Abstract
Introduction
Treatment patterns and costs were characterized among patients with overactive bladder (OAB) receiving later‐line target therapies (combination mirabegron/antimuscarinic, sacral nerve stimulation [SNS], percutaneous tibial nerve stimulation [PTNS], or onabotulinumtoxinA).
Methods
In a retrospective cohort study using 2013 to 2017 MarketScan databases, two partially overlapping cohorts of adults with OAB (“IPT cohort”: patients with incident OAB pharmacotherapy use; “ITT cohort,” incident target therapy) with continuous enrollment were identified; first use was index. Demographic characteristics, treatment patterns and costs over the 24‐month follow‐up period were summarized. Crude mean (standard deviation [SD]) OAB‐specific (assessed by OAB diagnostic code or pharmaceutical dispensation record) costs were estimated according to target therapy.
Results
The IPT cohort comprised 54 066 individuals (mean [SD] age 58.5 [15.0] years; 76% female), the ITT cohort, 1662 individuals (mean [SD] age 62.8 [14.9] years; 83% female). Seventeen percent of the IPT cohort were treated with subsequent line(s) of therapy after index therapy; among those, 73% received antimuscarinics, 23% mirabegron, and 1.4% a target therapy. For the ITT cohort, 32% were initially treated with SNS, 27% with onabotulinumtoxinA, 26% with combination mirabegron/antimuscarinic, and 15% with PTNS. Subsequently, one‐third of this cohort received additional therapies. Mean (SD) costs were lowest among patients receiving index therapy PTNS ($6959 [$7533]) and highest for SNS ($29 702 [$26 802]).
Conclusions
Costs for SNS over 24 months are substantially higher than other treatments. A treatment patterns analysis indicates that oral therapies predominate; first‐line combination therapy is common in the ITT cohort and uptake of oral therapy after procedural options is substantial.
Keywords: combination mirabegron/antimuscarinic therapy, costs and resource utilization, onabotulinumtoxinA, overactive bladder, percutaneous tibial nerve stimulation, sacral nerve stimulation
1. INTRODUCTION
Overactive bladder (OAB) is characterized by urinary urgency, with or without urge urinary incontinence, usually accompanied by frequency and nocturia. 1 To avoid adverse quality‐of‐life effects, 2 effective management is important; the American Urological Association/Society of Urodynamics and Female Pelvic Medicine (AUA/SUFU) guidelines propose a step‐wise algorithm for managing OAB. 1 While behavioral therapy and lifestyle modifications are recommended as initial therapy, 3 many patients require pharmacological treatment. Antimuscarinics and β3‐adrenoceptor agonists are similarly efficacious first‐line oral pharmacotherapies. 1 , 4 , 5 , 6 , 7 However, antimuscarinics are often used first despite higher adverse event and discontinuation rates. 8 , 9 , 10 , 11
For patients with inadequate response to oral therapies, procedures including sacral nerve stimulation (SNS), percutaneous tibial nerve stimulation (PTNS), or onabotulinumtoxinA injection are options. AUA/SUFU Guidelines consider the evidence strength for onabotulinumtoxinA to be Grade B but only recommend the treatment for carefully selected patients willing to perform intermittent catheterization for treatment induced retention. 1 While both SNS (evidence Grade C) and PTNS (evidence Grade B) have promising efficacy and generally mild adverse event profiles, these options require the willingness of patients to engage in surgeries for SNS or comply with the demanding treatment schedule associated with PTNS procedures. 12 Additionally, SNS, PTNS, and onabotulinumtoxinA injections may be viewed by patients as relatively invasive treatment options. 13
Recently, the US Food and Drug Administration (FDA) approved the use of combination mirabegron/solifenacin for patients with OAB 14 based on the results of the Phase 3 SYNERGY I, SYNERGY II, and BESIDE studies. 15 , 16 , 17 The treatment was incorporated in the AUA's 2019 guidelines for OAB (evidence Grade B) for use in subsequent lines of therapy after the failure of behavioural and oral monotherapies. 1 The three clinical trials demonstrated that combination mirabegron/solifenacin therapy offered improved efficacy over either monotherapy or placebo across most outcomes considered, with a similar safety profile. Mirabegron/antimuscarinic combination therapy may therefore offer a potential option for patients who are still symptomatic after monotherapy or want to avoid invasive procedural therapies. At present, there are few published studies characterizing the population receiving combination mirabegron/antimuscarinic treatment or procedural therapies for OAB. Real‐world data on how these therapies are used in clinical practice, and their attendant health care resource use (HCRU) and costs, are lacking. The objective of the present study was to characterize treatment patterns, HCRU and costs among patients with OAB on target therapies (combination mirabegron/antimuscarinic, SNS, PTNS, or onabotulinumtoxinA) in the United States.
2. METHODS
2.1. Study design and data source
A retrospective cohort study was performed using the IBM MarketScan claims databases from 2013 to 2017. These are large, nationally‐representative health care claims from encounters of patients insured through commercial or Medicare Supplemental plans. These databases contain individual linked data for over 84 million people, allowing characterization of patient populations, treatment patterns, clinical outcomes and HCRU and costs (including medication fills). 18
2.2. Study sample
To be eligible for inclusion, patients were more than or equal to 18 years of age on 1 January 2013; and had more than or equal to 1 OAB‐specific medication or procedural code (Appendix 1) claims during the study identification period (1 January 2014 to 31 December 2015). Additional requirements were continuous data availability more than or equal to 12 months before, and 24 months after, cohort entry (“index date”). From this group, two partially overlapping cohorts of patients with OAB were identified.
For the incident pharmacotherapy (IPT) cohort, individuals were enrolled at their first observed (index) dispensation for an OAB‐specific pharmacotherapy (antimuscarinic monotherapy, mirabegron monotherapy, or combination mirabegron/antimuscarinic therapy). For the incident target therapy (ITT) cohort, individuals were enrolled at the initiation of mirabegron in combination with any antimuscarinic, onabotulinumtoxinA, SNS, or PTNS. In the 12 months preceding index, IPT cohort patients were required to have no OAB‐specific pharmacotherapy dispensations and ITT patients, no target therapy dispensations.
Potential patients were excluded based on diagnosis or procedural codes indicative of neurogenic bladder, Parkinson's disease, multiple sclerosis, spinal cord injury, pregnancy, malignant neoplasm, renal impairment, hepatic insufficiency, trauma, or organ transplantation during the preindex or postindex period; as well as based on episodes of urinary retention during the preindex period (Appendix 1).
2.3. OAB treatment patterns
Target therapies were identified as follows (see Appendix 1 for codes; Figure 1 for study design schematic):
Combination mirabegron/antimuscarinic: Simultaneous or sequential fills for mirabegron and an antimuscarinic (in any order) within the days covered by the first medication.
OnabotulinumtoxinA: First recorded injection.
SNS: Initial device implantation and battery insertion or replacement within 30 days.
PTNS: More than or equal to 9 codes for PTNS observed over a 16‐week period.
Figure 1.
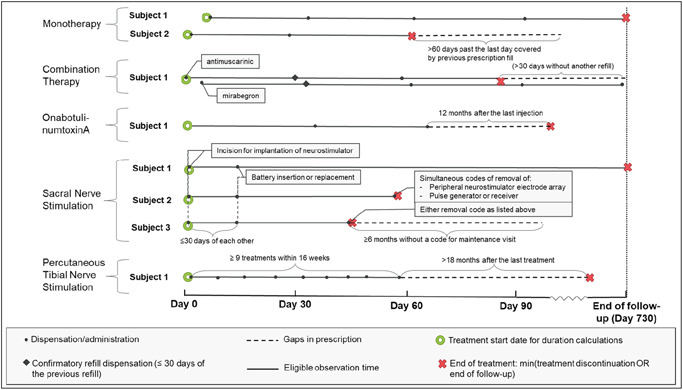
Study design schematic.1A confirmatory refill within 30 days of both mirabegron and any antimuscarinic were required to verify that the first observed instance was a combination therapy (and not a treatment switch). If the antimuscarinic continues after discontinuation of mirabegron (or vice versa), this is considered a de‐escalation and new line of therapy. 2Simultaneous codes of revision or removal of peripheral neurostimulator electrode array and pulse generator or receiver OR one of these revision/removal codes with no follow‐up maintenance code for more than 6 months were considered sacral nerve stimulation discontinuation
For identifying treatment patterns, a “line of therapy” was defined with respect to pharmacotherapy or procedures only, that is, “first‐line therapy” refers to the first OAB pharmacotherapy or procedure identified, independent of any prior behavioral therapies (or nontarget pharmacological therapies for the ITT cohort). The second line of therapy was the second pharmacotherapy or procedure observed, etc. Patients were tracked through up to four lines of therapy over the 24‐month follow‐up period. Oral and procedural therapies occurring concurrently were considered independent lines of therapy and ordered by start date. Treatment switches were identified by fills of a different OAB medication or initiation of a new therapy with no concurrent use of the original treatment. For combination mirabegron/antimuscarinic therapy, changing antimuscarinic medications was not considered a switch.
To assess treatment discontinuation, specific rules were applied for each therapy (see also Figure 1):
Monotherapy: Treatment gap of more than 60 days past the last day covered by prescription fill.
Combination mirabegron/antimuscarinic therapy: Treatment gap of more than 30 days past the last day covered by prescription fill for mirabegron and/or antimuscarinic (a more rigid treatment gap was applied to ensure concurrent treatment).
OnabotulinumtoxinA: more than 12 months without reinjection.
SNS: Device removal (further details in Appendix 1).
PTNS: A gap of more than 18 months after last treatment.
Treatment duration was calculated from the beginning to last available covered treatment date within the study period; specifically, for combination treatment, the latest covered date of either of the two prescriptions was used. For procedures, treatment duration was assessed at: (a) SNS removal, (b) 12 months after the last onabotulinumtoxinA injection, or (c) 18 months after last PTNS treatment.
2.4. HCRU and costs
HCRU and costs were characterized from the time of first target therapy among the ITT cohort and first OAB pharmacotherapy among the IPT cohort, to the end of the 24‐month follow‐up period. HCRU considered included medication dispensations (mean [SD] [standard deviation] number of distinct classes and distinct medications), inpatient hospitalizations (mean [SD] number of hospitalizations per 100 individuals and length of stay [LOS]), outpatient clinic visits (stratified by general practitioner, specialist, and other visits), and mean (SD) emergency room (ER) visits per 100 individuals. Medication use was categorized by National Drug Code (NDC) dispensations and days of use. Health care costs were estimated based on the total cost associated with each medical encounter or medication claim, including pharmaceutical, inpatient, outpatient costs.
2.5. Analysis
Demographic characteristics (age and sex) were summarized; the unweighted Elixhauser Index 19 was calculated over the 12 months before index and the percentage with specific comorbidities at baseline was calculated for the ITT cohort.
For the IPT cohort, the percentage of individuals transitioning to any subsequent line of therapy, as well as to a specific target therapy over the period, was calculated. A logistic regression model was used to explore the relationship between receipt of targeted therapy and patient characteristics, such as age, sex, and geographic region.
OAB‐specific HCRU and costs over the period were estimated; overall, and among those treated with a target therapy. For the ITT cohort, treatment patterns over the 24‐month follow‐up period were summarized, including durations of therapy by target therapy, the percentage of individuals transitioning to a subsequent line of therapy, and frequency of treatment switches or discontinuations. Crude mean (SD) OAB‐specific HCRU and costs were estimated according to index therapy from index until end of follow‐up. Generalized linear models (GLM) with a gamma distribution and log transformation were used to predict costs from index to end of follow‐up in the ITT cohort. Predicted total per‐patient‐per‐month (PPPM) costs were adjusted for sex, age at index, and baseline (in the 1 year before index) Elixhauser score and PPPM costs.
The definition used to classify PTNS (≥9 visits over 16 weeks) may have led to underestimation of costs by including those who had not completed their full PTNS treatment (12 treatments over 16 weeks) in the analysis. A sensitivity analysis was conducted to explore the proportion of PTNS‐treated patients among the ITT cohort who had completed the standard twelve treatments over the study period.
3. RESULTS
3.1. IPT cohort
The IPT cohort comprised 54 066 patients (mean [SD] age 58.5 [15] years; 76.3% female; Table 1). Among this cohort, 48 650 (90.0%) received an antimuscarinic as their index therapy, 5251 (9.7%) received mirabegron, and 165 (0.3%) received combination mirabegron/antimuscarinic therapy. Over the follow‐up period, 1.4% (n = 744) proceeded to (a minimum of one) target therapy (32.8% were treated with combination mirabegron/antimuscarinic, 32.8% with onabotulinumtoxinA, 25.3% with SNS, and 13.2% with PTNS). Older (≥65 years old), and female, patients were significantly more likely to have received a targeted therapy during their follow‐up; and patients from the South were significantly less likely to have received a targeted therapy (Appendix 2). Of patients in the IPT cohort, approximately 17% (n = 9386) proceeded to a second line of therapy, with 73.2% receiving an antimuscarinic and 22.8%, mirabegron (Figure 2). Few patients were treated with each of combination mirabegron/antimuscarinic (0.7%), SNS (1.3%), PTNS (0.7%), and onabotulinumtoxinA (1.4%) as a second round of therapy. Approximately 17% of those treated with a second‐line therapy (n = 1571) proceeded to a third line and 221 patients (14% of third‐line patients) proceeded to a fourth line (Figure 2). While few patients with an initial treatment of antimuscarinic proceeded to a second round of therapy within the follow‐up period, among those who did, 62.9% switched to at least one other antimuscarinic (data not shown).
Table 1.
Baseline demographic and clinical characteristics of the ITT cohort
| Incident target therapy cohort | |||||
|---|---|---|---|---|---|
| All (n = 1662) | Combi mira/AM (n = 427) | OnabotulinumtoxinAa (n = 456) | SNS (n = 535) | PTNSa (n = 245) | |
| Age in years | |||||
| Mean (SD) | 62.8 (14.9) | 65.5 (15.1) | 62.3 (15) | 59.4 (13.9) | 66.6 (14.6) |
| Median (IQR) | 61 (21) | 63 (23) | 61 (22.2) | 59 (19) | 68 (21) |
| Age category, y, n (%) | |||||
| 18 to <40 | 101 (6.1%) | 18 (4.2%) | 33 (7.2%) | 40 (7.5%) | 10 (4.1%) |
| 40 to <65 | 825 (49.6%) | 196 (45.9%) | 219 (48%) | 314 (58.7%) | 96 (39.2%) |
| 65 to <75 | 321 (19.3%) | 76 (17.8%) | 92 (20.2%) | 94 (17.6%) | 60 (24.5%) |
| 75+ | 415 (25%) | 137 (32.1%) | 112 (24.6%) | 87 (16.3%) | 79 (32.2%) |
| Sex, n (%) | |||||
| Female | 1385 (83.3%) | 312 (73.1%) | 400 (87.7%) | 477 (89.2%) | 197 (80.4%) |
| Male | 277 (16.7%) | 115 (26.9%) | 56 (12.3%) | 58 (10.8%) | 48 (19.6%) |
| Elixhauser score (# of categories; unweighted) | |||||
| Mean (SD) | 1.9 (1.7) | 2.0 (1.9) | 1.9 (1.7) | 2.0 (1.6) | 1.6 (1.5) |
| Median (IQR) | 2 (2) | 2 (2) | 1.5 (2) | 2 (2) | 1 (2) |
| Selected Elixhauser index comorbidities, n (%) | |||||
| Cardiac arrhythmias | 230 (13.8%) | 62 (14.5%) | 56 (12.3%) | 77 (14.4%) | 35 (14.3%) |
| Valvular disease | 134 (8.1%) | 39 (9.1%) | 34 (7.5%) | 39 (7.3%) | 22 (9.0%) |
| Peripheral vascular disorders | 155 (9.3%) | 49 (11.5%) | 38 (8.3%) | 35 (6.5%) | 33 (13.5%) |
| Hypertension, uncomplicated | 326 (19.6%) | 90 (21.1%) | 71 (15.6%) | 145 (27.1%) | 20 (8.2%) |
| Chronic pulmonary disease | 300 (18.1%) | 75 (17.6%) | 80 (17.5%) | 107 (20.0%) | 38 (15.5%) |
| Diabetes, uncomplicated | 125 (7.5%) | 37 (8.7%) | 32 (7.0%) | 44 (8.2%) | 12 (4.9%) |
| Hypothyroidism | 362 (21.8%) | 90 (21.1%) | 109 (23.9%) | 120 (22.4%) | 43 (17.6%) |
| Obesity | 244 (14.7%) | 72 (16.9%) | 60 (13.2%) | 74 (13.8%) | 38 (15.5%) |
| Depression | 411 (24.7%) | 103 (24.1%) | 110 (24.1%) | 152 (28.4%) | 46 (18.8%) |
| OAB symptoms, n (%) | |||||
| Incontinence without urgency or frequency | 154 (9.3%) | 46 (10.8%) | 31 (6.8%) | 52 (9.7%) | 25 (10.2%) |
| Incontinence with urgency and/or frequency | 1226 (73.8%) | 264 (61.8%) | 380 (83.3%) | 393 (73.5%) | 190 (77.6%) |
| Also with codes for nocturia | 424 (25.5%) | 105 (24.6%) | 122 (26.8%) | 125 (23.4%) | 72 (29.4%) |
Note: Not all individuals in the Incident pharmacotherapy cohort will experience a target therapy.
Abbreviations: AM, antimuscarinic; IQR, interquartile range; ITT, incident target therapy; mira, mirabegron; OAB, overactive bladder; PTNS, percutaneous tibial nerve stimulation; SD, standard deviation; SNS, sacral nerve stimulation.
One patient receiving PTNS and onabotulinumtoxinA concurrently at index, and is included in both group.
Figure 2.
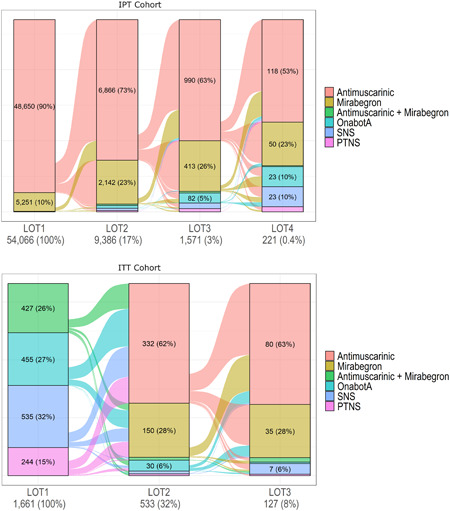
Sankey diagram of treatment pathways in the IPT and ITT cohorts. IPT, incident pharmacotherapy; ITT, incident target therapy; LOT, line of therapy; PTNS, percutaneous tibial nerve stimulation; SNS, sacral nerve stimulation
Mean (SD) OAB‐specific costs for the IPT cohort over the follow‐up period were $1787 ($4105 (Table 2). Among the subgroups who went onto target therapy, mean (SD) costs were $6626 ($5173) for those treated with PTNS, $7032 ($5854) for combination mirabegron/antimuscarinic, $10 183 ($13 725) for onabotulinumtoxinA and $39 954 ($28 113) for SNS (Table 2; medians, Appendix 3; resource use, Appendix 4).
Table 2.
Crude mean (SD) OAB‐specific US$ costs per patient from initiation of pharmacotherapy to end of study period, ITT cohort (n = 1662) and IPT cohort (n = 54 066; overall and among the subgroups who went on to target therapy)
| IPT Cohort | ITT Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Combi mira/AM | onabotulinumtoxinA (n = 244) | SNS | PTNS | All | Combi mira/AM | onabotulinumtoxinA | SNS | PTNS | |
| (n = 54,066) | (n = 245) | (n = 188) | (n = 98) | (n = 1,662) | (n = 427) | (n = 456) | (n = 535) | (n = 245) | ||
| Medications | ||||||||||
| Mean (SD) | 1203 (1872) | 5435 (3867) | 1670 (1872) | 1364 (1738) | 1846 (1954) | 2552 (4001) | 7706 (4093) | 754 (1867) | 580 (1729) | 1223 (1965) |
| Inpatient visits (hospitalizations) | ||||||||||
| Mean (SD) | 45 (1298) | 0 (0) | 214 (2515) | 61 (812) | 0 (0) | 64 (1190) | 34 (480) | 102 (1373) | 0 (8) | 187 (2386) |
| ER visits (inpatient and outpatient) | ||||||||||
| Mean (SD) | 7 (225) | 0 (0) | 14 (142) | 10 (142) | 0 (0) | 14 (342) | 0 (0) | 5 (55) | 3 (50) | 79 (884) |
| Outpatient visits | ||||||||||
| Mean (SD) | 538 (3312) | 1597 (5086) | 8299 (12 564) | 38 528 (27 895) | 4780 (4466) | 12 702 (20 229) | 1633 (5976) | 7670 (11 065) | 29 122 (26 590) | 5549 (6625) |
| Physician visits, mean (SD) | ||||||||||
| GP | 45 (327) | 81 (233) | 502 (3315) | 637 (2597) | 173 (1030) | 337 (1789) | 75 (310) | 245 (854) | 650 (2867) | 278 (1383) |
| Specialist | 206 (688) | 646 (1619) | 2415 (2490) | 4123 (4246) | 3547 (2953) | 1998 (2788) | 580 (1474) | 2100 (2510) | 2446 (3166) | 3328 (3141) |
| Other | 287 (2962) | 870 (3743) | 5382 (11 202) | 33 768 (26 803) | 1060 (2777) | 10 367 (19 376) | 978 (5380) | 5325 (10 272) | 26 026 (25 919) | 1942 (5250) |
| Procedures | ||||||||||
| Mean (SD) | 71 (1625) | 804 (4022) | 2883 (6838) | 13 867 (20 469) | 2844 (2116) | 5719 (13 091) | 486 (3178) | 2774 (4573) | 13 250 (20 322) | 3889 (3794) |
| Total, mean (SD) | 1787 (4105) | 7032 (5854) | 10 183 (13 725) | 39 954 (28 113) | 6626 (5173) | 15 318 (19 648) | 9373 (7112) | 8527 (11 333) | 29 702 (26 802) | 6959 (7533) |
Abbreviations: AM, antimuscarinic; ER, emergency room; GP, general practitioner; IPT, incident pharmacotherapy cohort; IQR, interquartile range; mira, mirabegron; OAB, overactive bladder; PPPS, per‐member‐per‐month; PTNS, percutaneous tibial nerve stimulation; SD, standard deviation; SNS, sacral nerve stimulation.
3.2. ITT cohort
The ITT cohort (n = 1662) was slightly older than the IPT cohort (mean [SD] age was 62.8 [14.9] years) and the proportion of females was higher (83.3%). The mean (SD) Elixhauser score was 1.9 (1.7), and depression (24.7%) and hypothyroidism (21.8%) were the most common comorbidities. Among the ITT cohort, 73.8% had codes for incontinence with urgency and/or frequency, and 25.5% of these also had codes for nocturia (Table 1).
At index, 427 patients (25.7%) were treated with combination mirabegron/antimuscarinic therapy, 455 (27.1%) with onabotulinumtoxinA, 244 (14.7%) with PTNS, 535 (32.2%) with SNS and 1 (0.1%) with both PTNS and onabotulinumtoxinA (Figure 2). The mean (SD) treatment duration was 644.5 (172.5) days for combination therapy, 623.5 (159.9) for onabotulinumtoxinA, 710.5 (37.9) for PTNS and 700.0 (116.5) for SNS.
Approximately a third of patients (n = 533) proceeded to a further therapy after their initial target therapy (Figure 2), 332 (62.3%) of whom received antimuscarinics and 150 (28.1%) mirabegron (both potentially concurrent with the patient's initial procedural therapy). In terms of follow‐up target therapies, 28 (5.2%) patients were treated with onabotulinumtoxinA, and a few with combination mirabegron/antimuscarinic, SNS, or PTNS. Similar trends were observed among the patients (n = 127; 8%) that proceeded to at least two lines of therapy after initial target therapy.
Mean (SD) OAB‐specific costs over the period were $6959 ($7533) for PTNS, $8527 ($11 333) for onabotulinumtoxinA, $9373 ($7112) for combination therapy and $29 702 ($26 802) for SNS (Table 2; medians, Appendix 3; resource use Appendix 4).
In the GLM, predicted mean monthly OAB‐specific costs were $698 for females and $551 for males. Predicted costs were highest among those treated with SNS (from $1202 to $1340 per month, depending on age and sex category). The lowest costs were observed among male PTNS‐treated patients aged 18 to 65 years ($255; Table 3).
Table 3.
Predicted mean PPPM OAB‐specific US$ costs, by age, sex, and target therapy
| By target therapy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All target therapies | Combi mira/AM | onabotulinumtoxinA | SNS | PTNS | |||||||
| (n = 1662) | (n = 427) | (n = 456) | (n = 535) | (n = 245) | |||||||
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | ||
| From index to end of follow‐up | (n = 1385) | (n = 277) | (n = 312) | (n = 115) | (n = 400) | (n = 56) | (n = 477) | (n = 58) | (n = 197) | (n = 48) | |
| Age | 18‐65 | 698 | 551 | 415 | 385 | 364 | 293 | 1202 | 1121 | 258 | 255 |
| 65+ | 614 | 487 | 382 | 354 | 362 | 298 | 1340 | 1215 | 310 | 333 | |
Abbreviations: AM, antimuscarinic; mira, mirabegron; OAB, overactive bladder; PPPM, per‐member‐per‐month; PTNS, percutaneous tibial nerve stimulation; SD, standard deviation; SNS, sacral nerve stimulation.
The sensitivity analysis demonstrated that the majority (85.3%) of PTNS‐treated patients in the ITT cohort completed a full course (12) of PTNS treatments over the study period.
4. DISCUSSION
This study provides insight into treatment patterns, HCRU and costs among patients with OAB treated with mirabegron in combination with any antimuscarinic, SNS, PTNS, or onabotulinumtoxinA in the United States. Evidence from the present study suggests that treatment with the procedural therapy SNS can be expensive, costing more than three times as much as the next closest therapy in both the IPT and ITT cohorts. Among the ITT cohort, mean OAB‐specific costs reached $30 000 for patients with index SNS therapy, compared with mean costs of $15 000 across all therapies and just over $9000 for the next most expensive therapy (combination mirabegron/antimuscarinic). Across both cohorts, PTNS was the least expensive option with costs of $6626 for the IPT cohort and $6959 for the ITT cohort. While treatment switching away from incident pharmacotherapy was relatively infrequent over the first 2 years of therapy—occurring in 17% of the IPT cohort—it is interesting that among that group, switching between traditional “first‐line” therapies (eg, from one antimuscarinic to another) was frequent. Switching to a target therapy over the follow‐up period was rare (observed in <2% of the cohort). While there are few published studies examining treatment patterns in later lines of therapy for OAB, these numbers are in line with findings over similar time periods from Linder et al 20 and two recently published abstracts 21 , 22 (1.1‐2.2%). 20 , 21 , 22 Despite being a relatively new therapy, a substantial portion of the ITT cohort (>26%) received combination therapy, which could be an indication that an oral therapy is preferable to a procedural therapy for some patients. Switching among members of the ITT cohort was more common, with approximately one‐third proceeding to a second line of therapy. While some use of target therapy was observed as a follow‐up to initial target therapy, treatment with antimuscarinics or mirabegron was more frequent, suggesting that retrying patients on oral pharmacotherapies, either in place of or in addition to further procedural therapies is relatively common.
To date, the body of evidence describing outcomes among patients with OAB who proceed beyond first‐line monotherapy to procedural therapies remains scant, with a predominance of studies with small sample sizes. 1 The finding that treatment with combination mirabegron/antimuscarinic, onabotulinumtoxinA, and SNS is more frequent than with PTNS is consistent with the results from a US database study of women with OAB. 20 Estimates of costs for SNS from the present study were substantially higher than those reported in a recently published observational study. 23 However, the latter used different costing methodology which hampers direct comparison.
There are several other methodologic points related that warrant discussion. First, while only OAB‐specific costs are presented here in the main analysis, bearing in mind that there is always some misclassification in disease‐specific costs, all‐cause costs were also calculated and were found to follow the same trajectory as the OAB‐specific costs. The definition for PTNS used here was implemented to be more sensitive to how PTNS may be administered in clinical practice; this may have led to underestimating costs by including those who had not completed their full PTNS treatment. However, the sensitivity analysis demonstrated that over 85% of patients completed a full course of treatment, thus, underestimation of costs should be minimal. As resource use and associated costs were a principal interest of this study, a conservative approach was taken for cohort identification so as to focus on patients who received treatment. As a result, some patients who were assessed for an SNS procedure but failed or had inconclusive stage I or peripheral nerve evaluation (PNE; ie, CPT code 64561) and thus did not receive treatment, were not included in the cohort. We acknowledge this could have an impact on the outcomes of the treatment patterns analysis. Additionally, because of the granularity of dispensations data available on prescription fills compared with procedural therapy data, a relatively strict definition for stopping combination mirabegron/antimuscarinic therapy could be imposed, compared with PTNS or onabotulinumtoxinA resulting in more opportunity to observe patients on combination mirabegron/antimuscarinic discontinuing therapy compared with patients on procedural therapies. Interestingly, approximately 30% of those who discontinued combination mirabegron/antimuscarinic therapy were treated with antimuscarinic or mirabegron monotherapy in follow‐up, the reasons for which cannot be determined from administrative data. While treatment with mirabegron has generally shown better adherence than antimuscarinic therapy in OAB, 8 , 9 , 20 , 24 , 25 evidence on adherence to combination mirabegron/antimuscarinic therapy is lacking. One retrospective study reported slightly higher adherence for patients treated with combination vs. mirabegron monotherapy; however, the sample size was relatively small. 8 Prospective studies specifically measuring adherence, and reasons for lack of compliance, may help fill this gap. In addition, future lines of research could include patient or clinician preference studies to better understand perceptions of burden and treatment effectiveness, which are difficult to characterize from claims data as well as real word evidence studies documenting reasons for treatment recommendations.
Finally, the relatively short follow‐up time available in MarketScan presents a number of challenges. Concurrent or subsequent use of oral OAB pharmacotherapy was common among patients treated with procedural therapies; occurring in one‐third of SNS patients and almost two‐thirds of those treated with PTNS. However, because of the long potential therapeutic durations of some procedural therapies (eg, PTNS), whether this use of oral pharmacotherapy represented a treatment failure versus a supplemental therapy is not possible to ascertain using claims data. Furthermore, due to the limited timescale, there is the potential that procedural therapies were not being compared over the full therapeutic duration of the treatment and thus they were adversely affected by up‐front costs in the comparison.
The fact that this is the first large observational study to describe and compare the use of procedural therapies and combination mirabegron/antimuscarinic treatment for OAB is a key strength of this study; allowing for the characterization of treatment patterns, HCRU and costs among patients with OAB treated with target therapies. The analyses were informed by data from IBM MarketScan, a large, generalizable and well‐characterized US claims data set suitable for addressing the study objectives and widely validated for clinical, pharmacoepidemiologic, and pharmacoeconomic research. 26 , 27 , 28 The study separately examined outcomes in the periods before and after initiation of target therapy, an advantageous approach which conserved the largest possible sample size of patients treated with the target therapies within the ITT cohort. In the IPT cohort, it provided a complete picture of treatment sequencing among incident OAB pharmacotherapy patients.
As most IPT cohort patients did not transition to a target therapy within the follow‐up period, outcomes among target therapy patients in the IPT cohort would only be generalizable to more rapidly‐progressing patients with OAB. Additionally, concurrent therapies occurring after procedural target therapy initiation were difficult to identify, as when an individual “stopped” a procedural therapy had to be assumed. The application of pre‐specified rules to classify discontinuations resulted in most patients on procedural therapies remaining on therapy for much of the follow‐up period (in the absence of a switch being observed). Indeed, estimates herein of treatment duration for onabotulinumtoxinA and PTNS are at the higher end of published values. 20 , 21 , 22 That administrative claims data are collected for billing, rather than research purposes may be a further study limitation, as misclassification may occur as coding may be driven by reimbursement (rather than clinical) factors and reasons for treatment discontinuation (treatment failure, success or other) cannot be discerned. Finally, given the sampling frame for the study (those with commercial or Medicare plans), the findings may not be reflective of those with other types of or without insurance.
5. CONCLUSIONS
This study characterized treatment patterns and costs of care among patients with OAB who transition beyond initial oral pharmacotherapeutic options. Costs were highest among the subgroup of patients treated with SNS and lowest among those receiving PTNS. Among patients with OAB who transition beyond initial oral monotherapy, uptake of combination mirabegron/antimuscarinic therapy was substantial and oral therapies dominated subsequent lines of therapy in this cohort. These findings help characterize treatment patterns and costs, and suggest promising, less expensive therapies for patients with OAB who are insufficiently managed on initial oral pharmacotherapy.
APPENDIX 1. CODES TO IDENTIFY OAB MEDICATIONS AND TREATMENTS, AND TO APPLY EXCLUSION CRITERIA
| OAB medication and treatment codes | ||
|---|---|---|
| Description | Code type | Code |
| Darifenacin | NDC | 0430‐0170, 0430‐0171, 0591‐4375, 0591‐4380, 10370‐170, 10370‐171, 13668‐202, 13668‐203, 35356‐272, 42291‐206, 42291‐207, 54868‐5363, 54868‐5704, 59746‐516, 59746‐517, 65862‐861, 65862‐862, 69097‐431, 69097‐432 |
| Fesoterodine | NDC | 0069‐0242, 00690244, 54868‐6156, 54868‐6175, 55154‐2737, 55154‐2738, 63539‐183, 63539‐242, 69189‐0242, 69189‐0244 |
| Oxybutynin | NDC | 0023‐5812, 0023‐5861, 0093‐5206, 0093‐5207, 0093‐5208, 01210671, 0179‐0187, 0378‐6015, 0378‐6605, 0378‐6610, 03786615, 0603‐1491, 0603‐4975, 0615‐3512, 0615‐7519, 0615‐7520, 0615‐7521, 0832‐0038, 0904‐2821, 0904‐6570, 10135‐609, 10135‐610, 10135‐611, 10147‐0761, 10147‐0771, 10147‐0781, 10544‐518, 10544‐559, 11523‐4311, 11523‐4322, 16729‐317, 16729‐318, 16729‐319, 17856‐1491, 21695‐406, 33261‐342, 35356‐909, 35356‐958, 35356‐991, 42291‐633, 42291‐634, 42291‐635, 43063‐145, 43353‐367, 43353‐769, 43353‐978, 50090‐0317, 50090‐0318, 50090‐2049, 50111‐456, 50268‐627, 50268‐628, 50268‐629, 50436‐4777, 50458‐805, 50458‐810, 50458‐815, 51079‐722, 51079‐723, 52544‐041, 52544‐084, 52544‐166, 52544‐920, 53808‐0618, 53808‐0747, 53808‐0873, 54569‐1990, 54838‐510, 54868‐2157, 54868‐4502, 54868‐4835, 54868‐5728, 54868‐5742, 54868‐5743, 54868‐6171, 55154‐0657, 55154‐5537, 55154‐6298, 55154‐6647, 55154‐7225, 60432‐092, 60760‐980, 61786‐605, 62175‐270, 62175‐271, 62175‐272, 63187‐749, 63629‐1354, 63629‐5484, 63629‐6355, 63629‐6434, 63739‐548, 64980‐209, 64980‐210, 64980‐211, 65162‐371, 65162‐372, 65162‐373, 66336‐604, 67296‐1175, 68071‐1875, 68071‐2013, 68084‐400, 68084‐480, 68084‐610, 68151‐3646, 68788‐6402, 69189‐0581, 69189‐5206, 69189‐6605, 69189‐6610, 70518‐0158, 70518‐0202, 76237‐216, 76237‐217, 76237‐218 |
| Solifenacin | NDC | 50090‐0972, 51248‐150, 51248‐151, 54569‐5790, 54868‐4705, 54868‐5398, 55154‐3875, 55154‐3876, 55154‐3877, 55154‐3878 |
| Tolterodine | NDC | 0009‐4541, 0009‐4544, 0009‐5190, 0009‐5191, 0093‐0010, 0093‐0018, 0093‐2049, 0093‐2050, 0093‐2055, 0093‐2056, 0093‐7163, 0093‐7164, 0378‐3402, 0378‐3404, 0378‐5445, 0378‐5446, 0904‐6592, 09046593, 13668‐189, 13668‐190, 16590‐959, 33342‐097, 33342‐098, 35356‐417, 51079‐197, 51079‐198, 51079‐235, 54868‐4514, 54868‐5126, 55154‐3933, 55154‐3935, 55289‐132, 59762‐0047, 59762‐0048, 59762‐0170, 59762‐0800, 60429‐825, 60429‐826, 60505‐3527, 60505‐3528, 63629‐5625, 68151‐2049, 68151‐4281, 69189‐3404, 69189‐5190 |
| Trospium | NDC | 0574‐0118, 0574‐0145, 0591‐3636, 23155‐530, 42291‐846, 60429‐098, 60429‐103, 60505‐3454, 68001‐228, 68462‐461, 69097‐912 |
| Mirabegron (Myrbetriq) | NDC | 00469‐2601, 00469‐2602 |
| OnabotulinumtoxinA | CPT | 52287 |
| Cystourethroscopy, with injections(s) for chemodenervation of the bladder | ||
| Percutaneous tibial nerve stimulation (PTNS)* | CPT | 64566 |
| Posterior tibial neurostimulation, percutaneous needle electrode, single treatment, includes programming | ||
| Sacral nerve stimulation (SNS) | ||
| Definition of incident SNS patients | ||
| Initial device implantation | 64581 | Incision for implantation of neurostimulator |
| AND | ||
| 64590 | Battery insertion or replacement, occurring on the same day or up to 30‐d apart | |
| Please note: codes 64581 AND 64590 can occur on the same day, or with 64590 occurring up to 30 d after 64581 to meet initial device implantation criteria; note that the date of 64581 will be identified as the index date. | ||
| Definitions of prevalent SNS patients | ||
| Maintenance visit | 95972 | Follow‐up analysis or programming visit |
| Battery replacement | 64590 | Battery insertion or replacement |
| WITHOUT | ||
| 64581 | Incision for implantation of neurostimulator | |
| Device revision | 64585 | Revision or removal of peripheral neurostimulator electrode array |
| OR | ||
| 64595 | Revision or removal of pulse generator or receiver | |
| AND | ||
| 95972 | Follow‐up analysis or programming visit, within 6‐mo following 64585 OR 64595 | |
| Definition of SNS treatment discontinuation | ||
| Device removal | 64585 | Revision or removal of peripheral neurostimulator electrode array |
| AND | ||
| 64595 | Revision or removal of pulse generator or receiver | |
| OR | One of 64585 or 64595, with no code for maintenance visit within the following 6‐mo | |
| Exclusion criteria diagnostic codes | ||
|---|---|---|
| Description | Code type | Code |
| Bladder‐related comorbidities to be excluded during pre‐index period only | ||
| Urinary retention | ICD‐9 | 788.2x |
| ICD‐10 | R33.9 | |
| Non‐indwelling bladder catheter (eg, straight catheterization for residual urine) | CPT | 51701 |
| Sterile intermittent catheter kit | HCPCS | A4353 |
| Indwelling bladder catheter (eg, Foley) | CPT | 51702 |
| Bladder‐related comorbidities to be excluded during preindex and postindex periods | ||
| Parkinson's disease | ICD‐9 | 332.xx |
| ICD‐10 | G20 | |
| Multiple sclerosis | ICD‐9 | 340 |
| ICD‐10 | G35 | |
| Spinal cord injury | ICD‐9 | 952.xx |
| ICD‐10 | S14, S24, S34 | |
| Neurogenic bladder | ICD‐9 | 596.54, 344.61, or 596.53 |
| ICD‐10 | N31.9, G83,4, N31.2 | |
| Non‐bladder related comorbidities to be excluded during preindex and postindex periods | ||
| Malignant neoplasms | ICD‐9 | 140.xx‐209.xx |
| ICD‐10 | C00.xx‐C96.xx | |
| Transplant | ICD‐9 | V42.0‐V42.9x, 996.80–996.89 |
| ICD‐10 | Z94.x, T86.x | |
| ICD‐9 (procedural) | 00.18, 00.91‐00.93, 07.94, 33.50‐33.52, 33.6, 37.51, 41.00‐41.09, 41.94, 46.97, 50.51, 50.59, 52.80‐52.86, 55.53, 55.61, 55.69 | |
| ICD‐10 (procedural) | 02YA0Z0‐02YA0Z2, 0BYC0Z0‐0BYC0Z2, 0BYD0Z0‐0BYD0Z2, 0BYF0Z0‐0BYF0Z2, 0BYG0Z0‐0BYG0Z2, 0BYH0Z0‐0BYH0Z2, 0BYJ0Z0‐0BYJ0Z2, 0BYK0Z0‐0BYK0Z2, 0BYL0Z0‐0BYL0Z2, 0BYM0Z0‐0BYM0Z2, 0TY00Z0‐0TY00Z2, 0TY10Z0‐0TY10Z2, 0DY80Z0‐0DY80Z2, 0DYE0Z0‐0DYE0Z2, 0FY00Z0‐0FY00Z2, 0FYG0Z0‐0FYG0Z2, 3E030U0‐3E030U1, 3E033U0‐3E033U1, 3E0J3U0‐3E0J3U1, 3E0J7U0‐3E0J7U1, 3E0J8U0‐3E0J8U1, 07YP0Z0‐07YP0Z2, 30230AZ, 30230G0‐30230G1, 30230×0‐ 30230×1, 30230Y0‐30230Y1, 30233AZ, 30233G0‐30233G1, 30233×0‐30233×1, 30233Y0‐30233Y1, 30240AZ, 30240G0‐30240G1, 30240×0‐30240×1, 30240Y0‐30240Y1, 30243AZ, 30243G0‐30243G1, 30243×0‐30243×1, 30243Y0‐30243Y1, 30250G0‐30250G1, 30250×0‐30250×1, 30250Y0‐30250Y1, 30253G0‐30253G1, 30253×0‐30253×1, 30253Y0‐30253Y1, 30260G0‐30260G1, 30260×0‐30260×1, 30260Y0‐30260Y1, 30263G0‐30263G1, 30263×0‐30263×1, 30263Y0‐30263Y1 | |
| CPT | 32851‐32854, 33935, 33945, 38240‐38242, 44135, 44136, 47135, 47136, 48160, 48554, 50360, 50365, 50380, 60512 | |
| Hepatic insufficiency | ICD‐9 | 570.xx, 571.xx, 572.xx |
| ICD‐10 | K70.xx‐K77.xx | |
| Renal impairment | ICD‐9 | 403.xx, 404.xx, 581.xx, 583.xx, 584.xx, 585.xx, 586.xx, 587.xx, 588.9x, V56.x |
| ICD‐10 | I12.xx, I13.xx, N04.xx, N05.xx, N17.xx – N19.xx, N25.9, N26.9, Z49.xx | |
| CPT | 90918‐90999 | |
| Pregnancy | ICD‐9 | 630.xx‐679.xx, V22.xx, and V23.xx; |
| ICD‐9 (procedural) | 72.xx, 73.xx, 74.xx; | |
| ICD‐10 | O00.xx‐O99.xx, O9A.xx, Z32.xx‐Z34.xx | |
| ICD‐10 (procedural) | 10x | |
| CPT | 59050, 59051, 59300, 59400, 59409, 59410, 59414, 59430, 59510, 59514, 59515, 59525, 59610, 59612, 59614, 59618, 59620, 59622, 59898, 59899 | |
| Trauma | ICD‐9 | E80.0‐E84.8, E90.8, E90.9, E91.6‐E92.8 |
| ICD‐10 | V00‐V99, W00‐W99, X00‐X58 | |
APPENDIX 2. THE ASSOCIATION BETWEEN PATIENT DEMOGRAPHIC CHARACTERISTICS AND THE USE OF TARGETED THERAPY
| OR | 95% CI | P value | |
|---|---|---|---|
| (Intercept) | 0.01 | (0.01‐0.02) | .0000 |
| Baseline age category | |||
| 18 to <40 | ref | … | … |
| 40 to <65 | 0.89 | (0.68‐1.19) | .4289 |
| 65 to <75 | 1.91 | (1.41‐2.62) | .0000 |
| 75+ | 1.47 | (1.09‐2.02) | .0125 |
| Sex | |||
| Male | ref | … | … |
| Female | 1.40 | (1.17‐1.69) | .0003 |
| Region | |||
| Northeast | ref | … | … |
| North Central | 0.89 | (0.72‐1.10) | .2634 |
| South | 0.80 | (0.66‐0.98) | .0326 |
| West | 0.91 | (0.70‐1.19) | .5029 |
| Unknowna | … | … | … |
Abbreviations: CI, confidence interval; OR, odds ratio.
Not estimable as there was only 66 patients in this group and none had gone on to use target therapies during their 24‐month follow‐up.
APPENDIX 3. CRUDE MEDIAN (IQR) TOTAL OAB‐SPECIFIC US$ COSTS PER PATIENT FROM INITIATION OF PHARMACOTHERAPY TO END OF STUDY PERIOD, IPT COHORT (N = 54 066) AND ITT (N = 1662), OVERALL AND AMONG THE PATIENTS WHO WENT ON TO TARGET THERAPY IN THE IPT COHORT
| IPT cohort | ITT cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 54 066) | Combi mira/AM (n = 245) | OnabotulinumtoxinA (n = 244) | SNS (n = 188) | PTNS (n = 98) | All (n = 1662) | Combi mira/AM (n = 427) | OnabotulinumtoxinA (n = 456) | SNS (n = 535) | PTNS (n = 245) | |
| Medications | ||||||||||
| Median (IQR) | 322 (72, 1308) | 5139 (2200, 7537) | 885 (325, 2558) | 725 (253, 1623) | 1023 (390, 2973) | 30 (0, 4253) | 7467 (4626, 10 551) | 0 (0, 462) | 0 (0, 0) | 81 (0, 1577) |
| Inpatient visits (hospitalizations) | ||||||||||
| Median (IQR) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| ER visits (inpatient and outpatient) | ||||||||||
| Median (IQR) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Outpatient visits | ||||||||||
| Median (IQR) | 74 (0, 297) | 201 (114, 865) | 3983 (2408, 7563) | 30 371 (22 386, 47 374) | 3861 (2651, 5481) | 3696 (1088, 19 454) | 329 (96, 895) | 3702 (1928, 8026) | 25 405 (8494, 38 262) | 3724 (2336, 5802) |
| Physician visits, median (IQR) | ||||||||||
| GP | 0 (0, 0) | 0 (0, 0) | 0 (0, 111) | 0 (0, 82) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 4) | 0 (0, 0) |
| Specialist | 0 (0, 169) | 114 (0, 201) | 1821 (821, 3024) | 3585 (1867, 5432) | 3138 (1987, 4463) | 1272 (206, 2766) | 201 (0, 560) | 1355 (423, 2906) | 1761 (927, 3102) | 2668 (1426, 4469) |
| Other | 0 (0, 0) | 0 (0, 89) | 1899 (9, 4923) | 26 946 (18 420, 42 880) | 5 (0, 595) | 713 (0, 15 586) | 0 (0, 68) | 1436 (0, 5748) | 22 900 (4332, 35 266) | 0 (0, 1544) |
| Procedures | ||||||||||
| Median (IQR) | 0 (0, 0) | 0 (0, 0) | 972 (411, 2559) | 5460 (1859, 19 346) | 2239 (1750, 3467) | 1549 (123, 4664) | 0 (0, 0) | 1250 (497, 3112) | 4911 (1777, 19 374) | 2780 (1775, 4706) |
| Total, median (IQR) | 595 (185, 1982) | 5564 (2519, 8073) | 6479 (3877, 9906) | 32 188 (23 190, 48 220) | 5823 (3810, 7732) | 7921 (3651, 20 846) | 8097 (5608, 11 922) | 4520 (2381, 9351) | 26 057 (9978, 38 514) | 5199 (2841, 8194) |
APPENDIX 4. CRUDE OAB‐SPECIFIC RESOURCE USE, OVER THE FOLLOW‐UP PERIOD
| Incident target therapy cohort | Incident pharmacotherapy cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All (n = 1662) | Combi mira/AM (n = 427) | OnabotulinumtoxinA (n = 456) | SNS (n = 535) | PTNSa (n = 245) | All (n = 54,066) | Combi mira/AM (n = 245) | OnabotulinumtoxinA (n = 244) | SNS (n = 188) | PTNS (n = 98) | |
| Total OAB‐specific resource useb | ||||||||||
| Medications | ||||||||||
| Number of distinct classes of medications dispensed, mean(SD) | 0.8 (0.9) | 1.9 (0.2) | 0.4 (0.6) | 0.3 (0.5) | 0.6 (0.7) | 1.1 (0.3) | 2.0 (0.0) | 1.3 (0.5) | 1.2 (0.4) | 1.3 (0.5) |
| Number of distinct medications dispensed, mean(SD) | 0.9 (1.0) | 2.2 (0.6) | 0.4 (0.7) | 0.3 (0.6) | 0.7 (0.8) | 1.2 (0.5) | 2.1 (0.3) | 1.7 (0.8) | 1.5 (0.7) | 1.6 (0.8) |
| Inpatient visits | ||||||||||
| n(%) requiring hospitalization | 19 (1.1) | 7 (1.6) | 7 (1.5) | 1 (0.2) | 4 (1.6) | 256 (0.5) | 0 (0.0) | 7 (2.9) | 2 (1.1) | 0 (0.0) |
| Mean (SD) hospitalizations per 100 individuals | 1.1 (10.6) | 1.6 (12.7) | 1.5 (12.3) | 0.2 (4.3) | 1.6 (12.7) | 0.5 (7.4) | 0.0 (0.0) | 2.9 (16.7) | 1.1 (10.3) | 0.0 (0.0) |
| Mean (SD) LOS per hosp. | 3.7 (2.6) | 3.9 (3.2) | 3.3 (2.1) | 1.0 (NA) | 4.8 (2.9) | 4.4 (6.1) | NA | 3.1 (2.7) | 3.0 (0.0) | NA |
| ER visits (inpatient and outpatient) | ||||||||||
| n (%) visiting ER | 15 (0.9) | 2 (0.5) | 6 (1.3) | 3 (0.6) | 4 (1.6) | 295 (0.5) | 0 (0.0) | 4 (1.6) | 1 (0.5) | 0 (0.0) |
| Mean (SD) ER visits per 100 individuals | 1.0 (10.4) | 0.5 (6.8) | 1.3 (11.4) | 0.6 (7.5) | 2.0 (16.8) | 0.6 (8.2) | 0.0 (0.0) | 1.6 (12.7) | 0.5 (7.3) | 0.0 (0.0) |
| Outpatient visits | ||||||||||
| n(%) visiting each physician type | ||||||||||
| GP | 357 (21.5) | 73 (17.1) | 107 (23.5) | 136 (25.4) | 41 (16.7) | 9142 (16.9) | 5 (20.0) | 73 (29.9) | 51 (27.1) | 19 (19.4) |
| Specialist | 1379 (83.0) | 296 (69.3) | 391 (85.7) | 463 (86.5) | 230 (93.9) | 20,477 (37.9) | 14 (56.0) | 218 (89.3) | 167 (88.8) | 95 (96.9) |
| Other | 1063 (64.0) | 132 (30.9) | 310 (68.0) | 505 (94.4) | 117 (47.8) | 9881 (18.3) | 7 (28.0) | 190 (77.9) | 186 (98.9) | 50 (51.0) |
| Mean (SD) outpatient visits | ||||||||||
| GP | 0.7 (2.2) | 0.5 (1.4) | 0.7 (1.9) | 0.9 (2.1) | 1.0 (3.6) | 0.3 (0.9) | 0.4 (1.0) | 1.1 (2.7) | 1.3 (3.0) | 0.7 (2.4) |
| Specialist | 5.4 (6.5) | 2.7 (3.5) | 4.6 (4.1) | 3.5 (3.1) | 15.6 (9.2) | 0.9 (1.9) | 2.3 (3.9) | 6.3 (4.7) | 6.5 (4.4) | 16.7 (7.9) |
| Other | 2.1 (3.8) | 0.8 (2.0) | 2.1 (2.9) | 2.3 (2.7) | 4.1 (7.3) | 0.4 (1.2) | 0.7 (1.4) | 2.3 (2.6) | 3.6 (2.9) | 3.8 (7.2) |
| n (%) with OAB procedures | 1272 (76.5) | 37 (8.7) | 456 (100.0) | 535 (100.0) | 245 (100.0) | 687 (1.3) | 1 (4.0) | 243 (99.6) | 188 (100.0) | 98 (100.0) |
| POS: Office | 646 (38.9) | 23 (5.4) | 229 (50.2) | 179 (33.5) | 216 (88.2) | 354 (0.7) | 1 (4.0) | 119 (48.8) | 61 (32.4) | 89 (90.8) |
| OnabotulinumtoxinA injection | 248 (14.9) | 10 (2.3) | 225 (49.3) | 7 (1.3) | 7 (2.9) | 113 (0.2) | 1 (4.0) | 110 (45.1) | 3 (1.6) | 0 (0.0) |
| PTNS | 231 (13.9) | 8 (1.9) | 7 (1.5) | 1 (0.2) | 216 (88.2) | 141 (0.3) | 0 (0.0) | 10 (4.1) | 2 (1.1) | 89 (90.8) |
| SNS | 190 (11.4) | 5 (1.2) | 8 (1.8) | 173 (32.3) | 4 (1.6) | 108 (0.2) | 0 (0.0) | 5 (2.0) | 59 (31.4) | 0 (0.0) |
| POS: Outpatient hospital | 757 (45.5) | 20 (4.7) | 229 (50.2) | 470 (87.9) | 39 (15.9) | 368 (0.7) | 1 (4.0) | 120 (49.2) | 166 (88.3) | 11 (11.2) |
| OnabotulinumtoxinA injection | 237 (14.3) | 11 (2.6) | 216 (47.4) | 7 (1.3) | 4 (1.6) | 110 (0.2) | 0 (0.0) | 108 (44.3) | 3 (1.6) | 1 (1.0) |
| PTNS | 31 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 31 (12.7) | 14 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 10 (10.2) |
| SNS | 510 (30.7) | 9 (2.1) | 25 (5.5) | 470 (87.9) | 6 (2.4) | 251 (0.5) | 1 (4.0) | 16 (6.6) | 165 (87.8) | 1 (1.0) |
| POS: Ambulatory Surgical Center | 201 (12.1) | 5 (1.2) | 76 (16.7) | 113 (21.1) | 7 (2.9) | 101 (0.2) | 0 (0.0) | 43 (17.6) | 38 (20.2) | 0 (0.0) |
| OnabotulinumtoxinA injection | 80 (4.8) | 2 (0.5) | 74 (16.2) | 1 (0.2) | 3 (1.2) | 41 (0.1) | 0 (0.0) | 40 (16.4) | 4 (2.1) | 0 (0.0) |
| PTNS | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| SNS | 123 (7.4) | 3 (0.7) | 2 (0.4) | 113 (21.1) | 5 (2.0) | 64 (0.1) | 0 (0.0) | 7 (2.9) | 37 (19.7) | 0 (0.0) |
| POS: Outpatient other | 36 (2.2) | 0 (0.0) | 9 (2.0) | 22 (4.1) | 5 (2.0) | 11 (0.0) | 0 (0.0) | 2 (0.8) | 4 (2.1) | 1 (1.0) |
| OnabotulinumtoxinA injection | 9 (0.5) | 0 (0.0) | 8 (1.8) | 1 (0.2) | 0 (0.0) | 2 (0.0) | 0 (0.0) | 2 (0.8) | 0 (0.0) | 0 (0.0) |
| PTNS | 5 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (2.0) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) |
| SNS | 23 (1.4) | 0 (0.0) | 2 (0.4) | 21 (3.9) | 0 (0.0) | 8 (0.0) | 0 (0.0) | 0 (0.0) | 4 (2.1) | 0 (0.0) |
Abbreviations: AM, antimuscarinic; ER, emergency room; GP, general practitioner; IQR, interquartile range; mira, mirabegron; OAB, overactive bladder; POS, place of service; PTNS, percutaneous tibial nerve stimulation; SD, standard deviation; SNS, sacral nerve stimulation.
Not estimable as there was only 66 patients in this group and none had gone on to use target therapies during their 24‐mo follow‐up.
OAB‐specific claims are identified with medical claims with one or more OAB International Classification of Diseases [ICD]‐9/10 codes, pharmacy claims for mirabegron or antimuscarinics, or OAB‐specific Current Procedural Terminology (CPT) codes.
Kraus SR, Shiozawa A, Szabo SM, Qian C, Rogula B, Hairston J. Treatment patterns and costs among patients with OAB treated with combination oral therapy, sacral nerve stimulation, percutaneous tibial nerve stimulation, or onabotulinumtoxinA in the United States. Neurourology and Urodynamics. 2020;39:2206–2222. 10.1002/nau.24474
REFERENCES
- 1. American Urological Association . Diagnosis and Treatment of Non‐Neurogenic Overactive Bladder (OAB) in Adults: an AUA/SUFU Guideline; 2019.
- 2. Bartoli S, Aguzzi G, Tarricone R. Impact on quality of life of urinary incontinence and overactive bladder: a systematic literature review. Urology. 2010;75(3):491‐500. [DOI] [PubMed] [Google Scholar]
- 3. Dumoulin C, Hay‐Smith J. Pelvic floor muscle training versus no treatment for urinary incontinence in women. A Cochrane systematic review. Eur J Phys Rehabil Med. 2008;44(1):47‐63. [PubMed] [Google Scholar]
- 4. Gormley EA, Lightner DJ, Faraday M, Vasavada SP. Diagnosis and treatment of overactive bladder (non‐neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015;193(5):1572‐1580. [DOI] [PubMed] [Google Scholar]
- 5. Kim TH, Lee KS. Persistence and compliance with medication management in the treatment of overactive bladder. Investig Clin Urol. 2016;57(2):84‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kennelly MJ. A comparative review of oxybutynin chloride formulations: pharmacokinetics and therapeutic efficacy in overactive bladder. Rev Urol. 2010;12(1):12‐19. [PMC free article] [PubMed] [Google Scholar]
- 7. Maman K, Aballea S, Nazir J, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol. 2014;65(4):755‐765. [DOI] [PubMed] [Google Scholar]
- 8. Pindoria N, Malde S, Nowers J, Taylor C, Kelleher C, Sahai A. Persistence with mirabegron therapy for overactive bladder: a real life experience. Neurourol Urodyn. 2017;36(2):404‐408. [DOI] [PubMed] [Google Scholar]
- 9. Wagg A, Franks B, Ramos B, Berner T. Persistence and adherence with the new beta‐3 receptor agonist, mirabegron, versus antimuscarinics in overactive bladder: early experience in Canada. Can Urol Assoc J. 2015;9(9‐10):343‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nitti VW, Rovner ES, Franks B, Muma G, Berner T, Fan A. Persistence with mirabegron versus tolterodine in patients with overactive bladder. Am J Pharm Benefits. 2016;8(2):e25‐e33. [Google Scholar]
- 11. Sussman D, Yehoshua A, Kowalski J, et al. Adherence and persistence of mirabegron and anticholinergic therapies in patients with overactive bladder: a real‐world claims data analysis. Int J Clin Pract. 2017;71(3‐4):e12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinson M, MacDiarmid S, Black E. Cost of neuromodulation therapies for overactive bladder: percutaneous tibial nerve stimulation versus sacral nerve stimulation. J Urol. 2013;189(1):210‐216. [DOI] [PubMed] [Google Scholar]
- 13. Wu JM, Fulton RG, Amundsen CL, Knight SK, Kuppermann M. Patient preferences for different severities of and treatments for overactive bladder. Female Pelvic Med Reconstr Surg. 2011;17(4):184‐189. [DOI] [PubMed] [Google Scholar]
- 14. Gassman MD. Audrey. Supplement Approval ‐ Co‐administration therapy of MYRBETRIQ® with solifenacin succinate for the treatment of overactive bladder In: Astellas Pharma US I, ed. Silver Spring MD 20993. Department of Health and Human Services ‐ Food and Drug Administration. [Google Scholar]
- 15. Herschorn S, Kohan A, Aliotta P, et al. The efficacy and safety of onabotulinumtoxinA or solifenacin compared with placebo in solifenacin naive patients with refractory overactive bladder: results from a multicenter, randomized, double‐blind phase 3b trial. J Urol. 2017;198(1):167‐175. [DOI] [PubMed] [Google Scholar]
- 16. Westhofen T, Magistro G, Stief C, Gratzke C. Long‐term safety and efficacy of mirabegron and solifenacin in combination compared with monotherapy in patients with overactive bladder: a randomised, multicentre phase 3 Study (SYNERGY II). Eur Urol. 2018;49:328‐333. [DOI] [PubMed] [Google Scholar]
- 17. Drake MJ, Chapple C, Esen AA, et al. Efficacy and safety of mirabegron add‐on therapy to solifenacin in incontinent overactive bladder patients with an inadequate response to initial 4‐week solifenacin monotherapy: a randomised double‐blind multicentre phase 3B study (BESIDE). Eur Urol. 2016;70(1):136‐145. [DOI] [PubMed] [Google Scholar]
- 18. IBM Watson Health . MarketScan Databases; 2018. http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases. Accessed August 18, 2020.
- 19. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8‐27. [DOI] [PubMed] [Google Scholar]
- 20. Linder BJ, Gebhart JB, Elliott DS, Van Houten HK, Sangaralingham LR, Habermann EB. National patterns of filled prescriptions and third‐line treatment utilization for privately insured women with overactive bladder. Female Pelvic Med Reconstr Surg. 2019. [published online ahead of print May 30, 2019]. 10.1097/SPV.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 21. Wolff* E, Cook T, Kirby A, Gore J. PD31‐03 proportion of women with overactive bladder who progress from second‐to third‐line treatment in a real‐world setting. J Urol. 2019;201(Suppl 4):e566. [Google Scholar]
- 22. Woff E, Cook T, Gore J, Kirby A, Gill* B. PD31‐05 overactive bladder in men: medication abandonment and lack of third‐line therapy. J Urol. 2019;201(Suppl 4):e567. [Google Scholar]
- 23. Chughtai B, Clemens JQ, Thomas D, Sun T, Ghomrawi H, Sedrakyan A. Real world performance of SNM and onabotulinumtoxinA for OAB: focus on safety and cost. J Urol. 2019;203:179‐184. 101097JU0000000000000462. [DOI] [PubMed] [Google Scholar]
- 24. Benner JS, Nichol MB, Rovner ES, et al. Patient‐reported reasons for discontinuing overactive bladder medication. BJU Int. 2010;105(9):1276‐1282. [DOI] [PubMed] [Google Scholar]
- 25. Chapple CR, Nazir J, Hakimi Z, et al. Persistence and adherence with mirabegron versus antimuscarinic agents in patients with overactive bladder: a retrospective observational study in UK clinical practice. Eur Urol. 2017;72(3):389‐399. [DOI] [PubMed] [Google Scholar]
- 26. Wu JM, Matthews CA, Conover MM, Pate V, Funk MJ. Lifetime risk of stress incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marsico M, Mehta V, Chastek B, Liaw K‐L, Derkay C. Estimating the incidence and prevalence of juvenile‐onset recurrent respiratory papillomatosis in publicly and privately insured claims databases in the United States. Sex Transm Dis. 2014;41(5):300‐305. [DOI] [PubMed] [Google Scholar]
- 28. Gauthier G, Guérin A, Zhdanava M, et al. Treatment patterns, healthcare resource utilization, and costs following first‐line antidepressant treatment in major depressive disorder: a retrospective US claims database analysis. BMC Psychiatry. 2017;17:222. [DOI] [PMC free article] [PubMed] [Google Scholar]


