Abstract
Background
Pembrolizumab plus platinum‐based chemotherapy has demonstrated improved clinical outcomes over chemotherapy alone in patients with previously untreated advanced/metastatic non–small cell lung cancer (NSCLC), regardless of tumor programmed death ligand 1 (PD‐L1) expression. This study pooled data from 3 randomized controlled trials to evaluate outcomes with pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced/metastatic NSCLC negative for PD‐L1 (ie, a tumor proportion score < 1%).
Methods
Individual patient data were pooled from KEYNOTE‐021 cohort G (nonsquamous; NCT02039674), KEYNOTE‐189 (nonsquamous; NCT02578680 and NCT03950674), and KEYNOTE‐407 (squamous; NCT02775435). Treatment comprised pembrolizumab plus chemotherapy (pemetrexed and platinum for nonsquamous histology and carboplatin and paclitaxel/nab‐paclitaxel for squamous histology) or chemotherapy alone. Responses were assessed according to Response Evaluation Criteria in Solid Tumors version 1.1 by blinded, independent, central review. No α was assigned to this descriptive, exploratory analysis.
Results
Four hundred forty‐four of the 1328 patients (33.4%) who were enrolled across the 3 trials had PD‐L1‒negative tumors (256 on pembrolizumab plus chemotherapy [nonsquamous, n = 155; squamous, n = 94; other, n = 7] and 188 on chemotherapy alone [nonsquamous, n = 83; squamous, n = 99; other, n = 6]). The median time from randomization to the data cutoff was 28.0 months (range, 14.7‐55.4 months). Pembrolizumab plus chemotherapy improved overall survival (OS; hazard ratio [HR], 0.63; 95% CI, 0.50‐0.79) and progression‐free survival (HR, 0.68; 95% CI, 0.56‐0.83) over chemotherapy. Sixteen patients in the pembrolizumab plus chemotherapy arm completed 2 years of treatment; the objective response rate was 87.5% (95% CI, 61.7%‐98.4%), and the 3‐year OS rate was 100%. Adverse events (AEs) were experienced by 99.2% of the patients who received pembrolizumab plus chemotherapy and by 98.9% of the patients who received chemotherapy alone, with grade 3 or higher AEs occurring in 71.4% and 72.0%, respectively; immune‐mediated AEs and infusion reactions were experienced by 29.0% and 12.4%, respectively.
Conclusions
Pembrolizumab plus chemotherapy demonstrated response and survival improvements with manageable safety in comparison with chemotherapy alone in PD‐L1‒negative advanced/metastatic NSCLC, and it is a standard‐of‐care first‐line therapy for patients with advanced NSCLC, regardless of PD‐L1 expression.
Lay Summary
Some tumors produce a protein called programmed death ligand 1 (PD‐L1), which interacts with the body's immune system and prevents an immune response against cancer.
Antibody therapies such as pembrolizumab block interactions between tumor PD‐L1 and the immune system and enable an immune response. Used alone, pembrolizumab provides benefit for patients with non–small cell lung cancer (NSCLC) tumors that produce PD‐L1. However, when it is combined with chemotherapy, which can stimulate anticancer immune responses, pembrolizumab provides a benefit, regardless of tumor PD‐L1 production.
This article shows that among patients with NSCLC whose tumors produce no PD‐L1, outcomes are better with pembrolizumab plus chemotherapy in comparison with chemotherapy alone.
Keywords: antineoplastic agents, combined drug therapy, non–small cell lung cancer, pembrolizumab, programmed cell death ligand 1 protein (human CD274 protein)
Short abstract
This pooled analysis of individual patient data from 3 randomized controlled trials showed a clinically meaningful benefit and a manageable safety profile with pembrolizumab plus platinum‐based chemotherapy versus chemotherapy alone in previously untreated advanced/metastatic non–small cell lung cancer (NSCLC) negative for programmed death ligand 1 (PD‐L1). Pembrolizumab plus platinum‐based chemotherapy is a standard‐of‐care first‐line therapy for patients with advanced squamous or nonsquamous NSCLC, including patients with PD‐L1–negative tumors.
Introduction
The anti–programmed death 1 (PD‐1) monoclonal antibody pembrolizumab first showed increasing clinical efficacy with higher levels of programmed death ligand 1 (PD‐L1) expression inpatients with advanced non–small cell lung cancer (NSCLC) in the phase 1b KEYNOTE‐001 study. 1 Results from the phase 3 studies KEYNOTE‐024 and KEYNOTE‐042 for patients with PD‐L1 expression on ≥50% of tumor cells (tumor proportion score [TPS] ≥ 50%) 2 and for patients with a PD‐L1 TPS ≥ 1%, 3 respectively, subsequently established pembrolizumab monotherapy as a first‐line standard of care for patients with PD‐L1‒positive advanced NSCLC and no EGFR or ALK genomic tumor aberrations. On the basis of results from KEYNOTE‐001, patients with PD‐L1‒negative tumors (PD‐L1 TPS < 1%) were not included in these studies, and they are not eligible for pembrolizumab monotherapy.
Chemotherapy agents have shown immunomodulatory properties, including directly and indirectly stimulating immune responses and increasing tumor immunogenicity. 4 , 5 Chemotherapy may also enhance the antitumor activity of immunotherapy 4 and thus increase the likelihood of a clinical benefit with pembrolizumab, regardless of tumor PD‐L1 expression. In cohort G of the multicohort, phase 1/2 KEYNOTE‐021 study, a significantly higher objective response rate (ORR) was observed with pembrolizumab plus pemetrexed and carboplatin versus chemotherapy alone in patients with previously untreated advanced nonsquamous NSCLC and no sensitizing EGFR or ALK alterations. 6 The randomized, placebo‐controlled, phase 3 KEYNOTE‐189 study, a larger confirmatory clinical trial, demonstrated significantly improved overall survival (OS) and progression‐free survival (PFS) with pembrolizumab plus pemetrexed and carboplatin or cisplatin versus placebo plus chemotherapy in patients with metastatic nonsquamous NSCLC without sensitizing EGFR or ALK alterations. 7 In addition, the randomized, placebo‐controlled, phase 3 KEYNOTE‐407 study showed significantly improved OS and PFS with pembrolizumab plus carboplatin and paclitaxel or nab‐paclitaxel versus placebo plus chemotherapy in patients with stage IV squamous NSCLC. 8 Each of these studies demonstrated that the combination of pembrolizumab and platinum‐based chemotherapy improved clinical outcomes both for patients with PD‐L1‒positive tumors (PD‐L1 TPS ≥ 1%) and for patients with PD‐L1‒negative tumors and had a manageable safety profile. 6 , 7 , 8
As a result of these findings, pembrolizumab plus platinum‐based chemotherapy is now a standard of care for the first‐line treatment of patients with metastatic NSCLC with no sensitizing EGFR or ALK alterations, regardless of histology or tumor PD‐L1 expression. 9 To further characterize the efficacy and safety of pembrolizumab plus chemotherapy versus chemotherapy alone in PD‐L1‒negative advanced NSCLC, we performed a pooled analysis among patients with PD‐L1‒negative tumors by using individual patient data from KEYNOTE‐021 cohort G, KEYNOTE‐189, and KEYNOTE‐407.
Materials and Methods
Patients
Individual patient data were pooled from 3 international, multicenter, clinical randomized controlled trials in NSCLC: cohort G of the phase 1/2, open‐label KEYNOTE‐021 study (stage IIIB/IV, nonsquamous; NCT02039674) and the phase 3, double‐blind, placebo‐controlled metastatic NSCLC studies KEYNOTE‐189 (nonsquamous; NCT02578680 and NCT03950674) and KEYNOTE‐407 (squamous; NCT02775435). 6 , 7 , 8
Eligibility criteria common to all 3 studies included age ≥ 18 years, histologically or cytologically confirmed advanced NSCLC, 1 or more measurable lesions according to Response Evaluation Criteria in Solid Tumors version 1.1 , 10 and an Eastern Cooperative Oncology Group performance status of 0 or 1. Exclusions included prior systemic treatment for advanced disease, symptomatic central nervous system metastases, active autoimmune disease requiring systemic immunosuppressive therapy ≤2 years before study treatment, and active interstitial lung disease or a history of pneumonitis that required glucocorticoid treatment. Enrollment in KEYNOTE‐021 cohort G and KEYNOTE‐189 also excluded patients with sensitizing EGFR or ALK genomic tumor aberrations. In all 3 studies, a tumor biopsy sample was required for the evaluation of PD‐L1 status. Further details of the eligibility criteria for each of these studies have been published previously. 6 , 7 , 8 For each study, procedures were approved by an appropriate institutional review committee.
Treatment
Patients in cohort G of KEYNOTE‐021 were randomized 1:1 either to pembrolizumab200 mg every 3 weeks for 35 cycles (ie, 2 years) plus pemetrexed 500 mg/m2 and carboplatin area under the curve (AUC) 5 mg/mL/min every 3 weeks for 4 cycles followed by pemetrexed maintenance therapy or to pemetrexed and carboplatin alone. 6 Randomization was stratified by PD‐L1 TPS (<1% vs ≥1%). 6
Patients in KEYNOTE‐189 were randomized 2:1 to receive pembrolizumab 200 mg or placebo every 3 weeks for 35 cycles. Both treatment groups also received 4 cycles of pemetrexed 500 mg/m2 and the investigator's choice of carboplatin AUC 5 mg/mL/min or cisplatin 75 mg/m2 followed by pemetrexed maintenance therapy. Randomization was stratified by PD‐L1 status (TPS < 1% vs TPS ≥ 1%), choice of platinum drug (carboplatin vs cisplatin), and smoking history (current/former vs never). 7
Patients in KEYNOTE‐407 were randomized 1:1 to receive pembrolizumab 200 mg or placebo every 3 weeks for 35 cycles. Both treatment groups also received 4 cycles of carboplatin AUC 6 mg/mL/min on day 1 and the investigator's choice of either paclitaxel 200 mg/m2 on day 1 or nab‐paclitaxel 100 mg/m2 on days 1, 8, and 15. Randomization was stratified by PD‐L1 TPS (<1% vs ≥1%), choice of taxane (paclitaxel vs nab‐paclitaxel), and geographic region (East Asia vs the rest of the world). 8
In each of the 3 studies, pembrolizumab treatment was continued for up to 35 cycles or until disease progression, unacceptable toxicity, a physician's decision to discontinue treatment, or withdrawal of patient consent. Patients in the chemotherapy alone groups with radiological disease progression confirmed by blinded, independent, central review in KEYNOTE‐189 and KEYNOTE‐407 were eligible to cross over to open‐label pembrolizumab monotherapy if protocol‐specified safety criteria were met. 6 , 7 , 8
Assessments
Across the 3 studies, PD‐L1 expression was evaluated centrally in formalin‐fixed tumor samples from newly biopsied tissue or archival specimens via the PD‐L1 IHC 22C3 pharmDx assay (Agilent Technologies, Carpinteria, California). 6 , 7 , 8 In KEYNOTE‐189 and KEYNOTE‐407, specimens provided after the diagnosis of metastatic disease were preferred. Investigators were blinded to PD‐L1 assessments.
Tumor imaging was performed at weeks 6 and 12 (and additionally at week 18 in KEYNOTE‐021 cohort G and KEYNOTE‐407); then every 9 weeks through the remainder of the first year for KEYNOTE‐021 cohort G, through week 48 for KEYNOTE‐189, andthrough week 45 for KEYNOTE‐407; and at 12‐week intervals thereafter. 6 , 7 , 8 In all 3 studies, response was assessed by blinded, independent, central radiological review. 6 , 7 , 8 , 10 During follow‐up, survival was assessed every 8 weeks in KEYNOTE‐021 cohort G and every 12 weeks in KEYNOTE‐189 and KEYNOTE‐407. Adverse events (AEs) and laboratory findings were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (version 4.03 for KEYNOTE‐407). 6 , 7 , 8
Endpoints
In KEYNOTE‐021 cohort G, the primary efficacy endpoint was ORR, and the key secondary endpoint was PFS. In KEYNOTE‐189 and KEYNOTE‐407, PFS and OS were dual primary endpoints, and ORR and duration of response (DOR) were key secondary endpoints. In all 3 studies, progression‐free survival 2 (PFS‐2), defined as the time from randomization to second/subsequent disease progression after the initiation of new anticancer therapy (including crossover to pembrolizumab) or death from any cause (whichever occurred first), was an exploratory endpoint. PFS‐2 events were characterized as follows: time of death for patients who did not receive or stopped second‐line therapy without progressive disease and did not start third‐line therapy, time of investigator‐assessed disease progression that led to discontinuation of second‐line therapy, and time of third‐line therapy initiation in patients who stopped second‐line therapy without disease progression. Patients without a PFS‐2 event at the time of the data cutoff were censored at the time they were last known to be alive and without disease progression after the initiation of new anticancer therapy.
Statistical Analysis
This analysis included patients from KEYNOTE‐021 cohort G, KEYNOTE‐189, and KEYNOTE‐407 who were determined to have PD‐L1–negative tumors; patients with nonevaluable PD‐L1 results were not included. Efficacy was analyzed in the pooled intention‐to‐treat population, and safety was analyzed in the pooled population of patients who received 1 or more doses of the study treatment. All analyses were descriptive. No α was assigned to this exploratory analysis.
The Kaplan‐Meier method was used to estimate OS, PFS, DOR, and PFS‐2. Hazard ratios (HRs) and 95% CIs for OS, PFS, and PFS‐2 were provided on the basis of an unstratified Cox regression model with treatment as a covariate. Subgroup analyses of OS and PFS were based on an unstratified Cox regression model with treatment as a covariate. Analyses were performed with SAS (version 9.4; SAS Institute, Inc., Cary, North Carolina). The data cutoff dates were August 19, 2019, for KEYNOTE‐021 cohort G; May 20, 2019, for KEYNOTE‐189; and May 9, 2019, for KEYNOTE‐407.
Results
Of the 1328 total patients enrolled in the KEYNOTE‐021 cohort G, KEYNOTE‐189, and KEYNOTE‐407 studies, 444 (33.4%) had PD‐L1‒negative NSCLC and were included in this analysis (256 on pembrolizumab plus chemotherapy [nonsquamous, n = 155; squamous, n = 94; other, n = 7] and 188 on chemotherapy alone [nonsquamous, n = 83; squamous, n = 99; other, n = 6]; Fig. 1). Overall, 68.0% of the patients in the pooled analysis population were men, and 61.9% had a baseline Eastern Cooperative Oncology Group performance status of 1 (Table 1). Baseline brain metastases and liver metastases were present in 15.5% and 18.5%, respectively. The percentage of patients with nonsquamous histology was higher in the pembrolizumab plus chemotherapy group than the chemotherapy alone group because of the 2:1 randomization ratio in KEYNOTE‐189; otherwise, baseline characteristics were generally similar between treatment groups (Table 1).
Figure 1.
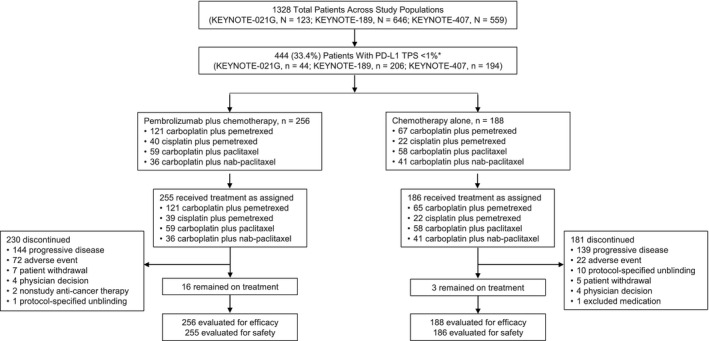
Summary of enrollment and patient disposition in the pooled analysis population of patients with advanced NSCLC and no PD‐L1 expression (TPS < 1%). Patients were enrolled into KEYNOTE‐021 cohort G (stage IIIB/IV nonsquamous NSCLC) between November 25, 2014, and January 25, 2016; into KEYNOTE‐189 (stage IV nonsquamous NSCLC) between February 26, 2016, and March 6, 2017 (global study), and between August 7, 2017, and May 11, 2018 (Japanese extension study); or into KEYNOTE‐407 (stage IV squamous NSCLC) between August 19, 2016, and December 28, 2017. *Patients with tumor samples that were nonevaluable for PD‐L1 expression were excluded from this analysis. NSCLC indicates non–small cell lung cancer; PD‐L1, programmed death ligand 1; TPS, tumor proportion score.
Table 1.
Demographics and Baseline Disease Characteristics in a Pooled Analysis of Patients With a PD‐L1 TPS < 1%
| Characteristic | Pembrolizumab + Chemotherapy (n = 256) | Chemotherapy Alone (n = 188) |
|---|---|---|
| Age | ||
| Median (range), y | 64.0 (34‐87) | 64.0 (37‐82) |
| <65 y, No. (%) | 131 (51.2) | 96 (51.1) |
| Male sex, No. (%) | 169 (66.0) | 133 (70.7) |
| Region of enrollment, No. (%) | ||
| Europe | 142 (55.5) | 89 (47.3) |
| North America | 60 (23.4) | 45 (23.9) |
| East Asia | 36 (14.1) | 29 (15.4) |
| Other | 18 (7.0) | 25 (13.3) |
| ECOG PS, No. (%) | ||
| 0 | 96 (37.5) | 72 (38.3) |
| 1 | 159 (62.1) | 116 (61.7) |
| 2 | 1 (0.4) | 0 |
| Smoking history, No. (%) | ||
| Current or former | 225 (87.9) | 174 (92.6) |
| Never | 31 (12.1) | 14 (7.4) |
| Histology, No. (%) | ||
| Squamous | 94 (36.7) | 99 (52.7) |
| Nonsquamous | 155 (60.5) | 83 (44.1) |
| Other | 7 (2.7) | 6 (3.2) |
| Brain metastases, No. (%) | 43 (16.8) | 26 (13.8) |
| Liver metastases, No. (%) | 38 (14.8) | 44 (23.4) |
| Previous therapy, No. (%) | ||
| Radiotherapy | 47 (18.4) | 41 (21.8) |
| Neoadjuvant | 4 (1.6) | 4 (2.1) |
| Adjuvant | 12 (4.7) | 8 (4.3) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PD‐L1, programmed death ligand 1; PS, performance status; TPS, tumor proportion score.
Among all 444 patients, 255 in the pembrolizumab plus chemotherapy group and 186 in the chemotherapy alone group received 1 or more doses of treatment (Fig. 1). Nine patients in the pembrolizumab plus chemotherapy group and 2 patients in the chemotherapy alone group completed treatment; 16 and 3 patients, respectively, remained on treatment at the data cutoff date. The most common reasons for discontinuation were disease progression (pembrolizumab plus chemotherapy, n = 144 [56.5%]; chemotherapy alone, n = 139 [74.7%]) and AEs (n = 72 [28.2%] and n = 22 [11.8%], respectively). When we excluded patients who remained on treatment, 105 of the 183 patients (57.4%) who initially received chemotherapy alone crossed over to pembrolizumab monotherapy on study and/or received anti‒PD‐[L]1 therapy outside of crossover (3 patients received both pembrolizumab on study and subsequent anti‒PD‐(L)1 therapy outside of crossover); 118 patients (64.5%) received any subsequent therapy. When we excluded patients who remained on treatment, 129 of 239 patients (54.0%) who initially received pembrolizumab plus chemotherapy received subsequent therapy. The median time from randomization to the data cutoff was 28.0 months (range, 14.7‐55.4 months).
Efficacy
At the time of the individual study data cutoffs, a total of 311 deaths had occurred among all 444 patients (70%) in the pooled PD‐L1‒negative population. Pembrolizumab plus chemotherapy improved OS over chemotherapy alone (HR, 0.63; 95% CI, 0.50‐0.79) with a median OS of 19.0 months (95% CI, 15.5‐22.8 months) in the pembrolizumab plus chemotherapy group and a median OS of 11.4 months (95% CI, 9.4‐13.5 months) in the chemotherapy alone group (Fig. 2A). The estimated 12‐month OS rates were 67.4% (95% CI, 61.3%‐72.8%) and 47.9% (95% CI, 40.6%‐54.9%), respectively, and the 24‐month rates were 41.0% (95% CI, 34.6%‐47.2%) and 25.4% (95% CI, 19.0%‐32.3%), respectively. HR point estimates for OS favored pembrolizumab plus chemotherapy versus chemotherapy alone across all subgroups analyzed (Fig. 2B).
Figure 2.
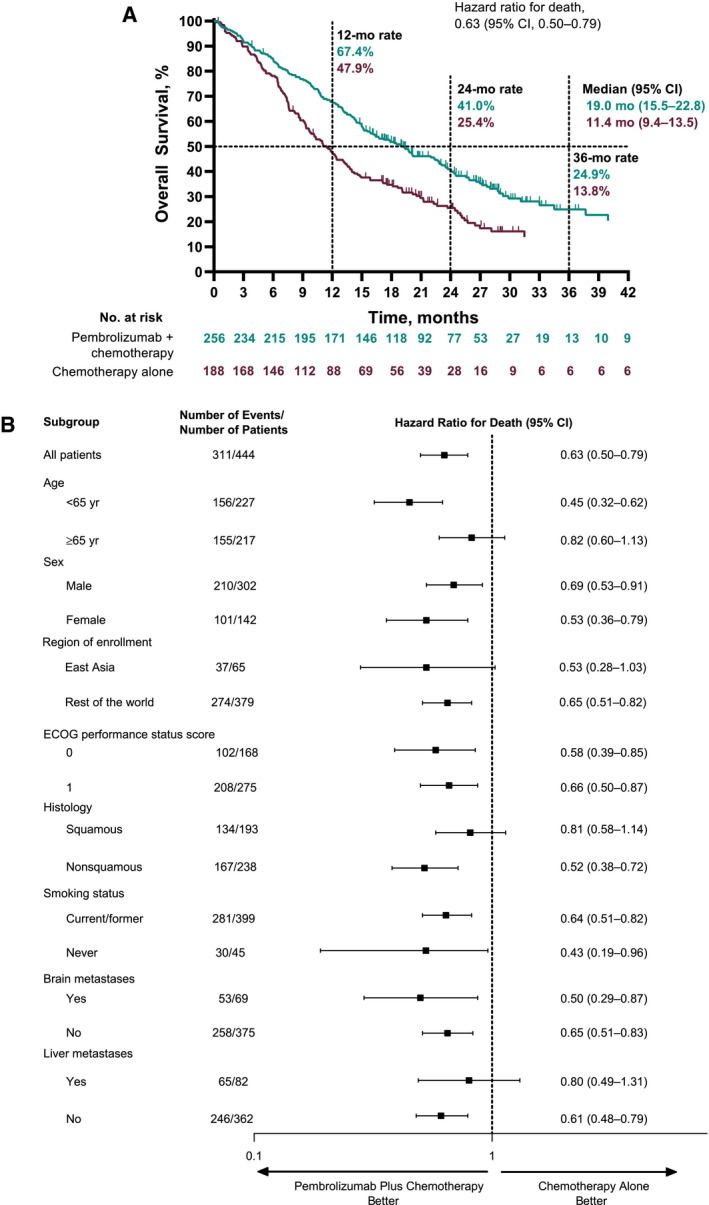
Kaplan‐Meier estimates of OS among patients with no PD‐L1 expression (TPS < 1%): (A) OS for patients who received pembrolizumab plus chemotherapy versus chemotherapy alone and (B) subgroup analysis of OS. ECOG indicates Eastern Cooperative Oncology Group; OS, overall survival; PD‐L1, programmed death ligand 1; TPS, tumor proportion score.
At the time of the data cutoff, events of disease progression or death had occurred in 386 of the 444 patients. Pembrolizumab plus chemotherapy improved PFS over chemotherapy alone (HR, 0.68; 95% CI, 0.56‐0.83). The median PFS was 6.9 months (95% CI, 6.2‐8.8 months) in the pembrolizumab plus chemotherapy group and 5.8 months (95% CI, 4.8‐6.2 months) in the chemotherapy alone group (Fig. 3A). The estimated 12‐month PFS rates were 32.8% (95% CI, 27.0%‐38.7%) and 19.3% (95% CI, 13.9%‐25.3%), respectively, and the 24‐month rates were 17.1% (95% CI, 12.5%‐22.4%) and 8.5% (95% CI, 4.8%‐13.5%), respectively.
Figure 3.
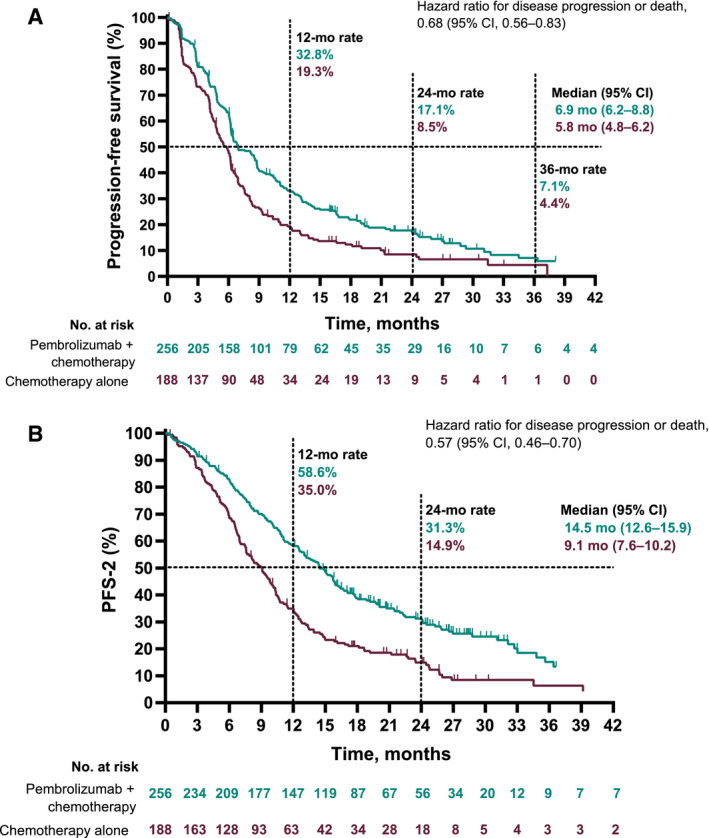
Kaplan‐Meier estimates of PFS among patients with no PD‐L1 expression (TPS < 1%): (A) PFS for patients who received pembrolizumab plus chemotherapy versus chemotherapy alone and (B) PFS‐2 for patients who received pembrolizumab plus chemotherapy versus chemotherapy alone. PD‐L1, programmed death ligand 1; PFS, progression‐free survival; PFS‐2, progression‐free survival 2; TPS, tumor proportion score.
Pembrolizumab plus chemotherapy improved PFS‐2 over chemotherapy alone (HR, 0.57; 95% CI, 0.46‐0.70). The median PFS‐2 was 14.5 months (95% CI, 12.6‐15.9 months) in the pembrolizumab plus chemotherapy group and 9.1 months (95% CI, 7.6‐10.2 months) in the chemotherapy alone group (Fig. 3B).
Among patients with PD‐L1‒negative tumors, the ORR was higher with pembrolizumab plus chemotherapy (50.0%; 95% CI, 43.7%‐56.3%) than chemotherapy alone (29.8%; 95% CI, 23.4%‐36.9%). The median DORs were 8.5 months (range, 1.1+ to 46.0 months) and 6.9 months (range, 1.4+ to 30.1+ months), respectively (Table 2).
Table 2.
Summary of Confirmed ORRs Assessed According to RECIST Version 1.1by Blinded, Independent, Central Review in a Pooled Analysis of Patients With a PD‐L1 TPS < 1%
| Pembrolizumab + Chemotherapy (n = 256) | Chemotherapy Alone (n = 188) | |
|---|---|---|
| ORR a | ||
| No. of patients | 128 | 56 |
| % (95% CI) | 50.0 (43.7‐56.3) | 29.8 (23.4‐36.9) |
| Best overall response, No. (%) | ||
| Complete response | 2 (0.8) | 5 (2.7) |
| Partial response | 126 (49.2) | 51 (27.1) |
| Stable disease | 90 (35.2) | 79 (42.0) |
| Progressive disease | 20 (7.8) | 32 (17.0) |
| Not evaluable b | 11 (4.3) | 12 (6.4) |
| No assessment c | 7 (2.7) | 9 (4.8) |
| Time to response, median (range), mo | 1.6 (1.2‐26.3) | 1.4 (1.2‐26.9) |
| DOR, median (range), mo d | 8.5 (1.1+ to 46.0) | 6.9 (1.4+ to 30.1+) |
| Ongoing response, No. (%) e | 20 (15.6) | 9 (16.1) |
Abbreviations: DOR, duration of response; ORR, objective response rate; PD‐L1, programmed death ligand 1; RECIST, Response Evaluation Criteria in Solid Tumors; TPS, tumor proportion score.
Includes confirmed complete responses plus partial responses.
Includes patients with 1 or more postbaseline tumor assessments, none of which were evaluable for response, and those with a postbaseline tumor assessment less than 6 weeks from randomization showing a complete response, a partial response, or stable disease.
No postbaseline assessment was available for a response evaluation.
A plus sign indicates no progressive disease at the time of the last disease assessment.
Includes patients who were alive and had not progressed, initiated new anticancer treatment, or been lost to follow‐up with a last adequate assessment less than 5 months before the data cutoff date.
Among the 73 patients who crossed over to pembrolizumab on study, the median OS from the time of the initiation of crossover pembrolizumab was 6.3 months (95% CI, 4.8‐7.7 months), and the median PFS was 2.6 months (95% CI, 2.0‐2.8 months).
Sixteen patients (6.3%) in the pembrolizumab plus chemotherapy group completed 2 years of treatment. The ORR among these patients was 87.5% (95% CI, 61.7%‐98.4%); 2 patients had a complete response, 12 had a partial response, and 2 had stable disease. The median DOR was 46.0 months (range, 7.6‐46.0 months), and the median PFS was 47.4 months (95% CI, 15.9‐47.4 months). The 3‐year OS rate was 100%; all patients remained alive at the data cutoff.
Safety
AEs of any grade, regardless of attribution to study treatment by the investigator, occurred in 253 of 255 patients (99.2%) in the pembrolizumab plus chemotherapy group and in 184 of 186 patients (98.9%) in the chemotherapy alone group (Table 3). The most common AEs in both the pembrolizumab plus chemotherapy group and the chemotherapy alone group were anemia and nausea. AEs of grade 3 or higher occurred in 182 patients (71.4%) in the pembrolizumab plus chemotherapy group and in 134 patients (72.0%) in the chemotherapy alone group; 27 (10.6%) and 12 (6.5%), respectively, had AEs that led to death (Table 3 and Supporting Table 1). Thirteen patients (5.1%) in the pembrolizumab plus chemotherapy group and 3 patients (1.6%) in the chemotherapy alone group experienced treatment‐related AEs leading to death (Table 3 and Supporting Table 1).
Table 3.
All‐Cause Adverse Events in Patients With a PD‐L1 TPS < 1%
| Pembrolizumab + Chemotherapy (n = 255), No. (%) | Chemotherapy Alone (n = 186), No. (%) | |||
|---|---|---|---|---|
| Any Grade | Grade 3‐5 | Any Grade | Grade 3‐5 | |
| Any event | 253 (99.2) | 182 (71.4) | 184 (98.9) | 134 (72.0) |
| Event leading to discontinuation of study drug | 93 (36.5) | 64 (25.1) | 31 (16.7) | 24 (12.9) |
| Event leading to death | 27 (10.6) | 27 (10.6) | 12 (6.5) | 12 (6.5) |
| Event leading to treatment‐related death | 13 (5.1) | 13 (5.1) | 3 (1.6) | 3 (1.6) |
| Event occurring in ≥20% of patients in either group | ||||
| Anemia | 132 (51.8) | 41 (16.1) | 105 (56.5) | 41 (22.0) |
| Nausea | 131 (51.4) | 5 (2.0) | 88 (47.3) | 5 (2.7) |
| Fatigue | 98 (38.4) | 15 (5.9) | 56 (30.1) | 6 (3.2) |
| Diarrhea | 88 (34.5) | 9 (3.5) | 48 (25.8) | 6 (3.2) |
| Constipation | 82 (32.2) | 1 (0.4) | 59 (31.7) | 3 (1.6) |
| Decreased appetite | 86 (33.7) | 2 (0.8) | 57 (30.6) | 0 |
| Neutropenia | 72 (28.2) | 38 (14.9) | 52 (28.0) | 37 (19.9) |
| Cough | 59 (23.1) | 1 (0.4) | 48 (25.8) | 0 |
| Thrombocytopenia | 65 (25.5) | 22 (8.6) | 46 (24.7) | 15 (8.1) |
| Vomiting | 61 (23.9) | 6 (2.4) | 31 (16.7) | 4 (2.2) |
| Alopecia | 55 (21.6) | 1 (0.4) | 43 (23.1) | 1 (0.5) |
| Asthenia | 53 (20.8) | 11 (4.3) | 36 (19.4) | 9 (4.8) |
| Rash | 53 (20.8) | 1 (0.4) | 23 (12.4) | 2 (1.1) |
| Dyspnea | 40 (15.7) | 3 (1.2) | 38 (20.4) | 1 (0.5) |
Abbreviations: PD‐L1, programmed death ligand 1; TPS, tumor proportion score.
Immune‐mediated AEs and infusion reactions of any grade occurred in 74 of 255 patients (29.0%) in the pembrolizumab plus chemotherapy group and in 23 of 186 patients (12.4%) in the chemotherapy alone group (Table 4). The most common immune‐mediated AEs in the pembrolizumab plus chemotherapy group were hypothyroidism, pneumonitis, and hyperthyroidism. Two patients (0.8%) in the pembrolizumab plus chemotherapy group had grade 5 events of pneumonitis; no patients in the chemotherapy group had grade 5 immune‐mediated AEs or infusion reactions.
Table 4.
Immune‐Mediated Adverse Events and Infusion Reactions in Patients With a PD‐L1 TPS < 1%
| Pembrolizumab + Chemotherapy (n = 255), No. (%) | Chemotherapy Alone (n = 186), No. (%) | |||
|---|---|---|---|---|
| Any Grade | Grade 3‐5 a | Any Grade | Grade 3‐5 a | |
| Any event | 74 (29.0) | 31 (12.2) | 23 (12.4) | 6 (3.2) |
| Hypothyroidism | 19 (7.5) | 1 (0.4) | 6 (3.2) | 0 |
| Pneumonitis | 18 (7.1) | 11 (4.3) | 4 (2.2) | 2 (1.1) |
| Hyperthyroidism | 14 (5.5) | 0 | 5 (2.7) | 0 |
| Infusion reactions | 12 (4.7) | 3 (1.2) | 4 (2.2) | 0 |
| Colitis | 5 (2.0) | 3 (1.2) | 1 (0.5) | 1 (0.5) |
| Nephritis | 5 (2.0) | 4 (1.6) | 1 (0.5) | 1 (0.5) |
| Hepatitis | 5 (2.0) | 4 (1.6) | 0 | 0 |
| Severe skin reactions | 4 (1.6) | 2 (0.8) | 3 (1.6) | 3 (1.6) |
| Hypophysitis | 3 (1.2) | 1 (0.4) | 0 | 0 |
| Thyroiditis | 2 (0.8) | 0 | 0 | 0 |
| Adrenal insufficiency | 2 (0.8) | 0 | 0 | 0 |
| Encephalitis | 1 (0.4) | 1 (0.4) | 0 | 0 |
| Guillain‐Barre syndrome | 1 (0.4) | 1 (0.4) | 0 | 0 |
| Pancreatitis | 1 (0.4) | 1 (0.4) | 0 | 0 |
| Myositis | 1 (0.4) | 0 | 0 | 0 |
Abbreviations: PD‐L1, programmed death ligand 1; TPS, tumor proportion score.
Events are included regardless of attribution to the study drug or immune relatedness by the investigator.
Two patients in the pembrolizumab plus chemotherapy group had events of pneumonitis that led to death. There were no immune‐mediated adverse events or infusion reactions leading to death in the chemotherapy group.
Discussion
This pooled analysis of 3 randomized controlled trials demonstrated a substantial clinical benefit and manageable safety profile with first‐line pembrolizumab plus platinum‐doublet chemotherapy versus chemotherapy alone in patients with PD‐L1‒negative NSCLC. Pembrolizumab plus chemotherapy substantially reduced the risk of death (HR for OS, 0.63) and improved the median OS by ~8 months (19.0 vs 11.4 months); it also improved PFS (HR, 0.68), PFS‐2 (HR, 0.57), and ORR (50.0% vs 29.8%). Patients who completed 2 years of treatment were among those who derived a long‐term benefit from pembrolizumab plus chemotherapy; in this group, ORR was high, and all patients remained alive at the data cutoff. Together, these results support pembrolizumab plus chemotherapy as a standard‐of‐care first‐line treatment for patients with PD‐L1‒negative advanced NSCLC without EGFR/ALK alterations.
Pembrolizumab plus platinum‐based chemotherapy arguably constitutes the standard of care for patients with PD‐L1‒negative advanced/metastatic NSCLC who are eligible for checkpoint inhibitors. In both the first‐line setting 2 , 3 and the second‐line setting, 11 pembrolizumab monotherapy has demonstrated improved OS only among patients with a PD‐L1 TPS ≥ 1%, with further benefits observed among those with PD‐L1 TPS ≥ 50%. 3 , 11 Previously, bevacizumab and necitumumab were the only biologics/targeted agents to have demonstrated an improvement in OS (by ~2 months) when combined with platinum chemotherapy in patients with advanced nonsquamous NSCLC (HR, 0.79) 12 and advanced squamous NSCLC (HR, 0.84), respectively. 13 Importantly, our results demonstrate that pembrolizumab plus chemotherapy provides a clinically meaningful OS benefit for patients with PD‐L1‒negative NSCLC, a population in need of better treatment options. Although cross‐trial comparisons should be approached with caution, the magnitude of benefit provided by pembrolizumab plus chemotherapy versus chemotherapy alone in this setting is substantially more pronounced than previously observed with other agents.
The activity of pembrolizumab (an anti‒PD‐1 monoclonal antibody) plus chemotherapy among patients whose tumors did not express PD‐L1 at the baseline demonstrated the combined effects of immunotherapy and chemotherapy in the NSCLC setting. As noted previously, chemotherapy agents can have immunomodulating effects that increase the immunogenicity of tumors; the addition of pembrolizumab may, therefore, enhance these immunomodulating effects, regardless of PD‐L1 expression. 4 , 5
In addition to the improvements in OS, PFS, and ORR, pembrolizumab plus chemotherapy improved PFS‐2 in comparison with chemotherapy alone, and this demonstrated that the benefits of first‐line pembrolizumab plus chemotherapy were maintained through the next line of therapy. The HR for PFS‐2 (0.57) was lower than that for PFS (0.68) despite the high rate of crossover to anti–PD‐(L)1 therapy (57.4%) among patients who initially received chemotherapy alone; this supports preferential use of first‐line pembrolizumab plus chemotherapy in PD‐L1‒negative NSCLC. The early and continued separation in both PFS curves over time indicates that early disease control with pembrolizumab plus chemotherapy is important in achieving a long‐term clinical benefit. In addition, the OS and PFS observed after on‐study crossover from chemotherapy to pembrolizumab monotherapy were modest (~6 and ~3 months, respectively), and this further supports the need to administer pembrolizumab as part of first‐line combination therapy.
In an exploratory analysis of part 1 of CheckMate 227, among patients with PD‐L1‒negative NSCLC, nivolumab plus chemotherapy improved PFS in comparison with chemotherapy alone (HR, 0.74; 95% CI, 0.58‐0.94), 14 and nivolumab plus ipilimumab improved OS in comparison with chemotherapy (HR, 0.62; 95% CI, 0.48‐0.78). 15 Subsequently, part 2 showed no OS benefit with nivolumab plus chemotherapy versus chemotherapy alone in the all‐comers nonsquamous NSCLC population. 16 Similarly, in IMpower‐132 and IMpower‐130, atezolizumab added to a platinum doublet showed benefit in patients with nonsquamous NSCLC, regardless of PD‐L1 status. 17 , 18
Overall, pembrolizumab plus chemotherapy had manageable toxicity, although the proportion with grade 5 treatment‐related AEs was higher in the pembrolizumab plus chemotherapy group than the chemotherapy alone group. No new safety signals were observed in this pooled population of patients with PD‐L1‒negative tumors with respect to AEs previously associated with the combination of pembrolizumab and platinum‐based chemotherapy in advanced NSCLC. 6 , 7 , 8
This pooled analysis of individual patient data provides a large population for an evaluation of the efficacy and safety of pembrolizumab plus platinum‐based chemotherapy in patients with PD‐L1‒negative tumors. In each of the parent studies, PD‐L1 was a prospectively evaluated stratification factor, and this allowed the retrospective pooled analysis to be conducted. Although the chemotherapy regimens differed between the studies, each study included standard platinum‐based regimens recommended according to NSCLC histology. 19 Because of the 2:1 randomization ratio in KEYNOTE‐189, the proportion of patients with nonsquamous histology was higher in the pembrolizumab plus chemotherapy group than the chemotherapy alone group. Notably, the direction of the treatment effect was similar in both patients with squamous NSCLC andpatients with nonsquamous NSCLC (albeit with a different magnitude). The HR for OS in the current pooled analysis favoring pembrolizumab plus chemotherapy over chemotherapy alone compared closely with results for the PD‐L1–negative subgroups of the primary analyses of the KEYNOTE‐189 and KEYNOTE‐407 studies 7 , 8 (HR point estimates for OS, 0.63 [pooled], 0.59 [KEYNOTE‐189; nonsquamous], and 0.61 [KEYNOTE‐407; squamous]). This pooled analysis represents the largest evaluation of outcomes with an immunotherapy plus chemotherapy combination in patients with PD‐L1‒negative advanced NSCLC, and the outcomes described provide an important benchmark for comparison with outcomes in future studies.
In conclusion, this pooled analysis showed clinically meaningful improvements in OS, PFS, and ORR and manageable safety with pembrolizumab plus chemotherapy versus chemotherapy alone in patients with PD‐L1‒negative advanced NSCLC. Pembrolizumab plus platinum‐based chemotherapy is a standard‐of‐care first‐line therapy for patients with advanced squamous or nonsquamous NSCLC, including patients with PD‐L1‒negative tumors.
Funding Support
Funding for this research was provided by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co., Inc. (Kenilworth, New Jersey).
Conflict of Interest Disclosures
Hossein Borghaei receives partial support from the National Institutes of Health (P30 CA006927); has received clinical trial research support from Millennium Pharmaceuticals, Merck, Celgene, Bristol‐Myers Squibb, and Lilly; has served on an advisory board or as a consultant for Bristol‐Myers Squibb, Lilly, Genentech, Celgene, Pfizer, Merck, EMD Serono, Boehringer Ingelheim, AstraZeneca, Novartis, Genmab, Regeneron, BioNTech, Cantargia AB, Amgen, AbbVie, Axiom, PharmaMar, Takeda, Huya Bioscience International, GLG Pharma, and Daiichi‐Sankyo; has served on data and safety monitoring boards for the University of Pennsylvania chimeric antigen receptor T‐cell program, Takeda, and Incyte; has served on scientific advisory boards for Sonnet BioTherapeutics and Rgenix; owns stock in Sonnet BioTherapeutics and Rgenix; and is an employee of Fox Chase Cancer Center. Corey J. Langer has served as a consultant for/received honoraria from Boehringer Ingelheim, Novocure, AstraZeneca, Takeda, Genentech/Roche, Merck, Gilead Sciences, GlaxoSmithKline, OncLive, Takai, and RTP; has received research funding from Advantagene, AstraZeneca, Genentech/Roche, Lilly, Takai, Trizell, Merck, Takeda, and Inovio Pharmaceuticals; has received medical writing support from Novartis; and has served on data and safety monitoring committees for Lilly, Amgen, and SWOG. Luis Paz‐Ares reports honoraria (to him/spouse) for scientific advice or speaking from Advanced Accelerator Applications (Adacap), Amgen, AstraZeneca, Bayer, Blueprint Medicines, Bristol‐Myers Squibb, Boehringer Ingelheim, Celgene, Incyte, Ipsen, Lilly, Merck, MSD, Novartis, PharmaMar, Pfizer, Roche, Sanofi, Servier, Takeda, and Sysmex; is a board member for Genomica and Altum Sequencing; and reports grants to his institution from AstraZeneca, Bristol‐Myers Squibb, MSD, and Pfizer. Delvys Rodríguez‐Abreu reports grants from Bristol‐Myers Squibb and has received honoraria for lectures and consulting from Bristol‐Myers Squibb, Genentech/Roche, MSD, AstraZeneca, Boehringer Ingelheim, Lilly, Roche, Novartis, and Pfizer. Balazs Halmos has received research funding from Amgen, Janssen, Advaxis, Merck, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Lilly, Novartis, GlaxoSmithKline, Pfizer, AbbVie, AstraZeneca, Mirati Therapeutics, Takeda, and Guardant Health; has served as a consultant for Merck, Novartis, Boehringer Ingelheim, AstraZeneca, Novartis, Pfizer, Guardant Health, Spectrum, Bristol‐Myers Squibb, and Genentech; and has received personal fees from Amgen and TPT. Marina C. Garassino has received grants and personal fees for clinical trials from AstraZeneca, Novartis, Bristol‐Myers Squibb, Roche, Pfizer, Celgene, Bayer, and MSD; grants from Turning Point, Ipsen, MedImmune, Exelixis, Incyte, Lilly, Otsuka Pharmaceutical, Tiziana Life Sciences, Clovis Oncology, Merck Serono, Merck KGaA, Blueprint Medicine, GlaxoSmithKline, United Therapeutics Corporation, and Spectrum Pharmaceuticals; personal fees from Seattle Genetics, Daiichi Sankyo, GlaxoSmithKline, Lilly, Janssen, Boehringer Ingelheim, Blueprint Medicine, Otsuka Pharmaceutical, Incyte, Inivata, Spectrum Pharmaceuticals, Takeda, and Sanofi‐Aventis; and nonfinancial support from MSD, Pfizer, and Lilly. Takayasu Kurata has received honoraria from AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Chugai, Lilly, MSD, and Ono Pharmaceutical and reports grants from AstraZeneca, MSD, Bristol‐Meyers Squibb, Novartis, Takeda, and Chugai. Jianxin Lin is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA. M. Catherine Pietanza is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA and owns stock in Merck & Co., Inc., Kenilworth, New Jersey, USA. Bilal Piperdi is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA. and owns stock in Merck & Co, Inc., Kenilworth, New Jersey, USA. Shirish M. Gadgeel has received personal fees from AstraZeneca, Daichii‐Sankyo, Genentech/Roche, Takeda/Ariad, Boehringer Ingelheim, Merck, Novocure, Bristol‐Myers Squibb, AbbVie, Jazz Pharmaceuticals, and Xcovery. The other authors made no disclosures.
Author Contributions
Hossein Borghaei: Investigation, resources, and writing–review and editing. Corey J. Langer: Investigation, resources, and writing–review and editing. Luis Paz‐Ares: Investigation, resources, and writing–review and editing. Delvys Rodríguez‐Abreu: Investigation, resources, and writing–review and editing. Balazs Halmos: Investigation, resources, and writing–review and editing. Marina C. Garassino: Investigation, resources, and writing–review and editing. Baerin Houghton: Investigation, resources, and writing–review and editing. Takayasu Kurata: Investigation, resources, and writing–review and editing. Ying Cheng: Investigation, resources, and writing–review and editing. Jianxin Lin: Formal analysis and writing‒review and editing. M. Catherine Pietanza: Conceptualization, investigation, and writing‒review and editing. Bilal Piperdi: Conceptualization, investigation, and writing–review and editing. Shirish M. Gadgeel: Investigation, resources, and writing–review and editing.
Supporting information
Supplementary Material
Borghaei H, Langer CJ, Paz‐Ares L, Rodríguez‐Abreu D, Halmos B, Garassino MC, Houghton B, Kurata T, Cheng Y, Lin J, Pietanza MC, Piperdi B, Gadgeel SM. Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non–small cell lung cancer without tumor PD‐L1 expression: A pooled analysis of 3 randomized controlled trials. Cancer. 2020. 10.1002/cncr.33142
We thank the patients and their families and caregivers for participating in the studies included in this pooled analysis along with all investigators and site personnel. Medical writing assistance was provided by Sheri Arndt, PharmD, and Mariana Ovnic, PhD, of ICON plc (North Wales, Pennsylvania). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Kenilworth, New Jersey).
Data Availability
The data sharing policy, including restrictions, of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Kenilworth, New Jersey), is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
References
- 1. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small‐cell lung cancer. N Engl J Med. 2015;372:2018‐2028. [DOI] [PubMed] [Google Scholar]
- 2. Reck M, Rodriguez‐Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD‐L1–positive non–small‐cell lung cancer. N Engl J Med. 2016;375:1823‐1833. [DOI] [PubMed] [Google Scholar]
- 3. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD‐L1–expressing, locally advanced or metastatic non–small‐cell lung cancer (KEYNOTE‐042): a randomised, open‐label, controlled, phase 3 trial. Lancet. 2019;393:1819‐1830. [DOI] [PubMed] [Google Scholar]
- 4. Kersten K, Salvagno C, de Visser KE. Exploiting the immunomodulatory properties of chemotherapeutic drugs to improve the success of cancer immunotherapy. Front Immunol. 2015;6:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galluzzi L, Zitvogel L, Kroemer G. Immunological mechanisms underneath the efficacy of cancer therapy. Cancer Immunol Res. 2016;4:895‐902. [DOI] [PubMed] [Google Scholar]
- 6. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non–small‐cell lung cancer: a randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol. 2016;17:1497‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gandhi L, Rodriguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med. 2018;378:2078‐2092. [DOI] [PubMed] [Google Scholar]
- 8. Paz‐Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non–small‐cell lung cancer. N Engl J Med. 2018;379:2040‐2051. [DOI] [PubMed] [Google Scholar]
- 9. Planchard D, Popat S, Kerr K, et al. Metastatic non–small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2019;30:863‐870. [DOI] [PubMed] [Google Scholar]
- 10. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 11. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1–positive, advanced non–small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet. 2016;387:1540‐1550. [DOI] [PubMed] [Google Scholar]
- 12. Sandler A, Gray R, Perry MC, et al. Paclitaxel‐carboplatin alone or with bevacizumab for non–small‐cell lung cancer. N Engl J Med. 2006;355:2542‐2550. [DOI] [PubMed] [Google Scholar]
- 13. Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first‐line therapy in patients with stage IV squamous non–small‐cell lung cancer (SQUIRE): an open‐label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763‐774. [DOI] [PubMed] [Google Scholar]
- 14. Borghaei H, Hellman MD, Paz‐Ares LG, et al. Nivolumab (nivo) + platinum‐doublet chemotherapy (chemo) vs chemo as first‐line (1L) treatment (Tx) for advanced non–small cell lung cancer (NSCLC) with <1% tumor PD‐L1 expression: results from CheckMate 227 [abstract 9001]. J Clin Oncol. 2018;36:9001. [Google Scholar]
- 15. Hellmann MD, Paz‐Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small‐cell lung cancer. N Engl J Med. 2019;381:2020‐2031. [DOI] [PubMed] [Google Scholar]
- 16. Paz‐Ares L, Ciuleanu TE, Yu X, et al. Nivolumab + platinum‐doublet chemotherapy vs chemo as first‐line treatment for advanced non–small cell lung cancer: CheckMate 227—part 2 final analysis [abstract LBA3]. Ann Oncol. 2019;30:LBA3. [Google Scholar]
- 17. Papadimitrakopoulou VA, Cobo M, Bordoni R, et al. IMpower132: PFS and safety results with 1L atezolizumab + carboplatin/cisplatin + pemetrexed in stage IV non‐squamous NSCLC. Paper presented at: World Conference on Lung Cancer; September 23‐26, 2018; Toronto, ON, Canada. [Google Scholar]
- 18. Cappuzzo F, McCleod M, Hussein M, et al. IMpower130: progression‐free survival (PFS) and safety analysis from a randomised phase III study of carboplatin + nab‐paclitaxel (CnP) with or without atezolizumab (atezo) as first‐line (1L) therapy in advanced non‐squamous NSCLC [abstract LBA53]. Ann Oncol. 2018;29(suppl 8):viii742. [Google Scholar]
- 19. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non–Small Cell Lung Cancer. Version 6.2020. Accessed August 10, 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data sharing policy, including restrictions, of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (Kenilworth, New Jersey), is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.


