Abstract
The dilution effect predicts increasing biodiversity to reduce the risk of infection, but the generality of this effect remains unresolved. Because biodiversity loss generates predictable changes in host community competence, we hypothesised that biodiversity loss might drive the dilution effect. We tested this hypothesis by reanalysing four previously published meta‐analyses that came to contradictory conclusions regarding generality of the dilution effect. In the context of biodiversity loss, our analyses revealed a unifying pattern: dilution effects were inconsistently observed for natural biodiversity gradients, but were commonly observed for biodiversity gradients generated by disturbances causing losses of biodiversity. Incorporating biodiversity loss into tests of generality of the dilution effect further indicated that scale‐dependency may strengthen the dilution effect only when biodiversity gradients are driven by biodiversity loss. Together, these results help to resolve one of the most contentious issues in disease ecology: the generality of the dilution effect.
Keywords: biodiversity, community structure, dilution effect, parasitism
The dilution effect predicts increasing biodiversity to reduce the risk of infection, but the generality of this effect remains unresolved. Here, by re‐analyzing the (often conflicting) results of previously published meta‐analyses, we show that ecological processes like habitat fragmentation, urbanisation, and agricultural intensification consistently lead to increases in infectious diseases, likely because these events drive concurrent losses of local biodiversity and predictable changes in host community composition.

INTRODUCTION
Increasing biodiversity is often associated with a reduction in the risk of infectious diseases, a phenomenon known as the dilution effect (Keesing et al., 2006, 2010; Civitello et al., 2015; Halliday and Rohr, 2019). Yet, despite more than three decades of empirical research, meta‐analyses, reviews, and syntheses, there remains polarising debate regarding the generality of this effect (Halsey, 2019; Rohr et al., 2020). A considerable body of research, initially grounded in pioneering studies of Lyme Disease (e.g., Allan et al. 2003; LoGiudice et al. 2003; Ostfeld & LoGiudice 2003), and later extended to other systems (e.g., Keesing et al. 2006, 2010; Ostfeld & Keesing 2012; Johnson et al. 2013, 2019; Joseph et al. 2013; Mihaljevic et al. 2014; Strauss et al. 2016; Liu et al. 2018; Halliday et al. 2019), provides a promising framework for resolving this debate, suggesting that changes in the structure of host communities, rather than biodiversity per se, can explain when a dilution effect should be observed. A commonality among these studies is a focus on biodiversity loss: the structure of host communities often shifts predictably when biodiversity is lost or recovered, particularly following disturbances, and often in a way that favours species with combinations of physiological traits associated with increased disease risk (LoGiudice et al. 2003; Ostfeld & LoGiudice 2003; Joseph et al., 2013; Mihaljevic et al., 2014; Johnson et al., 2015a). These predictable shifts suggest that there should be a strong relationship between biodiversity and disease risk following a loss of biodiversity. In contrast, such predictable changes are not expected over natural biodiversity gradients (Table 1).
Table 1.
Common drivers of local biodiversity loss and expected impacts on host community structure
| Effect on biodiversity | Effect on community structure and host community competence | Relationship between biodiversity and competence | |
|---|---|---|---|
| (A) Drivers of biodiversity gradients associated with biodiversity loss | |||
| Fragmentation | Increasing fragmentation reduces host diversity (Hanski, 2015) | Slow pace of life hosts, which tend to exhibit low competence (Cronin et al., 2010; Johnson et al., 2012), and tend to be poor dispersers, are among the first to be lost, while fast pace of life hosts, which tend to also be good dispersers, tend to resist fragmentation (Hanski et al., 2006; Gibbs and Van Dyck, 2010; Keinath et al., 2017; Ziv and Davidowitz, 2019). Habitat specialists tend to be lost more commonly than habitat generalists (Keinath et al., 2017). Parasites that specialise on one or a few hosts also tend to be lost more commonly than host generalists (Colwell et al., 2012; but see Farrell et al., 2015). | Negative (i.e., increasing biodiversity is associated with a reduction in host community competence) |
| Urbanisation | Increasing urbanisation reduces host diversity (McKinney, 2008) | Increasing urbanisation can be considered as a series of filters that select different species (Williams et al., 2009). Most of these filters appear to favour fast pace‐of‐life hosts and good dispersers and disfavour slow‐pace of life hosts and poor dispersers. For example, urbanisation often increases fragmentation and the frequency and duration of disturbances (Stenhouse, 2004; Hahs et al., 2009; Ramalho et al., 2014), which together tend to favour fast pace‐of‐life hosts (Tilman, 1990; Cadotte, 2007; Keinath et al., 2017; Lopez et al., 2018). Urban environmental effects include soil and atmospheric pollution, increased temperatures due to the urban heat island effect, and increased water stress (Pickett et al., 2001; Grimm et al., 2008), which also tend to favour hosts with fast‐pace‐of life and high dispersal abilities (Albrecht and Haider, 2013; Fay et al., 2015; Merckx et al., 2018; Heckman et al., 2019). | Negative |
| Agricultural intensification | Increasing agricultural intensification reduces host diversity (Beckmann et al., 2019) | Increasing agricultural intensification fragments host habitat, favouring fast pace of life, and highly competent hosts. Increasing nutrient supplies associated with agricultural intensification also tends to favour hosts with fast‐pace‐of life and low defence against enemies (Fay et al., 2015; Heckman et al., 2019). Similarly, pesticides often disproportionately harm large‐bodied, slow‐growing (and less competent) hosts (Wagner et al., 2015), and sublethal pesticide exposure can select for fast‐paced life‐history strategies (Debecker et al., 2016), and increase host exposure to parasites by shifting host behaviour (Gendron et al., 2003). | Negative |
| (B) Drivers of biodiversity gradients not associated with biodiversity loss | |||
| Environmental heterogeneity | Increasing heterogeneity within communities generally increases host richness (Stein et al., 2014). Among communities, environmental heterogeneity can generate variation in host diversity, though the direction of the effect depends on the source of heterogeneity (e.g., resource supply, soil type, temperature). | Change in composition is related to the underlying source of heterogeneity. For example, soil resource availability could generate variation based on growth‐defence tradeoffs (Heckman et al., 2019), whereas topographical heterogeneity might be harder to predict (see “Elevation”). | Positive, negative, or none |
| Island biogeography | Increasing distance and decreasing island size reduces host diversity (MacArthur and Wilson, 1967; Simberloff and Wilson, 1970; MacArthur, 1972) | Increasing distance and decreasing island size favours fast pace‐of‐life hosts, which also tend to be good dispersers (MacArthur and Wilson, 1967; Hanski et al., 2006; Gibbs and Van Dyck, 2010; Keinath et al., 2017; Ziv and Davidowitz, 2019), and might be more competent (Cronin et al., 2010; Johnson et al., 2012). | Negative |
| Elevation | Increasing elevation can increase host diversity, decrease host diversity, or generate unimodal diversity patterns, depending on characteristics of the ecosystem, habitat, host taxonomic group, and their interactions (Körner, 2007; Wohlgemuth et al., 2008; Altermatt et al., 2013; Peters et al., 2016; Laiolo et al., 2018) | High elevations may favour slow‐growing, long lived, well defended (and therefore, less competent) hosts due to limited resources and stressful environmental conditions (Nobis and Schweingruber, 2013). Alternatively, high elevations may favour more competent hosts due to reduced selection for resistance (Pellissier et al., 2014; Kergunteuil et al., 2019). Additionally, increasing elevation can change the intensity of biological interactions (Roslin et al., 2017; Hargreaves et al., 2019), thereby altering how individual host species contribute to host community competence (Benkman, 2013). | Positive, negative, or none |
| Latitude | Increasing latitude reduces host biodiversity (Wallace 1878, Hillebrand 2004) | Latitudinal gradients of host community structure are often idiosyncratic. For some taxa (e.g., birds), high latitudes favour fast pace‐of‐life hosts (Jetz et al., 2008), whereas for other taxa (e.g., some plants) high latitudes appear to favour slow‐growing, long lived, well defended (and therefore, less competent) hosts (Oleksyn et al., 2003). Additionally, increasing latitude can change the intensity of biological interactions (Roslin et al., 2017; Hargreaves et al., 2019), thereby altering how individual host species contribute to host community competence (Benkman, 2013). | Positive, negative, or none |
While many studies focus on measuring the diversity of host species in the context of disease, the structure of host communities can also be measured in the context of disease using characteristics of host species or host functional traits (Johnson et al., 2013; Halliday et al., 2019; Kirk et al., 2019), resulting in trait‐based measures of host community competence (Stewart Merrill and Johnson, 2020). This approach, which has rapidly gained traction in disease ecology, suggests that host species that are the best able to spread diseases (i.e., the most competent hosts), often share particular suites of physiological traits (Huang et al., 2013; Martin et al., 2019; Becker and Han, 2020). Thus, host community competence can be linked to distributions of important host traits across host communities (Johnson et al., 2015b; Liu et al., 2017). Importantly, several recent studies indicate that host community competence often covaries with host diversity, obscuring the true effect of host diversity, per se, on infectious disease risk (Johnson et al., 2015a; Young et al., 2017; Halliday et al., 2019). This covariance in host community competence and host diversity might, in turn, be driven by community disassembly or recolonisation associated with biodiversity loss (Johnson et al., 2019; Rohr et al., 2020).
We define biodiversity loss as a process through which host species are lost from local assemblages, due to changes in ecological parameters. These changes can be abrupt, such as droughts, fires, floods, and windstorms (Jentsch and White, 2019), or sustained, such as nutrient eutrophication, climate change, and land‐use change (Newbold et al., 2015). We contrast this with biodiversity gradients that are not associated with biodiversity loss, but result from differences in the way that communities assemble based on existing environmental filters (HilleRisLambers et al., 2012) or through ecological drift (Vellend, 2010), including variation in latitude, elevation, environmental heterogeneity, or habitat size. Importantly, the mechanisms through which these processes alter host communities are not mutually exclusive. For example, habitat fragmentation and island biogeography both generate variation in species richness due to changes in habitat size and isolation of habitat patches. The important difference is that habitat fragmentation predominantly changes species richness through the process of community disassembly, whereas island biogeography predominantly changes species richness through the processes of community assembly and ecological drift.
Biodiversity loss can drive the dilution effect because the most competent hosts also tend to be the species that remain or recolonise following biodiversity loss (Table 1A). One explanation for this pattern relates to host life history (Ostfeld and Keesing, 2000; Previtali et al., 2012). Specifically, hosts with life history strategies that favour growth, reproduction, and dispersal, over defence against parasites (e.g., hosts exhibiting a fast pace of life), often contribute the most to disease in the communities that they occupy (i.e., act as disease amplifiers; Cronin et al., 2010; Johnson et al., 2012; Sears et al., 2015). Similarly, in a study of 2277 vertebrate host species and 66 parasites, the best reservoir hosts (those with high abundance and diversity of parasites) were hosts with broad geographic ranges that invest heavily in reproduction and growth (Han et al., 2015b) (see also Luis et al., 2013). These fast pace‐of‐life hosts are also often the most resistant hosts to extinction (Hanski et al., 2006; Gibbs and Van Dyck, 2010; Albrecht and Haider, 2013; Fay et al., 2015; Keinath et al., 2017; Merckx et al., 2018; Ziv and Davidowitz, 2019). Consequently, as host communities become fragmented or disturbed and biodiversity is lost, these fast pace‐of‐life, amplifying hosts remain, while their slow pace‐of‐life counterparts are lost (Joseph et al., 2013; Mihaljevic et al., 2014; Johnson et al., 2015a), leading to covariance between host diversity and host community competence. This hypothesis has been borne out for amphibian (Johnson et al., 2013), mammal (Ostfeld and LoGiudice, 2003), and plant hosts (Liu et al., 2018). In two recent experiments, one using amphibian hosts (Johnson et al., 2019) and the other focused on plant hosts (Liu et al., 2018), dilution was not observed in communities that were disassembled randomly, but when communities disassembled naturally, biodiversity significantly reduced disease, lending further support to this hypothesis. Consequently, theory suggests that biodiversity gradients associated with biodiversity loss should result in dilution effects.
Whereas biodiversity loss is often linked to increased host community competence during community disassembly, the relationship between natural biodiversity gradients and host community competence is less clearly defined (Table 1B). For example, increasing elevation can increase host diversity, decrease host diversity, or generate unimodal diversity patterns, depending on characteristics of the ecosystem, habitat, host taxonomic group, and their interactions (Körner, 2007; Wohlgemuth et al., 2008; Altermatt et al., 2013; Peters et al., 2016; Laiolo et al., 2018). Similarly, increasing elevation can select for more poorly‐defended hosts when there is reduced selection for resistance at high elevations (Pellissier et al., 2014; Kergunteuil et al., 2019), but might also favour slow‐growing, long‐lived, well‐defended hosts due to limited resources and stressful environmental conditions at high elevation (Nobis and Schweingruber, 2013). Consequently using host competence to predict biodiversity‐disease relationships along elevational gradients is challenging.
Different drivers of biodiversity gradients might also influence whether and when contingencies arise in the strength and direction of biodiversity‐disease relationships (e.g., Halliday and Rohr, 2019). For example, it has been proposed that biodiversity‐disease relationships should be strongest at local scales and in tropical regions, where biotic interactions are strongest, and should weaken as spatial scale and (absolute values of) latitude increase and the strength of biotic interactions declines (Wood and Lafferty, 2013; Johnson et al., 2015a; Cohen et al., 2016; Halliday and Rohr, 2019; Liu et al., 2020; Rohr et al., 2020) (but see Magnusson et al., 2020). This effect might be particularly strong among studies that depend on biodiversity loss if biodiversity loss generates consistent patterns of host community competence, and might be weaker or even reverse among studies that do not depend on biodiversity loss depending on the relationship between biodiversity and host community competence (Table 1). Thus, moderation of the dilution effect might differ among studies that do not involve biodiversity loss and among studies that do.
In this study, we test whether the diluting effect of host diversity on disease risk varies between natural biodiversity gradients and biodiversity gradients that are associated with recent loss of host species. We test this by reanalysing four previously published meta‐analyses that came to contradictory conclusions regarding generality in the dilution effect. Re‐analysing these data in the context of biodiversity loss reveals a unifying pattern: dilution effects are inconsistently observed for biodiversity gradients that are not associated with the loss of biodiversity (e.g., latitudinal, elevation, and habitat size gradients, or environmental heterogeneity), but are very regularly observed for biodiversity gradients that are generated by disturbances that cause losses of biodiversity (Table 1). These patterns are robust to misclassification of as many as 50% of the biodiversity gradients in these two categories. Incorporating biodiversity loss into tests of generality in the dilution effect further helps to unify understanding of contingencies in the biodiversity–disease relationships, suggesting that scale‐dependency should weaken the dilution effect when biodiversity gradients do not involve biodiversity loss, but may strengthen the dilution effect when biodiversity gradients are driven by biodiversity loss. Together, these results help to resolve one of the most contentious issues in disease ecology: the generality of the dilution effect.
METHODS
Does biodiversity loss underlie the dilution effect of biodiversity?
To test whether biodiversity loss can explain generality in the relationship between biodiversity and disease risk, we reanalysed four previously published meta‐analyses. These four previously published studies used different selection criteria and modelling frameworks, focused on different subsets of host and parasite taxa, and came to different conclusions regarding the generality of the dilution effect (Table 2). Conclusions from these published syntheses were contradictory, suggesting that the dilution effect can be robust (Civitello et al., 2015; Magnusson et al., 2020), scale dependent (Halliday and Rohr, 2019), or dependent on latitude, habitat, and parasite life history (Liu et al., 2020).
Table 2.
Summary of key data syntheses studying generality in the relationship between biodiversity and disease risk
| Data types | Studies (unique) | Manuscripts (unique) | Moderators | Contingencies identified | Moderators that interact with biodiversity loss | |
|---|---|---|---|---|---|---|
| Civitello et al., (2015) | All studies | 208 (123) | 45 (21) | Parasite type, lifecycle, functional group, specialisation; Study type | None | Parasite type |
| Halliday & Rohr 2019 | Studies with more than three unique diversity measures | 217 (48) | 37 (6) | Spatial scale | Spatial scale | Spatial scale |
| Magnusson et al., (2020) | Observational studies | 120 (16) | 37 (9) | Spatial scale; Latitude; Geographic region | Stronger relationships in temperate regions | Spatial scale |
| Liu et al., (2020) | Studies of non‐agricultural plant communities | 136 (58) | 20 (13) | Parasite life history, symptom; Ecosystem type; Study design; Latitude | Ecosystem type; Study design; Parasite life history; Latitude | Parasite life history, symptom; Latitude |
Studies correspond to individual relationships between biodiversity and disease risk, with a unique effect size for each study. Manuscripts are the total number of manuscripts from which these effect sizes were calculated. Unique studies and manuscripts are those that were only included in a given meta‐analysis. For example, our reanalysis includes 208 effect sizes from Civitello et al., 2015, 85 of which are included in at least one additional meta‐analysis, and 123 of which are unique to the Civitello et al., 2015 dataset. Specific details of which manuscripts and studies were included in which dataset can be found in Table S1 and on Figshare (Halliday, 2020), respectively. The column titled, “Moderators that interact with biodiversity loss” summarises the tests of statistical interactions between biodiversity loss and previously‐hypothesised moderators of the dilution effect. Figures showing these tests are presented in Figures S1–S3. The following manuscripts were not included because the underlying source of the biodiversity gradient could not be identified: J. N. Mills. Archives of Virology, 45–57 (2005); A.T. Strauss, et al. Ecol Monogr 86(4):393–411, (2016); Zimmermann et al. Acta Parasitologica 62: 493–501 (2017); J. A. Lau, S. Y. Strauss. Ecology 86, 2990–2997 (2005). Sin Nombre Virus from unpublished data in D. J. Salkeld et. al. Ecology Letters 16, 679–686 (2013).
We obtained data and code (when available) from these four publications. For each study in each dataset, we assigned the driver of the underlying biodiversity gradient, and whether or not that driver was associated with biodiversity loss (presented in Table 1; Table S1) by reading the abstract and methods of each study. We could not identify the driver of biodiversity gradients in five studies (Table 2; Table S1), so those studies were omitted from our analysis. The resulting database from Civitello et al. (2015) included 208 effect sizes (Hedge’s G) from 45 manuscripts, the database from Halliday and Rohr (2019) included 217 effect sizes (Spearman Rank correlations) from 37 manuscripts, the database from Magnusson et al. (2020) included 120 effect sizes (Hedge’s G) from 37 manuscripts, and the database from Liu et al. (2020) included 136 effect sizes (Fisher’s Z) from 20 manuscripts.
The original studies by Civitello et al. (2015), Magnusson et al. (2020), and Liu et al. (2020) were all carried out using a common analytical framework, by performing mixed‐effects meta‐analyses using the R package metafor (Viechtbauer, 2010). In contrast, Halliday and Rohr (2019) constructed multilevel random effects models using R package lme4 (Bates et al., 2014) to analyse Spearman Rank correlations. So that all four datasets could be analysed using a common statistical framework, we transformed Spearman Rank correlations from Halliday and Rohr (2019) into Fisher’s Z following the methods provided in Liu et al (2020). Briefly, the Spearman rank correlation from each study was transformed into Fisher’s Z using the following equation: , and the variance of Fisher’s Z was defined as . One study included a Spearman Rank correlation of −1. We therefore subtracted 1e−5 from the Spearman Rank correlation when calculating Fisher’s Z.
We then performed mixed‐effects meta‐analyses using Hedge’s G values reported in Civitello et al. (2015) and Magnusson et al. (2020), Fisher’s Z values reported in Liu et al. (2020), and Fisher’s Z transformed from Spearman Rank correlations reported in Halliday and Rohr (2019). Models were constructed in concordance with the methods described in Civitello et al. (2015) and Magnusson et al. (2020), using the rma.mv function in the R package metafor (Viechtbauer, 2010). Each model included whether or not the biodiversity gradient was associated with biodiversity loss as a two‐level moderator of the dilution effect. Heterogeneity was estimated using restricted maximum likelihood, and separate random intercepts were assigned to the manuscript from which from which effect sizes originated and to parasite species to account for underlying heterogeneity and a lack of independence among effect sizes within a manuscript and across manuscripts that tested the same parasite species. The raw data and an annotated R Markdown script for these analyses are available on Figshare (Halliday, 2020).
Are biodiversity‐disease patterns robust to misclassification and whether or not studies included manipulative experiments?
We acknowledge that our classification of biodiversity gradients as being associated with biodiversity loss or not might be imprecise. For example, Rendón‐Franco et al. (2014) measured diseases of small mammals in three different vegetation types: short grassland, tall grassland, and mesquite shrub, with the aim of acquiring a gradient of host richness and diversity. The factors that determined these three different vegetation types was unclear from the manuscript, so we assigned the driver of this biodiversity gradient as environmental heterogeneity, which is not associated with biodiversity loss (Table 1, Table S1). However, it is equally possible that these three vegetation types were a reflection of different land‐use histories, which would be associated with biodiversity loss, and that we therefore misclassified the underlying biodiversity gradient in this study.
To test whether our results were sensitive to misclassification in how we assigned drivers of biodiversity gradients, we randomly selected a proportion of studies, then randomly assigned the driver of biodiversity gradients in those studies, and re‐analysed the data, permuting this misclassification analysis 200 times for each misclassification rate.
In addition to problems of misclassification, assigning the underlying driver of biodiversity gradients in experiments can be problematic. Most experimental designs involve some kind of biodiversity loss; however, whether that loss is a random artefact of experimental design or represents a realistic example of biodiversity loss in nature depends on how the experiment is conducted. Consequently, the relationship between biodiversity and disease risk in manipulative experiments is often sensitive to host composition (Venesky et al., 2014; Han et al., 2015a; Halliday et al., 2017). To the best of our knowledge, only two studies have compared random and realistic biodiversity loss experimentally, with both studies finding that realistic biodiversity loss produced the strongest and most consistent dilution effects (Liu et al., 2018; Johnson et al., 2019). We therefore next dropped experiments from all datasets and re‐analysed the data.
The debate surrounding the generality of the dilution effect often involves questions of whether or not it is appropriate to include manipulative experiments in tests of generality (e.g., Halliday and Rohr, 2019; Magnusson et al, 2020). We therefore conducted all remaining analyses on datasets that excluded experiments.
Does accounting for biodiversity loss explain inconsistencies among different data syntheses?
Finally, using the databasets, but excluding experiments, we tested whether inconsistencies among studies in the factors that modify the dilution effect could be explained by biodiversity loss. To this end, we re‐analysed the data, using each hypothesised moderator (i.e., explanatory variable) of the dilution effect that was tested in each original meta‐analysis (e.g., Parasite life history, Study type, Latitude, Spatial scale, etc.; Table 2) and an interaction between that moderator and whether or not the biodiversity gradient was associated with biodiversity loss. These tests were carried out as mixed‐effects meta‐analyses using Hedge’s G from Civitello et al. (2015) and Magnusson et al. (2020), Fisher’s Z from Liu et al. (2020), and Fisher’s Z from Halliday and Rohr (2019), using the rma.mv function in the R package metafor. Heterogeneity was estimated using restricted maximum likelihood, and separate random intercepts were assigned to the manuscript from which from which effect sizes originated and to parasite species to account for underlying heterogeneity and a lack of independence among effect sizes within a manuscript and across manuscripts that tested the same parasite species. The raw data and an annotated R Markdown script for these analyses are available on Figshare (Halliday, 2020).
RESULTS
Our reanalysis of the four previously published datasets revealed that biodiversity gradients associated with biodiversity loss consistently generated dilution effects (Civitello et al.: g = −1.08 ± 0.15 [mean ± SE], P < 0.0001; Halliday and Rohr: Z = −0.29 ± 0.08, P = 0.0002; Magnusson et al.: g = −0.69 ± 0.17, P < 0.0001; Liu et al.: Z = −0.32 ± 0.10, P = 0.001), whereas other biodiversity gradients inconsistently generated dilution effects (Civitello et al.: g = −0.78 ± 0.37, P = 0.036; Halliday and Rohr: Z = 0.13 ± 0.07, P = 0.082; Magnusson et al.: g = −0.29 ± 0.19, P < 0.14; Liu et al.: Z = −0.28 ± 0.20, P = 0.17 Fig. 1). These patterns were robust to misclassification of the underlying source of biodiversity gradients in as many as 50% of the studies (Fig. 2). Moreover the patterns were often robust to the exclusion of experimental studies, which can often test contrived community compositions. After excluding experiments in the Civitello et al., Halliday and Rohr, and Magnusson et al. datasets, biodiversity gradients associated with biodiversity loss still consistently generated dilution effects (g = −0.93 ± 0.22, P < 0.0001; Z = −0.25 ± 0.12, P = 0.042; g = −0.69 ± 0.17, P < 0.0001, respectively), whereas gradients not clearly associated with biodiversity loss still did not (g = −0.61 ± 0.33, P = 0.07; Z = −0.13 ± 0.08, P = 0.12; g = −0.29 ± 0.20, P = 0.14, respectively; Fig. 3). The exception was the Liu database (Table 2; Liu et al., 2020), where there was no significant dilution effect after excluding experiments (Biodiversity loss: Z = −0.33 ± 0.21, P = 0.12; No biodiversity loss: Z = −0.29 ± 0.27, P = 0.28).
Figure 1.
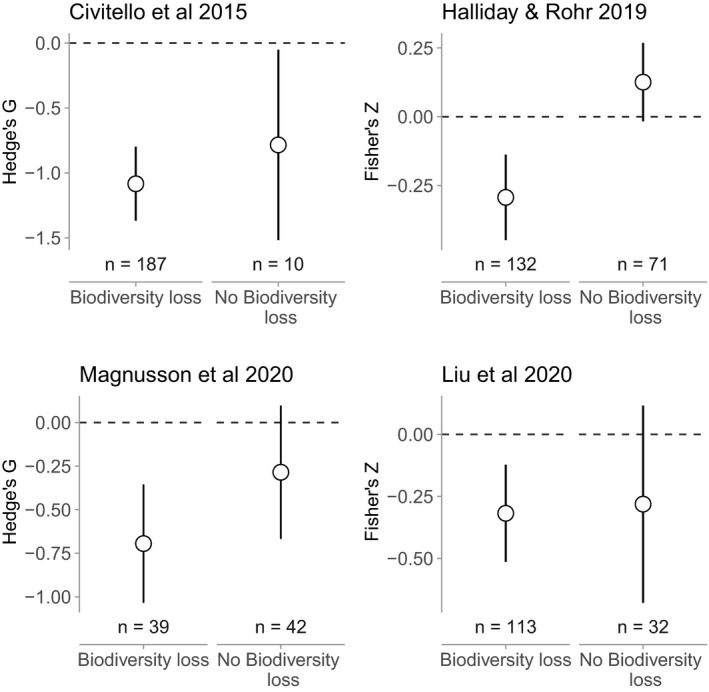
Effect of biodiversity loss on the dilution effect. Each panel corresponds to a separate meta‐analysis of the dilution effect. The y‐axis is a standardised effect size from the meta‐analysis, aimed at estimating the strength of the dilution effect, with values below zero corresponding to a negative effect of biodiversity on disease risk (i.e., dilution). Points are model‐estimated means, and error bars are model‐estimated 95% confidence intervals. The dilution effect is robust across biodiversity gradients driven by biodiversity loss, but this effect is idiosyncratic across diversity gradients that do not involve biodiversity loss.
Figure 2.
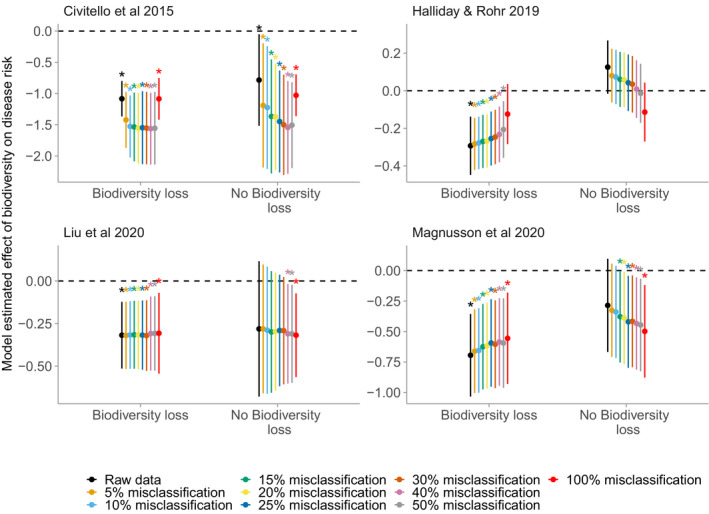
Effect of misclassification on moderation of the dilution effect by biodiversity loss. Each panel corresponds to a separate meta‐analysis of the dilution effect. The y‐axis is a standardised effect size from the meta‐analysis, aimed at estimating the strength of the dilution effect, with values below zero corresponding to a negative effect of biodiversity on disease risk (i.e., dilution). Points are the average model‐estimated mean, and error bars are the average model‐estimated 95% confidence intervals across 200 simulations. Asterisks correspond to misclassification rates in which the average 95% confidence interval did not overlap zero (i.e., in which tests identified significant dilution or amplification, on average, across the 200 simulations). The effect of biodiversity loss on the strength of the dilution effect is robus to misclassification of at least 10% and up to 50% of studies.
Figure 3.
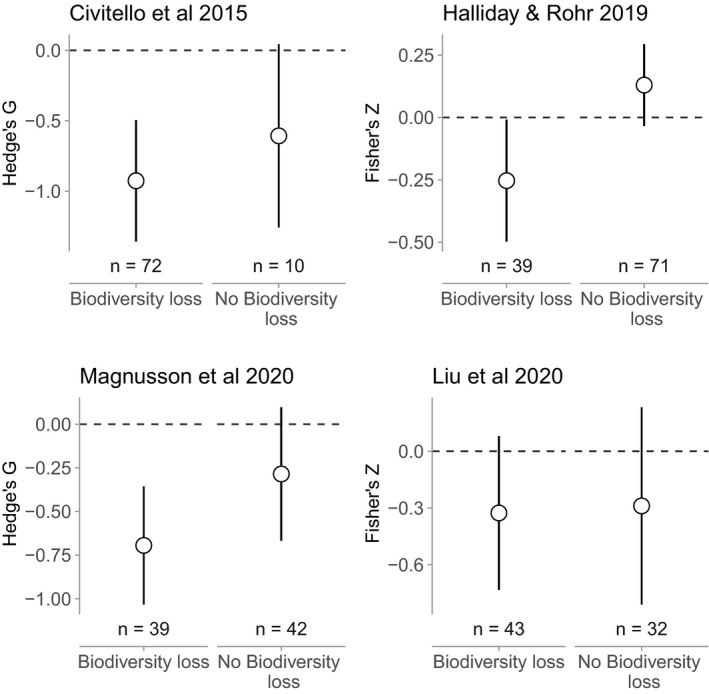
Effect of biodiversity loss on the dilution effect after excluding experiments. Panels correspond to different databases. Y‐axes are standardised effect sizes, with values below zero corresponding to negative effects (i.e., dilution). Points are model‐estimated means, and error bars are model‐estimated 95% confidence intervals. With the exception of Liu, which was sensitive to study design, the dilution effect is robust across biodiversity gradients driven by biodiversity loss, but this effect is idiosyncratic across diversity gradients that do not involve biodiversity loss, even after excluding experiments.
Finally, we tested whether statistical interactions between biodiversity loss and other hypothesised moderators of the dilution effect (e.g., Parasite life history, Study type, Latitude, Spatial scale, etc.) could explain inconsistencies among the four focal studies (Fig. S1–S3). The degree to which the four databases included gradients of biodiversity driven by biodiversity loss versus other factors resolved inconsistencies regarding spatial moderation of the dilution effect, but amplified discrepancies related to latitudinal gradients (Table 2; Fig. 4). Spatial scale significantly interacted with biodiversity loss in both studies that evaluated spatial scale (Halliday and Rohr: estimate = 0.099 ± 0.045, LRT 5.12, P = 0.024; Magnusson et al.: estimate = 0.099 ± 0.041, LRT 6.23, P = 0.013); the strength of dilution increased with scale for studies that involved biodiversity loss and weakened with scale for studies that did not (Fig. 4). In contrast to the consistency across studies in the scale patterns, we found a non‐significant (estimate = −0.015 ± 0.011, LRT 2.40, P = 0.12) and significant (estimate = −0.087 ± 0.039, LRT 5.54, P = 0.019) interaction between biodiversity loss and (absolute value of) latitude for the Magnusson et al. and Liu et al. datasets, respectively (Fig. 4). Moreover the direction of these effects were opposite; in Liu et al., dilution weakened with increasing (absolute values of) latitude for biodiversity‐loss studies, whereas in Magnusson et al., dilution strengthened with increasing (absolute values of) latitude for non‐biodiversity‐loss studies (Fig. 4).
Figure 4.
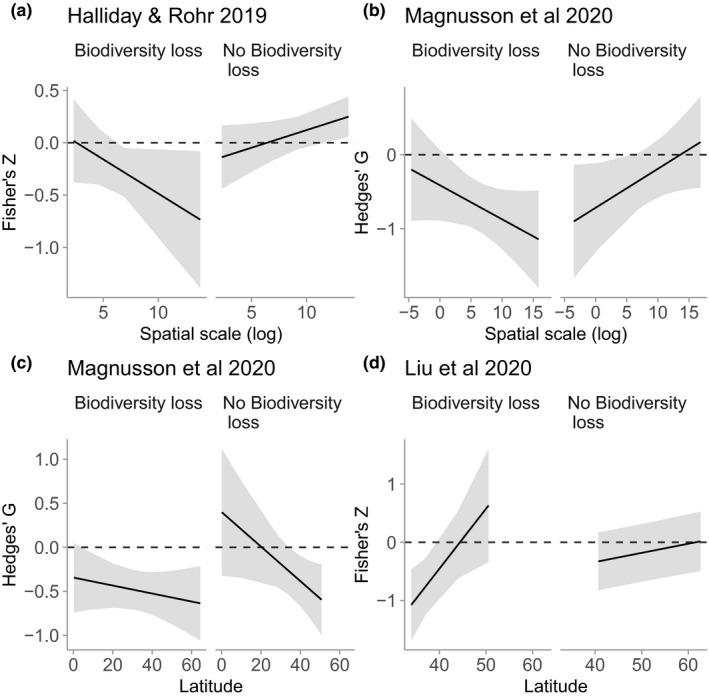
Effect of biodiversity loss on moderation of the dilution effect. Panels correspond to models of the interaction between biodiversity‐loss and spatial scale (a and b) or latitude (c and d) for different meta‐analyses, excluding experiments. The y‐axis is a standardised effect size from the meta‐analysis. Lines are model‐estimated means, and ribbons are model‐estimated 95% confidence intervals. Incorporating biodiversity loss resolves inconsistences in the effect of spatial scale, but not latitude.
DISCUSSION
This study shows broad evidence that biodiversity loss underlies the dilution effect. The effect of biodiversity loss on the dilution effect was robust to misclassification and whether or not studies included manipulative experiments. Furthermore, accounting for biodiversity loss explained some inconsistencies among prior data syntheses. Together, these results provide important context for understanding the role that biodiversity plays in protecting human wellbeing and ecosystem health, suggesting that preventing biodiversity loss can proactively reduce infectious disease risk (Rohr et al., 2020).
Because community disassembly often favours more competent hosts (Table 1), we expected that biodiversity loss would commonly result in dilution effects. Our reanalysis of four published datasets is consistent with this idea: dilution effects were commonly observed among biodiversity‐loss studies across all four datasets. However, we did not directly test whether dilution effects arise due to an increase in competent hosts, because most published studies do not report the identities or abundances of (potentially) diluting host species. Future studies should test for generality in this mechanism directly by comparing host community structure (including traits associated with host community competence and host biodiversity) across a variety of biodiversity drivers (e.g., Halliday et al., 2019), and in a variety of study systems.
Because the relationship between host community competence and biodiversity is often unpredictable along natural biodiversity gradients (Table 1), we expected that gradients not associated with biodiversity loss would inconsistently result in dilution effects. Our results support this idea: dilution effects were inconsistently observed among non‐biodiversity‐loss studies in three out of four datasets. However, these results do not suggest that dilution effects only occur when biodiversity gradients are associated with biodiversity loss. Importantly, even when there is no net association between host diversity and community competence, increasing biodiversity can still reduce disease risk of parasites that are specialised to infect a small number of host species by modulating host density (i.e., via encounter reduction; Mitchell et al., 2002; Keesing et al., 2006). Encounter reduction, in turn, might be particularly relevant when gradients include seasonality (e.g., latitude, elevation) that affects peak prevalence and the duration of the epidemic season. Thus, biodiversity gradients that are not associated with biodiversity loss could still generate consistent dilution effects via encounter reduction for specialist parasites. Understanding the degree to which biodiversity influences disease risk among specialists versus generalists in the context of biodiversity loss therefore remains an important topic for future studies.
Our prediction that biodiversity loss underlies the dilution effect was grounded in host community competence, because host communities become more competent as biodiversity is lost (e.g., Johnson et al., 2013; Liu et al., 2017); however, biodiversity loss could also influence the dilution effect by other potential mechanisms. As an example, biodiversity loss does not necessarily alter nutrient availability in the same way that nutrient availability influences biodiversity, with implications for higher trophic levels (Grace et al., 2016; Cappelli et al., 2020). Gradients that are or are not associated biodiversity loss could also differ in host abundance or density, connectivity of hosts and parasites, or host temporal turnover (Keesing et al., 2006, 2010; Young et al., 2014; Johnson et al., 2015a).
Our results also suggest that statistical interactions between biodiversity loss and spatial scale might be sufficient to explain inconsistencies among the four focal studies, but that interactions between biodiversity loss and latitude are not. However, as in prior studies on the dilution effect, we wish to emphasise that our analysis of spatial scale might be sensitive to the scarcity of studies conducted at the largest spatial scale and to a variety of study characteristics linked to spatial scale, including the metrics used to estimate diversity and disease, study design, and parasite type (Halliday and Rohr, 2019). Importantly, both datasets that tested spatial scale only included one global study where the underlying gradient involved biodiversity loss (Derne et al., 2011) and only one global study where the underlying gradient did not involve biodiversity loss (Wood et al., 2017). Consequently, we cannot rule out the possibility that these results could change if future studies filled these research gaps. Nevertheless, incorporating biodiversity loss resolved inconsistencies among studies related to spatial moderation of the dilution effect.
Even among datasets where biodiversity loss interacted with scale or latitude, the direction and magnitude of these interactions were not always consistent with theory. Specifically, theory predicts that increasing spatial scale and (absolute values of) latitude should weaken the dilution effect, because biotic interactions tend to weaken with increasing spatial scale and (absolute values of) latitude (Wood and Lafferty, 2013; Johnson et al., 2015a; Cohen et al., 2016; Halliday and Rohr, 2019; Liu et al., 2020; Rohr et al., 2020) (but see Magnusson et al., 2020). We therefore expected that if host community competence drives the dilution effect (Johnson et al., 2013), and this process occurs more commonly when biodiversity is lost (Table 1), then this moderating effect of latitude and spatial scale would be strongest among biodiversity‐loss studies. Consistent with this hypothesis, increasing latitude weakened the dilution effect in biodiversity‐loss studies, though this effect was only observed in one dataset (Liu et al., 2020). In contrast, increasing scale increased the strength of the dilution effect among biodiversity‐loss studies. We suggest that this result might be more statistical than biological: among non‐biodiversity‐loss studies where biodiversity is not associated with host community competence, large spatial scales can confound biodiversity gradients with changes in species pools, weakening dilution effects (Wood and Lafferty, 2013; Rohr et al., 2020). In contrast, among biodiversity‐loss studies where biodiversity is associated with community competence regardless of the underlying species pool, increasing scale could strengthen the dilution effect, particularly if large‐scale studies capture a larger portion of the biodiversity gradient than smaller‐scale studies, and their biodiversity‐disease relationships favour dilution over the majority of the gradient (i.e., they are right‐skewed; Halliday and Rohr, 2019; Rohr et al., 2020). These results highlight the need for studies that measure biodiversity gradients across spatial scales to better disentangle conditions under which spatial scale and latitude moderate the dilution effect.
This study was not designed to test whether changes in ecological parameters, such as those resulting from anthropogenic factors, cause increasing disease risk due to changes in local biodiversity. In other words, this study was not designed to test whether biodiversity loss is an intermediate cause of changes in disease risk. Changes in biodiversity resulting from anthropogenic drivers have been linked to subsequent changes in ecosystem functioning (Hautier et al., 2015). However, anthropogenic drivers do not universally reduce local biodiversity (Chase et al., 2019; Dornelas et al., 2019), complicating the linkages among anthropogenic drivers, biodiversity loss, and disease risk. Disentangling whether anthropogenic drivers alter disease risk due to changes in species richness, per se, is further complicated by delayed impacts of past events, which can interact with existing environmental filters during community reassembly (Jung et al 2019). However, despite these limitations, ecological theory does suggest that characteristics of host species that allow those species to resist or rapidly recover following changes in ecological parameters may also make those host species more competent, causing increases in disease risk following such events (Johnson et al., 2013, 2019; Joseph et al., 2013; Mihaljevic et al., 2014; Liu et al., 2018; Halliday et al., 2019). Our results lend some support to this assertion, suggesting that the link between biodiversity and disease risk is strongest when changes in ecological parameters cause biodiversity loss.
Together, the results of this study highlight the need to consider drivers of biodiversity gradients when predicting the role of biodiversity in influencing infectious disease. Specifically, our results suggest that dilution effects may occur less commonly for biodiversity gradients that are not associated with the loss of biodiversity, but occur regularly for biodiversity gradients that are generated by disturbances that cause losses of biodiversity. These results are consistent with a growing body of literature suggesting that the role of biodiversity in regulating ecosystem processes depends on characteristics of species or individuals present in those ecosystems (Mouillot et al., 2011; Allan et al., 2015; Leitão et al., 2016; Van de Peer et al., 2018et al., 2018; Bagousse‐Pinguet et al., 2019; Start and Gilbert, 2019; Heilpern et al., 2020). These results therefore provide clarity in an increasingly polarised debate. Specifically, because characteristics of host communities often predictably change with biodiversity loss, these results suggest that biodiversity loss generally exacerbates infectious disease risk.
AUTHOR CONTRIBUTIONS
FWH designed the study, analysed the data, and wrote the first draft. All authors contributed substantially to revising the manuscript.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ele.13590.
Supporting information
Fig S1‐S3
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank D. Civitello, M. Jalo, P. Wilfahrt, J. Chase, members of the Laine Lab, and two anonymous reviewers for insightful suggestions. This work was supported by the University of Zürich and by grants from the Academy of Finland (296686) to A‐LL and the European Research Council (Consolidator Grant RESISTANCE 724508) to A‐LL.
[Correction added on 31 August 2020, after first online publication: First paragraph of the Introduction and Reference list have been updated.]
DATA AVAILABILITY STATEMENT
The data and code supporting the results are archived on Figshare: https://dx.doi.org/10.6084/m9.figshare.12155562
References
- Albrecht, H. & Haider, S. (2013). Species diversity and life history traits in calcareous grasslands vary along an urbanization gradient. Biodivers. Conserv., 22, 2243–2267. [Google Scholar]
- Allan, B.F. , Keesing, F. & Ostfeld, R.S. (2003). Effect of Forest Fragmentation on Lyme Disease Risk. Conserv. Biol., 17, 267–272. [Google Scholar]
- Allan, E. , Manning, P. , Alt, F. , Binkenstein, J. , Blaser, S. , Blüthgen, N. et al (2015). Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett., 18, 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altermatt, F. , Seymour, M. & Martinez, N. (2013). River network properties shape α‐diversity and community similarity patterns of aquatic insect communities across major drainage basins. J. Biogeogr., 40, 2249–2260. [Google Scholar]
- Bagousse‐Pinguet, Y.L. , Soliveres, S. , Gross, N. , Torices, R. , Berdugo, M. & Maestre, F.T. (2019). Phylogenetic, functional, and taxonomic richness have both positive and negative effects on ecosystem multifunctionality. Proc. Natl Acad. Sci., 116, 8419–8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. & Walker, S. (2014). Fitting linear mixed‐effects models using lme4. J. Stat. Softw., 67, 1–48. [Google Scholar]
- Becker, D.J. & Han, B.A. (2020). The macroecology and evolution of avian competence for Borrelia burgdorferi. bioRxiv, 2020.04.15.040352. [Google Scholar]
- Beckmann, M. , Gerstner, K. , Akin‐Fajiye, M. , Ceaușu, S. , Kambach, S. , Kinlock, N.L. et al (2019). Conventional land‐use intensification reduces species richness and increases production: A global meta‐analysis. Glob. Chang. Biol., 25, 1941–1956. [DOI] [PubMed] [Google Scholar]
- Benkman, C.W. (2013). Biotic interaction strength and the intensity of selection. Ecol. Lett., 16, 1054–1060. [DOI] [PubMed] [Google Scholar]
- Cadotte, M.W. (2007). Competition‐colonization trade‐offs and disturbance effects at multiple scales. Ecology, 88, 823–829. [DOI] [PubMed] [Google Scholar]
- Cappelli, S.L. , Pichon, N.A. , Kempel, A. & Allan, E. (2020). Sick plants in grassland communities: a growth‐defense trade‐off is the main driver of fungal pathogen abundance. Ecology Letters. 10.1111/ele.13537 [DOI] [PubMed] [Google Scholar]
- Chase, J.M. , McGill, B.J. , Thompson, P.L. , Antão, L.H. , Bates, A.E. , Blowes, S.A. et al (2019). Species richness change across spatial scales. Oikos, 128, 1079–1091. [Google Scholar]
- Civitello, D.J. , Cohen, J. , Fatima, H. , Halstead, N.T. , Liriano, J. , McMahon, T.A. et al (2015). Biodiversity inhibits parasites: Broad evidence for the dilution effect. Proc. Natl Acad. Sci., 112, 8667–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J.M. , Civitello, D.J. , Brace, A.J. , Feichtinger, E.M. , Ortega, C.N. , Richardson, J.C. et al (2016). Spatial scale modulates the strength of ecological processes driving disease distributions. Proc. Natl Acad. Sci. USA, 113, E3359–E3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell, R.K. , Dunn, R.R. & Harris, N.C. (2012). Coextinction and persistence of dependent species in a changing world. Annu. Rev. Ecol. Evol. Syst., 43, 183–203. [Google Scholar]
- Cronin, J.P. , Welsh, M.E. , Dekkers, M.G. , Abercrombie, S.T. & Mitchell, C.E. (2010). Host physiological phenotype explains pathogen reservoir potential. Ecol. Lett., 13, 1221–1232. [DOI] [PubMed] [Google Scholar]
- Debecker, S. , Sanmartín‐Villar, I. , Guinea‐Luengo, M. , Cordero‐Rivera, A. & Stoks, R. (2016). Integrating the pace‐of‐life syndrome across species, sexes and individuals: covariation of life history and personality under pesticide exposure. J. Anim. Ecol., 85, 726–738. [DOI] [PubMed] [Google Scholar]
- Derne, B.T. , Fearnley, E.J. , Lau, C.L. , Paynter, S. & Weinstein, P. (2011). Biodiversity and leptospirosis risk: A case of pathogen regulation? Med. Hypotheses, 77, 339–344. [DOI] [PubMed] [Google Scholar]
- Dornelas, M. , Gotelli, N.J. , Shimadzu, H. , Moyes, F. , Magurran, A.E. & McGill, B.J. (2019). A balance of winners and losers in the Anthropocene. Ecol. Lett., 22, 847–854. [DOI] [PubMed] [Google Scholar]
- Farrell, M.J. , Stephens, P.R. , Berrang‐Ford, L. , Gittleman, J.L. & Davies, T.J. (2015). The path to host extinction can lead to loss of generalist parasites. J. Anim. Ecol., 84, 978–984. [DOI] [PubMed] [Google Scholar]
- Fay, P.A. , Prober, S.M. , Stanley Harpole, W. , Knops, J. , Bakker, J.D. , Borer, E.T. et al (2015). Grassland productivity limited by multiple nutrients. Nat. Plants, 1, 5. [DOI] [PubMed] [Google Scholar]
- Gendron, A.D. , Marcogliese, D.J. , Barbeau, S. , Christin, M.‐S. , Brousseau, P. , Ruby, S. et al (2003). Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae. Oecologia, 135, 469–476. [DOI] [PubMed] [Google Scholar]
- Gibbs, M. & Van Dyck, H. (2010). Butterfly flight activity affects reproductive performance and longevity relative to landscape structure. Oecologia, 163, 341–350. [DOI] [PubMed] [Google Scholar]
- Grace, J.B. , Anderson, T.M. , Seabloom, E.W. , Borer, E.T. , Adler, P.B. , Harpole, W.S. et al (2016). Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature, 529, 1–10. [DOI] [PubMed] [Google Scholar]
- Grimm, N.B. , Faeth, S.H. , Golubiewski, N.E. , Redman, C.L. , Wu, J. , Bai, X. et al (2008). Global change and the ecology of cities. Science, 319, 756–760. [DOI] [PubMed] [Google Scholar]
- Hahs, A.K. , McDonnell, M.J. , McCarthy, M.A. , Vesk, P.A. , Corlett, R.T. , Norton, B.A. et al (2009). A global synthesis of plant extinction rates in urban areas. Ecol. Lett., 12, 1165–1173. [DOI] [PubMed] [Google Scholar]
- Halliday, F.W. (2020). Data and code from “Biodiversity loss underlies the dilution effect of biodiversity”. [DOI] [PMC free article] [PubMed]
- Halliday, F.W. , Heckman, R.W. , Wilfahrt, P.A. & Mitchell, C.E. (2017). A multivariate test of disease risk reveals conditions leading to disease amplification. Proc. R. Soc. B Biol. Sci., 284, 20171340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday, F.W. , Heckman, R.W. , Wilfahrt, P.A. & Mitchell, C.E. (2019). Past is prologue: host community assembly and the risk of infectious disease over time. Ecology Letters, 22(1), 138–148. [DOI] [PubMed] [Google Scholar]
- Halliday, F.W. & Rohr, J.R. (2019). Measuring the shape of the biodiversity‐disease relationship across systems reveals new findings and key gaps. Nat. Commun., 10, 5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey, S. (2019). Defuse the dilution effect debate. Nat. Ecol. Evol., 3, 145–146. [DOI] [PubMed] [Google Scholar]
- Han, B.A. , Kerby, J.L. , Searle, C.L. , Storfer, A. & Blaustein, A.R. (2015a). Host species composition influences infection severity among amphibians in the absence of spillover transmission. Ecol. Evol., 5, 1432–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, B.A. , Schmidt, J.P. , Bowden, S.E. & Drake, J.M. (2015b). Rodent reservoirs of future zoonotic diseases. Proc. Natl Acad. Sci. USA, 112, 7039–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski, I. (2015). Habitat fragmentation and species richness. J. Biogeogr., 42, 989–993. [Google Scholar]
- Hanski, I. , Saastamoinen, M. & Ovaskainen, O. (2006). Dispersal‐related life‐history trade‐offs in a butterfly metapopulation. J. Anim. Ecol., 75, 91–100. [DOI] [PubMed] [Google Scholar]
- Hargreaves, A.L. , Suárez, E. , Mehltreter, K. , Myers‐Smith, I. , Vanderplank, S.E. , Slinn, H.L. et al (2019). Seed predation increases from the Arctic to the Equator and from high to low elevations. Sci. Adv., 5, eaau4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautier, Y. , Tilman, D. , Isbell, F. , Seabloom, E.W. , Borer, E.T. & Reich, P.B. (2015). Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science, 348, 336–340. [DOI] [PubMed] [Google Scholar]
- Heckman, R.W. , Halliday, F.W. & Mitchell, C.E. (2019). A growth–defense trade‐off is general across native and exotic grasses. Oecologia, 191, 1–12. [DOI] [PubMed] [Google Scholar]
- Heilpern, S.A. , Anujan, K. , Osuri, A. & Naeem, S. (2020). Positive correlations in species functional contributions drive the response of multifunctionality to biodiversity loss. Proc. R. Soc. B Biol. Sci., 287, 20192501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HilleRisLambers, J. , Adler, P.B. , Harpole, W.S. , Levine, J.M. & Mayfield, M.M. (2012). Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. Syst., 43, 227–248. [Google Scholar]
- Hillebrand, H. (2004). On the generality of the latitudinal diversity gradient. Am. Nat., 163, 192–211. [DOI] [PubMed] [Google Scholar]
- Huang, Z.Y.X. , de Boer, W.F. , van Langevelde, F. , Olson, V. , Blackburn, T.M. & Prins, H.H.T. (2013). Species’ life‐history traits explain interspecific variation in reservoir competence: A possible mechanism underlying the dilution effect. PLoS One, 8, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch, A. & White, P. (2019). A theory of pulse dynamics and disturbance in ecology. Ecology, 100, e02734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetz, W. , Freckleton, R.P. & McKechnie, A.E. (2008). Environment, migratory tendency, phylogeny and basal metabolic rate in birds. PLoS One, 3, e3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, P.T.J. , Calhoun, D.M. , Riepe, T. , McDevitt‐Galles, T. & Koprivnikar, J. (2019). Community disassembly and disease: realistic—but not randomized—biodiversity losses enhance parasite transmission. Proc. R. Soc. B Biol. Sci., 286, 20190260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, P.T.J. , Ostfeld, R.S. & Keesing, F. (2015a). Frontiers in research on biodiversity and disease. Ecol. Lett., 18, 1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, P.T.J. , Preston, D.L. , Hoverman, J.T. & Richgels, K.L.D. (2013). Biodiversity decreases disease through predictable changes in host community competence. Nature, 494, 230–233. [DOI] [PubMed] [Google Scholar]
- Johnson, P.T.J. , Rohr, J.R. , Hoverman, J.T. , Kellermanns, E. , Bowerman, J. & Lunde, K.B. (2012). Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol. Lett., 15, 235–242. [DOI] [PubMed] [Google Scholar]
- Johnson, P.T.J. , de Roode, J.C. & Fenton, A. (2015b). Why infectious disease research needs community ecology. Science (80‐., ), 349, 1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, M.B. , Mihaljevic, J.R. , Orlofske, S.A. & Paull, S.H. (2013). Does life history mediate changing disease risk when communities disassemble? Ecol. Lett., 16, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Keesing, F. , Belden, L.K. , Daszak, P. , Dobson, A. , Harvell, C.D. , Holt, R.D. et al (2010). Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature, 468, 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing, F. , Holt, R.D. & Ostfeld, R.S. (2006). Effects of species diversity on disease risk. Ecol. Lett., 9, 485–498. [DOI] [PubMed] [Google Scholar]
- Keinath, D.A. , Doak, D.F. , Hodges, K.E. , Prugh, L.R. , Fagan, W. , Sekercioglu, C.H. et al (2017). A global analysis of traits predicting species sensitivity to habitat fragmentation. Glob. Ecol. Biogeogr., 26, 115–127. [Google Scholar]
- Kergunteuil, A. , Röder, G. & Rasmann, S. (2019). Environmental gradients and the evolution of tri‐trophic interactions. Ecol. Lett., 22, 292–301. [DOI] [PubMed] [Google Scholar]
- Kirk, D. , Shea, D. & Start, D. (2019). Host traits and competitive ability jointly structure disease dynamics and community assembly. J. Anim. Ecol., 88, 1379–1391. [DOI] [PubMed] [Google Scholar]
- Körner, C. (2007). The use of ‘altitude’ in ecological research. Trends Ecol. Evol., 22, 569–574. [DOI] [PubMed] [Google Scholar]
- Laiolo, P. , Pato, J. & Obeso, J.R. (2018). Ecological and evolutionary drivers of the elevational gradient of diversity. Ecology Letters, 21(7), 1022–1032. [DOI] [PubMed] [Google Scholar]
- Leitão, R.P. , Zuanon, J. , Villéger, S. , Williams, S.E. , Baraloto, C. , Fortunel, C. et al (2016). Rare species contribute disproportionately to the functional structure of species assemblages. Proc. R. Soc. B Biol. Sci., 283, 20160084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Chen, F. , Lyu, S. , Sun, D. & Zhou, S. (2018). Random species loss underestimates dilution effects of host diversity on foliar fungal diseases under fertilization. Ecol. Evol., 8, 1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Chen, L. , Liu, M. , García‐Guzmán, G. , Gilbert, G.S. & Zhou, S. (2020). Dilution effect of plant diversity on infectious diseases: latitudinal trend and biological context dependence. Oikos, 129(4), 457–465. [Google Scholar]
- Liu, X. , Lyu, S. , Sun, D. , Bradshaw, C.J.A. & Zhou, S. (2017). Species decline under nitrogen fertilization increases community‐level competence of fungal diseases. Proceedings of the Royal Society B: Biological Sciences, 284(1847), 20162621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGiudice, K. , Ostfeld, R.S. , Schmidt, K.A. & Keesing, F. (2003). The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. U. S. A., 100, 567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, B.E. , Urban, D. & White, P.S. (2018). Testing the effects of four urbanization filters on forest plant taxonomic, functional, and phylogenetic diversity. Ecol. Appl., 28, 2197–2205. [DOI] [PubMed] [Google Scholar]
- Luis, A.D. , Hayman, D.T.S. , O’Shea, T.J. , Cryan, P.M. , Gilbert, A.T. , Pulliam, J.R.C. et al (2013). A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B Biol. Sci., 280, 20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur, R.H. (1972). Geographical Ecology: Patterns in the Distribution of Species. Princeton University Press, Princeton. [Google Scholar]
- MacArthur, R.H. & Wilson, E.O. (1967). Island biogeography. Princeton University Press, Princeton. [Google Scholar]
- Magnusson, M. , Fischhoff, I.R. , Ecke, F. , Hörnfeldt, B. & Ostfeld, R.S. (2020). Effect of spatial scale and latitude on diversity–disease relationships. Ecology, 101, e02955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, L.B. , Addison, B. , Bean, A.G.D. , Buchanan, K.L. , Crino, O.L. , Eastwood, J.R. et al (2019). Extreme competence: keystone hosts of infections. Trends Ecol. Evol., 34, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney, M.L. (2008). Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst., 11, 161–176. [Google Scholar]
- Merckx, T. , Souffreau, C. , Kaiser, A. , Baardsen, L.F. , Backeljau, T. , Bonte, D. et al (2018). Body‐size shifts in aquatic and terrestrial urban communities. Nature, 558, 113–116. [DOI] [PubMed] [Google Scholar]
- Mihaljevic, J.R. , Joseph, M.B. , Orlofske, S.A. , Paull, S.H. & Killilea, M. (2014). The scaling of host density with richness affects the direction, shape, and detectability of diversity‐disease relationships. PLoS One, 9, e97812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, C.E. , Tilman, D. & Groth, J.V. (2002). Effects of grassland plant species diversity, abundance, and composition on foliar fungal disease. Ecology, 83, 1713–1726. [Google Scholar]
- Mouillot, D. , Villéger, S. , Scherer‐Lorenzen, M. & Mason, N.W.H. (2011). Functional structure of biological communities predicts ecosystem multifunctionality. PLoS One, 6, e17476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold, T. , Hudson, L.N. , Hill, S.L.L. , Contu, S. , Lysenko, I. , Senior, R.A. et al (2015). Global effects of land use on local terrestrial biodiversity. Nature, 520, 45–50. [DOI] [PubMed] [Google Scholar]
- Nobis, M.P. & Schweingruber, F.H. (2013). Adult age of vascular plant species along an elevational land‐use and climate gradient. Ecography (Cop.), 36, 1076–1085. [Google Scholar]
- Oleksyn, J. , Reich, P.B. , Zytkowiak, R. , Karolewski, P. & Tjoelker, M.G. (2003). Nutrient conservation increases with latitude of origin in European Pinus sylvestris populations. Oecologia, 136, 220–235. [DOI] [PubMed] [Google Scholar]
- Ostfeld, R.S. & Keesing, F. (2000). Biodiversity series: The function of biodiversity in the ecology of vector‐borne zoonotic diseases. Can. J. Zool., 78, 2061–2078. [Google Scholar]
- Ostfeld, R.S. & LoGiudice, K. (2003). Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology, 84, 1421–1427. [Google Scholar]
- Ostfeld, R.S. & Keesing, F. (2012). Effects of Host Diversity on Infectious Disease. Annu. Rev. Ecol. Evol. Syst., 43, 157–182. [Google Scholar]
- Van de Peer, T. , Verheyen, K. , Ponette, Q. , Setiawan, N.N. & Muys, B. (2018). Overyielding in young tree plantations is driven by local complementarity and selection effects related to shade tolerance. J. Ecol., 106, 1096–1105. [Google Scholar]
- Pellissier, L. , Roger, A. , Bilat, J. & Rasmann, S. (2014). High elevation Plantago lanceolata plants are less resistant to herbivory than their low elevation conspecifics: is it just temperature? Ecography (Cop.), 37, 950–959. [Google Scholar]
- Peters, M.K. , Hemp, A. , Appelhans, T. , Behler, C. , Classen, A. , Detsch, F. et al (2016). Predictors of elevational biodiversity gradients change from single taxa to the multi‐taxa community level. Nat. Commun., 7, 13736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett, S.T.A. , Cadenasso, M.L. , Grove, J.M. , Nilon, C.H. , Pouyat, R.V. , Zipperer, W.C. et al (2001). Urban Ecological Systems: Linking terrestrial ecological, physical, and socioeconomic components of metropolitan areas. Annu. Rev. Ecol. Syst., 32, 127–157. [Google Scholar]
- Previtali, M.A. , Ostfeld, R.S. , Keesing, F. , Jolles, A.E. , Hanselmann, R. & Martin, L.B. (2012). Relationship between pace of life and immune responses in wild rodents. Oikos, 121, 1483–1492. [Google Scholar]
- Ramalho, C.E. , Laliberté, E. , Poot, P. & Hobbs, R.J. (2014). Complex effects of fragmentation on remnant woodland plant communities of a rapidly urbanizing biodiversity hotspot. Ecology, 95, 2466–2478. [Google Scholar]
- Rendón‐Franco, E. , Muñoz‐García, C.I. , Romero‐Callejas, E. , Moreno‐Torres, K.I. & Suzán, G. (2014). Effect of host species diversity on multiparasite systems in rodent communities. Parasitol. Res., 113, 447–450. [DOI] [PubMed] [Google Scholar]
- Rohr, J.R. , Civitello, D.J. , Halliday, F.W. , Hudson, P.J. , Lafferty, K.D. , Wood, C.L. et al (2020). Towards common ground in the biodiversity–disease debate. Nat. Ecol. Evol., 4, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roslin, T. , Hardwick, B. , Novotny, V. , Petry, W.K. , Andrew, N.R. , Asmus, A. et al (2017). Higher predation risk for insect prey at low latitudes and elevations. Science, 356, 742–744. [DOI] [PubMed] [Google Scholar]
- Sears, B.F. , Snyder, P.W. & Rohr, J.R. (2015). Host life history and host‐parasite syntopy predict behavioural resistance and tolerance of parasites. J. Anim. Ecol., 84, 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberloff, D.S. & Wilson, E.O. (1970). Experimental Zoogeography of Islands. A Two‐Year Record of Colonization. Ecology, 51, 934–937. [Google Scholar]
- Start, D. & Gilbert, B. (2019). Trait variation across biological scales shapes community structure and ecosystem function. Ecology, 100, e02769. [DOI] [PubMed] [Google Scholar]
- Stein, A. , Gerstner, K. & Kreft, H. (2014). Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett., 17, 866–880. [DOI] [PubMed] [Google Scholar]
- Stenhouse, R.N. (2004). Fragmentation and internal disturbance of native vegetation reserves in the Perth metropolitan area. Western Australia. Landsc. Urban Plan., 68, 389–401. [Google Scholar]
- Stewart Merrill, T.E. & Johnson, P.T.J. (2020). Toward a mechanistic understanding of competence: a missing link in diversity‐disease research. Parasitology, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, A.T. , Shocket, M.S. , Civitello, D.J. , Hite, J.L. , Penczykowski, R.M. , Duffy, M.A. et al (2016). Habitat, predators, and hosts regulate disease in Daphnia through direct and indirect pathways. Ecol. Monogr., 86, 393–411. [Google Scholar]
- Tilman, D. (1990). Constraints and tradeoffs: toward a predictive theory of competition and succession. Oikos, 58, 3–15. [Google Scholar]
- Vellend, M. (2010). Conceptual synthesis in community ecology. Q. Rev. Biol., 85, 183–206. [DOI] [PubMed] [Google Scholar]
- Venesky, M.D. , Liu, X. , Sauer, E.L. & Rohr, J.R. (2014). Linking manipulative experiments to field data to test the dilution effect. J. Anim. Ecol., 83, 557–565. [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor Package. J. Stat. Softw., 36, 1–48. [Google Scholar]
- Wagner, N. , Mingo, V. , Schulte, U. & Lötters, S. (2015). Risk evaluation of pesticide use to protected European reptile species. Biol. Conserv., 191, 667–673. [Google Scholar]
- Wallace, A.R. (1878). Tropical Nature, and Other Essays. Macmillan and Company, London. [Google Scholar]
- Williams, N.S.G. , Schwartz, M.W. , Vesk, P.A. , McCarthy, M.A. , Hahs, A.K. , Clemants, S.E. et al (2009). A conceptual framework for predicting the effects of urban environments on floras. J. Ecol., 97, 4–9. [Google Scholar]
- Wohlgemuth, T. , Nobis, M.P. , Kienast, F. & Plattner, M. (2008). Modelling vascular plant diversity at the landscape scale using systematic samples. J. Biogeogr., 35, 1226–1240. [Google Scholar]
- Wood, C.L. & Lafferty, K.D. (2013). Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends Ecol. Evol., 28, 239–247. [DOI] [PubMed] [Google Scholar]
- Wood, C.L. , McInturff, A. , Young, H.S. , Kim, D. & Lafferty, K.D. (2017). Human infectious disease burdens decrease with urbanization but not with biodiversity. Philos. Trans. R. Soc. B Biol. Sci., 372, 20160122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, H.S. , Dirzo, R. , Helgen, K.M. , McCauley, D.J. , Billeter, S.A. , Kosoy, M.Y. et al (2014). Declines in large wildlife increase landscape‐level prevalence of rodent‐borne disease in Africa. Proc. Natl Acad. Sci. USA, 111, 7036–7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, H.S. , Parker, I.M. , Gilbert, G.S. , Sofia Guerra, A. & Nunn, C.L. (2017). Introduced species, disease ecology, and biodiversity‐disease relationships. Trends Ecol. Evol., 32, 41–54. [DOI] [PubMed] [Google Scholar]
- Ziv, Y. & Davidowitz, G. (2019). When landscape ecology meets physiology: effects of habitat fragmentation on resource allocation trade‐offs. Front. Ecol. Evol., 7, 137. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S3
Supplementary Material
Data Availability Statement
The data and code supporting the results are archived on Figshare: https://dx.doi.org/10.6084/m9.figshare.12155562


