Abstract
Aims
The primary aim of the VIP‐HF study was to examine the incidence of sustained ventricular tachyarrhythmias (VTs) in heart failure (HF) with mid‐range (HFmrEF) or preserved ejection fraction (HFpEF). Secondary aims were to examine the incidence of non‐sustained VTs, bradyarrhythmias, HF hospitalizations and mortality.
Methods and results
This was an investigator‐initiated, prospective, multicentre, observational study of patients with HF and left ventricular ejection fraction (LVEF) >40%. Patients underwent extensive phenotyping, after which an implantable loop recorder was implanted. We enrolled 113 of the planned 250 patients [mean age 73 ± 8 years, 51% women, New York Heart Association class II/III 54%/46%, median N‐terminal pro B‐type natriuretic peptide 1367 (710–2452) pg/mL and mean LVEF 54 ± 6%; 75% had LVEF >50%]. Eighteen percent had non‐sustained VTs and 37% had atrial fibrillation on Holter monitoring. During a median follow‐up of 657 (219–748) days, the primary endpoint of sustained VT was observed in one patient. The incidence of the primary endpoint was 0.6 (95% confidence interval 0.2–3.5) per 100 person‐years. The incidence of the secondary endpoint of non‐sustained VT was 11.5 (7.1–18.7) per 100 person‐years. Five patients developed bradyarrhythmias [3.2 (1.4–7.5) per 100 person‐years], three were implanted with a pacemaker. In total, 23 patients (20%) were hospitalized for HF [16.3 (10.9–24.4) per 100 person‐years]. Fourteen patients (12%) died [8.7 (5.2–14.7) per 100 person‐years]; 10 due to cardiovascular causes, and four sudden deaths, one with implantable loop recorder‐confirmed bradyarrhythmias as terminal event, three others undetermined.
Conclusion
Despite the lower than expected number of included patients, the incidence of sustained VTs in HFmrEF/HFpEF was low. Clinically relevant bradyarrhythmias were more often observed than expected.
Keywords: Heart failure with preserved ejection fraction, Heart failure with mid‐range ejection fraction, Ventricular tachyarrhythmias, Sudden death, Bradyarrhythmias
Graphical abstract of the VIP‐HF study. HF, heart failure; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; VT, ventricular tachyarrhythmia. Adapted from Servier Medical Art licensed under CC BY 3.0.
Introduction
Heart failure (HF) with mid‐range (HFmrEF) or preserved ejection fraction (HFpEF) is an increasingly large medical and epidemiological problem. At present, no pharmacological or device treatment has convincingly been shown to improve outcome, and none of these interventions is recommended for this indication in the current European Society of Cardiology (ESC) HF guidelines. 1 , 2 Sudden death is a common cause of death in all patients with HF. 3
In the past, patients with HF with reduced ejection fraction (HFrEF) often underwent diagnostic testing to determine their arrhythmogenic risk profile and to examine the susceptibility to develop lethal ventricular tachyarrhythmias. This was mostly done by performing 24 h ambulatory Holter monitoring and some reports indicated that that the presence of ventricular tachyarrhythmias in HFrEF was associated with sudden death and all‐cause mortality, whereas others did not. 4 , 5 , 6 , 7 All studies showed that the severity of left ventricular systolic dysfunction was the dominant risk indicator for both sudden and all‐cause mortality in HFrEF. This has led to clear recommendations, based on left ventricular ejection fraction (LVEF), for implantable cardioverter‐defibrillator (ICD) therapy to prevent sudden death in HFrEF. 1 , 8 Detection of ventricular tachyarrhythmias is not part of the diagnostic work‐up of patients with HFrEF anymore. 1
In HFmrHF and HFpEF patients specifically, sudden death is one of the most prominent modes of death. 9 , 10 Studies have reported incidences between 20–30% of all deaths. 11 , 12 , 13 , 14 It is unknown, however, whether ventricular tachyarrhythmias are the cause of sudden death in patients with HFmrEF/HFpEF.
The primary objective of the Ventricular tachyarrhythmia detection by Implantable loop recording in Patients with Heart Failure and preserved ejection fraction (VIP‐HF) study was therefore to examine the incidence of sustained ventricular tachyarrhythmias by continuous rhythm recording using an implantable loop recorder (ILR) during 2 years of follow‐up in HFmrEF/HFpEF patients. Secondary objectives were to examine the incidence of non‐sustained ventricular tachyarrhythmias, bradyarrhythmias, HF hospitalizations and mortality.
Methods
Study design
The VIP‐HF study was an investigator‐initiated, prospective, multicentre, observational study conducted in The Netherlands (ClinicalTrials.gov NCT01989299). The patients were included between January 2015 and December 2019 in four centres in The Netherlands. All patients provided written informed consent, and the study was approved by the medical ethics committee in Groningen. In addition, the present study was in concordance with the principles outlined in the Declaration of Helsinki.
Study population
Patients were eligible for the VIP‐HF study if they were aged >18 years, had mild to moderate symptoms of HF [compatible with New York Heart Association (NYHA) functional class II–III], and a hospitalization or emergency room visit for HF, or symptom relief with diuretics within the previous 12 months. They could either be in sinus rhythm or in atrial fibrillation (AF) and had elevated levels of natriuretic peptides [i.e. an N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) >300 pg/mL if in sinus rhythm, or >900 pg/mL if in AF]. The LVEF was required to be >40% on echocardiography, and patients were required to have echocardiographic evidence of functional and/or structural alterations, including septal or posterior wall thickness ≥11 mm, and/or left ventricular diastolic dysfunction (mean septal and lateral e′ <9 cm/s, or E/e′ ≥13), and/or left atrial dilatation (left atrial volume index ≥34 mL/m2). 1 Key exclusion criteria included the presence of an ICD or pacemaker, or an indication for ICD therapy according to the ESC HF guidelines. 1 In addition, patients with a life expectancy of <1 year, as well as those with a myocardial infarction, percutaneous intervention or coronary artery bypass graft within the last 3 months were also excluded, as well as patients with complex congenital heart disease. All patients were enrolled in the outpatient clinic.
Study procedures
At baseline, a comprehensive medical history, including a family history of premature sudden death, coronary artery disease, and HF was obtained. In addition, patients underwent extensive phenotyping of HF, including echocardiography, 24 h ambulatory electrocardiographic (ECG) Holter recording, and blood sampling for the analysis of biomarkers, all using standard methods. Cardiac magnetic resonance imaging (MRI) was performed using a 1.5 Tesla scanner, and images were analysed off‐line using dedicated software, as recently described in detail. 15 In addition, late gadolinium enhancement (LGE) images were acquired 10 min after intravenous administration of 0.2 mmol/kg gadolinium‐based contrast agent (Dotarem, Gorinchem, The Netherlands, 0.2 mmol/kg; and Gadovist, Berlin, Germany, 0.2 mmol/kg). 16 , 17
After baseline diagnostics were obtained, all study participants received an Abbott® (Chicago, IL, USA) ILR, type Confirm® Model DM2102 or Confirm Rx® model DM3500. The algorithm used for rhythm monitoring was the same in ILR types, battery‐lifetime was minimal 2 years. The ILR was programmed with highest priority for tachyarrhythmias. The tachyarrhythmia cut‐off heart rate was the maximum heart rate on Holter monitoring plus 10 bpm, with a minimum of 150 bpm. All episodes lasting ≥10 beats were stored. AF episodes >30 s with heart rate below the tachyarrhythmia cut‐off, based on irregularity criteria, were stored separately as AF episodes. Bradyarrhythmias and AF were programmed with lower priority. The bradyarrhythmia cut‐off rate was 30 bpm or asystole >3 s.
Typically for ILRs is that when the maximum storage capacity of the ILR is reached, the oldest stored episode in its priority category is overwritten by the new episode. To minimize the risk of ILR noise capture, the sensitivity of the ILR was tested at every follow‐up visit using the R‐wave amplitude, and the ILR was adjusted accordingly.
After ILR implantation, all patients were seen for ILR device interrogation every 6 months, and yearly with the treating physician, with a maximum of 2 years (based on the ILR battery life). All potentially clinically relevant ILR events were communicated to the treating physician, and potential treatment adjustments were left to the discretion of the treating physician. Treatment of HF and associated comorbidities was recommended according to the ESC HF guidelines. 1
Endpoints
The primary endpoint was the incidence of sustained ventricular tachyarrhythmias in patients with HFmrEF/HFpEF. Sustained ventricular tachyarrhythmia was defined as lasting >30 s or with haemodynamic compromise. Secondary endpoints were the incidence of non‐sustained ventricular tachyarrhythmias, incidence of bradyarrhythmias, HF hospitalizations, all‐cause mortality and sudden death. An endpoint adjudication committee, consisting of two experienced electrophysiologists, assessed all ILR‐documented events, and classified tachyarrhythmias as ventricular tachyarrhythmias or AF. This committee was blinded to patient characteristics. If an ILR event was not unanimously adjudicated, a third independent reviewer was consulted.
Statistical analysis
The study was designed to assess the incidence of sustained ventricular tachyarrhythmias in patients with HFmrEF/HFpEF. A priori, we assumed a mortality rate of 15% per year based on I‐Preserve and CHARM, of which one third would be due to sudden death, rendering a sudden death rate of 5% per year. 11 , 12 The assumption that every episode of sustained ventricular tachyarrhythmias causes sudden death is not true. Previously, the DEFINITE study demonstrated that appropriate ICD shocks for sustained ventricular tachyarrhythmias occurred approximately twice as the number of sudden deaths in patients with HFrEF. 18 Therefore we assumed that the incidence of sustained ventricular tachyarrhythmias would be twice as high as the expected sudden death rate, i.e. 10% per year. During 2 years of follow‐up, the expected incidence rate of ventricular tachyarrhythmias would thus be 20% per 2 years. To estimate a frequency of 20% sustained ventricular tachyarrhythmias in a cohort of HFmrEF/HFpEF patients, with a confidence level of 95%, the calculated sample size was 246, and therefore the aim of the study was to include 250 patients. Due to the slower‐than‐expected enrolment, the independent data safety monitoring board advised to stop inclusion at 113 patients.
Data are presented as mean ± standard deviation if (visually inspected) distribution was normal, or median with interquartile ranges if the distribution of data was non‐normal. Categorical data are presented as number (%). The incidence rates of the primary endpoint of sustained ventricular tachyarrhythmias, and secondary endpoints of non‐sustained ventricular tachyarrhythmias, bradyarrhythmias, HF hospitalizations and all‐cause mortality with corresponding confidence intervals were calculated using the package epiR in R. Differences in continuous variables between groups were analysed using the independent samples t‐test or Wilcoxon rank test, depending on the distribution. Differences in categorical variables between groups were analysed using the Chi‐squared test or Fisher's exact test. The association between non‐sustained ventricular tachyarrhythmias on Holter recording and all‐cause mortality and the combined endpoint of all‐cause mortality and HF hospitalizations were assessed using a Cox proportional hazard regression model. A Kaplan–Meier plot with log‐rank test was used to display the relation between Holter‐detected non‐sustained ventricular tachyarrhythmias and all‐cause mortality, and the combined endpoint of all‐cause mortality and HF hospitalizations. Statistical analyses were performed using SPSS (version 23; Chicago, IL, USA) and R (version 3.3.3; Vienna, Austria). Statistical significance was considered achieved at a P‐value <0.05.
Results
Patient characteristics
We enrolled a total of 113 patients, of whom 28 (25%) had HFmrEF (LVEF 41–49%), and 85 patients had HFpEF (LVEF ≥50%). Mean age was 73 ± 8 years, 51% were women, and the patient characteristics are depicted in Table 1 . About half of the patients had mild HF (NYHA functional class II), and of the others had moderate HF (NYHA functional class III). Median NT‐proBNP was markedly elevated, and patients had a wide range of common comorbidities. On Holter recording, 20 of the 113 patients (18%) had non‐sustained ventricular tachyarrhythmia with a median duration of 3 (3–5) beats. Thirty‐eight percent of patients had AF at baseline. Of the 113 patients, 105 underwent a cardiac MRI. In eight patients MRI was not performed, mostly because of claustrophobia. Left ventricular systolic and diastolic volumes were significantly larger in patients with HFmrEF than in those with HFpEF (both P < 0.001) (Table 2 ). The characteristics of the patients with non‐sustained ventricular tachyarrhythmias on Holter recording and those without non‐sustained ventricular tachyarrhythmias are depicted in online supplementary Table SS1 . Patients with non‐sustained ventricular tachyarrhythmias were more often men as compared to those without non‐sustained ventricular tachyarrhythmias (75% vs. 43%, P = 0.009). In addition, the proportion of patients with non‐sustained ventricular tachyarrhythmias in NYHA functional class II was higher (75% vs. 50%, P = 0.04), had a lower LVEF (51% vs. 54%, P = 0.02), and more often had a high number of solitary premature ventricular complexes on the baseline Holter recording (45% vs. 20%, P = 0.02). On MRI, patients with non‐sustained ventricular tachyarrhythmias had larger left ventricular systolic and diastolic volumes than patients without non‐sustained ventricular tachyarrhythmias (P = 0.001 and P = 0.002, respectively).
Table 1.
Patient characteristics of the study population
| Total (n = 113) | HFpEF (n = 85, 75%) | HFmrEF (n = 28, 25%) | P‐value | |
|---|---|---|---|---|
| Age (years) | 72.6 ± 8.1 | 73.7 ± 8.0 | 69.2 ± 7.7 | 0.01 |
| Women | 58 (51%) | 50 (59%) | 8 (29%) | 0.005 |
| Body mass index (kg/m2) | 29.7 ± 5.7 | 30.3 ± 5.7 | 28.3 ± 5.5 | 0.12 |
| Comorbidities | ||||
| Hypertension | 89 (78%) | 70 (82%) | 19 (68%) | 0.10 |
| Previous myocardial infarction | 23 (20%) | 15 (18%) | 8 (29%) | |
| History of atrial fibrillation | 64 (57%) | 54 (64%) | 10 (36%) | 0.01 |
| Diabetes mellitus | 45 (40%) | 35 (41%) | 10 (36%) | 0.6 |
| Renal dysfunction a | 54 (48%) | 41 (48%) | 13 (46%) | 0.9 |
| COPD | 21 (19%) | 15 (18%) | 6 (21%) | 0.7 |
| NYHA HF class | 0.2 | |||
| II | 61 (54%) | 43 (51%) | 18 (64%) | |
| III | 52 (46%) | 42 (49%) | 10 (36%) | |
| Previous HF hospitalization | 48 (43%) | 35 (41%) | 13 (46%) | 0.6 |
| Baseline laboratory values | ||||
| NT‐proBNP (pg/mL) | 1367 (710–2452) | 1312 (687–2344) | 1611 (744–2843) | 0.9 |
| Current medication | ||||
| Beta‐blockers | 99 (88%) | 24 (86%) | 75 (88%) | 0.7 |
| ACEi/ARB | 72 (64%) | 52 (61%) | 20 (71%) | 0.3 |
| MRA | 42 (37%) | 30 (35%) | 12 (43%) | 0.5 |
| Diuretics | 102 (90%) | 76 (89%) | 26 (93%) | 0.6 |
| Holter data | ||||
| AF as basal rhythm | 42 (37%) | 35 (42%) | 7 (25%) | 0.12 |
| Mean heart rate (bpm) | 72 ± 13 | 73 ± 13 | 69 ± 12 | 0.3 |
| PVC ≥1000/24 h | 27 (24%) | 19 (23%) | 8 (29%) | 0.5 |
| Non‐sustained VT | 20 (18%) | 12 (15%) | 8 (29%) | 0.09 |
| Echocardiography | ||||
| LVEF (%) | 54 ± 6 | 56 ± 5 | 45 ± 2 | <0.001 |
| LA volume index (mL/m2) | 46 ± 16 | 46 ± 14 | 47 ± 21 | 0.8 |
| LV mass index (g/m2) | 102 ± 37 | 102 ± 39 | 103 ± 34 | 0.9 |
ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; PVC, premature ventricular complex; VT, ventricular tachyarrhythmia.
Defined as estimated glomerular filtration rate < 60 mL/min/1.73 m2.
Table 2.
Cardiac magnetic resonance imaging characteristics
| Total a (n = 105) | HFpEF (n = 77) | HFmrEF (n = 28) | P‐value | |
|---|---|---|---|---|
| Left ventricle | ||||
| End‐diastolic volume (mL/m2) | 89 ± 25 | 84 ± 22 | 104 ± 26 | <0.001 |
| End‐systolic volume (mL/m2) | 43 ± 17 | 39 ± 14 | 56 ± 19 | <0.001 |
| Ejection fraction (%) | 53 ± 8 | 54 ± 8 | 48 ± 9 | 0.001 |
| Mass (g/m2) | 57 ± 23 | 57 ± 23 | 59 ± 24 | 0.7 |
| Right ventricle | ||||
| End‐diastolic volume (mL/m2) | 81 ± 20 | 79 ± 19 | 87 ± 21 | 0.1 |
| End‐systolic volume (mL/m2) | 39 ± 15 | 38 ± 15 | 42 ± 15 | 0.3 |
| Ejection fraction (%) | 53 ± 10 | 52 ± 11 | 53 ± 9 | 0.9 |
| Ejection fraction <45% | 25 (24%) | 19 (25%) | 6 (21%) | 0.7 |
| Late gadolinium enhancement | (n = 91) | (n = 64) | (n = 27) | |
| Positive (%) | 30 (33%) | 19 (30%) | 11 (41%) | 0.3 |
| Ischaemic pattern (%) | 18 (20%) | 10 (16%) | 8 (30%) | 0.3 |
HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction.
Available in 105 of 113 patients.
Sustained ventricular tachyarrhythmias
During ILR monitoring for a median of 657 (219–748) days, one patient had a sustained ventricular tachyarrhythmia that deteriorated into ventricular fibrillation [incidence: 0.6 (95% confidence interval 0.2–3.5) per 100 person‐years] (Figure 1 ). This patient was a 55‐year‐old man with LVEF 50% and extensive coronary artery disease, for which he had undergone several interventions. He had no ventricular tachyarrhythmias on his Holter, but earlier on his ILR one relatively slow ventricular tachyarrhythmia of seven beats, 105 bpm was captured. After his cardiac arrest (after 1 year into the study), he received an ICD, and his ILR was removed.
Figure 1.
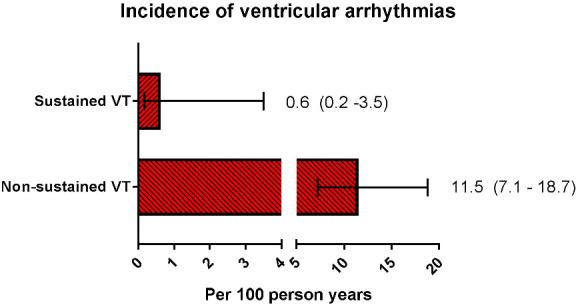
Bar graphs with 95% confidence intervals showing the incidence of sustained and non‐sustained ventricular tachyarrhythmias (VT).
Non‐sustained ventricular tachyarrhythmias
Non‐sustained ventricular tachyarrhythmias were observed on ILR in 16 patients with a median of 19 (14–24) beats. These non‐sustained ventricular tachyarrhythmias on ILR were observed after an average time of 7 months, two ventricular tachyarrhythmias were observed >1 year after implantation. The incidence of non‐sustained ventricular tachyarrhythmias was 11.5 (7.1–18.7) per 100 person‐years.
Bradyarrhythmias
Clinically relevant bradyarrhythmias were seen in five patients, of whom four had an indication for pacemaker therapy [3.2 (1.4–7.5) per 100 person‐years]. One patient developed dizziness, and had a third‐degree atrioventricular block, one patient had a near‐collapse and showed episodes of a slow ventricular escape rhythm (20 bpm), and another patient had a serious collapse with a sinus arrest of 21 s on ILR. All episodes were captured by the ILR.
Heart failure hospitalizations and all‐cause mortality
During the study, there were 23 HF hospitalizations [20%, 16.3 (10.9–24.4) per 100 person‐years]. There were 14 deaths [12%, incidence 8.7 (5.2–14.7) per 100 person‐years], of which 10 due to cardiovascular causes. Of the 10 cardiovascular deaths, four were sudden deaths and the other six were due to progressive HF. Of the four patients with sudden death, only two had non‐sustained ventricular tachyarrhythmias documented earlier on Holter/ILR. In one of these four patients with sudden death, bradyarrhythmias and asystole were seen in the terminal phase, but no sustained ventricular tachyarrhythmias. In the other three patients, the ILR was not analysable, and the terminal phase rhythm was unknown. There was no association between non‐sustained ventricular tachyarrhythmias on Holter recording and all‐cause mortality, or mortality and HF hospitalizations combined (Figure 2 ).
Figure 2.
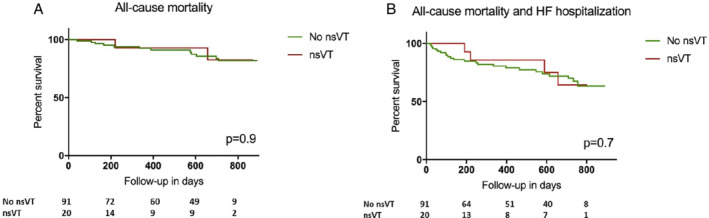
Time‐to‐first event analysis of non‐sustained ventricular tachycardia (nsVT) on Holter monitoring and all‐cause mortality (A), and composite of heart failure (HF) hospitalization and all‐cause mortality (B).
Implantable loop recorder‐related adverse events
During the study, there were four ILR‐related serious adverse events. Two patients (1.8%) had mild pocket infections, with only local inflammation and skin induration. In both patients, the ILR was explanted and both patients were discharged the same day. In one other patient, the ILR was removed at the request of the patient because of irritation at the site after an elective coronary artery bypass graft, with no signs of infection. In another patient the ILR pocket was expanded, also with no signs of infection. Four more patients had their ILR removed, three because of ICD or cardiac resynchronization therapy with defibrillator implantation, and one because of new breast cancer, most of them at patient's request.
Discussion
First, the VIP‐HF study had a lower than expected number of patients, due to slow inclusion rates. Despite this, the main finding of this study was the low incidence of sustained ventricular tachyarrhythmias in patients with HFpEF/HFmrEF, 10 times lower than anticipated. Second, clinically relevant bradyarrhythmias were more often observed than expected. Lastly, the incidence of HF hospitalizations and all‐cause mortality was high, but no association was seen with non‐sustained ventricular tachyarrhythmia on Holter monitoring.
Although it is increasingly recognized that non‐cardiovascular death is more common in patients with HFmrEF and HFpEF than in HF patients with reduced LVEF, sudden death is still one of the most common causes of death in this population. 10 , 11 , 19 It is unclear how many of these patients die from an arrhythmia, and if so, whether these are tachyarrhythmias or bradyarrhythmias. No ILR or Holter data are available to date. 19 To our knowledge, the VIP‐HF study is the first prospective study in HFmrEF/HFpEF patients attempting to determine the incidence of ventricular tachyarrhythmias and the rhythm directly before sudden death. In the VIP‐HF study, sustained ventricular tachyarrhythmias and sudden death were uncommon. Prediction of increased risk of sudden death in patients with HFmrEF/HFpEF has shown to be difficult if not impossible. 13 , 19 , 20 Attempts to create risk prediction models in patients with LVEF >40% found age, male sex, history of myocardial infarction, diabetes, presence of left bundle branch block and NT‐proBNP as risk indicators of sudden death. 21 , 22 In addition, earlier studies suggest that the mortality risk may be greater in ischaemic causes of HF, compared to HF due to non‐ischaemic causes. 3 The majority of patients in VIP‐HF did not have a previous myocardial infarction, which may have led to a lower sudden death rate.
In a recent paper focussing on renal patients, it was shown that in contrast to traditional thinking, sudden death is often not caused by ventricular tachyarrhythmias, but rather by bradyarrhythmias followed by asystole. 23 Interestingly, in VIP‐HF, we also observed a higher incidence of bradyarrhythmias than sustained ventricular tachyarrhythmias. In VIP‐HF, five patients had significant bradyarrhythmia and/or asystole on ILR, of whom four had an indication for pacemaker therapy, thereby potentially making it an important clinical issue. One study examined the value of ILR in patients with reduced LVEF and found clinical significant bradyarrhythmias in a substantial proportion of patients. 24 To our knowledge, no data on the incidence of bradyarrhythmias in HFpEF are currently available.
Use of an ILR in the present study led to an increased number of patients in whom non‐sustained ventricular tachyarrhythmias was detected (from 18% on baseline 24 h Holter monitoring to 27%). No association between non‐sustained ventricular tachyarrhythmias on Holter monitoring and HF hospitalization or mortality was observed. In a recent retrospective study of 85 HFpEF patients with a pacemaker, 45% of patients had episodes of non‐sustained ventricular tachyarrhythmias during 4 years of follow‐up. 25 In this study also no association was found between non‐sustained ventricular tachyarrhythmias and mortality. HF patients with a pacemaker are not comparable to those without a pacemaker since their prognosis is more impaired. 26
Study limitations
There are several limitations to this study. First, the number of patients in the present study was limited, and smaller than originally planned, and conclusions should be cautiously interpreted. Second, some inherent limitations come with ILR recording. The battery life of ILR is limited, with a maximum of 2‐year reliable monitoring. In case ILR memory is full, ILRs start overwriting already stored episodes. Establishing the cause of death with an ILR may therefore be difficult, because the recording of the causative episode may be replaced post‐death with newer recordings of asystole or noise. Absence of intracardiac leads, making distinction between low‐amplitude ventricular fibrillation and asystole difficult, increases also the risk for noise capture.
Conclusions
Despite the lower than expected number of included patients, the incidence of sustained ventricular tachyarrhythmias in HFmrEF/HFpEF was low. Clinically relevant bradyarrhythmias were more often observed than expected.
Funding
The VIP‐HF study (ClinicalTrials.gov identifier NCT01989299) was financially supported by an unrestricted grant from Abbott‐Netherlands to the University Medical Centre Groningen. Abbott‐Netherlands was neither involved in the conduction of the study, nor in the writing of the manuscript.
Conflict of interest: none declared.
Supporting information
Table S1. Characteristics of patients with and without non‐sustained ventricular tachyarrhythmia on Holter monitoring.
VIP‐HF principal investigator was D.J. van Veldhuisen (chair); and co‐chairs were M. Rienstra and I.C. van Gelder. Patients were enrolled at the University Medical Centre Groningen (n = 90), The Maastricht University Medical Centre (n = 18), the Erasmus Medical Centre Rotterdam (n = 4), and the Medical Spectre Twente Enschede (n = 1). Members of the Data Safety and Monitoring Board were G.C.M. Linssen (cardiologist, Almelo), P.J.A.M. Brouwers (Neurologist, Enschede), and H. ten Cate (Internist, Maastricht). The Endpoint Committee consisted of R.G. Tieleman (Electrophysiologist, Groningen) and A.H. Maass (Electrophysiologist, Groningen).
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Lam CS, Voors AA, de Boer RA, Solomon SD, van Veldhuisen DJ. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur Heart J 2018;39:2780–2792. [DOI] [PubMed] [Google Scholar]
- 3. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH; Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 4. Gradman A, Deedwania P, Cody R, Massie B, Packer M, Pitt B, Goldstein S. Predictors of total mortality and sudden death in mild to moderate heart failure. Captopril‐Digoxin Study Group. J Am Coll Cardiol 1989;14:564–572. [DOI] [PubMed] [Google Scholar]
- 5. Szabo BM, van Veldhuisen DJ, Crijns HJ, Wiesfeld AC, Hillege HL, Lie KI. Value of ambulatory electrocardiographic monitoring to identify increased risk of sudden death in patients with left ventricular dysfunction and heart failure. Eur Heart J 1994;15:928–933. [DOI] [PubMed] [Google Scholar]
- 6. Doval HC, Nul DR, Grancelli HO, Varini SD, Soifer S, Corrado G, Dubner S, Scapin O, Perrone SV. Nonsustained ventricular tachycardia in severe heart failure. Independent marker of increased mortality due to sudden death. GESICA‐GEMA Investigators. Circulation 1996;94:3198–3203. [DOI] [PubMed] [Google Scholar]
- 7. Teerlink JR, Jalaluddin M, Anderson S, Kukin ML, Eichhorn EJ, Francis G, Packer M, Massie BM. Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. PROMISE (Prospective Randomized Milrinone Survival Evaluation) Investigators. Circulation 2000;101:40–46. [DOI] [PubMed] [Google Scholar]
- 8. Priori SG, Blomstrom‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 9. Wellens HJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, Huikuri HV, Kaab S, La Rovere MT, Malik M, Myerburg RJ, Simoons ML, Swedberg K, Tijssen J, Voors AA, Wilde AA. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 2014;35:1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail 2013;15:604–613. [DOI] [PubMed] [Google Scholar]
- 11. Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJ, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pocock S, Pfeffer MA. Effect of candesartan on cause‐specific mortality in heart failure patients: the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation 2004;110:2180–2183. [DOI] [PubMed] [Google Scholar]
- 12. Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez‐Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE; I‐Preserve Investigators . Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I‐Preserve) trial. Circulation 2010;121:1393–1405. [DOI] [PubMed] [Google Scholar]
- 13. Vaduganathan M, Claggett BL, Chatterjee NA, Anand IS, Sweitzer NK, Fang JC, O'Meara E, Shah SJ, Hegde SM, Desai AS, Lewis EF, Rouleau J, Pitt B, Pfeffer MA, Solomon SD. Sudden death in heart failure with preserved ejection fraction: a competing risks analysis from the TOPCAT trial. JACC Heart Fail 2018;6:653–661. [DOI] [PubMed] [Google Scholar]
- 14. Desai AS, Vaduganathan M, Cleland JG, Claggett BL, Barkoudah E, Finn P, McCausland F, Yilmaz MB, Lefkowitz M, Shi V, Pfeffer MA, McMurray JJ, Solomon SD. Mode of death in patients with heart failure and preserved ejection fraction ≥45%: insights from PARAGON‐HF. J Am Coll Cardiol 2020;75:1415 (abstr). [DOI] [PubMed] [Google Scholar]
- 15. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail 2018;20:1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols . Standardized cardiovascular magnetic resonance imaging (CMR) protocols, Society for Cardiovascular Magnetic Resonance: Board of Trustees Task Force on Standardized Protocols. J Cardiovasc Magn Reson 2008;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Te Rijdt WP, Ten Sande JN, Gorter TM, van der Zwaag PA, van Rijsingen IA, Boekholdt SM, van Tintelen JP, van Haelst PL, Planken RN, de Boer RA, Suurmeijer AJ, van Veldhuisen DJ, Wilde AA, Willems TP, van Dessel PF, van den Berg MP. Myocardial fibrosis as an early feature in phospholamban p.Arg14del mutation carriers: phenotypic insights from cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2019;20:92–100. [DOI] [PubMed] [Google Scholar]
- 18. Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A; Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators . Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation 2006;113:776–782. [DOI] [PubMed] [Google Scholar]
- 19. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol 2017;69:556–569. [DOI] [PubMed] [Google Scholar]
- 20. Manolis AS, Manolis AA, Manolis TA, Melita H. Sudden death in heart failure with preserved ejection fraction and beyond: an elusive target. Heart Fail Rev 2019;24:847–866. [DOI] [PubMed] [Google Scholar]
- 21. Adabag S, Rector TS, Anand IS, McMurray JJ, Zile M, Komajda M, McKelvie RS, Massie B, Carson PE. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2014;16:1175–1182. [DOI] [PubMed] [Google Scholar]
- 22. Levine YC, Rosenberg MA, Mittleman M, Samuel M, Methachittiphan N, Link M, Josephson ME, Buxton AE. B‐type natriuretic peptide is a major predictor of ventricular tachyarrhythmias. Heart Rhythm 2014;11:1109–1116. [DOI] [PubMed] [Google Scholar]
- 23. Samanta R, Chan C, Chauhan VS. Arrhythmias and sudden cardiac death in end stage renal disease: epidemiology, risk factors, and management. Can J Cardiol 2019;35:1228–1240. [DOI] [PubMed] [Google Scholar]
- 24. Bloch Thomsen PE, Jons C, Raatikainen MJ, Moerch Joergensen R, Hartikainen J, Virtanen V, Boland J, Anttonen O, Gang UJ, Hoest N, Boersma LV, Platou ES, Becker D, Messier MD, Huikuri HV; Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) Study Group . Long‐term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: the Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. Circulation 2010;122:1258–1264. [DOI] [PubMed] [Google Scholar]
- 25. Ash J, Akdemir B, Gutierrez A, Chen J, Adabag S. Ventricular tachycardia is a common arrhythmia among patients with heart failure with preserved ejection fraction. Circulation 2019;140:A11658 (abstr). [Google Scholar]
- 26. Shen L, Jhund PS, Docherty KF, Petrie MC, Anand IS, Carson PE, Desai AS, Granger CB, Komajda M, McKelvie RS, Pfeffer MA, Solomon SD, Swedberg K, Zile MR, McMurray JJ. Prior pacemaker implantation and clinical outcomes in patients with heart failure and preserved ejection fraction. JACC Heart Fail 2019;7:418–427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of patients with and without non‐sustained ventricular tachyarrhythmia on Holter monitoring.


