Abstract
Background
International guidelines recommend hereditary thoracic aortic diseases (HTADs) to be managed in multidisciplinary aorta clinics.
Aim
To study HTAD patient's experiences with a aortopathy clinic in Norway and to review the literature on aortopathy clinics.
Methods
(a) A systematic scoping review of research on multidisciplinary clinics for HTADs. (b) A cross‐sectional postal questionnaire study to investigate patient experiences with the health‐services. Fifty consecutive patients from the aortopathy clinic and 50 controls in usual care were invited to participate.
Results
The review identified eight publications on aortopathy clinics. Although the papers were not judged for quality, these showed promising results from such clinics in terms of diagnostics and increased adherence to guideline‐directed therapy. The survey constituted thirty‐seven (74%) patients and 22 (44%) controls who responded to postal questionnaires. Both groups reported delays in diagnostics and follow‐up appointments prior to the start of the clinic. Patients indicated high satisfaction with the aortopathy clinic, whereas controls reported poor coordination of medical follow‐up. Individuals in both groups struggled with disease self‐management.
Conclusion
Norwegian patient experiences found the aortopathy clinic beneficial. According to studies included in the review, disease management in aortopathy clinics may improve patient satisfaction, diagnostics and follow‐up. Effect studies may further document the benefits of clinic organization, treatment, cost‐efficiency and patient experiences.
Keywords: cardiac genetics clinic, hereditary aortic dilation and dissection, hereditary aortic disease, Marfan clinic, multidisciplinary aortopathy clinic
1. INTRODUCTION
Hereditary thoracic aortic diseases (HTADs) are a heterogeneous group of disorders caused by many different genes (Milewicz & Regalado, 1993). Today, more than 30 different disease‐causing genes are known (Brownstein et al., 2018; De Backer et al., 2019). The disorders range from those mainly affecting the thoracic aorta (non‐syndromic) to conditions affecting multiple organ systems (syndromic). The latter group includes among others, Marfan syndrome (MFS), Loeys‐Dietz syndromes (LDS) and vascular Ehlers‐Danlos syndrome (vEDS) (De Backer et al., 2019). These conditions have overlapping features and diagnostics are challenging. For instance, in MFS a median time of 641 days from suspicion to diagnosis was documented in one study (Roll, 2012). The most serious and potentially life threatening symptoms in HTADs are aneurysms and dissections of the aorta and other large arteries (Meester et al., 2017). In addition, the involvement of other organ systems, including skin, musculoskeletal systems and lungs are common (Isselbacher, Lino Cardenas, & Lindsay, 2016). Eye involvement with lens luxation and risk of retinal detachment concerns mainly MFS (Loeys et al., 2010). In LDS, more severe vascular involvement with arterial tortuosity as well as a higher incidence of asthma and gastrointestinal symptoms has been reported (Johansen, Velvin, & Lidal, 2020; Krohg‐Sørensen et al., 2017; MacCarrick et al., 2014). In vEDS, there is a high risk of intestinal (colon) and peripartum uterine rupture and arterial involvement with rupture of branch arteries that might be preceded by aneurysm or dissection—or even occur spontaneously (Byers et al., 2017).
In order to avoid complications, strict follow‐up regimes for HTADs are highly recommended (Byers et al., 2017; Loeys et al., 2010; MacCarrick et al., 2014). Research on these rare disorders are still scarce and existing guidelines on follow‐up still largely consensus based, with significant differences between them (Rozado, Martin, Pascual, Hernandez‐Vaquero, & Moris, 2017). The most important treatment is prophylactic blood pressure control with anti‐hypertensive medications (Rozado et al., 2017), and timely aortic surgery to prevent dissection (Milewicz & Regalado, 1993). For MFS especially, ophthalmologic follow‐up and treatment of ectopia lentis is essential (Loeys et al., 2010). Despite this, some patients need emergency interventions, with higher risk of morbidity and complications compared to prophylactic treatment strategies (Bradley, Alvarez, & Horne, 2016).
Living with a potential life threatening inheritable disease can be a challenge for patients. Low health‐related quality of life has been reported in MFS, while research on other HTADs are lacking (Velvin, Wilhelmsen, Johansen, Bathen, & Geirdal, 2019). MFS patients' ability to activate their personal resources and manage their disease have been described to have large impact on life satisfaction (Stanišić et al., 2018).
International guidelines recommend that treatment of HTADs should be concentrated within “aorta clinics” (Erbel et al., 2014). Such clinics involve a multidisciplinary team consisting of many different medical specialties in the patient diagnostics‐ and follow‐up processes (Boodhwani et al., 2014; Erbel et al., 2014). The main benefit being better pathways for diagnostics, follow‐up and treatment of the patients, promoting better health outcomes and lower mortality (Boodhwani et al., 2014). It is also said to be more cost‐effective (Blankart, Milstein, Rybczynski, Schüler, & von Kodolitsch, 2016). Furthermore, a multidisciplinary team‐based approach has been shown to give quicker clinical decision‐making (Showkathali et al., 2014), help patients' innovation and coping skills (Hannemann‐Weber & Schultz, 2014) and increase patient satisfaction (Wen & Schulman, 2014).
Despite the fact that multidisciplinary aorta clinics are recommended in the care for patients with HTADs, there seems to be few studies describing the organization, patients' experiences and their satisfaction with such clinics.
While planning the establishment of an aortopathy clinic at a university hospital in Norway, a review of the research knowledge to inform on this topic was deemed necessary. Furthermore, with a new organization of health‐services, assessments of patient's experiences is a valuable part of the quality judgment and for further improvement of such a clinic. Therefore, the aim of this study was twofold:
To review and present the research literature about the organization and patient experiences with multidisciplinary clinics for patients with HTADs.
To explore patients' experiences and their satisfaction with diagnostics pathways and follow‐up before and after the establishment of an aortopathy clinic compared to controls in usual care.
2. METHODS AND MATERIALS
2.1. Literature review
2.1.1. Study design
Our aim was to get an overview of existing research on multidisciplinary clinics for persons with HTADs. Owing to the potential limited number of papers and the expected variety in study designs and methodologies, a scoping review method was chosen. A scoping review is a type of review that aims to systematically search for research findings to identify trends, concepts and research gaps. This is a suitable method to apply to broad review questions, also when very diverse findings make a systematic review approach with critical appraisal and meta‐analysis difficult (Peters et al., 2015). This manuscript was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses Extension for Scoping Reviews (PRISMA ScR) (Tricco et al., 2018). However, we have made one adjustment by looking into the findings of the included studies, and have indicated their overall results on organization and patient experiences with aortopathy clinics.
2.1.2. Eligibility criteria
The review inclusion criteria were: (a) Publications describing organization and experiences (including patient data and patient experiences) with multidisciplinary clinics for persons with HTADs. (b) All types of study‐designs, published in English, German, or Scandinavian languages were included. No exclusions were made on the basis of age groups, gender or ethnicities, neither for publication date.
2.1.3. Search strategy
Systematic searches were conducted in March 2019 in PubMed, AMED, EMBASE, CINAHL, and Google Scholar. A three‐stage search strategy was utilized. We first conducted a search using the following search terms: Marfan syndrome OR Loeys‐Dietz syndrome OR familial aortic aneurysm and dissection OR FTAAD OR vascular Ehlers‐Danlos syndrome OR aortopathy OR aortopathies OR aortic disease OR thoracic aortic disease OR congenital aortic disease OR genetic aortic disease (a total of 6,102 hits). Then another search was done in the same databases with the following terms: Cardiac genetics clinic OR genomic medicine OR aortopathy bundle of care OR guidelines OR healthcare team OR multidisciplinary OR cardiogenetics OR quality of care OR cost‐of‐illness OR economic impact (a total of 84,702 hits). Finally we combined searches 1 AND search 2, resulting in 120 hits after deleting duplicates and foreign language articles (ex. Japanese). Additional references were sought by examining the citations in included papers. The search strategy is presented in Figure 1.
FIGURE 1.
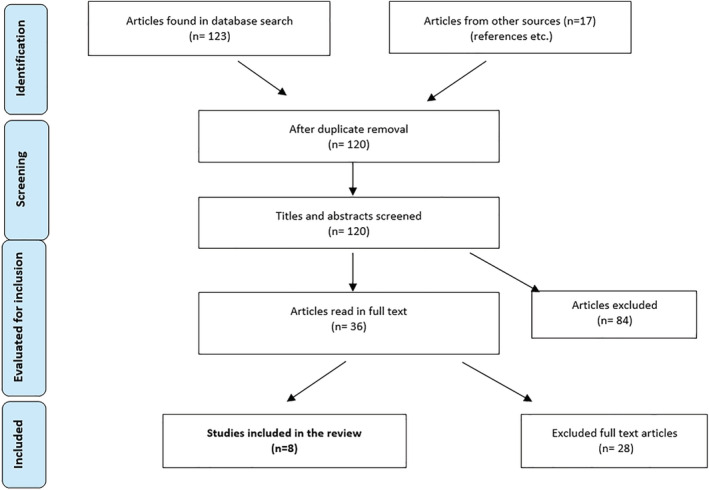
Flow‐chart of search strategy for literature on multidisciplinary clinics for persons with heritable thoracic artery diseases [Color figure can be viewed at wileyonlinelibrary.com]
Two reviewers (TB and IBL) independently screened the abstracts and/or articles from each reference identified through the search. When considered potentially eligible according to the inclusion criteria, the full‐text publications were obtained and reviewed for a final decision on inclusion by the same two reviewers.
2.1.4. Data extraction
Data from included studies were extracted, using an á priori scheme. The data presentation included the following information from each paper (Table 1): reference (title, author and publication year), study aim, design and methods, reported data on included patients (age, gender, diagnoses), data on patient recruitment and the organization of the clinic. In addition and not according to PRISMA‐ScR, we collected description of benefits or drawbacks with the clinic, patient experiences, and primary author's conclusion.
TABLE 1.
Data extraction from included articles on aortopathy clinics
| Authors, publication year, title | Aim of the study | Study design methods | Report patient data? | Describes organization of aorthopaty clinic? | Describes benefits and/or patients experiences of aorthopaty clinic? | Primary authors' conclusion |
|---|---|---|---|---|---|---|
|
Strider et al., 1996. Marfan's syndrome: a family affair |
To develop strategies for long‐term management of persons with MFS |
Prospective study During a 20‐month period between 1994–96, 112 persons From 15 different families were evaluated by an interdisciplinary team for the presence of clinical manifestations consistent with MFS. |
Report data on 112 persons from 15 different families with presence of MFS‐related traits. 24 already definite diagnosis of MFS (Berlin diagnostic criteria). Recruited from hearth Centre at one university hospital, USA | The interdisciplinary team included three thoracic cardiovascular surgeons, two cardiologists, an ophthalmologist, a medical geneticist, a pediatric endocrinologist, a dentist, a social worker, and three nurses who coordinated screening and provided patient education. |
MFS was present in seven of the 15 families, forty‐three patients (57.3%) demonstrated significant cardiovascular lesions, with 20 undergoing cardiac surgery. Thirty‐one patients (41.3%) were initially seen with significant ocular lesions, and 38 (50. 7%) displayed orthopedic deformities. The health care team developed strategies for long‐term management of persons with MFS, including antihypertensive therapy, periodic testing, risk‐factor modification, genetic counseling, and surgery for appropriate patients The interdisciplinary approach to the MFS population provides the flexibility and depth to meet the diverse health care needs of these individuals. |
The MFS screening project used an interdisciplinary health care team to provide the long‐term management to persons already diagnosed with MFS. The screening of first‐degree relatives provided a forum to teach patients and their families about the genetic basis, natural history, complications, medical management, and surgical interventions for MFS. |
|
Summers et al., 2006. Challenges in the diagnosis of Marfan syndrome |
To describe the potential range of diagnostic dilemmas faced by physicians who consider Marfan syndrome (MFS) as a provisional diagnosis for a patient or family. |
Report of clinical experience with case series. Evaluation at Marfan clinic routinely involves cardiological assessment including echocardiography, and ophthalmological review. Patients were assessed by a clinical geneticist and a genetic counselor. |
Report data on 4 patients with Homocysteinuria, Marfan syndrome (Ghent1), Marfan‐like, and FTAAD. Recruited from the Marfan Clinic at Prince Charles Hospital in Brisbane, Australia |
The Marfan clinic was staffed by a nurse coordinator, two cardiologists, an ophthalmologist, a pediatrician specializing in clinical genetics, two genetic counselors and a molecular geneticist. Referrals for assessment of Marfanoid habitus arise primarily from general practitioners and pediatricians. The clinic also receives referrals from cardiologists because of aortic dilatation or dissection and from ophthalmologists because of dislocated lenses. |
The clinic has assessed over 600 individuals from more than 300 families since 1995–2006. At least one individual in 22% of the families seen at the Prince Charles hospital Marfan clinic satisfied the international diagnostic criteria for MFS. Additionally, 18% of the families were given other diagnoses. |
We consider that the 22% diagnostic rate represents an appropriate referral pattern, because of the difficulties in diagnosis and because treatments are available. Full cardiovascular, ophthalmological and musculoskeletal evaluation of patients suspected of having Marfan syndrome ensures appropriate diagnosis and circumvents potential problems with improper treatment and surveillance. |
|
Andvik, Sherrah, & Jeremy, 2011 Improved Care for Thoracic Aortic Aneurysm—Two Decades Experience |
To describe experiences of an aortic disease clinic, royal prince albert hospital Australia from 1991–2011 |
Conference abstract, retrospective chart review Patient diagnosis, regular use of beta‐blockers or angiotensin‐blockers and outcomes are compared for first and second decades of clinic experience |
Report data on 176 Marfan patients, 153 isolated or familial aneurysm or dissection (TAAD), 53 bicuspid aortic valves, 23 other (Ehlers‐Danlos, LoeysDietz). Use of diagnostic criteria not specified. |
Organization and which professions are involved are not described. Patients had annual echo and clinical review and received medical therapy according to current guidelines. |
Over 20 years, 732 patients were enrolled. Although recognition of non‐syndromal TAAD has increased, diagnosis for all remains delayed. Use of beta‐blockers remains relatively low, but AgII blockers are increasingly used. Dissection and surgery rates have fallen for TAAD. |
Conclusions: Increased detection and follow‐up has improved outcomes in the last decade but uptake of medical therapy remains low and death rates are unchanged. |
|
McLean, 2012 Aortopathy: Developing a thoracic‐aorta clinic. |
Discuss the benefits of providing specialized and advanced health care for these patients, and development of a thoracic‐aorta clinic in Canada. |
Conference abstract, report of clinical experience |
No patient data are reported in abstract. |
The thoracic‐aorta clinic consists of a multi‐disciplinary team that includes a cardiac surgeon, cardiologist and a registered nurse, who is the clinic co‐ordinator. The aim is to provide quality care that is comprehensive and holistic. The role of the clinic coordinator is multi‐faceted. Educational materials, assessment and documentation tools, care plans, and a website page have been developed to facilitate the patients learning and coping strategies. Additionally, the coordinator plays a role in the emotional and supportive care for the patients and families. |
Not described in abstract |
Not described in abstract |
|
Zentner et al., 2015. The Cardiac Genetics Clinic: a model for multidisciplinary genomic medicine |
To describe patient characteristics, standard operating procedure, and uptake of genetic testing at the multidisciplinary Cardiac Genetics Clinic (CGC) at the Royal Melbourne Hospital during its first 6 years, 2007–2013 |
Retrospective chart review Database exploration of referral diagnoses, sex, number of clinic visits and incidence of genetic testing in a population of individuals attending the CGC. |
Dataextraction on 1,170 patients; referred for cardiomyopathy, aorthopathy (n = 303), arythmia disorders, other diagnoses. 170 individuals were seen for the first time over the 6‐year period; 57.5% made only one visit. Median age 39 years. Genetic testing was undertaken in 381 individuals (32.6%), and a pathogenic mutation was identified in 47.6% of tests, representing 15.3% of the total population. |
The CGC is a joint undertaking by the clinical genetics and cardiology units at the Royal Melbourne Hospital. It is managed by a cardiac trained nurse who performs telephone intake on all referrals, and as well as coordinating screening tests and collating relevant clinical information on individuals and their family members before the clinical appointment. As many patients travel long distances to visit us, we attempt to provide same‐day cardiac testing, before the clinical review. Clinics are preceded by a multidisciplinary planning meeting, then clinic consultation with cardiologist/electrophysiologist and clinical geneticist and/or genetic counselor, and post clinic plan and follow up. |
The clinical benefit achieved by the simultaneous review of patients by a cardiologist and a clinical geneticist includes the identification of rare diseases and accurate assessment of the utility of genetic testing. This is borne out by the high yield of mutation detection by genetic testing, which highlights the importance of a multidisciplinary clinic and the usefulness of the whole‐family approach. The ultimate intention of the CGC is to prevent adverse cardiac events through early identification and optimal management advice to at risk individuals. There are currently no long‐term data that show improved outcomes were achieved by this approach, and providing these data remained a long‐term aspiration of the service. Similarly, although it is anticipated that cost‐effectiveness can be achieved, largely by excluding from screening genotype‐negative individuals from high‐risk families, this remains to be confirmed. |
The CGC fulfills an important role in assisting clinicians and patients by reviewing genetic cardiac diagnoses. Clinical practice during the study period moved from a selected candidate gene approach to broader gene panel‐based testing. This move to next‐generation sequencing may increase the detection of mutations and variants of unknown significance. A major contribution by the clinic to the care of these individuals and their families is the provision (or negating) of a diagnosis, and of a plan for managing risks of predictable cardiac disease. |
|
Bradley & Bowdin, 2016. Multidisciplinary Aortopathy Clinics Should Now Be the Standard of Care in Canada |
To presents personal experience of >10 years in developing multidisciplinary aortopathy clinics in a pediatric and adult cardiology clinic setting, with the knowledge of the issues faced by similar clinics across Canada. |
Narrative review/ expert experience. Discussing their experiences and relevant literature. No search strategy is given. |
Does not report patientdata on patients with aorthopaties. Discuss this patient group in lieu of and experiences with multidisciplinary care in other chronic cardiovascular conditions |
Describes aspects that should be included: Gentic screening and counseling, cardiovascular imaging, specialized medical management (including patient education, guidelinedirected therapy, counseling on employment and lifestyle restrictions), specialized surgical management and specialized pregnancy management In the beginning consisting of cardiologist and thoracic – Surgeon, now many contributing professions are common; like imaging specialists, cardiac anaestiologists, intensivists, nurses, social workers, clinical and/ or research coordinators. Now multidisciplinary joint clinics in which the team see the patients at the same time now are common |
Discuss that in other chronic heart condition multidisciplinary hearth teams has a long tradition and are given class 1 recommendations in American and European guidelines. Benefits of this multidisciplinary “heart team”‐based approach include increased adherence to guideline‐directed therapy, reduced clinical decision‐making times, continuity of patient care, improved knowledge translation to patients and referring physicians, improved patient satisfaction and quality of life, improved physician satisfaction through opportunities for professional development, and opportunities for collection of data for research and more effective resource utilization. |
The authors conclude that multidisciplinary aortopathy clinics should now be the standard of care for the management of TAD in Canada and should implement best practice guidelines. |
|
von Kodolitch et al., 2016. The role of the multidisciplinary health care team in the management of patients with Marfan syndrome |
To give a personal account of each key team members contribution to management of patients with MFS at the multi‐disciplinary health care team at the Hamburg Marfan center |
Narrative review/ expert experience. Describe experiences from the Marfan clinic from 1998–2016 |
Mainly report professional experiences. Report data on a small survey (n = 77) through the German Marfan patient organization on patients views on what was important in a Marfan Centre. |
The Hamburg Marfan center for adults started 1998 and consists of: (a) the team of coordinators; a cardiologist, a scientist, a nurse, and a geneticist, (b) core disciplines (pediatrician, geneticist, cardiologist, heart surgeon, vascular surgeon, orthopedic surgeon, ophthalmologist, nurse), (c) auxiliary disciplines (forensic pathologist, radiologist, pulmonologist, sleep specialist, rythmologist, orthodontist/ dentist, neurologist, obstetric surgeon, psychologist, rehabilitation specialist). |
Describe that the first Marfan clinic started in the late 1960s in United States. The Hamburg Marfan Centre for adults started in 1996, for children in 2006. Services are given to persons with Marfan and related disorders The Marfan Centre is built on evidence‐based design criteria to support patient‐centered care: a) access and continuity of care, b) provide opportunities for patients to participate in the care process, c)provide self‐management support, d)coordinate care between settings The patient survey found that what patients most appreciated in a Marfan Centre was competence of the team (33.8%), multidisciplinary care (29.9%), and trusting the doctor, overcoming fear and getting explanations (15.6%). |
A multidisciplinary health care team is a means to maximize therapeutic success. Most importantly: The multidisciplinary approach for MFS provides more precise data for diagnosis and possible phenotype–genotype correlations. |
|
Wright et al., 2016. Definition and delivery of an aortopathy bundle of care (ABC): a tool for improving diagnosis and management of Marfan syndrome and related conditions |
To accelerate diagnosis and improve management of Marfan syndrome and related conditions, a multidisciplinary improvement science project was developed between cardiology and genomic medicine. | Innovation report |
Report experiences with follow up of Marfan patients. |
A monthly genetic aortopathy team clinic (GATC) was established, permitting multidisciplinary consultations with a cardiologist, clinical geneticist and genetic counselor. To optimize the utility of this new, resource‐intensive clinic, plan‐do‐study‐act cycles were embedded in each clinic, and increasing numbers of patients were seen each month. Current guidelines were reviewed to define key indices of effective management: The aortopathy bundle of care (ABC). The ABC consisted of 6 elements: Documentation of diagnosis according to revised Ghent criteria, clinical follow‐up, genetic counseling provision, appropriate imaging, blood pressure management and appropriate referral for cardiac surgical opinion. |
The proportion of patients whose diagnosis was confirmed and documented according to revised Ghent criteria rose from 25% to 100%. 100% of patients received appropriate imaging, compared to 25% previously. Patients reported high levels of satisfaction with the multidisciplinary approach. |
The multidisciplinary GATC was shown to be effective in achieving a diagnosis in fewer clinic visits than previously, and provides a means of delivery of appropriate and comprehensive care to this patient group. The ABC appears useful for measuring the effectiveness of care for patients with genetic aortopathies such as Marfan syndrome. |
2.2. Primary study of the experiences from the Oslo university hospital aortopathy clinic
2.2.1. Organization
The aortopathy clinic at Oslo University Hospital (OUH) started with a project period (2017–2019) funded by the Norwegian National Advisory Unit on Rare Disorders. The project was planned and organized by a reference group with representatives from each of the participating departments at OUH (i.e., thoracic surgery, cardiology, genetics, radiology, pediatric, physiatrist and ophthalmology), from TRS Resource Centre for Rare disorders (TRS) and with patient representatives from the Norwegian Marfan and EDS organizations. The department of thoracic surgery at OUH administered the project. A patient coordinator (nurse) was employed in a 50% position to organize patient contact and practicalities. This also ensured easy access to the clinic, as the coordinator could be contacted by patients without referral. A registry collecting data on all relevant organ systems for those patients giving their consent, was also organized as part of the clinic (not further presented in the article).
The aortopathy clinic was organized monthly from September 2017, with 4 to 5 patients on each occasion. If possible, visits were scheduled for 1 day (sometimes 2 days) with the patients meeting several professionals in one session (thoracic surgeon, patient coordinator, doctor from TRS, geneticist, physiatrist, and for children a pediatric cardiologist). When necessary, radiology and echocardiography were done shortly before the clinic schedule. At the clinic meeting, the patients underwent a clinical exam, results from radiology/− echocardiography were explained and genetic counseling was offered. If necessary blood samples for genetic testing were taken. After the patient consultation, the involved professionals had a joint meeting and agreed upon a further plan for diagnostics and follow‐up for the patient. The thoracic surgeon coordinated the writing of a discharge report with input from all the specialists, which was sent to the patient's primary care physician and/ or local hospital, with a copy to the patient. Further referrals, such as to ophthalmologist, special care during pregnancy or other needed health‐services, were done when necessary.
Before the establishment of the OUH aortopathy clinic, the care for patients with HTADs was not systematically coordinated. Children were followed‐up at the Department of Pediatric Cardiology at OUH, or sometimes in their local hospitals. At the age of 16–18 years, the follow‐up became even more fragmented: Some were transferred to the GUCH‐unit (Grown Up Congenital Heart disease unit) at OUH for follow‐up with echocardiography, others received services at the Department of Cardiothoracic Surgery, usually with MRI‐ or CT‐evaluations, while some were followed in cardiology departments at their local hospitals. For MFS patients, eye follow‐up was even more fragmented as patients were followed at OUH, at local eye doctors, or in local hospitals.
Some Norwegian consensus follow‐up guidelines for HTAD's exists, for instance cardiology guidelines for follow‐up of children with MFS (Holten‐Andersen, Holmstrøm, Neukamm, & Riise, 2018). Existing consensus is based on the European guidelines for aortic diseases and congenital grown up heart disease (Baumgartner et al., 2010; Erbel et al., 2014). However, with Norway as a large geographical area, many hospitals and primary care physicians involved, and turnover of medical staff, there was a risk that patients did not get adequate and coordinated treatment and follow‐up (Vanem et al., 2018).
A further challenge for both patients and primary care physicians, was that written reports from exams and test results was fragmented and not put together. As such, there was also a risk of diverging reports and recommendations. Neither responsibility for—nor methods and intervals for follow‐ups were coordinated and defined, and based on this unsatisfactory situation, a group of professionals started planning the OUH aortopathy clinic.
2.2.2. Evaluation of patient experiences
To evaluate patient experiences with diagnostics and follow‐up, the reference group decided to conduct a questionnaire study with patients attending the clinic from the start and onwards the first one and a half year. Importantly, the study design included a control group of patients receiving follow‐up as usual at the same hospital. The study was led and organized by TRS.
The study was planned as a prospective study with postal questionnaires. The study‐group consisted of the first consecutive 50 patients at the OUH aortopathy clinic, and were enrolled from September 2017 to November 2018. At their first visit at the clinic, they all got an information letter, the first out of two questionnaires, a consent form, and a prepaid return envelope. A control‐group with 50 HTAD patients in usual follow‐up was drawn from the electronic patient registry at the thoracic surgery department at the same hospital. The controls received the same information letter, questionnaire, consent form, and a prepaid return envelope by post in august 2017. If necessary, one reminder was sent to individuals in both groups. An additional follow‐up questionnaire was sent to both groups in March 2019. Adults answered for themselves, while parents answered and consented on behalf of children aged ˂18 years.
2.2.3. Ethical considerations
Participation in the study was voluntary and a written consent was obtained from all participants. The study information letter highlighted that both participation and non‐participation would have no consequences for further treatment or contact with OUH or TRS. Ethical approval was applied and given by the Data Protection officer at OUH.
2.2.4. Measurement methods
Due to the lack of a suitable validated instruments for the study purposes, two sets of study specific questionnaires were constructed in order to capture patient experiences. The literature review was used to inform the choices of questions. This included questions about: (a) patient's previous experiences with diagnostics (age at suspicion, age at final diagnosis, diagnostic time [years]), (b) patient's experiences with follow‐up previous to the establishment of the aortopathy clinic, (c) patient's experiences and satisfaction with follow‐up in usual care and with the aortopathy clinic. The questionnaire also had freetext/open‐ended options for additional comments.
One standardized questionnaire was included—The Effective Musculoskeletal Consumer Scale (EC‐17), which assesses patient perception of skills and behaviors important for effectively managing, participating in or leading their health care (Hamnes, Garratt, Kjeken, Kristjansson, & Hagen, 2010). The EC‐17 is a self‐administered instrument with 17 items covering five skill domains: (a) How to use health information, (b) How to clarify priorities, (c) Communication with others, (d) How to negotiate own role and take control, and (e) How to decide and take action. The items are summated and converted to produce a score from 0 to 100 with 100 as the best possible score. This instrument was chosen because it has been translated and validated into Norwegian (Hamnes et al., 2010) and the questions were deemed relevant by the reference group.
2.2.5. Analyzes
Patient data were de‐identified and entered into a customized database. The data was then processed using SPSS version 25. Due to the small sample sizes, only descriptive analyzes were used. Data are given as frequencies, percentages, medians and ranges. For comparison with other published studies, mean and SDs were used. Free‐text/open‐ended answers are presented as condensed meanings and quotes. Missing values in the EC‐17 questionnaire were handled as follows: Participants with >3 missing values (two persons on the first questionnaire, four on the second) were omitted from the analyses. For participants with ≤3 missing values (six persons on the first questionnaire, three on the second), the missing value was substituted by the item substitution method, replacing the missing item with the mean item value across subjects (Polit & Beck, 2008).
3. RESULTS
3.1. Results from the literature review
Thirty‐six potentially relevant papers were identified and read in full‐text (Figure 1). Only six peer‐reviewed papers (Bradley & Bowdin, 2016; Strider et al., 1996; Summers et al., 2006; von Kodolitsch et al., 2016; Wright et al., 2016; Zentner et al., 2015) and two conference abstracts (Andvik et al., 2011; McLean, 2012) met the eligibility criteria and were included in this review (Table 1). Excluded articles with reasons for exclusion are presented in Table S1. The included peer‐reviewed papers comprised a prospective study (Strider et al., 1996), one case series (Summers et al., 2006), one retrospective chart review (Zentner et al., 2015), one innovation report (Wright et al., 2016) and two narrative reviews/ expert experiences (Bradley & Bowdin, 2016; von Kodolitsch et al., 2016). Four studies (Andvik et al., 2011; Strider et al., 1996; Summers et al., 2006; Zentner et al., 2015) reported patient data on a total of 771 patients with MFS and other HTADs.
Except for a conference abstract (Andvik et al., 2011), all included papers described the organization of an aortopathy clinic/Marfan clinic/thoracic‐aortic clinic/cardiac‐ genetics clinic organized with a multidisciplinary team and a nurse as a patient coordinator. For simplicity, the term aortopathy clinic has been used for the rest of this article. Three papers reported benefits of consultations in settings where the patient‐meetings were organized with several professionals (multidisciplinary) at the same time. The advantages included for instance more appropriate and timely care and follow‐up, increased adherence with medical therapy, and reduced time to reach clinical decisions (Bradley & Bowdin, 2016; Wright et al., 2016; Zentner et al., 2015). Other documented benefits were better diagnostics and long‐term management of patients (Strider et al., 1996; Summers et al., 2006; Wright et al., 2016; Zentner et al., 2015). Andvik et al described a reduction in aortic dissection‐ and cardiovascular surgery rates (Andvik et al., 2011). One study found that the patients specifically appreciated the team competence and multidisciplinary care approach at the Marfan clinic (von Kodolitsch et al., 2016), while another reported high patient satisfaction with the multidisciplinary approach (Wright et al., 2016). None of the studies included control groups, but Andvik et al. compared results from their clinic from one decade with the next decade (Andvik et al., 2011).
3.2. Results from the evaluation of patient experiences
Thirty‐seven patients from the study‐group and 22 controls answered the first questionnaire, response‐rates of 74% and 44%, respectively. Twenty‐five people from the study‐group and 19 from the control‐group answered the second questionnaire as well. (Figure 2). The control‐group consisted of adults only, whereas the study‐group also included answers from parents on behalf of six children. Distribution of age, genders and places (region) of residence for both groups are shown in Table 2.
FIGURE 2.
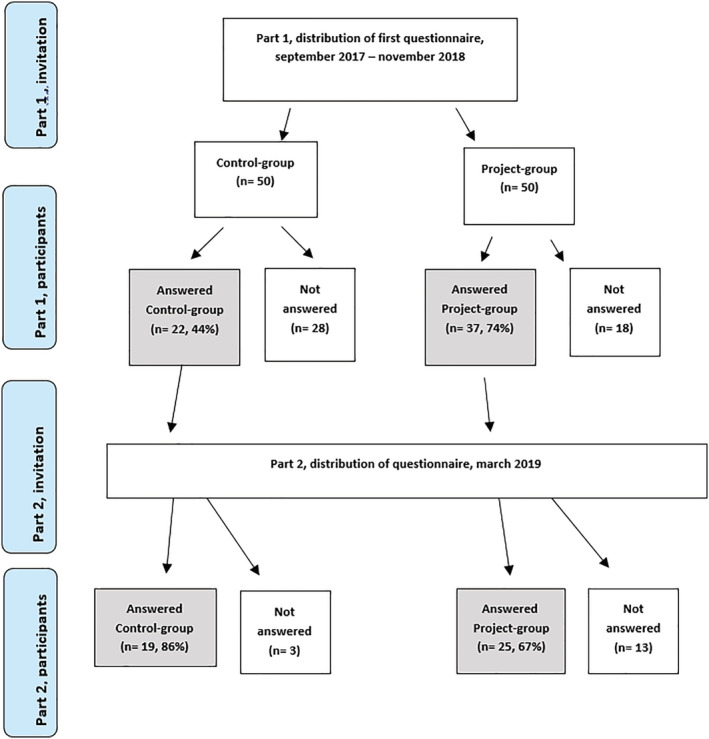
Flow‐chart of inclusion of patients in the evaluation of patient experiences [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Patient demographic data from Questionnaires 1 and 2
| First questionnaire | Follow‐up questionnaire | First questionnaire | Follow‐up questionnaire | ||
|---|---|---|---|---|---|
| Study‐group | Study‐group | Control‐group | Control‐group | ||
| (n = 37) | (n = 25) | (n = 22) | (n = 19) | ||
|
Age (mean (SD) Median (range)) |
36.14 (17.57) 36.00 (3–64) |
37.64 (19.17) 42 (9–64) |
49.82 (13.17) 53.00 (21–70) |
48.05 (22.71) 53 (21–68) |
|
| Gender (women) n (%) | 21 (56.8) | 16 (64%) | 12 (54.5) | 11 (57.9) | |
| Health‐region n (%) | |||||
| South eastern Norway | 29 (78.4) | 19 (76) | 18 (81.8) | 17 (89.5) | |
| Western‐Norway | 5 (13.5) | 5 (20) | 2 (9.1) | 1 (5.3) | |
| Middle‐Norway | 1 (2.7) | 0 | 1 (4.5) | 0 | |
| Northern‐Norway | 2 (5.4) | 1(4) | 1 (4.5) | 1 (5.3) | |
3.2.1. Patient experiences with diagnostics and follow‐up prior to the start of the aortopathy clinic
Patients in both the study‐ and the control‐group reported experiences of diagnostic delay, with a mean number of years from suspicion of diagnosis to final conclusion of 4.88 years (SD 7.69), median 1 year, range 0–32. There were some differences between the study‐group and control‐group, but this should be interpreted with caution as the groups were small. In 16 persons (12 with MFS), the diagnosis was changed from the originally suspected diagnosis, to another HTAD (Table 3). Median age at final diagnosis (for both groups) was 35.5 years, range 3–63.
TABLE 3.
First questionnaire, previous experiences with diagnostics
| Study‐group | Control‐group | Total | ||
|---|---|---|---|---|
| Number (%) | Number (%) | Number (%) | ||
| Suspected diagnosis | (n = 37) a | (n = 22) | (n = 59) | |
| Marfan syndrome | 24 (64.9) | 13 (59.1) | 37 (62.7) | |
| Loeys‐Dietz syndrome | 8 (21.6) | 3 (13.6) | 11 (18.6) | |
| Vascular Ehlers‐Danlos syndrome | 0 | 3 (13.6) | 3 (5.1) | |
| Other HTAD | 5 (13.5) | 3 (13.6) | 8 (13.6) | |
| Final diagnosis concluded? | (n = 36) | (n = 22) | (n = 58) | |
| Yes | 34 (94.4) | 22 (100) | 56 (96.5) | |
| No | 2 (5.6) | 0 | 2 (3.5) | |
| If concluded, which diagnosis? | (n = 34) | (n = 22) | (n = 56) | |
| Marfan syndrome | 16 (47) | 9 (40.9) | 25 (44.6) | |
| Loeys‐Dietz syndrome | 13 (38) | 8 (36.4) | 21 (37.5) | |
| Vascular Ehlers‐Danlos syndrome | 1 (2.9) | 3 (13.6) | 4 (7.2) | |
| Other HTAD | 4 (11.1) | 2 (9.1) | 6 (10.7) | |
| Changed diagnosis from suspected to final? | (n = 34) | (n = 22) | (n = 56) | |
| Yes | 10 (29.4) | 6 (27.3) | 16 (28.6) | |
| No | 24 (70.6) | 16 (72.7) | 40 (71.4) | |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | ||
| Age at suspected diagnosis |
(n = 36) 26.44 (21.99) 19.50 (0–63) |
(n = 20) 33.80 (19.06) 34.5 (2–59) |
(n = 56) 29.07 (21.12) 29.0 (0–63) |
|
| Age at final diagnosis |
(n = 27) 32.19 (20.20) 34 (3–63) |
(n = 17) 36.67 (16.11) 35 (5–60) |
(n = 44) 33.7 (18.76) 34.50 (3–63) |
|
| Number of years from suspicion to final diagnosis |
(n = 27) 6 (8.75)1 (0–32) |
(n = 13) 2.54 (SD 4.18) 1 (0–14) |
(n = 40) 4.88 (SD 7.69) 1 (0–32) |
|
Number of persons who have answered varies for each question; n is therefore given for each question and each group.
There were large variations in reported time since the last cardiovascular follow‐up or eye examination. The control‐group had a longer time since the last follow‐up compared to the study‐group (Table 4). Five of 27 MFS patients (from both groups) reported that they had never been examined by an ophthalmologist. However, four patients with LDS, one with vEDS and one with other HTAD, had been examined by ophthalmologist. All individuals reported to have had follow‐up visits with cardiologist or thoracic surgeon, but six reported to have had no MRI or CT investigations of the heart, aorta, or other blood vessels.
TABLE 4.
First questionnaire. How many years since your last follow‐up?
| Study‐group | Study‐group | Control group | Control group | |
|---|---|---|---|---|
| Mean (SD) | Follow‐up ≥3 years ago | Mean (SD) | Follow‐up ≥3 years ago | |
| Type of follow‐up | Median (range) | Number of persons | Median (range) | Number of persons |
| Eye‐doctor |
(n = 20) a 2.15 (3.13) 1 (0–12) |
6 |
(n = 11) 3.09 (3.75) 2 (1–14) |
4 |
| Thoracic‐surgeon or cardiologist |
(n = 32) 1.91 (2.35) 1 (1–14) |
5 |
(n = 21) 3.29 (3.33) 3.0 (1–10) |
13 |
| MRI or CT of aorta or other arteries |
(n = 28) 1.89 (2.47) 1.0 (1–14) |
2 |
(n = 22) 2.82 (1.14) 3.0 (2–7) |
12 |
Number of persons who have answered varies for each question. n is therefore given for each question and each group.
3.2.2. Patient satisfaction with specialist health‐care services
A large proportion of the patients in both groups (study‐group 83%, control‐group 77%) reported high or very high confidence with medical specialists. This finding was about the same in both questionnaires (Figure 3a). Regarding specialist's cooperation about disease follow‐up, a larger proportion of the control‐group reported “no—not at all”/“yes—but not good enough” compared to the study‐group (Figure 3b). This was also the same for the questions on organization of specialist services (Figure 3c) and counseling on how to cope with the disease (Figure 3d). In the follow‐up questionnaire, the study‐group had a drop in the percentage of persons answering “very good” (Figure 3b–d) and slightly more on “do not know” on these three questions. One person commented: “I have only been at the clinic once, so I do not know yet”.
FIGURE 3.
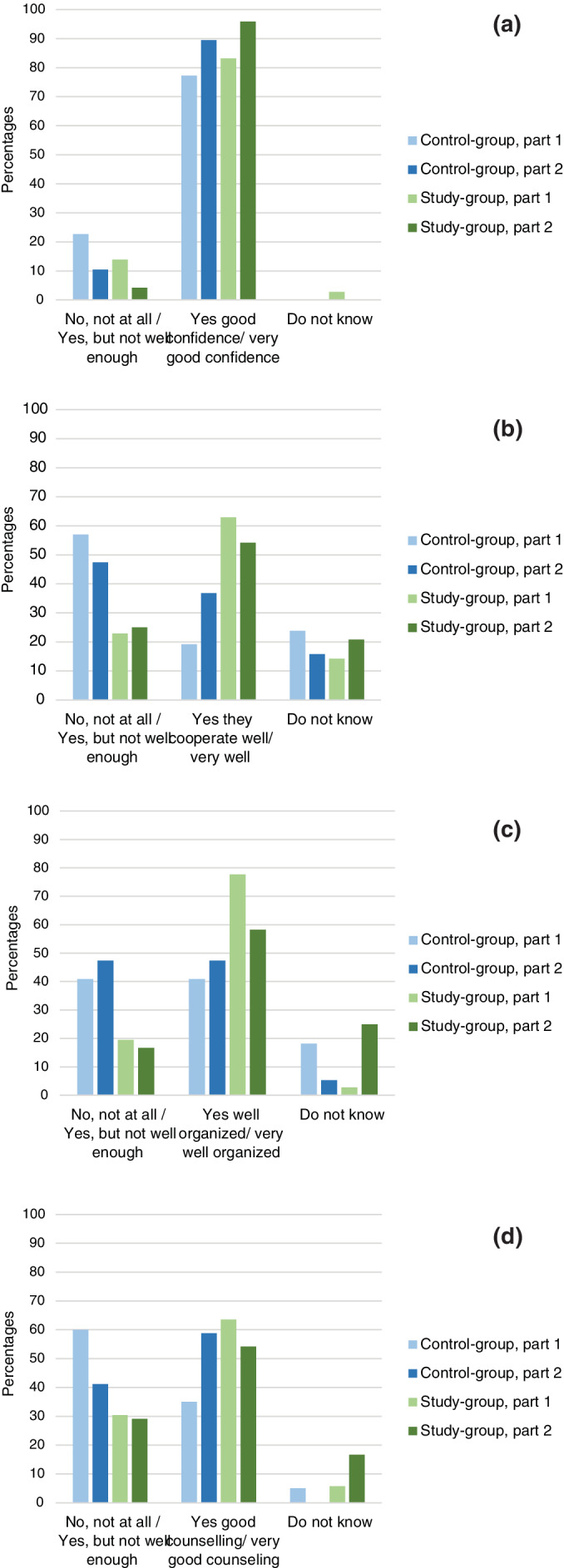
Patient experiences with follow‐up. (a) “Do you have confidence in the specialist's (OUH/ aortopathy clinic) professional competence?” (b) “Do you feel that the medical specialists (OUH/aortopathy clinic) cooperate well in the follow‐up of you and your disease?” (c) “Do you feel that the specialist services (OUH/aortopathy clinic) are well organized?” (d) “Do you feel that the specialists (OUH/aortopathy clinic) gave you adequate counseling to cope with your disease?” [Color figure can be viewed at wileyonlinelibrary.com]
The follow‐up questionnaire also asked the control‐group how they considered the coordination of their specialist follow‐up (on a scale from 0 (not at all) to 10 (very well)). Thirteen of 19 answered with a median of 4 (range 0–10). Eight of 19 elaborated these challenges in free‐text, mainly describing a lack of coordination, exemplified by these two statements:
I do not know, I guess that my primary physician receive the exam results. I have to keep order on getting my schedules for exams on time myself.
Severe lack of coordination, I do not get summons to important exams according to plan.
Fifteen of 19 individuals in the control‐group answered the open‐ended questioned on how they wished the services for their follow‐up to be organized. Three main themes were described: (a) Better coordination of the health‐services, and having a coordinator to contact with questions. (b) A multidisciplinary unit that sees the whole patient and disease picture. (c) Coordination of appointments to specialists and examinations at the same time/ day, diminishing travel time and making waiting time for the test results shorter.
The study‐group participants were asked to evaluate the OUH aortopathy clinic on a scale of 0 (not good) to 10 (very good): Median 9 (range 3–10). Twenty‐two of 36 persons also answered the open‐ended question on what they were especially satisfied with e most at the OUH aortopathy clinic visit. The comments mainly encompassed three themes:
1. Good information:
They are easily accessible, give good and thorough answers to questions, and are also available for contact after the appointment.
2. Multidisciplinary meeting with professionals with expert knowledge:
It is reassuring to see that the different specialties and professionals can cooperate and view the disease in a holistic perspective, it makes me believe that the system is moving forwards, and is important for me as I have children with the diagnosis.
3. Better coordination of appointments:
We got to meet all the specialists we needed that day, good to have all schedules on one day.
Eleven patients gave suggestions for further improvement of the clinic. These ranged from “keep up the good work”, to “a need for more focus on other issues” (i.e., pain, fatigue, mental health) and “coping with the aspects of the disease other than the affected organ‐systems”.
3.2.3. Patient's self‐management of disease, EC‐17
Patients in the control‐group had an EC‐17 mean score of 61.9 (SD 21.3) in the first questionnaire, with small changes at follow‐up (62.3, SD 22.1). The study‐group scored slightly higher on both occasions with 69.3 (SD 17.9) and 67.71 (SD 15.3), respectively. Comparisons between the two groups and reported mean values for a Norwegian group of rheumatoid arthritis patients are shown in Figure 4. The EC 17 items with the lowest mean scores were item 12: “I can be assertive to get what I need to meet my health needs” and item 13: “I feel a sense of control over my disease”.
FIGURE 4.
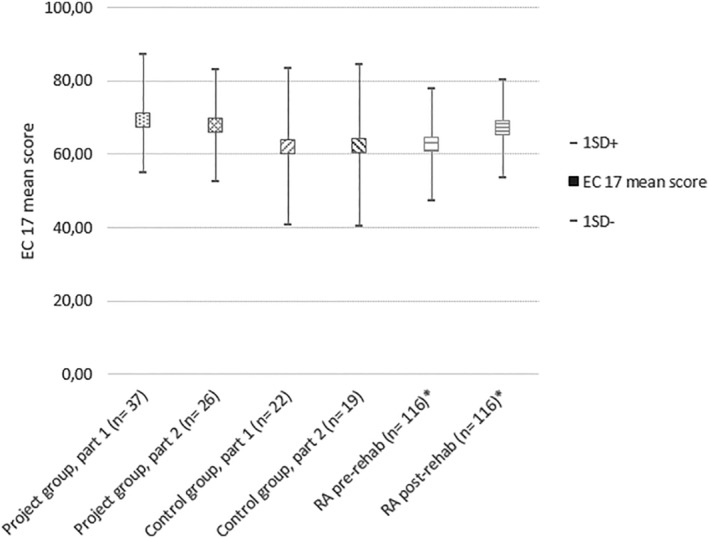
Mean score (±1 SD) of Effective musculoskeletal consumer scale 17 (EC17) for: Project‐group questionnaire 1 and 2, control group questionnaire 1 and 2, reported values for a Norwegian group of Rheumatoid arthritis patients before and after rehabilitation *Hamnes et al., 2010
Patients in both study‐group and control‐group gave free‐text comments on the EC‐17 questions (19 and 21 comments, respectively). The comments illustrated two main themes: challenges with coping with the disease, but also that some questions were not perceived as relevant for patients who did not view themselves as ill. This was by two patients' comments::
How to cope with this disease depends on how I feel from day to day, it is challenging to “always” cope with this disease.
These questions are not relevant for me and difficult to answer, as I do not perceive myself as ill or having a disease.
4. DISCUSSION
The literature review found only a few published articles on experiences with aortopathy clinics. However published research literature on aortopathy clinics indicated promising results when it comes to diagnostics and follow‐up routines. Our study of patient experiences indicated high satisfaction with the multidisciplinary aortopathy clinic. However both the literature review and our study of patient experiences highlighted that the diagnostic process and follow‐up routines are challenging in HTADs.
4.1. Review of the literature on multidisciplinary clinics for patients with HTADs
Only six papers (Bradley & Bowdin, 2016; Strider et al., 1996; Summers et al., 2006; von Kodolitsch et al., 2016; Wright et al., 2016; Zentner et al., 2015) and two conference abstracts (Andvik et al., 2011; McLean, 2012) were included in the scoping review. Despite thorough literature searches, we were surprised to find few scientific publications on the organization of aortopathy clinics, and very little concerning the patient experiences with such clinics. We may have lost relevant articles published with other search terms and purposes. The articles we found indicated that multidisciplinary aortopathy clinics provide better diagnostics and follow‐up services for patients with genetic aortic diseases. However, the published data is on less than a 1,000 patients, and the study designs were not suitable to conclude on the effects of such clinics.
Only two papers described patient experiences (von Kodolitsch et al., 2016; Wright et al., 2016). In one study, an e‐mail questionnaire was sent to patients, and results showed that the responders (19%) appreciated the competence of the medical team, multidisciplinary care, trusting the doctor, overcoming fear, and explanations (von Kodolitsch et al., 2016). In the study by Wright et al, patients reported high levels of satisfaction with the multidisciplinary approach (Wright et al., 2016). As Zentner et al stated, there seems to be need for more research to evaluate patient outcomes and cost‐effectiveness of aortopathy clinics (Zentner et al., 2015).
4.2. Diagnostic delay
In our study of patient experiences, patients in both the control‐ and study‐group reported a diagnostic period with a mean of 4.88 years from the first suspicion of diagnosis to conclusion. One patient waited a total of 32 years to have the diagnosis confirmed. In comparison, Roll et al found that 55% of German sickness‐insured MFS patients had a diagnostic delay with an average time to diagnosis of 607 days, maximum 3 years. They argued that an enhanced cooperation between physicians is necessary to improve diagnostic processes in MFS, preferably in multidisciplinary teams across sectors (Roll, 2012). A total of 16 of our patients reported that they had a change of diagnosis from suspicion to conclusion, this also illustrate that diagnostics is challenging in these patient groups. The clinical overlap between HTAD's and diagnostic criteria is substantial. As Pope et al found, the interpretation of genetic sequence variants is a substantial challenge in HTAD's (Pope et al., 2019). Our literature review found four studies describing that multidisciplinary aortopathy clinics seemed to have contributed to more accurate and quicker diagnostics (Strider et al., 1996; Summers et al., 2006; Wright et al., 2016; Zentner et al., 2015). More research seems to be needed to show if aortopathy clinics give better diagnostic services pathways for these patient groups. The timeframe in our study was too short to answer this research question.
4.2.1. Follow‐up
Median follow‐up time for eye and aortic issues varied between 1 and 3 years in both groups and might be explained by individual considerations and health status. It is however surprising that 13/22 persons in the control‐group and 5/37 in the study‐group reported their last exam by thoracic surgeon/cardiologist 3 years ago (or longer). This seems not to be in accordance with the existing European guidelines, which for instance recommend that stable MFS patients need a yearly visit with echocardiography (Baumgartner et al., 2010). For other HTAD's there still is a scarcity of research and clear consensus guidelines, for instance for vascular EDS were case to case multidisciplinary discussions is recommended (Erbel et al., 2014).
Explanations to our findings may include recall bias or the patient may have misinterpreted the study question. It may also illustrate that patients may have trouble both understanding and remembering information about follow‐up, and need help coordinating follow‐up of these very complex disorders. As many patients live with some distance away from Oslo, some are also followed‐up at their local hospital. This may cause challenges with cooperation and coordinated follow‐up. Nevertheless, Vanem et al. did a 10‐year follow‐up study and found that Norwegian MFS patients had significantly reduced life expectancy compared to the general Norwegian population and that 32% of the survivors were not followed‐up and treated according to given recommendations and guidelines (Vanem et al., 2018).
The results from the EC‐17 questionnaire illustrated that self‐management of these diagnoses are challenging for the patients. Some regarded themselves as healthy, whereas others struggled with multiple consequences of the disease and a very uncontrollable health situation. This highlights the need for coordinated multidisciplinary follow‐up. We assume that in the longer term, documentation from our clinic will collect relevant data to decide the impact of the multidisciplinary approach. As the time between first questionnaire and follow‐up was short for some of the patients we could not investigate if follow‐up time was improved due to the OUH aortopathy clinic.
4.2.2. Patient satisfaction
Patients and controls reported high confidence in the specialists' competence. The similar findings in the two groups are not surprising as mainly the same professionals examined the patients, both in the control‐ and study‐group. The study‐group had a slightly higher satisfaction with the specialist health‐services (i.e., organization, cooperation and counseling), compared to the control‐group. The numbers are small and results must be interpreted with caution. However, the study‐group showed very high satisfaction with the OUH aortopathy clinic and highlighted the benefit of better coordination and meeting different specialists at the same time. Our results therefore support findings from other research, both in aortopathies and other disorders, that multidisciplinary team follow‐up improve patient satisfaction (Hannemann‐Weber & Shultz, 2014; Wen & Schulman, 2014; Wright et al., 2016). Investigating patient experiences with aortopathy clinics seems to be an important area for further research.
4.2.3. Self‐management of disease
We chose to include a measure of self–management of disease, the EC‐17. Patients in the project‐group had slightly higher scores on both occasions than the control‐group. Patients in both groups scored much in the same range as reported values for patients with rheumatoid arthritis (Hamnes et al., 2010), illustrating that self‐management of disease can be challenging for patients with HTAD's. Self‐management of a disease include having the confidence to deal with medical‐, role‐ and emotional management of a condition (Packer et al., 2018). This is a tall order in diseases where finding and understanding advice may be difficult, and evidence‐based advice on follow‐up and treatment still is inadequate. Stanišić et al. concluded that it is especially important to help patients with aortic diseases to support their resources in managing their health‐condition, as this can improve life satisfaction and have a positive effect on treatment and recovery processes (Stanišić et al., 2018). Patient's self‐management of disease might be an important issue to investigate further in patients with HTAD's, also to evaluate if follow‐up in an aortopathy clinic will support self‐management.
4.2.4. Limitations and strengths
The literature review inclusion criteria restricted to English, German and Nordic languages may have lost important references, however the broad search in several databases may have compensated somewhat for this. The PRISMA‐ScR was followed, except for the addition of presenting overall findings of the included studies. Since this is not normally part of a scoping review, we want to underline this addition. Included articles were not assessed regarding risk of bias and results should be interpreted with caution.
The study of patient experiences has several potential biases. We did not have the possibility or permission to do a case matching on project‐group and control‐group participants, therefore between group differences may have influenced results. We were also unable to do an analysis on responders versus non‐responders and therefore do lack information on whether our study‐group and control‐group responders were significantly different from non‐responders. However the relatively high response rate, especially in the project group is a strength.
The questions on past experiences might have introduced recall bias to the results. Both the literature review and the questionnaire study might suffer from selection bias. The use of a self‐constructed nonvalidated questionnaire is a limitation. However, the construction of a questionnaire was deemed necessary, as no validated questionnaire exist for this purpose in these patient groups. Furthermore, the small sample precludes statistical comparisons, and the results must be interpreted with caution.
5. CONCLUSION
Our study of patient experiences, indicate that an aortopathy clinic is important for the management of patients with HTADs. The findings are supported by eight publications included in the literature review which also indicate that aortopathy clinics can improve diagnostics, follow‐up and treatment. There is a need for further studies to document the benefits of aortopathy clinics, including organization, treatment benefits, cost‐efficiency, patient and primary care physician satisfaction. Studies applying designs to document effects are suggested.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
The authors Trine Bathen, Kirsten Krogh‐Sørensen and Ingeborg B. Lidahl have contributed to the manuscript as follows: Contributions to conception and design: Trine Bathen, Kirsten Krogh‐Sørensen, Ingeborg B. Lidahl. Acquisition of data, analysis and interpretation of data: Trine Bathen, Ingeborg B. Lidahl. Drafting the manuscript and revising it critically for important intellectual content: Trine Bathen, Kirsten Krogh‐Sørensen, Ingeborg B. Lidahl. Given final approval of the version to be published: Trine Bathen, Kirsten Krogh‐Sørensen, Ingeborg B. Lidahl. Agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Trine Bathen, Kirsten Krogh‐Sørensen, Ingeborg B. Lidahl.
Supporting information
Table S1 list of excluded papers
ACKNOWLEDGMENTS
The authors want to thank all the patients who participated in the study. A special thank also to the leadership of participating departments at Oslo University Hospital and at TRS National Resource center and to colleagues at the aortopathy clinic, and at TRS. A special thank to the National Advisory Unit for Rare Disorders for financial support.
Bathen T, Krohg‐Sørensen K, Lidal IB. Multidisciplinary aortopathy clinics: A systematic scoping review of the literature and evaluation of patient experiences from a newly started clinic in Norway. Am J Med Genet Part A. 2020;182A:2552–2569. 10.1002/ajmg.a.61827
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Andvik, S. , Sherrah, A. , & Jeremy, R. (2011). Improved care for thoracic aortic aneurysm‐ two decades experience. Heart Lung and Circulation, 2, S224–S225. [Google Scholar]
- Baumgartner, H. , Bonhoeffer, P. , De Groot, N. M. , de Haan, F. , Deanfield, J. E. , Galie, N. , & Walma, E. (2010). ESC Guidelines for the management of grown‐up congenital heart disease (new version 2010). European Heart Journal, 31(23), 2915–2957. 10.1093/eurheartj/ehq249 [DOI] [PubMed] [Google Scholar]
- Blankart, C. R. , Milstein, R. , Rybczynski, M. , Schüler, H. , & von Kodolitsch, Y. (2016). Economic and care considerations of Marfan syndrome. Expert Review of Pharmacoeconomics Outcomes Research, 16(5), 591–598. [DOI] [PubMed] [Google Scholar]
- Boodhwani, M. , Andelfinger, G. , Leipsic, J. , Lindsay, T. , McMurtry, M. S. , Therrien, J. , & Siu, S. C. (2014). Canadian cardiovascular society position statement on the management of thoracic aortic disease. The Canadian Journal of Cardiology, 30(6), 577–589. 10.1016/j.cjca.2014.02.018 [DOI] [PubMed] [Google Scholar]
- Bradley, T. J. , Alvarez, N. A. M. , & Horne, S. G. (2016). A practical guide to clinical management of thoracic aortic disease. Canadian Journal of Cardiology, 32(1), 124–130. [DOI] [PubMed] [Google Scholar]
- Bradley, T. J. , & Bowdin, S. C. (2016). Multidisciplinary aortopathy clinics should now be the standard of care in Canada. The Canadian Journal of Cardiology, 32(1), 8–12. 10.1016/j.cjca.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Brownstein, A. J. , Kostiuk, V. , Ziganshin, B. A. , Zafar, M. A. , Kuivaniemi, H. , Body, S. C. , … Elefteriades, J. A. (2018). Genes associated with thoracic aortic aneurysm and dissection: 2018 update and clinical implications. Aorta (Stamford), 6(1), 13–20. 10.1055/s-0038-1639612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers, P. H. , Belmont, J. , Black, J. , De Backer, J. , Frank, M. , Jeunemaitre, X. , … Wheeldon, N. (2017). Diagnosis, natural history, and management in vascular Ehlers‐Danlos syndrome. American Journal of Medical Genetics. Part C: Seminars in Medical Genetics, 175(1), 40–47. 10.1002/ajmg.c.31553 [DOI] [PubMed] [Google Scholar]
- De Backer, J. , Bondue, A. , Budts, W. , Evangelista, A. , Gallego, P. , Jondeau, G. , … Roos Hesselink, J. (2019). Genetic counselling and testing in adults with congenital heart disease: A consensus document of the ESC Working Group of Grown‐Up Congenital Heart Disease, the ESC Working Group on Aorta and Peripheral Vascular Disease and the European Society of Human Genetics. European Journal of Preventive Cardiology, 2047487319854552, 204748731985455 10.1177/2047487319854552 [DOI] [PubMed] [Google Scholar]
- Erbel, R. , Aboyans, V. , Boileau, C. , Bossone, E. , Bartolomeo, R. D. , Eggebrecht, H. , … Vrints, C. J. (2014). 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). European Heart Journal, 35(41), 2873–2926. 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- Hamnes, B. , Garratt, A. , Kjeken, I. , Kristjansson, E. , & Hagen, K. B. (2010). Translation, data quality, reliability, validity and responsiveness of the Norwegian version of the effective musculoskeletal consumer scale (EC‐17). BMC Musculoskeletal Disorders, 11, 21 10.1186/1471-2474-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannemann‐Weber, H. , & Shultz, C. (2014). The impact of health care professionals' service orientation on patients' innovative behavior. Health Care Management Review, 39(4), 329–339. 10.1097/HMR.0b013e31829d534c. [DOI] [PubMed] [Google Scholar]
- Holten‐Andersen, M. N. , Holmstrøm, H. , Neukamm, C. , & Riise, N. (2018). Kardiologisk oppfølging av barn med Marfan Syndrom, i Pediatriveiledere Fra Norsk barnelegeforening, Oslo, Norway: The Norwegian Health Library; Helsebiblioteket; Retrieved from https://www.helsebiblioteket.no/pediatriveiledere?menuitemkeylev1=5962&menuitemkeylev2=5970&key=262181. [Google Scholar]
- Isselbacher, E. M. , Lino Cardenas, C. L. , & Lindsay, M. E. (2016). Hereditary influence in thoracic aortic aneurysm and dissection. Circulation, 133(24), 2516–2528. 10.1161/circulationaha.116.009762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen, H. , Velvin, G. , & Lidal, I. (2020). Adults with Loeys‐Dietz syndrome and vascular Ehlers‐Danlos syndrome: A cross‐sectional study of health burden perspectives. American Journal of Medical Genetics. Part A, 182(1), 137–145. 10.1002/ajmg.a.61396 [DOI] [PubMed] [Google Scholar]
- Krohg‐Sørensen, K. , Lingaas, P. S. , Lundblad, R. , Seem, E. , Paus, B. , & Geiran, O. R. (2017). Cardiovascular surgery in Loeys‐Dietz syndrome types 1‐4. European Journal of Cardio‐Thoracic Surgery, 52(6), 1125–1131. 10.1093/ejcts/ezx147 [DOI] [PubMed] [Google Scholar]
- Loeys, B. L. , Dietz, H. C. , Braverman, A. C. , Callewaert, B. L. , De Backer, J. , Devereux, R. B. , … De Paepe, A. M. (2010). The revised Ghent nosology for the Marfan syndrome. Journal of Medical Genetics, 47(7), 476–485. 10.1136/jmg.2009.072785 [DOI] [PubMed] [Google Scholar]
- MacCarrick, G. , Black, J. H., 3rd , Bowdin, S. , El‐Hamamsy, I. , Frischmeyer‐Guerrerio, P. A. , Guerrerio, A. L. , … Dietz, H. C., 3rd. (2014). Loeys‐Dietz syndrome: A primer for diagnosis and management. Genetics in Medicine, 16(8), 576–587. 10.1038/gim.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean, K. (2012). Aortopathy: Developing a thoracic‐aorta clinic. Canadian Journal of Cardiology, 1, S429. [Google Scholar]
- Meester, J. A. N. , Verstraeten, A. , Schepers, D. , Alaerts, M. , Van Laer, L. , & Loeys, B. L. (2017). Differences in manifestations of Marfan syndrome, Ehlers‐Danlos syndrome, and Loeys‐Dietz syndrome. Ann Cardiothorac Surg, 6(6), 582–594. 10.21037/acs.2017.11.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz, D. M. , & Regalado, E. (1993). Heritable thoracic aortic disease overview In Adam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., Bean L. J. H., Stephens K., & Amemiya A. (Eds.), GeneReviews((R)). Seattle (WA): University of Washington, Seattle; GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved. [Google Scholar]
- Packer, T. L. , Fracini, A. , Audulv, Å. , Alizadeh, N. , van Gaal, B. G. , Warner, G. , & Kephart, G. (2018). What we know about the purpose, theoretical foundation, scope and dimensionality of existing self‐management measurement tools: A scoping review. Patient Education and Counseling, 101(4), 579–595. [DOI] [PubMed] [Google Scholar]
- Peters, M. D. , Godfrey, C. M. , Khalil, H. , McInerney, P. , Parker, D. , & Soares, C. B. (2015). Guidance for conducting systematic scoping reviews. International Journal of Evidence‐Based Healthcare, 13(3), 141–146. 10.1097/xeb.0000000000000050 [DOI] [PubMed] [Google Scholar]
- Polit, D. F. , & Beck, C. T. (2008). Nursing research. Generating and Assessing Evidence for Nursing Practice: Lippincott Williams & Wilkins. [Google Scholar]
- Pope, M. K. , Ratajska, A. , Johnsen, H. , Rypdal, K. B. , Sejersted, Y. , & Paus, B. (2019). Diagnostics of hereditary connective tissue disorders by genetic next‐generation sequencing. Genetic Testing and Molecular Biomarkers., 23(11), 783–790. [DOI] [PubMed] [Google Scholar]
- Roll, K. (2012). The influence of regional health care structures on delay in diagnosis of rare diseases: The case of Marfan syndrome. Health Policy, 105(2–3), 119–127. 10.1016/j.healthpol.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Rozado, J. , Martin, M. , Pascual, I. , Hernandez‐Vaquero, D. , & Moris, C. (2017). Comparing American, European and Asian practice guidelines for aortic diseases. Journal of Thoracic Disease, 9(Suppl 6), S551–s560. 10.21037/jtd.2017.03.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showkathali, R. , Chelliah, R. , Brickham, B. , Dworakowski, R. , Alcock, E. , Deshpande, R. , … Byrne, J. J. I. j. o. c. (2014). Multi‐disciplinary clinic: Next step in “heart team” approach for TAVI. Heart, 174(2), 453–455. [DOI] [PubMed] [Google Scholar]
- Stanišić, M.‐G. , Rzepa, T. , Gawrońska, A. , Kubaszewski, P. , Putowski, M. , Stefaniak, S. , & Perek, B. (2018). Personal resources and satisfaction with life in Marfan syndrome patients with aortic pathology and in abdominal aortic aneurysm patients. Kardiochirurgia i torakochirurgia polska = Polish Journal of Cardio‐thoracic Surgery, 15(1), 27–30. 10.5114/kitp.2018.74672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strider, D. , Moore, T. , Guarini, J. , Fallin, B. , Ivey, J. , & Kron, I. (1996). Marfan's syndrome: A family affair. Journal of Vascular Nursing, 14(4), 91–98. [DOI] [PubMed] [Google Scholar]
- Summers, K. M. , West, J. A. , Peterson, M. M. , Stark, D. , McGill, J. J. , & West, M. J. (2006). Challenges in the diagnosis of Marfan syndrome. The Medical Journal of Australia, 184(12), 627–631. [DOI] [PubMed] [Google Scholar]
- Tricco, A. C. , Lillie, E. , Zarin, W. , O'Brien, K. K. , Colquhoun, H. , Levac, D. , … Weeks, L. J. A. o. i. m. (2018). PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Annals of Internal Medicine, 169(7), 467–473. [DOI] [PubMed] [Google Scholar]
- Vanem, T. T. , Geiran, O. R. , Krohg‐Sorensen, K. , Roe, C. , Paus, B. , & Rand‐Hendriksen, S. (2018). Survival, causes of death, and cardiovascular events in patients with Marfan syndrome. Molecular Genetics & Genomic Medicine, 6(6), 1114–1123. 10.1002/mgg3.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velvin, G. , Wilhelmsen, J. E. , Johansen, H. , Bathen, T. , & Geirdal, A. O. (2019). Systematic review of quality of life in persons with hereditary thoracic aortic aneurysm and dissection diagnoses. Clinical Genetics, 95(6), 661–676. 10.1111/cge.13522 [DOI] [PubMed] [Google Scholar]
- von Kodolitsch, Y. , Rybczynski, M. , Vogler, M. , Mir, T. S. , Schüler, H. , Kutsche, K. , … Pyeritz, R. E. (2016). The role of the multidisciplinary health care team in the management of patients with Marfan syndrome. Journal of Multidisciplinary Healthcare, 9, 587–614. 10.2147/jmdh.s93680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, J. , & Schulman, K. A. J. P. O. (2014). Can team‐based care improve patient satisfaction? A systematic review of randomized controlled trials. PLoS One, 9(7), e100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, E. B. , Henriques, S. , Houghton, C. , Clarke, B. , Keavney, B. , & Venetucci, L. (2016). Definition and delivery of an aortopathy bundle of care (ABC): A tool for improving diagnosis and management of Marfan syndrome and related conditions. Clinical Medicine, 16(Suppl 3), s30–s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner, D. , Thompson, T. N. , James, P. A. , Trainer, A. , Adès, L. C. , Macciocca, I. , … Lewis, N. (2015). The cardiac genetics clinic: A model for multidisciplinary genomic medicine. The Medical Journal of Australia, 203(6), 261–261.e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 list of excluded papers
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


