Abstract
Single risk factors, such as hypertension and dyslipidemia, can combine to exacerbate the development and severity of cardiovascular disease. Treatment goals may be more effectively achieved if multiple disease factors are targeted with combination treatment. We enrolled 202 patients who were randomly divided into the following three groups: telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg, telmisartan 80 mg + rosuvastatin 20 mg, and telmisartan/amlodipine 80/5 mg. The primary efficacy variables were changes from baseline in mean sitting systolic blood pressure (MSSBP) between telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan 80 mg + rosuvastatin 20 mg at 8 weeks, and the percent changes from baseline in low‐density lipoprotein (LDL) cholesterol between telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan/amlodipine 80/5 mg at 8 weeks. The secondary efficacy variables were changes in MSSBP, mean sitting diastolic blood pressure (MSDBP), LDL cholesterol and other lipid levels at 4 weeks and 8 weeks, as well as observed adverse events during follow‐up. There were no significant differences between the three groups in demographic characteristics and no significant difference among the three groups in terms of baseline characteristics for the validity evaluation variables. The mean overall treatment compliance in the three groups was, respectively, 98.42%, 96.68%, and 98.12%, indicating strong compliance for all patients. The Least‐Square (LS) mean (SE) for changes in MSSBP in the two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan 80 mg + rosuvastatin 20 mg) groups were −19.3 (2.68) mm Hg and −6.69 (2.76) mm Hg. The difference between the two groups was significant (−12.60 (2.77) mm Hg, 95% CI −18.06 to −7.14, P < .0001). The LS Mean for the percent changes from baseline in LDL cholesterol in the two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan/amlodipine 80/5 mg) groups were −52.45 (3.23) % and 2.68 (3.15) %. The difference between the two groups was significant (−55.13 (3.20) %, 95% CI −61.45 to −48.81, P < .0001). There were no adverse events leading to discontinuation or death. Combined administration of telmisartan/amlodipine 80/5 mg and rosuvastatin 20 mg for the treatment of hypertensive patients with dyslipidemia significantly reduces blood pressure and improves lipid control. ClinicalTrials.gov identifier: NCT03067688.
Keywords: amlodipine, dyslipidemia, hypertension, rosuvastatin, telmisartan
1. INTRODUCTION
A joint study of six hospitals affiliated with the US Department of Veterans Affairs showed that 30.4% of all‐cause inpatients had dyslipidemia with hypertension. 1 Single risk factors, such as hypertension and dyslipidemia, were synergistic in the development and exacerbation of cardiovascular disease, so treatment goals should be targeted to treat combinations of all factors. 2 Nevertheless, patient compliance with combination therapy is low, and improved drug compliance is an important factor in achieving treatment goals. 3
The 2017 American College of Cardiology/American Heart Association hypertension guideline recommended initiating two antihypertensive agents from different classes in adult patients with stage 2 hypertension, which is defined as an average systolic blood pressure (BP) of at least 140 mm Hg or an average diastolic BP of at least 90 mm Hg. 4 The guideline recommended thiazide diuretics, CCBs, and ACEIs or ARBs as first‐line agents and 2 first‐line agents with different mechanisms of action for stage 2 patients. Telmisartan and amlodipine are a representative angiotensin receptor blocker (ARB) and a calcium channel blocker (CCB), respectively. Combination treatment with the two components has been shown to lower blood pressure significantly compared to single doses. 5 In a previous study, combination of telmisartan plus amlodipine showed significant BP control by reducing the systolic BP and diastolic BP by 26.4 and 20.1 mm Hg, respectively. 6 Telmisartan not only inhibits the renin‐angiotensin‐aldosterone system but also partially acts on peroxisome proliferator activated receptor‐γ (PPAR‐γ), giving it a number of pleiotropic effects beyond lowering blood pressure. 7
Over the last 20 years, accumulating evidence has shown a dramatic reduction in cardiovascular risk using 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors (“statins”) to lower levels of low‐density lipoprotein (LDL) cholesterol. In general, a 21% reduction in the risk of major cardiovascular events is associated with every 1 mmol/L (39 mg/dL) reduction in LDL cholesterol. 8 Rosuvastatin is a selective inhibitor of HMG‐CoA reductase. The cholesterol‐lowering effect of the drug manifests by blocking 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase, a rate controlling enzyme in the metabolic pathway that produces cholesterol. Of particular note, rosuvastatin has the highest affinity and highest inhibitory effect against HMG‐CoA reductase compared to other statin drugs, effectively reducing LDL cholesterol and increasing HDL cholesterol. 9
In previous ARB or CCB with rosuvastatin combination studies, telmisartan 80 mg plus rosuvastain 20 mg and amlodipine 10 mg plus rosuvastatin 20 mg showed good efficacy and safety profile. 10 , 11 Therefore, a combination of ARB and CCB with rosuvastatin was expected to show excellent efficacy and safety in patients with hypertension and dyslipidemia. The objective of this study was designed to compare the efficacy and safety of telmisartan/amlodipine plus rosuvastatin versus telmisartan plus rosuvastatin or telmisartan/amlodipine in patients with hypertension and dyslipidemia.
2. METHODS
2.1. Overall study design
This study was conducted as a randomized, double‐blind, active‐controlled, multicenter Phase III trial to evaluate the efficacy and safety of telmisartan/amlodipine and rosuvastatin in patients with hypertension and dyslipidemia. The study protocol was approved by the institutional review boards of each participating center, and written informed consent was provided by all study patients.
Patients who met the screening criteria were asked to discontinue prescriptions for previous antihypertensive or lipid lowering drugs, instructed on lifestyle modifications, and given telmisartan 80 mg once daily for 4 weeks run‐in period. Subjects receiving antihypertensive drugs (eg, beta‐blockers, patients with central nervous system antihypertensives) that require gradual reduction were tapered for 1‐2 weeks before the start of the run‐in period. Eligible patients were randomly assigned to receive telmisartan/amlodipine 80/5 mg plus rosuvastatin 20 mg (Tel/Aml 80/5 mg + Ros 20 mg), telmisartan 80 mg plus rosuvastatin 20 mg (Tel 80 mg + Ros 20 mg), or telmisartan/amlodipine 80/5 mg (Tel/Aml 80/5 mg) using an interactive web‐based system in a 1:1:1 ratio. A double‐dummy technique was used to maintain a double‐blind study. All patients took 3 tablets of investigational products once daily. ‘Tel/Aml 80/5 mg + Ros 20 mg’ group received 1 active tablet of telmisartan/amlodipine 80/5 mg fixed‐dose combination (FDC), 1 active tablet of rosuvastatin 20 mg, and 1 placebo tablet of telmisartan 80 mg. ‘Tel 80 mg + Ros 20 mg’ group received 1 active tablet of telmisartan 80 mg, 1 active tablet of rosuvastatin 20 mg, and 1 placebo tablet of telmisartan/amlodipine 80/5 mg FDC. ‘Tel/Aml 80/5 mg’ group received 1 active tablet of telmisartan/amlodipine 80/5 mg FDC, 1 placebo tablet of telmisartan 80 mg, and 1 placebo tablet of rosuvastatin 20 mg.
2.2. Study population
The eligible subjects in present were as follows: (a) aged ≥19 years old in both gender, (b) drugs‐free patients with hypertension and dyslipidemia (defined as mean sitting systolic blood pressure [MSSBP] ≥160 mm Hg and <180 mm Hg, an LDL‐C level of ≤250 mg/dL, and a triglyceride level<400 mg/dL 12 ) at the time of screening, (c) and had uncontrolled hypertension and dyslipidemia (defined as MSSBP ≥140 mm Hg and <180 mm Hg, or ≥130 mm Hg and <180 mm Hg in patients with diabetic or chronic kidney disease, an LDL‐C level of ≤250 mg/dL, and a triglyceride level <400 mg/dL 12 ) were included in the study.
Subjects with any of following criteria were excluded if: (a) women of childbearing potential, who were unable or unwilling to use appropriate contraceptive methods to avoid pregnancy for the entire trial period, women were pregnant or lactating, women with positive results on a pregnancy test; (b) suspected secondary hypertension or secondary hypertension; (c) orthostatic hypotensive patients with symptoms; (d) concomitant diseases, which were severe ventricular tachycardia, atrial fibrillation, atrial flutter, hypertrophic obstructive cardiomyopathy, ischemic heart disease (unstable angina and myocardial infarction), aortic stenosis, hemodynamically significant aortic valve or mitral stenosis, peripheral vascular disease, severe heart failure (NYHA class III or IV), prior percutaneous coronary angioplasty or coronary artery bypass grafting, severe cerebrovascular disorders (cerebral infarction, cerebral hemorrhage, transient ischemic attack) within the previous 6 months; (e) adverse drug reactions and drug allergies.
After 4 weeks run‐in period, patients who fulfilled the randomization criteria were eligible for 8 weeks treatment period: (a) 140 mm Hg ≤ MSSBP <180 mm Hg measured at randomization, or 130 mm Hg ≤ MSSBP <180 mm Hg for patients with diabetes or chronic kidney disease, (b) LDL‐C levels and TG criteria measured at the time of randomization corresponding to one of the following criteria according to cardiovascular risk factors; 2‐1) cardiovascular risk factors ≤1; 160 ≤ LDL‐C ≤ 250 mg/dL; TG < 400 mg/dL; 2‐2) cardiovascular risk factors ≥2; 10‐year risk <10%; 160 ≤ LDL‐C ≤ 250 mg/dL; TG < 400 mg/dL; 2‐3) cardiovascular risk factors ≥ 2; 10% ≤10‐year risk ≤ 20%; 130 ≤ LDL‐C ≤ 250 mg/dL; TG < 400 mg/dL; 2‐4) Coronary artery disease or equivalent; 100 ≤ LDL‐C ≤ 250 mg/dL; TG < 400 mg/dL (Appendix S1).
2.3. Efficacy variables
The primary end points were the change from baseline in MSSBP between telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan 80 mg + rosuvastatin 20 mg at 8 weeks; and the percent changes from baseline of LDL cholesterol between telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan/amlodipine 80/5 mg at 8 weeks. The secondary end points included the changes in MSSBP at 4 and 8 weeks after administration compared to baseline (excluding primary efficacy variable); changes in MSDBP at 4 and 8 weeks after administration compared to baseline; percent changes of LDL‐C at 4 and 8 weeks after administration compared to baseline (excluding primary efficacy variable); percent changes of the other lipid profiles (TC, HDL‐C, TG) at 4 and 8 weeks after administration compared to baseline.
The BP was measured when the patient was relaxed for at least 3 minutes using the same arm and electronic sphygmomanometer provided by the sponsor. BP was measured three times at the interval of >1 minute in the selected arm. If the gap of the last two diastolic BP was >5 mm Hg, BP was measured once more. Mean BP was calculated from the last two of the three or more measured BPs. Blood samples were drawn from each patient after 8 hours of fasting. The lipid profiles (LDL‐C, TC, HDL‐C, TG) for efficacy analysis were analyzed at the central laboratory.
2.4. Safety variables
Safety was assessed based on adverse events (AE) monitored and recorded by the investigators. AEs were rearranged by treatment group and encoded to system‐organ class (SOC) and preferred term (PT) according to the Medical Dictionary for Regulatory Activities (MedDRA), version 20.0.
2.5. Statistical analysis
The safety analysis population for the assessment of safety included all patients who took the investigational products at least once. The efficacy analysis population for the assessment of efficacy included all patients in the safety population who assessed efficacy at least once. The efficacy assessments for continuous such as change in MSBP were performed by using a MMRM included visit, baseline value, treatment group, the stratification factor (cardiovascular risk category‐group 1/ 2/ 3), visit‐by‐baseline value interaction and visit‐by‐treatment group interaction. The efficacy assessments for proportion such as a responder rate in MSBP were performed by using a logistic regression model included treatment group and the stratification factor (cardiovascular risk category‐group 1/ 2/ 3).
2.6. Sample size
Since the efficacy variables of both hypotheses must be satisfied, the significance level was set to 5% on both sides without multiplicity correction. Assuming a mean (SD) difference of 7.8 (12.4) mm Hg for change from baseline in MSSBP between the treatment groups, we calculated a sample size of 54 per group to satisfy a significance level of 5% (two‐sided) and power of 90%. And assuming a mean (SD) difference of 48 (25) % for percent change from baseline in LDL‐C between treatment groups, 54 per group to satisfy a significance level of 5% (two‐sided) and power of over 99%. The total sample size was set to 180 (60 per group) patients in consideration of a 10% dropout rate.
3. RESULTS
3.1. Study population
A total of 408 patients from 28 clinical centers were screened after providing written consent, while 206 patients were screened out of the study, leaving 202 patients eligible for randomization according to criteria (Figure 1, Appendix S1). There were no significant differences between the three groups in terms of demographic and baseline characteristics of the efficacy evaluation variables (Table 1). The mean overall treatment compliance for the three groups was 98.42%, 96.68%, and 98.12%, respectively, indicating strong compliance for all patients (Table 2).
FIGURE 1.
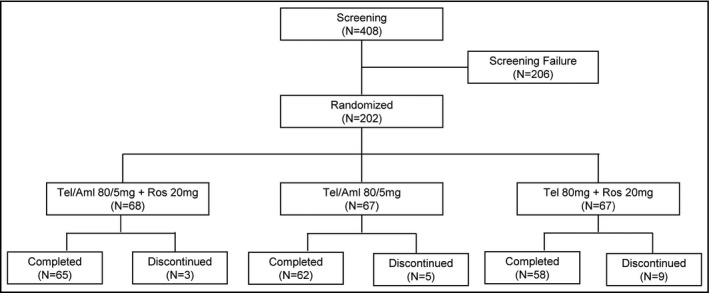
Subject disposition
TABLE 1.
Demographics and baseline characteristics of the patients
| Tel/Aml 80/5 mg + Ros 20 mg (N = 66) | Tel/Aml 80/5 mg (N = 66) | Tel 80 mg + Ros 20 mg (N = 65) | Total (N = 197) | |
|---|---|---|---|---|
| Age (years) | 68.33 ± 8.31 | 66.09 ± 10.43 | 64.89 ± 9.11 | 66.45 ± 9.39 |
| Sex, Male, n (%) | 45 (68.18) | 48 (72.73) | 46 (70.77) | 139 (70.56) |
| Height (cm) | 163.56 ± 8.65 | 164.27 ± 9.21 | 163.10 ± 8.11 | 163.65 ± 8.64 |
| Weight (kg) | 70.56 ± 12.58 | 72.13 ± 10.78 | 70.97 ± 11.56 | 71.22 ± 11.62 |
| BMI (kg/m2)CHD risk factors, n (%) | 26.26 ± 3.40 | 26.67 ± 2.81 | 26.60 ± 3.23 | 26.51 ± 3.14 |
| Current Smoker | 10 (15.15) | 15 (22.73) | 11 (16.92) | 36 (18.27) |
| DBP ≥90 mm Hg | 24 (36.36) | 33 (50.00) | 37 (56.92) | 94 (47.72) |
| HDL‐C <40 mg/dL | 16 (24.24) | 16 (24.24) | 12 (18.46) | 44 (22.34) |
| Family history of premature CHD | 6 (9.09) | 2 (3.03) | 2 (3.08) | 10 (5.08) |
| (−) HDL‐C ≥60 mg/dL | 11 (16.67) | 16 (24.24) | 12 (18.46) | 39 (19.80) |
| Diabetes mellitus* | 28 (42.42) | 29 (43.94) | 32 (49.23) | 89 (45.18) |
| Coronary and peripheral arterial disease | 40 (60.61) | 38 (57.58) | 39 (60.00) | 117 (59.39) |
| 10‐y risk >20% | 31 (46.97) | 30 (45.45) | 21 (32.31) | 82 (41.62) |
| Cardiovascular risk category | ||||
| Group 1 (risk factor 0‐1) | 1 (1.52) | 2 (3.03) | 2 (3.08) | 5 (2.54) |
| Group 2 (risk factor ≥2 and 10‐y risk ≤20%) | 6 (9.09) | 6 (9.09) | 5 (7.69) | 17 (8.63) |
| Group 3 (CHD or CHD equivalence or 10‐y risk >20%) | 59 (89.39) | 58 (87.88) | 58 (89.23) | 175 (88.83) |
| Chronic kidney disease, n (%) | 3 (4.55) | 5 (7.58) | 1 (1.54) | 9 (4.57) |
| Baseline values | ||||
| MSSBP (mm Hg) | 155.40 ± 12.12 | 155.00 ± 12.09 | 154.42 ± 12.86 | 154.94 ± 12.30 |
| MSDBP (mm Hg) | 87.60 ± 8.61 | 89.49 ± 9.99 | 88.54 ± 11.17 | 88.54 ± 9.95 |
| LDL‐C (mg/dL) | 160.12 ± 32.34 | 153.41 ± 31.30 | 153.88 ± 36.73 | 155.81 ± 33.49 |
| Total cholesterol (mg/dL) | 225.55 ± 34.29 | 218.17 ± 36.91 | 221.20 ± 38.74 | 221.64 ± 36.62 |
| Triglyceride (mg/dL) | 170.68 ± 78.79 | 159.27 ± 67.87 | 174.26 ± 84.14 | 168.04 ± 77.06 |
| HDL‐C (mg/dL) | 46.38 ± 10.89 | 47.80 ± 12.04 | 47.89 ± 12.88 | 47.36 ± 11.92 |
| LDL‐C/HDL‐C | 3.59 ± 0.94 | 3.38 ± 0.98 | 3.42 ± 1.16 | 3.46 ± 1.03 |
| TC/HDL‐C | 5.05 ± 1.08 | 4.79 ± 1.19 | 4.89 ± 1.41 | 4.91 ± 1.23 |
Data are mean ± SD or n (%).Percentage denominator is the number of patients in each column.
Abbreviations: Aml, Amlodipine; BMI, Body Mass Index; CHD, Coronary Heart Disease; DBP, Diastolic Blood Pressure; HDL‐C, High Density Lipoprotein Cholesterol; LDL‐C, Low‐Density Lipoprotein Cholesterol; MSDBP, Mean Sitting Diastolic Blood Pressure; MSSBP, Mean Sitting Systolic Blood Pressure; Ros, Rosuvastatin; TC, Total Cholesterol; Tel, Telmisartan.
Patients with FPG (fasting plasma glucose) ≥ 126 mg/dL or HbA1c ≥6.5% at screening.
TABLE 2.
Treatment compliance
| Variables | Tel/Aml 80/5 mg + Ros 20 mg (N = 67) | Tel/Aml 80/5 mg (N = 67) | Tel 80 mg + Ros 20 mg (N = 65) | Total (N = 199) |
|---|---|---|---|---|
| Compliance at week 4 (week 0 ~ week 4) | 98.61 ± 3.25 | 97.39 ± 4.50 | 98.24 ± 5.12 | 98.09 ± 4.36 |
| Subjects with compliance ≥80% at week 4, n (%) | 66 (100.00) | 63 (98.44) | 62 (98.41) | 191 (98.96) |
| Compliance at week 8 (week 4 ~ week 8) | 98.32 ± 3.28 | 98.89 ± 5.04 | 98.19 ± 5.12 | 98.47 ± 4.52 |
| Subjects with compliance ≥80% at week 8, n (%) | 65 (100.00) | 62 (100.00) | 57 (98.28) | 184 (99.46) |
| Overall compliance | 98.42 ± 2.57 | 96.68 ± 11.70 | 98.12 ± 4.85 | 97.73 ± 7.50 |
| Subjects with compliance ≥ 80%, n (%) | 66 (98.51) | 66 (98.51) | 64 (98.46) | 196 (98.49) |
Abbreviations: Aml, Amlodipine; Ros, Rosuvastatin; Tel, Telmisartan.
3.2. Primary end points
The least‐square mean values (LS Means, SE) of the MSSBP in the two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan 80 mg + rosuvastatin 20 mg) groups were −19.3 (2.68) mm Hg and −6.69 (2.76) mm Hg at 8 weeks. The difference between the two groups was significant (−12.60 (2.77) mm Hg, 95% CI −18.06 to −7.14, P < .0001) (Table 3, Figure 2). The LS Means for LDL cholesterol reduction in the two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan/amlodipine 80/5 mg) groups were −52.45 (3.23) % and 2.68 (3.15) % at 8 weeks. The difference between the two groups was significant (−55.13 (3.20) %, 95% CI −61.45 to −48.81, P < .0001) (Table 3, Figure 2).
TABLE 3.
Change from baseline in MSSBP and LDL‐C at week 4 and week 8
| Tel/Aml 80/5 mg + Ros 20 mg (N = 66) | Tel/Aml 80/5 mg (N = 66) | Tel80 mg + Ros20 mg (N = 65) | |
|---|---|---|---|
| MSSBP (mm Hg) | |||
| Baseline | 155.4 ± 12.12 | 155.0 ± 12.09 | 154.42 ± 12.86 |
| Week 4 | 136.44 ± 13.47 | 141.57 ± 14.61 | 148.55 ± 1 8.58 |
| MMRM | |||
| LS Mean (SE) | −19.61 (2.63) | −13.57 (2.61) | −5.66 (2.64) |
| Difference vs Tel/Aml80/5 mg + Ros 20 mg | −6.04 (2.57) | −13.95 (2.54) | |
| 95% CI | [−11.12, −0.96] | [−18.97, −8.93] | |
| P‐value | .0201 | <.0001 | |
| Week 8 | 135.16 ± 13.72 | 141.87 ± 14.74 | 146.27 ± 17.75 |
| MMRM | |||
| LS Mean (SE) | −19.30 (2.68) | −12.99 (2.64) | −6.69 (2.76) |
| Difference vs Tel/Aml80/5 mg + Ros 20 mg | −6.30 (2.67) | −12.60 (2.77) | |
| 95% CI | [−11.58, −1.03] | [−18.06, −7.14] | |
| P‐value | .0194 | <.0001 | |
| LDL‐C (mg/dL) | |||
| Baseline | 160.12 ± 32.34 | 153.41 ± 31.3 | 153.88 ± 36.73 |
| Week 4 | 72.83 ± 19 | 153.64 ± 38.45 | 69.72 ± 23.52 |
| MMRM | |||
| LS Mean (SE) | −51.68 (2.95) | 2.42 (2.88) | −51.99 (2.92) |
| Difference vs Tel/Aml80/5 mg + Ros 20 mg | −54.10 (2.63) | 0.31 (2.61) | |
| 95%CI | [−59.29, −48.91] | [−4.84, 5.45] | |
| P‐value | <.0001 | .9065 | |
| Week 8 | 73.3 ± 22.18 | 154.77 ± 36.94 | 69.63 ± 29.09 |
| MMRM | |||
| LS Mean (SE) | −52.45 (3.23) | 2.68 (3.15) | −51.89 (3.26) |
| Difference vs Tel/Aml80/5 mg + Ros 20 mg | −55.13 (3.20) | −0.56 (3.29) | |
| 95% CI | [−61.45, −48.81] | [−7.06, 5.94] | |
| P‐value | <.0001 | .8661 | |
Abbreviations: Aml, Amlodipine; CI, Confidence Interval; LDL‐C, Low‐Density Lipoprotein Cholesterol; LS Mean (SE), Least‐Square Mean (Standard Error); MMRM, Mixed effect Models for Repeated Measures; MSSBP, Mean Sitting Systolic Blood Pressure; Ros, Rosuvastatin; Tel, Telmisartan.
FIGURE 2.
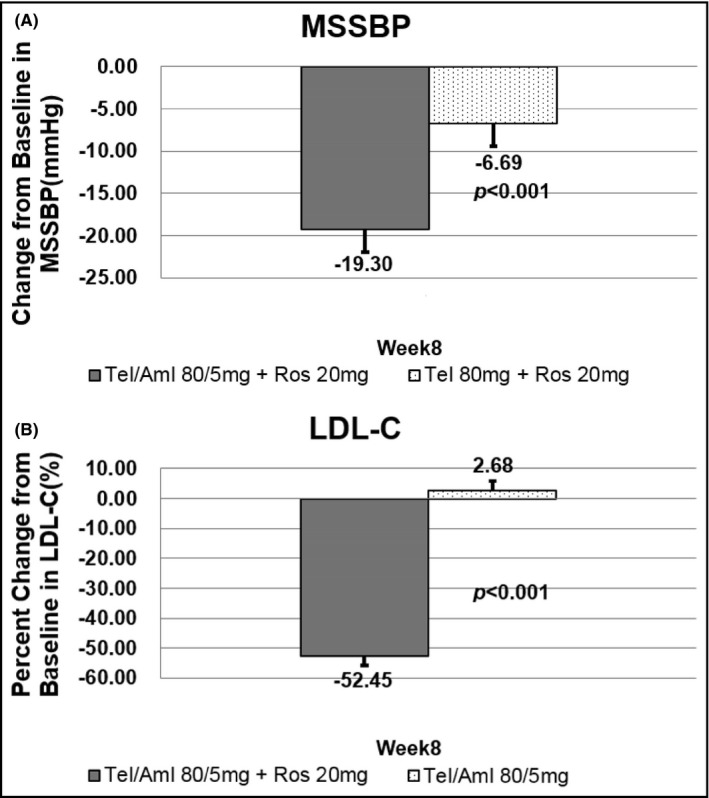
Primary end points: MSSBP and LDL‐C changes at week 8. A, the least‐square mean values (LS Means, SE) of the MSSBP in the two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan 80 mg + rosuvastatin 20 mg) groups. B, the LS Means for LDL cholesterol reduction in the two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan/amlodipine 80/5 mg) groups
3.3. Secondary end points
The LS mean (SE) of the MSSBP changes in the three (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg group, the telmisartan/amlodipine 80/5 mg group and telmisartan 80 mg + rosuvastatin 20 mg) groups were −19.61 (2.63) mm Hg, −13.57 (2.61) mm Hg and −5.66 (2.64) mm Hg at 4 weeks, respectively. The difference between the two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan 80 mg + rosuvastatin 20 mg) groups was significant (−13.95 (2.54) mm Hg, 95% CI −18.97 to −8.93, P < .0001). The difference between the other two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan/amlodipine 80/5 mg) groups was also significant (−6.04 (2.57) mm Hg, 95% CI −11.12 to −0.96, P = .0201) (Table 3). The LS mean (SE) of the MSDBP changes in the three (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg group, the telmisartan/amlodipine 80/5 mg group and telmisartan 80 mg + rosuvastatin 20 mg) groups were −7.26 (1.23) mm Hg, −7.13 (1.22) mm Hg and −0.99 (1.23) mm Hg at 4 weeks, and −7.89 (1.31) mm Hg, −6.31 (1.29) mm Hg and −1.83 (1.35) mm Hg at 8 weeks, respectively. The difference between the two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan 80 mg + rosuvastatin 20 mg) groups was significant at 4 weeks (−6.27 (1.16) mm Hg, 95% CI −8.56 to −3.98, P < .0001) and 8 weeks (−6.06 (1.38) mm Hg, 95% CI −8.78 to −3.33, P < .0001). However, the difference between the other two (telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan/amlodipine 80/5 mg) groups was not significant at all time points. The LS mean (SE) for the percent changes from baseline in LDL cholesterol in the telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg group and the telmisartan/amlodipine 80/5 mg group were −51.68 (2.95) % and 2.42 (2.88) % at 4 weeks, and the LS mean difference (SE) in the LDL‐C reduction between the two groups was −54.10 (2.63) % (95% CI −59.29 to −48.91, P < .0001) (Table 3, Figure 3). In the telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg group, total cholesterol levels were decreased at all time points after administration, whereas the telmisartan/amlodipine 80/5 mg group had total cholesterol increased at all time points, with the LSM difference (SE) for percent change in total cholesterol between the two treatment groups at each time point being −37.81 (1.96) % (95% CI −41.67 to −33.94, P < .0001) at 4 weeks, and −38.17 (2.36) (95% CI −42.82 to −33.52, P < .0001) % at 8 weeks. The telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg group showed higher triglyceride reduction than the telmisartan/amlodipine 80/5 mg group, which was statistically significant; and the LSM Difference (SE) for percent change from baseline in triglyceride was −19.39 (4.71) % (95% CI −28.68 to −10.11, P < .0001) at 4 weeks and −16.95 (5.25) % (95% CI −27.30 to −6.59, P = .0015) at 8 weeks, respectively (Figure 3). HDL cholesterol increased at all time points in both telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg and telmisartan/amlodipine 80/5 mg groups, with higher increase observed in the former group, and the LSM difference (SE) for percent changes from baseline in HDL‐C was 11.59 (2.96) % (95% CI 5.75 to 17.43, P = .0001) at 4 weeks and 11.19 (3.29) % (95% CI 4.69 to 17.69, P = .0008) at 8 weeks, respectively (Figure 3).
FIGURE 3.
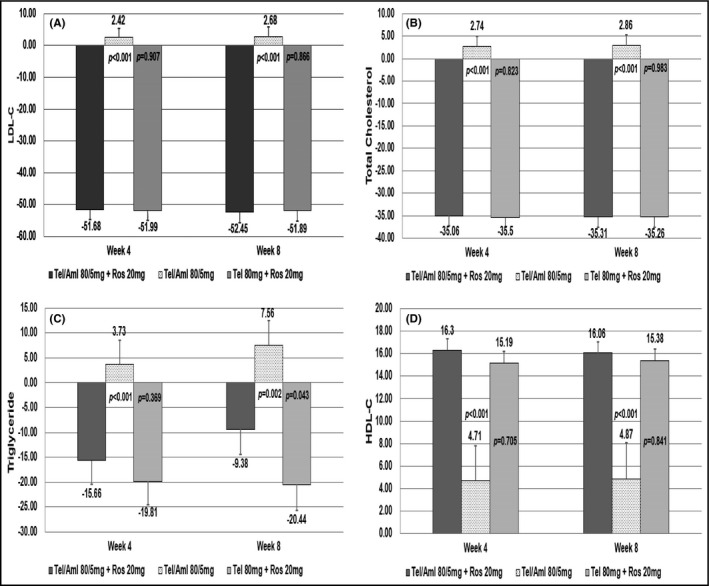
Secondary end points at week 4 and week 8. A, the LS Means for LDL cholesterol reduction in all groups. B, the LS Means for total cholesterol reduction in all groups. C, the LS Means for triglyceride reduction in all groups. D, the LS Means for HDL cholesterol increase in all groups
3.4. Safety assessments
Safety analysis was performed on patients who took at least 1 dose of the double‐blind investigational drug. Treatment‐emergent adverse events occurred in a total of 30/199 (15.08%) patients: 12/67 (17.91%) in the telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg group, 10/67 (14.93%) in the telmisartan/amlodipine 80/5 mg group, and 8/65 (12.31%) in the telmisartan 80 mg + rosuvastatin 20 mg group. Serious TEAEs occurred in 2/199 (1.01%) patients, involving severe spinal column stenosis in a patient of the telmisartan/amlodipine 80/5 mg + rosuvastatin 20 mg group and severe chest pain in a patient of the telmisartan 80 mg + rosuvastatin 20 mg group. No serious TEAE occurred in the telmisartan/amlodipine 80/5 mg group (Table 4).
TABLE 4.
Overall summary of TEAEs
| Tel/Aml 80/5 mg + Ros 20 mg (N = 67) | Tel/Aml 80/5 mg (N = 67) | Tel 80 mg + Ros 20 mg (N = 65) | |
|---|---|---|---|
| Subjects with TEAEs | 12 (17.91) | 10 (14.93) | 8 (12.31) |
| Subjects with ADRs | 5 (7.46) | 6 (8.96) | 6 (9.23) |
| Dizziness | 2 (2.99) | 1 (1.49) | 0 |
| Headache | 0 | 0 | 2 (3.08) |
| Essential tremor | 0 | 0 | 1 (1.54) |
| Asthenia | 0 | 1 (1.49) | 1 (1.54) |
| Chest pain | 0 | 0 | 1 (1.54) |
| Oedema peripheral | 1 (1.49) | 0 | 0 |
| Helicobacter gastritis | 0 | 1 (1.49) | 0 |
| Upper respiratory tract infection | 0 | 0 | 1 (1.54) |
| Viral upper respiratory tract infection | 0 | 0 | 1 (1.54) |
| Palpitations | 0 | 0 | 1 (1.54) |
| Tachycardia | 0 | 0 | 1 (1.54) |
| Abdominal pain | 1 (1.49) | 0 | 0 |
| Constipation | 0 | 1 (1.49) | 0 |
| Arthralgia | 1 (1.49) | 0 | 0 |
| Myalgia | 0 | 1 (1.49) | 0 |
| Alanine aminotransferase increased | 1 (1.49) | 0 | 0 |
| Aspartate aminotransferase increased | 1 (1.49) | 0 | 0 |
| Blood alkaline phosphatase increased | 1 (1.49) | 0 | 0 |
| Gamma‐glutamyl transferase increased | 1 (1.49) | 0 | 0 |
| Gout | 0 | 1 (1.49) | 0 |
| Orthostatic hypotension | 0 | 0 | 1 (1.54) |
| Subjects with SAEs | 1 (1.49) | 0 | 1 (1.54) |
| Subjects with Serious ADRs | 0 | 0 | 0 |
| Subjects with TEAEs Leading to Discontinuation | 0 | 0 | 0 |
| Subjects with TEAEs Leading to Death | 0 | 0 | 0 |
| Subjects with ADRs Leading to Discontinuation | 0 | 0 | 0 |
| Subjects with ADRs Leading to Death | 0 | 0 | 0 |
Abbreviations: ADR, Adverse Drug Reaction; Aml, Amlodipine; Ros, Rosuvastatin; SAE, Serious Adverse Event; TEAE, Treatment Emergent Adverse Events; Tel, Telmisartan.
4. DISCUSSION
The treatment of chronic diseases such as hypertension and dyslipidemia can be negatively impacted by poor levels of patient compliance with medication. For such patients, reducing the number of pills and improving convenience could improve outcomes. 13 , 14 , 15
Telmisartan is an angiotensin receptor antagonist that effectively reduces blood pressure, while amlodipine is a dihydropyridine CCB that reduces peripheral vascular resistance and blood pressure with a long half‐life of 35 hours. 16 These two drugs are the most common components of CCB and ARB fixed‐dose combinations. Such combination approaches can effectively lower blood pressure through different mechanisms. 5
Other studies 17 , 18 have shown that compared to baseline values, the percent changes in lipid levels such as LDL‐C, total cholesterol and triglyceride in the Aml/Los/Ros 5/100/20 mg group are superior to the Aml/Los 5/100 mg group, while the MSSBP changes exceed the Aml/Ros 5/20 mg group, similar to the findings in our study. Briasoulis et al, 19 performed a meta‐analysis evaluating data from a total of 40 prospective randomized controlled trials of statin therapy, finding small but statistically significant reductions in SBP (−2.62 and −3.07 mm Hg in patients taking statins and in hypertensive patients, respectively). Although the mechanism (s) involved and the extent of BP reduction with statin use remain to be clearly elucidated, it is believed that statins lower BP by increasing nitric oxide bioavailability and improving arterial compliance. 19
Our results show that HDL‐C changes in the triple combination group were higher than in the dual combination group, but other studies 20 , 21 , 22 reported contrary results, although the underlying mechanism remains unclear. Several factors, including genetic variation, sex, baseline HDL/triglyceride levels and medications may be involved.
Our phase 1 trial have shown no statistically significant effect of co‐administration on telmisartan pharmacokinetics. 23 The geometric least‐square mean (GLSM) ratios and 90% CIs of telmisartan were 0.9829 (0.8334‐1.1590) for C max,ss and 1.0003 (0.9342‐1.0710) for AUCτ,ss. There was no statistically significant effect of co‐administration on amlodipine pharmacokinetics; the GLSM ratios and 90% CIs of amlodipine were 0.9908 (0.9602‐1.0223) for C max,ss and 1.0081 (0.9758‐1.0413) for AUCτ,ss. The GLSM ratios and 90% CIs of rosuvastatin were 2.2762 (2.0113‐2.5758) for C max,ss and 1.3261 (1.2385‐1.4198) for AUCτ,ss. Both the C max,ss and AUCτ,ss of rosuvastatin showed statistically significant changes and their 90% CIs did not meet the equivalence criteria. The change in the absorption phase of rosuvastatin is evident with a shortened T max,ss, and the mechanism of this change was speculated to be due to intervention in hepatic uptake and a possible change to biliary excretion transporters. 24
Telmisartan and its metabolites can also compete for breast cancer resistance protein (BCRP), which may explain the increase in plasma rosuvastatin exposure through the reduced efflux of rosuvastatin into bile. BCRP can also be inhibited by amlodipine, 25 which could further increase plasma rosuvastatin exposure. The pharmacokinetic parameters of telmisartan and amlodipine met the pharmacokinetic equivalent criteria, but the pharmacokinetic parameters of rosuvastatin were affected when telmisartan/amlodipine was administered in combination.
5. CONCLUSION
Telmisartan/amlodipine 80/5 mg and rosuvastatin 20 mg combination administration for the treatment of hypertensive patients with dyslipidemia simultaneously elicited a significant reduction in blood pressure and improved lipid control. If developed as a combination drug in future, it would be expected to not only increase the effect of blood pressure control, but also contribute to the therapeutic goal of higher compliance over the long term through ease of use and cost‐effectiveness.
CONFLICT OF INTEREST
Moo‐Yong Rhee has received lecture honoraria from Pfizer Inc, LG Life Sciences Ltd, Boehringer Ingelheim Pharma GmbH & Co. KG., Hanmi Pharm. Co. Ltd., Yuhan Co. Ltd., and Boryung Pharmaceutical Co. Ltd.; fees for consulting from Hanmi Pharm. Co. Ltd. and Shin Poong Pharma. Co. Ltd.; and research grants from Boryung Pharmaceutical Co. Ltd. and Dong‐A Pharmaceutical Co. Ltd. Hana Lee and Yoonhwa Cho are salaried employees of Yuhan Corporation. The other authors have indicated that they have no other conflicts of interest regarding the content of this article. The sponsor, Yuhan Corporation supported the supply of the study drug, laboratory tests, data collection, and data analysis. The sponsor had no role in data interpretation, the writing of the original draft of manuscript, or the decision to submit the article for publication.
AUTHOR CONTRIBUTIONS
Xuan Jin and Moo Hyun Kim: wrote the original draft preparation and visualization. Ki Hoon Han, Soon Jun Hong, Jeong‐Cheon Ahn, Jung‐Hoon Sung, Jin‐Man Cho, Han Cheol Lee, So‐Yeon Choi, Kyounghoon Lee, Woo‐Shik Kim, Moo‐Yong Rhee, Ju Han Kim, Seung Pyo Hong, Byung Su Yoo, Eun Joo Cho, Jae‐Hwan Lee, Pum‐Joon Kim, Chang‐Gyu Park, Min Su Hyon, Jin Ho Shin, Sang Hyun Lee, Ki Chul Sung, Jinyong Hwang, Kihwan Kwon, In‐Ho Chae, Jeong‐Sook Seo, and Hyungseop Kim: involved in investigation. Hana Lee and Yoonhwa Cho: Wrote the review and editing. Hyo‐Soo Kim: involved in conceptualization, methodology, supervision, and investigation. All authors approved the final version of the manuscript, including the authorship list.
Supporting information
App S1
ACKNOWLEDGEMENTS
We would like to thank the patients and their families, the investigators at all study sites.
Jin X, Kim MH, Han KH, et al. Efficacy and safety of co‐administered telmisartan/amlodipine and rosuvastatin in subjects with hypertension and dyslipidemia. J Clin Hypertens. 2020;22:1835–1845. 10.1111/jch.13893
Xuan Jin and Moo Hyun Kim equally contributed.
Funding information
This study was funded by Yuhan Corporation.
REFERENCES
- 1. Johnson ML, Pietz K, Battleman DS, et al. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Heart Dis. 2004;2:3. [PubMed] [Google Scholar]
- 2. Jackson R, Lawes CMM, Bennett DA, et al. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual's absolute cardiovascular risk. Lancet. 2005;365(9457):434‐441. [DOI] [PubMed] [Google Scholar]
- 3. Dezii CM. A retrospective study of persistence with single‐pill combination therapy vs. concurrent two‐pill therapy in patients with hypertension. Manag Care (Langhorne, PA). 2000;9(9 Suppl):2‐6. [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;71:e127ee248. [DOI] [PubMed] [Google Scholar]
- 5. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta‐analysis on 11,000 participants from 42 trials. Am J Med. 2009;122(3):290‐300. [DOI] [PubMed] [Google Scholar]
- 6. Littlejohn TW III, Majul CR, Olvera R, et al. Results of treatment with telmisartan‐amlodipine in hypertensive patients. J Clin Hypertens (greenwich). 2009;11:207‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benson SC, Pershadsingh HA, Ho CI, et al. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPAR gamma‐modulating activity. Hypertension. 2004;43:993‐1002. [DOI] [PubMed] [Google Scholar]
- 8. Baigent C. Cholesterol Treatment Trialists'(CTT) Collaborators: Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomized trials of statins. Lancet. 2005;366:1267‐1278. [DOI] [PubMed] [Google Scholar]
- 9. Rubba P, Marotta G, Gentile M. Efficacy and safety of rosuvastatin in the management of dyslipidemia. Vasc Health Risk Manag. 2009;5:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gyu Chul OH, Han J‐K, Kim H‐S, et al. Efficacy and safety of fixed‐dose combination therapy with telmisartan and rosuvastatin in korean patients with hypertension and dyslipidemia: TELSTA‐YU (TELmisartan‐rosuvaSTAtin from YUhan), a multicenter, randomized, 4‐arm, double‐blind, placebo‐controlled, phase III study. Clin Ther. 2018;40(5):676‐691.e1. [DOI] [PubMed] [Google Scholar]
- 11. Kim W, Park CG, Chang K, et al. A randomized, double‐blind clinical trial to evaluate the efficacy and safety of a fixed‐dose combination of amlodipine/rosuvastatin in patients with dyslipidemia and hypertension. J Clin Hypertens. 2020;00:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reiner Ž, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidemias: the Task Force for the management of dyslipidemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769‐1818. [DOI] [PubMed] [Google Scholar]
- 13. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed‐dose combinations of antihypertensive agents: a meta‐analysis. Hypertension. 2010;55(2):399‐407. [DOI] [PubMed] [Google Scholar]
- 14. Clark LT. Improving compliance and increasing control of hypertension: needs of special hypertensive populations. Am Heart J. 1991;121(2):664‐669. [DOI] [PubMed] [Google Scholar]
- 15. Neutel JM. Low‐dose antihypertensive combination therapy: its rationale and role in cardiovascular risk management. Am J Hypertens. 1999;12(S5):73S‐79S. [DOI] [PubMed] [Google Scholar]
- 16. Haria M, Wagstaff AJ. Amlodipine. Drugs. 1995;50(3):560‐586. [DOI] [PubMed] [Google Scholar]
- 17. Lee HY, Kim SY, Choi KJ, et al. A randomized, multicenter, double‐blind, placebo‐controlled study to evaluate the efficacy and the tolerability of a triple combination of amlodipine/losartan/rosuvastatin in patients with comorbid essential hypertension and hyperlipidemia. Clin Ther. 2017;39(12):2366‐2379. [DOI] [PubMed] [Google Scholar]
- 18. Hong SJ, Jeong HS, Han SH, et al. Comparison of fixed‐dose combinations of amlodipine/losartan potassium/chlorthalidone and amlodipine/losartan potassium in patients with stage 2 hypertension inadequately controlled with amlodipine/losartan potassium: a randomized, double‐blind, multicenter, phase III study. Clin Ther. 2017;39(10):2049‐2060. [DOI] [PubMed] [Google Scholar]
- 19. Briasoulis A, Agarwal V, Valachis A, et al. Antihypertensive effects of statins: a meta‐analysis of prospective controlled studies. J Clin Hypertens. 2013;15(5):310‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karlson BW, Palmer MK, Nicholls SJ, et al. Effects of age, gender and statin dose on lipid levels: Results from the VOYAGER meta‐analysis database. Atherosclerosis. 2017;265:54‐59. [DOI] [PubMed] [Google Scholar]
- 21. Postmus I, Warren HR, Trompet S, et al. Meta‐analysis of genome‐wide association studies of HDL cholesterol response to statins. J Med Genet. 2016;53(12):835‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McTaggart F, Jones P. Effects of statins on high‐density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther. 2008;22(4):321‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moon SJ, Jeon J‐Y, Jang K, et al. Pharmacokinetic interactions between telmisartan/amlodipine and rosuvastatin after multiple oral administrations in healthy Korean male subjects. Drug Des Devel Ther. 2019;13:2533‐2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Son M, Guk J, Kim Y, et al. Pharmacokinetic interaction between rosuvastatin, telmisartan, and amlodipine in healthy male Korean subjects: a randomized, open‐label, multiple‐dose, 2‐period crossover study. Clin Ther. 2016;38(8):1845‐1857. [DOI] [PubMed] [Google Scholar]
- 25. Takara K, Matsubara M, Yamamoto K, et al. Differential effects of calcium antagonists on ABCG2/BCRP‐mediated drug resistance and transport in SN‐38‐resistant HeLa cells. Mol Med Rep. 2012;5(3):603‐609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


