MS [6, 7, 8], whilst other case–control studies have reported negligible findings [9, 10, 11]. A well‐powered epidemiological study estimating the influence of stress on the risk of MS is lacking.
To improve our knowledge of the biological mechanisms underlying the development of MS, the aim was to conduct the hitherto largest population‐based case–control study on such topics. Extensive questionnaire data collected on 10 important life events that occurred before disease onset, available for 9100 incident MS cases and controls, were used to understand the impact of life events on the risk of MS.
Methods
Study base
The present research was carried out capitalizing on existing data from a large‐scale population‐based study initiated in 2005, the Epidemiological Investigation of Multiple Sclerosis (EIMS), a Swedish case–control study which enables researchers to investigate various genetic and environmental risk factors of MS. The study base comprises both men and women, 16–70 years, recruited from all over Sweden. Informed consent was received for our study and the institutional review broad of Karolinska University Hospital approved our research protocol.
Identification of cases and controls
Neurologists examined newly diagnosed cases fulfilling the McDonald criteria [12] at the participating units of collaborating hospitals (N = 42). When an MS patient was identified at the clinic, the doctor or nurse registered the year and month when the first symptoms of MS started, and this time point was considered as time of disease onset (the year therefore as the index year). For each case, controls were randomly selected from the continuously updated national population register shortly after case identification and were matched to the case on age, sex and residential area. The controls received information about EIMS, together with a questionnaire, by mail. In total, 3185 cases and 9208 controls were invited to participate in the study, of whom 2930 cases and 6170 controls responded (a participation rate of 92% amongst cases and 67% amongst controls) [13].
Collection of data
Subjects were asked to answer an extensive self‐administered questionnaire, comprising questions on demography, lifestyle and psychosocial factors. Regarding the 10 specific life events (serious conflict with a spouse or partner; serious conflict with a close relative or friend; sickness or accident occurring to a spouse, partner or child; death of a spouse, partner or child; death of a close relative or friend; poor economy; conflict at work; divorce or equivalent; marriage or equivalent; become unemployed), the following questions were asked: (i) Have you during the last 10 years been involved in any of the following events? (ii) If yes, can you state the year and mark how important this was to you when it occurred? An individual was considered to be exposed only if he or she reported an event before symptom onset (e.g. before the index year). Any event happening after symptom onset or at the same year as symptom onset was not considered as an exposure and the data were not used in our analysis.
Statistical analysis
The individual life events were to some extent related to each other. To reduce statistical burden, the 10 events were first combined into several clusters according to their dissimilarity structure. Spearman’s rank‐order correlation coefficients were calculated for each life event with all other life events and pairwise coefficients were mapped into a heat map. Due to non‐responders, not each MS case would have a matched control, and not every control would have a matched case. Therefore, to maximize statistical power, it was decided to break the matching and include all available cases and controls instead. The association of life event clusters with the risk of MS was assessed by unconditional logistic regressions, estimating odds ratios (ORs) with 95% confidence intervals (CIs) comparing exposed versus non‐exposed individuals. In all analyses, adjustments were made for the matching variables age, sex and residential area. In addition, other confounders such as smoking (pack‐year), alcohol consumption (g/day), body mass index (BMI) (kg/m2) and education (university degree) at diagnosis were also considered. Analysis was performed for MS overall as well as for men and women separately to investigate the sex‐specific effect.
Furthermore, the same analysis was performed looking into each individual life event to complement with main findings. To reduce bias from reverse causality, i.e. an already predisposed yet undiagnosed MS would affect one’s psychiatric or psychological status, a sensitivity analysis was performed based on when in time the event happened (≤ or >5 years prior to MS onset). P values < 0.05 were considered statistically significant.
Results
Table 1 shows the characteristics for MS cases and controls. Consistent with previous findings, MS cases were significantly more likely to be heavier in weight (BMI 25.1 kg/m2 vs. 24.9 kg/m2, P = 0.03), to be ever‐smokers (55% vs. 45%, P < 0.0001), to smoke more heavily (8.1 vs. 7.2 pack‐years, P = 0.002) and to drink less (29.6 vs. 30 g/week, P < 0.0001) (note that the differences observed for BMI and alcohol drinking were minimal). No difference in levels of education was observed (P = 0.94).
Table 1.
Characteristics of the MS cases and controls
| Exposure | MS cases | Controls |
|---|---|---|
| Age at onset a , mean (SD) | 34.6 (10.6) | 34.6 (10.7) |
| Body mass index, mean (SD) | 25.1 (4.8) | 24.9 (4.3) |
| Sex | ||
| Female (%) | 2105 (0.72) | 4401 (0.71) |
| Male (%) | 825 (0.28) | 1769 (0.29) |
| Smoking status | ||
| Ever (%) | 1603 (0.55) | 2730 (0.45) |
| Never (%) | 1296 (0.45) | 3366 (0.55) |
| Pack‐years of smoking (SD) | 8.1 (9.3) | 7.2 (8.6) |
| Higher education | ||
| Yes (%) | 1323 (0.45) | 2786 (0.45) |
| No (%) | 1601 (0.55) | 3360 (0.55) |
| Alcohol consumption | ||
| Ever | 2562 (0.88) | 5329 (0.87) |
| Never | 351 (0.12) | 774 (0.13) |
| Median consumption (g) | 29.6 (0–60) | 30.0 (0–64.7) |
Controls do not have an age of onset but rather the index year received from their matched cases.
Table S1 presents the distribution of life events amongst cases and controls. 86% of our study population (86.2% cases, 85.7% controls) experienced at least one important life event during the past 10 years. Compared with individuals who reported no life event, the risk of MS increased as the number of total (any) events increased [OR (95% CI) 0.88 (0.74–1.04) for 1–2 events; OR (95% CI) 1.09 (0.91–1.31) for 3–5 events; OR (95% CI) 1.53 (1.14–2.05) for >6 events; P value for linear trends 0.001] (Table S2).
The 10 individual life events were modestly related with each other with correlation coefficients ranging from 21% to 58% (Fig. 1a). According to the dissimilarity structure, the life events were combined into four broad clusters (Fig. 1b). Cluster 1 contained a single component, ‘death of a close relative or friend’, indicating that an unfortunate event happened to extended family members or surroundings. Cluster 2 contained two components, ‘sickness or accident of a spouse, partner or child’ and ‘death of a spouse, partner or child’, indicating that an unfortunate event happened to core family members. Cluster 3 contained three components, ‘marriage’, ‘divorce’ and ‘conflict with a spouse or partner’, indicating major changes of an intimate or romantic relationship. Cluster 4 contained four components, ‘poor economy’, ‘become unemployed’, ‘conflict at work’ and ‘serious conflict with a close relative or friend’, indicating major changes of a working relationship or social support. An individual was considered as non‐exposed only if he or she reported to have experienced none of the component event(s) within that cluster (otherwise, exposed). These clusters are mutually exclusive, and relatives do not include spouse, partner or children.
Figure 1.
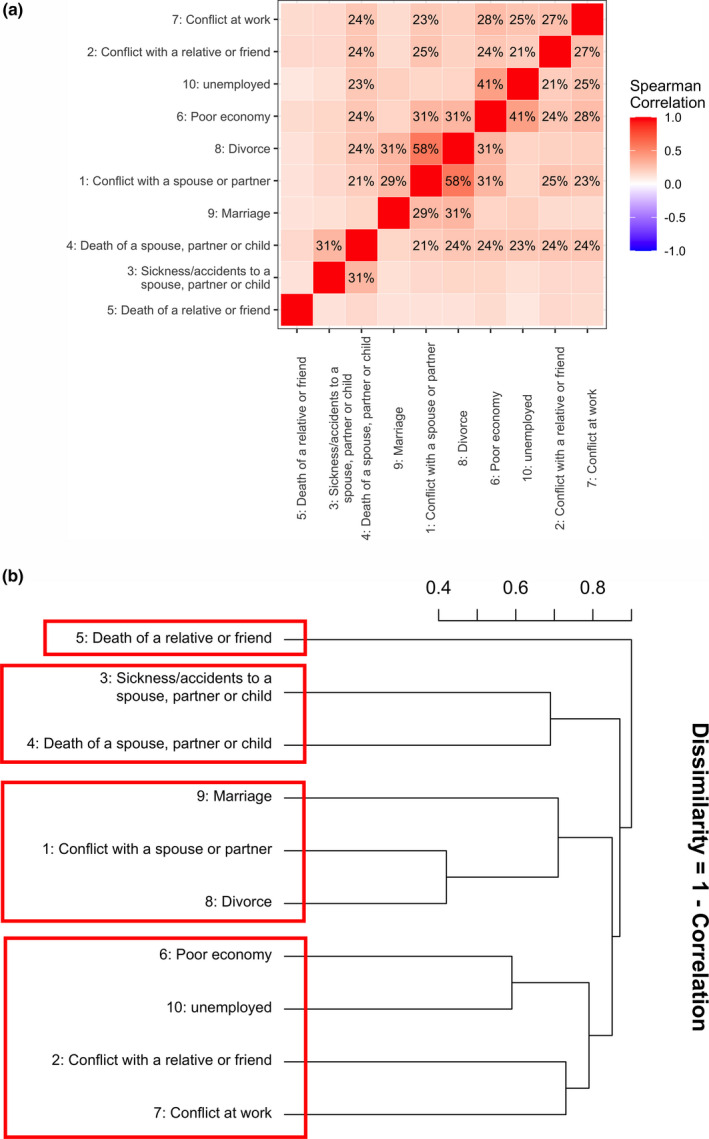
The correlation and dissimilarity structure amongst 10 life events. (a) Correlation matrix of the 10 life events. Spearman’s rank correlation coefficients for each event with all other events were calculated and these pairwise coefficients were mapped into a heat map. The color of each checker represents the magnitude of correlation. A darker color represents stronger correlation. (b) Dissimilarity structure of the 10 life events. Agglomerative hierarchical cluster analysis was performed (using complete linkage) to identify subgroups of the 10 life events. The y‐axis represents the dissimilarity (1 – correlation)
A significant association between life events and the risk of MS was found (Table 2 and Fig. 2). Compared to controls, cases were more likely to experience negative life events such as sickness, accidents or death of a core family member (cluster 2, 12% vs. 9%), major changes to a romantic relationship (cluster 3, 48% vs. 44%) and major changes to a working relationship (cluster 4, 44% vs. 39%). All of these posed a 14%–26% significantly increased risk of MS (cluster 2, OR 1.26, 95% CI 1.08–1.47, P = 0.007; cluster 3, OR 1.14, 95% CI 1.03–1.27, P = 0.014; cluster 4, OR 1.16, 95% CI 1.04–1.30, P = 0.007). These events seem to influence women more severely than men, since the significant effects from all three clusters were confined to women (cluster 2, OR = 1.37, P = 0.001; cluster 3, OR = 1.16, P = 0.018; cluster 4, OR = 1.15, P = 0.032) but not men (cluster 2, OR = 0.98, P = 0.90; cluster 3, OR = 1.09, P = 0.39; cluster 4, OR = 1.19, P = 0.12). However, when analyzing the sex‐by‐event interaction effect by adding an interaction term into the logistic regression model, no significant interactions were identified (all P values >0.05, presented in the last column of Table 2). Analyzing the associations using conditional logistic regression yielded highly consistent results (Table S3). When splitting the exposure into three levels (none, 1 event, ≥ 2 events), it was found that across all clusters the risk of MS increased (although not necessarily significantly) as the number of exposures increased (Table S4).
Table 2.
The association between clustered life events and risk of multiple sclerosis, stratified by sex
| Event | Population | Exposure | MS cases | Controls | OR (95% CI) | P value | P value for interaction |
|---|---|---|---|---|---|---|---|
| Cluster 1: Death of a close friend or relative | Overall | No event | 1555 (0.70) | 2951 (0.69) | 1.00 ref | ||
| Any event | 661 (0.30) | 1351 (0.31) | 0.90 (0.80–1.01) | 0.08 | 0.89 | ||
| Female | No event | 1113 (0.7) | 2085 (0.68) | 1.00 ref | |||
| Any event | 479 (0.3) | 975 (0.32) | 0.89 (0.78–1.02) | 0.10 | NA | ||
| Male | No event | 442 (0.71) | 866 (0.7) | 1.00 ref | |||
| Any event | 182 (0.29) | 376 (0.3) | 0.90 (0.72–1.13) | 0.36 | NA | ||
| Cluster 2: Sickness, accident or death of a spouse, partner or child | Overall | No event | 2255 (0.88) | 4792 (0.91) | 1.00 ref | ||
| Any event | 302 (0.12) | 503 (0.09) | 1.26 (1.08–1.47) | 0.007 | 0.06 | ||
| Female | No event | 1578 (0.87) | 3360 (0.9) | 1.00 ref | |||
| Any event | 239 (0.13) | 373 (0.1) | 1.37 (1.14–1.63) | 0.001 | NA | ||
| Male | No event | 677 (0.91) | 1432 (0.92) | 1.00 ref | |||
| Any event | 63 (0.09) | 130 (0.08) | 0.98 (0.70–1.36) | 0.90 | NA | ||
| Cluster 3: Serious conflict with a spouse or partner, divorce, marriage | Overall | No event | 1191 (0.52) | 2515 (0.56) | 1.00 ref | ||
| Any event | 1105 (0.48) | 1989 (0.44) | 1.14 (1.03–1.27) | 0.014 | 0.61 | ||
| Female | No event | 823 (0.5) | 1753 (0.54) | 1.00 ref | |||
| Any event | 822 (0.5) | 1480 (0.46) | 1.16 (1.03–1.32) | 0.018 | NA | ||
| Male | No event | 368 (0.57) | 762 (0.6) | 1.00 ref | |||
| Any event | 283 (0.43) | 509 (0.4) | 1.09 (0.89–1.34) | 0.39 | NA | ||
| Cluster 4: Conflict at work, conflict with a close relative or friend, unemployment, poor economy | Overall | No event | 1188 (0.56) | 2561 (0.61) | 1.00 ref | ||
| Any event | 921 (0.44) | 1637 (0.39) | 1.16 (1.04–1.30) | 0.007 | 0.81 | ||
| Female | No event | 791 (0.53) | 1699 (0.57) | 1.00 ref | |||
| Any event | 711 (0.47) | 1269 (0.43) | 1.15 (1.01–1.31) | 0.032 | NA | ||
| Male | No event | 397 (0.65) | 862 (0.7) | 1.00 ref | |||
| Any event | 210 (0.35) | 368 (0.3) | 1.19 (0.96–1.47) | 0.12 | NA |
CI, confidence interval; OR, odds ratio.
Results adjusted for age, sex (for overall population), residential area, pack‐years of smoking, alcohol consumption in grams, levels of education. P values for interaction present P values for the event‐by‐sex interaction calculated in the overall population by adding an interaction term to the logistic model. Bold values indicate results with statistical significance, that is, a P‐value <0.05
Figure 2.
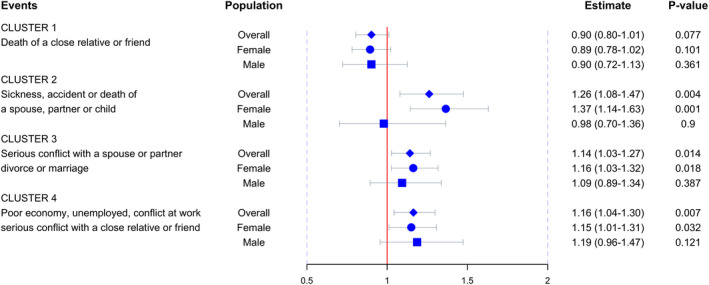
The effect size of clustered life events on MS. Blue diamonds and horizontal bars represent the odds ratios and confidence intervals of each cluster with the risk of MS. Effect sizes in females and males are presented by circles and squares, respectively
Next the influence from individual component events within each cluster was investigated. Since no significant effect was observed from cluster 1 which involved only one single component, analysis was performed focusing on the remaining clusters. As shown in Table 3 and Fig. 3, within cluster 2, both components seem to matter. Sickness or accident of a spouse, partner or child increased overall MS risk by 25% [1.25 (1.06–1.46), P = 0.007] and affected women (OR = 1.38, P = 0.001) more markedly than men (OR = 0.90, P = 0.53). Similarly, the rare event of death of a spouse, partner or child (reported by < 1% of the study population) elevated MS risk by 36%, although not reaching statistical significance [1.36 (0.82–2.28), P = 0.24]. Likewise, within cluster 3, all three components increased MS risk. For example, serious conflict with a spouse or partner increased disease risk by 18% [1.18 (1.05–1.34), P = 0.007] and affected men [1.17 (0.91–1.50), P = 0.21] and women [1.19 (1.03–1.37), P = 0.017] equally. Divorce increased disease risk by 28% [1.28 (1.12–1.47), P = 0.0003] and influenced similarly men [1.23 (0.93–1.63), P = 0.15] and women [1.29 (1.10–1.50), P = 0.001]. Marriage appeared to increase disease risk by 17% [1.17 (1.05–1.31), P = 0.005) and only within women [1.23 (1.08–1.41), P = 0.002] rather than amongst men [1.03 (0.83–1.28), P = 0.78]. Finally, within cluster 4, unemployment did not seem to contribute a significant effect to disease risk probably due to the good social welfare of Sweden. However, poor economy [1.19 (1.03–1.38), P = 0.02], serious conflict at work [1.30 (1.12–1.50), P = 0.001] or with a close relative or friend [1.23 (1.07–1.42), P = 0.007] all increased disease risk by ~20%–30%. Conflicts, in particular, struck women more severely (23%–33% significantly increased risk) than men (19%–21% non‐significant effect). Despite the difference on effect size between men and women, no significant sex‐by‐event interaction effect was observed except for sickness or accident of a spouse, partner or child (P for interaction 0.02). Analyzing the associations using conditional logistic regression yielded highly consistent results (Table S5). The exposure was then explored using three levels (none, 1 event, ≥2 events); as expected, across all components, the risk of MS increased as the number of events increased (Table S6).
Table 3.
The association between each component event and risk of multiple sclerosis, stratified by sex
| Cluster | Component event | Population | Exposure | MS cases | Controls | OR (95% CI) | P value | P value for interaction |
|---|---|---|---|---|---|---|---|---|
| Cluster 2 | Sickness or accident of a spouse, partner or child | Overall | No event | 2278 (0.89) | 4843 (0.91) | 1.00 ref | 0.007 | 0.02 |
| Any event | 286 (0.11) | 480 (0.09) | 1.25 (1.06–1.46) | |||||
| Female | No event | 1593 (0.87) | 3405 (0.91) | 1.00 ref | 0.001 | NA | ||
| Any event | 229 (0.13) | 354 (0.09) | 1.38 (1.15–1.65) | |||||
| Male | No event | 685 (0.92) | 1438 (0.92) | 1.00 ref | 0.53 | NA | ||
| Any event | 57 (0.08) | 126 (0.08) | 0.90 (0.63–1.26) | |||||
| Death of a spouse, partner or child | Overall | No event | 2864 (0.99) | 5998 (0.99) | 1.00 ref | 0.24 | 0.17 | |
| Any event | 24 (0.01) | 40 (0.01) | 1.36 (0.82–2.28) | |||||
| Female | No event | 2053 (0.99) | 4266 (0.99) | 1.00 ref | 0.83 | NA | ||
| Any event | 15 (0.01) | 32 (0.01) | 1.07 (0.58–1.99) | |||||
| Male | No event | 811 (0.99) | 1732 (1.00) | 1.00 ref | 0.051 | NA | ||
| Any event | 9 (0.01) | 8 (0.00) | 2.67 (1.00–7.13) | |||||
| Cluster 3 | Serious conflict with a spouse or partner | Overall | No event | 1974 (0.78) | 4135 (0.81) | 1.00 ref | 0.007 | 0.91 |
| Any event | 543 (0.22) | 939 (0.19) | 1.18 (1.05–1.34) | |||||
| Female | No event | 1376 (0.77) | 2887 (0.80) | 1.00 ref | 0.017 | NA | ||
| Any event | 413 (0.23) | 717 (0.20) | 1.19 (1.03–1.37) | |||||
| Male | No event | 598 (0.82) | 1248 (0.85) | 1.00 ref | 0.21 | NA | ||
| Any event | 130 (0.18) | 222 (0.15) | 1.17 (0.91–1.50) | |||||
| Divorce or equivalent | Overall | No event | 2221 (0.84) | 4805 (0.87) | 1.00 ref | 0.0003 | 0.85 | |
| Any event | 417 (0.16) | 691 (0.13) | 1.28 (1.12–1.47) | |||||
| Female | No event | 1558 (0.83) | 3376 (0.86) | 1.00 ref | 0.001 | NA | ||
| Any event | 322 (0.17) | 533 (0.14) | 1.29 (1.10–1.50) | |||||
| Male | No event | 663 (0.87) | 1429 (0.90) | 1.00 ref | 0.15 | NA | ||
| Any event | 95 (0.13) | 158 (0.10) | 1.23 (0.93–1.63) | |||||
| Marriage or equivalent | Overall | No event | 1628 (0.68) | 3441 (0.72) | 1.00 ref | 0.005 | 0.16 | |
| Any event | 761 (0.32) | 1344 (0.28) | 1.17 (1.05–1.31) | |||||
| Female | No event | 1147 (0.67) | 2452 (0.71) | 1.00 ref | 0.002 | NA | ||
| Any event | 567 (0.33) | 978 (0.29) | 1.23 (1.08–1.41) | |||||
| Male | No event | 481 (0.71) | 989 (0.73) | 1.00 ref | 0.78 | NA | ||
| Any event | 194 (0.29) | 366 (0.27) | 1.03 (0.83–1.28) | |||||
| Cluster 4 | Serious conflict with a close relative or friend | Overall | No event | 2176 (0.85) | 4634 (0.88) | 1.00 ref | 0.005 | 0.87 |
| Any event | 374 (0.15) | 614 (0.12) | 1.23 (1.07–1.42) | |||||
| Female | No event | 1490 (0.83) | 3179 (0.86) | 1.00 ref | 0.009 | NA | ||
| Any event | 310 (0.17) | 506 (0.14) | 1.23 (1.05–1.45) | |||||
| Male | No event | 686 (0.91) | 1455 (0.93) | 1.00 ref | 0.27 | NA | ||
| Any event | 64 (0.09) | 108 (0.07) | 1.21 (0.86–1.69) | |||||
| Poor economy | Overall | No event | 2115 (0.86) | 4529 (0.88) | 1.00 ref | 0.018 | 0.53 | |
| Any event | 346 (0.14) | 598 (0.12) | 1.19 (1.03–1.38) | |||||
| Female | No event | 1484 (0.85) | 3174 (0.87) | 1.00 ref | 0.079 | NA | ||
| Any event | 255 (0.15) | 458 (0.13) | 1.16 (0.98–1.38) | |||||
| Male | No event | 631 (0.87) | 1355 (0.91) | 1.00 ref | 0.12 | NA | ||
| Any event | 91 (0.13) | 140 (0.09) | 1.26 (0.94–1.69) | |||||
| Conflict at work | Overall | No event | 2125 (0.86) | 4461 (0.89) | 1.00 ref | 0.001 | 0.42 | |
| Any event | 349 (0.14) | 565 (0.11) | 1.30 (1.12–1.50) | |||||
| Female | No event | 1489 (0.84) | 3135 (0.88) | 1.00 ref | 0.001 | NA | ||
| Any event | 274 (0.16) | 430 (0.12) | 1.33 (1.12–1.57) | |||||
| Male | No event | 636 (0.89) | 1326 (0.91) | 1.00 ref | 0.26 | NA | ||
| Any event | 75 (0.11) | 135 (0.09) | 1.19 (0.88–1.63) | |||||
| Unemployed | Overall | No event | 2242 (0.88) | 4733 (0.89) | 1.00 ref | 0.17 | 0.98 | |
| Any event | 313 (0.12) | 579 (0.11) | 1.11 (0.96–1.29) | |||||
| Female | No event | 1599 (0.88) | 3371 (0.89) | 1.00 ref | 0.27 | NA | ||
| Any event | 224 (0.12) | 418 (0.11) | 1.10 (0.93–1.32) | |||||
| Male | No event | 643 (0.88) | 1362 (0.89) | 1.00 ref | 0.46 | NA | ||
| Any event | 89 (0.12) | 161 (0.11) | 1.11 (0.84–1.49) |
CI, confidence interval; OR, odds ratio.
Results adjusted for age, sex (for overall population), residential area, pack‐years of smoking, alcohol consumption in grams, levels of education. P values for interaction present P values for the event‐by‐sex interaction calculated in the overall population by adding an interaction term to the logistic model. Bold values indicate results with statistical significance, that is, a P‐value <0.05
Figure 3.
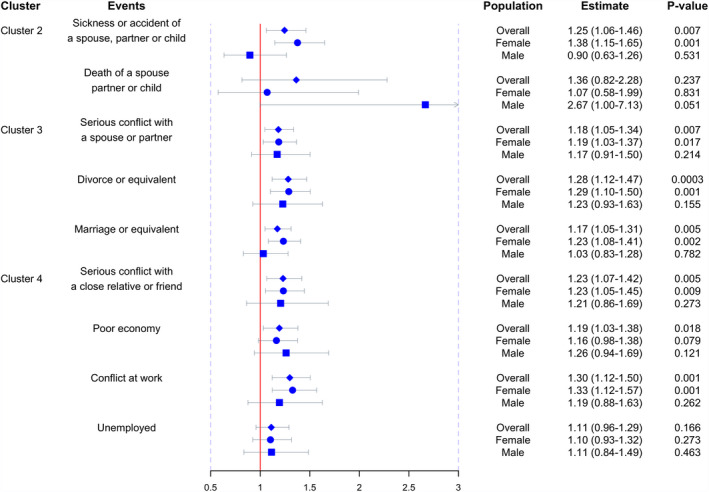
The effect size of individual life events on MS. Blue diamonds and horizontal bars represent the odds ratios and confidence intervals of each individual life event with the risk of MS. Effect sizes in females and males are presented by circles and squares, respectively
Multiple sclerosis cases might be convinced that negative life events play a role in their disease risk which may lead to over‐reporting or improved recall amongst cases. It was therefore attempted to understand if cases would recall events that happened longer ago than controls. It was found that, amongst cases, most of the events happened at a median of 3.99 years prior to MS onset, whilst the corresponding figure was 3.88 years amongst controls. Indeed, cases were able to recall events that occurred a slightly longer time ago (1.5 months more) than controls; no significant difference was observed, however (P = 0.08). This held true for both women and men, and across all 10 events (Fig. S1). A sensitivity analysis was also conducted where events were stratified into ‘within 5 years’ and ‘beyond 5 years’ prior to disease onset. Most events happening up to 5 years ago were significantly associated with MS risk. Yet, most events happening more than 5 years ago were not significantly associated with MS risk (most probably due to a lack of power for this stratum); their effect sizes attenuated slightly (Table S7).
Discussion
A large‐scale population‐based case–control study was conducted on stressful life events and the risk of MS, using extensive questionnaire information on demographic and lifestyle environmental factors, available for a total of 2930 incident MS cases and 6170 controls. Overall, 86% of our study population experienced at least one important life event during the past 10 years. Compared with individuals who reported no life event, the risk of MS increased as the number of total (any) events increased, indicating a dose–response relationship (P value for linear trends 0.001). After accounting for the effect of potential confounders, compelling evidence was found for a link between major life events and risk of MS – most life events that happened prior to disease onset were significantly associated with an increased disease risk of 15%–30%. It was further observed that women were more vulnerable under certain stressful scenarios such as conflict at work or within families, marriage, sickness or accident of family members. Despite an overall lack of event‐by‐sex interaction effect, our results indicate that women are usually affected at least to a comparable extent to (if not more than) men.
Despite a growing number of epidemiological investigations examining the role of stress in MS, earlier studies were mostly small in sample size, prone to selection bias, and did not control for confounding, all of which influence the consistency of results [14]. Nevertheless, findings remain controversial even for recently conducted well‐designed prospective studies. For example, in 2011, Riise et al. followed 238 371 female nurses and found no increased risk of MS (<400 MS cases) associated with severe stress at home (hazard ratio 0.85, 95% CI 0.32–2.26) or with self‐reported severe physical or sexual abuse in childhood or adolescence [15]. Nielsen et al., in contrast, followed a nationwide birth cohort of 2.9 million Danes and found a significantly elevated risk of MS (N = 4760) with childhood exposure to parental divorce (relative risk 1.13, 95% CI 1.04–1.23) [16] yet adults who lost a child, were divorced or widowed did not seem to have any changed risk of MS [17]. A recently published population‐ and sibling‐matched retrospective cohort study conducted in Sweden, with 106 464 patients exposed to stress‐related disorders, reported an overall significantly increased risk of MS amongst those exposed to stress‐related disorders (total number of events 1775, including 200 MS cases amongst 911 000 exposed person‐years vs. 1575 MS cases amongst 9 675 000 non‐exposed person‐years; relative risk 1.22, 95% CI 1.05–1.43) [5]. Our results have confirmed and extended previous findings through a systematic interrogation involving 10 different life events within and beyond families. It was found that most life events occurring prior to disease onset, including divorce, conflicts, sickness and accidents, significantly increase MS risk by 15%–30%, and women are usually affected at least to a comparable extent to (if not more than) men. One study has assumed a 12‐month period prior to the first demyelination event as a critical window in MS development [18]. Our results have demonstrated that this window may well be longer given that life events occurring within 5 years preceding disease onset significantly influence MS risk.
Biological mechanisms underlying such associations remain to be elucidated. Previous work has identified an increased comorbidity for psychiatric/stress‐related disorders in MS and vice versa [19]. Our recent work has analyzed multiple genome‐wide association study data on >200 000 patients for 25 brain‐associated disorders and found a shared yet very weak genetic correlation between psychiatric/stress‐related disorders and neurological diseases (e.g. MS) [20]. Numerous empirical studies have also shown that psychological challenges are capable of directly altering various features of human immune response, such as increasing the level of circulating natural killer cells and antibodies to the latent Epstein–Barr virus [21]. Stress is known to trigger the release of a wide variety of substances via sympathetic nerve fibers that could influence immune response as well as dysregulate the hypothalamic‐pituitary‐adrenal (HPA) axis, and MS patients have a hyperresponsive HPA axis [22, 23, 24]. Moreover, stressful life events may affect disease susceptibility through environmental modifications, e.g. behavioral changes including increased tobacco use, reduced physical activity or other hazards.
Our study has several strengths. Under the current sample size (2930 cases and 6170 controls) and assuming a prevalence of exposure of 20% (a lower bound of the real‐world situation), our study has 80% power to detect an OR of 1.17, 92% power to detect an OR of 1.20 and 99% power to detect an OR of 1.30, at an alpha level of 0.05. With such a power, stratified analyses by sex as well as to explore dose–response relationships could be conducted, whereas previous studies did not have this opportunity. As a chronic progressive autoimmune disease, MS affects approximately 2.5 million people worldwide [25] and 1.89/1000 of the nationwide population in Sweden [26]; our study may provide novel insights into the etiology of MS by disentangling the relationship between stressful life events and MS.
There are some caveats. The observational nature and retrospective design of our study make it open to the criticism that validity of results could be plagued by measurement error or recollection bias. Our strategy to counteract such an impact was to involve objective events such as divorce, marriage, unemployment, death or accidents of family members, which are very unlikely to be mistakenly recalled or classified, and of which some are also independent of responders’ activities. The fact that effect sizes are of similar magnitude across objective and subjective life events further supports the validity of our results. Although in our study, on comparing the recollection time of events between cases and controls, the difference seems to be small and the likelihood of recall bias seems to be minor (3.99 vs. 3.88, P = 0.08), such a bias could probably still not be fully ruled out. Concern can also be raised for reverse causality in that individuals predisposed to MS might have altered neurological or psychiatric status years prior to disease onset, leading to increased level of conflicts. Sensitivity analysis has identified similarly significant effects for most events as far as 5 years prior to disease onset minimizing the likelihood of reverse causality. However, recent work on the MS prodrome have suggested that the 5‐year period before MS onset is not an etiologically relevant period for the start of the process eventually leading to MS and that it is necessary to look even further back [27]. If so, it would seem that the results presented here are probably more consistent with an acceleration of bringing the already existing pathological process to a clinical breakthrough point. The recruitment of cases and controls may introduce selection bias. However, the Swedish healthcare system provides free of charge access to all Swedish residents and almost all cases of MS are referred to hospital‐based neurological units. In total, 42 study centers reported cases of MS to our study, and a satisfactory participation rate of 92% was received; it is therefore believed that the cases recruited to EIMS are representative of the overall MS case population in Sweden. Whereas the focus was on stressful life events and all 10 life events were analyzed in a non‐differential manner, it is acknowledged that it is an older approach in which events are identified as being likely to cause stress, rather than examining the perceived stress associated with events. Positive and negative events were also not separated, which can potentially have different effects on our outcome. Family history of psychiatric disorder could be an important confounder or mediator here in our analysis. Unfortunately there are no extra data on these factors. Finally, our study did not take into consideration the distinct genetic backgrounds of our individuals. Future studies may focus on the complex gene–environment interplay in MS, to better understand the impact of major life changes on disease risk for those genetically vulnerable individuals.
Disclosure of conflicts of interest
None.
Supporting information
Table S1. Distribution of major life events amongst all subjects as well as cases and controls.
Table S2. The association between number of total life events (summing up all 10 life events) and risk of multiple sclerosis.
Table S3. The association between four life event clusters and risk of multiple sclerosis; results from conditional logistic regression analysis.
Table S4. The association between numbers of clustered life events and risk of multiple sclerosis, stratified by sex.
Table S5. The association between 10 individual life events and risk of multiple sclerosis; results from conditional logistic regression analysis.
Table S6. The association between numbers of individual life events and risk of multiple sclerosis.
Table S7. The association between each component event and risk of multiple sclerosis, stratified by year of occurrence.
Acknowledgements
The authors want to thank all the multiple sclerosis patients and controls who have participated in our study. Funding information: Dr Jiang is supported by a starting package from the Swedish Research Council (Vetenskapsrådet 2018 Starting package/NA).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 2. International Multiple Sclerosis Genetics Consortium . Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019;365: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O’Gorman C, Lucas R, Taylor B. Environmental risk factors for multiple sclerosis: a review with a focus on molecular mechanisms. Int J Mol Sci 2012; 13: 11718–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glaser R, Kiecolt‐Glaser JK. Stress‐induced immune dysfunction: implications for health. Nat Rev Immunol 2005; 5(3): 243–251. [DOI] [PubMed] [Google Scholar]
- 5. Song H, Fang F, Tomasson G, et al Association of stress‐related disorders with subsequent autoimmune disease. JAMA 2018; 319: 2388–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grant I, Brown GW, Harris T, McDonald WI, Patterson T, Trimble MR. Severely threatening events and marked life difficulties preceding onset or exacerbation of multiple sclerosis. J Neurol Neurosurg Psychiatry 1989; 52: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu XJ, Ye HX, Li WP, Dai R, Chen D, Jin M. Relationship between psychosocial factors and onset of multiple sclerosis. Eur Neurol 2009; 62: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warren S, Greenhill S, Warren KG. Emotional stress and the development of multiple sclerosis: case–control evidence of a relationship. J Chronic Dis 1982; 35: 821–831. [DOI] [PubMed] [Google Scholar]
- 9. Mei‐Tal V, Meyerowitz S, Engel GL. The role of psychological process in a somatic disorder: multiple sclerosis. 1. The emotional setting of illness onset and exacerbation. Psychosom Med 1970; 32: 67–86. [DOI] [PubMed] [Google Scholar]
- 10. Palumbo R, Fontanillas L, Salmaggi A, La Mantia L, Milanese C. Stressful life events and multiple sclerosis: a retrospective study. Ital J Neurol Sci 1998; 19: 259–260. [DOI] [PubMed] [Google Scholar]
- 11. Pratt RTC. An investigation of the psychiatric aspects of disseminated sclerosis. J Neurol Neurosurg Psychiatry 1951; 14: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson AJ, Montalban X, Barkhof F, et al Diagnostic criteria for primary progressive multiple sclerosis: a position paper. Ann Neurol 2000; 47: 831–835. [PubMed] [Google Scholar]
- 13. Hedström AK, Bäärnhielm M, Olsson T, Alfredsson L. Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology 2009; 73: 696–701. [DOI] [PubMed] [Google Scholar]
- 14. Briones‐Buixassa L, Milà R, Ma Aragonès J, Bufill E, Olaya B, Arrufat FX. Stress and multiple sclerosis: a systematic review considering potential moderating and mediating factors and methods of assessing stress. Health Psychol Open 2015; 2: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riise T, Mohr DC, Munger KL, Rich‐Edwards JW, Kawachi I, Ascherio A. Stress and the risk of multiple sclerosis. Neurology 2011; 76: 1866–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nielsen NM, Pedersen BV, Stenager E, Koch‐Henriksen N, Frisch M. Stressful life‐events in childhood and risk of multiple sclerosis: a Danish nationwide cohort study. Mult Scler Houndmills Basingstoke Engl 2014; 20(12): 1609–1615. [DOI] [PubMed] [Google Scholar]
- 17. Nielsen NM, Bager P, Simonsen J, et al Major stressful life events in adulthood and risk of multiple sclerosis. J Neurol Neurosurg Psychiatry 2014; 85: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 18. Saul A, Ponsonby A‐L, Lucas RM, et al Stressful life events and the risk of initial central nervous system demyelination. Mult Scler Houndmills Basingstoke Engl 2017; 23(7): 1000–1007. [DOI] [PubMed] [Google Scholar]
- 19. Johansson V, Lundholm C, Hillert J, et al Multiple sclerosis and psychiatric disorders: comorbidity and sibling risk in a nationwide Swedish cohort. Mult Scler Houndmills Basingstoke Engl 2014; 20: 1881–1891. [DOI] [PubMed] [Google Scholar]
- 20. Brainstorm Consortium , Anttila V, Bulik‐Sullivan B, et al Analysis of shared heritability in common disorders of the brain. Science 2018; 360: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta‐analytic study of 30 years of inquiry. Psychol Bull 2004; 130: 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huitinga I, Erkut ZA, van Beurden D, Swaab DF. Impaired hypothalamus‐pituitary‐adrenal axis activity and more severe multiple sclerosis with hypothalamic lesions. Ann Neurol 2004; 55: 37–45.14705110 [Google Scholar]
- 23. Huitinga I, Erkut ZA, van Beurden D, Swaab DF. The hypothalamo‐pituitary‐adrenal axis in multiple sclerosis. Ann N Y Acad Sci 2003; 992: 118–128. [DOI] [PubMed] [Google Scholar]
- 24. Melief J, de Wit SJ, van Eden CG, et al HPA axis activity in multiple sclerosis correlates with disease severity, lesion type and gene expression in normal‐appearing white matter. Acta Neuropathol (Berl) 2013; 126(2): 237–249. [DOI] [PubMed] [Google Scholar]
- 25. Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun 2014; 49: 134–142. [DOI] [PubMed] [Google Scholar]
- 26. Ahlgren C, Odén A, Lycke J. High nationwide incidence of multiple sclerosis in Sweden. PLoS One 2014; 9: e108599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wijnands JM, Zhu F, Kingwell E, et al Five years before multiple sclerosis onset: phenotyping the prodrome. Mult Scler Houndmills Basingstoke Engl 2019; 25: 1092–1101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Distribution of major life events amongst all subjects as well as cases and controls.
Table S2. The association between number of total life events (summing up all 10 life events) and risk of multiple sclerosis.
Table S3. The association between four life event clusters and risk of multiple sclerosis; results from conditional logistic regression analysis.
Table S4. The association between numbers of clustered life events and risk of multiple sclerosis, stratified by sex.
Table S5. The association between 10 individual life events and risk of multiple sclerosis; results from conditional logistic regression analysis.
Table S6. The association between numbers of individual life events and risk of multiple sclerosis.
Table S7. The association between each component event and risk of multiple sclerosis, stratified by year of occurrence.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


