Abstract
Background
The risks of local recurrence and treatment‐related morbidity need to be balanced after local excision of early rectal cancer. The aim of this meta‐analysis was to determine oncological outcomes after local excision of pT1–2 rectal cancer followed by no additional treatment (NAT), completion total mesorectal excision (cTME) or adjuvant (chemo)radiotherapy (aCRT).
Methods
A systematic search was conducted in PubMed, Embase and the Cochrane Library. The primary outcome was local recurrence. Statistical analysis included calculation of the weighted average of proportions.
Results
Some 73 studies comprising 4674 patients were included in the analysis. Sixty‐two evaluated NAT, 13 cTME and 28 aCRT. The local recurrence rate for NAT among low‐risk pT1 tumours was 6·7 (95 per cent c.i. 4·8 to 9·3) per cent. There were no local recurrences of low‐risk pT1 tumours after cTME or aCRT. The local recurrence rate for high‐risk pT1 tumours was 13·6 (8·0 to 22·0) per cent for local excision only, 4·1 (1·7 to 9·4) per cent for cTME and 3·9 (2·0 to 7·5) per cent for aCRT. Local recurrence rates for pT2 tumours were 28·9 (22·3 to 36·4) per cent with NAT, 4 (1 to 13) per cent after cTME and 14·7 (11·2 to 19·0) per cent after aCRT.
Conclusion
There is a substantial risk of local recurrence in patients who receive no additional treatment after local excision, especially those with high‐risk pT1 and pT2 rectal cancer. The lowest recurrence risk is provided by cTME; aCRT has outcomes comparable to those of cTME for high‐risk pT1 tumours, but shows a higher risk for pT2 tumours.
This meta‐analysis showed that patients who undergo no additional treatment after local excision of pT1–2 rectal cancer have a high risk of local recurrence, especially those with high‐risk pT1 and pT2 lesions. The risk of local recurrence after adjuvant (chemo)radiotherapy for high‐risk pT1 tumours seems to be similar to that after completion TME. For pT2 tumours, adjuvant (chemo)radiotherapy seems less effective than radical surgery.
![]()
No additional therapy after local excision of pT1‐2 associated with high risk of local recurrence
Antecedentes
Tras una resección temprana de un cáncer de recto localizado, hay que considerar el equilibrio entre el riesgo de recidiva local y la morbilidad relacionada con el tratamiento. El objetivo de este metaanálisis era determinar los resultados oncológicos tras la resección de un cáncer de recto pT1‐T2 seguida de ningún tratamiento adicional (no additional treatment, NAT), escisión total del mesorrecto (completion total mesorectal excision, cTME) o quimiorradioterapia adyuvante (adjuvant chemoradiotherapy, aCRT).
Methods
Se llevó a cabo una búsqueda sistemática en PubMed, Embase y biblioteca Cochrane. La variable principal de resultado era la recidiva local (local recurrence, LR). En el análisis estadístico se calcularon las medias ponderadas de proporciones.
Resultados
Se incluyeron en el análisis 76 estudios con un total de 4.793 pacientes. NAT fue evaluada en 72 estudios, cTME en 13 y aCRT en 28. La tasa de LR para NAT en tumores pT1 de bajo riesgo era de 6,7% (i.c. del 95% 4,8‐9,3). No se observaron casos de LR en tumores pT1 de bajo riesgo tras cTME o aCRT. La tasa de LR para tumores pT1 de alto riesgo fue de 13,6% (i.c. del 95% 8,0‐22,0) para la resección local como único tratamiento, 4,1% (i.c. del 95% 1,7‐9,4) para cTME y 3,9% (i.c. del 95% 2,0‐7,5) para aCRT. La tasa de LR para tumores pT2 fue de 28,9% (i.c. del 95% 22,3‐36,4) para NAT, 4,3% (i.c. del 95% 1,4‐12,5) para cTME y 14,7% (i.c. del 95% 11,2‐19,0) para aCRT.
Conclusión
Tras la resección local de cáncer pT1 de alto riesgo y pT2, existe un riesgo sustancial de recidiva local en ausencia de tratamiento adicional. La escisión total del mesorrecto se asocia con el menor riesgo de recidiva. La quimiorradioterapia adyuvante ofrece resultados similares a la escisión total del mesorrecto en tumores pT1 de alto riesgo, pero presenta un mayor riesgo en tumores pT2.
Introduction
Screening programmes for bowel cancer have resulted in a substantial shift towards earlier stages of colorectal cancer 1 , 2 . Apart from low‐risk pT1 tumours, the current standard treatment for rectal cancer is total mesorectal excision (TME) with or without neoadjuvant (chemo)radiotherapy depending on tumour stage 3 . This radical approach is associated with morbidity, long‐term functional impairment and consequently a decrease in quality of life 4 , 5 . The increased incidence of early lesions, treatment‐related morbidity and the impact of treatment on quality of life create a clinical need for organ preservation, especially in patients with early rectal cancer 3 , 6 .
Clinical staging by endoscopy, MRI and endoscopic ultrasonography (EUS) has low accuracy in distinguishing low‐risk T1 from high‐risk T1 or early T2 rectal cancers 7 , 8 . Therefore, local excision as an initial diagnostic procedure is an attractive approach in early rectal cancer. This might turn out to be therapeutic in selected patients based on the histopathological results, and is associated with low morbidity and good functional outcomes 9 . Local excision is not considered oncologically safe for high‐risk pT1 tumours because of a higher risk of recurrence 3 , 10 , 11 . Despite the recommendations of guidelines, patients and physicians often refrain from completion TME (cTME) for high‐risk tumours 12 . Clinical data supporting this strategy are scarce and relatively high recurrence rates have been reported 11 , 13 , 14 , 15 , 16 .
A promising organ‐sparing alternative after local excision is adjuvant (chemo)radiotherapy (aCRT), which is being evaluated in trials 17 . Long‐term outcome data for all treatment options are essential in developing a valid clinical decision‐making algorithm for both patients and physicians.
The aim of this meta‐analysis was to provide an update on a previous meta‐analysis, and to evaluate the increasing amount of data for the three treatment strategies after local excision of pT1–2 rectal cancer: no additional treatment (NAT), cTME and aCRT 18 . Local recurrence rates, distant recurrence rates and both disease‐free (DFS) and overall survival (OS) rates were evaluated for these treatment strategies.
Methods
Search strategy
The study was performed according to the PRISMA guidelines 19 . Comprehensive searches regarding the treatment options were performed in the bibliographic databases of PubMed, Embase and the Cochrane Library for NAT ( Appendix S1, supporting information) and for cTME and aCRT ( Appendix S2, supporting information). In contrast to the previous meta‐analysis 18 , NAT was added as a treatment option and an additional subgroup analysis was performed for low‐ and high‐risk pT1 tumours. Literature searches were carried out on 26 August 2019 and contained all available records to the date of the search. Studies were reviewed for eligibility by two independent researchers and a third in the event of discrepancies.
Studies were considered eligible if pT1–2 rectal carcinomas were included, treated with local excision followed by either NAT, cTME or aCRT, and met the following inclusion criteria: local recurrence rates were reported, a minimum of ten patients were included, articles were published since 1990 in the English language, and median length of follow‐up was at least 36 months. Exclusion criteria were neoadjuvant treatment, distant metastasis at the time of local excision and studies that included patients with suspected nodal metastases on MRI. Studies that did not describe pT category, treatment modality or the distinction between colonic or rectal cancer were considered ineligible. Animal studies, studies with overlapping data, reviews and letters were excluded.
Quality assessment
To assess the quality of the included studies, the Methodological Index for Non‐Randomized Studies (MINORS) instrument was used 20 . Each item was scored independently by two authors from 0 to 2 points: 0, not reported; 1, reported inadequately; and 2, reported adequately. In addition to the eight established elements, an item considering allocation bias was added to evaluate whether the treatment was chosen according to a protocol or surgeon's preference, or whether the rationale was not described.
Outcome measures and statistical analysis
The primary outcome was local recurrence, defined as endoluminal recurrence or nodal recurrence in the pelvis. This included patients with isolated local recurrence as well as those with distant metastases. Secondary outcomes were distant metastasis, DFS and OS. Subgroup analyses were performed to differentiate outcomes by tumour category (pT1 versus pT2). A subgroup analysis for low‐ and high‐risk pT1 tumours was also included. Low‐ and high‐risk tumours were analysed separately if the presence of risk factors was described. High‐risk tumours were defined as lesions with at least one of the following histopathological risk factors: lymphovascular invasion, poor differentiation, deep submucosal invasion (sm3, Haggitt 4 or at least 1000 μm), tumour budding or positive resection margins (margin less than 1 mm or tumour in resection plane) 21 , 22 . These factors had to be absent for tumours to be considered low risk. A weighted average of proportions was calculated using the generic inverse‐variance method and a random‐effects model. After natural log transformation of the individual proportions, the final results were back‐transformed.
Heterogeneity was assessed by means of the I 2 statistic; an I 2 value of 75–100 per cent represented considerable heterogeneity, so 75 per cent was used as the cut‐off value 23 . One pooled analysis of NAT in pT2 tumours showed statistically significant heterogeneity (I 2 = 55 per cent, P < 0·010) but was retained in the analyses. Owing to heterogeneous and scarce reporting of OS and DFS, no weighted averages were determined for these outcomes, but the range is presented. Survival rates were not incorporated if studies included patients with tumours other than pT1–2 lesions, without specified survival rates. DFS was defined as survival without local or distant recurrence.
Results
Included studies
A total of 73 cohort studies were included in this systematic review and meta‐analysis, compared with 19 in the previous meta‐analysis 18 (Figs 1 and 2 ). Sixty‐two publications 11 , 13 , 14 , 15 , 16 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 on local excision only were included, comprising 3050 patients with pT1 and 545 with pT2 disease (Table S1, supporting information). Thirteen studies 11 , 33 , 35 , 44 , 56 , 59 , 60 , 61 , 73 , 78 , 81 , 82 , 83 reported outcomes of local excision followed by cTME, comprising 180 patients with pT1 and 70 with pT2 tumours (Table S2, supporting information). Finally, 28 studies 24 , 28 , 29 , 32 , 34 , 35 , 38 , 41 , 42 , 48 , 52 , 54 , 59 , 60 , 63 , 64 , 67 , 72 , 74 , 75 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 of aCRT were included, and contained 385 patients with pT1 and 444 with pT2 lesions (Table S3, supporting information). For the subgroup analysis of low‐risk pT1 tumours, a total of 29 studies described no additional treatment, one cTME and one aCRT. Among studies of high‐risk pT1 tumours, NAT was administered in 19, cTME in seven and aCRT in 12. Twenty‐nine of the 62 studies of local excision alone reported active surveillance during follow‐up. For aCRT, 14 of the 28 studies reported close follow‐up schemes. Ten of the 73 included studies (14 per cent) were prospective cohort studies. Of these, eight concerned NAT after local excision, one included patients who underwent cTME and three evaluated aCRT.
Fig. 1.
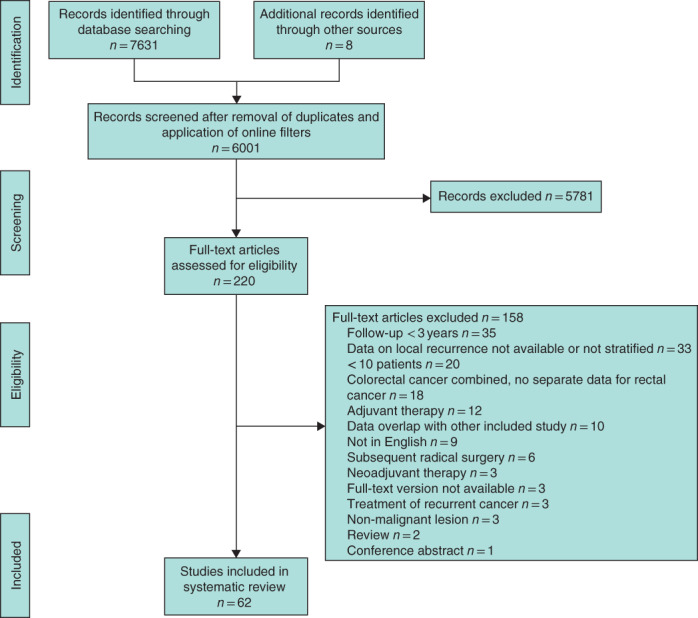
PRISMA flow chart showing selection of articles concerning local excision of early rectal cancer without additional treatment
Fig. 2.
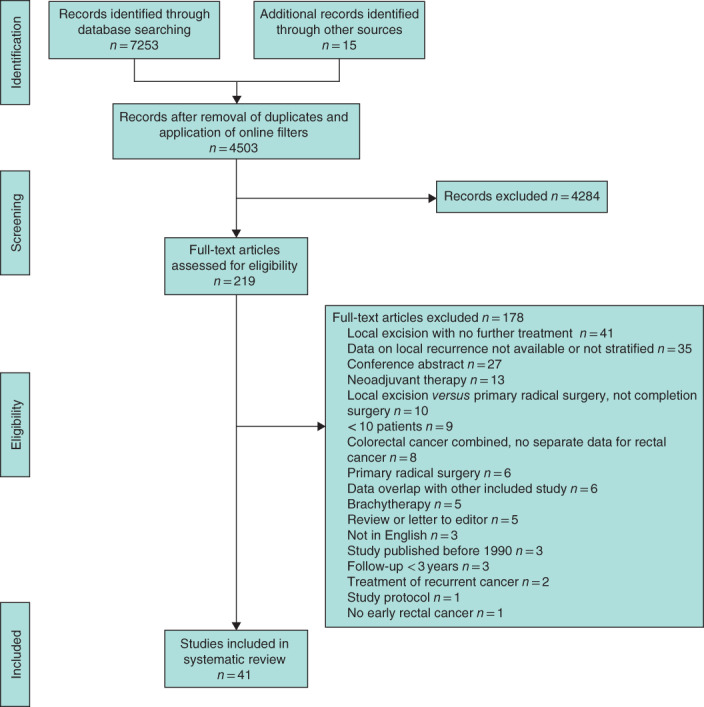
PRISMA flow chart showing selection of articles concerning adjuvant (chemo)radiotherapy or completion total mesorectal excision after local excision of early rectal cancer
Different local excision techniques were used in the included studies. In the NAT group, 52 of the 62 studies evaluated surgical modalities of local excision, five investigated endoscopic local excisions, and five studied either both or did not describe the local excision technique. For cTME, nine of 13 studies evaluated surgical local excision, three investigated endoscopic local excision, and one included both surgical and endoscopic techniques. Among studies of aCRT, 24 of 28 evaluated surgical excision techniques, whereas four investigated both surgical and endoscopic techniques or did not describe the excision method used (Tables S1–S3, supporting information).
Local recurrence
For patients with a pT1 tumour, the weighted local recurrence rate was 8·1 (95 per cent c.i. 6·6 to 9·9) per cent without additional treatment, 2·8 (1·2 to 6·5) for those who had cTME and 4·8 (2·3 to 9·8) among patients who received aCRT (Table 1 ). For low‐risk pT1 tumours, NAT was associated with a local recurrence rate of 6·7 (4·8 to 9·3) per cent. No local recurrences were reported after cTME or aCRT in patients with low‐risk pT1 lesions, based on one study for each treatment strategy. Weighted local recurrence rates for high‐risk pT1 tumours were 13·6 (8·0 to 22·0), 4·1 (1·7 to 9·4) and 3·9 (2·0 to 7·5) per cent for NAT, cTME and aCRT respectively.
Table 1.
Weighted average local recurrence rates
| Local recurrence | ||||||
|---|---|---|---|---|---|---|
| NAT | cTME | aCRT | ||||
| Proportion of patients | Weighted average (%) | Proportion of patients | Weighted average (%) | Proportion of patients | Weighted average (%) | |
| pT1 | 268 of 3050 | 8·1 (6·6, 9·9) | 5 of 180 | 2·8 (1·2, 6·5) | 24 of 385 | 4·8 (2·3, 9·8) |
| Low risk | 75 of 1019 | 6·7 (4·8, 9·3) | 0 of 28* | 0 | 0 of 1* | 0 |
| High risk | 44 of 282 | 13·6 (8·0, 22·0) | 5 of 123 | 4·1 (1·7, 9·4) | 10 of 254 | 3·9 (2·0, 7·5) |
| pT2 | 136 of 545 | 28·9 (22·3, 36·4) | 3 of 70 | 4 (1, 13) | 66 of 444 | 14·7 (11·2, 19·0) |
Values in parentheses are 95 per cent confidence intervals.
Results from a single study. NAT, no additional treatment; cTME, completion total mesorectal excision; aCRT, adjuvant (chemo)radiotherapy.
In patients with a pT2 tumour, NAT was associated with a local recurrence rate of 28·9 (22·3 to 36·4) per cent. This analysis showed significant heterogeneity (I 2 = 55 per cent; P < 0·010) (Fig. S1, supporting information). For pT2 tumours, the local recurrence rate was 4 (1 to 13) per cent after cTME and 14·7 (11·2 to 19·0) per cent following aCRT.
Patients with local recurrences were defined as patients with either local recurrence only, or those with both local and distant recurrence (Table S4, supporting information). Study‐specific local recurrence rates are reported in Tables S5–S10 and Figs S1–S6 (supporting information).
Distant metastasis
The weighted distant metastasis rate in patients with pT1 tumours was 3·4 (95 per cent c.i. 2·5 to 4·6) per cent in those who had NAT, 4·9 per cent (2·4 to 9·4) per cent after cTME and 5·0 (3·0 to 8·3) per cent after aCRT (Table 2 ; Tables S5–S7 and Figs S7–S9, supporting information). Weighted average distant metastasis rates for pT2 tumours were 6·2 (2·8 to 13·0) per cent for NAT, 7 (3 to 18) per cent for cTME and 5·8 (2·7 to 11·9) per cent for aCRT.
Table 2.
Weighted average distant recurrence rates
| Distant recurrence | ||||||
|---|---|---|---|---|---|---|
| NAT | cTME | aCRT | ||||
| Proportion of patients | Weighted average (%) | Proportion of patients | Weighted average (%) | Proportion of patients | Weighted average (%) | |
| pT1 | 101 of 2658 | 3·4 (2·5, 4·6) | 8 of 165 | 4·9 (2·4, 9·4) | 14 of 280 | 5·0 (3·0, 8·3) |
| Low risk | 25 of 783 | 3·2 (2·2, 4·7) | 1 of 28* | 4 | 0 of 1* | 0 |
| High risk | 20 of 233 | 7·2 (3·6, 13·9) | 6 of 108 | 5·6 (2·5, 11·8) | 8 of 208 | 3·9 (1·9, 7·5) |
| pT2 | 28 of 398 | 6·2 (2·8, 13·0) | 4 of 55 | 7 (3, 18) | 17 of 254 | 5·8 (2·7, 11·9) |
Values in parentheses are 95 per cent confidence intervals.
Results from a single study. NAT, no additional treatment; cTME, completion total mesorectal excision; aCRT, adjuvant (chemo)radiotherapy.
Survival
Five‐year DFS for local excision without additional treatment of pT1 tumours was reported in eight publications (range 67–97 per cent), with two of these studies reporting a 5‐year DFS rate above 85 per cent (Table 3 ; Tables S5–S7, supporting information). Only one study reported a 5‐year DFS rate after cTME, which was 81 per cent. For aCRT of pT1 tumours, the 5‐year DFS rate was reported in six studies (range 59–100 per cent), of which five reported a rate of more than 85 per cent. For pT2 tumours, the 5‐year DFS with NAT was reported in three publications as 65, 81 and 93 per cent. One study reported a 3‐year DFS of 100 per cent after cTME. Four studies reported 5‐year DFS rates for treatment of pT2 tumours with aCRT, which ranged from 58 to 78 per cent.
Table 3.
Five‐year overall and disease‐free survival rates
| NAT | cTME | aCRT | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Survival rate (%) | Reported survival > 85% | n | Survival rate (%) | n | Survival rate (%) | Reported survival > 85% | |
| pT1 | ||||||||
| Disease‐free survival | 8 | 67–97 | 2 of 8 | 1 | 81 | 6 | 59–100 | 5 of 6 |
| Overall survival | 15 | 65–100 | 5 of 15 | 1 | 92 | 6 | 63–98 | 3 of 6 |
| pT2 | ||||||||
| Disease‐free survival | 3 | 65–93 | 1 of 3 | 1 | 100* | 4 | 58–78 | 0 of 4 |
| Overall survival | 7 | 30–95 | 2 of 7 | n.r. | 5 | 58–93 | 2 of 5 | |
Three‐year disease‐free survival. NAT, no additional treatment; cTME, completion total mesorectal excision, aCRT, adjuvant (chemo)radiotherapy; n, number of studies reporting this value; n.r., not reported.
The 5‐year OS rate after NAT of pT1 tumours was reported in 15 studies (range 65–100 per cent) and exceeded 85 per cent in one‐third of these (Table 3 ; Tables S5–S7, supporting information). After cTME, the 5‐year OS rate was 92 per cent in one study. Five‐year OS following aCRT was reported in six studies (range 63–98 per cent), half of which showed a rate of over 85 per cent. For pT2 tumours, the 5‐year OS rate in patients without additional treatment was reported in seven publications (range 30–95 per cent), and two of these studies described an OS rate of more than 85 per cent. Five studies reported 5‐year OS after aCRT for pT2 tumours (range 58–93 per cent); the rate exceeded 85 per cent in two of these studies.
Study quality assessment
Study assessment according to the MINORS checklist revealed that almost 90 per cent of the included studies were carried out retrospectively (Tables S11–S12, supporting information).
Discussion
This study showed that patients who undergo NAT after local excision of pT1–2 rectal cancer have a high risk of local recurrence, especially those with high‐risk pT1 and pT2 lesions. For high‐risk pT1 tumours, the risk of local recurrence after aCRT seems similar to that for cTME. For pT2 tumours, aCRT seems less effective than radical surgery. The study findings could be used to support both patients and clinicians in decision‐making.
A recent review 92 of local recurrence rates for pT1 colorectal tumours excised endoscopically without additional treatment identified an overall recurrence rate of 9 per cent for rectal cancer. The present data showed that high‐risk pT1 is associated with a relatively high risk of recurrence of 13·6 per cent after local excision alone, which is consistent with previous findings 46 , 53 . An older large cohort study 10 reported an even higher rate of 19 per cent local recurrence for pT1 tumours. The relatively high local recurrence rate of 29 per cent for locally excised pT2 tumours in the same study corresponds to the present findings. Other studies 93 , 94 have confirmed high local recurrence rates for pT2 cancer, and cTME is recommended.
Data on aCRT after local excision of early rectal cancer are scarce. Most series are hampered by a lack of standardized histopathological evaluation distinguishing low‐ from high‐risk pT1 lesions. One of the largest cohort studies 86 of 83 patients reported a 3·6 per cent rate of local recurrence. In a review by Cutting and colleagues 95 , local recurrence rates were comparable to those of the present analysis: 5·8 per cent for pT1 and 13·8 per cent for pT2 tumours. An earlier meta‐analysis by the present study group 18 , which did not incorporate patients without additional treatment, reported a higher local recurrence rate of 10 per cent for pT1 tumours, and a similar rate of 15 per cent for pT2 tumours. One of the largest series of tumours resected endoscopically followed by cTME, reported by Tamaru and co‐workers 73 , included 56 pT1 tumours and showed a local recurrence rate of 4 per cent. Borschitz et al. 11 described the largest number of cTMEs after transanal endoscopic microsurgery, and reported a local recurrence rate of 5 per cent for high‐risk pT1 tumours and 10 per cent for pT2 tumours, which are higher than the pooled rates reported here.
The occurrence of distant metastases was comparable for the three treatment strategies, with weighted average rates ranging between 3·4 and 5·0 per cent for pT1 lesions, and from 5·8 to 7 per cent for pT2 lesions. This is lower than rates reported elsewhere. In a study 96 of locally excised pT2–3 rectal cancers, distant metastases were observed in 16 per cent at 3 years of follow‐up of patients who underwent NAT or transanal endoscopic microsurgery followed by cTME. In the previous review 18 , the weighted average distant recurrence rate was 9 per cent in patients treated with aCRT or cTME.
The type of treatment is not expected to influence the occurrence of distant metastasis. However, aspects such as tumour biology and the development of local recurrence may influence the risk of distant metastasis. These hypotheses cannot be confirmed based on the present review, but are in line with the findings of other studies 97 , 98 .
The intensity of surveillance of patients who received NAT varied among the studies. About half of the studies reported endoscopic, MRI and/or EUS surveillance every 3–4 months during the first 2 or 3 years after local excision. A large proportion of the studies (31 of 73) did not report specific follow‐up schemes. Active surveillance of both local and distant recurrences is crucial in an organ‐preserving strategy for high‐risk tumours. Unfortunately, the type (endoluminal or nodal) and stage of local recurrences were not reported in the majority of the included studies. Few studies have reported eligibility and outcomes of salvage treatment in the event of local recurrence after local excision 99 , 100 , 101 . Based on this limited evidence, the proportion of patients deemed eligible for salvage surgery ranges from 73 to 93 per cent 13 , 100 , 101 , 102 , 103 . Salvage surgery is associated with more extensive procedures and low rates of sphincter preservation. Weiser and colleagues 104 described a cohort of 50 patients who underwent salvage surgery, of whom 55 per cent required extended pelvic resection. In three studies 100 , 101 , 105 of salvage treatment, the sphincter could not be preserved in approximately two‐thirds of the patients who underwent salvage surgery. Moreover, survival rates are low in patients eligible for curative salvage surgery. Several studies 100 , 104 , 106 have reported 5‐year OS rates of around 50 per cent after salvage treatment. Limited data are available on cancer‐specific survival following salvage treatment. Doornebosch et al. 13 reported a 3‐year cancer‐specific survival rate of 58 per cent, and Vaid and colleagues 103 a 5‐year cancer‐specific survival rate of 53 per cent. A systematic review 99 also reported a disappointing 5‐year OS rate of 50 per cent after salvage surgery, presumably owing to the increased incidence of distant metastasis. Conceivably, with adequate follow‐up, local recurrences might be detected at an early stage. If clear resection margins were achieved, the 5‐year OS rate was estimated at 59 per cent by Weiser and colleagues 104 , compared with 0 per cent for incomplete resections.
Although the present data seem more robust than those in previous reports, there remains a lack of high‐quality data and appropriate reporting of long‐term outcomes of local treatment for early rectal cancer, which emphasizes the need for clinical trials 18 . The advantages and disadvantages (morbidity, function and oncological outcomes) of the three treatment options should be considered for each patient individually. The increase in risk of recurrence that is acceptable in order to preserve the rectum is unclear, and may differ between patients and physicians. Eventually, the decision regarding rectum‐preserving treatment depends on both patient preferences and tumour characteristics, and should be based on shared decision‐making.
An alternative strategy to accomplish organ preservation is the use of neoadjuvant chemoradiotherapy, which has been shown to downsize tumours and even lead to complete remission in over 50 per cent of patients 107 , 108 . However, patients without complete remission require TME surgery. This implies that neoadjuvant chemoradiotherapy led to overtreatment of patients with non‐responding or partially responding tumours, and likely resulted in increased morbidity. More importantly, as clinical staging by imaging has been shown to lack accuracy, this treatment strategy also incorporates patients with low‐risk tumours, who could have been treated with local excision only 7 , 8 . For this reason, a strategy comprising local excision of small lesions without signs of risk factors on preoperative imaging seems more attractive. Based on histopathological risk factors, additional treatment can be tailored to the individual patient.
The present meta‐analysis is based on extensive data from 73 studies, compared with 19 in the previous meta‐analysis 18 . Besides the newly added third treatment strategy, NAT, for which 62 studies were included, the number of studies evaluating cTME and aCRT was doubled to 13 and 28 respectively. Yet, the present analysis was limited by the heterogeneity of the included studies and selection bias in allocated treatment. Variation in follow‐up protocols, duration of follow‐up, sample size and type of adjuvant treatment was observed. In some studies, patients underwent radiotherapy without concurrent chemotherapy. Patients unfit for surgery and those who refused additional treatment were often allocated to NAT, presumably leading to selection bias. Owing to the variability in follow‐up, local recurrence rates were not correlated with follow‐up duration or protocols. Despite these methodological differences, it was decided to perform a pooled analysis. Quality assessment according to the MINORS checklist revealed that nearly 90 per cent of the included studies were retrospective. Many studies did not describe the histopathological inclusion criteria in detail, and definitions of histopathological risk factors varied; for example, some studies reported a margin of less 1 mm as a risk factor, whereas others defined a positive resection margin by the presence of carcinoma in the resection plane. Moreover, deep submucosal infiltration was determined to be a histopathological risk factor. However, more recent evidence shows that deep submucosal invasion alone is not a strong risk factor for lymph node metastases in multivariable analysis 109 . Nevertheless, subgroup analysis for low‐ and high‐risk pT1 tumours was undertaken because it provides important information for clinical decision‐making, and reporting only overall local recurrence rates would have led to additional bias. The data on pT2 tumours are heterogeneous, and probably include a proportion of patients with nodal disease as a result of under‐reporting of inclusion criteria and suspected nodal involvement on preoperative imaging. Furthermore, patients with unidentified nodal disease might have been included in studies of NAT and aCRT, whereas such patients were excluded from analyses of cTME. These issues may have influenced the outcomes. Survival data were not reported sufficiently to allow pooling, and might have been influenced by the selection of patients for each treatment strategy. For these reasons, only ranges could be described and no conclusions could be drawn based on the available data. A potential confounder is the method of local excision. The majority of included studies evaluated surgical local excision techniques. Further research is needed to explore differences in outcomes within and between surgical and endoscopic techniques for local excision 110 , 111 . In addition, the location of local recurrence (endoluminal, mesorectal or lymph node involvement) was generally not reported, but is of value in decision‐making for salvage treatment. Despite these limitations, an attempt was made to minimize heterogeneity by applying strict inclusion criteria and reporting data for the included subgroups only.
Supporting information
Appendix S1 Search details of local excision without additional treatment
Appendix S2 Search details of adjuvant (chemo)radiation and completion TME following local excision
Table S1 Characteristics of studies on local excision without additional treatment for early rectal cancer
Table S2 Characteristics of studies on local excision followed by completion TME for early rectal cancer
Table S3 Characteristics of studies on local excision followed by adjuvant (chemo)radiation for early rectal cancer
Table S4 Proportions of local recurrence, either local recurrence only or local recurrence and distant metastases.
Table S5 Outcome data of local excision without additional treatment
Table S6 Outcome data of local excision followed by completion total mesorectal excision
Table S7 Outcome data of local excision followed by adjuvant (chemo)radiation
Table S8 Outcome data of local excision without additional treatment, subgroup analysis low‐ and high‐risk pT1.
Table S9 Outcome data of local excision followed by completion total mesorectal excision, subgroup analysis low‐ and high‐risk pT1
Table S10 Outcome data of local excision followed by adjuvant (chemo)radiotherapy, subgroup analysis low‐ and high‐risk pT1
Table S11 Quality assessment of studies on no additional treatment after local excision
Table S12 Quality assessment of studies on completion total mesorectal excision and adjuvant (chemo)radiation following local excision
Figure S1 Forest plots of overall local recurrence of local without additional treatment in patients with a) pT1 and b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S2 Forest plots of overall local recurrence of local excision followed by completion total mesorectal excision in patients with a) pT1 b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S3 Forest plots of overall local recurrence of local excision followed by adjuvant (chemo)radiotherapy in patients with a) pT1 b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S4 Forest plots of overall local recurrence of local excision without additional treatment, subgroup analysis low‐ and high‐risk T1 tumours a) low‐risk pT1 and b) high‐risk pT1 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S5 Forest plots of overall local recurrence of local excision followed by completion total mesorectal excision, subgroup analysis high‐risk pT1 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S6 Forest plots of overall local recurrence of local excision followed by adjuvant (chemo)radiotherapy, subgroup analysis high‐risk pT1 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S7 Forest plots of overall distant recurrence of local excision without additional treatment in patients with a) pT1, b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals.
Figure S8 Forest plots of overall distant recurrence of local excision followed by completion total mesorectal excision in patients with a) pT1 and b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S9 Forest plots of overall distant recurrence of local excision followed by adjuvant (chemo)radiotherapy in patients with a) pT1 and b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Acknowledgements
S.E.v.O. and L.J.H.S. contributed equally to this article. No preregistration exists for the studies reported in this article.
Disclosure: The authors declare no conflict of interest.
References
- 1. Steele RJC, McClements P, Watling C, Libby G, Weller D, Brewster DH et al Interval cancers in a FOBT‐based colorectal cancer population screening programme: implications for stage, gender and tumour site. Gut 2012; 61: 576–581. [DOI] [PubMed] [Google Scholar]
- 2. Morris EJA, Whitehouse LE, Farrell T, Nickerson C, Thomas JD, Quirke P et al A retrospective observational study examining the characteristics and outcomes of tumours diagnosed within and without of the English NHS Bowel Cancer Screening Programme. Br J Cancer 2012; 107: 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borstlap WAA, van Oostendorp SE, Klaver CEL, Hahnloser D, Cunningham C, Rullier E et al; research committee of the European Society of Coloproctology . Organ preservation in rectal cancer: a synopsis of current guidelines. Colorectal Dis 2018; 20: 201–210. [DOI] [PubMed] [Google Scholar]
- 4. Bennis M, Parc Y, Lefevre JH, Chafai N, Attal E, Tiret E. Morbidity risk factors after low anterior resection with total mesorectal excision and coloanal anastomosis: a retrospective series of 483 patients. Ann Surg 2012; 255: 504–510. [DOI] [PubMed] [Google Scholar]
- 5. Lezoche E, Paganini AM, Fabiani B, Balla A, Vestri A, Pescatori L et al Quality‐of‐life impairment after endoluminal locoregional resection and laparoscopic total mesorectal excision. Surg Endosc 2014; 28: 227–234. [DOI] [PubMed] [Google Scholar]
- 6. Morino M, Risio M, Bach S, Beets‐Tan R, Bujko K, Panis Y et al; European Association for Endoscopic Surgery, European Society of Coloproctology . Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc 2015; 29: 755–773. [DOI] [PubMed] [Google Scholar]
- 7. O'Connell E, Galvin R, McNamara DA, Burke JP. The utility of preoperative radiological evaluation of early rectal neoplasia: a systematic review and meta‐analysis. Colorectal Dis 2020; 10.1111/codi.15015 [E‐pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Detering R, van Oostendorp SE, Meyer VM, van Dieren S, Bos ACRK, Dekker JWT et al; Dutch ColoRectal Audit Group. MRI cT1–2 rectal cancer staging accuracy: a population‐based study. Br J Surg 2020; 10.1002/bjs.11590 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allaix ME, Rebecchi F, Giaccone C, Mistrangelo M, Morino M. Long‐term functional results and quality of life after transanal endoscopic microsurgery. Br J Surg 2011; 98: 1635–1643. [DOI] [PubMed] [Google Scholar]
- 10. Bach SP, Hill J, Monson JRT, Simson JNL, Lane L, Merrie A et al; Association of Coloproctology of Great Britain and Ireland Transanal Endoscopic Microsurgery (TEM) Collaboration. A predictive model for local recurrence after transanal endoscopic microsurgery for rectal cancer. Br J Surg 2009; 96: 280–290. [DOI] [PubMed] [Google Scholar]
- 11. Borschitz T, Gockel I, Kiesslich R, Junginger T. Oncological outcome after local excision of rectal carcinomas. Ann Surg Oncol 2008; 15: 3101–3108. [DOI] [PubMed] [Google Scholar]
- 12. van Groningen JT, van Hagen P, Tollenaar RAEM, Tuynman JB, Marang‐van de Mheen PJ, Doornebosch PG et al; Dutch Colorectal Audit. Evaluation of a completion total mesorectal excision in patients after local excision of rectal cancer: a word of caution. J Natl Compr Canc Netw 2018; 16: 822–828. [DOI] [PubMed] [Google Scholar]
- 13. Doornebosch PG, Ferenschild FTJ, de Wilt JHW, Dawson I, Tetteroo GWM, de Graaf EJR. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum 2010; 53: 1234–1239. [DOI] [PubMed] [Google Scholar]
- 14. Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long‐term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum 2004; 47: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 15. Elmessiry MM, Van Koughnett JAM, Maya A, DaSilva G, Wexner SD, Bejarano P et al Local excision of T1 and T2 rectal cancer: proceed with caution. Colorectal Dis 2014; 16: 703–709. [DOI] [PubMed] [Google Scholar]
- 16. Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, Garcia‐Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum 2000; 43: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 17. Borstlap WAA, Tanis PJ, Koedam TWA, Marijnen CAM, Cunningham C, Dekker E et al A multi‐centred randomised trial of radical surgery versus adjuvant chemoradiotherapy after local excision for early rectal cancer. BMC Cancer 2016; 16: 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Borstlap WAA, Coeymans TJ, Tanis PJ, Marijnen CA, Cunningham C, Bemelman WA et al Meta‐analysis of oncological outcomes after local excision of pT1–2 rectal cancer requiring adjuvant (chemo)radiotherapy or completion surgery. Br J Surg 2016; 103: 1105–1116. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG; Group PRISMA . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 20. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 21. Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y et al; Japanese Society for Cancer of the Colon and Rectum . Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2018; 23: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A et al; EGW Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013; 24: 64–72. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). https://www.training.cochrane.org/handbook [accessed 18 February 2020].
- 24. Allaix ME, Arezzo A, Caldart M, Festa F, Morino M. Transanal endoscopic microsurgery for rectal neoplasms: experience of 300 consecutive cases. Dis Colon Rectum 2009; 52: 1831–1836. [DOI] [PubMed] [Google Scholar]
- 25. Amann M, Burghardt J, Stratz C, Buess GF, Modabber A. Transanal endoscopic microsurgery in treatment of small rectal T1 high‐risk, T2 and T3 carcinomas combined with radiochemotherapy. Eur Surg 2015; 47: 226–237. [Google Scholar]
- 26. Amann M, Modabber A, Burghardt J, Stratz C, Falch C, Buess GF et al Transanal endoscopic microsurgery in treatment of rectal adenomas and T1 low‐risk carcinomas. World J Surg Oncol 2012; 26: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Araki Y, Isomoto H, Shirouzu K. Video‐assisted gasless transanal endoscopic microsurgery: a review of 217 cases of rectal tumors over the past 10 years. Dig Surg 2003; 20: 48–52. [DOI] [PubMed] [Google Scholar]
- 28. Bacić D, Durut I, Bukvić N, Cepić I. Transanal endoscopic microsurgery (TEM) –alternative or a method of choice in treating tumors of the rectum with appropriately selected patients? Coll Antropol 2014; 38: 1127–1130. [PubMed] [Google Scholar]
- 29. Balyasnikova S, Read J, Tait D, Wotherspoon A, Swift I, Cunningham D et al The results of local excision with or without postoperative adjuvant chemoradiotherapy for early rectal cancer among patients choosing to avoid radical surgery. Colorectal Dis 2017; 19: 139–147. [DOI] [PubMed] [Google Scholar]
- 30. Bleday R, Breen E, Jessup JM, Burgess A, Sentovich SM, Steele G Jr. Prospective evaluation of local excision for small rectal cancers. Dis Colon Rectum 1997; 40: 388–392. [DOI] [PubMed] [Google Scholar]
- 31. Budhoo H. Transanal excision of early rectal carcinoma – review of a personal series. Colorectal Dis 2000; 2: 73–76. [DOI] [PubMed] [Google Scholar]
- 32. Chakravarti A, Compton CC, Shelito PC, Wood WC, Landry J, Machuta SR et al Long‐term follow‐up of patients with rectal cancer managed by local excision with and without adjuvant irradiation. Ann Surg 1999; 230: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, Jeong SY. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: a prospective cohort study. Dis Colon Rectum 2009; 52: 438–445. [DOI] [PubMed] [Google Scholar]
- 34. Coco C, Magistrelli P, Netri G, Cogliandolo S, Carbone L, Morganti AG et al Combined modality therapy in low risk (T2 N0) rectal cancer. Rays 1995; 20: 156–164. [PubMed] [Google Scholar]
- 35. Duek SD, Issa N, Hershko DD, Krausz MM. Outcome of transanal endoscopic microsurgery and adjuvant radiotherapy in patients with T2 rectal cancer. Dis Colon Rectum 2008; 51: 379–384. [DOI] [PubMed] [Google Scholar]
- 36. Endreseth BH, Myrvold HE, Romundstad P, Hestvik UE, Bjerkeset T, Wibe A; Norwegian Rectal Cancer Group . Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum 2005; 48: 1380–1388. [DOI] [PubMed] [Google Scholar]
- 37. Floyd ND, Saclarides TJ. Transanal endoscopic microsurgical resection of pT1 rectal tumors. Dis Colon Rectum 2006; 49: 164–168. [DOI] [PubMed] [Google Scholar]
- 38. Ganai S, Kanumuri P, Rao RS, Alexander AI. Local recurrence after transanal endoscopic microsurgery for rectal polyps and early cancers. Ann Surg Oncol 2006; 13: 547–556. [DOI] [PubMed] [Google Scholar]
- 39. Garcia‐Aguilar J, Mellgren A, Sirivongs P, Buie D, Madoff RD, Rothenberger DA. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg 2000; 231: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gonzalez QH, Heslin MJ, Shore G, Vickers SM, Urist MM, Bland KI. Results of long‐term follow‐up for transanal excision for rectal cancer. Am Surg 2003; 69: 675–678. [PubMed] [Google Scholar]
- 41. Gopaul D, Belliveau P, Vuong T, Trudel J, Vasilevsky CA, Corns R et al Outcome of local excision of rectal carcinoma. Dis Colon Rectum 2004; 47: 1780–1788. [DOI] [PubMed] [Google Scholar]
- 42. Greenberg JA, Shibata D, Herndon JE II, Steele GD Jr, Mayer R, Bleday R. Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum 2008; 51: 1185–1194. [DOI] [PubMed] [Google Scholar]
- 43. Guerrieri M, Gesuita R, Ghiselli R, Lezoche G, Budassi A, Baldarelli M. Treatment of rectal cancer by transanal endoscopic microsurgery: experience with 425 patients. World J Gastroenterol 2014; 20: 9556–9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heintz A, Mörschel M, Junginger T. Comparison of results after transanal endoscopic microsurgery and radical resection for T1 carcinoma of the rectum. Surg Endosc 1998; 12: 1145–1148. [DOI] [PubMed] [Google Scholar]
- 45. Huh JW, Park YA, Lee KY, Kim SA, Sohn SK. Recurrences after local excision for early rectal adenocarcinoma. Yonsei Med J 2009; 50: 704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N et al Long‐term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 2013; 144: 551–559. [DOI] [PubMed] [Google Scholar]
- 47. Im YC, Kim CW, Park S, Kim JC. Oncologic outcomes and proper surveillance after local excision of rectal cancer. J Korean Surg Soc 2013; 84: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones HJS, Hompes R, Mortensen N, Cunningham C. Modern management of T1 rectal cancer by TEM: a 10‐year single‐centre experience. Colorectal Dis 2018; 20: 586–592. [DOI] [PubMed] [Google Scholar]
- 49. Junginger T, Goenner U, Hitzler M, Trinh TT, Heintz A, Wollschlaeger D, Blettner M. Long‐term oncologic outcome after transanal endoscopic microsurgery for rectal carcinoma. Dis Colon Rectum 2016; 59: 8–15. [DOI] [PubMed] [Google Scholar]
- 50. Kouyama Y, Kudo SE, Miyachi H, Ichimasa K, Matsudaira S, Misawa M et al Risk factors of recurrence in T1 colorectal cancers treated by endoscopic resection alone or surgical resection with lymph node dissection. Int J Colorectal Dis 2018; 33: 1029–1038. [DOI] [PubMed] [Google Scholar]
- 51. Kwakye G, Curran T, Uegami S, Finne CO III, Lowry AC, Madoff RD et al Locally excised T1 rectal cancers: need for specialized surveillance protocols. Dis Colon Rectum 2019; 62: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 52. Lamont JP, McCarty TM, Digan RD, Jacobson R, Tulanon P, Lichliter WE. Should locally excised T1 rectal cancer receive adjuvant chemoradiation? Am J Surg 2000; 180: 402–406. [DOI] [PubMed] [Google Scholar]
- 53. Lebedyev A, Tulchinsky H, Rabau M, Klausner JM, Krausz M, Duek SD. Long‐term results of local excision for T1 rectal carcinoma: the experience of two colorectal units. Tech Coloproctol 2009; 13: 231–236. [DOI] [PubMed] [Google Scholar]
- 54. Lee S, Woo CG, Lee HJ, Kim KJ, Ye BD, Byeon JS et al Effectiveness of adjuvant radiotherapy after local excision of rectal cancer with deep submucosal invasion: a single‐hospital, case–control analysis. Surg Endosc 2015; 29: 3231–3238. [DOI] [PubMed] [Google Scholar]
- 55. Lee W, Lee D, Choi S, Chun H. Transanal endoscopic microsurgery and radical surgery for T1 and T2 rectal cancer. Surg Endosc 2003; 17: 1283–1287. [DOI] [PubMed] [Google Scholar]
- 56. Lee WY, Lee WS, Yun SH, Shin SH, Chun HK. Decision for salvage treatment after transanal endoscopic microsurgery. Surg Endosc 2007; 21: 975–979. [DOI] [PubMed] [Google Scholar]
- 57. Luglio G, Tarquini R, Sivero L, Giglio MC, De Werra C, Formisano C et al Functional and oncological outcomes after transanal local excision for rectal cancer. A prospective study. Chirurgia 2013; 26: 337–340. [Google Scholar]
- 58. Maslekar S, Pillinger SH, Monson JR. Transanal endoscopic microsurgery for carcinoma of the rectum. Surg Endosc 2007; 21: 97–102. [DOI] [PubMed] [Google Scholar]
- 59. Min BS, Kim NK, Ko YT, Lee KY, Baek SH, Cho CH et al Long‐term oncologic results of patients with distal rectal cancer treated by local excision with or without adjuvant treatment. Int J Colorectal Dis 2007; 22: 1325–1330. [DOI] [PubMed] [Google Scholar]
- 60. Morino M, Allaix ME, Caldart M, Scozzari G, Arezzo A. Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc 2011; 25: 3683–3690. [DOI] [PubMed] [Google Scholar]
- 61. Nakagoe T, Ishikawa H, Sawai T, Tsuji T. Long‐term outcomes of radical surgery after gasless video endoscopic transanal excision of T1/T2 rectal cancers. Eur J Surg Oncol 2004; 30: 638–642. [DOI] [PubMed] [Google Scholar]
- 62. Oka S, Tanaka S, Kanao H, Ishikawa H, Watanabe T, Igarashi M et al Mid‐term prognosis after endoscopic resection for submucosal colorectal carcinoma: summary of a multicenter questionnaire survey conducted by the colorectal endoscopic resection standardization implementation working group in Japanese Society for Cancer of the Colon and Rectum. Dig Endosc 2011; 23: 190–194. [DOI] [PubMed] [Google Scholar]
- 63. O'Neill CH, Platz J, Moore JS, Callas PW, Cataldo PA. Transanal endoscopic microsurgery for early rectal cancer: a single‐center experience. Dis Colon Rectum 2017; 60: 152–160. [DOI] [PubMed] [Google Scholar]
- 64. Paty PB, Nash GM, Baron P, Zakowski M, Minsky BD, Blumberg D et al Long‐term results of local excision for rectal cancer. Ann Surg 2002; 236: 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peng J, Chen W, Sheng W, Xu Y, Cai G, Huang D et al Oncological outcome of T1 rectal cancer undergoing standard resection and local excision. Colorectal Dis 2011; 13: e14–e19. [DOI] [PubMed] [Google Scholar]
- 66. Ptok H, Marusch F, Meyer F, Schubert D, Koeckerling F, Gastinger I et al; Colon/Rectal Cancer (Primary Tumor) Study Group. Oncological outcome of local vs radical resection of low‐risk pT1 rectal cancer. Arch Surg 2007; 142: 649–656. [DOI] [PubMed] [Google Scholar]
- 67. Ramirez JM, Aguilella V, Valencia J, Ortego J, Gracia JA, Escudero P et al Transanal endoscopic microsurgery for rectal cancer. Long‐term oncologic results. Int J Colorectal Dis 2011; 26: 437–443. [DOI] [PubMed] [Google Scholar]
- 68. Restivo A, Zorcolo L, D'Alia G, Cocco F, Cossu A, Scintu F, Casula G. Risk of complications and long‐term functional alterations after local excision of rectal tumors with transanal endoscopic microsurgery (TEM). Int J Colorectal Dis 2016; 31: 257–266. [DOI] [PubMed] [Google Scholar]
- 69. Russell AH, Harris J, Rosenberg PJ, Sause WT, Fisher BJ, Hoffman JP et al Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long‐term results of radiation therapy oncology group protocol 89‐02. Int J Radiat Oncol Biol Phys 2000; 46: 313–322. [DOI] [PubMed] [Google Scholar]
- 70. Serra‐Aracil X, Vallverdú H, Bombardó‐Junca J, Pericay‐Pijaume C, Urgelles‐Bosch J, Navarro‐Soto S. Long‐term follow‐up of local rectal cancer surgery by transanal endoscopic microsurgery. World J Surg 2008; 32: 1162–1167. [DOI] [PubMed] [Google Scholar]
- 71. Stornes T, Wibe A, Nesbakken A, Myklebust TA, Endreseth BH. National early rectal cancer treatment revisited. Dis Colon Rectum 2016; 59: 623–629. [DOI] [PubMed] [Google Scholar]
- 72. Sun G, Tang Y, Li X, Meng J, Liang G. Analysis of 116 cases of rectal cancer treated by transanal local excision. World J Surg Oncol 2014; 12: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tamaru Y, Oka S, Tanaka S, Nagata S, Hiraga Y, Kuwai T et al Long‐term outcomes after treatment for T1 colorectal carcinoma: a multicenter retrospective cohort study of Hiroshima GI Endoscopy Research Group. J Gastroenterol 2017; 52: 1169–1179. [DOI] [PubMed] [Google Scholar]
- 74. Taylor RH, Hay JH, Larsson SN. Transanal local excision of selected low rectal cancers. Am J Surg 1998; 175: 360–363. [DOI] [PubMed] [Google Scholar]
- 75. Tsai BM, Finne CO, Nordenstam JF, Christoforidis D, Madoff RD, Mellgren A. Transanal endoscopic microsurgery resection of rectal tumors: outcomes and recommendations. Dis Colon Rectum 2010; 53: 16–23. [DOI] [PubMed] [Google Scholar]
- 76. Turza KC, Brien T, Porbunderwala S, Bell CM, Lorenzo‐Rivero S, Moore RA et al The Ferguson operating anoscope for resection of T1 rectal cancer. Am Surg 2016; 82: 1105–1108. [PubMed] [Google Scholar]
- 77. Winde G, Blasius G, Herwig R, Lügering N, Keller R, Fischer R. Benefit in therapy of superficial rectal neoplasms objectivized: transanal endoscopic microsurgery (TEM) compared to surgical standards. Minim Invas Ther Allied Technol 1997; 6: 315–323. [Google Scholar]
- 78. Wykypiel H, Conrad F, Klingler A, Mittermair R, Tschmelitsch J. Local excision of rectal tumors. Coloproctology 2002; 24: 203–208. [Google Scholar]
- 79. You YN, Baxter NN, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg 2007; 245: 726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zacharakis E, Freilich S, Rekhraj S, Athanasiou T, Paraskeva P, Ziprin P et al Transanal endoscopic microsurgery for rectal tumors: the St. Mary's experience. Am J Surg 2007; 194: 694–698. [DOI] [PubMed] [Google Scholar]
- 81. Antonelli G, Berardi G, Rampioni Vinciguerra GL, Brescia A, Ruggeri M, Mercantini P et al Clinical management of endoscopically resected pT1 colorectal cancer. Endosc Int Open 2018; 6: E1462–E1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hahnloser D, Wolff BG, Larson DW, Ping J, Nivatvongs S. Immediate radical resection after local excision of rectal cancer: an oncologic compromise? Dis Colon Rectum 2005; 48: 429–437. [DOI] [PubMed] [Google Scholar]
- 83. Ortenzi M, Ghiselli R, Paolucci A, Guerrieri M. The feasibility of laparoscopic rectal resection in patients undergoing reoperation after transanal endoscopic microsurgery (TEM). Surg Endosc 2017; 32: 2020–2025. [DOI] [PubMed] [Google Scholar]
- 84. Benson R, Wong CS, Cummings BJ, Brierley J, Catton P, Ringash J et al Local excision and postoperative radiotherapy for distal rectal cancer. Int J Radiat Oncol Biol Phys 2001; 50: 1309–1316. [DOI] [PubMed] [Google Scholar]
- 85. Jeong JU, Nam TK, Kim HR, Shim HJ, Kim YH, Yoon MS et al Adjuvant chemoradiotherapy instead of revision radical resection after local excision for high‐risk early rectal cancer. Radiat Oncol 2016; 11: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rackley TP, Ma RM, Brown CJ, Hay JH. Transanal local excision for patients with rectal cancer: can radiation compensate for what is perceived as a nondefinitive surgical approach? Dis Colon Rectum 2016; 59: 173–178. [DOI] [PubMed] [Google Scholar]
- 87. Sasaki T, Ito Y, Ohue M, Kanemitsu Y, Kobatake T, Ito M et al Postoperative chemoradiotherapy after local resection for high‐risk T1 to T2 low rectal cancer: results of a single‐arm, multi‐institutional, phase II clinical trial. Dis Colon Rectum 2017; 60: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stipa F, Burza A, Lucandri G, Ferri M, Pigazzi A, Ziparo V et al Outcomes for early rectal cancer managed with transanal endoscopic microsurgery: a 5‐year follow‐up study. Surg Endosc 2006; 20: 541–545. [DOI] [PubMed] [Google Scholar]
- 89. Suzuki T, Sadahiro S, Tanaka A, Okada K, Saito G, Miyakita H et al Outcomes of local excision plus chemoradiotherapy in patients with T1 rectal cancer. Oncology 2018; 95: 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Valentini VM, Morganti AG, De Santis M, Vecchio FM, Coco C, Picciocchi A et al Local excision and external beam radiotherapy in early rectal cancer. Int J Radiat Oncol Biol Phys 1996; 35: 759–764. [DOI] [PubMed] [Google Scholar]
- 91. Wagman R, Minsky BD, Cohen AM, Saltz L, Paty PB, Guillem JG. Conservative management of rectal cancer with local excision and postoperative adjuvant therapy. Int J Radiat Oncol Biol Phys 1999; 44: 841–846. [PubMed] [Google Scholar]
- 92. Antonelli G, Vanella G, Orlando D, Angeletti S, Di Giulio E. Recurrence and cancer‐specific mortality after endoscopic resection of low‐ and high‐risk pT1 colorectal cancers: a meta‐analysis. Gastrointest Endosc 2019; 90: 559–569. [DOI] [PubMed] [Google Scholar]
- 93. Xu ZS, Cheng H, Xiao Y, Cao JQ, Cheng F, Xu WJ et al Comparison of transanal endoscopic microsurgery with or without neoadjuvant therapy and standard total mesorectal excision in the treatment of clinical T2 low rectal cancer: a meta‐analysis. Oncotarget 2017; 8: 115681–115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Halverson AL, Morris AM, Cleary RK, Chang GJ. For patients with early rectal cancer, does local excision have an impact on recurrence, survival, and quality of life relative to radical resection? Ann Surg Oncol 2019; 26: 2497–2506. [DOI] [PubMed] [Google Scholar]
- 95. Cutting JE, Hallam SE, Thomas MG, Messenger DE. A systematic review of local excision followed by adjuvant therapy in early rectal cancer: are pT1 tumours the limit? Colorectal Dis 2018; 20: 854–863. [DOI] [PubMed] [Google Scholar]
- 96. Leijtens JWA, Koedam TWA, Borstlap WAA, Maas M, Doornebosch PG, Karsten TM et al Transanal endoscopic microsurgery with or without completion total mesorectal excision for T2 and T3 rectal carcinoma. Dig Surg 2019; 36: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Junginger T, Goenner U, Trinh TT, Heintz A, Lollert A, Blettner M et al The link between local recurrence and distant metastases in patients with rectal cancer. Anticancer Res 2019; 39: 3079–3088. [DOI] [PubMed] [Google Scholar]
- 98. van den Brink M, Stiggelbout AM, van den Hout WB, Kievit J, Klein Kranenbarg E, Marijnen CA et al Clinical nature and prognosis of locally recurrent rectal cancer after total mesorectal excision with or without preoperative radiotherapy. J Clin Oncol 2004; 22: 3958–3964. [DOI] [PubMed] [Google Scholar]
- 99. Jones HJS, Cunningham C, Nicholson GA, Hompes R. Outcomes following completion and salvage surgery for early rectal cancer: a systematic review. Eur J Surg Oncol 2018; 44: 15–23. [DOI] [PubMed] [Google Scholar]
- 100. Bikhchandani J, Ong GK, Dozois EJ, Mathis KL. Outcomes of salvage surgery for cure in patients with locally recurrent disease after local excision of rectal cancer. Dis Colon Rectum 2015; 58: 283–287. [DOI] [PubMed] [Google Scholar]
- 101. Christoforidis D, Cho HM, Dixon MR, Mellgren AF, Madoff RD, Finne CO. Transanal endoscopic microsurgery versus conventional transanal excision for patients with early rectal cancer. Ann Surg 2009; 249: 776–782. [DOI] [PubMed] [Google Scholar]
- 102. Stipa F, Giaccaglia V, Burza A. Management and outcome of local recurrence following transanal endoscopic microsurgery for rectal cancer. Dis Colon Rectum 2012; 55: 262–269. [DOI] [PubMed] [Google Scholar]
- 103. Vaid S, Park JS, Sinnott RJ. Outcomes of recurrent rectal cancer after transanal excision. Am Surg 2016; 82: 152–155. [PubMed] [Google Scholar]
- 104. Weiser MR, Landmann RG, Wong WD, Shia J, Guillem JG, Temple LK et al Surgical salvage of recurrent rectal cancer after transanal excision. Dis Colon Rectum 2005; 48: 1169–1175. [DOI] [PubMed] [Google Scholar]
- 105. Friel CM, Cromwell JW, Madoff RD, Rothenberger DA, Garcia‐Aguílar J. Salvage radical surgery after failed local excision for early rectal cancer. Dis Colon Rectum 2002; 45: 875–879. [DOI] [PubMed] [Google Scholar]
- 106. Baron PL, Enker WE, Zakowski MF, Urmacher C. Immediate vs. salvage resection after local treatment for early rectal cancer. Dis Colon Rectum 1995; 38: 177–181. [DOI] [PubMed] [Google Scholar]
- 107. Stijns RCH, de Graaf EJR, Punt CJA, Nagtegaal ID, Nuyttens J, van Meerten E et al; CARTS Study Group . Long‐term oncological and functional outcomes of chemoradiotherapy followed by organ‐sparing transanal endoscopic microsurgery for distal rectal cancer: the CARTS study. JAMA Surg 2019; 154: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stijns RCH, Tromp MR, Hugen N, de Wilt JHW. Advances in organ preserving strategies in rectal cancer patients. Eur J Surg Oncol 2018; 44: 209–219. [DOI] [PubMed] [Google Scholar]
- 109. Yasue C, Chino A, Takamatsu M, Namikawa K, Ide D, Saito S et al Pathological risk factors and predictive endoscopic factors for lymph node metastasis of T1 colorectal cancer: a single‐center study of 846 lesions. J Gastroenterol 2019; 54: 708–717. [DOI] [PubMed] [Google Scholar]
- 110. Dekkers N, Boonstra JJ, Moons LMG, Hompes R, Bastiaansen BA, Tuynman JB et al Transanal minimally invasive surgery (TAMIS) and endoscopic submucosal dissection (ESD) for resection of non‐pedunculated rectal lesions (TRIASSIC study): study protocol of a European multicenter randomised controlled trial. BMC Gastroenterol 2020; 20: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lezoche G, Guerrieri M, Lezoche E. Pyramidal excision for early rectal cancer and special closure techniques In Transanal Minimally Invasive Surgery (TAMIS) and Transanal Total Mesorectal Excision (taTME), Atallah S. (ed.). Springer International Publishing: Cham, 2019; 97–111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search details of local excision without additional treatment
Appendix S2 Search details of adjuvant (chemo)radiation and completion TME following local excision
Table S1 Characteristics of studies on local excision without additional treatment for early rectal cancer
Table S2 Characteristics of studies on local excision followed by completion TME for early rectal cancer
Table S3 Characteristics of studies on local excision followed by adjuvant (chemo)radiation for early rectal cancer
Table S4 Proportions of local recurrence, either local recurrence only or local recurrence and distant metastases.
Table S5 Outcome data of local excision without additional treatment
Table S6 Outcome data of local excision followed by completion total mesorectal excision
Table S7 Outcome data of local excision followed by adjuvant (chemo)radiation
Table S8 Outcome data of local excision without additional treatment, subgroup analysis low‐ and high‐risk pT1.
Table S9 Outcome data of local excision followed by completion total mesorectal excision, subgroup analysis low‐ and high‐risk pT1
Table S10 Outcome data of local excision followed by adjuvant (chemo)radiotherapy, subgroup analysis low‐ and high‐risk pT1
Table S11 Quality assessment of studies on no additional treatment after local excision
Table S12 Quality assessment of studies on completion total mesorectal excision and adjuvant (chemo)radiation following local excision
Figure S1 Forest plots of overall local recurrence of local without additional treatment in patients with a) pT1 and b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S2 Forest plots of overall local recurrence of local excision followed by completion total mesorectal excision in patients with a) pT1 b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S3 Forest plots of overall local recurrence of local excision followed by adjuvant (chemo)radiotherapy in patients with a) pT1 b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S4 Forest plots of overall local recurrence of local excision without additional treatment, subgroup analysis low‐ and high‐risk T1 tumours a) low‐risk pT1 and b) high‐risk pT1 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S5 Forest plots of overall local recurrence of local excision followed by completion total mesorectal excision, subgroup analysis high‐risk pT1 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S6 Forest plots of overall local recurrence of local excision followed by adjuvant (chemo)radiotherapy, subgroup analysis high‐risk pT1 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S7 Forest plots of overall distant recurrence of local excision without additional treatment in patients with a) pT1, b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals.
Figure S8 Forest plots of overall distant recurrence of local excision followed by completion total mesorectal excision in patients with a) pT1 and b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals
Figure S9 Forest plots of overall distant recurrence of local excision followed by adjuvant (chemo)radiotherapy in patients with a) pT1 and b) pT2 tumours. An inverse‐variance random‐effects model. Proportions with 95 per cent confidence intervals


