Abstract
Enemy‐risk effects, often referred to as non‐consumptive effects (NCEs), are an important feature of predator–prey ecology, but their significance has had little impact on the conceptual underpinning or practice of biological control. We provide an overview of enemy‐risk effects in predator–prey interactions, discuss ways in which risk effects may impact biocontrol programs and suggest avenues for further integration of natural enemy ecology and integrated pest management. Enemy‐risk effects can have important influences on different stages of biological control programs, including natural enemy selection, efficacy testing and quantification of non‐target impacts. Enemy‐risk effects can also shape the interactions of biological control with other pest management practices. Biocontrol systems also provide community ecologists with some of the richest examples of behaviourally mediated trophic cascades and demonstrations of how enemy‐risk effects play out among species with no shared evolutionary history, important topics for invasion biology and conservation. We conclude that the longstanding use of ecological theory by biocontrol practitioners should be expanded to incorporate enemy‐risk effects, and that community ecologists will find many opportunities to study enemy‐risk effects in biocontrol settings.
Keywords: Agricultural ecology, behavioural ecology, biological control, enemy‐risk effects, natural enemies, non‐consumptive effects, pest management, predation risk, predator–prey ecology, trophic cascades
We provide a synthetic review of enemy‐risk effects in biological control of arthropod pests. We provide a conceptual overview of enemy‐risk effects, review the current body of literature documenting risk effects in biocontrol systems and describe in detail ways in which risk effects may impact the implementation of biocontrol programs.

INTRODUCTION
Biological control (or biocontrol) is the use of an organism to reduce or prevent the unwanted impact of another organism, typically through an exploitative interaction (Eilenberg et al., 2001). While competitive relationships are sometimes utilised (Tyndale‐Biscoe and Vogt, 1996), most biological control agents, including predators, parasitoids, pathogens and herbivores, are consumers of pest organisms (Heimpel and Mills, 2017). Perhaps the best‐known form of biological control is ‘classical’ or importation biological control, where a natural enemy is imported from a region other than the target area, often from the native home range of the pest. Today, this involves a rigorous process of enemy selection, efficacy testing and non‐target testing (Bigler et al., 2006), since history is filled with examples of exotic enemies wreaking havoc on naïve, native communities (Simberloff and Stiling, 1996). Inundative and inoculative releases of natural enemies, collectively referred to as ‘augmentative control’, involve the release of large numbers of enemies, either to bolster existing populations or to provide a short pulse of control without long‐term establishment. In contrast, conservation biological control is the attempt to increase the effectiveness of already‐present enemies. Methods include the provision of alternative resources for biocontrol agents (e.g. extrafloral or floral nectar, pollen), changes in landscape complexity and the preservation of natural areas beneficial to enemies (Bianchi et al., 2006; Tscharntke et al., 2007, 2016). Altogether, these various methods of biological control provide significant ecosystem services in both natural and agricultural ecosystems (Losey and Vaughan, 2006; Zhang and Swinton, 2012; Naranjo et al., 2015).
Biological control and predator–prey/parasitoid‐host (‘natural enemy’) ecology have a long relationship (Hassell and Varley, 1969; McMurtry et al., 1970; Murdoch et al., 1985). Early theory in natural enemy ecology was heavily influenced by examples of classical biological control, and broader natural enemy ecology has served to inform biocontrol practice. However, biological control has lagged behind natural enemy ecology by not recognising the impact and importance of enemy‐risk effects, often referred to as non‐consumptive effects (NCEs), fear effects, risk effects, non‐lethal effects or trait‐mediated effects. Biocontrol typically focuses on direct lethal effects of enemies on pests, whether through consumption or parasitism (which we refer to as consumptive effects or CEs) or through infection. However, natural enemy ecology has long recognised the importance of enemy‐risk effects (Abrams et al., 1996; Werner and Anholt, 1996; Schmitz, 1998; Werner and Peacor, 2003). Enemies induce behavioural, physiological, morphological or life‐history changes in their prey that can lead to significant changes in individual fitness, population dynamics and community dynamics. Meta‐analyses and reviews have noted that even when natural enemies kill relatively few prey or hosts, they can have major impacts via enemy‐risk effects (Preisser et al., 2005; Peckarsky et al., 2008; Preisser and Bolnick, 2008; Schmitz et al., 2008; Sih et al., 2010; Buchanan et al., 2017). While numerous studies have demonstrated major enemy‐risk effects in many biological control systems, this knowledge has not been implemented in standard thinking about biocontrol. Several ways that enemy‐risk effects connect to biocontrol include understanding: (1) the dynamics of trophic cascades where natural enemies have positive impacts on plants not only by killing pests (CEs), but also by altering pest traits; (2) the role of risk effects in governing interactions in biocontrol systems with multiple enemies, intraguild predation (IGP) and bottom‐up effects; (3) the impacts of enemy‐induced pest dispersal on the spatiotemporal ecology of biocontrol; and (4) how effects of natural enemies differ on coevolved versus naïve prey, as is common for target versus non‐target prey respectively. Insights about enemy‐risk effects can thus help to better guide agent selection, non‐target testing, integrated pest management (IPM) programs and other biocontrol practices. Conversely, biocontrol systems are ideal for the general study of enemy‐risk effects, offering opportunities to study risk at multiple scales, across multiple trophic levels, with varying levels of co‐evolution, and in systems amenable to experimental manipulation.
We provide a systematic overview of insights gained from integrating enemy‐risk effects into the ecology of biocontrol, focusing on management of arthropod pests. We begin with a conceptual overview of current literature on enemy‐risk effects, including work outside of biocontrol systems, then review studies of enemy‐risk effects in biocontrol and finish by demonstrating and discussing in some detail how a conceptual knowledge of risk effects can inform and improve pest management and biocontrol programs (see Box 1 for a well‐studied example).
Box 1. Enemy‐risk effects and the biological control of the red imported fire ant.
The red imported fire ant, Solenopsis invicta, was inadvertently introduced from South America into the port city of Mobile, Alabama in the 1930s. Expanding its range across much of the southern United States, it achieved exceptionally high densities (5–10 times greater than in their native range), displacing native ants, damaging agricultural production and creating a sting hazard for anyone active outdoors (Porter and Gilbert, 2004; Oi et al., 2015). After a massive and controversial insecticide‐based eradication effort failed, attention turned to classical biological control. Studies in the native range of the ants revealed over 20 species of parasitoid flies in the genus Pseudacteon (family Phoridae), most of which appeared to be host specific and thus to be potentially acceptable in terms of low risk of non‐target impacts. Pseudacteon spp. parasitoids lay eggs in adult worker ants, the resulting parasitoid larvae completing their development in the heads of their host ants, which fall off as the larvae develop (hence their common name: decapitating flies).
Early investigations of Pseudacteon spp. in the native ranges of the fire ants concluded, however, that they were poor candidates for effective biological control, because they achieved very low rates of parasitism (Jouvenaz et al. 1981). Extensive year‐long sampling across multiple sites confirmed that parasitism was indeed rare, with only 0.24% of workers parasitised on average (Calcaterra et al., 2008). Retrospective analyses of the extensive literature on the introductions of parasitoids as classical biological control agents by Hawkins et al. (1993) and Hawkins and Cornell (1994) suggested that a threshold for success exists: parasitoids that fail to achieve maximum parasitism rates of >32% in their native ranges, or >33‐36% in their introduced ranges, have been unable to produce economically acceptable levels of pest suppression. Because the entire Pseudacteon spp. complex exerted a maximum of only 2.81% parasitism in the native range (Calcaterra et al., 2008), the suggestion that these flies would be of ‘dubious value’ for biological control (Jouvenaz et al. 1981) was not hard to understand.
However, as argued by Feener and Brown (1992) and Porter and Gilbert (2004), a reliance on parasitism rates alone might lead us to grossly underestimate the potential value of Pseudacteon spp. parasitoids as control agents. Earlier studies had shown that phorid parasitoids attacking a different ant, while also generating little parasitism, elicited dramatic anti‐predator defences. Ants responded to the presence of flies by fleeing back to the nest or by sheltering from fly attacks in the leaf litter, causing the ants to lose their status as competitive dominants in their interactions with other ants (Feener, 1981). Subsequent studies of S. invicta revealed a similar pattern: in response to a fly’s arrival, workers retreated underground, took cover below sticks or pebbles, or adopted stereotypic defensive postures with their sting‐bearing gasters raised (Orr et al., 1995; Porter et al., 1995). This eliminated their ability to recruit foragers to food sources, with other ants immediately exploiting the now‐available resources. Just a single parasitoid could arrest the foraging activity of hundreds of fire ant workers (Porter et al., 1995). Thus, S. invicta display dramatic and costly anti‐predator defences, and the non‐consumptive effects of phorid flies on fire ants may allow native ants to compete effectively with these invaders.
Thus, recognition of the potential importance of enemy‐risk effects of Pseudacteon spp. motivated the decision to import these species as classical biological control agents. Six species have been introduced to the United States to date, with different species attacking different subsets of worker ants, based on ant size, time of activity or foraging location (at the nest or at foraging trails; reviewed by Oi et al., 2015). Importantly, host‐range testing included assessments not only of parasitism of non‐targets, but also the attraction to worker ants and expression of the hovering attacks that elicit defensive responses (Porter and Gilbert, 2004). Whether the enemy‐risk effects will prove to be sufficient to control S. invicta in its invasive range remains, however, an open question, as Pseudacteon spp. continue to build their populations and expand their ranges while monitoring continues (Chen and Fadamiro, 2018; Oi et al., 2019).
ENEMY‐RISK EFFECTS: A BRIEF CONCEPTUAL OVERVIEW
Many organisms exhibit responses to natural enemies (predators and parasitoids; we frequently use ‘predator/prey’ as a catchall that includes parasitoid/host relationships), including within‐generation changes in behaviour (e.g reduced activity, increased refuge use, increased group size; Lima, 1998), physiology (Hawlena and Schmitz, 2010; Clinchy et al., 2013), morphology (Bourdeau and Johansson, 2012; Hulthén et al., 2014) and life history (Miner et al., 2005; LaManna and Martin, 2016; Relyea et al., 2018). These responses typically have costs in terms of reduced feeding and growth rates and, ultimately, reduced fitness (Kerfoot and Sih, 1987; Stamps, 2007; Orrock et al., 2013) and population growth rates (Creel and Christianson, 2008). Because these responses often involve niche shifts (e.g. in prey diets or habitat use), they also affect prey interactions with other species (Werner and Peacor, 2003). For example, anti‐predator responses can alter competition among prey (Werner and Anholt, 1996), increase exposure to other predators (Sih et al., 1998; Fouzai et al., 2019) or to diseases (Duffy et al., 2011; Shang et al., 2019) and alter impacts on their own resources (Schmitz et al., 2004). Notably, if prey exhibit strong, effective anti‐enemy responses, predators might actually kill few prey (i.e. have weak consumptive effects, CEs) but instead have large impacts on prey fitness and prey interactions with the rest of their community (Preisser et al., 2005). These three levels of effect (individual response, impacts on fitness/populations and community effects) are best defined as enemy‐induced trait responses, non‐consumptive effects and trait‐mediated indirect effects (Peacor et al., 2020). Box 2 discusses this terminology in greater detail.
Box 2. Categorising enemy‐risk effects.
The term NCE is frequently used to describe processes at three levels: the enemy‐induced trait response (e.g. increased refuge use), the consequences for the individual prey/host (e.g. reduced growth rate) or the consequences at the prey/host population level (e.g. increased emigration). Referring to all three levels as NCEs reduces the important distinctions between them, we advocate for a more explicit framework (Fig. 1), and clearer terminology (also see Peacor et al., 2020). We will use the terms enemy‐risk effect to refer to the overall process, enemy‐induced trait response to refer to the mechanism of response, NCE to refer to fitness/population consequences and trait‐mediated indirect effect to refer to effects cascading to trophic levels below the prey/host. A complementary way of conceptualising enemy‐risk effects is to take a more phenomenological approach, focusing on the aspects of a pest population: its per capita impact, abundance and distribution (box shading in Fig. 1).
Behavioural shifts are a commonly studied trait responses in arthropods, and are generally the most rapid and reversible. Examples include changes in time spent feeding (Thaler and Griffin, 2008; Jandricic et al., 2016; Ingerslew and Finke, 2017), food source (Schmitz et al., 1997), microhabitat and refuge use (Lucas et al., 2000; Lawson‐Balagbo et al., 2007; Penfold et al., 2017), oviposition rate (Deas and Hunter, 2013; Hermann and Thaler, 2018), oviposition site selection (Angelon and Petranka, 2002; Vonesh and Blaustein, 2010; Silberbush and Blaustein, 2011), short‐distance escape (Tamaki et al., 1970; Nelson, 2007; Fill et al., 2012) and dispersal (Höller et al., 1994; Henry et al., 2010; Welch and Harwood, 2014; Otsuki and Yano, 2014b).
Physiological shifts can be direct responses to risk, but they are often the consequences of behavioural shifts. For example, a reduction in individual growth rate (physiological) is often a result of reduced foraging effort (behavioural). This can make physiological shifts difficult to categorise within the framework shown in Fig. 1. Examples include changes in growth rate (Kaplan et al., 2014), development time (Bellamy and Alto, 2018) and assimilation efficiency (Thaler et al., 2014).
Morphological shifts are generally slower to appear and more difficult to reverse than behavioural or even physiological shifts. They have been less described in terrestrial arthropods, but thoroughly studied in systems such as Daphnia pulex, where predator cues trigger production of carapace protrusions that decrease vulnerability to predation (Havel and Dodson, 1984; Tollrian, 1995; Rabus and Laforsch, 2011). Life‐history shifts frequently occur over a long timescale and are irreversible for an individual prey/host. They include changes in timing of reproduction or metamorphosis (Ims, 1990; Benard, 2004; Relyea, 2007), quality and quantity of offspring produced (Map pes et al., 1997) and production of winged morphs (Sloggett and Weisser, 2002; Kunert and Weisser, 2003).
Trait responses carry costs for individuals, and we can categorise NCEs based on these costs. These costs are ultimately tied to individual fitness, including reduced fecundity (Map pes et al., 1997) and reduced survival (Walzer and Schausberger, 2009).
Both responses and consequences at the individual level can cascade to affect the entire prey/host population. Finally, community‐level impacts include both trait‐mediated indirect effects, wherein an NCE reduces the prey population such that they have a smaller effect on a lower trophic level, and interaction modifications, wherein a trait response causes an existing interaction with another species to change. As seen in Fig. 1, these community effects can occur via different pathways that may not be captured equally in all studies.
Experiments to evaluate the relative strength of CEs and NCEs typically contrast the total effect of actual predators (CE + NCEs) with the effect of constrained predators (e.g. predators caged or artificially manipulated to prevent use of mouthparts) or predator cues (NCEs only) on prey. A meta‐analysis of these experiments found that the importance of NCEs was highly variable, but on average roughly the same magnitude as CEs (Preisser et al., 2005). For biocontrol, trait‐mediated indirect effects cascading to the plant may be even more relevant. Enemies frequently have very strong positive effects on plants due to trait shifts by herbivores, even when CEs are relatively small (Schmitz et al., 2004; Creel and Christianson, 2008).
Behavioural ecology theory and experiments suggest that prey typically exhibit stronger trait responses when perceived risk is higher and when the marginal costs of response are lower (Lima, 1998). When perceived risk reflects actual risk, predators that are more dangerous in the absence of prey defences can induce such strong anti‐predator responses that they kill fewer prey (but cause stronger NCEs) than less dangerous predators. Thus, predation rate is often not a good measure of predation risk, and therefore not always a good indicator of total effect on prey (CEs + NCEs). Perceived risk, however, is not always proportional to actual risk. Perceived risk depends on not just the type of predator and its attack success, but also on the type and strength of predator cues or prey alarm cues (Kats and Dill, 1998; Stankowich and Blumstein, 2005; Ferrari et al., 2010), on the habitat per se (Verdolin, 2006; Thaker et al., 2011) and on prey sensory/cognitive capacities (Kats and Dill, 1998; Ferrari et al., 2010; Bedoya‐Perez et al., 2019). Predators that are not very dangerous, but difficult to locate and assess (e.g. ambush predators) can induce strong anti‐predator responses and thus strong NCEs (Sih, 1992; Preisser et al., 2007). Prey may even respond to an organism that is incapable of killing them if the cues are sufficiently close to those of a dangerous enemy (Fill et al., 2012). Box 3 discusses how prey perceive risk in more detail and implications for enemy‐risk effects and biocontrol.
LITERATURE REVIEW OF BIOLOGICAL CONTROL ENEMY‐RISK EFFECT STUDIES
We carried out a systematic review of empirical studies on enemy‐risk effects in biocontrol systems using combinations of the search terms “biological control”, “biocontrol”, and “pest” with the terms “non‐consumptive”, “nonconsumptive”, “non‐lethal”, “nonlethal”, “sub‐lethal”, “sublethal”, “risk effect*”, “anti‐predator”, or “anti‐predator.” Studies were included if they were on arthropod pests, investigated some stage of the enemy‐risk effect pathway depicted in Fig. 1, and demonstrated some relevance to pest control. Our review of the literature yields several takeaway messages: (1) enemy‐risk effects are prevalent in arthropod pest systems, (2) enemy‐induced trait shifts can interact with other aspects of agroecosystems, such as plant defences, trap crops and plant pathogen transmission, (3) risk effects produced by predators have been studied more extensively than those produced by parasitoids, (4) the importance of enemy‐risk effects on non‐target species has received little attention and (5) few studies have examined the consequences of enemy‐risk effects for plant damage in the field.
Figure 1.
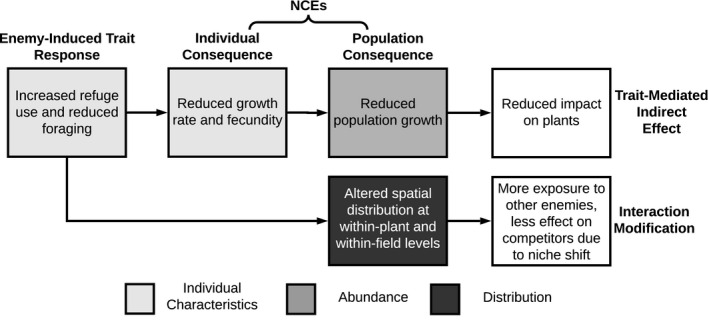
Demonstration of a particular enemy‐risk effect fitting in to the broader framework we describe in Box 2. An enemy‐risk effect is described by both the stage, beginning with individual response and ending with community effects, as well as by the effects on the abundance, distribution and characteristics of a pest population.
We organised papers in Table 1 according to the ‘level’ of study, ranging from documentation of enemy‐induced trait responses to explicit measure of NCEs on pest control and trait‐mediated indirect effects on crops (see Supporting Information for expanded table format). This categorisation is not meant to rank the quality or usefulness of studies, but rather to demonstrate where research has been focused and where room for growth remains. Fifty‐four per cent of studies (32 of 59) aimed to assess the strength of pest responses, which is a critical step in the inclusion of enemy‐risk effects in the design and implementation of biocontrol programs. Many of these studies incorporated other aspects relevant to pest management, such as variation in spatial scale (Lee et al., 2014), ability to transmit plant pathogens (Tholt et al., 2018), interactions with trap cropping (Lee et al., 2011) and plant defence (Thaler et al., 2014). Of the 27 remaining studies, about half documented demographic consequences for pests, and half documented the levels of pest damage. Four studies measured changes in plant damage in the field (Griffin and Thaler, 2006; Thaler and Griffin, 2008; Steffan and Snyder, 2010; Hermann and Thaler, 2018). Only two studies measured the risk effects of enemies on non‐target species (Walzer and Schausberger, 2009; Fill et al., 2012); these effects are likely overlooked in many evaluations of host range, as we discuss in the following section.
TABLE 1.
Table of biocontrol enemy‐risk effect studies, organised according to the level of study
| ‘Highest’ level of study | Other aspects | Citation |
|---|---|---|
| Behavioural/Physiological/Morphological Response (32) | None (8) | Angelon and Petranka, (2002), Silberbush et al. (2010), Silberbush and Blaustein, (2011), Warburg et al. (2011), Thaler et al. (2012), Fischhoff et al. (2018), Dupuy and Ramirez, (2019), La‐Spina et al. (2019) |
| Variation among agents (8) | Pallini et al. (1999), Wuellner et al. (2002), Ramirez et al. (2010), Hoki et al. (2014), Otsuki and Yano, (2014b), Dias et al. (2016), Jacobsen et al. (2016), Staats et al. (2016) | |
| Variation among pests (1) | Wilson and Leather, (2012) | |
| Variation among agents and pests (3) | Nelson and Rosenheim, (2006), Ingerslew and Finke, (2017), Francesena et al.(2019) | |
| Interaction with competition (1) | Stav et al. (2010) | |
| Variation of cues (2) | Ninkovic et al. (2013), Hermann and Thaler, (2014) | |
| Variation among agents, pests, cues (1) | Roberts, (2014) | |
| Interaction with resources (2) | Wasserberg et al. (2013), Silberbush et al. (2014) | |
| Interaction with plant defence (1) | Thaler et al. (2014) | |
| Variation of spatial scales (1) | Lee et al. (2014) | |
| Interaction with trap cropping, variation among agents (1) | Lee et al. (2011) | |
| Variation in plant variety (1) | Cuny et al. (2019) | |
| Ability to transmit plant pathogen (1) | Tholt et al. (2018) | |
| Indirect effects on other pest (1) | Prasad et al. (2018) | |
| Individual Fitness Consequences (3) | None (1) | Matsumoto et al. (2003) |
| Variation in agents and cues (1) | Gyuris et al. (2017) | |
| Effects of enemy on survival while infected with pathogen (1) | Ugine and Thaler, (2020) | |
| Demographic Consequences (11) | None (2) | Nelson, (2007), Xiong et al. (2015) |
| Interaction with temperature (1) | Bannerman et al. (2011) | |
| Variation among agents (1) | Folgarait and Gilber, (1999) | |
| Variation among agents and pests (1) | Weisser et al. (1999) | |
| Variation of NCE pathways (1) | Fievet et al. (2008) | |
| Interaction with plant defence (2) | Kaplan and Thaler, (2012), Kersch‐Becker and Thaler, (2015) | |
| Non‐target effects (1) | Fill et al. (2012) | |
| Multiple‐enemy effects (1) | Bilu and Coll, (2007) | |
| Effects driven by commensal species (1) | Jensen and Toft, (2020) | |
| Plant Damage (13) | None (4) | Snyder and Wise, (2000), Maanak et al. (2013), Jandricic et al. (2016), Rendon et al. (2016) |
| Variation among agents (2) | Hlivko and Rypstra, (2003), Hogg et al. (2014) | |
| Variation among pests and agents (1) | Rypstra and Buddle, (2012) | |
| Interaction with plant defence (1) | Kaplan and Thaler, (2010) | |
| Non‐target effects, variation among agents (1) | Walzer and Schausberger, (2009) | |
| In field (4) | Griffin and Thaler, (2006), Thaler and Griffin, (2008), Steffan and Snyder, (2010), Hermann and Thaler, (2018) |
It can be difficult to scale up enemy‐risk effect studies from measuring pest responses to the measures of biocontrol efficacy, including effects on pest population dynamics or crop yield, as these typically require longer timescales and broader spatial scales (Hermann and Landis, 2017). However, when moving from pest–agent interactions to the harvest and sale of a crop, there are many steps where the enemy‐risk effects may attenuate (Hamburg and Hassell, 1984; Godfray and Waage, 1991; Collier and Van Steenwyk, 2004; Kaplan et al., 2014). Additionally, there may be many interacting effects on pests and crop yield, ranging from environmental factors to pesticide applications. Due to these complications, enemy‐risk effect studies that do not measure outcomes beyond pest responses may not fully capture the relevance of enemy‐risk effects in pest management.
Some of the most fruitful areas for further research include (1) separating NCEs and CEs to improve predictions of pest population dynamics (see Box 4), (2) considering enemy‐risk effects that include qualitative shifts, such as spatiotemporal location, and how they interact with agricultural practices in ways that differentiate them from CEs, (3) including enemy‐risk effects in assessment of agent efficacy and non‐target impacts, (4) expanding taxonomic breadth to include more parasitoids and (5) expanding scales of study to better understand the impacts on crop production. We believe ongoing empirical work would be well served by incorporating theory from the broader study of enemy‐risk effects, which would facilitate predictions about when and where risk effects may play an important role in the efficacy of pest management programs.
Box 4. Consequences of NCEs vs. CEs for prey and predator population dynamics.
A central difference between CEs and NCEs is their consequences for natural enemy reproduction (Abram et al., 2019). CEs, on the one hand, generally lead to an increase in natural enemy birth rates: an immature parasitoid develops to the reproductive adult stage by attacking and killing a host, and a predator survives and reproduces by consuming prey. NCEs, on the other hand, do not result in any increase in the natural enemy’s population, and if they reduce the victim population through increased mortality or decreased fecundity, they actually shrink the resource pool available to the natural enemy. A natural enemy that induced NCEs only would eventually go extinct, as it would never be able to reproduce. It is worth noting that a generalist enemy may impose strictly NCEs on some of its prey taxa, as long as it is able to consume other species of prey or engage in omnivory. If NCEs are not explicitly accounted for, a gap between high pest mortality and low enemy reproduction may be erroneously attributed to other causes, such as poor assimilation efficiency or natural enemy mortality.
CEs and NCEs may also vary in how their overall magnitudes at the population level are influenced by predator density. A large decrease in the number of predators may lead to a large decrease in consumption of prey, but the small number of predators may still be enough to induce significant NCEs (Carpenter et al., 1987). The strength of NCEs can also be linked to CEs, creating potential feedbacks between the two effect pathways (Weissburg and Beauvais, 2015). Understanding the perception of risk and thresholds prey use to make decisions can help determine how NCE strength may vary with enemy population compared to CE strength (see Box 3 for a more thorough discussion of prey perception and risk management).
The inclusion of enemy‐risk effects in models has varying effects on the resulting dynamics, ranging from increased to decreased stability, the appearance of population cycles and even the reversal of predicted trophic cascades (Abrams and Matsuda, 1997; Abrams, 2008; Peckarsky et al., 2008; Larsen, 2012). The classic example of predator–prey dynamics involving lynx and snowshoe hares has discrepancies between observed data and CE‐only predictions, but the inclusion of enemy‐risk effects can help improve the match between prediction and observation (Hik, 1995; Boonstra et al., 1998). The relative contributions of NCEs and CEs to population dynamics can vary with environmental factors and the spatiotemporal scales of study, so these interactions must be accounted for if possible. Considering enemy‐risk effects in population dynamics is not simply the addition of ecological complexity for its own sake, but a way to improve predictions of population modelling.
ENEMY‐RISK EFFECTS AND THE EVALUATION OF BIOLOGICAL CONTROL AGENTS
A primary task of biocontrol researchers is evaluating the impact of biological control agents on target and non‐target organisms. Evaluations occur during each stage of a biocontrol project, whether the program is classical, augmentative or conservation biocontrol. First, the initial step in most biocontrol programs is to describe, as quantitatively as possible, the natural enemy community associated with a target pest; for invasive species, this may involve describing food webs in both the native and invaded ranges. Second, as part of classical biological control programs, and in some cases augmentative biological control, candidate agents need to be screened for host/prey specificity to assess the risks of non‐target impacts and to identify the most promising agent(s) for mass‐rearing and release. Finally, after classical biocontrol agents are released and established, it is important to evaluate efficacy, including effects on targets and non‐targets. The methods used in each of these stages of assessment are overlapping, and different methods can be complementary (Barratt et al., 2010; Furlong, 2015; Macfadyen et al., 2015; Van Driesche, 2016; Lövei and Ferrante, 2017). However, as shown in Table 2, many methods, especially increasingly popular sequencing‐based methods, capture only consumptive effects, and either partially or completely fail to record enemy‐risk effects. Although a narrower focus on CEs is compatible with efforts to describe trophic webs, methods that reflect both CEs and enemy‐risk effects will provide better efficacy assessments and measures of non‐target effects. As noted in the literature review section, there are relatively few documented cases of enemy‐risk effects on non‐target species, but this likely reflects a failure to investigate them. The increasing prevalence of methods like immunoassays and sequencing‐based approaches to detect direct parasitism or consumption may only exacerbate this lack of documentation. Because there is no guarantee that the magnitudes of CEs are strongly correlated with the magnitudes of risk effects, both must be included in evaluation methods, if not measured separately.
TABLE 2.
Methods used, either singly or in combination, to evaluate the impact of biological control agents on target and non‐target organisms
| Method | Useful for predators, parasitoids or both | Measures consumptive effects? | Measures non‐consumptive effects? |
|---|---|---|---|
| Artificial sentinel prey models (e.g. clay caterpillars) evaluated for removal or marks of attack | Mostly Predators | ✓ | ✗ |
| Live tethered or outplanted sentinel prey/hosts (usually immobile stages, like eggs or pupae; but also confined larval stages) | Both | ✓ | ✗ |
| Post hoc assessment of natural enemy impact via detection of bite‐marks or other physical damage to prey | Predators | ✓ | ✗ |
| Post hoc assessment of natural enemy impact via detection of distinctive host remains, host‐feeding tubes or damage, remains of developing parasitoids (egg chorions, larval or pupal exuvia, meconia, cocoons), or distinctive parasitoid or host emergence holes | Parasitoids | ✓ | ✗ |
| Dissection of hosts to record parasitoid eggs, larvae or pupae; or rearing of hosts | Parasitoids | ✓ | ✗ |
| Monoclonal antibody‐ELISA or DNA‐based assays of hosts to detect internally developing parasitoids | Parasitoids | ✓ | ✗ |
| Gut content analyses – detection of prey remains using simple dissections and visual inspection | Predators | ✓ | ✗ |
| Monoclonal antibody‐ELISA, immunomarking or DNA‐based assays of consumer gut contents | Predators and host‐feeding parasitoids | ✓ | ✗ |
| Focal observations of prey/hosts, using human observers or video cameras | Both | ✓ | Partially* |
| Field life table construction by repeated sampling of a cohort of developing hosts/prey to quantify survival and rate of development from eggs to adults; often used with immobile hosts/prey | Both | ✓ | Partially† |
| Short‐term (i.e. too short for prey reproduction) mesocosm assays using hand removal or caging treatments to contrast the effects of natural enemy presence/absence; response variable = prey survival | both | ✓ | Partially† |
| Long‐term (i.e. long enough to permit substantial prey reproduction) mesocosm assays using hand removal or caging treatments to contrast the effects of natural enemy presence/absence; response variable = prey population size or growth rate | both | ✓ | ✓ |
| Experimental removal of natural enemy populations using selective insecticides; response variable = prey/host population size or growth rate | both | ✓ | ✓ |
| Experimental addition of natural enemy populations by controlling ants that otherwise exclude the nature enemy; response variable = prey/host population size or growth rate | both | ✓ | ✓ |
| Observational field methods comparing natural enemy present vs. absent (e.g. in classical biocontrol settings: pre‐ vs. post‐release, or release site vs. non‐release site); response variable = prey/host population size or growth rate‡ | both | ✓ | ✓ |
Focal observations might reveal some NCEs related to the expression of anti‐predator behaviours, although would be unlikely to quantify the costs of such behaviours.
This method could capture the costs of some NCEs if those costs were expressed through a reduction in developmental survival rates.
Purely correlative studies examining associations between densities of predators and prey or hosts and parasitoids are also sometimes reported. But, without additional evidence of a causal link (and support for the direction of causality) such studies are often open to multiple interpretations. Thus, we omit them from the current discussion.
Projecting the non‐target impacts of a candidate classical biological control agent is quite challenging. It is difficult to canvas what is often a very broad array of possible non‐target species, each of which needs to be brought into quarantine, reared and tested for vulnerability to attack. Furthermore, it is increasingly acknowledged that not just direct impacts, but also possible indirect effects of an introduction should be assessed, including the potential for competition, IGP and apparent competition effects (Hajek et al., 2016; Heimpel and Mills, 2017). To this already imposing prospect, we add that it may be important to consider enemy‐risk effects. In some cases, even the simple in‐quarantine host‐range testing protocols using small and simplified microcosms and short exposures to natural enemies can reveal some evidence of enemy‐risk effects. For example, host‐range tests of candidate parasitoid species may reveal elevated mortality of individual hosts that do not produce parasitoid offspring (e.g. Abram et al., 2016; Bulgarella et al., 2017); in these cases, hosts may die following parasitoid probes without oviposition, or parasitoid oviposition may lead to early mortality of both the host and the parasitoid eggs prior to any consumption of the host. In some cases, such parasitoid‐generated host mortality has been found in host species on which parasitoids never successfully produce offspring (Hoddle and Pandey, 2014; Valente et al., 2017), emphasising that parasitism rates alone may not suffice to capture non‐target effects (Abram et al., 2019). Depending on response variables measured in target or non‐target hosts or prey, including altered movement or microhabitat selection, development rates, feeding behaviour or reproduction, other risk effects could potentially also be detected in a quarantine setting, but current host‐range testing generally sidesteps the possible importance of these effects. Like indirect effects, however, many possible enemy‐risk effects, including those expressed via longer range movements, are not readily evaluated within a quarantine facility.
More encouragingly, many widely used protocols for assessing the efficacy of biological control measure either target (or non‐target) population density as the primary response variables (Table 2), thereby capturing the combined influences of CEs and NCEs. This is particularly true in studies of conservation biocontrol, which also frequently incorporate larger spatiotemporal scales and whole communities of enemies. Although it may sometimes be of academic interest to separate the roles of CEs and NCEs (but see Box 4), these protocols accomplish the central objective of capturing the full range of pathways through which natural enemies may contribute to herbivore population suppression.
ECO‐EVOLUTIONARY EXPERIENCE AND RESPONSES TO BIOLOGICAL CONTROL AGENTS
Recent research on prey responses to exotic enemies emphasises the importance of prey’s eco‐evolutionary experience (EEE) with enemies (Blumstein, 2006; Cox and Lima, 2006; Sih et al., 2010; Saul and Jeschke, 2015; Trimmer et al., 2017; Carthey and Blumstein, 2018; Ehlman et al., 2019). By ‘eco‐evolutionary experience’ we mean either an evolutionary history, or an earlier ecological (developmental) history that allowed prey to either evolve or learn to cope with a predator. Naïve prey that lack previous experience with a novel predator often respond insufficiently, suffering heavy predation (high CEs). Examples include the devastating impacts of novel predators (humans, other mammals, brown tree snakes) on naïve island prey, or of novel predatory fish on naïve prey in previously fishless lakes (Cox and Lima, 2006). For classical biological control, the expectation is that if the target pest has had an extensive evolutionary history with the imported enemy, it will likely exhibit adaptive responses (and thus NCEs) that reduce CEs. In contrast, non‐target prey that have not had previous EEE with the biocontrol agent might exhibit much weaker, if any, anti‐predator response. If the predator can attack these non‐target prey, then the biocontrol agent might prefer and exert strong CEs on non‐target prey and less consumptive impact on the targeted pest.
Some naïve prey, however, exhibit appropriate responses to novel predators. One key factor is the prey’s past history not with the specific novel predator, but with predation pressure in general. Prey that have experienced little predation pressure of any sort tend to be bolder and thus exhibit weaker response to novel predators, as compared to those that have evolved with moderate to heavy predation pressure (Ferrari et al., 2015). Therefore, non‐target prey should be particularly vulnerable to novel biocontrol agents if those prey species have evolved with little predation (demonstrated by novel invasive social insects in Hawaii; Wilson et al., 2009; Krushelnycky et al., 2017). Recent work adds that if prey have experienced persistent high predation risk, then they should also be bold, not cautious. If predators are persistently present, prey cannot hide indefinitely, and should only respond strongly to cues that indicate particularly high impending risk (Trimmer et al., 2017; Ehlman et al., 2019). Additionally, though we focus on arthropod pests, work on invasive plants suggests that invasive species facing no top‐down pressure may evolve to devote fewer resources to anti‐enemy responses and more to competitive ability (Blossey and Notzold, 1995). This process may be rapidly reversed upon the reintroduction of natural enemies through biological control programs, with invasive species rapidly developing anti‐enemy responses that could drastically change the initial CE–NCE ratio (Stastny and Sargent, 2017).
Another key factor in predicting prey response to an introduced biocontrol agent is its similarity to familiar, native predators. Even if non‐target prey have never experienced the particular novel predator, the ‘cue similarity’ hypothesis posits that if the introduced predator resembles familiar predators, ‘naïve’ prey are likely to respond (Sih et al., 2010; Saul and Jeschke, 2015). Understanding the sensory/cognitive ecology of how target versus non‐target prey perceive risk from biocontrol agents is then key (see Box 3). Even if prey correctly perceive the risk and respond, they can still suffer heavy predation if they show an inappropriate response (e.g. freeze when they should flee) or if their response is ineffective (e.g. they flee but the predator is too fast; Sih et al., 2010; Carthey and Blumstein, 2018). Sih et al. (2010) suggested that the effectiveness of naïve prey responses to novel predators should depend on the functional ‘attack mode’ similarity of novel and familiar predators, and on whether prey rely on generalised responses (that work well against a broad range of predators) or specialised ones (that work very well, but only with specific predators). If the novel predator exhibits cue similarity but attack mode dissimilarity to familiar predators, it might induce both strong but ineffective responses that result in high CEs and high NCEs. This scenario could be ideal for suppressing target prey, but disastrous if it applies to non‐target prey.
Box 3. Prey perception of risk.
A large literature in behavioural and sensory ecology has examined prey perception of danger based on cues that provide information on the levels of enemy risk (Weissburg et al., 2014; Ehlman et al., 2019). Arthropods perceive risk using chemical (both airborne and via direct contact; Dicke and Grostal, 2001; Sitvarin and Rypstra, 2012; Hermann and Thaler, 2014), visual (Gonçalves‐Souza et al., 2008), vibratory (Castellanos and Barbosa, 2006), auditory (Skals, 2005) and tactile cues (Castellanos et al., 2011; Okada and Akamine, 2012). Organisms often use multiple cue modalities, which can vary depending on prey perceptual ability and the types of enemies.
A primary source of risk cues is the enemy itself, whether directly as sounds, vibrations, chemical cues or visual presence, or indirectly as chemical footsteps, faeces, molts and silk. Organisms can also respond to indicators of risk before they actually detect enemies; for example by responding to ‘alarm cues’ associated with other prey being attacked, injured or killed (Schoeppner and Relyea, 2005; Vandermoten et al., 2012). Alarm cues can induce a range of responses and can even be shared across species (Agarwala et al., 2003; Goodale and Nieh, 2012). Another cue may be habitat or microhabitat type. If certain habitat types are associated with enemy risk, then risk avoidance may drive habitat selection, regardless of direct cues from enemies or even conspecifics (Lucas et al., 2000).
Cues can vary widely in spatiotemporal extent, affecting different numbers of prey over varying timescales. For example, because chemical cues can spread widely and remain detectable for long periods, they can cause risk effects to persist long after enemies have left an area (Wilson and Leather, 2012; Ninkovic et al., 2013). Theory suggests that because the cost of under‐responding to risk (i.e. getting killed) is often much greater than the cost of over‐responding (e.g. hiding unnecessarily and losing feeding opportunities), when cues provide imprecise information about the presence (versus absence) of predators, this uncertainty can induce strong enemy‐risk effects even when predators are only occasionally present (Sih, 1992). This may be true for many prey facing the risk of attack by ambush predators. In contrast, seeing or coming into physical contact with an enemy is usually a more definitive risk indicator.
The links between cue generation, detection and anti‐enemy response are complex, involving multiple steps and interactions. Environmental context can strongly affect both the strength and detection of a cue (e.g. wind may disperse a chemical cue) and the perception of risk upon detection (e.g. perceived risk may be lower if a refuge is nearby). Response to risk can be highly state dependent; a starving organism may be more likely to accept higher risk to avoid starvation, and a larger, faster individual may assess risk differently than a smaller, more vulnerable organism. In some cases, it can take a combination of multiple cues to trigger a response (Gish et al., 2011). Recent theoretical work has suggested that cues indicating risk should be integrated with other cues indicating safety to shape responses (Trimmer et al., 2017; Ehlman et al., 2019), and supporting evidence has emerged from recent studies with desert isopods (Zaguri and Hawlena, 2019).
A key insight from signal detection theory is that all cues are imperfect indicators. Cues can vary in strength; a chemical cue can be diluted or concentrated, a visual cue can be obscured by other objects and an auditory cue can be disrupted by ambient sounds. On top of variance in cue strength, the specificity of cue modalities can vary. The visual cue of a looming shape could come from a dangerous enemy or a harmless passing organism, the chemical and tactile cue of a parasitoid could come from a species that parasitises the pest or another, closely related parasitoid that does not (Fill et al., 2012), and cues that elicit stress and reduce population growth can come from activity of commensal organisms (Jensen and Toft, 2020). The reliability of cues may change with the introduction of novel organisms (Ehlman et al., 2019) or through habituation to the cue. The consistent application of synthetic alarm pheromone may cause decreased sensitivity of aphids to the cue, but this insensitivity may in turn increase CEs by coccinellid predators (de Vos et al., 2010). Finally, synthetic predator kairomones can increase mosquito mortality synergistically with Bacillus thuringiensis applications, even when completely decoupled from real predators (Op de Beeck et al., 2016; Delnat et al., 2020). Biocontrol practices might benefit from deeper understanding of pest perception of cues associated with enemy risk.
Marginal costs of enemy‐induced trait responses are higher (and prey exhibit weaker trait responses) if prey are energy stressed (hungry), resources or mates are abundant but more accessible only if prey show little anti‐predator response, or if prey have high reproductive value (more to lose; Houston et al., 1999; Clark, 1994). For herbivores, the strength of the enemy‐risk effect depends on, among other things, plant abundance and quality, herbivore condition and life‐history stage (McArthur et al., 2012; Stephan et al., 2017).
The role of enemy‐risk effects in community dynamics becomes more complex when we consider multiple enemies and IGP, common occurrences in biocontrol systems. With multiple agents of mortality, enemy‐risk effects can often blend into CEs where a trait response to an enemy (e.g. a shift in microhabitat use) increases mortality from another enemy (Sih et al., 1998), environmental stressors (Schmitz et al., 1997) or even pesticides (Janssens and Stoks, 2013). With IGP, predators are also potentially prey, and thus also exhibit enemy‐induced trait responses and NCEs. The mix of CEs and NCEs involving multiple species then influences community outcomes including biocontrol efficacy. We discuss this in more detail in a later section.
Many of these predictions about enemy‐risk effects assume that prey exhibit adaptive responses to enemies that they have coevolved with. Prey lacking evolutionary (or developmental) history with enemies (or specific enemies) often exhibit much weaker anti‐enemy responses and thus suffer heavy mortality (strong CEs) when novel enemies appear (e.g. island prey or prey in fishless ponds; Cox and Lima, 2006; Carthey and Blumstein, 2018). This depends on the cue or functional similarity of new enemies to the prey’s familiar enemies (Sih et al., 2010; Carthey and Banks, 2014; Saul and Jeschke, 2015). Given that biocontrol often involves introducing enemies that have a co‐evolutionary history with the target pest, but not with non‐target organisms, the effect of evolutionary history on CEs versus NCEs is clearly a salient issue that we discuss in more detail below.
A community‐level prediction is that prey should be more likely to respond well to a novel predator if the prey have EEE with a greater diversity of predator archetypes (Blumstein, 2006; Cox and Lima, 2006; Ehlman et al., 2019). If prey have EEE with only one main type of predator, they might exhibit predator‐specific defences. In contrast, if either target or non‐target prey have EEE with a broad range of predators, they should be more likely to exhibit a diversity of specialised and generalised defences that could be effective against novel biocontrol agents.
Finally, it is possible that contemporary evolution could occur during a long‐term biocontrol relationship. While there are examples of evolved resistance to parasitism through enhanced immune responses (Berberet et al., 2003), we know of no cases where arthropod pests evolve anti‐enemy responses to biocontrol agents. Hufbauer and Roderick (2005) thoroughly reviewed microevolution in biocontrol, which may provide insights along with those gleaned from evolution of prey responses to invasive predators. Studying this directly in biocontrol systems would require measuring enemy‐risk effects over long timescales, which could become a routine part of long‐term efficacy studies.
SPATIOTEMPORAL ASPECTS OF ENEMY‐RISK EFFECTS
Enemy‐risk effects and direct consumptive effects frequently occur on different spatiotemporal scales, with many risk effects occurring over larger areas and longer times than CEs. This means that many studies focusing on CEs lack the scale necessary to capture enemy‐risk effects, a topic that has been reviewed elsewhere (Hermann and Landis, 2017) and covered with respect to biological control in Table 2. Beyond expanding the scales of biocontrol enemy‐risk effect research in the future, current theory and evidence from the broader literature may help biocontrol practitioners conceptualise and predict how enemy risk affects pest abundance and interactions with other pest management measures in time and space.
Just as pests act within a ‘landscape of fear’ shaped by enemy cues that are heterogeneous through time and space (Laundré et al., 2001), agricultural landscapes exhibit spatiotemporal variability across multiple scales. Agroecosystems are spatially heterogeneous at the within‐plant, between‐plant, within‐field and between‐field scales, especially when farmers use practices such as intercropping or planting hedgerows. They also change throughout time, as many crops undergo a relatively predictable growth pattern, changing in vulnerability to various pests and in their spatial structure. Farmers apply pesticides, irrigate and harvest crops according to schedules, creating temporal patterns of disturbance. By superimposing the temporally variable landscape of risk and the temporally variable agricultural landscape, we may be able to integrate enemy‐risk effects into predictions on interactions between biocontrol agents and other IPM strategies. We outline specific ways in which enemy‐risk effects in space and time may interact with agricultural practices in the following sections.
Enemy‐Risk Effects in Space
At smaller spatial scales, enemy risk may alter microhabitat use as pests seek refuges or move to lower quality parts of the plant (Lee et al., 2011; Paterson et al., 2013; Calvet et al., 2018). Pest fitness may be affected by decreased foraging time due to refuge use or consistent foraging on lower quality resources. Some pests, particularly aphids, will drop off a plant in response to enemy risk (Humphreys and Ruxton, 2019). This behaviour incurs significant costs, as dropping reduces feeding time (Nelson and Rosenheim, 2006; Nelson, 2007). It may also expose pests to a new set of mortality sources, such as ground‐dwelling enemies or increased exposure to extreme temperatures. Conversely, increased refuge use due to enemy risk may decrease pesticide exposure (Jallow and Hoy, 2005; Martini et al., 2012). Additionally, shifts in microhabitat use by pests may reduce the reliability of field sampling methods based on the inspections of certain parts of the plant (Southwood and Henderson, 2000).
At larger spatial scales, enemies may influence pest dispersal and habitat selection at within‐field and between‐field scales. Foraging models, such as the Ideal Free Distribution (IFD), are often used to predict pest movement and abundance within a patchy habitat, but the inclusion of mobile enemies and prey perception of enemies can drastically alter those predictions (Sih et al., 1998; Brown and Kotler, 2004; Fraker and Luttbeg, 2012). Natural enemies can change the threshold at which pests disperse, either increasing dispersal by making patches riskier, or decreasing dispersal by making the movement between patches riskier (Sih and Wooster, 1994; Hammill et al., 2015). Modelling work has shown that this can lead to seemingly counterintuitive results at the metapopulation level; if prey immigration is not affected by enemy presence, but emigration is reduced by it, then prey density can be higher in patches with enemies. Whether or not natural enemy distributions match the distributions of their prey can depend on mobility of the pests and enemies, the resource needs of each and other density‐dependent effects for each population (Winder et al., 2001; Nachman, 2006; Pearce and Zalucki, 2006). In general, understanding how natural enemies affect spatial patterns of pest abundance, such as higher density near field borders, may allow for more precise pest sampling and pesticide spraying, increasing the efficacy and cost effectiveness of these methods. Boxes 5, 6 and 7 all describe particular cases in which enemy‐induced dispersal aids or hinders specific pest management goals, including disease transmission, pesticide resistance and trap cropping.
Box 5. Enemy‐risk effects and biological control of vectors of plant disease.
One of the most damaging ways that insect herbivores affect their host plants is by acting as vectors of plant pathogens. Biological control agents can clearly slow the spread of vectored pathogens by suppressing vector population densities; as both consumptive and non‐consumptive effects can depress population growth rates of insect vector populations, both can contribute to this ecosystem service (Landis and Van der Werf, 1997; Moore et al., 2009; Finke, 2012; Long and Finke, 2015; Clark et al., 2019).
However, it is now widely recognised that enemy‐risk effects may also have a somewhat counterintuitive and unhelpful influence on the epidemiology of insect‐vectored pathogens: in some cases, anti‐enemy behaviours may involve increased movement of insect vectors on both local and regional scales, accelerating disease transmission. Thus, the net effect of biological control on disease prevalence can be negative, neutral or positive, depending on the relative magnitudes of consumptive effects and enemy‐risk effects and the details of the interactions (Finke, 2012; Crowder et al., 2019). The empirical record has shown that outcomes can depend on the identity of the biocontrol agents, the herbivore and the pathogen (Nelson and Rosenheim, 2006; Belliure et al., 2011; Dumont et al., 2015; Clark et al., 2019); in particular, predator–prey interactions that result in strong prey dispersal in response to predation risk or actual predator attacks often result in short‐term increases in disease transmission any time pathogen acquisition and transmission by the vector is not interrupted by the decision to leave a feeding site.
The empirical literature shows that a widespread response of insect vectors of plant disease to predator presence and, especially actual predator attacks is to move away from the attack site via local movements (Weber et al., 2006; Belliure et al., 2011; Hodge et al., 2011; Dáder et al., 2012; Long and Finke, 2015). Aphids, which vector more than half of all plant viruses, release alarm pheromones when attacked by predators, causing clone‐mates to run away or, in some cases, to drop from the host plant (Vandermoten et al., 2012). Especially in cases where disease transmission requires rapid movement between two host plants (common for viruses that are transmitted via transient contamination of aphid mouthparts), this can accelerate disease transmission.
Predators can also shape longer distance movements via two potentially offsetting processes. First, many herbivores show density‐dependent induction of winged morphs or other forms of density‐dependent dispersal (Denno and Peterson, 1995; Pepi et al., 2016); in this case, suppression of vector population densities via consumptive or non‐consumptive effects has the potential to slow disease spread (Michaud and Belliure, 2001). Second, however, many herbivores also induce winged forms in response to detection of predator cues, including, for aphids, alarm pheromones (Weisser et al., 1999; Mondor et al., 2005; Vandermoten et al., 2012), potentially leading to substantial increases in potential for disease transmission over larger spatial scales. Although experimental studies have demonstrated the potential for both of these effects, how this plays out in nature is unknown.
The preponderance of evidence from experimental studies supports the hypothesis that natural enemies accelerate disease transmission in crop plant populations (Long and Finke, 2015). However, because most published studies are quite short duration, they can reveal the immediate effects of increased vector movement, but may underestimate the importance of vector population suppression, which often requires multiple generations of predator–herbivore interactions. Also, because most studies have been performed in laboratory or greenhouse settings, the importance of predators as elicitors of vector movement may be exaggerated relative to its true effect in the field, where many other factors can trigger the same trivial movements (e.g. effects of wind, mechanical disturbances and contacts with other herbivores; Bailey et al., 1995; Nelson and Rosenheim, 2006). Nevertheless, it is clear that biological control can be a double‐edged sword when directed against disease vectors.
Box 6. Enemy‐risk effects, between‐plant movement and insecticide resistance management.
Predator‐induced between‐plant movement by herbivores can disrupt schemes that are intended to delay the evolution of resistance to insecticides. A significant recent change in agricultural pest management has been the introduction of crop plants genetically engineered to produce their own insecticidal proteins, derived from the bacterium Bacillus thuringiensis (‘Bt’; Tab ashnik et al., 2013). Although Bt crops can reduce the need for widespread applications of insecticides, planting a crop that constitutively produces an insecticidal toxin is a recipe for rapid evolution of resistance. To reduce this risk, evolutionary biologists working with regulators and seed companies designed and implemented the ‘high dose, refuge’ strategy of resistance management. Assuming a monogenic basis for resistance with susceptible allele S and resistance‐conferring allele R, a ‘high dose’ means that both susceptible homozygotes (genotype SS) and heterozygotes (RS) are killed on Bt plants. Only the rare resistant homozygotes (RR) can survive. The ‘refuge’ refers to a planted block of non‐Bt plants, which are expected to produce relatively large numbers of SS individuals. The rare RR homozygotes surviving on Bt plants are then expected to mate with one of the abundant SS individuals developing in the refuge, and the offspring (genotype RS) are subsequently killed on the Bt crops, removing R alleles from the population. In this way, the models suggest, resistance can be dramatically delayed (Tab ashnik et al., 2013).
A key problem, however, has been farmer compliance with planting the block of non‐Bt refuge plants (Carroll et al., 2012; Garcia et al., 2016). In response to this, seed companies have introduced the notion of a ‘refuge in a bag’: planting seed is sold as a mixture of Bt and non‐Bt seed, which generates a field with spatially interspersed Bt and non‐Bt plants. This approach is now being adopted on a global scale (Tab ashnik et al., 2013; Carrière et al., 2016). But if pests move frequently between plants in response to unsuccessful predator attacks, two problems are introduced (Mallet and Porter, 1992; Carroll et al., 2012; Carrière et al., 2016). First, the efficacy of the refuge may be eroded. The refuge in a bag idea relies on the expectation that individual non‐Bt plants, surrounded by Bt plants, can still support the development of SS individuals. If, however, SS individuals move between plants, individuals beginning their development on a non‐Bt refuge plant may move to a Bt plant and be killed (Head et al., 2014). Second, the efficacy of the high dose may be eroded. RS heterozygotes, which must be killed under the high‐dose strategy, can survive, favouring a rapid increase in R allele frequency, in either of two ways. First, herbivores may begin their lives on a non‐Bt plant, where the highly vulnerable early developmental instars can be passed safely, and then move to Bt plants as later instar larvae, which are often more tolerant of Bt toxins, allowing RS individuals to survive (e.g. Head et al., 2014). Second, young RS individuals who start their feeding on a Bt plant may be exposed to toxins, but if they move to non‐Bt plants before they ingest a lethal dose they may survive. Thus, enemy‐risk effects of predators that cause increases in herbivore movement, even on the very small spatial scale required to move between adjacent plants, can have major effects on the evolutionary trajectory of pest populations.
Box 7. NCEs, trap crops and push‐pull systems.
Enemy‐induced dispersal can create large‐scale shifts in spatiotemporal pest distribution, a phenomenon that may be put to use to improve pest management programs. For example, enemies that induce stable, predictable spatiotemporal pest patterns may allow for more precisely targeted pesticide applications. Another potential route is to use enemy‐induced dispersal in tandem with trap cropping or push‐pull systems. Trap cropping is the use of highly attractive ‘trap’ plants to lure pests out of the main crop, whereas push–pull systems add a repellent ‘push’ intercrop to the ‘pull’ trap crop (Cook et al., 2007). Enemies may be utilised as a second ‘push’, driving pests out of the main crop and into the trap crop. This effect was studied by Lee et al. (2011) who demonstrated an increased level of whitefly dispersal from poinsettia into the cucumber trap crop when natural enemies were present in poinsettia. Whiteflies preferred settling in cucumber over poinsettia, but once settled in poinsettia, they did not tend to move to cucumber. Of the three natural enemies tested, only one increased whitefly dispersal into cucumber, demonstrating the importance of the specific pest and enemy pairing in this scenario.
Predictable and stable movement of pests from the main crop into the trap crop may be more likely with certain combinations of enemy, pest and plant traits. Ideally, enemies would primarily occupy the main crop, making it more dangerous than the trap crop and inducing pest dispersal into the trap crop. This could occur when enemies are habitat specialists with a strong preference for the main crop, due to plant chemical cues (Reddy, 2002), oviposition site preferences (Coll, 1996; Lundgren and Fergen, 2006) or omnivorous needs (Coll, 1996; Kopta et al., 2012). It could also occur if enemies are relatively immobile and can be released solely into the trap crop, which could be possible with inundative or inoculative biological control. Reduction of natural enemy dispersal has been a goal in other contexts, such as releasing wingless ladybirds to prevent them from leaving the focal field (Lommen et al., 2008), and it is possible that similar efforts could work at a within‐field scale as well.
Complications may arise if enemies do not primarily occupy the main crop, instead preferring the trap crop, the spaces between crops or matching pest abundance. If the enemy prefers the trap crop, it may have the opposite effect as intended, reducing pest preference for the trap crop and increasing abundance in the main crop. However, if enemies prefer the trap crop, but pests still disperse into it, the trap crop may still be effective, and enemies may then have strong effects on the pests that establish there. If enemies, perhaps ground‐dwelling predators, prefer spaces between crops, then they may increase the risk of dispersal in any direction, reducing effectiveness of the trap crop. Finally, if enemies track pest distribution, they may induce dispersal both into and out of the trap crop. This could have a range of effects, depending on the timing of dispersal, cost of dispersal and amount of trap crop. For example, if enemies track pests, forcing them to move back and forth between trap and main crops, but dispersal is very costly, the repeated dispersal may have high fitness costs for the pest. In this case, the lack of unidirectional movement into the trap crop may be more than made up for.
Just as multiple enemies may have additive, synergistic or disruptive effects on pests, so too might natural enemies and trap cropping techniques. Pest management outcomes may be optimised with a careful consideration of pest, enemy and crop combinations, necessitating more research on this topic beyond the promising existing studies.
Arthropod movement between fields is of particular interest when considering field‐scale implementation of biocontrol. Under a classical biocontrol program, where the goal is typically for an agent to disperse widely and match the pest range, enemy‐induced dispersal may not be a cause for alarm, as the enemy would be predicted to follow its prey. However, if enemy dispersal does not match pest dispersal, certain augmentative biocontrol releases may simply result in the pest problem being pushed from one farm to another. For example, flightless morphs of ladybeetles have been shown to control aphid populations more effectively due to their longer residency time in the crop (Koch, 2003). However, some ladybeetles can induce strong increases in alate production (Kaplan and Thaler, 2012) and aphid dispersal, potentially exporting the pest problem.
Finally, oviposition site selection can be strongly influenced by enemy presence. Many arthropods can detect enemies when making oviposition choices and prefer low‐risk sites (Kraus and Vonesh, 2010; Livingston et al., 2017), which may lead to heterogeneous patterns within or between fields. If natural enemies are in fields prior to oviposition, they may even completely deter pest establishment, referred to as biotic resistance (Gruner, 2005; Wanger et al., 2011). This would be more likely to occur with generalist predators, since their populations may be sustained by other species prior to the arrival of the target pest. Conservation biological control, being most focused on supporting native enemy populations, utilises biotic resistance most strongly, though any natural enemy with sufficient density prior to pest establishment may help prevent establishment.
Enemy‐Risk Effects in Time
Temporal scaling of enemy‐risk effects is complex, since pests can respond to enemies on multiple scales, and consequences of those responses can appear at multiple scales as well. Short‐term behavioural changes by pests can lead to two main categories of outcomes: there may be a long‐term fitness consequence of short‐term changes, or there may be compensation for the short‐term effect in the long‐term. Other pest responses occur only over a longer timescale, such as changes in life‐history events. The goals of a biocontrol program affect the importance of different enemy‐risk effects across time.
Short‐term behavioural responses may lead to long‐term fitness consequences. The accumulation of small fitness losses, such as reduced feeding, mating opportunities or increased energy expenditure, can lead to long‐term reductions in population growth. Short‐term reductions in feeding rate during a vulnerable life stage may also delay development, which may lead to increased pest mortality due to high CEs (Uesugi, 2015). Furthermore, if the focus of a study is solely on short‐term effects, these long‐term changes may not be measured. Similarly, if long‐term population growth is studied without looking at short‐term mechanisms, NCEs might be missed entirely, and the change in growth rate may be attributed solely to CEs (see Hermann and Landis, 2017 for a more in depth discussion of appropriate timescales).
Pests may also compensate for short‐term enemy‐induced trait responses in the long‐term, leading to no NCEs and little impact on the pest population as a whole. If enemy risk is variable, pests that suffer losses in feeding or mating during high‐risk periods may be able to compensate during periods of low risk (Houston et al., 1993). Compensatory mortality can also occur in biological control systems, as when density‐dependent mortality is replaced by enemy‐induced mortality, leading to no overall difference in mortality (Cloutier and Bauduin, 1995; Suh et al., 2000). While this has been demonstrated in CEs, the same could occur for NCEs, where strong effects during one life stage lead to no difference in later population size.
Short‐term behavioural shifts alone may have a significant impact on biocontrol outcomes if they can be aligned with periods of crop vulnerability. Pests are often only damaging during a particular crop or pest growth stage (Hokkanen, 1991; Wiedenmann and Smith, 1997). The use of temporal asynchrony between crop and pest stages, achieved through precise timing of crop production, can exploit the narrowness of the crop vulnerability window to reduce pest impact (Letourneau and Bruggen, 2006). Similarly, if pest pressure can be reduced during that time through enemy‐induced behavioural responses, crop damage may be decreased regardless of impacts on pest population growth.
Some trait responses to natural enemies only occur in the long‐term, and as such, their consequences only appear in the long‐term as well. Pests can shift their life history in response to enemy risk, including increasing developmental rate (Thaler et al., 2012; Elliott et al., 2016; Rendon et al., 2016). Speeding up the development of a vulnerable life stage may reduce overall exposure to natural enemies, but incur costs later on. If shorter development means less time in a crop‐damaging life stage (e.g. less time spent as a crop‐feeding caterpillar), this may be beneficial to the crop, though it may also increase the rate of pest population growth. Different pests, even within the same order, may allocate risk avoidance behaviour to different life stages, either exhibiting oviposition site selection or juvenile enemy‐avoidance behaviour (Stav et al., 2000, 2010; Kiflawi et al., 2003; Brown and Kotler, 2004; Blaustein et al., 2005).
It is important to consider the goals of the biocontrol program when addressing temporal components of enemy‐risk effects. In a classical biocontrol program, where the goal is the long‐term establishment of the natural enemy, some level of CEs is necessary to sustain the enemy population, even if NCEs are initially very high. However, with an augmentative release, high enemy densities are expected to remain for only a short time. In this case, strong short‐term behavioural changes, such as temporarily reduced feeding, or short‐term behaviours that lead to long‐term fitness consequences may be enough to significantly impact the pest, though the enemy does not establish. For example, if an augmentative release of enemies leads to a large reduction in pest feeding during a week of high crop vulnerability, then long‐term impacts on pest population may be of little concern since the damaging behaviour itself was prevented.
ENEMY‐RISK EFFECTS WITH MULTIPLE BIOCONTROL AGENTS
Effects of multiple enemies on pests
An extensive literature has established that combinations of multiple predator species can have any of three outcomes on prey suppression: (1) additive, independent effects; (2) greater than additive, or synergistic effects; or (3) less than additive, or disruptive effects (Jonsson et al., 2017). Much of this literature has emphasised consumptive effects as the drivers of these outcomes; thus, synergistic effects may be generated by various forms of complementarity, including complementary use of space (e.g. consuming prey in different microhabitats) or time (e.g. consuming prey during different times of day or seasons), or differences in the host/prey stages or species attacked (Finke and Snyder, 2008; Straub and Snyder, 2008; Northfield et al., 2010), whereas disruptive effects may be generated by IGP or various forms of competitive interference (Vance‐Chalcraft et al., 2007).
Enemy‐risk effects may, however, also play important roles in shaping non‐additive effects of multiple predators (Sih et al., 1998). In particular, when prey defensive responses to one predator increase vulnerability to a second predator (‘risk enhancement’), the outcome is often predator facilitation and synergistic impacts on prey mortality. This is the case when pea aphids are attacked by combinations of the ladybird beetle Coccinella septempunctata and the carabid beetle Harpalus pennsylvanicus. Pea aphids drop off plants when threatened by the foliage‐foraging C. septempunctata, and despite adaptations for re‐grasping the plant as they fall (Meresman et al., 2017), some still reach the ground, where they are attacked by the strictly ground‐foraging H. pennsylvanicus (Losey and Denno, 1998). Similarly, strong risk enhancement is seen when Tetranychus kanzawai spider mites are driven out of their web refuges by specialised predatory mites Neoseiulus womersleyi, only to fall prey to ants that forage only outside of their webbing (Otsuki and Yano, 2014a).
Enemy‐risk effects can also contribute to predator interference. If defensive responses to one predator also confer protection against a second predator (‘risk reduction’), then total predation may be less than expected when both predators are present (Vance‐Chalcraft and Soluk, 2005). Alternatively, even when defensive responses appear to conflict, the presence of multiple predators may sometimes improve prey survival. For example, Meadows et al. (2017) showed that Culex mosquito larvae respond to a complex of mesopredators by diving towards the bottom of water bodies; however, in the presence of top predators, dragonfly larvae, which forage lower in the water column, diving responses by Culex are suppressed. Because the diving behaviour is costly, suppression of this response doubled the survival of larval mosquitoes to pupation. Thus, enemy‐risk effects often play key roles in shaping the emergent non‐additive impacts of multiple predators.
Enemy‐risk effects and predator–predator interactions
Insect herbivores face the dual challenge of well‐defended host plants and natural enemies (Polis, 1999). It has become increasingly well established that predators must also forage for defended food resources (their prey) under the risk of predation. Enemy risk can stem from specialist higher order enemies (e.g. obligate hyperparasitoids); intraguild predators (competitors that also engage in uni‐ or bidirectional predation with the focal predator); or cannibalistic conspecifics (Polis, 1981; Polis et al., 1989; Rosenheim et al., 1995; Rosenheim, 1998; Schausberger, 2003; Wise, 2006). And, just as for herbivores, the impacts of higher order predators, intraguild predators and cannibals can be both consumptive and non‐consumptive (reviewed by Snyder and Ives, 2008; Frago, 2016). Although enemy‐risk effects expressed by predators reacting to other predators are generally viewed as adaptations to reduce their own risk of predation, in most cases it is difficult to separate benefits from reducing the costs of predation versus reducing the costs of competition, or even other costs of high density, such as transmission of diseases that have broad host ranges. Predation risk reduction can, however, be clearly identified as the driver when competition and disease can be ruled out, such as when a primary parasitoid abandons host patches where it detects pheromones produced by an obligate hyperparasitoid (Höller et al., 1994).
Natural enemies express a broad array of responses to their own predators. A common response is to move away from areas where predator risk is perceived; this may be measured experimentally as shorter patch residency times (Nakashima and Senoo, 2003; Meisner et al., 2011; Frago and Godfray, 2014), reduced oviposition or prey consumption (Agarwala et al., 2003; Magalhães et al., 2004; Meisner et al., 2011; Choh et al., 2015) or outright avoidance of patches where predators or predator‐associated cues are detected (Magalhães et al., 2004; Choh et al., 2015; Cotes et al., 2015; Seiter and Schausberger, 2015). Occasionally, parasitoids have been found to increase, rather than decrease, their oviposition activity in host patches with elevated predation risk, likely due to high patch quality even considering predator presence (e.g. Velasco‐Hernández et al., 2013). Other common responses include modulation of overall foraging activity (either increased or decreased; Magalhães et al., 2004; Bucher et al., 2014; Walzer et al., 2015; Hentley et al., 2016) and increased use of refuges (Venzon et al., 2000). Developmental effects include increased mortality, delayed (or sometimes accelerated) development, decreased (or sometimes increased) adult body size and shortened pre‐oviposition periods for adults (Walzer et al., 2015; Michaud et al., 2016). Compensatory growth has been recorded following the periods of elevated predation risk that slowed growth (Walzer et al., 2015). In many cases, predators respond not to reduce their own risk of predation, but rather to reduce the likelihood that their more vulnerable offspring will be attacked. Transgenerational phenotypic plasticity in response to predation risk has been recorded (Seiter and Schausberger, 2015), and in cases where predator–prey role reversals are possible, adult predators that witness a heterospecific predator attacking juvenile members of its own species may subsequently be more aggressive in reciprocal attacks on juveniles of the attacking species (Choh et al., 2014). Predators may even invade central locations within colonies of their prey to secure the predation risk‐reduction benefits of a selfish herd (Dumont et al., 2015).
What influence these responses have on the overall success of biological control is uncertain. Much of the literature is framed around the idea that anti‐enemy behaviour of intraguild prey ameliorate the impact of IGP, potentially facilitating the coexistence of multiple natural enemies, and presumably enhancing the suppression of pest populations. In the short‐term, however, anti‐predator responses that reduce potential IGP or cannibalism often results in reduced overall consumption of prey (Sih et al., 1998; Vance‐Chalcraft et al., 2007). Localised loss of contributions to biological control ascribed to non‐consumptive effects of intraguild predators or hyperparasitoids has indeed been reported (Höller et al., 1994; Raymond et al., 2000; Meisner et al., 2011; Frago and Godfray, 2014). But it is easier to record the potential erosion of biocontrol in a focal patch of prey than to document the possibly enhanced biocontrol elsewhere (for one study that investigated but did not find such an outcome, see Frago and Godfray, 2014). Predators that abandon patches of rich host/prey resources due to the presence of other natural enemies presumably weaken biocontrol in those patches, but may strengthen biocontrol elsewhere. Furthermore, consumptive and non‐consumptive effects have not been separated in these studies, and doing so while still assessing the overall level of biocontrol success would not be easy: treatments (e.g. mouthpart manipulations) that could be applied to an intraguild predator to eliminate CEs imposed on an intermediate predator would also, unfortunately, eliminate CEs on the shared herbivore prey. Studies of hyperparasitoids could avoid this problem. In some cases the herbivores themselves have been shown to recognise localised enemy‐free space generated by hyperparasitoids and to respond with elevated per capita reproductive output, perhaps as a consequence of reduced expression of costly anti‐predator defences (Van Veen et al. 2001). To our knowledge, no one has attempted to measure or model the global effects of fear‐mediated redistribution of natural enemies (but see Northfield et al. 2017 for a model that could provide a useful framework for such an investigation).
ENEMY‐RISK EFFECTS AND BOTTOM‐UP EFFECTS
Interactions between top‐down and bottom‐up pressures have received much attention in broader natural enemy ecology, but specific breakdown of CEs and NCEs has been less common (but see Kaplan and Thaler, 2010, 2012; Thaler et al., 2014). A general framework for understanding the role of plant defences in altering the CE:NCE ratio focuses on the cost‐benefit ratio of engaging in anti‐enemy behaviour. An enemy‐avoidance behaviour that reduces foraging time may have a higher relative cost if food quality is low, leading to a reduction in that behaviour and resulting NCEs. The degree to which plant defences shift the trade‐off between foraging and enemy avoidance can depend on whether the pest is a generalist or specialist (Kaplan et al., 2014). Though a reduction in only NCEs would shift the CE:NCE ratio towards consumptive effects, plant defences can also affect the rates of enemy consumption. Generalist enemies may reduce consumption of a particular prey if plant quality or defences reduce prey biomass, prey quality or the chemical cues used by enemies to locate prey (Kersch‐Becker et al., 2017).
Bottom‐up effects do not always affect anti‐enemy behaviours simply by changing the cost‐benefit ratio of those behaviours. Additive effects may be possible if pests respond to plant defences and enemy risk in qualitatively different ways. For example, phytohormones have been shown to reduce aphid population growth, while natural enemies induce the production of winged morphs (Kaplan and Thaler, 2012). Here, the pathways operate independently, leading to additive effects of anti‐enemy behaviour and plant defence. In other studies, short‐distance dispersal and plant defences have been shown to interact strongly, with low plant quality and natural enemies synergistically increasing aphid dispersal (Kersch‐Becker and Thaler, 2015). Additionally, the effects of reduced plant quality and NCEs may occur on longer timescales than CEs. Pests can exploit these longer timescales by engaging in compensatory mechanisms to reduce the overall negative effects. Caterpillars facing predation risk can reduce their feeding rate but temporarily increase conversion efficiency to maintain a normal growth rate (Thaler et al., 2012). However, this cannot continue forever and may be dependent on the threat duration (Kaplan et al., 2014).
Finally, many biocontrol agents are omnivorous, meaning plant defences may affect their fitness directly. If high‐quality plants increase omnivorous enemy populations, consumption of prey may increase. However, high‐quality plants may also reduce the omnivore’s need to forage for prey, reducing per capita consumptive rates and NCEs. The interactions between plant defences and natural enemies are numerous, including risk effect pathways and others not discussed here, which have been more thoroughly reviewed elsewhere (Pappas et al., 2017). Due to these complexities, studies aiming to assess enemy‐risk effects in the field should consider what interactions with bottom‐up effects may occur.
CONCLUSION
The study of enemy‐risk effects has advanced greatly in the past two decades, developing into a more fully realised field, incorporating theoretical frameworks, many experimental methods and even predictive models. However, the field of biological control is still catching up to broader natural enemy ecology, and the incorporation of enemy‐risk effects into the biocontrol framework is still in its infancy. There is a significant body of research documenting the importance of risk effects in biocontrol systems, but there is much room to grow beyond this. We have outlined several areas in which risk effect literature may provide insight into biocontrol practice, and hope that further studies will investigate specific interactions between enemy‐risk effects and IPM programs more thoroughly.
Community ecologists likewise can find, in biological control systems, rich examples where the consequences of risk effects play out in well‐characterised predator–prey systems, including both coevolved versus novel predator–prey associations. Agricultural systems provide ideal settings for examining both the shorter‐ and the longer term consequences of risk effects, on both smaller and larger spatial scales. Opportunities exist to examine how risk effects shape trophic cascades, the distributions of prey populations in space and even microevolutionary responses to plant defensive traits.
One of the most crucial aspects of the merging of the fields will be broadly considering biocontrol of arthropods as an inherently behavioural issue. A focus on preventing unwanted and damaging pest behaviour, whether through killing pests or changing their behaviour, broadens the scope of interactions that may be utilised in biological control. The historical focus on population density is no longer sufficient in light of research demonstrating the importance of enemy‐risk effects and how they can cascade to the level of plants.
Studies of risk effects in biocontrol systems should also include more holistic studies of the numerous interactions, either synergistic or antagonistic, between pest behaviour and broader IPM practices. Studies in this area can simultaneously investigate core ecological concepts and provide more concrete suggestions for biocontrol practitioners.
Finally, we recognise that it may not be feasible to investigate all possible enemy‐risk effects in a given agroecosystem when attempting to predict the effects of a biocontrol agent, which is why we propose the incorporation of theory and predictive models from risk effect research into biocontrol decision‐making processes. By considering the evolutionary history of the pest, bottom‐up effects of the crop and spatiotemporal dynamics of the agroecosystem, pest management programs may be able to predict the relative importance of various types of risk effects and how they may interact with management practices. Just as other detailed aspects of pest and agent biology are incorporated into management decisions, we advocate for the inclusion of enemy‐risk effect knowledge as well.
AUTHORSHIP
All the authors discussed ideas and structure, wrote sections of the manuscript, reviewed literature and contributed substantially to revisions.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ele.13601.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge Sara Hermann, Paul Abram and Lea Pollack for their thoughtful comments on drafts of the manuscript and members of the Rosenheim Lab for insightful discussions on various topics covered in the manuscript. MCM was supported by a USDA NIFA Predoctoral Fellowship (Award 2019‐67011‐29710) during stages of writing the manuscript. We dedicate this paper in the memory of Leon Blaustein, an important contributor to the field of enemy risk ecology and former UC Davis student, who passed away during the preparation of this manuscript.
DATA AVAILABILITY STATEMENT
No original data were used in the manuscript, and all search terms and articles from the literature review are described and cited.
References
- Abram, P.K. , Brodeur, J. , Burte, V. & Boivin, G. (2016). Parasitoid‐induced host egg abortion: An underappreciated component of biological control services provided by egg parasitoids. Biol. Control, 98, 52–60. [Google Scholar]
- Abram, P.K. , Brodeur, J. , Urbaneja, A. & Tena, A. (2019). Nonreproductive effects of insect parasitoids on their hosts. Annu. Rev. Entomol., 64, 259–276. [DOI] [PubMed] [Google Scholar]
- Abrams, P.A. (2008). Measuring the population‐level consequences of predator‐induced prey movement. Evol. Ecol. Res., 10, 333–350. [Google Scholar]
- Abrams, P.A. & Matsuda, H. (1997). Prey adaptation as a cause of predator‐prey cycles. Evolution, 51, 10. [DOI] [PubMed] [Google Scholar]
- Abrams, P.A. , Menge, B.A. , Mittelbach, G.G. , Spiller, D.A. & Yodzis, P. (1996). The role of indirect effects in food webs In: Food Webs (eds Polis G.A. & Winemiller K.O.). Springer, Boston, MA, pp. 371–395. [Google Scholar]
- Agarwala, B.K. , Yasuda, H. & Kajita, Y. (2003). Effect of conspecific and heterospecific feces on foraging and oviposition of two predatory ladybirds: role of fecal cues in predator avoidance. J. Chem. Ecol., 29, 357–376. [DOI] [PubMed] [Google Scholar]
- Angelon, K.I.M.A. & Petranka, J.W. (2002). Chemicals of predatory mosquitofish (Gambusia affinis) influence selection of oviposition sites by Culex mosquitoes. J. Chem. Ecol. 28, 797–807. [DOI] [PubMed] [Google Scholar]
- Bailey, S.M. , Irwin, M.E. , Kampmeier, G.E. , Eastman, C.E. & Hewings, A.D. (1995). Physical and biological perturbations: their effect on the movement of apterous Rhopalosiphum padi (Homoptera: Aphididae) and localized spread of barley yellow dwarf virus. Environ. Entomol., 24, 24–33. [Google Scholar]
- Bannerman, J.A. , Gillespie, D.R. & Roitberg, B.D. (2011). The impacts of extreme and fluctuating temperatures on trait‐mediated indirect aphid‐parasitoid interactions. Ecol. Entomol., 36, 490–498. [Google Scholar]
- Barratt, B.I.P. , Howarth, F.G. , Withers, T.M. , Kean, J.M. & Ridley, G.S. (2010). Progress in risk assessment for classical biological control. Biol. Control, 52, 245–254. [Google Scholar]
- Bedoya‐Perez, M.A. , Smith, K.L. , Kevin, R.C. , Luo, J.L. , Crowther, M.S. & McGregor, I.S. (2019). Parameters that affect fear responses in rodents and how to use them for management. Front. Ecol. Evol., 7, 136. [Google Scholar]
- Bellamy, S.K. & Alto, B.W. (2018). Mosquito responses to trait‐ and density‐mediated interactions of predation. Oecologia, 187, 233–243. [DOI] [PubMed] [Google Scholar]
- Belliure, B. , Amorós‐Jiménez, R. , Fereres, A. & Marcos‐García, M.Á. (2011). Antipredator behaviour of Myzus persicae affects transmission efficiency of Broad bean wilt virus 1. Virus Res., 159, 206–214. [DOI] [PubMed] [Google Scholar]
- Benard, M.F. (2004). Predator‐induced phenotypic plasticity in organisms with complex life histories. Annu. Rev. Ecol. Evol. Syst., 35, 651–673. [Google Scholar]
- Berberet, R.C. , Zarrabi, A.A. , Payton, M.E. & Bisges, A.D. (2003). Reduction in Effective Parasitism of Hypera postica (Coleoptera: Curculionidae) by Bathyplectes curculionis (Hymenoptera: Ichneumonidae) Due to Encapsulation. Environ. Entomol., 32, 1123–1130. [Google Scholar]
- Bianchi, F.J.J. , Booij, C.J.H. & Tscharntke, T. (2006). Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc. Biol. Sci., 273, 1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babendreier, D. , Bigler, F. & Kuhlmann, U. (2006). Current status and constraints in the assessment of non‐target effects Environmental impact of invertebrates for biological control of arthropods: methods and risk assessment (eds Babendreier D., Bigler F. & Kuhlmann U.). CABI Publishing, Cambridge, MA. [Google Scholar]
- Bilu, E. & Coll, M. (2007). The importance of intraguild interactions to the combined effect of a parasitoid and a predator on aphid population suppression. Biocontrol, 52, 753–763. [Google Scholar]
- Blaustein, L. , Blaustein, J. & Chase, J. (2005). Chemical detection of the predator Notonecta irrorata by ovipositing Culex mosquitoes. J. Vector Ecol., 30, 3. [PubMed] [Google Scholar]
- Blossey, B. & Notzold, R. (1995). Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. J. Ecol., 83, 887. [Google Scholar]
- Blumstein, D.T. (2006). The multipredator hypothesis and the evolutionary persistence of antipredator behavior. Ethology, 112, 209–217. [Google Scholar]
- Boonstra, R. , Hik, D. , Singleton, G.R. & Tinnikov, A. (1998). The impact of predator‐induced stress on the snowshoe hare cycle. Ecol. Monogr., 68, 371–394. [Google Scholar]
- Bourdeau, P.E. & Johansson, F. (2012). Predator‐induced morphological defences as by‐products of prey behaviour: A review and prospectus. Oikos, 121, 1175–1190. [Google Scholar]
- Brown, J.S. & Kotler, B.P. (2004). Hazardous duty pay and the foraging cost of predation. Ecol. Lett., 7, 999–1014. [Google Scholar]
- Buchanan, A.L. , Hermann, S.L. , Lund, M. , & Szendrei, Z. (2017). A meta‐analysis of non‐consumptive predator effects in arthropods: the influence of organismal and environmental characteristics. Oikos, 126, 1233–1240. 10.1111/oik.04384 [DOI] [Google Scholar]
- Bucher, R. , Binz, H. , Menzel, F. & Entling, M.H. (2014). Effects of spider chemotactile cues on arthropod behavior. J. Insect Behav., 27, 567–580. [Google Scholar]
- Bulgarella, M. , Quiroga, M.A. , Boulton, R.A. , Ramírez, I.E. , Moon, R.D. , Causton, C.E. et al (2017). Life cycle and host specificity of the parasitoid Conura annulifera (Hymenoptera: Chalcididae), a potential biological control agent of Philornis downsi (Diptera: Muscidae) in the Galápagos Islands. Ann. Entomol. Soc. Am., 110, 317–328. [Google Scholar]
- Calcaterra, L.A. , Delgado, A. & Tsutsui, N.D. (2008). Activity patterns and parasitism rates of fire ant‐decapitating flies (Diptera: Phoridae: Pseudacteon spp.) in their native Argentina. Ann. Entomol. Soc. Am., 101, 539–550. [Google Scholar]
- Calvet, É.C. , Lima, D.B. , Melo, J.W.S. & Gondim, M.G.C. (2018). Chemosensory cues of predators and competitors influence search for refuge in fruit by the coconut mite Aceria guerreronis . Exp. Appl. Acarol., 74, 249–259. [DOI] [PubMed] [Google Scholar]
- Carpenter, S.R. , Kitchell, J.F. , Hodgson, J.R. , Cochran, P.A. , Elser, J.J. , Elser, M.M. et al (1987). Regulation of lake primary productivity by food web structure. Ecology, 68, 1863–1876. [DOI] [PubMed] [Google Scholar]
- Carrière, Y. , Fabrick, J.A. & Tabashnik, B.E. (2016). Can pyramids and seed mixtures delay resistance to Bt crops? Trends Biotechnol., 34, 291–302. [DOI] [PubMed] [Google Scholar]
- Carroll, M.W. , Head, G. & Caprio, M. (2012). When and where a seed mix refuge makes sense for managing insect resistance to Bt plants. Crop Prot., 38, 74–79. [Google Scholar]
- Carthey, A.J. & Blumstein, D.T. (2018). Predicting predator recognition in a changing world. Trends Ecol. Evol., 33, 106–115. [DOI] [PubMed] [Google Scholar]
- Carthey, A.J.R. & Banks, P.B. (2014). Na ï vet ´ e in novel ecological interactions: lessons from theory and experimental evidence. Biol. Rev. Camb. Philos. Soc., 89, 932–949. [DOI] [PubMed] [Google Scholar]
- Castellanos, I. & Barbosa, P. (2006). Evaluation of predation risk by a caterpillar using substrate‐borne vibrations. Anim. Behav., 72, 461–469. [Google Scholar]
- Castellanos, I. , Barbosa, P. , Zuria, I. , Tammaru, T. & Christman, M.C. (2011). Contact with caterpillar hairs triggers predator‐specific defensive responses. Behav. Ecol., 22, 1020–1025. [Google Scholar]
- Chen, L. & Fadamiro, H.Y. (2018). Pseudacteon phorid flies: Host specificity and impacts on solenopsis fire ants. Annu. Rev. Entomol., 63, 47–67. [DOI] [PubMed] [Google Scholar]
- Choh, Y. , Sabelis, M.W. & Janssen, A. (2015). Distribution and oviposition site selection by predatory mites in the presence of intraguild predators. Exp. Appl. Acarol., 67, 477–491. [DOI] [PubMed] [Google Scholar]
- Choh, Y. , Takabayashi, J. , Sabelis, M.W. & Janssen, A. (2014). Witnessing predation can affect strength of counterattack in phytoseiids with ontogenetic predator–prey role reversal. Anim. Behav., 93, 9–13. [Google Scholar]
- Clark, C.W. (1994). Antipredator behavior and the asset‐protection principle. Behav. Ecol., 5, 159–170. [Google Scholar]
- Clark, R.E. , Basu, S. , Lee, B.W. & Crowder, D.W. (2019). Tri‐trophic interactions mediate the spread of a vector‐borne plant pathogen. Ecology, 100, 1–8. [DOI] [PubMed] [Google Scholar]
- Clinchy, M. , Sheriff, M.J. & Zanette, L.Y. (2013). Predator‐induced stress and the ecology of fear. Funct. Ecol., 27, 56–65. [Google Scholar]
- Cloutier, C. & Bauduin, F. (1995). Biological control of the colorado potato beetle leptinotarsa decemlineata (Coleoptera: Chrysomelidae) In Quebec By Augmentative Releases Of The Two‐spotted Stinkbug Perillus bioculatus (Hemiptera: Pentatomidae). Can. Entomol., 127, 195–212. [Google Scholar]
- Coll, M. (1996). Feeding and ovipositing on plants by an omnivorous insect predator. Oecologia, 105, 214–220. [DOI] [PubMed] [Google Scholar]
- Collier, T. & Van Steenwyk, R. (2004). A critical evaluation of augmentative biological control. Biol. Control, 31, 245–256. [Google Scholar]
- Cook, S.M. , Khan, Z.R. , Pickett, J.A. (2007). The use of push‐pull strategies in integrated pest management. Annu. Rev. Entomol., 52, 375–400. [DOI] [PubMed] [Google Scholar]
- Cotes, B. , Rännbäck, L.‐M. , Björkman, M. , Norli, H.R. , Meyling, N.V. , Rämert, B. et al (2015). Habitat selection of a parasitoid mediated by volatiles informing on host and intraguild predator densities. Oecologia, 179, 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, J.G. & Lima, S.L. (2006). Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends Ecol. Evol., 21, 674–680. [DOI] [PubMed] [Google Scholar]
- Creel, S. & Christianson, D. (2008). Relationships between direct predation and risk effects. Trends Ecol. Evol., 23, 194–201. [DOI] [PubMed] [Google Scholar]
- Crowder, D.W. , Li, J. , Borer, E.T. , Finke, D.L. , Sharon, R. , et al (2019). Species interactions affect the spread of vector‐borne plant pathogens independent of transmission mode. Ecology, 100, 1–10–. 10.1002/ecy.2782 [DOI] [PubMed] [Google Scholar]
- Cuny, M.A.C. , Traine, J. , Bustos‐Segura, C. & Benrey, B. (2019). Host density and parasitoid presence interact and shape the outcome of a tritrophic interaction on seeds of wild lima bean. Sci. Rep., 9, 10.1038/s41598-019-55143-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dáder, B. , Moreno, A. , Viñuela, E. & Fereres, A. (2012). Spatio‐temporal dynamics of viruses are differentially affected by parasitoids depending on the mode of transmission. Viruses, 4, 3069–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deas, J.B. & Hunter, M.S. (2013). Delay, avoidance and protection in oviposition behaviour in response to fine‐scale variation in egg parasitism risk. Anim. Behav., 86, 933–940. [Google Scholar]
- Delnat, V. , Janssens, L. & Stoks, R. (2020). Effects of predator cues and pesticide resistance on the toxicity of a (bio)pesticide mixture. Pest Manag. Sci., 76, 1448–1455. [DOI] [PubMed] [Google Scholar]
- Denno, R.F. & Peterson, M.A. (1995). Density‐dependent dispersal and its consequences for population dynamics Population Dynamics: New Approaches and Synthesis (eds Cappuccino N. & Price P.W.). Academic Press, San Diego, CA, pp.113–130. [Google Scholar]
- Dias, C.R. , Bernardo, A.M.G. , Mencalha, J. , Freitas, C.W.C. , Sarmento, R.A. , Pallini, A. et al (2016). Antipredator behaviours of a spider mite in response to cues of dangerous and harmless predators. Exp. Appl. Acarol., 69, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicke, M. & Grostal, P. (2001). Chemical detection of natural enemies by arthropods: An ecological perspective. Annu. Rev. Ecol. Syst., 32, 1–23. [Google Scholar]
- Duffy, M.A. , Housley, J.M. , Penczykowski, R.M. , Caceres, C.E. & Hall, S.R. (2011). Unhealthy herds: indirect effects of predators enhance two drivers of disease spread. Funct. Ecol., 25, 945–953. [Google Scholar]
- Dumont, F. , Lucas, E. & Brodeur, J. (2015). Do furtive predators benefit from a selfish herd effect by living within their prey colony? Behav. Ecol. Sociobiol., 69, 971–976. [Google Scholar]
- Dupuy, M.M. & Ramirez, R.A. (2019). Consumptive and non‐consumptive effects of predatory arthropods on billbug (Coleoptera: Dryophthoridae) pests in turfgrass. Biol. Control, 129, 136–147. [Google Scholar]
- Ehlman, S.M. , Trimmer, P.C. & Sih, A. (2019). Prey responses to exotic predators: Effects of old risks and new cues. Am. Nat., 193, 575–587. [DOI] [PubMed] [Google Scholar]
- Eilenberg, J. , Hajek, A. & Lomer, C. (2001). Suggestions for unifying the terminology in biological control. Biocontrol, 46, 387–400. [Google Scholar]
- Elliott, K.H. , Betini, G.S. , Dworkin, I. & Norris, D.R. (2016). Experimental evidence for within‐ and cross‐seasonal effects of fear on survival and reproduction. J. Anim. Ecol., 85, 507–515. [DOI] [PubMed] [Google Scholar]
- Feener, D.H. (1981). Competition between ant species: outcome controlled by parasitic flies. Science, 214, 815–817. [DOI] [PubMed] [Google Scholar]
- Feener, D.H. Jr. & Brown, B.V. (1992). Reduced foraging of Solenopsis geminata (Hymenoptera: Formicidae) in the presence of parasitic Pseudacteon spp. (Diptera: Phoridae). Ann. Entomol. Soc. Am., 85, 80–84. [Google Scholar]
- Ferrari, M.C. , Wisenden, B.D. & Chivers, D.P. (2010). Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool., 88, 698–724. [Google Scholar]
- Ferrari, M.C.O. , McCormick, M.I. , Meekan, M.G. & Chivers, D.P. (2015). Background level of risk and the survival of predator‐naive prey: can neophobia compensate for predator naivety in juvenile coral reef fishes? Proc. R. Soc. B Biol. Sci., 282, 20142197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet, V. , Lhomme, P. & Outreman, Y. (2008). Predation risk cues associated with killed conspecifics affect the behavior and reproduction of prey animals. Oikos, 117, 1380–1385. [Google Scholar]
- Fill, A. , Long, E.Y. & Finke, D.L. (2012). Non‐consumptive effects of a natural enemy on a non‐prey herbivore population. Ecol. Entomol., 37, 43–50. [Google Scholar]
- Finke, D.L. (2012). Contrasting the consumptive and non‐consumptive cascading effects of natural enemies on vector‐borne pathogens. Entomol. Exp. Appl., 144, 45–55. [Google Scholar]
- Finke, D.L. & Snyder, W.E. (2008). Niche partitioning increases resource exploitation by diverse communities. Science, 321, 1488–1490. [DOI] [PubMed] [Google Scholar]
- Fischhoff, I.R. , Burtis, J.C. , Keesing, F. & Ostfeld, R.S. (2018). Tritrophic interactions between a fungal pathogen, a spider predator, and the blacklegged tick. Ecol. Evol., 8, 7824–7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgarait, P.J. & Gilber, L.E. (1999). Phorid parasitoids affect foraging activity of Solenopsis richteri under different availability of food in Argentina. Ecol. Ent, 24, 163–173. [Google Scholar]
- Fouzai, N. , Opdal, A.F. , Jørgensen, C. & Fiksen, Ø. (2019). Dying from the lesser of three evils: facilitation and non‐consumptive effects emerge in a model with multiple predators. Oikos, 128, 1307–1317. [Google Scholar]
- Frago, E. (2016). Interactions between parasitoids and higher order natural enemies: intraguild predation and hyperparasitoids. Curr. Opin. Insect Sci., 14, 81–86. [DOI] [PubMed] [Google Scholar]
- Frago, E. & Godfray, H.C.J. (2014). Avoidance of intraguild predation leads to a long‐term positive trait‐mediated indirect effect in an insect community. Oecologia, 174, 943–952. [DOI] [PubMed] [Google Scholar]
- Fraker, M.E. & Luttbeg, B. (2012). Predator–prey space use and the spatial distribution of predation events. Behaviour, 149, 555–574. [Google Scholar]
- Francesena, N. , Rocca, M. , Rizzo, E. , Arneodo, J.D. & Greco, N.M. (2019). Potential of predatory Neotropical ladybirds and minute pirate bug on strawberry aphid. Anais da Academia Brasileira de Ciências, 91, 1–11. [DOI] [PubMed] [Google Scholar]
- Furlong, M.J. (2015). Knowing your enemies: integrating molecular and ecological methods to assess the impact of arthropod predators on crop pests. Insect Sci., 22, 6–19. [DOI] [PubMed] [Google Scholar]
- Garcia, A.G. , Ferreira, C.P. , Cônsoli, F.L. & Godoy, W.A. (2016). Predicting evolution of insect resistance to transgenic crops in within‐field refuge configurations, based on larval movement. Ecol. Complex., 28, 94–103. [Google Scholar]
- Gish, M. , Dafni, A. & Inbar, M. (2011). Avoiding incidental predation by mammalian herbivores: accurate detection and efficient response in aphids. Naturwissenschaften, 98, 731–738. [DOI] [PubMed] [Google Scholar]
- Godfray, H.C.J. & Waage, J.K. (1991). Predictive modelling in biological control: The mango mealy bug (Rastrococcus invadens) and its parasitoids. J. Appl. Ecol., 28, 434. [Google Scholar]
- Gonçalves‐Souza, T. , Omena, P.M. , Souza, J.C. & Romero, G.Q. (2008). Trait‐mediated effects on flowers: artificial spiders deceive pollinators and decrease plant fitness. Ecology, 89, 2407–2413. [DOI] [PubMed] [Google Scholar]
- Goodale, E. & Nieh, J.C. (2012). Public use of olfactory information associated with predation in two species of social bees. Anim. Behav., 84, 919–924. [Google Scholar]
- Griffin, C.A.M. & Thaler, J.S. (2006). Insect predators affect plant resistance via density‐ and trait‐mediated indirect interactions. Ecol. Lett., 9, 335–343. [DOI] [PubMed] [Google Scholar]
- Gruner, D.S. (2005). Biotic resistance to an invasive spider conferred by generalist insectivorous birds on Hawai’i Island. Biol. Invasions, 7, 541–546. [Google Scholar]
- Gyuris, E. , Szép, E. , Kontschán, J. , Hettyey, A. & Tóth, Z. (2017). Behavioural responses of two‐spotted spider mites induced by predator‐borne and prey‐borne cues. Behav. Processes, 144, 100–106. [DOI] [PubMed] [Google Scholar]
- Hajek, A.E. , Hurley, B.P. , Kenis, M. , Garnas, J.R. , Bush, S.J. , Wingfield, M.J. et al (2016). Exotic biological control agents: a solution or contribution to arthropod invasions? Biol. Invasions, 18, 953–969. [Google Scholar]
- Hamburg, H.V. & Hassell, M.P. (1984). Density dependence and the augmentative release of egg parasitoids against graminaceous stalk borers. Ecol. Entomol., 9, 101–108. [Google Scholar]
- Hammill, E. , Fitzjohn, R.G. & Srivastava, D.S. (2015). Conspecific density modulates the effect of predation on dispersal rates. Oecologia, 178, 1149–1158. [DOI] [PubMed] [Google Scholar]
- Hassell, M.P. & Varley, G.C. (1969). New inductive population model for insect parasites and its bearing on biological control. Nature, 223, 1133–1137. [DOI] [PubMed] [Google Scholar]
- Havel, J.E. & Dodson, S.I. (1984). Chaoborus predation on typical and spined morphs of Daphnia pulex: Behavioral observations. Limnol. Oceanogr., 29, 487–494. [Google Scholar]
- Hawkins, B.A. & Cornell, H.V. (1994). Maximum parasitism rates and successful biological control. Science, 266, 1886–1887. [DOI] [PubMed] [Google Scholar]
- Hawkins, B.A. , Thomas, M.B. & Hochberg, M.E. (1993). Refuge theory and biological control. Science, 262, 1429–1432. [DOI] [PubMed] [Google Scholar]
- Hawlena, D. & Schmitz, O.J. (2010). Physiological stress as a fundamental mechanism linking predation to ecosystem functioning. Am. Nat., 176, 537–556. [DOI] [PubMed] [Google Scholar]
- Head, G. , Campbell, L.A. , Carroll, M. , Clark, T. , Galvan, T. , Hendrix, W.M. et al (2014). Movement and survival of corn rootworm in seed mixtures of SmartStax® insect‐protected corn. Crop Prot., 58, 14–24. [Google Scholar]
- Heimpel, G.E. & Mills, N.J. (2017). Biological Control: Ecology and Applications. Cambridge University Press, Cambridge. [Google Scholar]
- Henry, L.M. , Bannerman, J.A. , Gillespie, D.R. & Roitberg, B.D. (2010). Predator identity and the nature and strength of food web interactions. J. Anim. Ecol., 79, 1164–1171. [DOI] [PubMed] [Google Scholar]
- Hentley, W.T. , Vanbergen, A.J. , Beckerman, A.P. , Brien, M.N. , Hails, R.S. , Jones, T.H. et al (2016). Antagonistic interactions between an invasive alien and a native coccinellid species may promote coexistence. J. Anim. Ecol., 85, 1087–1097. [DOI] [PubMed] [Google Scholar]
- Hermann, S.L. & Landis, D.A. (2017). Scaling up our understanding of non‐consumptive effects in insect systems. Curr. Opin. Insect Sci., 20, 54–60. [DOI] [PubMed] [Google Scholar]
- Hermann, S.L. & Thaler, J.S. (2014). Prey perception of predation risk: volatile chemical cues mediate non‐consumptive effects of a predator on a herbivorous insect. Oecologia, 176, 669–676. [DOI] [PubMed] [Google Scholar]
- Hermann, S.L. & Thaler, J.S. (2018). The effect of predator presence on the behavioral sequence from host selection to reproduction in an invulnerable stage of insect prey. Oecologia, 188, 945–952. [DOI] [PubMed] [Google Scholar]
- Hik, D. (1995). Does risk of predation influence population dynamics? Evidence from the cyclic decline of snowshoe hares. Wildl. Res., 22, 115–129. [Google Scholar]
- Hlivko, J.T. & Rypstra, A.L. (2003). Spiders reduce herbivory: Nonlethal effects of spiders on the consumption of soybean leaves by beetle pests. Ann. Entomol. Soc. Am., 96, 914–919. [Google Scholar]
- Hoddle, M.S. & Pandey, R. (2014). Host range testing of Tamarixia radiata (Hymenoptera: Eulophidae) sourced from the Punjab of Pakistan for classical biological control of Diaphorina citri (Hemiptera: Liviidae: Euphyllurinae: Diaphorinini) in California. J. Econ. Entomol., 107, 125–136. [DOI] [PubMed] [Google Scholar]
- Hodge, S. , Hardie, J. & Powell, G. (2011). Parasitoids aid dispersal of a nonpersistently transmitted plant virus by disturbing the aphid vector. Agric. For. Entomol., 13, 83–88. [Google Scholar]
- Hogg, B.N. , Wang, X. , Mills, N.J. & Daane, K.M. (2014). Resident spiders as predators of the recently introduced light brown apple moth, Epiphyas postvittana . Entomol. Exp. Appl., 151, 65–74. [Google Scholar]
- Hoki, E. , Losey, J. & Ugine, T.A. (2014). Comparing the consumptive and non‐consumptive effects of a native and introduced lady beetle on pea aphids (Acyrthosiphon pisum). Biol. CONTROL, 70, 78–84. [Google Scholar]
- Hokkanen, H.M.T. (1991). Trap cropping in pest management. Annu. Rev. Entomol., 36, 20. [DOI] [PubMed] [Google Scholar]
- Höller, C. , Micha, S.G. , Schulz, S. , Francke, W. & Pickett, J.A. (1994). Enemy‐induced dispersal in a parasitic wasp. Experientia, 50, 182–185. [Google Scholar]
- Houston, A.I. , McNamara, J.M. & Hutchinson, J.M.C. (1993). General results concerning the trade‐off between gaining energy and avoiding predation. Philos. Trans. R. Soc. B‐Biol. Sci., 341, 375–397. [Google Scholar]
- Hufbauer, R.A. & Roderick, G.K. (2005). Microevolution in biological control: Mechanisms, patterns, and processes. Biol. Control, 35, 227–239. [Google Scholar]
- Hulthén, K. , Chapman, B.B. , Nilsson, P.A. , Hollander, J. & Brönmark, C. (2014). Express yourself: bold individuals induce enhanced morphological defences. Proc. R. Soc. B Biol. Sci., 281, 20132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys, R.K. & Ruxton, G.D. (2019). Dropping to escape: a review of an under‐appreciated antipredator defence: Dropping to escape. Biol. Rev., 94, 575–589. [DOI] [PubMed] [Google Scholar]
- Ims, R.A. (1990). On the adaptive value of reproductive synchrony as a predator‐swamping strategy. Am. Nat., 136, 15. [Google Scholar]
- Ingerslew, K.S. & Finke, D.L. (2017). Mechanisms underlying the nonconsumptive effects of parasitoid wasps on aphids. Environ. Entomol., 46, 75–83. [DOI] [PubMed] [Google Scholar]
- Jacobsen, S.K. , Alexakis, I. & Sigsgaard, L. (2016). Antipredator responses in Tetranychus urticae differ with predator specialization. J. Appl. Entomol., 140, 228–231. [Google Scholar]
- Jallow, M.F. & Hoy, C.W. (2005). Phenotypic variation in adult behavioral response and offspring fitness in Plutella xylostella (Lepidoptera: Plutellidae) in response to permethrin. J. Econ. Entomol., 98, 2195–2202. [DOI] [PubMed] [Google Scholar]
- Jandricic, S.E. , Schmidt, D. , Bryant, G. & Frank, S.D. (2016). Non‐consumptive predator effects on a primary greenhouse pest: Predatory mite harassment reduces western flower thrips abundance and plant damage. Biol. Control, 95, 5–12. [Google Scholar]
- Janssens, L. & Stoks, R. (2013). Synergistic effects between pesticide stress and predator cues: Conflicting results from life history and physiology in the damselfly Enallagma cyathigerum . Aquat. Toxicol., 132–133, 92–99. [DOI] [PubMed] [Google Scholar]
- Jensen, K. & Toft, S. (2020). Fly disturbance suppresses aphid population growth. Ecol. Entomol., 45, 901–903. [Google Scholar]
- Jonsson, M. , Kaartinen, R. & Straub, C.S. (2017). Relationships between natural enemy diversity and biological control. Curr. Opin. Insect Sci., 20, 1–6. [DOI] [PubMed] [Google Scholar]
- Jouvenaz, D.P. , Lofgren, C.S. & Banks, W.A. (1981) Biological control of imported fire ants: A review of current knowledge. Bull. ESA, 27, 203–209. [Google Scholar]
- Kaplan, I. , McArt, S.H. & Thaler, J.S. (2014). Plant defenses and predation risk differentially shape patterns of consumption, growth, and digestive efficiency in a guild of leaf‐chewing insects. PLoS One, 9, e93714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, I. & Thaler, J.S. (2010). Plant resistance attenuates the consumptive and non‐consumptive impacts of predators on prey. Oikos, 119, 1105–1113. [Google Scholar]
- Kaplan, I. & Thaler, J.S. (2012). Phytohormone‐mediated plant resistance and predation risk act independently on the population growth and wing formation of potato aphids. Macrosiphum euphorbiae. Arthropod‐Plant Interact., 6, 181–186. [Google Scholar]
- Kats, L.B. & Dill, L.M. (1998). The scent of death: Chemosensory assessment of predation risk by prey animals. Écoscience, 5, 361–394. [Google Scholar]
- Kerfoot, W.C. & Sih, A. (1987). Predation: Direct and Indirect Impacts on Aquatic Communities. University Press of New England, Lebanon, NH. [Google Scholar]
- Kersch‐Becker, M.F. , Kessler, A. & Thaler, J.S. (2017). Plant defences limit herbivore population growth by changing predator–prey interactions. Proc. R. Soc. B Biol. Sci., 284, 20171120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersch‐Becker, M.F. & Thaler, J.S. (2015). Plant resistance reduces the strength of consumptive and non‐consumptive effects of predators on aphids. J. Anim. Ecol., 84, 1222–1232. [DOI] [PubMed] [Google Scholar]
- Kiflawi, M. , Blaustein, L. & Mangel, M. (2003). Oviposition habitat selection by the mosquito Culiseta longiareolata in response to risk of predation and conspecific larval density. Ecol. Entomol., 28, 168–173. [Google Scholar]
- Koch, R.L. (2003). The multicolored Asian lady beetle, Harmonia axyridis: A review of its biology, uses in biological control, and non‐target impacts. J. Insect Sci., 3, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopta, T. , Pokluda, R. & Psota, V. (2012). Attractiveness of flowering plants for natural enemies. Horticultural Science,, 39, 8. [Google Scholar]
- Kraus, J.M. & Vonesh, J.R. (2010). Feedbacks between community assembly and habitat selection shape variation in local colonization. J. Anim. Ecol., 79, 795–802. [DOI] [PubMed] [Google Scholar]
- Krushelnycky, P.D. , Ogura‐Yamada, C.S. , Kanegawa, K.M. , Kaneshiro, K.Y. & Magnacca, K.N. (2017). Quantifying the effects of an invasive thief ant on the reproductive success of rare Hawaiian picture‐winged flies. Biol. Conserv., 215, 254–259. [Google Scholar]
- Kunert, G. & Weisser, W.W. (2003). The interplay between density‐ and trait‐mediated effects in predator‐prey interactions: A case study in aphid wing polymorphism. Oecologia, 135, 304–312. [DOI] [PubMed] [Google Scholar]
- LaManna, J.A. & Martin, T.E. (2016). Costs of fear: behavioural and life‐history responses to risk and their demographic consequences vary across species. Ecol. Lett., 19, 403–413. [DOI] [PubMed] [Google Scholar]
- Landis, D. & Van der Werf, W. (1997). Early‐season predation impacts the establishment of aphids and spread of beet yellows virus in sugar beet. Entomophaga, 42, 499–516. [Google Scholar]
- Larsen, A.E. (2012). Modeling multiple nonconsumptive effects in simple food webs: a modified Lotka – Volterra approach. Behav. Ecol., 23, 1115–1125. [Google Scholar]
- La‐Spina, M. , Jandricic, S.E. & Buitenhuis, R. (2019). Short‐term increases in aphid dispersal from defensive dropping do not necessarily affect long‐term biological control by parasitoids. J. Econ. Entomol., 112, 1552–1559. [DOI] [PubMed] [Google Scholar]
- Laundré, J.W. , Hernández, L. & Altendorf, K.B. (2001). Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, U.S.A. Can. J. Zool., 79, 1401–1409. [Google Scholar]
- Lawson‐Balagbo, L.M. Jr , Gondim, M.G.C. & Moraes, G.J.D. (2007). Refuge use by the coconut mite Aceria guerreronis: Fine scale distribution and association with other mites under the perianth, 43, 102–110. [Google Scholar]
- Lee, D.H. , Nyrop, J.P. & Sanderson, J.P. (2011). Avoidance of natural enemies by adult whiteflies, Bemisia argentifolii, and effects on host plant choice. Biol. Control, 58, 302–309. [Google Scholar]
- Lee, D.H. , Nyrop, J.P. & Sanderson, J.P. (2014). Non‐consumptive effects of the predatory beetle Delphastus catalinae ( Coleoptera : Coccinellidae ) on habitat use patterns of adult whitefly Bemisia argentifolii ( Hemiptera : Aleyrodidae ). Appl. Entomol. Zool., 49, 599–606. [Google Scholar]
- Letourneau, D. & van Bruggen, A. (2006). Crop protection in organic agriculture In: Organic Agriculture: A Global Perspective (eds Kristiansen P., Taji A., & Reganold J.). CABI, Wallingford, pp. 93–121. [Google Scholar]
- Lima, S.L. (1998). Nonlethal effects in the ecology of predator‐prey interactions. Bioscience, 48, 25–34. [Google Scholar]
- Livingston, G. , Fukumori, K. , Provete, D.B. , Kawachi, M. , Takamura, N. & Leibold, M.A. (2017). Predators regulate prey species sorting and spatial distribution in microbial landscapes. J. Anim. Ecol., 86, 501–510. [DOI] [PubMed] [Google Scholar]
- Lommen, S.T.E. , Middendorp, C.W. , Luijten, C.A. , van Schelt, J. , Brakefield, P.M. & de Jong, P.W. (2008). Natural flightless morphs of the ladybird beetle Adalia bipunctata improve biological control of aphids on single plants. Biol. Control, 47, 340–346. [Google Scholar]
- Long, E.Y. & Finke, D.L. (2015). Predators indirectly reduce the prevalence of an insect‐vectored plant pathogen independent of predator diversity. Oecologia, 177, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Losey, J.E. & Denno, R.F. (1998). Positive predator–predator interactions: enhanced predation rates and synergistic suppression of aphid populations. Ecology, 79, 2143–2152. [Google Scholar]
- Losey, J.E. & Vaughan, M. (2006). The economic value of ecological services provided by insects. Bioscience, 56, 311. [Google Scholar]
- Lövei, G.L. & Ferrante, M. (2017). A review of the sentinel prey method as a way of quantifying invertebrate predation under field conditions. Insect Sci., 24, 528–542. [DOI] [PubMed] [Google Scholar]
- Lucas, É. , Coderre, D. & Brodeur, J. (2000). Selection of molting and pupation sites by Coleomegilla maculata (Coleoptera: Coccinellidae): avoidance of intraguild predation. Environ. Entomol., 29, 454–459. [Google Scholar]
- Lundgren, J.G. & Fergen, J.K. (2006). The oviposition behavior of the predator orius insidiosus: acceptability and preference for different plants. Biocontrol, 51, 217–227. [Google Scholar]
- Maanak, V. , Nordenhem, H. , Bjorklund, N. , Lenoir, L. & Nordlander, G. (2013). Ants protect conifer seedlings from feeding damage by the pine weevil Hylobius abietis . Agric. For. Entomol., 15, 98–105. [Google Scholar]
- Macfadyen, S. , Davies, A.P. & Zalucki, M.P. (2015). Assessing the impact of arthropod natural enemies on crop pests at the field scale. Insect Sci., 22, 20–34. [DOI] [PubMed] [Google Scholar]
- Magalhães, S. , Tudorache, C. , Montserrat, M. , van Maanen, R. , Sabelis, M.W. & Janssen, A. (2004). Diet of intraguild predators affects antipredator behavior in intraguild prey. Behav. Ecol., 16, 364–370. [Google Scholar]
- Mallet, J. & Porter, P. (1992). Preventing insect adaptation to insect‐resistant crops: are seed mixtures or refugia the best strategy? Proc. R. Soc. Lond. B Biol. Sci., 250, 165–169. [Google Scholar]
- Mappes, J. , Mappes, T. & Lappalainen, T. (1997). Unequal maternal investment in offspring quality in relation to predation risk. Evol. Ecol., 11, 7. [Google Scholar]
- Martini, X. , Kincy, N. & Nansen, C. (2012). Quantitative impact assessment of spray coverage and pest behavior on contact pesticide performance. Pest Manag. Sci., 68, 1471–1477. [DOI] [PubMed] [Google Scholar]
- Matsumoto, T. , Itioka, T. & Nishida, T. (2003). Rapid change in the settling behavior of the arrowhead scale Unaspis yanonensis as an avoidance mechanism against introduced parasitoids, Aphytis yanonensis and Coccobius fulvus . Entomol. Exp. Appl., 107, 105–113. [Google Scholar]
- McArthur, C. , Orlando, P. , Banks, P.B. & Brown, J.S. (2012). The foraging tightrope between predation risk and plant toxins: a matter of concentration. Funct. Ecol., 26, 74–83. [Google Scholar]
- McMurtry, J.A. , Huffaker, C.B. & van de Vrie, M. (1970). Ecology of tetranychid mites and their natural enemies: a review. Hilgardia, 40, 391–458. [Google Scholar]
- Meadows, A.J. , Owen, J.P. & Snyder, W.E. (2017). Keystone nonconsumptive effects within a diverse predator community. Ecol. Evol., 7, 10315–10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner, M. , Harmon, J.P. , Harvey, C.T. & Ives, A.R. (2011). Intraguild predation on the parasitoid Aphidius ervi by the generalist predator Harmonia axyridis: The threat and its avoidance. Entomol. Exp. Appl., 138, 193–201. [Google Scholar]
- Meresman, Y. , Ben‐ari, M. & Inbar, M. (2017). Turning in mid‐air allows aphids that flee the plant to avoid reaching the risky ground. Integr. Zool., 12, 409–420. [DOI] [PubMed] [Google Scholar]
- Michaud, J. & Belliure, B. (2001). Impact of syrphid predation on production of migrants in colonies of the brown citrus aphid, Toxoptera citricida (Homoptera: Aphididae). Biol. Control, 21, 91–95. [Google Scholar]
- Michaud, J.P. , Barbosa, P.R.R. , Bain, C.L. & Torres, J.B. (2016). Extending the “Ecology of Fear” Beyond Prey: Reciprocal nonconsumptive effects among competing aphid predators. Environ. Entomol., 45, 1398–1403. [DOI] [PubMed] [Google Scholar]
- Miner, B.G. , Sultan, S.E. , Morgan, S.G. , Padilla, D.K. & Relyea, R.A. (2005). Ecological consequences of phenotypic plasticity. Trends Ecol. Evol., 20, 685–692. [DOI] [PubMed] [Google Scholar]
- Mondor, E.B. , Rosenheim, J.A. & Addicott, J.F. (2005). Predator‐induced transgenerational phenotypic plasticity in the cotton aphid. Oecologia, 142, 104–108. [DOI] [PubMed] [Google Scholar]
- Moore, G.G. , Singh, R. , Horn, B.W. & Carbone, I. (2009). Recombination and lineage‐specific gene loss in the aflatoxin gene cluster of Aspergillus flavus . Mol. Ecol., 18, 4870–4887. [DOI] [PubMed] [Google Scholar]
- Murdoch, W.W. , Chesson, J. & Chesson, P.L. (1985). Biological control in theory and practice. Am. Nat., 125, 344. [Google Scholar]
- Nachman, G. (2006). The effects of prey patchiness, predator aggregation, and mutual interference on the functional response of Phytoseiulus persimilis Feeding on Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Exp. Appl. Acarol., 38, 87–111. [DOI] [PubMed] [Google Scholar]
- Nakashima, Y. & Senoo, N. (2003). Avoidance of ladybird trails by an aphid parasitoid Aphidius ervi: active period and effects of prior oviposition experience. Entomol. Exp. Appl., 109, 163–166. [Google Scholar]
- Naranjo, S.E. , Ellsworth, P.C. & Frisvold, G.B. (2015). Economic value of biological control in integrated pest management of managed plant systems. Annu. Rev. Entomol., 60, 621–645. [DOI] [PubMed] [Google Scholar]
- Nelson, E.H. (2007). Predator avoidance behavior in the pea aphid: Costs, frequency, and population consequences. Oecologia, 151, 22–32. [DOI] [PubMed] [Google Scholar]
- Nelson, E.H. & Rosenheim, J.A. (2006). Encounters between aphids and their predators: the relative frequencies of disturbance and consumption. Entomol. Exp. Appl., 118, 211–219. [Google Scholar]
- Ninkovic, V. , Feng, Y. , Olsson, U. & Pettersson, J. (2013). Ladybird footprints induce aphid avoidance behavior. Biol. Control, 65, 63–71. [Google Scholar]
- Northfieldm T.D. , Barton, B.T. , & Schmitz, O.J. (2017). A spatial theory for emergent multiple predator‐prey interactions in food webs. Ecol. Evol., 7 , 6935–6948. 10.1002/ece3.3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northfield, T.D. , Snyder, G.B. , Ives, A.R. & Snyder, W.E. (2010). Niche saturation reveals resource partitioning among consumers. Ecol. Lett., 13, 338–348. [DOI] [PubMed] [Google Scholar]
- Oi, D. , Porter, S. & Valles, S. (2015). A review of the biological control of fire ants. Myrmecol. News, 21, 101–116. [Google Scholar]
- Oi, D. , Valles, S. , Porter, S. , Cavanaugh, C. , White, G. & Henke, J. (2019). Introduction of fire ant biological control agents into the coachella valley of california. Fla. Entomol., 102, 284–286. [Google Scholar]
- Okada, J. & Akamine, S. (2012). Behavioral response to antennal tactile stimulation in the field cricket Gryllus bimaculatus. J. Comp. Physiol. A, 198, 557–565. [DOI] [PubMed] [Google Scholar]
- Op de Beeck, L. , Janssens, L. & Stoks, R. (2016). Synthetic predator cues impair immune function and make the biological pesticide Bti more lethal for vector mosquitoes. Ecol. Appl., 26, 355–366. [DOI] [PubMed] [Google Scholar]
- Orr, M. , Seike, S. , Benson, W. & Gilbert, L.E. (1995). Flies suppress fire ants. Nature, 373, 292. [Google Scholar]
- Orrock, J.L. , Preisser, E.L. , Grabowski, J.H. & Trussell, G.C. (2013). The cost of safety: refuges increase the impact of predation risk in aquatic systems. Ecology, 94, 573–579. [DOI] [PubMed] [Google Scholar]
- Otsuki H., & Yano S. (2014a). Functionally different predators break down antipredator defenses of spider mites. Entomol. Exp. Appl., 151, 27–33. 10.1111/eea.12164 [DOI] [Google Scholar]
- Otsuki, H. & Yano, S. (2014b). Potential lethal and non‐lethal effects of predators on dispersal of spider mites. Exp. Appl. Acarol., 64, 265–275. [DOI] [PubMed] [Google Scholar]
- Pallini, A. , Janssen, A. & Sabelis, M.W. (1999). Spider mites avoid plants with predators. Exp. Appl. Acarol., 23, 803–815. [Google Scholar]
- Pappas, M.L. , Broekgaarden, C. , Broufas, G.D. , Kant, M.R. , Messelink, G.J. , Steppuhn, A. et al (2017). Induced plant defences in biological control of arthropod pests: a double‐edged sword. Pest Manag. Sci., 73, 1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, R.A. , Pritchard, D.W. , Dick, J.T.A. , Alexander, M.E. , Hatcher, M.J. & Dunn, A.M. (2013). Predator cue studies reveal strong trait‐mediated effects in communities despite variation in experimental designs. Anim. Behav., 86, 1301–1313. [Google Scholar]
- Peacor, S.D. , Barton, B.T. , Kimbro, D.L. , Sih, A. & Sheriff, M.J. (2020). A framework and standardized terminology to facilitate the study of predation‐risk effects. Ecology, [DOI] [PubMed] [Google Scholar]
- Pearce, S. & Zalucki, M.P. (2006). Do predators aggregate in response to pest density in agroecosystems? Assessing within‐field spatial patterns. J. Appl. Ecol., 43, 128–140. [Google Scholar]
- Peckarsky, B.L. , Abrams, P.A. , Bolnick, D.I. & Dill, L.M. (2008). Revisiting the classics: Considering nonconsumptive effects in textbook examples of predator – prey interactions. Ecology, 89, 2416–2425. [DOI] [PubMed] [Google Scholar]
- Penfold, S. , Dayananda, B. & Webb, J.K. (2017). Chemical cues influence retreat‐site selection by flat rock spiders. Behaviour, 154, 149–161. [Google Scholar]
- Pepi, A.A. , Broadley, H.J. & Elkinton, J.S. (2016). Density‐dependent effects of larval dispersal mediated by host plant quality on populations of an invasive insect. Oecologia, 182, 499–509. [DOI] [PubMed] [Google Scholar]
- Polis, G.A. (1981). The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Syst., 12, 225–251. [Google Scholar]
- Polis, G.A. (1999). Why are parts of the world green? Multiple factors control productivity and the distribution of biomass. Oikos, 86, 3–15. [Google Scholar]
- Polis, G.A. , Myers, C.A. & Holt, R.D. (1989). The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu. Rev. Ecol. Syst., 20, 297–330. [Google Scholar]
- Porter, S.D. & Gilbert, L.E. (2004). Assessing host specificity and field release potential of fire ant decapitating flies (Phoridae: Pseudacteon). Assess. Host Ranges Parasit. Predat. Used Class. Biol. Control Guide Best Pract . For. Health Technol. Enterp. Team FHTET Publ., 3, 152–176. [Google Scholar]
- Porter, S.D. , Meer, R.K.V. , Pesquero, M.A. , Campiolo, S. & Fowler, H.G. (1995). Solenopsis (Hymenoptera: Formicidae) fire ant reactions to attacks of Pseudacteon flies (Diptera: Phoridae) in southeastern Brazil. Ann. Entomol. Soc. Am., 88, 570–575. [Google Scholar]
- Prasad, R.P. , Snyder, W.E. , Prasad, R.P. & Snyder, W.E. (2018). A non‐trophic interaction chain links predators in different spatial niches. Oecologia, 162(3), 747–753. [DOI] [PubMed] [Google Scholar]
- Preisser, E.L. & Bolnick, D.I. (2008). The many faces of fear: comparing the pathways and impacts of nonconsumptive predator effects on prey populations. PLoS One, 3, 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisser, E.L. , Bolnick, D.I. & Benard, M.F. (2005). Scared to death? The effects of intimidation and consumption in predator‐prey interactions. Ecology, 86, 501–509. [Google Scholar]
- Preisser, E.L. , Orrock, J.L. & Schmitz, O.J. (2007). Predator hunting mode and habitat domain alter nonconsumptive effects in predator‐prey interactions. Ecology, 88(11), 2744–2751. [DOI] [PubMed] [Google Scholar]
- Southwood, T.R.E. & Henderson, P. (2000). Ecological Methods, 3rd edn.
- Rabus, M. & Laforsch, C. (2011). Growing large and bulky in the presence of the enemy: Daphnia magna gradually switches the mode of inducible morphological defences. Funct. Ecol., 25, 1137–1143. [Google Scholar]
- Ramirez, R.A. , Crowder, D.W. , Snyder, G.B. , Strand, M.R. & Snyder, W.E. (2010). Antipredator behavior of Colorado potato beetle larvae differs by instar and attacking predator. Biol. Control, 53, 230–237. [Google Scholar]
- Raymond, B. , Darby, A. & Douglas, A. (2000). Intraguild predators and the spatial distribution of a parasitoid. Oecologia, 124, 367–372. [DOI] [PubMed] [Google Scholar]
- Reddy, G.V.P. (2002). Plant volatiles mediate orientation and plant preference by the predator Chrysoperla carnea Stephens (Neuroptera: Chrysopidae). Biol. Control, 25, 49–55. [Google Scholar]
- Relyea, R.A. (2007). Getting out alive: how predators affect the decision to metamorphose. Oecologia, 152, 389–400. [DOI] [PubMed] [Google Scholar]
- Relyea, R.A. , Stephens, P.R. , Barrow, L.N. , Blaustein, A.R. , Bradley, P.W. , Buck, J.C. et al (2018). Phylogenetic patterns of trait and trait plasticity evolution: Insights from amphibian embryos. Evolution, 72, 663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon, D. , Whitehouse, M.E.A. & Taylor, P.W. (2016). Consumptive and non‐consumptive effects of wolf spiders on cotton bollworms. Entomol. Exp. Appl., 158, 170–183. [Google Scholar]
- Roberts, D. (2014). Mosquito larvae change their feeding behavior in response to kairomones from some predators. J. Med. Entomol., 51, 368–374. [DOI] [PubMed] [Google Scholar]
- Rosenheim, J.A. , Kaya, H.K. , Ehler, L.E. , Marois, J.J. & Jaffee, B.A. (1995). Intraguild predation among biological control agents – Theory and evidence. Biol. Control, 5, 303–335. [Google Scholar]
- Rosenheim, J.A. (1998). Higher‐order predators and the regulation of insect herbivore populations. Annu. Rev. Entomol., 43, 421–447. [DOI] [PubMed] [Google Scholar]
- Rypstra, A.L. & Buddle, C.M. (2012). Spider silk reduces insect herbivory. Biol. Lett., 9, 20120948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul, W.‐C. & Jeschke, J.M. (2015). Eco‐evolutionary experience in novel species interactions. Ecol. Lett., 18, 236–245. [DOI] [PubMed] [Google Scholar]
- Schausberger, P. (2003). Cannibalism among phytoseiid mites: a review. Exp. Appl. Acarol., 29, 173–191. [DOI] [PubMed] [Google Scholar]
- Schmitz, O.J. (1998). Direct and indirect effects of predation and predation risk in old‐field interaction webs. Am. Nat., 151, 327–342. [DOI] [PubMed] [Google Scholar]
- Schmitz, O.J. , Beckerman, A.P. & O’Brien, K.M. (1997). Behaviorally mediated trophic cascades: Effects of predation risk on food web interactions. Ecology, 78, 1388–1399. [Google Scholar]
- Schmitz, O.J. , Grabowski, J.H. , Peckarsky, B.L. , Preisser, E.L. , Trussell, G.C. & Vonesh, J.R. (2008). From individuals to ecosystem function: towards an integration of evolutionary and ecosystem ecology. Ecology, 89, 2436–2445. [DOI] [PubMed] [Google Scholar]
- Schmitz, O.J. , Krivan, V. & Ovadia, O. (2004). Trophic cascades: The primacy of trait‐mediated indirect interactions. Ecol. Lett., 7, 153–163. [Google Scholar]
- Schoeppner, N.M. & Relyea, R.A. (2005). Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences: Damage, digestion, and defence. Ecol. Lett., 8, 505–512. [DOI] [PubMed] [Google Scholar]
- Seiter, M. & Schausberger, P. (2015). Maternal intraguild predation risk affects offspring anti‐predator behavior and learning in mites. Sci. Rep., 5, 15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, G.‐Z. , Zhu, Y.‐H. , Wu, Y. , Cao, Y.‐F. & Bian, J.‐H. (2019). Synergistic effects of predation and parasites on the overwinter survival of root voles. Oecologia, 191, 83–96. [DOI] [PubMed] [Google Scholar]
- Sih, A. (1992). Prey uncertainty and the balancing of antipredator and feeding. Am. Nat., 139, 1052–1069. [Google Scholar]
- Sih, A. , Bolnick, D.I. , Luttbeg, B. , Orrock, J.L. , Peacor, S.D. , Pintor, L.M. et al (2010). Predator‐prey naivete, antipredator behavior, and the ecology of predator invasions. Oikos, 119, 610–621. [Google Scholar]
- Sih, A. , Englund, G. & Wooster, D. (1998). Emergent impacts of multiple predators on prey. Trends Ecol. Evol., 13, 350–355. [DOI] [PubMed] [Google Scholar]
- Sih, A. & Wooster, D.E. (1994). Prey behavior, prey dispersal, and predator impacts on stream prey. Ecology, 75, 1199–1207. [Google Scholar]
- Silberbush, A. & Blaustein, L. (2011). Mosquito females quantify risk of predation to their progeny when selecting an oviposition site. Funct. Ecol., 25, 1091–1095. [Google Scholar]
- Silberbush, A. , Markman, S. , Lewinsohn, E. , Bar, E. , Cohen, J.E. & Blaustein, L. (2010). Predator‐released hydrocarbons repel oviposition by a mosquito. Ecol. Lett., 13, 1129–1138. [DOI] [PubMed] [Google Scholar]
- Silberbush, A. , Tsurim, I. , Margalith, Y. & Blaustein, L. (2014). Interactive effects of salinity and a predator on mosquito oviposition and larval performance. Oecologia, 175, 565–575. [DOI] [PubMed] [Google Scholar]
- Simberloff, D. & Stiling, P. (1996). How risky is biological control? Ecology, 77, 1965–1974. [Google Scholar]
- Sitvarin, M.I. & Rypstra, A.L. (2012). Sex‐specific response of Pardosa milvina (Araneae: Lycosidae) to experience with a chemotactile predation cue. Ethology, 118, 1230–1239. [Google Scholar]
- Skals, N. (2005). Her odours make him deaf: crossmodal modulation of olfaction and hearing in a male moth. J. Exp. Biol., 208, 595–601. [DOI] [PubMed] [Google Scholar]
- Sloggett, J.J. & Weisser, W.W. (2002). Parasitoids induce production of the dispersal morph of the pea aphid, Acyrthosiphon pisum . Oikos, 98, 323–333. [Google Scholar]
- Snyder, W.E. & Ives, A.R. (2008). Behavior influences whether intra‐guild predation disrupts herbivore suppression by parasitoids Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications (eds Wajnberg E., Bernstein C. & van Alphen J.). Blackwell Publishing, Malden, MA, pp. 71–91. [Google Scholar]
- Snyder, W.E. & Wise, D.H. (2000). Antipredator behavior of spotted cucumber beetles (Coleoptera: Chrysomelidae) in response to predators that pose varying risks. Environ. Entomol., 29, 35–42. [Google Scholar]
- Staats, E.G. , Agosta, S.J. & Vonesh, J.R. (2016). Predator diversity reduces habitat colonization by mosquitoes and midges. Biol. Lett., 12, 20160580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamps, J.A. (2007). Growth‐mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett., 10, 355–363. [DOI] [PubMed] [Google Scholar]
- Stankowich, T. & Blumstein, D.T. (2005). Fear in animals: a meta‐analysis and review of risk assessment. Proc. R. Soc. B Biol. Sci., 272, 2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny, M. & Sargent, R.D. (2017). Evidence for rapid evolutionary change in an invasive plant in response to biological control. J. Evol. Biol., 30, 1042–1052. [DOI] [PubMed] [Google Scholar]
- Stav, G. , Blaustein, L. & Margalit, Y. (2000). Influence of nymphal Anax imperator (Odonata: Aeshnidae) on oviposition by the mosquito Culiseta longiareolata (Diptera: Culicidae) and community structure in temporary pools. J. Vector Ecol., 25, 190–202. [PubMed] [Google Scholar]
- Stav, G. , Kotler, B.P. & Blaustein, L. (2010). Foraging response to risks of predation and competition in artificial pools. Isr. J. Ecol. Evol., 56, 9–20. [Google Scholar]
- Steffan, S.A. & Snyder, W.E. (2010). Cascading diversity effects transmitted exclusively by behavioral interactions. Ecology, 91, 2242–2252. [DOI] [PubMed] [Google Scholar]
- Stephan, J.G. , Stenberg, J.A. & Björkman, C. (2017). Consumptive and nonconsumptive effect ratios depend on interaction between plant quality and hunting behavior of omnivorous predators. Ecol. Evol., 7, 2327–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, C.S. & Snyder, W.E. (2008). Increasing enemy biodiversity strengthens herbivore suppression on two plant species. Ecology, 89, 1605–1615. [DOI] [PubMed] [Google Scholar]
- Suh, C.‐P.‐C. , Orr, D.B. & Van Duyn, J.W. (2000). Trichogramma releases in north carolina cotton: Why releases fail to suppress heliothine pests. J. Econ. Entomol., 93, 1137–1145. [DOI] [PubMed] [Google Scholar]
- Tabashnik, B.E. , Brévault, T. & Carrière, Y. (2013). Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol., 31, 510. [DOI] [PubMed] [Google Scholar]
- Tamaki, G. , Eric, J.E. & Hathaway, D.O. (1970). Dispersal and reduction of colonies of pea aphids by Aphidius smithi (Hymenoptera: Aphidiidae). Ann Entomol Soc Am, 63, 973–980. [Google Scholar]
- Thaker, M. , Vanak, A.T. , Owen, C.R. , Ogden, M.B. , Niemann, S.M. & Slotow, R. (2011). Minimizing predation risk in a landscape of multiple predators: effects on the spatial distribution of African ungulates. Ecology, 92, 398–407. [DOI] [PubMed] [Google Scholar]
- Thaler, J.S. , Contreras, H. & Davidowitz, G. (2014). Effects of predation risk and plant resistance on Manduca sexta caterpillar feeding behaviour and physiology. Ecol. Entomol., 39, 210–216. [Google Scholar]
- Thaler, J.S. & Griffin, C.A.M. (2008). Relative importance of consumptive and non‐consumptive effects of predators on prey and plant damage: the influence of herbivore ontogeny. Entomol. Exp. Appl., 128, 34–40. [Google Scholar]
- Thaler, J.S. , McArt, S.H. & Kaplan, I. (2012). Compensatory mechanisms for ameliorating the fundamental trade‐off between predator avoidance and foraging. Proc. Natl Acad. Sci., 109, 12075–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholt, G. , Kis, A. , Medzihradszky, A. , Szita, É. , Tóth, Z. , Havelda, Z. et al (2018). Could vectors’ fear of predators reduce the spread of plant diseases? Sci. Rep., 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollrian, R. (1995). Predator‐induced morphological defenses: Costs, life history shifts, and maternal effects in daphnia pulex. Ecology, 76, 1691–1705. [Google Scholar]
- Trimmer, P.C. , Ehlman, S.M. & Sih, A. (2017). Predicting behavioural responses to novel organisms: state‐dependent detection theory. Proc. R. Soc. B Biol. Sci., 284, 20162108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharntke, T. , Bommarco, R. , Clough, Y. , Crist, T.O. , Kleijn, D. , Rand, T.A. et al (2007). Conservation biological control and enemy diversity on a landscape scale. Biol. Control, 43, 294–309. [Google Scholar]
- Tscharntke, T. , Karp, D.S. , Chaplin‐Kramer, R. , Batáry, P. , DeClerck, F. , Gratton, C. et al (2016). When natural habitat fails to enhance biological pest control – Five hypotheses. Biol. Cons., 204, 449–458. [Google Scholar]
- Tyndale‐Biscoe, M. & Vogt, W.G. (1996). Population status of the bush fly, Musca vetustissima (Diptera: Muscidae), and native dung beetles (Coleoptera: Scarabaeinae) in south‐eastern Australia in relation to establishment of exotic dung beetles. Bull. Entomol. Res., 86, 183. [Google Scholar]
- Uesugi, A. (2015). The slow‐growth high‐mortality hypothesis: direct experimental support in a leafmining fly. Ecol. Entomol., 40, 221–228. [Google Scholar]
- Ugine, T.A. & Thaler, J.S. (2020). Insect predator odors protect herbivore from fungal infection. Biol. Control, 143, 104186. [Google Scholar]
- Valente, C. , Afonso, C. , Gonçalves, C.I. , Alonso‐Zarazaga, M.A. , Reis, A. & Branco, M. (2017). Environmental risk assessment of the egg parasitoid Anaphes inexpectatus for classical biological control of the Eucalyptus snout beetle, Gonipterus platensis . Biocontrol, 62, 457–468. [Google Scholar]
- Van Driesche, R.G. (2016). Methods for evaluation of natural enemy impacts on invasive pests of wildlands Integrating Biological Control into Conservation Practice (eds Van Driesche R.G., Simberloff D., Blossey B., Causton C., Hoddle M.S., Wagner D.L., Marks C.O., Heinz K.M. & Warner K.D.). Wiley, West Sussex, UK, pp. 189–207. [Google Scholar]
- Van Veen, F.J.F. , Rajkumar, A. , Muller, C.B. & Godfray, H.C.J. (2001). Increased reproduction by pea aphids in the presence of secondary parasitoids. Ecol. Entomol., 26, 425–429. [Google Scholar]
- Vance‐Chalcraft, H.D. , Rosenheim, J.A. , Vonesh, J.R. , Osenberg, C.W. & Sih, A. (2007). The influence of intraguild predation on prey suppression and prey release: a meta‐analysis. Ecology, 88, 2689–2696. [DOI] [PubMed] [Google Scholar]
- Vance‐Chalcraft, H.D. & Soluk, D.A. (2005). Estimating the prevalence and strength of non‐independent predator effects. Oecologia, 146, 452–460. [DOI] [PubMed] [Google Scholar]
- Vandermoten, S. , Mescher, M.C. , Francis, F. , Haubruge, E. & Verheggen, F.J. (2012). Aphid alarm pheromone: an overview of current knowledge on biosynthesis and functions. Insect Biochem. Mol. Biol., 42, 155–163. [DOI] [PubMed] [Google Scholar]
- Velasco‐Hernández, M.C. , Ramirez‐Romero, R. , Cicero, L. , Michel‐Rios, C. & Desneux, N. (2013). Intraguild predation on the whitefly parasitoid Eretmocerus eremicus by the generalist predator Geocoris punctipes: a behavioral approach. PLoS One, 8, e80679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venzon, M. , Janssen, A. , Pallini, A. & Sabelis, M.W. (2000). Diet of a polyphagous arthropod predator affects refuge seeking of its thrips prey. Anim. Behav., 60, 369–375. [DOI] [PubMed] [Google Scholar]
- Verdolin, J.L. (2006). Meta‐analysis of foraging and predation risk trade‐offs in terrestrial systems. Behav. Ecol. Sociobiol., 60, 457–464. [Google Scholar]
- Vonesh, J.R. & Blaustein, L. (2010). Predator‐induced shifts in mosquito oviposition site selection: A meta‐analysis and implications for vector control. Isr. J. Ecol. Evol., 56, 263–279. [Google Scholar]
- de Vos, M. , Cheng, W.Y. , Summers, H.E. , Raguso, R.A. & Jander, G. (2010). Alarm pheromone habituation in Myzus persicae has fitness consequences and causes extensive gene expression changes. Proc. Natl Acad. Sci., 107, 14673–14678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer, A. , Lepp, N. & Schausberger, P. (2015). Compensatory growth following transient intraguild predation risk in predatory mites. Oikos, 124, 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer, A. & Schausberger, P. (2009). Non‐consumptive effects of predatory mites on thrips and its host plant. Oikos, 118, 934–940. [Google Scholar]
- Wanger, T.C. , Wielgoss, A.C. , Motzke, I. , Clough, Y. , Brook, B.W. , Sodhi, N.S. et al (2011). Endemic predators, invasive prey and native diversity. Proc. R. Soc. B Biol. Sci., 278, 690–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg, A. , Faiman, R. , Shtern, A. , Silberbush, A. , Markman, S. , Cohen, J.E. et al (2011). Oviposition habitat selection by anopheles gambiae in response to chemical cues by notonecta maculata oviposition habitat selection by anopheles gambiae in response to chemical cues by notonecta maculata. J. Vector Ecol., 36, 421–425. [DOI] [PubMed] [Google Scholar]
- Wasserberg, G. , White, L. , Bullard, A. , King, J. & Maxwell, R. (2013). Oviposition site selection in Aedes albopictus (Diptera: Culicidae): are the effects of predation risk and food level independent? J Med Entomol, 50, 1159–1164. [DOI] [PubMed] [Google Scholar]
- Weber, D.C. , Rowley, D.L. , Greenstone, M.H. & Athanas, M.M. (2006). Prey preference and host suitability of the predatory and parasitoid carabid beetle, Lebia grandis, for several species of Leptinotarsa beetles. J. Insect Sci., 6, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissburg, M. & Beauvais, J. (2015). The smell of success: the amount of prey consumed by predators determines the strength and range of cascading non‐consumptive effects. PeerJ, 3, e1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissburg, M. , Smee, D.L. & Ferner, M.C. (2014). The sensory ecology of nonconsumptive predator effects. Am. Nat., 184, 141–157. [DOI] [PubMed] [Google Scholar]
- Weisser, W.W. , Braendle, C. & Minoretti, N. (1999). Predator‐induced morphological shift in the pea aphid. Proc. R. Soc. B Biol. Sci., 266, 1175–1181. [Google Scholar]
- Welch, K.D. & Harwood, J.D. (2014). Temporal dynamics of natural enemy‐pest interactions in a changing environment. Biol. Control, 75, 18–27. [Google Scholar]
- Werner, E.E. & Anholt, B.R. (1996). Predator‐induced behavioral indirect effects: consequences to competitive interactions in anuran larvae. Ecology, 77, 157–169. [Google Scholar]
- Werner, E.E. & Peacor, S.D. (2003). A review of trait‐ mediated indirect interactions in ecological communities. Ecology, 84, 1083–1100. [Google Scholar]
- Wiedenmann, R.N. & Smith, J.W. (1997). Attributes of natural enemies in ephemeral crop habitats. Biol. Control, 10, 16–22. [Google Scholar]
- Wilson, E.E. , Mullen, L.M. & Holway, D.A. (2009). Life history plasticity magnifies the ecological effects of a social wasp invasion. Proc. Natl Acad. Sci., 106, 12809–12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M.R. & Leather, S.R. (2012). The effect of past natural enemy activity on host‐plant preference of two aphid species. Entomol. Exp. Appl., 144, 216–222. [Google Scholar]
- Winder, L. , Alexander, C.J. , Holland, J.M. , Woolley, C. & Perry, J.N. (2001). Modelling the dynamic spatio‐temporal response of predators to transient prey patches in the field. Ecol. Lett., 4, 568–576. [Google Scholar]
- Wise, D.H. (2006). Cannibalism, food limitation, intraspecific competition, and the regulation of spider populations. Annu. Rev. Entomol., 51, 441–465. [DOI] [PubMed] [Google Scholar]
- Wuellner, A.C.T. , Aglio‐Holvorcem, C.G.D. , Benson, W.W. & Gilbert, E. (2002). Phorid Fly ( Diptera : Phoridae ) oviposition behavior and fire ant ( Hymenoptera : Formicidae ) reaction to attack differ according to phorid species phorid fly ( Diptera : Phoridae ) oviposition behavior and fire ant ( Hymenoptera : Formicidae ) reactio. Ann. Entomol. Soc. Am., 95, 257–266. [Google Scholar]
- Xiong, X. , Michaud, J. P. , Li, Z. , Wu, P. , Chu, Y. , et al. (2015). Chronic, predator‐induced stress alters development and reproductive performance of the cotton bollworm, Helicoverpa armigera. BioControl, 60, 827–837. 10.1007/s10526-015-9689-9 [DOI] [Google Scholar]
- Zaguri, M. & Hawlena, D. (2019). Bearding the scorpion in his den: desert isopods take risks to validate their ‘landscape of fear’ assessment. Oikos, 128, 1458–1466. [Google Scholar]
- Zhang, W. & Swinton, S.M. (2012). Optimal control of soybean aphid in the presence of natural enemies and the implied value of their ecosystem services. J. Environ. Manage., 96, 7–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
No original data were used in the manuscript, and all search terms and articles from the literature review are described and cited.


