Summary
Morphological features of eosinophils in patients with reactive eosinophilia (28 patients) and clonal eosinophilia (26 patients) have been compared with each other and with the eosinophil characteristics of healthy volunteers (three subjects) and of patients with the idiopathic hypereosinophilic syndrome (three patients). Morphological features, assessed in isolation from other haematological abnormalities, were found to have poor specificity for a myeloid neoplasm. The most useful feature was the presence of basophilic granules in mature eosinophils, which was associated particularly with acute myeloid leukaemia with inv(16). Marked reduction in granules occurred more often in some subsets of the myeloid neoplasm group but nevertheless was lacking in specificity since it was not infrequently seen in reactive eosinophilia. Although experienced morphologists more often considered that a myeloid neoplasm was likely in patients in whom this was the diagnosis (69%), myeloid neoplasia was also considered likely in a considerable proportion (39%) of patients with reactive eosinophilia. Morphological abnormalities of eosinophils therefore cannot be assessed in isolation in seeking to make a diagnosis of a myeloid neoplasm. Morphology is, however, needed and should be integrated with the results of other investigations.
Keywords: eosinophil, eosinophilia, eosinophil dysplasia, eosinophilic leukaemia, myeloid neoplasia, reactive eosinophilia, morphology
The detection of neutrophil and megakaryocyte dysplasia 1 has an important role in the diagnosis and classification of haematological neoplasms, particularly in the diagnosis of myelodysplastic syndromes and in the recognition of acute myeloid leukaemia (AML) with myelodysplasia‐related changes. The detection of dyserythropoiesis can also be important as long as cases with erythroid dysplasia that is not the result of a haematological neoplasm are recognised. 2 The significance of morphological abnormalities of eosinophils is much less understood. This study was undertaken to identify the morphological abnormalities that occur in eosinophils in myeloid neoplasms and to determine whether they permit conditions with clonal eosinophilia to be distinguished from reactive eosinophilia and the idiopathic hypereosinophilic syndrome (iHES). For convenience we have referred to morphological abnormalities of eosinophils as ‘eosinophil dysplasia’ (DysEo), but recognising that such changes may occur in neoplastic and reactive conditions.
Materials and methods
A planning group of the The International Working Group on Morphology of MDS (IWGM‐MDS) (JG, JB and BB) met in London in August 2018 to examine blood films from healthy controls and from patients with eosinophilia or an eosinophil‐related disorder using a multiheaded microscope. Electronic images were also examined. Cases were selected on the basis of appropriate investigations to establish a diagnosis having been carried out and on the availability of a well‐stained peripheral blood film (May‒Grünwald‒Giemsa or Wright‒Giemsa). Selection was not based on whether or not DysEo was present. All cases had an eosinophil count of at least 1·5 × 109/l, with the exception of three healthy control subjects, five cases of AML with inv(16) and one case of mild reactive eosinophilia in acute lymphoblastic leukaemia. Brief descriptions of normal and abnormal eosinophils were agreed; these served, together with appropriate illustrative images, which were circulated electronically, as a consensus guideline to aid in the subsequent evaluation of blood films. Diagnosis and classification of haematological neoplasms was according to the 2016 World Health Organization classification. 3
All films were examined and photographed in one of two centres under oil immersion with a ×63 or ×100 objective and images of 100 eosinophils were digitised, for all except the three controls with lower counts; in order to digitise sufficient eosinophils from the normal controls, four blood films on each of these subjects were evaluated. In order to assess DysEo without bias, care was taken to exclude from the images provided any cells of other lineages that might inadvertently have provided a clue to a diagnosis of a myeloid neoplasm or to an alternative diagnosis (e.g. blast cells, dysplastic neutrophils or lymphoma cells). The digitised images were sent to all seven haematology investigators by internet and were evaluated for each of the dysplastic features shown in Table I and illustrated in a tutorial circulated to investigators (see Supplementary Material), without knowledge of the original diagnosis. At the end of the morphological evaluation of each case, investigators were asked to grade the DysEo as absent, mild, moderate or severe (according to their own experience) and to answer the question: ‘Do you think this patient is likely to have a myeloid neoplasm?’ A total of 5594 eosinophils were evaluated, using the codification that is explained in Table I. Each cell could be assigned more than one code when necessary. All cells were then classified according to the majority (≥4/7) of evaluations.
Table I.
Consensus guideline for evaluation of eosinophil morphology.
| Code | Explanation |
|---|---|
| Code 0 | Normal: nucleus has two or three lobes, the cytoplasm is not degranulated and there are few if any vacuoles; any vacuoles are generally small |
| Code 1a | Nonlobated nucleus: mature eosinophil with no lobation, nucleus in the form of a band |
| Code 1b | Nonlobated nucleus: mature eosinophil with no lobation, nucleus round or oval |
| Code 2 | Hyperlobated nucleus: four or more nuclear lobes |
| Code 3 | Ring nucleus |
| Code 4 | Binuclearity: cell clearly has two nuclei; cells with two round nuclear lobes close together with joining filament not clearly visualised are not included |
| Code 5 | Moderately hypogranular but agranular cytoplasm is less than 25% of cytoplasmic area |
| Code 6 | Markedly hypogranular: agranular cytoplasm is 25% or more of cytoplasmic area |
| Code 7 | Moderately vacuolated but vacuoles occupy less than 25% of cytoplasm |
| Code 8 | Markedly vacuolated: vacuoles occupy 25% or more of cytoplasm |
| Code 9 | Some granules with basophilic staining characteristics |
Statistical methods
All data were centralised and statistical analysis was performed using the open software R (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). A descriptive analysis was performed: it consists in point estimates (absolute numbers and percentages for qualitative variables and means and standard deviations for quantitative variables).
Results
A total of 60 cases were selected of which three were healthy control subjects with no history of allergy, 26 had a myeloid neoplasm, 28 had reactive eosinophilia and three had a diagnosis of iHES made after detailed investigations to exclude known causes of eosinophilia (Table II).
Table II.
Summary of cases selected.
| Category | Diagnosis | Number of cases |
|---|---|---|
| Myeloid neoplasm (n = 26) | Myeloid neoplasm with PDGFRA rearrangement | 5 |
| Myeloid neoplasm with PCM1‐JAK2 | 1 | |
| Myeloproliferative neoplasm | ||
| Polycythaemia vera | 1 | |
| Essential thrombocythaemia | 1 | |
| Chronic myeloid leukaemia, BCR‐ABL1‐positive | 1 | |
| Primary myelofibrosis | 2 | |
| Chronic eosinophilic leukaemia, NOS | 5 | |
| Myelodysplastic syndrome | 1 | |
| Systemic mastocytosis | 1 | |
| Atypical chronic myeloid leukaemia | 1 | |
| Acute myeloid leukaemia | ||
| With inv(16)(p13.1q22) | 5 | |
| Therapy‐related | 1 | |
| NOS | 1 | |
| Reactive eosinophilia (n = 28) | Parasitic or fungal infection (loiasis, strongyloidiasis, histoplasmosis) | 3 |
| Lymphoma (Sézary syndrome, angioimmunoblastic T‐cell lymphoma, Hodgkin lymphoma) | 4 | |
| Acute lymphoblastic leukaemia | 2 | |
| Other neoplasm (carcinoma, mesenchymal tumour, neuroblastoma) | 3 | |
| Connective tissue disorder (eosinophilic granuloma with polyangiitis, Crohn's disease, ulcerative colitis, allergic rhinitis and oesophagitis) | 8 | |
| Interleukin 2 therapy | 2 | |
| Allergic reaction to drug | 2 | |
| Post‐transplant | 2 | |
| Necrotising enteritis in premature neonate | 2 | |
| iHES | 3 | |
iHES, idiopathic hypereosinophilic syndrome; NOS, not otherwise specified.
The frequency with which codes were assigned in the four groups is shown in Table III and Fig 1 (a cell can be assigned more than one code). Among the 5594 eosinophils evaluated, 459 cells did not meet a consensus (≥4/7) between the seven evaluators. The global consensus was 91·8%.
Table III.
For each of the four groups, mean percentage and standard deviation of cells for which agreement between haematology investigators was ≥4/7.
| Group number of cases (cells evaluated) | Control, n = 3 (220 cells) | iHES, n = 3 (300 cells) | Neoplastic, n = 26 (2274 cells) | Reactive, n = 28 (2800 cells) | Description | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Code 0 | 73·2 | 7·2 | 34·3 | 6·5 | 17·2 | 15·9 | 25·6 | 23·1 | Normal |
| Code 1a | 0·4 | 0·7 | 5·0 | 3·5 | 11·3 | 11·0 | 10·3 | 9·5 | Nonlobated, band form nucleus |
| Code 1b | 0·3 | 0·6 | 6·6 | 8·8 | 2·6 | 7·8 | Nonlobated, round or oval nucleus | ||
| Code 2 | 3·8 | 3·3 | 1·3 | 0·6 | 5·5 | 10·4 | 3·9 | 4·8 | Hyperlobated |
| Code 3 | 0·7 | 1·2 | 0·6 | 1·0 | 1·0 | 1·9 | Ring nucleus | ||
| Code 4 | 0·5 | 2·0 | 0·2 | 0·5 | Binucleated | ||||
| Code 5 | 13·1 | 3·2 | 40·0 | 15·5 | 26·2 | 13·3 | 31·0 | 12·5 | Moderately hypogranular |
| Code 6 | 1·0 | 1·0 | 19·9 | 24·4 | 17·1 | 26·7 | Markedly hypogranular | ||
| Code 7 | 8·6 | 5·9 | 20·3 | 4·7 | 30·2 | 21·0 | 21·3 | 21·4 | Moderately vacuolated |
| Code 8 | 8·3 | 14·8 | 1·1 | 4·0 | Markedly vacuolated | ||||
| Code 9 | 2·3 | 3·0 | 6·0 | 8·7 | 7·6 | 13·7 | 4·6 | 6·2 | Basophilic granules |
Bold indicates findings of particular note.
iHES, idiopathic hyper‐eosinophilic syndrome; SD, standard deviation.
Fig 1.
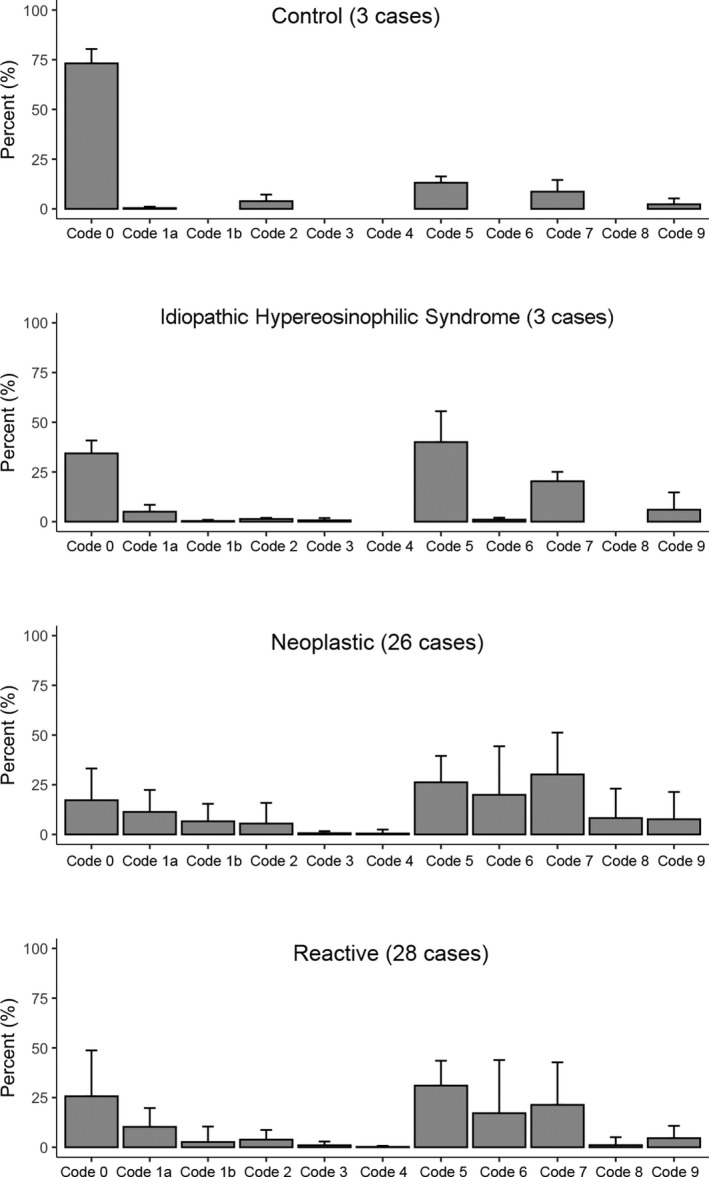
Histogram showing the frequency with which codes were assigned in the four groups: control, idiopathic hypereosinophilic syndrome, neoplastic and reactive; mean and standard deviation.
The following four groups have some notable characteristics (bold):
The control group was characterised by a very high percentage of normal eosinophils (code 0) but with some cells (13%) showing a reduction of eosinophil granules (but always <25% hypogranular cytoplasm) and some (8·6%) showing vacuolation (but always <25% of cytoplasm vacuolated). A few cells (2·3%) showed some basophilic granules. These findings may be considered to demonstrate the profile for normal eosinophils.
The most important characteristic of the iHES group was some reduction of granules and some vacuolation; 40% of cells showed moderate hypogranularity of the cytoplasm (<25% hypogranular) and 20·3% of cells showed moderate vacuolation (<25% of cytoplasm vacuolated). It may be noted that 5% of the cells had a band nucleus (code 1a).
Neoplastic cases had the lowest rate of normal eosinophils (17·2%) and the highest percentage of nonlobated nuclei — band form, codes 1a (11·3%) and round or oval nucleus, code 1b (6·6%). They also had 46·1% of cells showing moderate or marked reduction of granules (26·2% + 19·9%) and 38·5% showing moderate or marked vacuolation (30·2% + 8·3%). This group had the highest rate (7·6%) of cells with basophilic granules, linked to the cases with inv(16), as is demonstrated below.
Cases from the reactive group had some similarities with the neoplastic group (48·1% of cells with reduction of granules). The main difference was that code 8 (≥25% vacuolation) was assigned to 8·3% of cells in the neoplastic group and only 1·1% in the reactive group. In addition, code 1b (nonlobated, round or oval nucleus) was assigned to 6·6% of cells in the neoplastic group and only 2·6% in the reactive.
Figures 2 and 3, prepared after the diagnoses were revealed, show examples of the morphological features observed in the neoplastic and reactive groups respectively, and illustrate the similarity of the features in the two groups.
Fig 2.
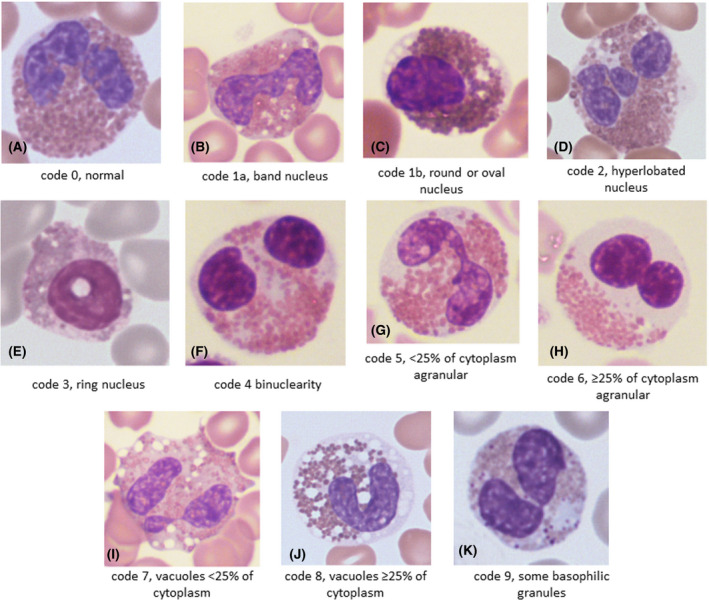
Representative examples of morphological features in patients with a myeloid neoplasm: (A) a normal eosinophil (code 0) from a patient with atypical chronic myeloid leukaemia evolving into acute myeloid leukaemia (AML); (B) a band eosinophil (code 1a) from a patient with FIP1L1‐PDGFRA; (C) an eosinophil with an oval nucleus (code 1b) from a patient with AML and inv(16); (D) an eosinophil with a hyperlobated nucleus (code 2) from a patient with FIP1L1‐PDGFRA; (E) an eosinophil with a ring nucleus (code 3) from a patient with AML and inv(16); (F) a binucleated eosinophil (code 4) from a patient with primary myelofibrosis, postsplenectomy; (G) a moderately hypogranular eosinophil (code 5) from a patient with polycythaemia vera; (H) a markedly hypogranular eosinophil (code 6) from a patient with primary myelofibrosis; (I) a moderately vacuolated eosinophil (code 7) from a patient with FIP1L1‐PDGFRA; (J) a markedly vacuolated eosinophil (code 8) from a patient with primary myelofibrosis; (K) an eosinophil with basophilic granules (code 9) from a patient with AML and inv(16). [Colour figure can be viewed at wileyonlinelibrary.com]
Fig 3.
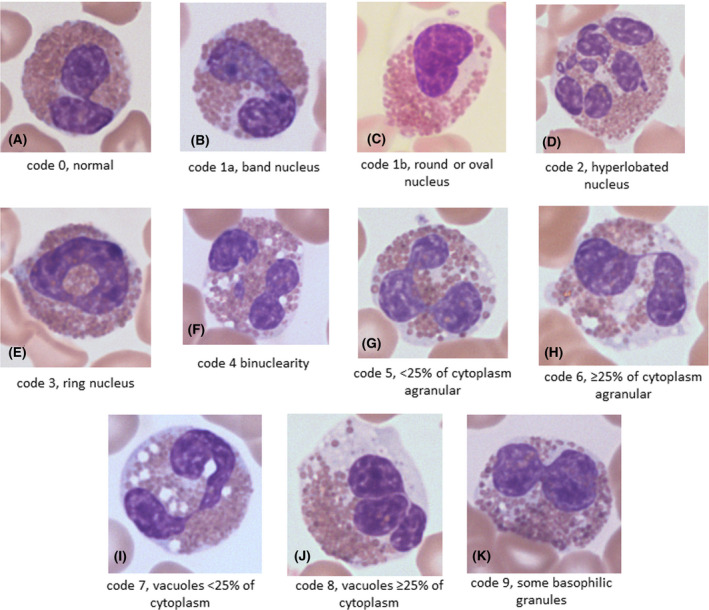
Representative examples of morphological features in cases of reactive eosinophilia: (A) a normal eosinophil (code 0) from a patient with allergic rhinitis; (B) a band eosinophil (code 1a) from a patient with necrotising enteritis; (C) an eosinophil with an oval nucleus (code 1b) from a patient with Sézary syndrome; (D) an eosinophil with a hyperlobated nucleus (code 2) from a patient with histoplasmosis; (E) an eosinophil with a ring nucleus (code 3) from a patient with histoplasmosis; (F) a binucleated eosinophil (code 4) from a patient with a neuroblastoma; (G) a moderately hypogranular eosinophil (code 5) from a patient with a leukaemoid reaction to carcinoma; (H) a markedly hypogranular eosinophil (code 6) from a patient with a leukaemoid reaction to carcinoma; (I) a moderately vacuolated eosinophil (code 7) from a patient with a drug reaction; (J) a markedly vacuolated eosinophil (code 8) from a patient with histoplasmosis; (K) an eosinophil with basophilic granules (code 9) from a patient with histoplasmosis. [Colour figure can be viewed at wileyonlinelibrary.com]
Comparing the frequency of morphological features in the different neoplastic subgroups (Table IV, highlighted bold) shows:
Code 1a (band form nucleus) is observed particularly in the ‘other leukaemia’ and miscellaneous subgroups.
Code 1b (round or oval nucleus) is a prominent characteristic of the cases associated with inv(16).
Code 2 (hyperlobated) was observed particularly in the ‘other leukaemia’ and chronic eosinophilic leukaemia, not otherwise specified (CEL, NOS) subgroups.
The lowest frequency of codes 5 and 6 (<25% and ≥25% reduction of granules respectively) was in the inv(16) group.
Code 6 (markedly hypogranular) was much less frequent in the miscellaneous group [myeloproliferative neoplasm (MPN)/mastocytosis cases] than in the FIPL1‐PDGFRA or CEL, NOS groups.
Code 9 (basophilic granules) was strongly associated with the inv(16) subgroup, being seen in almost a quarter of cells.
The FIP1L1‐PDGFRA group did not have any distinctive morphological features.
Table IV.
For each subgroup of the neoplastic group, mean percentage and standard deviation of cells for which agreement between haematology investigators was ≥4/7 (26 cases, 2274 cells).
| Inv(16), n = 5 | FIP1L1‐PDGRFA, n = 5 | CEL, NOS,* n = 3 | Other leukaemia,† n = 5 | Miscellaneous,‡ n = 8 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Code 0 | 17·9 | 22·6 | 14·8 | 14·5 | 7·0 | 8·9 | 19·4 | 18·5 | 20·8 | 14·5 |
| Code 1a | 6·7 | 6·5 | 7·6 | 9·9 | 7·0 | 4·4 | 16·2 | 19·8 | 15·2 | 7·7 |
| Code 1b | 18·5 | 12·1 | 2·2 | 2·5 | 8·3 | 9·3 | 3·0 | 2·8 | 3·5 | 4·9 |
| Code 2 | 1·3 | 1·7 | 4·4 | 2·5 | 17·0 | 19·3 | 11·2 | 16·4 | 0·9 | 1·2 |
| Code 3 | 0·7 | 1·5 | 0·8 | 1·1 | 1·3 | 1·5 | 0·4 | 0·5 | 0·4 | 0·7 |
| Code 4 | 0·3 | 0·6 | 0·4 | 0·5 | 1·3 | 3·5 | ||||
| Code 5 | 11·3 | 7·9 | 28·4 | 5·1 | 38·7 | 13·6 | 20·8 | 13·9 | 33·0 | 10·6 |
| Code 6 | 6·2 | 6·2 | 30·2 | 20·6 | 30·3 | 31·6 | 28·0 | 39·9 | 13·1 | 17·8 |
| Code 7 | 43·4 | 24·1 | 43·8 | 15·0 | 27·3 | 17·8 | 21·4 | 24·0 | 20·0 | 16·3 |
| Code 8 | 11·3 | 7·9 | 7·2 | 6·9 | 9·3 | 8·6 | 16·0 | 31·9 | 1·8 | 3·8 |
| Code 9 | 23·4 | 25·5 | 2·4 | 4·8 | 7·7 | 10·7 | 3·8 | 5·0 | 3·5 | 3·6 |
Chronic eosinophilic leukaemia, not otherwise specified.
Acute myeloid leukaemia without inv(16), atypical chronic myeloid leukaemia, myeloid neoplasm with PCM1‐JAK2.
Polycythaemia vera, essential thrombocythaemia, primary myelofibrosis, mastocytosis.
Comparing the frequency of morphological features in the different reactive subgroups (Table V) shows:
Code 0 (normal eosinophils) has the lowest percentage in the RL subgroup (two patients receiving interleukin 2 treatment) and in the RN subgroup (reactive to neoplasia).
Code 1b (nonlobated, round or oval nucleus) was most frequent (7·6%) in the RN subgroup.
Codes 2, 3 and 4 showed only minor variation between subgroups.
Code 5 showed the highest percentage of cells with a reduction (<25%) of granules in the 2 patients in the RL subgroup.
Code 6 (reduction of granules ≥25%) was most frequent in the RL and RN subgroups. If Codes 5 and 6 are pooled, the total percentage of cells with reduced granules is 76·5% in RL and 69·3% in RN, a notable difference from other subgroups.
The frequency of code 7 (presence of vacuolation <25%) is generally low, with the exception of the RN subgroup (35·7%).
Code 9 (basophilic granules) was relatively infrequent (5·7%), being comparable to other groups with the exception of cases with inv(16) (23·4%, see Table IV).
Table V.
For each subgroup of the reactive group, mean percentage and standard deviation of cells for which agreement between haematology investigators was ≥4/7 (28 cases, 2800 cells).
| Item | RI (n = 3) | RL (n = 2) | RN (n = 9) | RP (n = 3) | RR (n = 6) | Miscellaneous (n = 5) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Code 0 | 48·3 | 33·0 | 3·5 | 2·1 | 8·0 | 15·0 | 33·0 | 26·9 | 31·0 | 17·7 | 41·8 | 10·0 |
| Code 1a | 13·3 | 9·0 | 9·0 | 2·8 | 15·1 | 12·6 | 8·3 | 8·5 | 6·3 | 7·7 | 6·0 | 5·1 |
| Code 1b | 0·7 | 1·2 | 0·5 | 0·7 | 7·6 | 12·7 | 0·3 | 0·6 | 0·3 | 0·5 | ||
| Code 2 | 0·3 | 0·6 | 3·5 | 2·1 | 3·4 | 4·6 | 6·0 | 7·2 | 5·5 | 6·9 | 3·6 | 3·1 |
| Code 3 | 1·3 | 2·3 | 1·5 | 0·7 | 1·2 | 2·2 | 2·7 | 3·8 | 0·4 | 0·9 | ||
| Code 4 | 0·7 | 0·7 | ||||||||||
| Code 5 | 28·3 | 17·6 | 49·5 | 2·1 | 28·3 | 13·0 | 37·7 | 13·8 | 32·7 | 6·9 | 24·0 | 11·0 |
| Code 6 | 0·3 | 0·6 | 27·0 | 7·1 | 41·0 | 35·9 | 6·7 | 9·9 | 4·8 | 4·1 | 1·4 | 1·1 |
| Code 7 | 5·7 | 4·7 | 13·5 | 13·4 | 35·7 | 29·1 | 5·7 | 5·1 | 18·2 | 14·4 | 21·2 | 13·7 |
| Code 8 | 3·0 | 6·9 | 0·7 | 1·2 | 0·2 | 0·4 | ||||||
| Code 9 | 3·7 | 4·7 | 6·5 | 9·2 | 3·4 | 3·1 | 6·0 | 7·9 | 5·5 | 9·8 | 4·4 | 7·0 |
Bold indicates findings of particular note.
RI, reactive to infections or allergy (3 cases); RL, reactive to interleukin 2 (2 cases); RN, reactive to neoplasia (9 cases); RP, reactive to parasites or fungi (3 cases); RR, reactive to rectocolitis or Churg–Straus syndrome (6 cases).
In conclusion, eosinophilia related to neoplasia (nine cases) and to interleukin 2 treatment appeared to have somewhat different characteristics, particularly in relation to the higher frequency of hypogranularity in both and of vacuolation in the neoplastic group.
Based only on the morphology, the global opinion of haematology investigators was that control and iHES generally had mild (or absent) morphological abnormalities, whereas more notable abnormalities were generally observed in neoplastic and reactive groups (Table VI). Pooling ‘absent’ with ‘mild’, and ‘moderate’ with ‘severe’ (Table VII) it is clear that morphological abnormalities were more often evaluated as absent or mild in the control and iHES groups than in the neoplastic and reactive groups. In addition, the proportion of cases graded moderate or severe was higher in the neoplastic group (P = 0·0002 or P = 0·0001 when patients with no consensus were included) (Table VII).
Table VI.
Grade of morphological abnormality in the four groups: absent, mild, moderate, severe).
| Absent | Mild | Moderate | Severe | No consensus | |
|---|---|---|---|---|---|
| Control | 1 | 2 | 0 | 0 | 0 |
| iHES | 0 | 2 | 1 | 0 | 0 |
| Neoplastic | 0 | 5 | 9 | 10 | 2 |
| Reactive | 1 | 10 | 8 | 4 | 5 |
Main results that lead to the discussion are marked in bold.
iHES, idiopathic hypereosinophilic syndrome.
Table VII.
Grade of morphological abnormality in the four groups, pooling ‘absent’ with ‘mild’, and ‘moderate’ with ‘severe’; number (percentage of group).
| Group | Absent + mild, n (%) | Moderate + severe, n (%) | No consensus |
|---|---|---|---|
| Control | 3/3 (100) | 0 | |
| iHES | 2/3 (67) | 1 (33·3) | |
| Neoplastic | 5/26 (19) | 20/26 (73) | 2/26 (8) |
| Reactive | 11/28 (39) | 11/28 (43) | 5/28 (18) |
Main results that lead to the discussion are marked in bold.
iHES, idiopathic hypereosinophilic syndrome.
The opinion of evaluators was that they were able, on the basis of eosinophil morphology, to identify a probable myeloid neoplasm in only 69% of the confirmed neoplasms while 39% of the reactive cases would have been similarly identified as likely to represent a myeloid neoplasm (Table VIII, P = 0·00002).
Table VIII.
Responses to the question ‘Do you think the patient is likely to have a myeloid neoplasm?’; number (percentage).
| Group | No | Yes |
|---|---|---|
| Control | 3/3 (100) | 0 |
| iHES | 3/3 (100) | 0 |
| Neoplastic | 8/26 (31) | 18/26 (69) |
| Reactive | 17/28 (61) | 11/28 (39) |
Main results that lead to the discussion are marked in bold.
iHES, idiopathic hypereosinophilic syndrome.
Discussion
Healthy volunteers without any history of allergy can have eosinophils showing morphological features usually recognised as ‘abnormal’, particularly moderate reduction of granules and moderate vacuolation. These features, in a minority of cells, must therefore be recognised as being within the spectrum of ‘normal’. Hyperlobated nuclei are seen in only a small minority of cells in healthy volunteers and are seen in a similar proportion in patients with reactive or clonal eosinophilia; their presence is thus not diagnostically useful. Basophilic granules are seen in only a small minority of eosinophils in healthy volunteers and their presence is more likely to be diagnostically significant since they are more frequently observed in patients with reactive or clonal eosinophilia.
In patients with clonal or reactive eosinophilia, morphological abnormalities are much more frequent with the neoplastic group showing, on average, a greater degree of abnormality. The specific features observed in the two groups overlap, however, although normal eosinophils are generally more common in the reactive group and moderate and marked vacuolation are more a feature of the neoplastic group. Basophilic granules in mature eosinophils are more often recognised in the neoplastic group but this is attributable largely to their presence in association with inv(16) and to a lesser extent with CEL, NOS. Hyperlobated nuclei, binuclearity and ring nuclei are uncommon in both groups and not strongly associated with either reactive or clonal cases; their presence is thus not likely to be diagnostically useful. Hypogranularity is common but is similarly lacking in specificity. However marked reduction in granules is much less likely in the miscellaneous (MPN or systemic mastocytosis) and inv(16) groups.
The only feature that points to a specific neoplasm is the presence of basophilic granules in mature eosinophils, this being seen in about a quarter of eosinophils in acute myeloid leukaemia with inv(16). Some patients with AML with inv(16) have significant eosinophilia (eosinophil count at least 1·5 × 109/l) but this is not necessarily so. Nevertheless the eosinophils are part of the neoplastic clone and their cytological features can point to the diagnosis.
The small number of cases of idiopathic hypereosinophilic syndrome generally showed less morphological abnormality than the clonal and reactive groups.
We have observed that although experienced morphologists, using morphology of mature eosinophils alone, suspect a myeloid neoplasm with clonal eosinophils more often in cases that are actually neoplastic, the sensitivity of this is not high and specificity is low since a myeloid neoplasm was also suspected in more than a third of cases of reactive eosinophilia.
In conclusion, an important observation of this study was that, although there were some differences in the frequency of certain specific morphological abnormalities between the clonal and reactive groups (particularly a higher frequency of vacuolation in the former), it is not possible to identify a myeloid neoplasm reliably on the basis of morphological features of eosinophils alone. Nevertheless, examination of a blood film is of importance since blast cells, lymphoma cells, mast cells and even parasites may be observed, and eosinophil morphology retains some utility as one feature in the overall evaluation of the patient. The presence of basophilic granules is the most useful feature pointing to possible neoplasia while marked vacuolation is characteristic of some subsets of clonal eosinophilia but is not specific for neoplasia.
Author contributions
JG, JB, BB and RB planned the study. JG and BB took the photographs. JG, JB, BB, RB, M‐TV, MT and GZ evaluated the images. CL carried out the statistical analysis. All authors contributed to the writing of the paper and approved the final manuscript.
Conflict of interest
No author has a conflict of interests.
Supporting information
Supplementary Material
References
- 1. Goasguen JE, Bennett JM, Bain BJ, Brunning RD, Vallespí M‐T, Tomonaga M, et al.; The International Working Group on Morphology of MDS IWGM‐MDS . Quality control initiative on the evaluation of the dysmegakaryopoiesis in myeloid neoplasms: difficulties in the assessment of dysplasia. Leuk Res. 2016;45:75–81. [DOI] [PubMed] [Google Scholar]
- 2. Goasguen JE, Bennett JM, Bain BJ, Brunning R, Vallespi MT, Tomonaga M, et al.; The International Working Group on Morphology of MDS . Dyserythropoiesis in the diagnosis of the myelodysplastic syndromes and other myeloid neoplasms: problem areas. Br J Haematol. 2018;182:526–33. [DOI] [PubMed] [Google Scholar]
- 3. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. (eds). World Health Organization classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


