SUMMARY
Polyamines, such as putrescine, spermidine and spermine (Spm), are low‐molecular‐weight polycationic molecules present in all living organisms. Despite their implication in plant cellular processes, little is known about their molecular mode of action. Here, we demonstrate that polyamines trigger a rapid increase in the regulatory membrane lipid phosphatidylinositol 4,5‐bisphosphate (PIP2), and that this increase is required for polyamine effects on K+ efflux in Arabidopsis roots. Using in vivo 32Pi‐labelling of Arabidopsis seedlings, low physiological (μm) concentrations of Spm were found to promote a rapid PIP2 increase in roots that was time‐ and dose‐dependent. Confocal imaging of a genetically encoded PIP2 biosensor revealed that this increase was triggered at the plasma membrane. Differential 32Pi‐labelling suggested that the increase in PIP2 was generated through activation of phosphatidylinositol 4‐phosphate 5‐kinase (PIP5K) activity rather than inhibition of a phospholipase C or PIP2 5‐phosphatase activity. Systematic analysis of transfer DNA insertion mutants identified PIP5K7 and PIP5K9 as the main candidates involved in the Spm‐induced PIP2 response. Using non‐invasive microelectrode ion flux estimation, we discovered that the Spm‐triggered K+ efflux response was strongly reduced in pip5k7 pip5k9 seedlings. Together, our results provide biochemical and genetic evidence for a physiological role of PIP2 in polyamine‐mediated signalling controlling K+ flux in plants.
Keywords: Arabidopsis; phosphoinositide signalling; phosphatidylinositol 4,5‐bisphosphate (PIP2); phosphatidylinositol 4‐phosphate 5‐kinase (PIP5K); phosphatidic acid (PA); phospholipids; polyamines; K+ flux
Significance Statement
Polyamines, such as putrescine, spermidine and spermine, are low‐molecular‐weight polycations present in all living organisms. Despite their involvement in various plant cellular processes, little is known about their molecular mode of action. Here, we demonstrate that polyamines trigger a rapid increase in the signalling lipid PIP2 at the plasma membrane of Arabidopsis roots by activating two lipid kinases, PIP5K7 and PIP5K9, and that this lipid response is required for the K+ efflux that is triggered downstream.
INTRODUCTION
Development and adaptation of plants to a changing environment involve numerous cellular signalling pathways. So far, the interactions between signalling pathways and their integration are not well understood. The links between biochemical cascades and their hierarchical interplay are an important and current field of research. Our study focuses on the role of phosphatidylinositol 4,5‐bisphosphate (PIP2), an important lipid signalling molecule in plants (Munnik and Nielsen, 2011; Heilmann, 2016b; Noack and Jaillais, 2017; Gerth et al., 2017a; Colin and Jaillais, 2020). This minor lipid is formed by phosphorylation of phosphatidylinositol 4‐phosphate (PIP), catalysed by the enzyme PIP 5‐kinase (PIP5K). Vascular plants typically contain very low levels of PIP2 per gram fresh weight, being 30‐ to 100‐fold lower than in animal cells (Munnik et al., 1998a; Meijer and Munnik, 2003). In plants, PIP2 can be detected by metabolic in vivo labelling using radioactive phosphate (e.g., 32Pi) (Munnik and Zarza, 2013). Alternatively, genetically encoded fluorescent biosensors are available to visualise PIP2 in living cells (van Leeuwen et al., 2007; Simon et al., 2014). In response to salt or heat stress, a clear plasma membrane localisation of PIP2 biosensors was previously observed, which coincided with the formation of 32P‐PIP2 in a time‐ and dose‐dependent fashion (van Leeuwen et al., 2007; Simon et al., 2014).
The physiological function of PIP2 in plants is evident from the phenotypes of Arabidopsis mutants with genetic lesions in PIP5K genes. The Arabidopsis genome encodes a relatively large PIP5K gene family of 11 members, which can be categorised into two subfamilies (PIP5K1‐11; Mueller‐Roeber and Pical, 2002). Reversed genetics revealed that individual PIP5Ks exhibit specific and distinct roles in plant signalling and development. Some PIP5K isoenzymes are expressed in pollen tubes (PIP5K4–6, 10 and 11) (Sousa et al., 2008; Ischebeck et al., 2008; Zhao et al., 2010; Ischebeck et al., 2011) or root hairs (PIP5K3 and 4) (Stenzel et al., 2008; Kusano et al., 2008a; Wada et al., 2015) and are involved in polar tip growth. The ubiquitously expressed PIP5K1 and PIP5K2 have functions in growth and development in various Arabidopsis tissues (Mei et al., 2012; Ischebeck et al., 2013; Tejos et al., 2014; Marhava et al., 2020), in line with a pronounced polarised distribution of PIP2 and PIP5Ks in different cell types and several stages of development (Ischebeck et al., 2013; Tejos et al., 2014). As a consequence, pip5k1 pip5k2 double mutants exhibit severe defects in embryogenesis, vascular development and meristem formation in roots and shoots (Tejos et al., 2014; Marhava et al., 2020). PIP2 has also been implicated in protophloem differentiation (Rodriguez‐Villalon et al., 2015; Gujas et al., 2017; Marhava et al., 2020), where PIP5K1, PIP5K2 and PIP5K7 seem to be involved (Bauby et al., 2007; Marhava et al., 2020). Estradiol‐inducible overexpression of a human PIP5K in Arabidopsis dramatically increased PIP2 levels at the cost of PIP, and severely affected phloem and xylem differentiation (Gujas et al., 2017). Mutants in 5‐phosphatases (5‐PTases; i.e. CVP2, CVL1) that normally degrade PIP2 back into phosphatidylinositol 4‐phosphate (PI4P) also exhibit vascular differentiation defects (Rodriguez‐Villalon et al., 2015), and these genes were previously characterised for their role in cotyledon vascular patterning (Carland and Nelson, 2009). Independently, these findings highlight a role for PIP2 in development as well as stress signalling. Despite the obvious and prominent roles of PIP2, only little is known about how PIP2 production is triggered by upstream signalling pathways and how the lipid mediates its downstream effects.
In this work, we address the physiological function of PIP2 that is triggered in Arabidopsis roots by polyamines. Polyamines are small and versatile organic cations, present in all living organisms (Michael, 2016). The most common polyamines are putrescine (Put), spermidine (Spd) and spermine (Spm), representing a di‐, tri‐ and tetraamine, respectively. In plants, polyamines are implicated in stress responses, like drought, salinity and heat (Tiburcio et al., 2014; Michael, 2016). The molecular mechanisms behind polyamine perception and signalling are largely unknown, though several downstream components of polyamine action have been identified, including protein kinases, transcription factors, reactive oxygen species (ROS) and Ca2+ and K+ fluxes (Takahashi et al., 2003; Yoda, 2006; Kusano et al., 2008b; Wu et al., 2010; Bitrián et al., 2012; Moschou et al., 2012; Pál et al., 2015; Sagor et al., 2015; Pegg, 2016; Zarza et al., 2019). Polyamines have also recently been linked to the perception of salt stress through SOS1 (Chai et al., 2020). As polyamines appear to be involved in a range of plant stresses that also trigger PIP2 responses (Tiburcio et al., 2014; Heilmann, 2016a) and affect similar targets, including K+ channels (Liu et al., 2005; Ma et al., 2009; Wigoda et al., 2010), we hypothesised that polyamines are linked to the metabolism of phosphoinositides. This notion was supported by earlier studies, reporting effects of polyamine treatment on signalling phospholipids in spinach (Spinacia oleracea) hypocotyls (Dureja‐Munjal et al., 1992) and coffee (Coffea arabica) cells (Echevarría‐Machado et al., 2005).
Here, we show that polyamines, in particular Spm, trigger a rapid increase in PIP2 in the plasma membrane of Arabidopsis root cells. We further provide biochemical and genetic evidence that PIP2 is generated through activation of PIP5K7 and PIP5K9, and has a physiological role in regulating K+ fluxes.
RESULTS
Polyamines trigger rapid PIP2 responses in Arabidopsis roots
The effect of polyamines on the formation of membrane phospholipids was analysed by in vivo radiolabelling using intact Arabidopsis seedlings, which were pre‐labelled overnight with 32Pi and treated the next day for 30 min with physiological concentrations (60 µm) of Put, Spd or Spm (Tassoni et al., 2000). As shown in Figure 1a, a substantial increase in PIP2 was found with Spd and Spm, but not with Put, which only induced a PIP2 response at much higher (mm) concentrations (Figure 1b). In contrast, Spm already induced a PIP2 response at 15 µm, whereas for Spd, concentrations of ≥60 µm were required. (Figure 1a,b). Thermospermine (tSpm), an isomer of Spm, was equally potent as Spm (Figure 1b), while the diamine diaminopropane (Dap) behaved similarly to Put (Figure 1b). These results indicate that the capacity of polyamines to trigger PIP2 is a function of the number of amine groups (hence, positive charge) rather than their molecular structure, with Spm4+ = tSpm4+ > Spd3+ >> Put2+ ≈ Dap2+.
Figure 1.
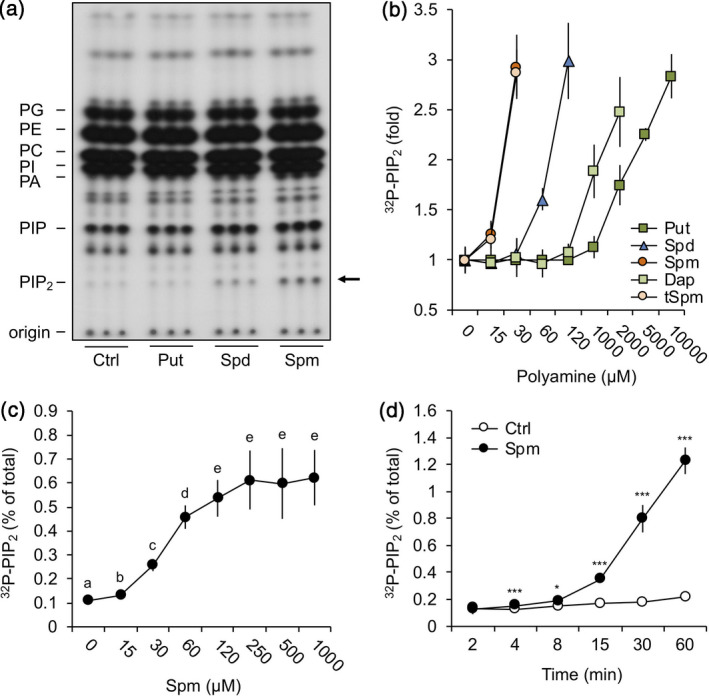
Polyamines trigger the formation of PIP2 in Arabidopsis seedlings.
32Pi‐pre‐labelled seedlings were treated for 30 min with 60 µm of putrescine (Put), spermidine (Spd) or spermine (Spm), or with buffer alone (control, Ctrl), after which their lipids were extracted, separated by thin‐layer chromatography and visualised by autoradiography.
(a) Autoradiograph of typical TLC, containing three samples per treatment. Abbreviations: PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PIP, phosphatidylinositol phosphate; PIP2, phosphatidylinositol 4,5‐bisphosphate. (b) Quantified 32P‐PIP2 response after 30 min treatment with Put, Spd, Spm, diaminopropane (Dap) or thermospermine (tSpm) at the indicated concentrations, calculated as fold increase compared with control. Data are presented as the mean ± SD (n = 6). (c) Dose–response with Spm for 30 min and (d) time‐course with 60 µm Spm or buffer alone, showing the percentage of 32P‐PIP2 with respect to the total of 32P‐labelled phospholipids. In all cases, data are presented as the mean ± SD (n = 6).
So far, only hyperosmotic stress, heat and wounding have been shown to trigger PIP2 responses (Pical et al., 1999; DeWald et al., 2001; Heilmann et al., 2001; van Leeuwen et al., 2007; König et al., 2007, 2008; Mosblech et al., 2008; Mishkind et al., 2009). To explore the effect of polyamines in more detail, Spm was used, being the most potent compound. As shown in Figure 1c, a dose‐dependent PIP2 increase was observed when seedlings were treated for 30 min with different concentrations of Spm. PIP2 was already detectable at low µm concentrations and reached a maximum of ~4.5‐fold increase with 60 µm Spm. Time‐course experiments using 60 µm Spm revealed a rapid PIP2 increase, starting within 4 min after treatment and continuing exponentially until at least 60 min (Figure 1d).
As Spm can be catabolised by polyamine oxidases, which are known to cause H2O2 and NO accumulation (Tun, 2006; Moschou et al., 2008a; Zarza et al., 2019), we investigated whether Spm itself or one of its downstream oxidation products induced the PIP2 response using the ROS and NO scavengers DMTU and cPTIO, respectively. As shown in Figure S1, neither scavenger affected the Spm‐induced PIP2 response, while the accumulation of H2O2 and NO was reduced (Zarza et al., 2019). These results suggest that Spm itself rather than its metabolites triggered the formation of PIP2.
Earlier, we discovered that Spm triggered a rapid increase in phosphatidic acid (PA) levels, generated through phospholipase Dδ (PLDδ; Zarza et al., 2019). Determining both lipid responses in time‐course and dose–response experiments revealed a coordinated pattern (Figure S2a,b), which may reflect an interdependency since PLDδ has been suggested to be regulated by PIP2 (Li et al., 2009; Hong et al., 2016).
Spm‐induced PIP2 is triggered by PIP5K activation
Theoretically, the accumulation of PIP2 could result from three enzymatic routes: (i) through stimulation of its synthesis by PIP5K, (ii) through inhibition of its breakdown by PLC or (iii) through inhibition of its breakdown by PIP2 phosphatase. To distinguish between these possibilities, a differential 32Pi‐labelling protocol was carried out that enhances the effect of kinase activity (Munnik et al., 1998b; Arisz and Munnik, 2013). The experimental approach exploits the fact that when 32Pi is added to seedlings, radiolabelled phosphate is rapidly incorporated into ATP, and hence into lipids that are phosphorylated in an ATP‐dependent fashion by kinases (i.e. PIP2 via PIP5K). When seedlings were short‐time labelled for 15 min and then treated with Spm, a massive increase in 32P‐PIP2 was observed, indicating that PIP2 was formed via PIP5K activation (Figure 2a). Similarly, pulse‐chase experiments in which seedlings were first treated with Spm, then labelled for 5 min with 32Pi and subsequently chased with non‐radioactive Pi revealed a quick rise in 32P‐PIP2 after stimulation, which then decreased again due to the rapid incorporation of non‐radioactive Pi (Figure 2b). This pattern is consistent with the notion that the Spm‐triggered PIP2 increase is a result of enhanced PIP5K activity, and not of inhibited PIP2 breakdown by PLC or 5‐PTase, because in those cases PIP2 levels would have remained high.
Figure 2.
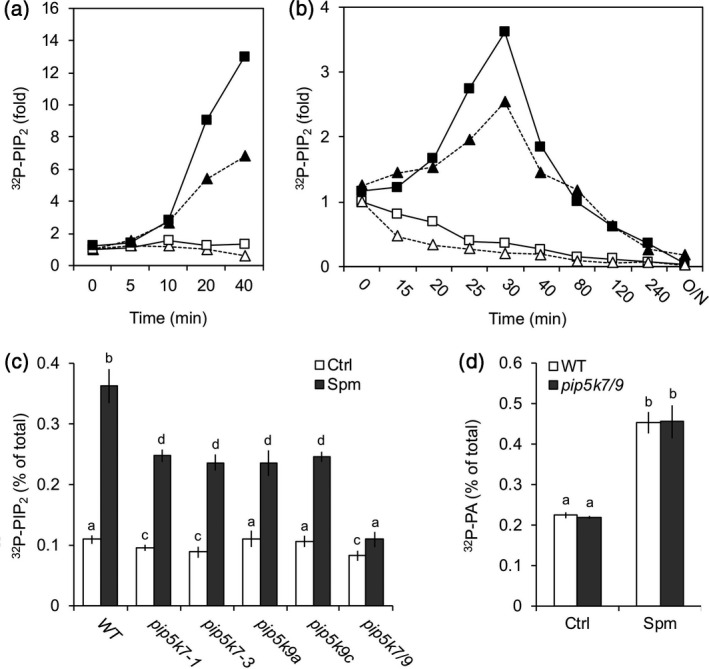
Spm‐induced PIP2 is triggered by activation of PIP5K, not through inhibition of PLC or PIP2 phosphatase.
(a) Seedlings were pulse‐labelled with 32Pi for 15 min and then treated with Spm or buffer alone (Ctrl) for the times indicated. The PIP2 fold increase of two independent experiments is shown (squares and triangles). (b) Pulse‐chase experiment where seedlings were treated with buffer ± Spm for 15 or 30 min (triangles and squares, respectively), labelled with 32Pi for 5 min and then chased (t = 0) with non‐radioactive Pi in the presence or absence of Spm for the times indicated. Values in (a) and (b) were normalised to 32P‐PI and expressed with respect to Ctrl, 0 min. Open symbols, Ctrl; closed symbols, Spm. (c) 32P‐PIP2 response to Spm of WT and pip5k7, pip5k9 and pip5k7 pip5k9 (pip5k7/9) double mutants. Seedlings were 32Pi‐labelled overnight and treated for 30 min with 60 μm Spm. (d) 32P‐PA response in WT and pip5k7/9 mutant plans. Data show that the PA response is independent of PIP2. In all cases, data are presented as the mean ± SD (n = 3).
Spm‐induced PIP2 is generated by PIP5K7 and PIP5K9
The Arabidopsis genome encodes 11 PIP5Ks (Mueller‐Roeber and Pical, 2002). To identify potential PIP5Ks involved, transfer DNA (T‐DNA) insertion mutants were tested for their 32P‐PIP2 response after Spm treatment (Figure S3a). A substantially reduced PIP2 response was found for T‐DNA mutants in PIP5K7 or PIP5K9 (Figure 2c). Individual knockout (KO) alleles of pip5k9a, pip5k9c or pip5k7‐1, as well as the knockdown line pip5k7‐3 (Figure S3b,c), all showed a ~45% reduction in Spm‐induced PIP2, whereas a pip5k7‐1 pip5k9c double KO mutant (in figures abbreviated as pip5k7/9) typically lost ≥90% of its PIP2 response compared to wild type (WT) plants (Figure 2c). These results again confirm that the increase of PIP2 in response to polyamines is generated through PIP5K, and PIP5K7 and PIP5K9 are identified as the main isoforms involved. Despite suppressed PIP2 responses, the pip5k mutants exhibited WT PA responses (Figure 2d). Similarly, we found that the pldδ mutant revealed normal PIP2 responses upon Spm treatment (Figure S4). Together, these observations have three important implications, namely (i) the Spm‐induced PIP2 response does not reflect PA via the PLC/DGK pathway, in agreement with the finding that PA is predominantly generated by PLDδ (Zarza et al., 2019); (ii) the increase in PIP2 does not function as an activator of PLDδ, a notion based on in vitro findings and suggested to function in vivo (Pappan et al., 1997; Qin et al., 1997; Munnik and Testerink, 2009); (iii) PIP2 is likely involved in signalling by itself.
PIP5K7 and PIP5K9 expression and regulation by Spm
To test expression patterns and transcriptional activation of the PIP5K7 and PIP5K9 genes by Spm, transgenic Arabidopsis lines expressing promoter–GUS fusions of PIP5K7 or PIP5K9 were generated and GUS activity was visualised using histochemistry (Figure 3). In untreated seedlings, both ProPIP5K7::GUS and ProPIP5K9::GUS reporters revealed predominant promoter activity in the vasculature of cotyledons (Figure 3a,e) and roots (Figure 3b,f) and in most cells of the root meristem (Figure 3d,h), which was consistent with an earlier promoter–reporter gene fusion of PIP5K7 (Bauby et al., 2007). In root elongation and differentiation zones (Figure 3b,f), PIP5K7 expression appeared to be restricted to the stele, pericycle and phloem, with companion cells showing the highest expression levels together with metaphloem and procambium (Figure 3c). By contrast, PIP5K9 seemed to be expressed in all cells of the elongation and differentiation zones, except in the endodermis, where its expression was limited to cells adjacent to the xylem pole pericycle (Figure 3g). Interestingly, discontinuous expression of PIP5K9 was detected in the epidermal cell layer (Figure 3g).
Figure 3.
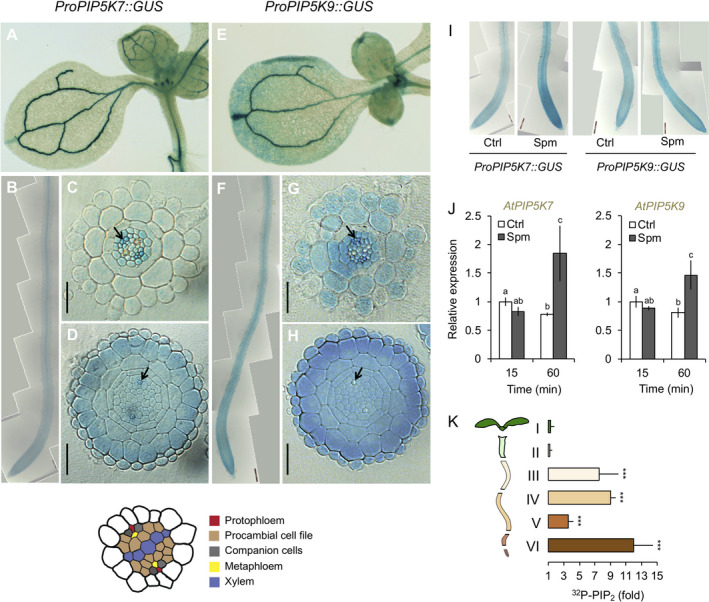
Expression of PIP5K7 and PIP5K9 in Arabidopsis seedlings.
Histological GUS analyses of 5‐day‐old transgenic lines expressing (a–d) ProPIP5K7::GUS or (e–h) ProPIP5K9::GUS. Pictures show (a, e) cotyledons, (b, f) a general overview of the root and cross‐sections of (c, g) the root differentiation zone and (d, h) the division zone (root meristem). In (c, d, g, h), black arrowheads mark the protophloem cells. Results were confirmed in three independent transgenic lines. Bars represent 25 µm. (i, j) Effects of Spm on the expression of PIP5K7 and PIP5K9 genes using (i) a GUS reporter system and (j) RT‐PCR. (k) 32P‐PIP2 response in different sections of Arabidopsis seedlings. Type of section/length: (I) root tip, 2 mm, (II) root tip, 3 mm, (III) root tip, 5 mm, (IV) root tip, 5–7 mm, (V) hypocotyl and (VI) cotyledons. Results are expressed as the fold increase of PIP2 with respect to control treatment of each section. In all cases, data are presented as the mean ± SD (n = 3).
Spm strongly induced GUS staining of the ProPIP5K7::GUS reporter in the root tip meristem and stele, whereas only a slight increase in the activity of the PIP5K9 promoter was observed after 30 min (Figure 3i). Quantitative real‐time PCR (qRT‐PCR) analyses confirmed the results from the promoter–GUS fusions, where between 15 and 60 min after Spm treatment an increased transcript abundance was detected for both genes (Figure 3j). These results indicate that enhanced PIP5K7/PIP5K9 expression may also contribute to the increased PIP2 formation upon Spm treatment.
To correlate PIP5K expression and PIP2 formation in more detail, 32Pi‐labelling and Spm treatment were performed as before, but now tissues were dissected after sample fixation, and the PIP2 responses were determined in the different sections (Figure 3k). Interestingly, Spm only triggered a PIP2 response in the root, not in the shoot or hypocotyl, in contrast to the clear vascular expression of both genes (Figure 3a,e). Within the root, the strongest PIP2 responses were found near the tip and maturation zone (sections III, IV, VI; Figure 3k), which correlated well with the PIP5K7 and PIP5K9 expression in these tissues (Figure 3i). Repeating the experiment with higher Spm concentrations gave similar results (Figure S5a). This lack of responsiveness in hypocotyl and cotyledons could be a consequence of tissue‐specific Spm perception and/or transport, or the absence of auxiliary signalling elements required for post‐transcriptional PIP5K activation. We did observe PIP2 responses in isolated leaf discs of mature (3‐week‐old) plants (Figure S5b).
Phenotypic characterisation of pip5k7 pip5k9 double mutants
No obvious growth or developmental phenotypes were observed in pip5k7 pip5k9 double mutants, on either agar plates or soil (Figure S6a,b), which is in strong contrast to the severe developmental defects reported for pipk5k1 pip5k2 (Ischebeck et al., 2013; Tejos et al., 2014; Marhava et al., 2020). The phenotypic differences between the pip5k7 pip5k9 and pip5k1 pip5k2 double mutants are likely due to local and/or temporal versus sustained changes in PIP2 concentrations, since the basal levels of PIP2 in pip5k7 pip5k9 seedlings were only marginally reduced (Figure 2), while those in pipk5k1 pip5k2 mutants were even very similar to WT (Figure S3a). Since PIP5K7 and PIP5K9 were strongly expressed in the stele, a more detailed analysis of the vascular tissue was performed. While no effect on phloem or xylem differentiation was found (Figure S6c), we did observe defects in the continuity of the veins of the cotyledons. While 2.4% of the WT seedlings contained discontinuous veins, for pip5k7 pip5k9 mutants this increased up to 20%, which seemed to be mainly caused by pip5k9 (24%), with only 10.9% discontinuous veins for pip5k7 (Figure S6d). Overall, the data suggest that the PIP2 formation by PIP5K7 and/or PIP5K9 has only minor roles in basal plant development, but is dynamically induced in response to polyamines.
Spm triggers the PIP2 response at the plasma membrane
To better understand the dynamic changes of PIP2 upon polyamine treatment, the subcellular localisation of the response in vivo was analysed using a fluorescent PIP2 biosensor (ProUBQ10::YFP‐PHPLCδ1) (Van Leeuwen et al., 2007; Simon et al., 2014; Tejos et al., 2014). Confocal imaging was focused on the cortex cells in the transition zone of the root tip because these cells exhibit sufficient contrast of the biosensor signal between the plasma membrane and cytosol and because PIP2 responses are particularly high in that zone (Figure 3i).
At control conditions, most of the biosensor was located in the cytosol (Figure 4a) due to the low basal concentrations of PIP2 in the membrane (van Leeuwen et al., 2007; Vermeer and Munnik, 2013). However, in response to Spm, the biosensor was clearly recruited to the plasma membrane, resulting in pronounced colabelling with FM4‐64 (Figure 4a). By contrast, this response was strongly reduced in the pip5k7 pip5k9 mutant (Figure 4b), confirming the involvement of PIP5K7 and PIP5K9 in the Spm‐induced PIP2 increase at the plasma membrane.
Figure 4.
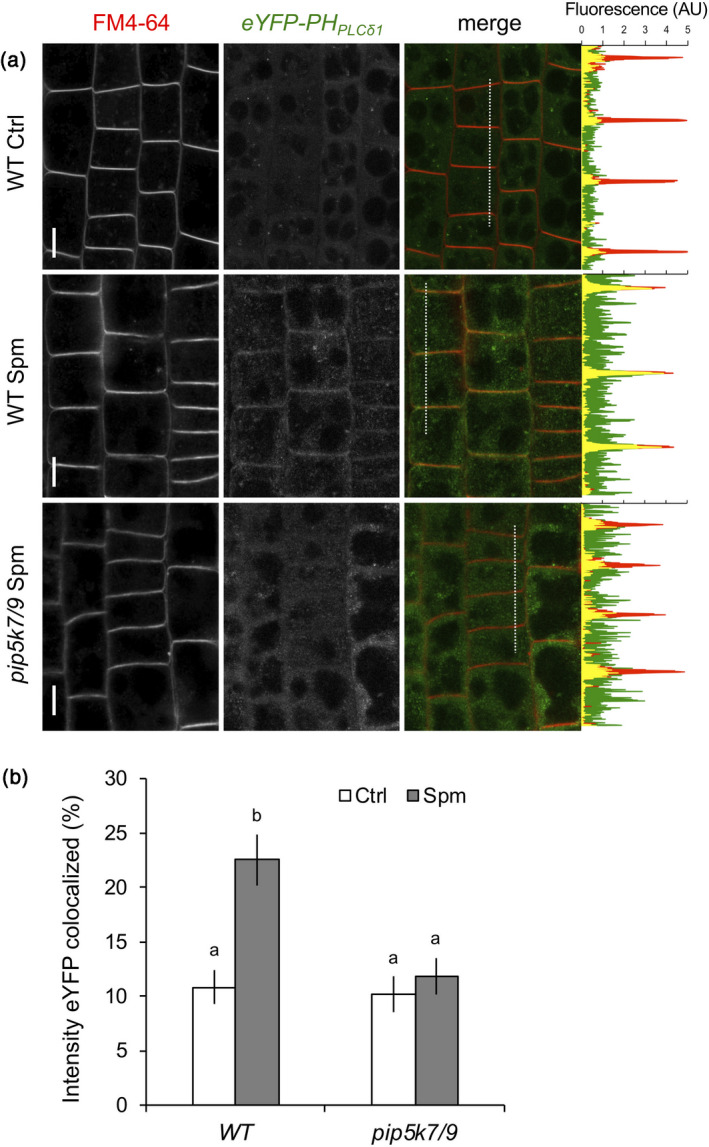
Spm‐induced PIP2 is generated at the plasma membrane.
(a) Confocal images of WT and pip5k7/9 seedlings expressing the PIP2 biosensor ProUBQ10::eYFP‐PHPLCδ1 treated with buffer ± Spm at 30 min. Images show cortex cells in the root transition zone (left) and a representative plot‐profile analysis (right) indicating differences in membrane association (FM4‐64, red) of eYFP‐PHPLCδ1 (green). Units are expressed as arbitrary units (AUs). Bars represent 10 µm. (b) To quantify the colocalisation of eYFP signal with FM4‐64, the entire cell (including plasma membrane) was selected as the region of interest and the percentage of fluorescence intensity colocalisation was determined. Data are presented as the mean ± SD (n = 20).
Spm‐induced K+ efflux requires PIP2 production by PIP5K7 and PIP5K9
Due to their positive charge at physiological and acidic pH, polyamines are known to trigger a depolarisation of the plasma membrane, with a consequent efflux of K+ observed in several plant roots, including pea (Pisum sativum), maize (Zea mays) and Arabidopsis (Pandolfi et al., 2010; Zarza et al., 2019). In mammalian cells, almost all K+ channels are regulated by PIP2 (Dickson and Hille, 2019), and also in plants there are indications that it can regulate K+ transport (Liu et al., 2005; Ma et al., 2009; Wigoda et al., 2010). To investigate a potential link between polyamine‐triggered PIP2 and K+ flux, we used gadolinium (GdCl3), which is an inhibitor of Spm uptake across the plasma membrane (Pistocchi et al., 1988; Ditomaso et al., 1992b; Pottosin and Shabala, 2014). Pre‐treatment of Arabidopsis seedlings with gadolinium blocked the Spm‐induced K+ efflux (Zarza et al., 2019), and inhibited the Spm‐induced PIP2 response (Figure 5a). This indicates that the effect of exogenous Spm on K+ currents and PIP2 may be triggered from the cytosolic side of internalised Spm, consistent with other reports (e.g. Liu et al., 2000). Part of the Spm appears to be transported via resistant to methyl viologen 1 (RMV1), a plasma membrane‐localised Spm uptake transporter (Fujita et al., 2012), since reduced PIP2 responses were observed in Arabidopsis rmv1 mutants and increased PIP2 responses when RMV1 was overexpressed (Figure 5b). These results link polyamine uptake and PIP2 responses.
Figure 5.
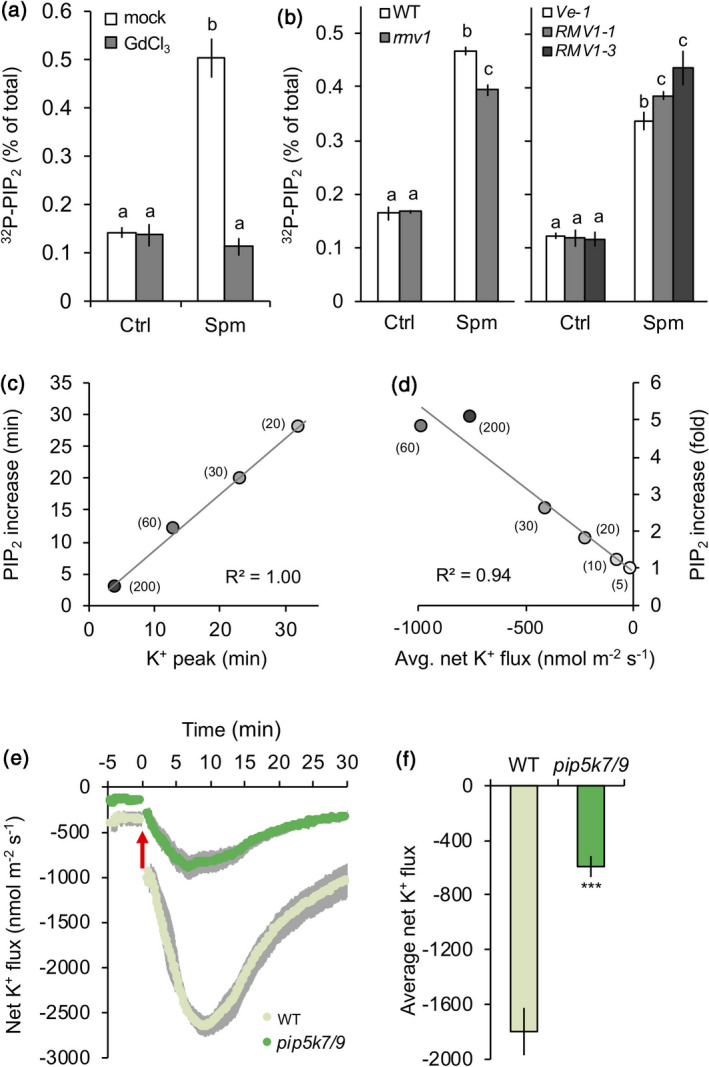
Spm‐induced K+ efflux relies on the formation of PIP2 and Spm transport across the plasma membrane.
(a) The Spm‐induced PIP2 response is gadolinium (GdCl3)‐sensitive. Pre‐treatment with gadolinium, a well‐known polyamine uptake inhibitor, completely blocks the Spm‐induced PIP2 response. 32P‐pre‐labelled WT seedlings were pre‐treated for 60 min with 100 µm GdCl3, washed and then treated with or without 60 µm Spm for 30 min. (b) The polyamine uptake transporter RMV1 is required for a full PIP2 response. WT, rmv1 and two RMV1‐OE lines were 32P‐labelled and tested for their PIP2 response in the absence or presence of 60 µm Spm for 30 min. For both cases, data are presented as the mean ± SD (n = 4). (c) Correlation between K+ efflux peak time and the PIP2 response at different Spm concentrations. (d) Correlation between net K+ efflux and the PIP2 response using different Spm concentrations (t = 30 min). (e) MIFE K+ flux kinetics in WT and pip5k7/9 seedlings when 60 µm Spm was added (red arrow). (f) MIFE average K+ flux in WT and pip5k7/9 mutant plants upon 60 µm Spm treatment. For all MIFE data, data are shown as the mean ± SE (n = 6–7); negative values represent net efflux of ions across the plasma membrane into the apoplast.
As polyamines influenced both changes in PIP2 and K+ flux, we next tested whether pip5k7 pip5k9 mutants were affected in K+ transport. Using non‐invasive microelectrode ion flux measurement (MIFE), Spm was found to induce a dose‐ and time‐dependent efflux of K+ in WT plants (Zarza et al., 2019). Comparing the loss of K+ with the onset of a significant PIP2 accumulation in time (Figure 5c) or with the intensity of the response (fold increase) after 30 min (Figure 5d), strong correlations were observed (R 2 = 1.00 and 0.94, respectively). Importantly, ~70% of the Spm‐triggered K+ efflux response was lost in pip5k7 pip5k9 mutants compared to WT (Figure 5e,f). Together, these results indicate (i) that Spm must be taken up into the cell to exert its function; (ii) that PIP2 is an element of the signalling cascade that links Spm with the efflux of K+ at the plasma membrane; and (iii) that an Spm‐induced increase of PIP2 at the plasma membrane functions upstream of inducing this K+ efflux.
Pip5k7 pip5k9 double mutants display enhanced sensitivity to Spm or KCl
Since the application of polyamines has previously been shown to affect root growth in seedlings (Couée et al., 2004; Jancewicz et al., 2016; Zarza et al., 2019), the effect in pip5k7 pip5k9 mutants was analysed. Five‐day‐old WT and pip5k7 pip5k9 double mutant seedlings were transferred to agar plates containing increasing concentrations of polyamines, and after 4 days, a substantially higher degree of root growth inhibition was observed for the double mutants compared to WT (Figure 6a,b). Due to the high potassium permeability of the plasma membrane (resulting from the presence of membrane channels), the cytoplasmic potassium concentration is very sensitive to changes in the membrane potential (Maathuis and Sanders, 1997). Conversely, when the apoplastic potassium level increases, a large influx of this cation will cause a reduction in the membrane potential, which, if not corrected, can cause deleterious effects on other transporters driven by the membrane potential (Haruta and Sussman, 2012). To test whether the increased sensitivity was related to the decreased capacity of the mutant to maintain K+ homeostasis, we increased the external K+ levels by adding 50 mm KCl to the agar plates. As shown in Figures 6c,d, while WT seedlings showed no apparent phenotype, and pip5k7 pip5k9 mutants showed a hypersensitive response revealed by a clear reduction in root growth, which is a typical response to higher KCl concentrations (Haruta and Sussman, 2012). These results further support a link between PIP2 production by PIP5K7 and PIP5K9 and the regulation of K+ transport.
Figure 6.
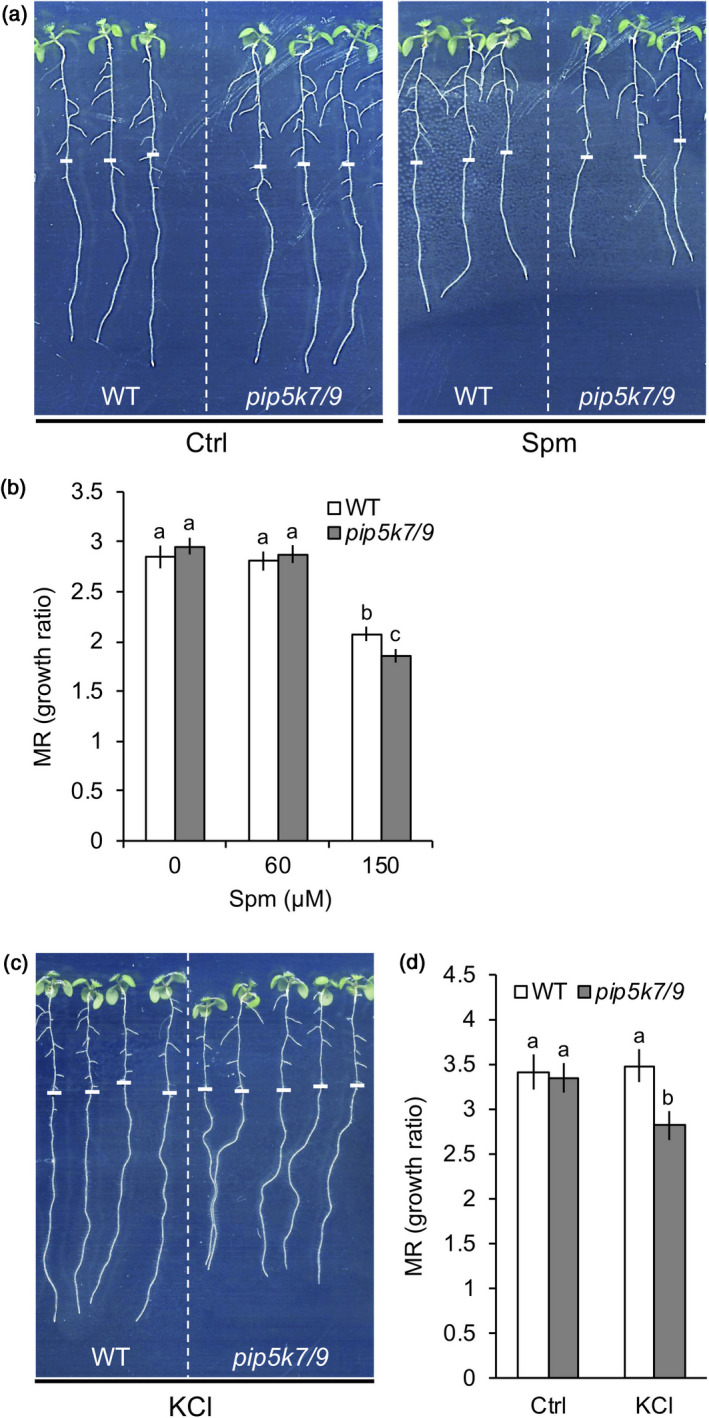
Effects of Spm and KCl on seedling root growth in WT and pip5k7/9 mutant plants.
Five‐day‐old seedlings were transferred to plates supplemented with (a, b) Spm or (c, d) KCl and grown for 4 more days. (a) Phenotype of WT and mutant seedlings grown with and without 150 µm Spm. (b) Effects of Spm on main root (MR) growth of WT and pip5k7/9 seedlings. (c, d) Phenotype of WT and mutant seedlings grown with and without of 50 mm KCl. Results are expressed as the MR growth ratio. Data are presented as the mean ± SD (n = 40). White dashes indicate the position of the root tip when seedlings were transferred.
DISCUSSION
Polyamines are naturally occurring polycationic molecules involved in multiple processes along a cell’s lifespan (Tiburcio et al., 2014; Miller‐Fleming et al., 2015). The elucidation of their mode of action to understand their pleiotropic effects has become a major challenge in biology. Here, we obtained biochemical and genetic evidence (i) that polyamines trigger a rapid PIP2 response in the plasma membrane of root cells through activation of PIP5K7 and PIP5K9 and (ii) that the increase of PIP2 modulates the flux of K+ across the plasma membrane. This polyamine‐induced PIP2 response was time‐, dose‐ and charge‐dependent, where the effects were ordered as follows: Spm4+ = t‐Spm4+ > Spd3+ > Put2+ = Dap2+ (Figures 1 and S1). Together with earlier observations in spinach hypocotyls (Dureja‐Munjal et al., 1992) and coffee cells (Echevarría‐Machado et al., 2005) and ample evidence in animal systems (Coburn, 2009), these results indicate the effect of polyamines on phosphoinositide signalling may have been conserved across kingdoms.
Polyamines are secreted in response to environmental cues such as drought and salt stress and in response to abscisic acid (Moschou et al., 2008b; Toumi et al., 2010). Once in the apoplast, they can be metabolised by polyamine oxidases, generating ROS and triggering downstream effects (Takahashi et al., 2003; Moschou et al., 2008a; Toumi et al., 2010; Pottosin and Shabala, 2014). However, their transport across the plasma membrane of cells distal from where the polyamines were secreted represents another important event (Ditomaso et al., 1992a; Angelini et al., 2010; Campestre et al., 2011; Moschou et al., 2012). The uptake of polyamines is very rapid, reaching saturation at µm concentrations and causing intracellular polyamine concentrations to rise by 10–1000 µm min−1 (Pistocchi et al., 1987, 1988; Ditomaso et al., 1992a; Echevarría‐Machado et al., 2005). In that regard, several polyamine uptake transporters (PUTs) have been recently identified, including RMV1/PUT3, which transports Spm across the plasma membrane (Fujita et al., 2012; Martinis et al., 2016). Our data indicate that RMV1/PUT3 is involved in the Spm uptake route that triggers PIP2 (Figure 5). This pathway is likely redundant as there are several members of the PUT and amino acid‐polyamine‐choline transporter family that could transport polyamines (Verrey et al., 2004; Rentsch et al., 2007; Mulangi et al., 2012; Fujita and Shinozaki, 2014), as well as other types, including the nitrogen transporter family (Tong et al., 2016).
Importantly, Spm concentrations found to be effective in this work (low µm range) are significantly lower than the basal polyamine levels in plant cells (Galston and Kaur‐Sawhney, 1995), and much lower than concentrations frequently used in other studies, where typically high µm/low mm concentrations have been used (Sagor et al., 2015; Marco et al., 2019; Tajti et al., 2019). In our hands, the effective concentration of Spm to induce PIP2 revealed a clear saturation response at low µm concentrations (Figure 1) and correlated with the dose‐dependent inhibition of root growth (Figure 6; Zarza et al., 2019). Interestingly, PIP2 hyperaccumulation and root growth inhibition are also observed in the Arabidopsis sac9 mutant, a 5‐PTase involved in PIP2 degradation (Williams et al., 2005), and in seedlings where PIP2 synthesis was boosted by overexpression of a human PIP5K (Im et al., 2014; Gujas et al., 2017).
In contrast to animal systems, phosphorylation of PI4P at the 5‐position of the inositol ring is the only route to synthesise PIP2 in plants (Munnik and Testerink, 2009, Munnik and Vermeer, 2010; Heilmann, 2016a). Radiolabelling and pulse‐chase experiments (Figures 1 and 2) indicated that the increase in PIP2 upon polyamine treatment was caused by PIP5K activation, and this was confirmed by a complementary reverse‐genetics approach, which identified PIP5K7 and PIP5K9 as the main enzymes involved. Expression of the PIP5K7 and PIP5K9 genes was also induced upon polyamine treatment (Figure 3); however, the rapid formation of PIP2 within minutes is more in favour of a post‐translational mode of activation, for which the mechanism is still unknown. PIP5K activity can be regulated by protein phosphorylation (Westergren et al., 2001; Hempel et al., 2017; Menzel et al., 2019). Checking the PhosPhAt database (Durek et al., 2010) revealed that PIP5K7 could be phosphorylated on serine‐224, but information on PIP5K9 is lacking. In principle, polyamines could increase PIP5K activity by interacting directly with the kinase and ATP‐Mg2+ (Meksuriyen et al., 1998). In vitro, human PIP5K can be activated by Spm and Spd (Bazenet et al., 1990; Singh et al., 1995; Chen et al., 1998), but whether this is also true for plant enzymes is unknown. Why this would be specific for PIP5K7 and PIP5K9 and leave other PIP5Ks expressed in the same tissues (e.g. PIP5K1–4) unaffected is also unknown. Indirect effects of polyamines could occur, for example, by modulating protein effectors such as 14‐3‐3 proteins (Athwal and Huber, 2002; Garufi et al., 2007) or stimulating Ca2+ influx (Takahashi et al., 2003). In animal cells polyamines can also regulate the activity of Rho‐kinase (Rao et al., 2003), which can stimulate PIP5K activity too (Oude Weernink et al., 2000). Plant PIP5Ks may be regulated differently, however, as indicated by the multiple Membrane Occupation and Recognition Nexus (MORN) motifs that are present in PIP5K1–9 from Arabidopsis, but lacking in animal and yeast PIP5Ks (Ma et al., 2006). Part of the increase in PIP5K activity may additionally be explained by the upregulation of PIP5K7 and PIP5K9 expression after prolonged Spm stimulation (Figure 3i,j). However, the rapid and specific activation of PIP5K7 and PIP5K9 is likely a more direct response to polyamines, possibly via a rapid post‐translational modification, as has been suggested previously for other plant PIP5Ks (Westergren et al., 2001; Heilmann and Heilmann, 2015; Heilmann, 2016b).
While PIP5K7 and PIP5K9 appear to be expressed in both shoot and root vasculature (Figure 3), the Spm‐induced PIP2 response was only observed in the root, with the highest activity near the tip (Figure 3k). This pattern suggests differences in perception and/or transport of Spm, or in the signalling pathway linking polyamines with the PIP2 increase in these tissues. Interestingly, the endogenous synthesis of Spm by spermine synthase (SPMS) and of tSpm by ACAULIS5 (ACL5) is also restricted to the vasculature, that is, phloem and xylem, respectively (Vera‐Sirera et al., 2015; Zarza et al., 2019). Indeed, plants with increased endogenous levels of Spm by overexpressing SPMS (Sagor et al., 2013) contained significantly higher basal amounts of PIP2 (Figure S7), supporting the idea of an intrinsic SPMS/PIP5K module that controls developmental aspects of root formation or root responses to environmental stresses. This is also supported by the fact that the Arabidopsis acl5 mutant is heavily perturbed in xylem differentiation (Muñiz et al., 2008) and that overexpression of human PIP5K in Arabidopsis seedlings caused severe defects in phloem and xylem differentiation (Gujas et al., 2017). Regulation of protophloem differentiation and vascular development is also known to involve phosphoinositides (Carland and Nelson, 2004; Carland and Nelson, 2009; Rodriguez‐Villalon et al., 2015; Gujas et al., 2017; Marhava et al., 2020; Figure S6).
While pip5k7 pip5k9 mutants displayed subtle defects in vein continuity in cotyledons, no macroscopic plant phenotypes were observed (Figure S6), which is in huge contrast to the dramatic root and developmental phenotypes found in pip5k1 pip5k2 mutants (Ischebeck et al., 2013; Tejos et al., 2014), root hair phenotypes found in pip5k3 and pip5k4 (Stenzel et al., 2008; Kusano et al., 2008a; Wada et al., 2015) and developmental disorders found in pollen tubes in pip5k4–6 and pip5k10–11 mutants (Sousa et al., 2008; Ischebeck et al., 2008; Zhao et al., 2010; Ischebeck et al., 2011). The fact that flowering plants have so many specific PIP5Ks and PIP2‐dependent cellular responses despite their low PIP2 levels is fascinating in itself. Evidently, PIP2 increases will be very transient and/or localised, and may be triggered by upstream signalling pathways according to particular cellular requirements. Advanced imaging of Arabidopsis cells of the root transition zone showed that Spm triggered the recruitment of the PIP2 biosensor to the plasma membrane, and that this recruitment was strongly reduced in the pip5k7 pip5k9 double mutant (Figure 4). Salt and heat stress also induced the accumulation of PIP2 at the plasma membrane (van Leeuwen et al., 2007; König et al., 2008; Mishkind et al., 2009; Simon et al., 2014; Menzel et al., 2019), suggesting a potential link to polyamines in stress conditions at which polyamines have also been shown to increase.
So far, the function of these dynamic stress‐induced PIP2 responses at the plasma membrane remains unclear, even though a role for clathrin‐mediated endocytosis has been proposed (König et al., 2008; Zhao et al., 2010; Mei et al., 2012; Ischebeck et al., 2013; Tejos et al., 2014; Menzel et al., 2019). Studies on tip‐growing cells suggested that PIP2 does not freely diffuse from its site of production, but may be channelled toward specific downstream effectors by processes depending on the interaction of PIP5K with these targets (Saavedra et al., 2012; Stenzel et al., 2012; Heilmann and Heilmann, 2013; Tejos et al., 2014). This channelling hypothesis is consistent with the notion that PIP2 colocalises with PIP5Ks in pollen tubes (Sousa et al., 2008; Ischebeck et al., 2008; Zhao et al., 2010; Ischebeck et al., 2011; Stenzel et al., 2012; Ugalde et al., 2016), root hairs and certain root cells (Stenzel et al., 2008; Thole et al., 2008; Kusano et al., 2008a; Vermeer et al., 2009; Tejos et al., 2014). In that sense, it is tempting to speculate that the accumulation of PIP2 at the plasma membrane in response to polyamines results from plasma membrane‐localised PIP5K7 and PIP5K9. Transient expression of PIP5K9‐GFP in tobacco (Nicotiana benthamiana) mesophyll cells and onion epidermal cells revealed fluorescence at the plasma membrane and nucleus (Lou et al., 2007). However, we never observed nuclear accumulation of the PIP2 biosensor upon Spm treatment, while we did observe this after prolonged heat stress (Mishkind et al., 2009). Arabidopsis PIP5Ks can contain functional nuclear localisation sequences (Gerth et al., 2017b), so it is possible that dynamic re‐localisation of PIP5Ks between cytoplasm, plasma membrane, nucleus and possibly other subcellular locations may occur to meet specific cellular requirements. So far, the complexity of plant PIP2 distribution and its functions are just emerging, and even the data available must be reviewed with caution, because different model systems have been used, including heterologous expression in different plant species, transient expression in cultured cells and stable expression in transgenic Arabidopsis plants.
Polyamine uptake in plant roots cause the plasma membrane to depolarise (Ozawa et al., 2010; Pottosin et al., 2014), resulting in a net efflux of K+ (Pandolfi et al., 2010; Zepeda‐Jazo et al., 2011). Besides its important role as counter‐ion for keeping the membrane potential in the cellular compartments (Dreyer and Uozumi, 2011), K+ is also a major cation that osmotically steers various turgor‐driven processes (Maathuis, 2009). In that sense, both K+ and polyamines have been speculated to play similar physiological roles, acting as a mutual counter‐balance, for a long time (Richards and Coleman, 1952). Using MIFE, a strong and transient efflux of K+ from epidermal cells of the root elongation zone was found upon Spm induction (Figure 5e; Zarza et al., 2019). The imbalance of the homeostatic‐K+ control caused by the efflux of K+ may lead to multiple downstream effects due to potassium’s capacity to activate K+‐sensitive enzymes (Marschner, 1995; Wu et al., 2018), or to function as a signalling agent itself (Demidchik, 2014; Shabala, 2017). Importantly, the loss of K+ during 30 min Spm treatment strongly coincided in time and intensity with the PIP2 response (Figure 5c,d). A causal effect of PIP2 on K+ fluxes can also be deduced from the observation that pip5k7 pip5k9 seedlings exhibited a lower basal K+ efflux than WT at control conditions before the application of Spm (Figure 5e). This difference became much more pronounced upon Spm treatment, where ~70% less K+ was excluded from pip5k7 pip5k9 than from WT (Figure 5e,f). Mutant seedlings were also more sensitive to Spm and KCl on plate growth assays (Figure 6), supporting a role for PIP5K7 and PIP5K9 in K+ homeostasis and placing PIP2 upstream of K+ flux, and providing a link with the inhibitory effect of Spm on root growth. In agreement, the tetraamine‐depleted acl5 spms double mutant is hypersensitive to KCl (Yamaguchi et al., 2006), reinforcing the idea of a polyamine/PIP2 module. In animal cells, most K+ channels are regulated by PIP2 (Dickson and Hille, 2019), and there are some indications for plants as well (Liu et al., 2005; Ma et al., 2009; Wigoda et al., 2010); here, genetic evidence for such a model is provided. An important next step will be to identify the K+ channels involved and whether the effect of PIP2 is direct or indirect. For example, the clustering of the Arabidopsis K+ channel GORK, which parallels its gating activity (Eisenach et al., 2014), could be PIP2‐dependent, or the suppression of the inward rectifier KAT1 (Eisenach et al., 2014), by removing it from the plasma membrane.
Future research on how PIP5K7 and PIP5K9 are activated, together with advanced electrophysiology experiments on pip5k7 pip5k9 mutants and polyamine transporters, should provide further knowledge on the molecular mechanisms that underlie these newly identified cellular and physiological responses.
EXPERIMENTAL PROCEDURES
Plant material and growth conditions
Arabidopsis thaliana pip5k7‐1 (SALK_151429), pip5k7‐3 (SALK_107796), pip5k8‐2 (SALK_040022) and pip5k9c (SALK_013602) T‐DNA insertion mutants and plants with the transposon allele pip5k9a (SM_3_39157) were obtained from the Nottingham Arabidopsis Stock Centre. Arabidopsis rmv1, pip5k1 pip5k2, pip5k3‐2, pip5k3‐4, pip5k4 pip5k5, pip5k6 and pip5k10 pip5k11 mutant null alleles and Pro35S::RMV1, Pro35S::SPMS‐1, Pro35S::SPMS‐15 and ProUBQ10::YFP‐PHPLCδ1 transgenic lines were described previously (Ischebeck et al., 2008; Kusano et al., 2008a; Zhao et al., 2010; Ischebeck et al., 2011; Fujita et al., 2012; Sagor et al., 2013; Ischebeck et al., 2013; Simon et al., 2014). The pip5k7/9 double mutant was obtained by crossing pip5k7‐1 with pip5k9c. The pip5k7/9 line was, in turn, crossed into a ProUBQ10:YFP‐PHPLCδ1 line for confocal studies. In most cases, A. thaliana ecotype Col‐0 was used as WT, except for the rmv1 and Pro35S::RMV1 lines, in which Ler ecotype and Col‐0 empty vector, Ve‐1, were used as WTs, respectively.
Seeds were surface‐sterilised using chlorine gas and sown under sterile conditions on square Petri dishes containing standard growth medium consisting of ½ MS medium with Gamborg B5 vitamins (pH 5.7; KOH), 1% (w/v) sucrose and 1% (w/v) agar. Plates were vernalised (4°C, 48 h) and placed vertically, under an angle of 70° in a growth chamber (16 h/8 h light/dark cycle, 110–130 µmol m−2 sec−1) at 22°C. Five‐day‐old seedlings were transferred to either 2 ml safe‐lock Eppendorf tubes for 32Pi‐labelling experiments or to small round Petri dishes for incubations with polyamines and chemicals. Glass was avoided since polyamines tend to stick to glass surfaces. For gene expression analyses, seeds were germinated on agar plates containing a nylon mesh (Ø 43 μm) to facilitate the transfer of the seedlings. Mature plants were grown on soil, under the same light and temperature regime as above.
Identification of pip5k7, pip5k9 and pip5k7/9 double mutants
Genotyping of pip5k7‐1, pip5k7‐3, pip5k9a, pip5k9c and pip5k7‐1/pip5k9c and isolation of homozygous mutant lines was performed by PCR using a combination of gene‐ and T‐DNA‐specific (SALK‐LB) primers (Table S1). For gene expression analyses, RNA from WT and mutants was extracted using TRIzol reagent (Invitrogen, Waltham, MA, USA) and treated with Turbo DNase (Ambion, Waltham, MA, USA). cDNA was synthesised using the RevertAid Synthesis Kit (Fermentas, Waltham, MA, USA), and transcript levels of AtPIP5K7 and AtPIP5K9 were determined by semi‐quantitative RT‐PCR using gene‐specific primers (Table S1) and the following PCR conditions: 95°C 5 min, followed by 40 cycles of 95°C 30 sec, 52°C 30 sec and 72°C 2 min and a final elongation step at 72°C 6 min. SAND (AT2G28390) was used as a reference gene since it was shown to be very consistently expressed under various conditions (Hong et al., 2010).
Reporter constructs
Promoter–GUS fusion constructs were generated as described previously (Stenzel et al., 2008). In brief, the GUSPlus gene was amplified from pCAMBIA1305.1 (AF354045) using primers described in Table S1, and the PCR product was introduced as NotI‐SacI fragment into pGreen0029, yielding pGreenGUSPlus. As promoters, 1500 bp genomic sequences upstream of coding sequences of PIP5K7 or PIP5K9 were amplified from BAC clones T19D16 and F8A24, respectively, using primers described in Table S1. PCR products were moved directionally as SalI‐NotI fragments into pGreenGUSPlus, and resulting plasmids transformed were into Agrobacterium tumefaciens strain EHA105 for transformation into Arabidopsis.
32Pi‐phospholipid labelling, extraction and analysis
Phospholipid responses were measured as described earlier (Munnik and Zarza, 2013). Briefly, three seedlings per sample were metabolically labelled overnight in 2 ml Eppendorf safe‐lock tubes containing 200 µl incubation buffer (2.5 mm MES‐KOH, pH 5.7, 1 mm KCl) and 2.5–10 µCi 32PO4 3− (1 µl stock 32Pi; carrier‐free, 10 µCi µl−1; Perkin‐Elmer), in continuous light. For mature plants, leaf discs (∅ 5 mm) were taken from 3‐week‐old plants and labelled using the same conditions. Treatments started by adding 200 µl of a 2× solution and were stopped by adding 5% (v/v) perchloric acid. In general, treatments of 30 min and 60 µm Spm were used, unless indicated otherwise. Lipids were extracted and analysed by thin‐layer chromatography using an alkaline solvent (Munnik et al., 1994) or a water‐saturated ethyl acetate solvent system for PA analyses (Munnik and Laxalt, 2013). Radioactivity was visualised by autoradiography and quantified by phosphoimaging (Typhoon FLA 7000; GE Healthcare, Chicago, IL, USA).
For certain experiments, a modified 32Pi‐labelling protocol was used: (i) for short‐labelling experiments, 32Pi was added 30 min prior to treatment; (ii) for pulse‐chase experiments, seedlings were first treated with Spm for 15 or 30 min and then labelled with 32Pi for 5 min, after which 1 mm Pi buffer (K2HPO4/KH2PO4, pH 5.7) was added in the presence or absence of Spm; (iii) for tissue dissection experiments, seedlings were treated and fixed as described above, after which they were carefully cut into sections with a scalpel and every section was extracted separately.
GUS analysis
GUS staining was performed as described in Depuydt et al. (2013). Briefly, seedlings were incubated for 15 min in GUS staining buffer and mounted in chloral hydrate for immediate visualisation with a Leica compound microscope. For root cross‐sections, GUS‐stained seedlings were fixed and embedded in HistoresinTM (Leica Instruments GmbH; Wetzlar, Germany) and sectioned on a Leica microtome (Rodriguez‐Villalon et al., 2014). Sections were mounted in water and visualised with a 40× magnification objective of a compound microscope.
Quantitative real‐time PCR gene expression analysis
For gene expression analysis, WT seedlings were grown on a nylon mesh on top of normal agar medium plates. After 5 days, seedlings were transferred via nylon mesh to Petri dishes containing incubation buffer and left for 2 h to recover. Seedlings were then treated for indicated times; samples were collected of ~50 seedlings in 2 ml safe‐lock Eppendorf tubes and immediately frozen in liquid nitrogen. Total RNA extraction and cDNA synthesis were performed, as indicated previously. qRT‐PCR was performed using HOT FIREPol EvaGreen qPCR mix Plus (ROX) (Solis Biodyne, Tartu, Estonia) and the ABI 7500 Real‐Time PCR system (Applied Biosystems, Waltham, MA, USA). The following PCR conditions were used: 50°C 2 min, 95°C 15 min, followed by 45 cycles of 95°C 15 sec and 60°C 1 min. Primers used for gene expression analyses are listed in Table S1. qRT‐PCR analyses were performed on three biological replicates with two technical replicates, using two reference genes, namely SAND (At2g28390) and EXPRS (At2g32170), which were shown to be very consistently expressed in different conditions by Hong et al. (2010).
Confocal laser scanning microscopy
Arabidopsis transgenic lines containing the ProUbi10:YFP‐PHPLCδ1 construct were grown for 5 days and then transferred for 30 min to small Petri dishes containing different treatments. Seedlings were then rinsed briefly with buffer, stained for 5 min with 2 µm FM4‐64 (Invitrogen), rinsed again twice with buffer and then mounted on a microscopy slide for analysis with a Zeiss LSM510 confocal microscope. eYFP and FM4‐64 were synchronously excited at 488 nm and 561 nm, respectively, and imaged using an HFT 405/488/561 nm major beam splitter and a 505 to 550 nm band‐pass filter and a 650 nm long‐pass filter, respectively. Images were converted to 8‐bit in ImageJ (www.imagej.net) for better visualisation of the eYFP and FM4‐64 signals. Plot profile analysis was performed and the region of interest based on single cells was selected for colocalisation measurements in ImageJ.
Ion flux measurement
Net K+ flux was measured using non‐invasive MIFE (Shabala et al., 2006; Zarza et al., 2019). Five‐day‐old seedlings were immobilised in a 30 ml measuring chamber containing basic salt medium (BSM; 0.5 mm KCl, 0.2 mm CaCl2, 5 mm MES, 2 mm Tris base, pH 6.0). Roots were immobilised in a horizontal position (Bose et al., 2014) and pre‐incubated in BSM for at least 30 min. Electrodes were positioned 40 µm from the root surface in the elongation zone (less than 2 mm from the root cap junction). First, steady‐state ion fluxes were recorded over a period of 5 min; thereafter Spm was added and the net ion flux was measured for another 30 min.
Root phenotyping assay on plates
Arabidopsis seedlings were grown on vertical plates containing standard sterile growth medium for 5 days and then transferred to medium supplemented with KCl or 0.22 µm filter‐sterilised Spm. Plates were scanned 4 days after transfer (4 DAT) using an Epson Perfection V700 Scanner at 300 dpi resolution. Root measurements were performed using EZ‐Rhizo software (Armengaud et al., 2009). Main root growth was expressed as growth ratio (MRlength/MRlength at 0 DAT).
Statistical analysis
SPSS was used for statistical analysis. For paired comparisons, the Student–Newman–Keuls test at P < 0.05 was used, were different letters indicate significantly different values. Student’s t‐test was used in comparisons with control treatments, where asterisks indicate significant differences: *P < 0.05, **P < 0.01, ***P < 0.005. Data shown represent the mean ± SD. The results obtained were confirmed by at least three independent experiments unless otherwise indicated.
ACCESSION NUMBERS
PIP5K7 (At1g10900); PIP5K9 (At3g09920); SAND (At3g28390); EXPRS (At2g32170).
AUTHOR CONTRIBUTIONS
XZ, SS, ARV and TM designed the experiments. LS performed MIFE analyses, AH GUS cross‐sectioning, ML phenotyping and XZ the rest, with assistance of RvW. ARV, AT, SS and IH added materials, ideas and discussions. XZ, IH and TM wrote the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interests.
Supporting information
Table S1. Oligonucleotides used in this work.
Figure S1. Spm‐induced PIP2 response is not caused by breakdown products of Spm.
Figure S2. PA and PIP2 responses occur simultaneously.
Figure S3. Spm‐induced PIP2 responses in Arabidopsis PIP5K T‐DNA insertion mutants.
Figure S4. PIP2 levels in the pldδ mutant.
Figure S5. PIP2 levels in response to Spm in seedlings and mature leaves.
Figure S6. No obvious phenotypes are apparent in pip5k7 pip5k9 mutants.
Figure S7. PIP2 levels are higher in SPMS overexpression lines.
Data S1. Supplementary methods.
ACKNOWLEDGEMENTS
We thank Ludek Tikovsky and Harold Lemereis for their assistance in the greenhouse, Dr. Dorus Gadella jr. for discussion and confocal microscopy support, Dr. T. Takahashi (Okayama University, Japan) for supplying tSpm, Dr. Igor Pottosin (University of Colima, Mexico) for discussion on MIFE and Dr. Michel Haring for early comments on the manuscript. This work was supported by the Spanish Ministerio de Ciencia e Innovación (BIO2011‐29683 & CSD2007‐00036 to XZ), the Generalitat de Catalunya (SGR2009‐1060 & BE DGR 2011 to XZ), the Swiss National Foundation (SNF_31003A_160201 to ARV), the Australian Research Council (LS & SS) and the Netherlands Organisation for Scientific Research (NWO 867.15.020 to TM).
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in the article and its supplementary information files. Plasmids and mutants are available upon request.
REFERENCES
- Angelini, R. , Cona, A. , Federico, R. , Fincato, P. , Tavladoraki, P. and Tisi, A. (2010) Plant amine oxidases “on the move”: An update. Plant Physiol. Biochem. 48, 560–564. [DOI] [PubMed] [Google Scholar]
- Anschütz, U. , Becker, D. and Shabala, S. (2014) Going beyond nutrition: Regulation of potassium homeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 171, 670–687. [DOI] [PubMed] [Google Scholar]
- Arisz, S.A. and Munnik, T. (2013) Distinguishing phosphatidic acid pools from de novo synthesis, PLD, and DGK. Methods Mol. Biol. 1009, 55–62. [DOI] [PubMed] [Google Scholar]
- Armengaud, P. , Zambaux, K. , Hills, A. , Sulpice, R. , Pattison, R.J. , Blatt, M.R. and Amtmann, A. (2009) EZ‐Rhizo: integrated software for the fast and accurate measurement of root system architecture. Plant J. 57, 945–956. [DOI] [PubMed] [Google Scholar]
- Athwal, G.S. and Huber, S.C. (2002) Divalent cations and polyamines bind to loop 8 of 14‐3‐3 proteins, modulating their interaction with phosphorylated nitrate reductase. Plant J. 29, 119–129. [DOI] [PubMed] [Google Scholar]
- Bauby, H. , Divol, F. , Truernit, E. , Grandjean, O. and Palauqui, J.‐C. (2007) Protophloem differentiation in early Arabidopsis thaliana development. Plant Cell Physiol. 48, 97–109. [DOI] [PubMed] [Google Scholar]
- Bazenet, C.E. , Ruano, A.R. , Brockman, J.L. and Anderson, R.A. (1990) The human erythrocyte contains two forms of phosphatidylinositol‐4‐phosphate 5‐kinase which are differentially active toward membranes. J. Biol. Chem. 265, 18012–18022. [PubMed] [Google Scholar]
- Bitrián, M. , Zarza, X. , Altabella, T. , Tiburcio, A.F. and Alcázar, R. (2012) Polyamines under abiotic stress: metabolic crossroads and hormonal crosstalks in plants. Metabolites, 2, 516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose, J. , Shabala, L. , Pottosin, I. , Zeng, F. , Velarde‐Buendía, A.‐M. , Massart, A. , Poschenrieder, C. , Hariadi, Y. and Shabala, S. (2014) Kinetics of xylem loading, membrane potential maintenance, and sensitivity of K(+) ‐permeable channels to reactive oxygen species: physiological traits that differentiate salinity tolerance between pea and barley. Plant Cell Environ. 37, 589–600. [DOI] [PubMed] [Google Scholar]
- Campestre, M.P. , Bordenave, C.D. , Origone, A.C. , Menéndez, A.B. , Ruiz, O.A. , Rodríguez, A.A. and Maiale, S.J. (2011) Polyamine catabolism is involved in response to salt stress in soybean hypocotyls. J. Plant Physiol. 168, 1234–1240. [DOI] [PubMed] [Google Scholar]
- Carland, F.M. and Nelson, T. (2004) Cotyledon vascular pattern2‐mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs. Plant Cell, 16, 1263–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carland, F. and Nelson, T. (2009) CVP2‐ and CVL1‐mediated phosphoinositide signaling as a regulator of the ARF GAP SFC/VAN3 in establishment of foliar vein patterns. Plant J. 59, 895–907. [DOI] [PubMed] [Google Scholar]
- Chai, H. , Guo, J. , Zhong, Y. , Hsu, C.C. , Zou, C. , Wang, P. , Zhu, J.K. and Shi, H. (2020) The plasma‐membrane polyamine transporter PUT3 is regulated by the Na+/H+ antiporter SOS1 and protein kinase SOS2. New Phytol. 226, 785–797. [DOI] [PubMed] [Google Scholar]
- Chen, H. , Baron, C.B. , Griffiths, T. , Greeley, P. and Coburn, R.F. (1998) Effects of polyamines and calcium and sodium ions on smooth muscle cytoskeleton‐associated phosphatidylinositol (4)‐phosphate 5‐kinase. J. Cell. Physiol. 177, 161–173. [DOI] [PubMed] [Google Scholar]
- Coburn, R.F. (2009) Polyamine effects on cell function: possible central role of plasma membrane PI(4,5)P2 . J. Cell. Physiol. 221, 544–551. [DOI] [PubMed] [Google Scholar]
- Colin, L.A. and Jaillais, Y. (2020) Phospholipids across scales: lipid patterns and plant development. Curr. Opin. Plant Biol. 53, 1–9. 10.1016/j.pbi.2019.08.007 [DOI] [PubMed] [Google Scholar]
- Couée, I. , Hummel, I. , Sulmon, C. , Gouesbet, G. and El Amrani, A. (2004) Involvement of polyamines in root development. Plant Cell Tissue Organ Cult. 76, 1–10. [Google Scholar]
- Demidchik, V. (2014) Mechanisms and physiological roles of K+ efflux from root cells. J. Plant Physiol. 171, 696–707. [DOI] [PubMed] [Google Scholar]
- Depuydt, S. , Rodriguez‐Villalon, A. , Santuari, L. , Wyser‐Rmili, C. , Ragni, L. and Hardtke, C.S. (2013) Suppression of Arabidopsis protophloem differentiation and root meristem growth by CLE45 requires the receptor‐like kinase BAM3. Proc. Natl. Acad. Sci. USA, 110, 7074–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWald, D.B. , Torabinejad, J. , Jones, C.A. , Shope, J.C. , Cangelosi, A.R. , Thompson, J.E. , Prestwich, G.D. and Hama, H. (2001) Rapid accumulation of phosphatidylinositol 4,5‐bisphosphate and inositol 1,4,5‐trisphosphate correlates with calcium mobilization in salt‐stressed Arabidopsis. Plant Physiol. 126, 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, E.J. and Hille, B. (2019) Understanding phosphoinositides: rare, dynamic, and essential membrane phospholipids. Biochem. J. 476, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditomaso, J.M. , Hart, J.J. and Kochian, L.V. (1992a) Transport kinetics and metabolism of exogenously applied putrescine in roots of intact maize seedlings. Plant Physiol. 98, 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditomaso, J.M. , Hart, J.J. , Linscott, D.L. and Kochian, L.V. (1992b) Effect of inorganic cations and metabolic inhibitors on putrescine transport in roots of intact maize seedlings. Plant Physiol. 99, 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer, I. and Uozumi, N. (2011) Potassium channels in plant cells. FEBS J. 278, 4293–4303. [DOI] [PubMed] [Google Scholar]
- Dureja‐Munjal, I. , Acharya, M.K. and Guha‐Mukherjee, S. (1992) Effect of hormones and spermidine on the turnover of inositolphospholipids in Brassica seedlings. Phytochemistry, 31, 1161–1163. [Google Scholar]
- Durek, P. , Schmidt, R. , Heazlewood, J.L. , Jones, A. , MacLean, D. , Nagel, A. , Kersten, B. and Schulze, W.X. (2010) PhosPhAt: the Arabidopsis thaliana phosphorylation site database. An update. Nucleic Acids Res. 38(suppl_1), D828–D834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarría‐Machado, I. , Ramos‐Díaz, A. , Brito‐Argáez, L. , Racagni‐Di Palma, G. , Loyola‐Vargas, V.M. and Hernández‐Sotomayor, S.M.T. (2005) Polyamines modify the components of phospholipids‐based signal transduction pathway in Coffea arabica L. cells. Plant Physiol. Biochem. 43, 874–881. [DOI] [PubMed] [Google Scholar]
- Eisenach, C. , Papanatsiou, M. , Hillert, E.K. and Blatt, M.R. (2014) Clustering of the K+ channel GORK of Arabidopsis parallels its gating by extracellular K+ . Plant J. 78, 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, M. and Shinozaki, K. (2014) Identification of polyamine transporters in plants: Paraquat transport provides crucial clues. Plant Cell Physiol. 55, 855–861. [DOI] [PubMed] [Google Scholar]
- Fujita, M. , Fujita, Y. , Iuchi, S. , Yamada, K. , Kobayashi, Y. , Urano, K. , Kobayashi, M. , Yamaguchi‐Shinozaki, K. and Shinozaki, K. (2012) Natural variation in a polyamine transporter determines paraquat tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA, 109, 6343–6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galston, A.W. and Kaur‐Sawhney, R. (1995). Polyamines as endogenous growth regulators In Plant Hormones(Davies P.J. ed.). Dordrecht: Springer, Netherlands, pp. 158–178. [Google Scholar]
- Garufi, A. , Visconti, S. , Camoni, L. and Aducci, P. (2007) Polyamines as physiological regulators of 14‐3‐3 interaction with the plant plasma membrane H+‐ATPase. Plant Cell Physiol. 48, 434–440. [DOI] [PubMed] [Google Scholar]
- Gerth, K. , Lin, F. , Menzel, W. , Krishnamoorthy, P. , Stenzel, I. , Heilmann, M. and Heilmann, I. (2017a) Guilt by association: a phenotype‐based view of the plant phosphoinositide network. Annu. Rev. Plant Biol. 68, 349–374. [DOI] [PubMed] [Google Scholar]
- Gerth, K. , Lin, F. , Daamen, F. , Menzel, W. , Heinrich, F. and Heilmann, M. (2017b) Arabidopsis phosphatidylinositol 4‐phosphate 5‐kinase 2 contains a functional nuclear localization sequence and interacts with alpha‐importins. Plant J. 92, 862–878. [DOI] [PubMed] [Google Scholar]
- Gujas, B. , Cruz, T.M.D. , Kastanaki, E. , Vermeer, J.E.M. , Munnik, T. and Rodriguez‐Villalon, A. (2017) Perturbing phosphoinositide homeostasis oppositely affects vascular differentiation in Arabidopsis thaliana roots. Development, 144, 3578–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta, M. and Sussman, M.R. (2012) The effect of a genetically reduced plasma membrane protonmotive force on vegetative growth of Arabidopsis. Plant Physiol. 158, 1158–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann, I. (2016a) Plant phosphoinositide signaling ‐ dynamics on demand. Biochim. Biophys. Acta, 1861, 1345–1351. [DOI] [PubMed] [Google Scholar]
- Heilmann, I. (2016b) Phosphoinositide signaling in plant development. Development, 143, 2044–2055. [DOI] [PubMed] [Google Scholar]
- Heilmann, M. and Heilmann, I. (2013) Arranged marriage in lipid signalling? The limited choices of PtdIns(4,5)P2 in finding the right partner. Plant Biol. (Stuttg), 15, 789–797. [DOI] [PubMed] [Google Scholar]
- Heilmann, M. and Heilmann, I. (2015) Plant phosphoinositides‐complex networks controlling growth and adaptation. Biochim. Biophys. Acta, 1851, 759–769. [DOI] [PubMed] [Google Scholar]
- Heilmann, I. , Perera, I.Y. , Gross, W. and Boss, W.F. (2001) Plasma membrane phosphatidylinositol 4,5‐bisphosphate levels decrease with time in culture. Plant Physiol. 126, 1507–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel, F. , Stenzel, I. , Heilmann, M. et al (2017) MAPKs influence pollen tube growth by controlling the formation of phosphatidylinositol 4,5‐bisphosphate in an apical plasma membrane domain. Plant Cell, 29, 3030–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, S.M. , Bahn, S.C. , Lyu, A. , Jung, H.S. and Ahn, J.H. (2010) Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant Cell Physiol. 51, 1694–1706. [DOI] [PubMed] [Google Scholar]
- Hong, Y. , Zhao, J. , Guo, L. , Kim, S.‐C. , Deng, X. , Wang, G. , Zhang, G. , Li, M. and Wang, X. (2016) Plant phospholipases D and C and their diverse functions in stress responses. Prog. Lipid Res. 62, 55–74. [DOI] [PubMed] [Google Scholar]
- Im, Y. , Smith, C. , Phillippy, B. , Strand, D. , Kramer, D. , Grunden, A. and Boss, W. (2014) Increasing phosphatidylinositol (4,5)‐bisphosphate biosynthesis affects basal signaling and chloroplast metabolism in Arabidopsis thaliana . Plants, 3, 27–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck, T. , Stenzel, I. and Heilmann, I. (2008) Type B phosphatidylinositol‐4‐phosphate 5‐kinases mediate Arabidopsis and Nicotiana tabacum pollen tube growth by regulating apical pectin secretion. Plant Cell, 20, 3312–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischebeck, T. , Stenzel, I. , Hempel, F. , Jin, X. , Mosblech, A. and Heilmann, I. (2011) Phosphatidylinositol‐4,5‐bisphosphate influences Nt‐Rac5‐mediated cell expansion in pollen tubes of Nicotiana tabacum . Plant J. 65, 453–468. [DOI] [PubMed] [Google Scholar]
- Ischebeck, T. , Werner, S. , Krishnamoorthy, P. et al (2013) Phosphatidylinositol 4,5‐bisphosphate influences PIN polarization by controlling clathrin‐mediated membrane trafficking in Arabidopsis. Plant Cell, 25, 4894–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancewicz, A.L. , Gibbs, N.M. and Masson, P.H. (2016) Cadaverine’s functional role in plant development and environmental response. Front. Plant Sci. 7, 870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König, S. , Mosblech, A. and Heilmann, I. (2007) Stress‐inducible and constitutive phosphoinositide pools have distinctive fatty acid patterns in Arabidopsis thaliana . FASEB J. 21, 1958–1967. [DOI] [PubMed] [Google Scholar]
- König, S. , Ischebeck, T. , Lerche, J. , Stenzel, I. and Heilmann, I. (2008) Salt‐stress‐induced association of phosphatidylinositol 4,5‐bisphosphate with clathrin‐coated vesicles in plants. Biochem. J. 415, 387–399. [DOI] [PubMed] [Google Scholar]
- Kusano, H. , Testerink, C. , Vermeer, J.E.M. , Tsuge, T. , Shimada, H. , Oka, A. , Munnik, T. and Aoyama, T. (2008a) The Arabidopsis phosphatidylinositol phosphate 5‐kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell, 20, 367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano, T. , Berberich, T. , Tateda, C. and Takahashi, Y. (2008b) Polyamines: essential factors for growth and survival. Planta, 228, 367–381. [DOI] [PubMed] [Google Scholar]
- Li, M. , Hong, Y. and Wang, X. (2009) Phospholipase D‐ and phosphatidic acid‐mediated signaling in plants. Biochim. Biophys. Acta Mol. Cell Biol. Lipids, 1791, 927–935. [DOI] [PubMed] [Google Scholar]
- Liu, K. , Fu, H. , Bei, Q. and Luan, S. (2000) Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 124, 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Li, L. and Luan, S. (2005) An essential function of phosphatidylinositol phosphates in activation of plant shaker‐type K+ channels. Plant J. 42, 433–443. [DOI] [PubMed] [Google Scholar]
- Lou, Y. , Gou, J.‐Y. and Xue, H.‐W. (2007) PIP5K9, an Arabidopsis phosphatidylinositol monophosphate kinase, interacts with a cytosolic invertase to negatively regulate sugar‐mediated root growth. Plant Cell, 19, 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, H. , Lou, Y. , Lin, W.H. and Xue, H.W. (2006) MORN motifs in plant PIPKs are involved in the regulation of subcellular localization and phospholipid binding. Cell Res. 16, 466–478. [DOI] [PubMed] [Google Scholar]
- Ma, X. , Shor, O. , Diminshtein, S. , Yu, L. , Im, Y.J. , Perera, I. , Lomax, A. , Boss, W.F. and Moran, N. (2009) Phosphatidylinositol (4,5)bisphosphate inhibits K+‐efflux channel activity in NT1 tobacco cultured cells. Plant Physiol. 149, 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis, F.J. (2009) Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 12, 250–258. [DOI] [PubMed] [Google Scholar]
- Maathuis, F.J.M. and Sanders, D. (1997) Regulation of K+ absorption in plant root cells by external K+: interplay of different plasma membrane K+ transporters. J. Exp. Bot. 48, 451–458. [DOI] [PubMed] [Google Scholar]
- Marco, F. , Busó, E. , Lafuente, T. and Carrasco, P. (2019) Spermine confers stress resilience by modulating abscisic acid biosynthesis and stress responses in Arabidopsis plants. Front. Plant Sci. 10, 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhava, P. , Aliaga Fandino, A.C. , Koh, S.W.H. et al (2020) Plasma membrane domain patterning and self‐reinforcing polarity in Arabidopsis. Dev. Cell, 52, 223–235.e5. [DOI] [PubMed] [Google Scholar]
- Marschner, H. (1995) Mineral nutrition of higher plants. Cambridge, Massachusetts, USA: Acad Press. [Google Scholar]
- Martinis, J. , Gas‐Pascual, E. , Szydlowski, N. , Crèvecoeur, M. , Gisler, A. , Bürkle, L. and Fitzpatrick, T.B. (2016) Long‐distance transport of thiamine (vitamin B1) is concomitant with that of polyamines. Plant Physiol. 171, 542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, Y. , Jia, W.‐J. , Chu, Y.‐J. and Xue, H.‐W. (2012) Arabidopsis phosphatidylinositol monophosphate 5‐kinase 2 is involved in root gravitropism through regulation of polar auxin transport by affecting the cycling of PIN proteins. Cell Res. 22, 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer, H.J.G. and Munnik, T. (2003) Phospholipid‐based signaling in plants. Annu. Rev. Plant Biol. 54, 265–306. [DOI] [PubMed] [Google Scholar]
- Meksuriyen, D. , Fukuchi‐Shimogori, T. , Tomitori, H. , Kashiwagi, K. , Toida, T. , Imanari, T. , Kawai, G. and Igarashi, K. (1998) Formation of a Complex Containing ATP, Mg2+, and Spermine: structural evidence and biological significance. J. Biol. Chem. 273, 30939–30944. [DOI] [PubMed] [Google Scholar]
- Menzel, W. , Stenzel, I. , Helbig, L.M. , Krishnamoorthy, P. , Neumann, S. , Eschen‐Lippold, L. , Heilmann, M. , Lee, J. and Heilmann, I. (2019) A PAMP‐triggered MAPK cascade inhibits phosphatidylinositol 4,5‐bisphosphate production by PIP5K6 in Arabidopsis thaliana . New Phytol. 224, 833–847. [DOI] [PubMed] [Google Scholar]
- Michael, A.J. (2016) Polyamines in eukaryotes, bacteria, and archaea. J. Biol. Chem. 291, 14896–14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller‐Fleming, L. , Olin‐Sandoval, V. , Campbell, K. and Ralser, M. (2015) Remaining mysteries of molecular biology: the role of polyamines in the cell. J. Mol. Biol. 427, 3389–3406. [DOI] [PubMed] [Google Scholar]
- Mishkind, M. , Vermeer, J.E.M. , Darwish, E. and Munnik, T. (2009) Heat stress activates phospholipase D and triggers PIP2 accumulation at the plasma membrane and nucleus. Plant J. 60, 10–21. [DOI] [PubMed] [Google Scholar]
- Mosblech, A. , König, S. , Stenzel, I. , Grzeganek, P. , Feussner, I. and Heilmann, I. (2008) Phosphoinositide and inositolpolyphosphate signalling in defense responses of Arabidopsis thaliana challenged by mechanical wounding. Mol. Plant, 1, 249–261. [DOI] [PubMed] [Google Scholar]
- Moschou, P.N. , Delis, I.D. , Paschalidis, K.A. and Roubelakis‐Angelakis, K.A. (2008a) Transgenic tobacco plants overexpressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiol. Plant. 133, 140–156. [DOI] [PubMed] [Google Scholar]
- Moschou, P.N. , Paschalidis, K.A. , Delis, I.D. , Andriopoulou, A.H. , Lagiotis, G.D. , Yakoumakis, D.I. and Roubelakis‐Angelakis, K.A. (2008b) Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell, 20, 1708–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschou, P.N. , Wu, J. , Cona, A. , Tavladoraki, P. , Angelini, R. and Roubelakis‐Angelakis, K.A. (2012) The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J. Exp. Bot. 63, 5003–5015. [DOI] [PubMed] [Google Scholar]
- Mueller‐Roeber, B. and Pical, C. (2002) Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide‐specific phospholipase C. Plant Physiol. 130, 22–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangi, V. , Chibucos, M.C. , Phuntumart, V. and Morris, P.F. (2012) Kinetic and phylogenetic analysis of plant polyamine uptake transporters. Planta, 236, 1261–1273. [DOI] [PubMed] [Google Scholar]
- Muñiz, L. , Minguet, E.G. , Singh, S.K. , Pesquet, E. , Vera‐Sirera, F. , Moreau‐Courtois, C.L. , Carbonell, J. , Blázquez, M.A. and Tuominen, H. (2008) ACAULIS5 controls Arabidopsis xylem specification through the prevention of premature cell death. Development, 135, 2573–2582. [DOI] [PubMed] [Google Scholar]
- Munnik, T. and Laxalt, A.M. (2013) Measuring PLD activity in vivo. Methods Mol. Biol. 1009, 219–231. [DOI] [PubMed] [Google Scholar]
- Munnik, T. and Testerink, C. (2009) Plant phospholipid signaling: “in a nutshell”. J. Lipid Res. 50(Supplement), S260–S265. 10.1194/jlr.r800098-jlr200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik, T. and Zarza, X. (2013) Analyzing plant signaling phospholipids through 32Pi‐labeling and TLC. Methods Mol. Biol. 1009, 3–15. [DOI] [PubMed] [Google Scholar]
- Munnik, T. , Musgrave, A. and de Vrije, T. (1994) Rapid turnover of polyphosphoinositides in carnation flower petals. Planta, 193, 89–98. [Google Scholar]
- Munnik, T. , Irvine, R.F. and Musgrave, A. (1998a) Phospholipid signalling in plants. Biochim. Biophys. Acta, 1389, 222–272. [DOI] [PubMed] [Google Scholar]
- Munnik, T. and Vermeer, J.E.M. (2010) Osmotic stress‐induced phosphoinositide and inositol phosphate signalling in plants. Plant Cell Environ. 33(4), 655–669. 10.1111/j.1365-3040.2009.02097.x [DOI] [PubMed] [Google Scholar]
- Munnik, T. , Van Himbergen, J.A.J. , Ter Riet, B. , Braun, F.J. , Irvine, R.F. , Van Den Ende, H. and Musgrave, A. (1998b) Detailed analysis of the turnover of polyphosphoinositides and phosphatidic acid upon activation of phospholipases C and D in Chlamydomonas cells treated with non‐permeabilizing concentrations of mastoparan. Planta, 207, 133–145. [Google Scholar]
- Noack, L.C. and Jaillais, Y. (2017) Precision targeting by phosphoinositides: how PIs direct endomembrane trafficking in plants. Curr. Opin. Plant Biol. 40, 22–33. [DOI] [PubMed] [Google Scholar]
- Oude Weernink, P.A. , Schulte, P. , Guo, Y. et al (2000) Stimulation of phosphatidylinositol‐4‐phosphate 5‐kinase by Rho‐kinase. J. Biol. Chem. 275, 10168–10174. [DOI] [PubMed] [Google Scholar]
- Ozawa, R. , Bertea, C.M. , Foti, M. , Narayana, R. , Arimura, G.‐I. , Muroi, A. , Maffei, M.E. and Takabayashi, J. (2010) Polyamines and jasmonic acid induce plasma membrane potential variations in Lima bean. Plant Signal. Behav. 5, 308–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál, M. , Szalai, G. and Janda, T. (2015) Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 237, 16–23. [DOI] [PubMed] [Google Scholar]
- Pandolfi, C. , Pottosin, I. , Cuin, T. , Mancuso, S. and Shabala, S. (2010) Specificity of polyamine effects on NaCl‐induced ion flux kinetics and salt stress amelioration in plants. Plant Cell Physiol. 51, 422–434. [DOI] [PubMed] [Google Scholar]
- Pappan, K. , Zheng, S. and Wang, X. (1997) Identification and characterization of a novel plant phospholipase D that requires polyphosphoinositides and submicromolar calcium for activity in Arabidopsis. J. Biol. Chem. 272, 7048–7054. [DOI] [PubMed] [Google Scholar]
- Pegg, A.E. (2016) Functions of polyamines in mammals. J. Biol. Chem. 291, 14904–14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pical, C. , Westergren, T. , Dove, S.K. , Larsson, C. and Sommarin, M. (1999) Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4,5‐bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J. Biol. Chem. 274, 38232–38240. [DOI] [PubMed] [Google Scholar]
- Pistocchi, R. , Bagni, N. and Creus, J.A. (1987) Polyamine uptake in carrot cell cultures. Plant Physiol. 84, 374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistocchi, R. , Keller, F. , Bagni, N. and Matile, P. (1988) Transport and subcellular localization of polyamines in carrot protoplasts and vacuoles. Plant Physiol. 87, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin, I. and Shabala, S. (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 5(154). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottosin, I. , Velarde‐Buendía, A.M. , Bose, J. , Fuglsang, A.T. and Shabala, S. (2014) Polyamines cause plasma membrane depolarization, activate Ca2+‐, and modulate H+‐ATPase pump activity in pea roots. J. Exp. Bot. 65, 2463–2472. [DOI] [PubMed] [Google Scholar]
- Qin, W. , Pappan, K. and Wang, X. (1997) Molecular heterogeneity of phospholipase D (PLD). Cloning of PLDgamma and regulation of plant PLDgamma, ‐beta, and ‐alpha by polyphosphoinositides and calcium. J. Biol. Chem. 272, 28267–28273. [DOI] [PubMed] [Google Scholar]
- Rao, J.N. , Guo, X. , Liu, L. , Zou, T. , Murthy, K.S. , Yuan, J.‐X.‐J. and Wang, J.‐Y. (2003) Polyamines regulate Rho‐kinase and myosin phosphorylation during intestinal epithelial restitution. Am. J. Physiol. Cell Physiol. 284(4), C848–C859. [DOI] [PubMed] [Google Scholar]
- Rentsch, D. , Schmidt, S. and Tegeder, M. (2007) Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett. 581, 2281–2289. [DOI] [PubMed] [Google Scholar]
- Richards, F.J. and Coleman, R.G. (1952) Occurrence of putrescine in potassium‐deficient barley. Nature, 170, 460. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Villalon, A. , Gujas, B. , Kang, Y.H. , Breda, A.S. , Cattaneo, P. , Depuydt, S. and Hardtke, C.S. (2014) Molecular genetic framework for protophloem formation. Proc. Natl. Acad. Sci. USA, 111, 11551–11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Villalon, A. , Gujas, B. , van Wijk, R. , Munnik, T. and Hardtke, C.S. (2015) Primary root protophloem differentiation requires balanced phosphatidylinositol‐4,5‐biphosphate levels and systemically affects root branching. Development, 142, 1437–1446. [DOI] [PubMed] [Google Scholar]
- Saavedra, L. , Mikami, K. , Malhó, R. and Sommarin, M. (2012) PIP kinases and their role in plant tip growing cells. Plant Signal. Behav. 7, 1302–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagor, G.H.M. , Berberich, T. , Takahashi, Y. , Niitsu, M. and Kusano, T. (2013) The polyamine spermine protects Arabidopsis from heat stress‐induced damage by increasing expression of heat shock‐related genes. Transgenic Res. 22, 595–605. [DOI] [PubMed] [Google Scholar]
- Sagor, G.H.M. , Chawla, P. , Kim, D.W. , Berberich, T. , Kojima, S. , Niitsu, M. and Kusano, T. (2015) The polyamine spermine induces the unfolded protein response via the MAPK cascade in Arabidopsis. Front. Plant Sci. 6, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala, S. (2017) Signalling by potassium: another second messenger to add to the list? J. Exp. Bot. 68, 4003–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala, L. , Ross, T. , McMeekin, T. and Shabala, S. (2006) Non‐invasive microelectrode ion flux measurements to study adaptive responses of microorganisms to the environment. FEMS Microbiol. Rev. 30, 472–486. [DOI] [PubMed] [Google Scholar]
- Simon, M.L.A. , Platre, M.P. , Assil, S. , van Wijk, R. , Chen, W.Y. , Chory, J. , Dreux, M. , Munnik, T. and Jaillais, Y. (2014) A multi‐colour/multi‐affinity marker set to visualize phosphoinositide dynamics in Arabidopsis. Plant J. 77, 322–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S.S. , Chauhan, A. , Brockerhoff, H. and Chauhan, V.P. (1995) Differential effects of spermine on phosphatidylinositol 3‐kinase and phosphatidylinositol phosphate 5‐kinase. Life Sci. 57, 685–694. [DOI] [PubMed] [Google Scholar]
- Sousa, E. , Kost, B. and Malhó, R. (2008) Arabidopsis phosphatidylinositol‐4‐monophosphate 5‐kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell, 20, 3050–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel, I. , Ischebeck, T. , König, S. , Hołubowska, A. , Sporysz, M. , Hause, B. and Heilmann, I. (2008) The type B phosphatidylinositol‐4‐phosphate 5‐kinase 3 is essential for root hair formation in Arabidopsis thaliana . Plant Cell, 20, 124–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel, I. , Ischebeck, T. , Quint, M. and Heilmann, I. (2012) Variable regions of PI4P 5‐kinases direct PtdIns(4,5)P2 toward alternative regulatory functions in tobacco pollen tubes. Front. Plant Sci. 2(114). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajti, J. , Hamow, K.Á. , Majláth, I. , Gierczik, K. , Németh, E. , Janda, T. and Pál, M. (2019). Polyamine‐induced hormonal changes in eds5 and sid2 mutant Arabidopsis plants. Int. J. Mol. Sci. 20, 5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y. , Berberich, T. , Miyazaki, A. , Seo, S. , Ohashi, Y. and Kusano, T. (2003) Spermine signalling in tobacco: activation of mitogen‐activated protein kinases by spermine is mediated through mitochondrial dysfunction. Plant J. 36, 820–829. [DOI] [PubMed] [Google Scholar]
- Tassoni, A. , Van Buuren, M. , Franceschetti, M. , Fornalè, S. and Bagni, N. (2000) Polyamine content and metabolism in Arabidopsis thaliana and effect of spermidine on plant development. Plant Physiol. Biochem. 38, 383–393. [Google Scholar]
- Tejos, R. , Sauer, M. , Vanneste, S. et al (2014) Bipolar plasma membrane distribution of phosphoinositides and their requirement for auxin‐mediated cell polarity and patterning in Arabidopsis. Plant Cell, 26, 2114–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole, J.M. , Vermeer, J.E.M. , Zhang, Y. , Gadella, T.W.J. and Nielsen, E. (2008) Root hair defective4 encodes a phosphatidylinositol‐4‐phosphate phosphatase required for proper root hair development in Arabidopsis thaliana . Plant Cell, 20, 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio, A.F. , Altabella, T. , Bitrián, M. and Alcázar, R. (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta, 240, 1–18. [DOI] [PubMed] [Google Scholar]
- Tong, W. , Imai, A. , Tabata, R. , Shigenobu, S. , Yamaguchi, K. , Yamada, M. , Hasebe, M. , Sawa, S. , Motose, H. and Takahashi, T. (2016) Polyamine resistance is increased by mutations in a nitrate transporter gene NRT1.3 (AtNPF6.4) in Arabidopsis thaliana . Front. Plant Sci. 7, 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toumi, I. , Moschou, P.N. , Paschalidis, K.A. , Bouamama, B. , Ben Salem‐Fnayou, A. , Ghorbel, A.W. , Mliki, A. and Roubelakis‐Angelakis, K.A. (2010) Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J. Plant Physiol. 167, 519–525. [DOI] [PubMed] [Google Scholar]
- Tun, N.N. (2006) Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 47, 346–354. [DOI] [PubMed] [Google Scholar]
- Ugalde, J.‐M. , Rodriguez‐Furlán, C. , Rycke, R.D. , Norambuena, L. , Friml, J. , León, G. and Tejos, R. (2016) Phosphatidylinositol 4‐phosphate 5‐kinases 1 and 2 are involved in the regulation of vacuole morphology during Arabidopsis thaliana pollen development. Plant Sci. 250, 10–19. [DOI] [PubMed] [Google Scholar]
- van Leeuwen, W. , Vermeer, J.E.M. , Gadella, T.W.J. and Munnik, T. (2007) Visualization of phosphatidylinositol 4,5‐bisphosphate in the plasma membrane of suspension‐cultured tobacco BY‐2 cells and whole Arabidopsis seedlings. Plant J. 52, 1014–1026. [DOI] [PubMed] [Google Scholar]
- Vera‐Sirera, F. , De Rybel, B. , Úrbez, C. et al (2015) A bHLH‐based feedback loop restricts vascular cell proliferation in plants. Dev. Cell, 35(4), 432–443. 10.1016/j.devcel.2015.10.022 [DOI] [PubMed] [Google Scholar]
- Vermeer, J.E.M. and Munnik, T. (2013) Using genetically encoded fluorescent reporters to image lipid signalling in living plants. Methods Mol. Biol. 1009, 283–289. [DOI] [PubMed] [Google Scholar]
- Vermeer, J.E.M. , Thole, J.M. , Goedhart, J. , Nielsen, E. , Munnik, T. and Gadella, T.W.J. Jr (2009) Imaging phosphatidylinositol 4‐phosphate dynamics in living plant cells. Plant J. 57, 356–372. [DOI] [PubMed] [Google Scholar]
- Verrey, F. , Closs, E.I. , Wagner, C.A. , Palacin, M. , Endou, H. and Kanai, Y. (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. Eur. J. Physiol. 447, 532–542. [DOI] [PubMed] [Google Scholar]
- Wada, Y. , Kusano, H. , Tsuge, T. and Aoyama, T. (2015) Phosphatidylinositol phosphate 5‐kinase genes respond to phosphate deficiency for root hair elongation in Arabidopsis thaliana . Plant J. 81, 426–437. [DOI] [PubMed] [Google Scholar]
- Westergren, T. , Dove, S.K. , Sommarin, M. and Pical, C. (2001) AtPIP5K1, an Arabidopsis thaliana phosphatidylinositol phosphate kinase, synthesizes PtdIns(3,4)P2 and PtdIns(4,5)P2 in vitro and is inhibited by phosphorylation. Biochem. J. 359, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigoda, N. , Ma, X. and Moran, N. (2010) Phosphatidylinositol 4,5‐bisphosphate regulates plant K+ channels. Biochem. Soc. Trans. 38, 705–709. [DOI] [PubMed] [Google Scholar]
- Williams, M.E. , Torabinejad, J. , Cohick, E. , Parker, K. , Drake, E.J. , Thompson, J.E. , Hortter, M. and Dewald, D.B. (2005) Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress‐response pathway. Plant Physiol. 138, 686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Shang, Z. , Wu, J. , Jiang, X. , Moschou, P.N. , Sun, W. , Roubelakis‐Angelakis, K.A. and Zhang, S. (2010) Spermidine oxidase‐derived H₂O₂ regulates pollen plasma membrane hyperpolarization‐activated Ca(2+) ‐permeable channels and pollen tube growth. Plant J. 63, 1042–1053. [DOI] [PubMed] [Google Scholar]
- Wu, H. , Zhang, X. , Giraldo, J.P. and Shabala, S. (2018) It is not all about sodium: revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil, 431, 1–17. [Google Scholar]
- Yamaguchi, K. , Takahashi, Y. , Berberich, T. , Imai, A. , Miyazaki, A. , Takahashi, T. , Michael, A. and Kusano, T. (2006) The polyamine spermine protects against high salt stress in Arabidopsis thaliana . FEBS Lett. 580, 6783–6788. [DOI] [PubMed] [Google Scholar]
- Yoda, H. (2006) Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol. 142, 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarza, X. , Shabala, L. , Fujita, M. , Shabala, S. , Haring, M.A. , Tiburcio, A.F. and Munnik, T. (2019) Extracellular spermine triggers a rapid intracellular phosphatidic acid response in Arabidopsis, involving PLDδ activation and stimulating ion flux. Front. Plant Sci. 10, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepeda‐Jazo, I. , Velarde‐Buendía, A.M. , Enríquez‐Figueroa, R. , Bose, J. , Shabala, S. , Muñiz‐Murguía, J. and Pottosin, I.I. (2011) Polyamines interact with hydroxyl radicals in activating Ca(2+) and K(+) transport across the root epidermal plasma membranes. Plant Physiol. 157, 2167–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Yan, A. , Feijó, J.A. , Furutani, M. , Takenawa, T. , Hwang, I. , Fu, Y. and Yang, Z. (2010) Phosphoinositides regulate clathrin‐dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell, 22, 4031–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Oligonucleotides used in this work.
Figure S1. Spm‐induced PIP2 response is not caused by breakdown products of Spm.
Figure S2. PA and PIP2 responses occur simultaneously.
Figure S3. Spm‐induced PIP2 responses in Arabidopsis PIP5K T‐DNA insertion mutants.
Figure S4. PIP2 levels in the pldδ mutant.
Figure S5. PIP2 levels in response to Spm in seedlings and mature leaves.
Figure S6. No obvious phenotypes are apparent in pip5k7 pip5k9 mutants.
Figure S7. PIP2 levels are higher in SPMS overexpression lines.
Data S1. Supplementary methods.
Data Availability Statement
All data generated or analysed during this study are included in the article and its supplementary information files. Plasmids and mutants are available upon request.


