Abstract
Objective
To establish novel facial characteristics unique to congenital rubella syndrome (CRS) as prediagnostic criteria to supplement disease diagnosis in patients with or without a history of maternal rubella infection.
Design
An analysis of 115 CRS case series (2018–2020) based on the presence of any of the triad features.
Setting
Outpatient department of a tertiary care referral cardiac hospital in Dhaka, Bangladesh.
Participants
In total, 115 participants (53.1% men) were enrolled. Participants underwent echocardiography if they presented with suspected cardiac symptoms along with deafness, cataract or microcephaly.
Main outcome measures
Age, sex and socioeconomic status of the participants; history of maternal vaccination and infection; facial characteristics unique to CRS (triangular face, prominent nose, wide forehead and a whorl on either side of the anterior hairline) named ‘rubella facies’ and frequency of systemic involvements in CRS.
Results
The median patient age was 2 years. The income of 50.4% of the participating families was <US$1500. Further, 32 mothers (27.8%) were infected with rubella during the first trimester of pregnancy, 15 (13.0%) during the second trimester and 3 (2.6%) during the third trimester. The remainder (65.2%) recalled no history of infection during pregnancy. Rubella facies presented as a triangular-shaped face in 95 (82.6%) cases, a broad forehead in 88 (76.5%) and a prominent nose in 75 (65.2%). A rubella whorl was present on the right or left side of the anterior hairline in 80% and 18.2% of cases, respectively. IgG and IgM antibodies were present in 91.3% and 8.6% of children, respectively. Cataract, deafness, microcephaly, and congenital heart disease were detected in 53.0%, 75.6%, 68.6% and 98.2% of cases, respectively.
Conclusions
Rubella facies, a set of unique facial characteristics, can support early CRS diagnosis and treatment and may supplement the existing CRS triad.
Keywords: cardiology, deafness, data collection, ophthalmology, syndrome
What is known about the subject?
Clinical diagnosis of congenital rubella syndrome is possible from a characteristic triad: cataract, deafness and congenital heart disease.
There are also some minor criteria such as microcephaly, splenomegaly, thrombocytopenia, developmental delay and failure to thrive.
In low-income and middle/low-income countries, pregnant women and neonates are not screened routinely for rubella.
What this study adds?
This study reports novel facial characteristics (triangular face, wide forehead, prominent nose and hair whorl on the anterior hairline) as supplementary findings in early recognition and diagnostic confirmation.
None of the mothers had received a rubella vaccine.
The median age of diagnosis was 2 years.
Introduction
Rubella virus belongs to the Togaviridae family and is a teratogenic agent that can cross the placenta and cause fetal infection at a risk rate dependent on gestational age.1 If acquired in the first trimester, such an infection can lead to congenital rubella syndrome (CRS), which is characterised by a triad consisting of congenital deafness, cataract and congenital heart disease (CHD).2 Sir Norman Gregg, an Australian ophthalmologist, first identified the relationship between gestational rubella infection and cataract in 1941 during a rubella outbreak in Australia.3 This discovery was followed by the identification of other complications associated with rubella infection, including microcephaly, low birth weight, hepatosplenomegaly, bone lesions, dental defects, hypospadias, cryptorchidism and inguinal hernia; additionally, interstitial pneumonitis, thyroid dysfunction, cerebral calcification, diabetes mellitus and micrognathia may also be present.4 CRS is associated with high mortality rates and significant morbidity.5 It can be prevented by an anti-rubella vaccine, which has been available since 1969.6
However, CRS remains common in countries where access to the anti-rubella vaccine is restricted; moreover, its incidence is often under-reported or underestimated. Owing to insufficient vaccination coverage among women of reproductive age in countries such as Bangladesh, Nigeria, Vietnam and Ethiopia, the incidence of rubella and CRS in these countries remains high.7 8 In Bangladesh, the anti-rubella vaccination was launched in January 2014 and included in the expanded programme for immunisation for children.9 Girls and women who are currently of reproductive age (15–49 years) and at risk were not included in the programme.10 The serological status with respect to rubella antibodies is rarely assessed during pregnancy, and serial screening is not advised in these countries. Additionally, screening for hearing defects in neonates is not routinely performed, further delaying the diagnosis of CRS.
Rubella virus infection in children and adults usually causes a mild, self-limiting, rash-like illness with minor complications. However, if a rubella infection is contracted during pregnancy, particularly during the first trimester, it may lead to significant complications. One such complication is CRS, which is challenging to diagnose owing to variability in signs and symptoms at presentation. Variable factors include the time elapsed between maternal infection and the birth of an infant, an unclear history of maternal infection, restricted access to hearing tests at birth and varied availability of echocardiography-based screening among babies of mothers with a suspected history of gestational rubella.2 7 11 Moreover, most women infected with rubella do not present with classic symptoms such as fever and rash.
Since the discovery of the rubella vaccine, the incidence of CRS has been significantly reduced in high-income countries, resulting in very few studies being conducted in this field of medicine in recent times. However, owing to the aforementioned reasons, CRS is still a pressing public health issue in the low-income and lower/middle-income countries, and it is essential to establish an easier and faster diagnostic method.
Several congenital syndromes are associated with facial characteristics such as those observed in Down’s, Noonan’s and Turner’s syndromes, among others, and could allow early diagnosis and management.12 Forrest and Menser13 had identified elfin-like facies in CRS (ADC 1970). This study examined the facial characteristics of patients with CRS and identified and reported unique features of ‘rubella facies’ which came from observation of a treating cardiologist that they all possessed similar facial characteristics. It aimed to characterise the facial morphology of patients with CRS from a visual impression and to establish these novel facial characteristics as a prediagnostic tool that may aid in earlier diagnosis and subsequent management of CRS.
Methods
Subjects
Patients were referred from ophthalmologists, otorhinolaryngologists and paediatricians for cardiac evaluation of suspected or clinically confirmed CRS. These patients showed one or more components of the rubella characteristic triad. All the patients had undergone clinical examination and echocardiography as part of their cardiac evaluation. Therefore, after obtaining written informed consent from the parents of these patients, their photographs with Samsung Galaxy S8 (Samsung Electronics, South Korea) camera were taken and stored with the patients’ clinical records for further analysis.
All children referred with suspected CRS aged between 3 months and 14 years were included in the study. Nineteen patients with other syndromes who were also associated with particular facial characteristics such as those in Noonan’s and Turner’s syndromes were excluded from the study. It was not possible for these patients to participate in the design or planning of the study since all were under 18 years and did not have the mental capacity to participate in such activities.
Setting
The study was conducted in the outpatient department of a paediatric cardiology unit in a tertiary cardiac hospital in Dhaka, Bangladesh (January 2018–January 2020).
Data collection
Demographic characteristics, such as age, sex, socioeconomic status, presence of one or more components of the rubella triad, maternal vaccination status, and history of rubella infection during pregnancy were documented, reviewed and analysed. The rubella antibody (IgG and IgM) status of the patients was obtained from medical records. Paired maternal antibody was not recommended for them.
Photographs were taken when the patients visited the hospital for echocardiography. Photographs were collected for this study since January 2018. The facial characteristics were visually examined in person during the patients’ visits and later analysed using the photographs. The patterns of patients’ heart diseases were recorded from their echocardiography reports.
Case definition of CRS
Clinically compatible case: a patient with two major components of the triad or one major and another minor component, such as microcephaly, splenomegaly, developmental delay, failure to thrive, thrombocytopenia and meningoencephalitis.
Probable case: a patient with heart disease and suspected hearing impairment, or at least one eye defect (cataract, glaucoma, pigmentary retinopathy, microphthalmos, optic atrophy, strabismus, nystagmus).
Laboratory-confirmed case: infant having at least one major component and meeting one of the following laboratory criteria: (a) detection of rubella IgM antibody or (b) sustained level of rubella IgG antibodies, as determined at age 6–12 months in the absence of rubella vaccination.
Major criteria of CRS
Deafness: as confirmed by the distraction test in the paediatric cardiology outpatient clinic and otoacoustic emission (OAE) and auditory brainstem response (ABR) in the otolaryngology department.
Eye findings (cataract and other findings): as confirmed by a detailed eye examination, including examination of the lens, ocular pressure (tonometry), examination of the retina and refraction test, under general anaesthesia by an ophthalmologist.
CHD: as identified on clinical examination and confirmed on echocardiography.
Minor criteria of CRS
Microcephaly: as confirmed by measurement of the occipitofrontal circumference. If the circumference is >2 SDs smaller than the mean for the patient’s age and sex.
Others: splenomegaly, developmental delay, failure to thrive, thrombocytopenia, meningoencephalitis and jaundice.
Definition of rubella facies
A triangular face, a prominent nose, a wide or broad forehead, and the presence of a whorl on the right or left side of the anterior hairline were components of rubella facies. The whorl on the hairline was named ‘rubella whorl’. All these characteristics were checked on visual examination. These findings were further analysed using photographs of the patients.
Triangular face: the forehead was considered as the base and the chin as the apex of the triangle.
Prominent nose: an increased distance between the subnasale and pronasale.14 The nasal bridge was sharp and formed a straight line with the tip of the nose.
Wide forehead: the frontal hairline was retreated, resulting a wide space above the eyebrow.
Whorl in the anterior hairline: the circular distribution of the hair on the scalp that revolves around an axis as determined by the direction of the growing follicle.
Facial characteristics overlapping with those of other diseases were excluded by thorough analysis.
Statistical analysis
Continuous data are expressed as means±SDs; categorical data are expressed as frequencies and percentages. Since this was a single-variant study, no comparative tests were needed.
Patient and public involvement
Patients were recruited from paediatric cardiology outpatient department. Written informed consent for participation in the study was obtained from all the parents of the patients, and the reason for taking their photographs was thoroughly explained. Photographs were taken to establish novel facial characteristics of patients with CRS.
As this study was conducted in a cardiac centre, the patients were followed up for their cardiac symptoms. This study did not intend to evaluate treatment outcome, rather it aimed to investigate only observation of new facial characteristics; hence, there was no active participation other than requiring consent to use patients’ photograph for analysis and publication. When the paper gets published, participants will be informed.
Results
From January 2018 to January 2020, 115 confirmed and suspected cases of CRS were reported to the Paediatric Cardiology Department of Labaid Cardiac Hospital for cardiac evaluation and management. Owing to the lack of a routine screening programme for pregnant women and newborns, the median age of detection in the study population was 2 years which was high. A poor socioeconomic status of most patients (50.4%) resulted in delayed reporting to the treatment facility. It took time for the parents themselves to identify any abnormality and to seek treatment. Clinically compatible cases in the series were 62.7%, probable cases were 28.7% and laboratory-confirmed cases were 8.6%. None of the mothers were immunised with the rubella vaccine. Some mothers were able to report a history of fever and rash in the first trimester (27.8%), the most critical time for teratogenicity due to rubella. Most mothers (65.2%) had either no history or an unknown history of such an illness (table 1).
Table 1.
Demographic characteristics of the children and mothers included in the study
| Variables | Mean±SD/no (%) |
| Patient age (range 3 months–14 years, median 2 years) | 4.4±2.8 years |
| Case definition | |
| Clinically compatible case | 72 (62.7) |
| Probable case | 33 (28.7) |
| Laboratory-confirmed case | 10 (8.6) |
| Sex | |
| Male | 61 (53.0) |
| Female | 54 (46.9) |
| Socioeconomic status | |
| Income <US$1500/year | 58 50.4) |
| Income US$1500–4500/year | 49 (42.6) |
| Income >US$4500/year | 9 (7.8) |
| Maternal vaccination status | 0 |
| Maternal infection status | |
| 1st trimester | 32 (27.8) |
| 2nd trimester | 15 (13.0) |
| 3rd trimester | 3 (2.6) |
| Unknown/no history | 75 (65.2) |
Serological tests identified acute infection in children owing to the presence of IgM in 10 (8.6%) cases. All were aged <1 year and were capable of infecting pregnant women, thereby transmitting the infection (figure 1).
Figure 1.

Frequency of rubella antibodies in patients with congenital rubella syndrome (N=115).
Among systemic manifestations of CRS and established major components of the triad, 98.2% of cases reported CHD. All patients were suspected of having CRS or were diagnosed with it during referral. Otolaryngologists referred 87 (75.6%) cases and ophthalmologists referred 61 (53.1) cases. Microcephaly cases (68.6%) were referred from paediatricians (figure 2).
Figure 2.
Distribution of systemic involvements among patients with congenital rubella syndrome (N=115).
Rubella facies is composed of four different components. Triangular face was found in 95 cases (82.6%), broad forehead in 88 (76.5%), prominent nose in 75 (65.2%), and whorl on the right or left side of the anterior hairline in 92 (80%) and 21 (18.2%) cases, respectively. In the later part of the study period, many cases were suspected following face observation on first sight. A triangular face with a whorl on the hairline was the most frequent combination (table 2, figures 3–5).
Table 2.
Frequency of facial characteristics (rubella facies) observed among patients with congenital rubella syndrome (N=115)
| Rubella facies | No (%) |
| Triangular face | 95 (82.6) |
| Broad forehead | 88 (76.5) |
| Whorl in hairline on right side of forehead (rubella whorl) | 92 (80) |
| Whorl in hairline on left side of forehead (rubella whorl) | 21 (18.2) |
| Prominent nose | 75 (65.2) |
Figure 3.
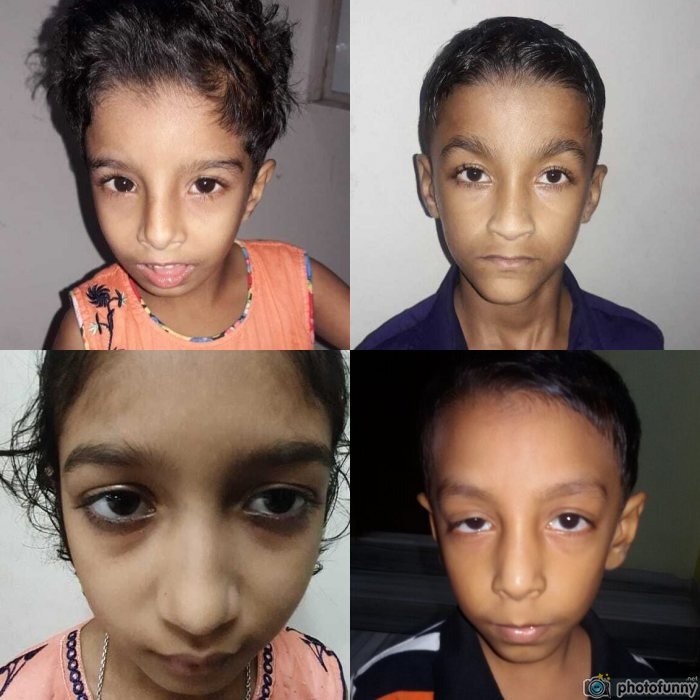
Triangular face in patients with congenital rubella syndrome.
Figure 4.

Prominent nose and wide forehead observed in patients with congenital rubella syndrome.
Figure 5.

Photographs of patients with congenital rubella syndrome exhibiting hair whorl on their anterior hairline, either on left or right side.
The patterns of CHD in the study population were analysed. Patent ductus arteriosus (PDA) was the most common isolated and combined lesion (76.5%) with other valvular diseases (table 3).
Table 3.
Types of congenital heart diseases (CHDs) (N=115) in CRS cases revealed from echocardiography
| Echocardiography findings | No | Percentage | |
| PDA (isolated and with other lesions) | 88 | 76.5 | Total CHD |
| Valvular stenosis, AS, PS (isolated and in combination with other CHDs) | 44 | 38.2 | 98.2 |
| RPA, LPA stenosis (isolated and combined) | 17 | 14.7 | |
| VSD (isolated or with PS) | 3 | 2.6 | |
| ASD (isolated or with PS) | 10 | 8.6 | |
| COA (isolated and combined) | 6 | 5.2 | |
| Cor triatriatum | 1 | 0.8 | |
| Normal heart | 2 | 1.7 | |
AS, aortic stenosis; ASD, atrial septal defect; COA, coarctation of the aorta; CRS, congenital rubella syndrome; LPA, left pulmonary artery; PDA, patent ductus arteriosus; PS, pulmonary stenosis; RPA, right pulmonary artery; VSD, ventricular septal defect.
Discussion
In this study, four components of facial characteristics emerged, named ‘rubella facies.’ The four components were triangular face (figure 3), wide forehead (figure 4), prominent nose (figure 4), and whorl on the right or left side of the anterior hairline (figure 5). Not all patients demonstrated all four components of rubella facies, but most patients showed a combination of these components (table 2). Cardiac involvement was 98.2% as patients were referred from other departments for cardiac evaluation after suspicion.
There were no age-related differences in the facial characteristics themselves (ie, the characteristics were not different between older and younger patients); however, the features were more prominent in older participants.
This study also found that there was a wide variation in the age of the participants (3 months–14 years, median 2 years). Therefore, even though this is a congenital disease, the wide age range of the study population indicates the lack of screening for pregnant women and infants using laboratory tests (rubella IgG and IgM antibody and reverse transcription-PCR for virus detection), thus delaying the diagnosis of patients with CRS.
As this study was conducted in a paediatric cardiology clinic, the pattern of heart disease in CRS was also analysed. PDA was found to be the most common cardiac condition associated with CRS.
In an epidemiological study conducted in the USA, 60% of the infants and children were diagnosed with CRS at birth or before 1 month of age and 16% were not diagnosed until 3–16 months of age. These patients also presented with PDA, hepatomegaly and thrombocytopenic purpura.15 In another study, the mean age of patients at diagnosis of CRS was 3 months.8 In our study, the mean age of the participants was 4.42±2.8 years, and median was 2 years which indicated a more delayed identification of these cases than those in the aforementioned studies.
Facial dysmorphism is the hallmark of many genetic and congenital disorders. The most frequently encountered chromosomal abnormality with distinct facial characteristics is Down’s syndrome.16 Turner’s syndrome is another disease that can be identified by a triangular face.17 18 Children with fragile X syndrome have long, narrow faces, prominent ears, joint hypermobility and flat feet, which become more obvious with increasing age and may help with this syndrome’s diagnosis.19 Noonan’s syndrome is a genetic disorder, characterised by mildly unusual facial features, such as long forehead, hypertelorism, down slanting palpebral fissures, short and broad nose, deep philtrum, widely spaced eyes, thick hooded eyelids and excessive nuchal skin with low posterior hairline.20 21
Rubella facies is a novel set of characteristics established in this study, which can serve as a hallmark such as those in the aforementioned syndromes. However, the components of rubella facies are found in different combinations in many other syndromes. ‘Triangular face’ has been defined as a hypoplastic face with prominent zygomatic arches, orbital hypertelorism, sunken cheeks, downturned mouth, a characteristic of Mulibrey nanism.22 Triangular face is also a characteristic feature of the Russel-Silver syndrome, Noonan’s syndrome, the Prader-Willi syndrome and the Angelman syndrome, among others.23
A wide or broad forehead is associated with some rare disorders such as facial dysmorphism-immunodeficiency-livedo-short stature syndrome.24
A prominent nose is found in patients with Traboulsi syndrome, heart diseases and autism spectrum disorder, among other diseases.25–27
Another component of rubella facies was the hair whorl on the anterior hairline, also known as a hair crown or cowlick. Abnormal hair whorls are also present in many other diseases, such as microcephaly, Apert’s syndrome and Crouzon’s syndrome, among others.28
Components of rubella facies were found unique after excluding other possibilities mentioned.
Facial features of CRS, named rubella facies, can be used as a landmark for the detection of CRS in the low-income and low/middle-income countries where the universal availability of the rubella vaccine, especially for girls and women of reproductive age, remains low.7 8 Most women infected during pregnancy were overlooked or missed because they showed only mild symptoms. Additionally, serological screening is not a part of antenatal check-ups in these countries (eg, Bangladesh, Nigeria, India, Vietnam, Ethiopia).29 Acute symptoms, such as thrombocytopenic purpura, hepatomegaly and PDA with heart failure are not always seen in neonates15; neonatal hearing assessment using OAE and ABR is not performed in low-income and low/middle-income countries routinely, and therefore, diagnosis is often missed.30 Therefore, some infants and children are permanently blind or deaf at the time of diagnosis. Many others die from heart failure due to PDA. Rubella facies will be a valuable diagnostic clue informing clinicians and parents in these countries to seek medical care available there.
Limitations of this study exist primarily in the design. First, photographs were taken by non-professional photographers; in most cases, the echocardiographer or cardiologist photographed the patient using a mobile phone with a two-dimensional camera. Second, three-dimensional (3D) digital anthropometric analysis was not performed. This restricted an advanced, detailed analysis of the face, such as measuring the accurate circumference of the forehead or the exact distance between prenasale and subnasale. 3D photographic methods have been used for facial analysis or anthropometric measurement of various races to compare them with other races and genders.31 32 Therefore, exact measurements of faces, noses and foreheads were not taken; hence, comparison with normative data was not possible. Normative data for Bangladeshi Asian populations were also not available. Third, this was a preliminary study on the findings of rubella facies by visual impression alone; no comparison was done with facial features of unaffected children (ie, a patient’s wide forehead was not measured or compared with the forehead of a normal child). This study was conducted on patients with CRS who were referred for cardiac evaluation. Therefore, many cases with milder symptoms in the community were not included. Hence, the epidemiology in this study does not represent the true status.
Identification of rubella facies will help clinicians and parents suspect CRS earlier in children both at a primary healthcare level or at home. Initial suspicion will lead to subsequent investigation and management, thus preventing permanent visual and hearing disabilities in several children. Early identification and treatment of CHD will prevent mortality due to heart failure in CRS cases.
Future research by dysmorphologist is necessary to compare patients with rubella facies with control group and to compare the influence of the triad on components of facies. In this study, no age-related influence on the facial characteristics was observed. Further studies are necessary to observe the variation of rubella facies with age (ie, to compare affected neonates, infants, adolescents and adults). Correlation of rubella facies with mental retardation and microcephaly may also be studied.
Recommendations for low-income and lower/middle-income countries
Achieving complete anti-rubella vaccination coverage for uncovered adolescent girls and women of childbearing age along with universal immunisation programme containing measles, mumps and rubella vaccine is paramount.
Rubella antibody screening for all pregnant women.
Screening of all infants for hearing impairment is encouraged.
Sustainable birth defect surveillance (eg, screening of all children with CHD, cataract, microcephaly, congenital deafness, unexplained hepatosplenomegaly or thrombocytopenia) for rubella and correlating them with rubella facies (which is evident at first sight) is crucial.
Conclusion
Rubella facies has the potential to serve as a landmark prediagnostic criterion of CRS, especially in low-income and lower/middle-income countries, where rubella remains a major public health concern with high morbidity and mortality rates. Using facial features to identify suspected cases of CRS with minimal or unrevealed criteria may help achieve earlier diagnoses and treatment.
Supplementary Material
Acknowledgments
I would like to thank the Paediatric Cardiology Department of Labaid for their help with patient care, Mashiyat Mayisha Ahmad (Pharmacology) for her help with editing the paper and Dr Abdullah Al Amin for helping with the administrative work.
Footnotes
Contributors: The corresponding author came up with the idea and design of the study. They observed the unique facial characteristics in patients with CRS, and planned and conducted the study, including taking photographs of the patients, analysing the data, writing the manuscript and editing it.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: There are no competing interests.
Patient consent for publication: Parental/guardian consent obtained.
Ethics approval: This study was conducted as per the Declaration of Helsinki and was approved by the Ethics Committee of Labaid Cardiac Hospital, Dhaka (Protocol number: 200716/01).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information. This paper contains identifiable photographs of some participants from whom written consent was taken to publish their photographs. Other data such as clinical records may be obtained from the author, if needed to understand the study. The author is Nurun Nahar Fatema and ORCID identifier number is 0000-0002-2169-0659.
References
- 1. Miller E, Cradock-Watson JE, Pollock TM. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 1982;2:781–4. 10.1016/S0140-6736(82)92677-0 [DOI] [PubMed] [Google Scholar]
- 2. Toda K, Reef S, Tsuruoka M, et al. Congenital rubella syndrome (CRS) in Vietnam 2011-2012--CRS epidemic after rubella epidemic in 2010-2011. Vaccine 2015;33:3673–7. 10.1016/j.vaccine.2015.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sever JL. The epidemiology of rubella. Arch Ophthalmol 1967;77:427–9. 10.1001/archopht.1967.00980020429002 [DOI] [PubMed] [Google Scholar]
- 4. Webster WS. Teratogen update: congenital rubella. Teratology 1998;58:13–23. [DOI] [PubMed] [Google Scholar]
- 5. Murhekar M, Verma S, Singh K, et al. Epidemiology of congenital rubella syndrome (CRS) in India, 2016-18, based on data from sentinel surveillance. PLoS Negl Trop Dis 2020;14:e0007982. 10.1371/journal.pntd.0007982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klein JO, Maldonado YA, Nizet V, et al. Current concepts of infections of the fetus and newborn infant : Remington JS, Klein JO, Infectious disease of the fetus and newborn infant. Philadelphia, PA: Saunders, 2006: 1–19. [Google Scholar]
- 7. Fatema NN, Razzaque AKM, Haque A. Pattern of heart disease in congenital rubella syndrome patient: analysis of cases over one year. Bangladesh J Cardiol 2010;2:275–8. [Google Scholar]
- 8. George IO, Frank-Briggs AI, Oruamabo RS. Congenital rubella syndrome: pattern and presentation in a southern Nigerian tertiary hospital. World J Pediatr 2009;5:287–91. 10.1007/s12519-009-0054-x [DOI] [PubMed] [Google Scholar]
- 9. Government of the People’s Republic of Bangladesh, Directorate General of Health Services Prime minister Sheikh Hasina formally opens vaccination campaign. Available: https://dghs.gov.bd/index.php/en/component/content/article/117-english-root/image-caption/798-prime-minister-sheikh-hasina-formally-opens-vaccination-campaign
- 10. Nessa A, Tabassum S, Akther T, et al. Rubella antibody prevalence and immunogenicity of single dose rubella vaccine among 16 – 25 years girls from Bangladesh. Bangladesh Med Res Counc Bull 2016;42:84–9. 10.3329/bmrcb.v42i2.32052 [DOI] [Google Scholar]
- 11. Begum NNF. Device closure of patent ductus arteriosus in complicated patient. J. Armed Forces Med. Coll. 2011;7:43–5. 10.3329/jafmc.v7i1.8627 [DOI] [Google Scholar]
- 12. Kava MP, Tullu MS, Muranjan MN, et al. Down syndrome: clinical profile from India. Arch Med Res 2004;35:31–5. 10.1016/j.arcmed.2003.06.005 [DOI] [PubMed] [Google Scholar]
- 13. Forrest JM, Menser MA. Congenital rubella in schoolchildren and adolescents. Arch Dis Child 1970;45:63–9. 10.1136/adc.45.239.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Budisteanu M, Musat GC, Sarafoleanu C, et al. Noses in dysmorphology - do we know all about the nose? Rom J Rhinol 2013;3:147–54. [Google Scholar]
- 15. Schluter WW, Reef SE, Redd SC, et al. Changing epidemiology of congenital rubella syndrome in the United States. J Infect Dis 1998;178:636–41. 10.1086/515384 [DOI] [PubMed] [Google Scholar]
- 16. Jayaratne YSN, Elsharkawi I, Macklin EA, et al. The facial morphology in Down syndrome: a 3D comparison of patients with and without obstructive sleep apnea. Am J Med Genet A 2017;173:3013–21. 10.1002/ajmg.a.38399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawrence K, Kuntsi J, Coleman M, et al. Face and emotion recognition deficits in Turner syndrome: a possible role for X-linked genes in amygdala development. Neuropsychology 2003;17:39–49. 10.1037/0894-4105.17.1.39 [DOI] [PubMed] [Google Scholar]
- 18. Babić M, Glisić B, Sćepan I. Mandibular growth pattern in Turner's syndrome. Eur J Orthod 1997;19:161–4. 10.1093/ejo/19.2.161 [DOI] [PubMed] [Google Scholar]
- 19. Garber KB, Visootsak J, Warren ST. Fragile X syndrome. Eur J Hum Genet 2008;16:666–72. 10.1038/ejhg.2008.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allanson JE. Objective studies of the face of Noonan, cardio-facio-cutaneous, and Costello syndromes: a comparison of three disorders of the Ras/MAPK signaling pathway. Am J Med Genet A 2016;170:2570–7. 10.1002/ajmg.a.37736 [DOI] [PubMed] [Google Scholar]
- 21. Allanson JE, Bohring A, Dörr H-G, et al. The face of Noonan syndrome: does phenotype predict genotype. Am J Med Genet A 2010;152A:1960–6. 10.1002/ajmg.a.33518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Free Dictionary [Internet] "triangular face". Segen’s Medical Dictionary, 2011. Available: https://medical-dictionary.thefreedictionary.com/Triangular+Face
- 23. Eggermann T. Russell-Silver syndrome. Am J Med Genet C Semin Med Genet 2010;154C:355–64. 10.1002/ajmg.c.30274 [DOI] [PubMed] [Google Scholar]
- 24. Pachlopnik Schmid J, Lemoine R, Nehme N, et al. Polymerase ε1 mutation in a human syndrome with facial dysmorphism, immunodeficiency, livedo, and short stature ("FILS syndrome"). J Exp Med 2012;209:2323–30. 10.1084/jem.20121303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kulkarni N, Lloyd IC, Ashworth J, et al. Traboulsi syndrome due to Asph mutation: an under-recognised cause of ectopia lentis. Clin Dysmorphol 2019;28:184–9. 10.1097/MCD.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 26. Pehlivan T, Pober BR, Brueckner M, et al. GATA4 haploinsufficiency in patients with interstitial deletion of chromosome region 8p23.1 and congenital heart disease. Am J Med Genet 1999;83:201–6. [DOI] [PubMed] [Google Scholar]
- 27. Mullegama SV, Rosenfeld JA, Orellana C, et al. Reciprocal deletion and duplication at 2q23.1 indicates a role for MBD5 in autism spectrum disorder. Eur J Hum Genet 2014;22:57–63. 10.1038/ejhg.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith DW, Gong BT. Scalp hair patterning as a clue to early fetal brain development. J Pediatr 1973;83:374–80. 10.1016/S0022-3476(73)80258-6 [DOI] [PubMed] [Google Scholar]
- 29. Deka D, Rustgi R, Singh S. Diagnosis of acute rubella infection during pregnancy. J Obstet Gynecol India 2006;56:44–6. [Google Scholar]
- 30. Eckel HE, Richling F, Streppel M, et al. [Early detection of profound hearing loss in children. Results of screening students in Rhine schools for the deaf and hearing impaired in Cologne]. Laryngorhinootologie 1998;77:125–30. 10.1055/s-2007-996946 [DOI] [PubMed] [Google Scholar]
- 31. Amirav I, Masumbuko CK, Hawkes MT, et al. 3D analysis of child facial dimensions for design of medical devices in low-middle income countries (LMIC). PLoS One 2019;14:e0216548. 10.1371/journal.pone.0216548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Othman SA, Majawit LP, Wan Hassan WN, et al. Anthropometric study of three-dimensional facial morphology in Malay adults. PLoS One 2016;11:e0164180. 10.1371/journal.pone.0164180 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



