Abstract
Background
Post‐surgery disabling scars are frequent after surgical interventions.
Aim
We evaluated a new strategy for scars management.
Methods
A woman with a postsurgery disabling scar, consequent to an accident that needed surgical intervention, had serious difficulties to walk and perform normal daily activities. A few months after the intervention, she was treated with a combined therapy consisting of polydeoxyribonucleotide (PDRN) vials 5.625 mg/3 mL (administered subcutaneously as a biostimulant treatment through the scars and throughout the whole atrophic area), associated with nucleotide administered topically and as a food supplement. The patient was treated with an additional topical nighttime treatment cream based on nucleotides, hyaluronic acid, Allium cepa extract, and vitamin E, plus a daily treatment with a cream containing nucleotides, and a nutraceutical systemic treatment with 25 mg/cps of nucleotides and 5 mg/cps of Q10‐coenzyme (1 cps/d).
Results
This reasonably cheap treatment was effective and safe for this disabling scar at the right foot. One year after starting treatment, the patient confirmed her complete satisfaction. This is the first case report describing an unexpectedly successful outcome while using this combination therapy on a woman with a postsurgery disabling scar.
Keywords: grafting, minimally invasive, patient satisfaction, scar
1. BACKGROUND
Polydeoxyribonucleotide (PDRN) is a mixture of deoxyribonucleotides with molecular weights varying from 50 to 1500 kDa derived from gonads of Oncorhynchus mykiss. 1 In vitro tests show that PDRN has a great affinity with the adenosine 2A receptor, which might explain its effect on cell proliferation and regeneration. The improvement of the skin repair process and enhanced wound breaking strength of PDRN was observed in diabetic rats 2 and mice with deep‐dermal second‐degree burn injuries, 3 in the healing of autologous skin graft donor sites, 4 and, later, as antioxidant skin protector against UV, 5 in a randomized trial on diabetic foot lesions, 6 in preventing scar formation in rats 7 and other inflammatory and degenerative diseases, with an appreciable level of safety. 1
Polydeoxyribonucleotide has never been used for the treatment of post‐traumatic or postsurgery scars; to our knowledge, this is the first case describing this use in the scientific literature.
2. CASE PRESENTATION
A woman, aged 56, 160 cm tall, and weighing 62 kg, was hospitalized in the orthopedic ward on September 26, 2016, following an accident at her place of work. The diagnosis was blunt trauma on the right foot, with partial post‐traumatic loss of soft tissue, with a closed fracture of the tarsus, metatarsus and phalanges, and instep and medial peri‐malleolar region skin necrosis (Figure 1). The first and second toes of the right foot were severed on October 13 due to necrosis. A free skin graft was performed on December 20, followed by a subcutaneous (SC) infiltration of triamcinolone 40 mg, as required by standard hospital procedure followed in the treatment and prophylaxis of thickened scars. One month later, 20 mg of triamcinolone (TAC) was administered as 1 mL SC injections; the dosage of TAC was increased to 30 mg for the subsequent injections performed 2 and 3 months after the surgery. During this period, a topical silicone dressing was applied daily.
FIGURE 1.
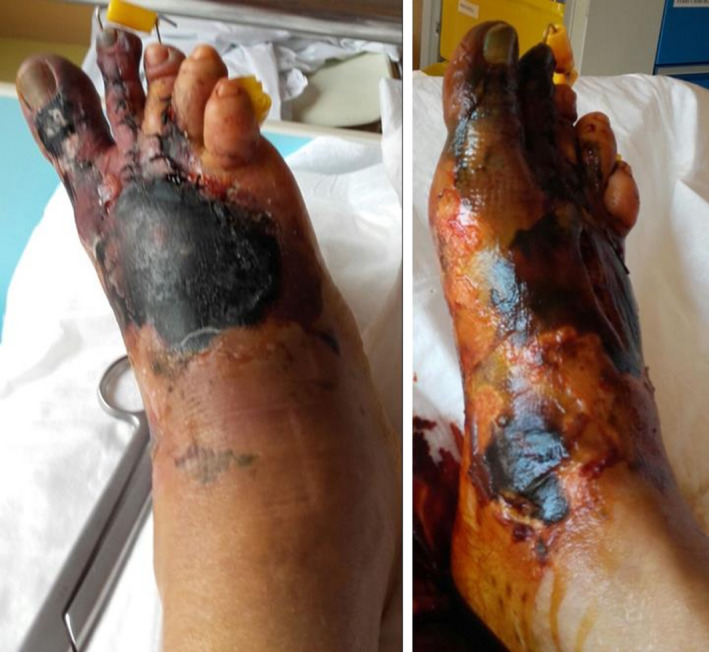
Patient's foot at hospitalization
The patient was diagnosed with prediabetes after the third injection of TAC; dietary control was therefore started, and TAC was discontinued. Before the hospitalization, the patient was in excellent health and had never suffered from diabetes or other disorders that could impair the wound healing process.
Because of her surgery and despite the steroid and silicone treatments while in hospital, the patient had a retracting hypertrophic scar, reaching from her instep to her malleolar area, with severe cutaneous atrophy on the instep, preventing her from walking and performing her usual daily activities, so that she was partially disabled (Figures 2, 3, 4). After her discharge from the hospital, she came to our outpatient clinic searching for a solution for her problem. Injections of 5‐fluorouracil and laser therapy were suggested to the patient but were refused because they were invasive and expensive.
FIGURE 2.
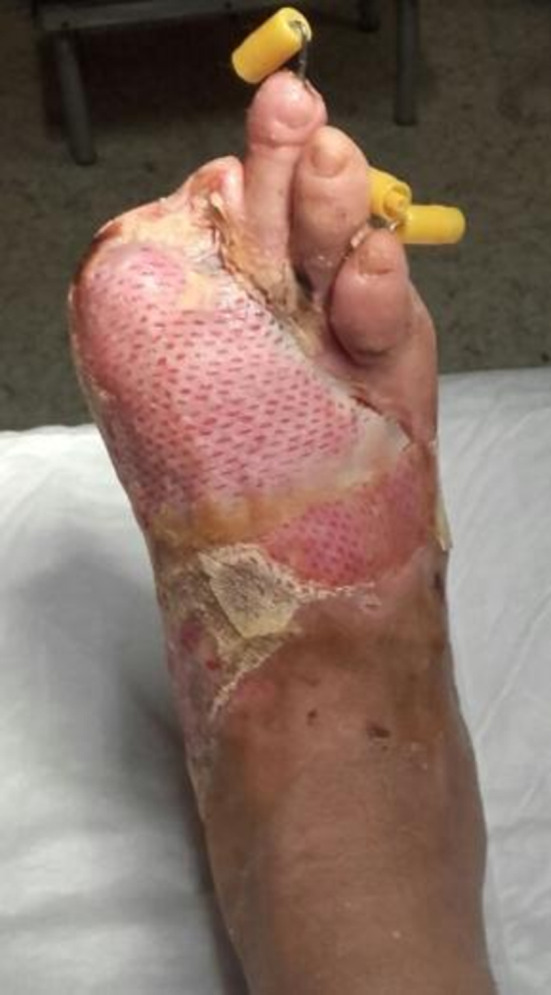
Patient's foot after surgery
FIGURE 3.
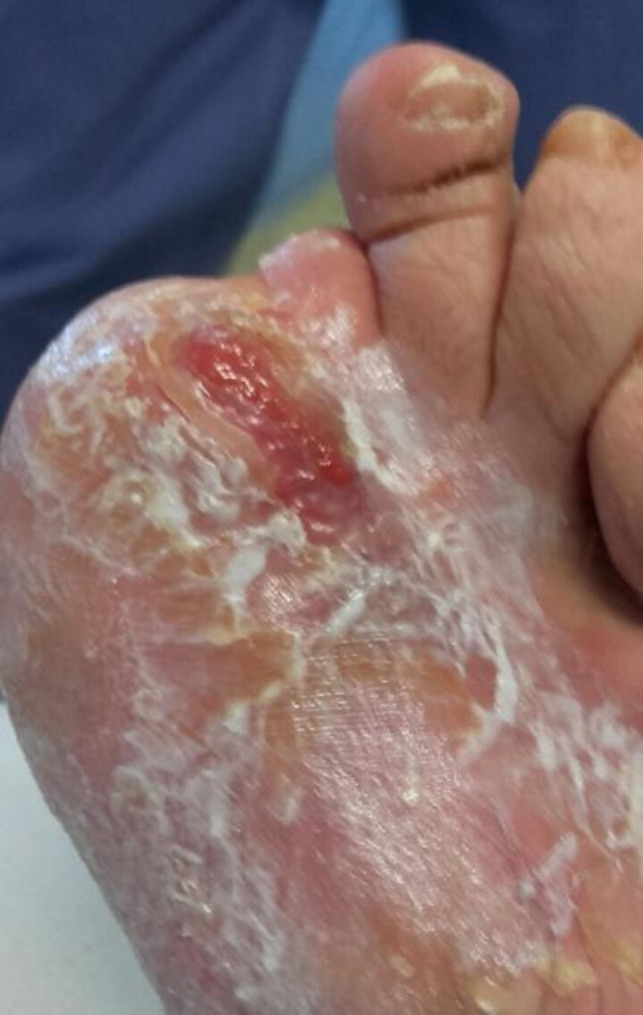
Patient's foot at discharge
FIGURE 4.
Patient's foot 7 mo after surgery
A new treatment regimen with PDRN and nucleotides was undertaken. The topical treatment included a cream containing 0.5% of nucleotides, 1% of hyaluronic acid, 10% Allium cepa extract, and 1% of vitamin E (Makeskin®—Mastelli SRL) 8 as nighttime occlusive treatment, plus a daily treatment with a 0.2% nucleotides cream (Turnover® cream—Mastelli SRL). 9 PDRN vials 5.625 mg/3 mL (Placentex®—Mastelli SRL) 10 were administered to her as an outpatient as biostimulant subcutaneous treatment through the scars and the whole atrophic area (Figure 5). PDRN was injected subcutaneously into the whole area of the scar. The micropuncture technique was performed using a 30‐G needle to inject each dose at 0.5‐1.0 cm from each other. Subcutaneous PDRN was administered monthly for 6 months. Furthermore, nutraceutical systemic treatment (NST) with 25 mg/cps of nucleotides and 5 mg/cps of Q10‐coenzyme (1 cps/d) (Turnover® tablets—Mastelli SRL) 11 was added to the topical therapy to improve cutaneous atrophy. 12
FIGURE 5.
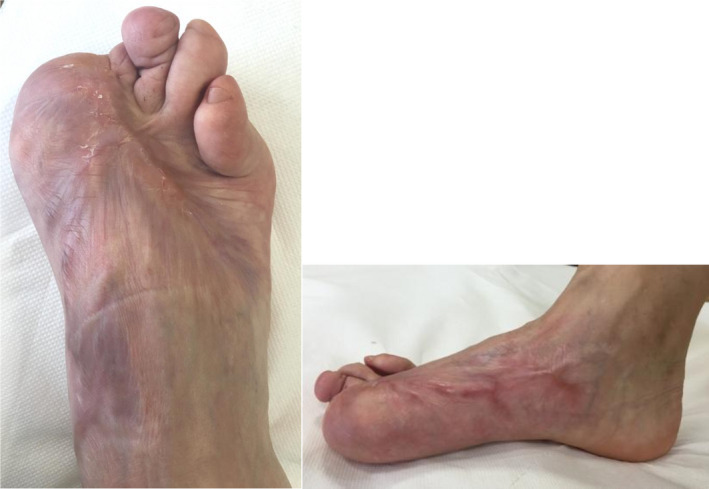
Patient's foot 1 mo after starting treatment
All treatments were continued for the following 6 months; they were noninvasive and had reasonable costs.
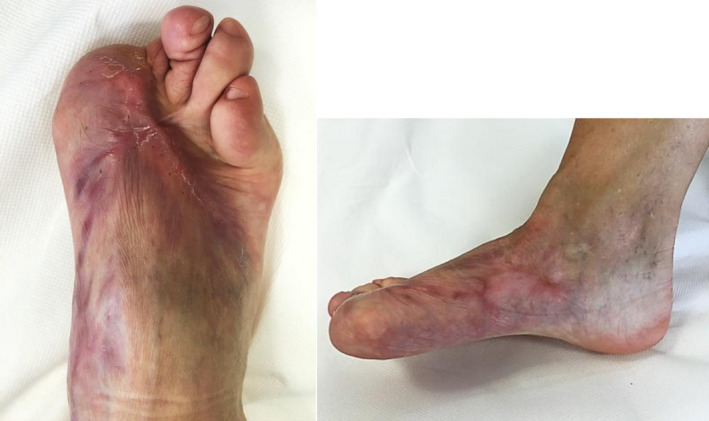
Steroid infiltrations and silicone topical treatment were never used during the treatment period. The patient showed clinically significant aesthetic and functional improvement; the cutaneous texture was restored in the dorsal and malleolar areas of the foot (Figure 6). The pain and prediabetes recovered; the patient was able to walk autonomously, reporting a significant improvement in her subjective quality of life.
FIGURE 6.
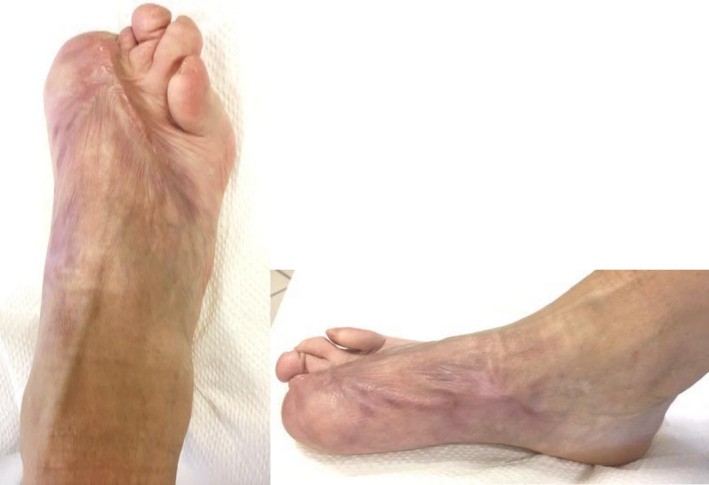
Patient's foot 6 mo after starting treatment
The patient was fully compliant with the treatment and showed no treatment‐related adverse events.
3. OUTCOME AND FOLLOW‐UP
The patient did not show any worsening at a 1‐year follow‐up (Figure 7).
FIGURE 7.
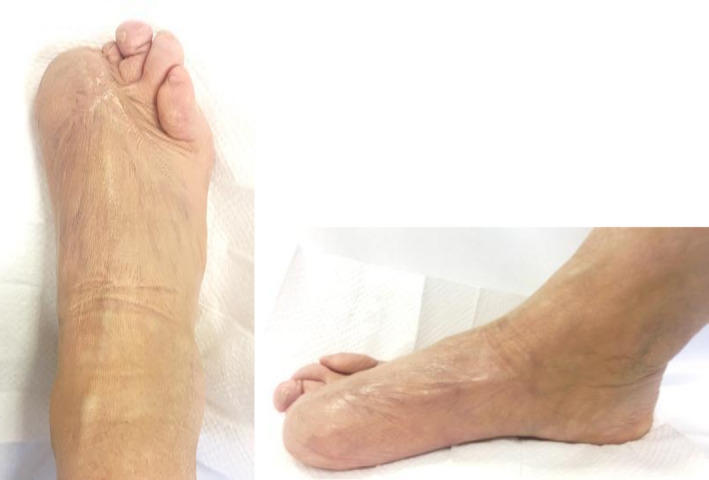
Patient's foot 12 mo after starting treatment
4. DISCUSSION
The combined use of subcutaneous PDRN and topical creams with nucleotides plus hyaluronic acid and Allium cepa extract, in association with the systemic oral administration of nucleotides and Q10‐coenzyme, showed a positive effect on severe tissue damage without side effects.
Polydeoxyribonucleotide was shown to increase collagen I expression, 13 , 14 through the activation of membrane‐bound adenosine receptors (A2ARs) 15 , 16 and to stimulate cell growth and angiogenesis. 3 , 17
Hyaluronic acid was shown to have a protecting, hydrating, and emollient action. 18 Allium cepa extract and vitamin E improve hypertrophic and keloids scars via multiple mechanisms. 19 , 20 NST has an antioxidant action, supporting repair processes and sustaining fibroblast activity. 21
The lack of effects on the immune system is one of the possible factors determining the good safety profile of PDRN. Differently from other DNA‐derived drugs, its selectivity vs A2A might support this hypothesis 14 ; caution is therefore required when extending the pharmacological properties of PDRN to other DNA‐derived products. 1
In a previous randomized double‐blind clinical trial on more than 100 patients, investigating PDRN use in diabetic patients showing hard‐to‐heal ulcers (Wagner grade 1 or 2), PDRN was injected five times a week for 8 weeks by the intramuscular route and twice a week for 8 weeks by perilesional SC injections. The frequency of complete healing was significantly higher with PDRN treatment than with placebo. 6 A new clinical trial “Efficacy and Safety of PLACENTEX® i.m. in Patients with Scleroderma Diseases (NCT03388255)” is evaluating the efficacy of PDRN in reducing fibrosis and fibrosclerotic skin lesions during the inactive stage of scleroderma disease.
The combination of the subcutaneous administration of PDRN with the topical administration of nucleotides, hyaluronic acid, Allium cepa extract, and vitamin E, together with the systemic oral administration of nucleotides and Q10‐coenzyme, was reasonably cheap, effective, and safe in the treatment of this disabling scar on the right foot.
These results were observed up to 1 year after the beginning of the treatment.
Further, properly designed, controlled clinical trials are needed to confirm the effectiveness of this combination in a wider population and the role of the different treatments.
5. PATIENT PERSPECTIVE
I am completely satisfied with the outcomes of this treatment, considering that the skin of the foot was purple, very tight, and with no sensitivity before starting it. The foot has continuously improved bringing considerable relief, and the skin has recovered.
STATEMENT OF CONSENT
The patient gave her informed consent to use her anonymized data for this study.
ACKNOWLEDGMENTS
The author thanks Dr Carla Benci for medical writing and Mastelli SrL., who financed only Dr Benci's medical writing.
Belmontesi M. Polydeoxyribonucleotide for the improvement of a hypertrophic retracting scar—An interesting case report. J Cosmet Dermatol. 2020;19:2982–2986. 10.1111/jocd.13710
[Corrections added on October 18, 2020, after online publication: the copyright line has been changed to "© 2020 The Authors. Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.".]
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Squadrito F, Bitto A, Irrera N, et al. Pharmacological activity and clinical use of PDRN. Front Pharmacol. 2017;8:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galeano M, Bitto A, Altavilla D, et al. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008;16(2):208‐217. [DOI] [PubMed] [Google Scholar]
- 3. Bitto A, Galeano M, Squadrito F, et al. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit Care Med. 2008;36(5):1594‐1602. [DOI] [PubMed] [Google Scholar]
- 4. Valdatta L, Thione A, Mortarino C, Buoro M, Tuinder S. Evaluation of the efficacy of polydeoxyribonucleotides in the healing process of autologous skin graft donor sites: a pilot study. Curr Med Res Opin. 2004;20(3):403‐408. [DOI] [PubMed] [Google Scholar]
- 5. Stanghellini E, De Aloe G, Carcagni ML, Cattarini GFM. Evaluation of antioxidant activity of a topical cream. in Fifth International Workshop on Photodermatology. 30–31 Maggio. 2003.
- 6. Squadrito F, Bitto A, Altavilla D, et al. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: results of a clinical trial [published correction appears in. J Clin Endocrinol Metab. 2015;100(2):763. [DOI] [PubMed] [Google Scholar]
- 7. Jeong W, Yang CE, Roh TS, et al. Scar prevention and enhanced wound healing induced by polydeoxyribonucleotide in a rat incisional wound‐healing model. Int J Mol Sci. 2017;18(8):1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klinger M, Forcellini D, Cornegliani G, Maione L.Trattamento topico delle cicatrici – Hi Tech Dermo – Makeskin Mastelli. hi.tech dermo 68. 2011. https://www.makeskin‐mastelli.com/it/trattamento‐topico‐cicatrici/. Accessed December 5, 2019.
- 9. https://shop.mastelli.com/turnover/19‐turnover‐integratore‐crema‐dermatologica.html. Accessed 13 September, 2020.
- 10. https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000325_004905_RCP.pdf&retry=0&sys=m0b1l3. Accessed 13 September, 2020.
- 11. https://shop.mastelli.com/turnover/36‐turnover‐integratore‐compresse.html. Accessed 13 September, 2020.
- 12. Sterczala AJ, DuPont WH, Comstock BA, et al. Physiological effects of nucleotide supplementation on resistance exercise stress in men and women. J Strength Cond Res. 2016;30(2):569‐578. [DOI] [PubMed] [Google Scholar]
- 13. Gennero L, De Siena R, Denysenko T, et al. A novel composition for in vitro and in vivo regeneration of skin and connective tissues. Cell Biochem Funct. 2011;29(4):311‐333. [DOI] [PubMed] [Google Scholar]
- 14. Sini P, Denti A, Cattarini G, et al. Effect of polydeoxyribonucleotides on human fibroblasts in primary culture. Cell Biochem Funct. 1999;17(2):107‐114. [DOI] [PubMed] [Google Scholar]
- 15. Perez‐Aso M, Mediero A, Cronstein BN. Adenosine A2A receptor (A2AR) is a fine‐tune regulator of the collagen1:collagen3 balance. Purinergic Signal. 2013;9(4):573‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perez‐Aso M, Fernandez P, Mediero A, et al. Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 2014;28(2):802‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altavilla D, Squadrito F, Polito F, et al. Activation of adenosine A2A receptors restores the altered cell‐cycle machinery during impaired wound healing in genetically diabetic mice. Surgery. 2011;149(2):253‐261. [DOI] [PubMed] [Google Scholar]
- 18. Guizzardi S, Uggeri J, Belletti S, et al. Hyaluronate increases polynucleotides effect on human cultured fibroblasts. J Cosmet Dermatol Sci Appl. 2013;03:124‐128. [Google Scholar]
- 19. Hosnuter M, Payasli C, Isikdemir A, et al. The effects of onion extract on hypertrophic and keloid scars. J Wound Care. 2007;16:251‐254. [DOI] [PubMed] [Google Scholar]
- 20. Perez OA, Viera MH, Patel JK, et al. A comparative study evaluating the tolerability and efficacy of two topical therapies for the treatment of keloids and hypertrophic scars. J Drugs Dermatol. 2010;9(5):514‐518. [PubMed] [Google Scholar]
- 21. Tan BL, Norhaizan ME, Liew WP, Sulaiman Rahman H. Antioxidant and oxidative stress: a mutual interplay in age‐related diseases. Front Pharmacol. 2018;9:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NCT03388255. https://clinicaltrials.gov/ct2/show/NCT03388255?term=placentex&draw=2&rank=1. Accessed 13 September, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


