Abstract
This study compared initiation of insulin and other antihyperglycaemic agents (AHAs) with canagliflozin versus placebo for participants with type 2 diabetes and a history/high risk of cardiovascular disease in the CANagliflozin cardioVascular Assessment Study (CANVAS) Program. After 1 year, fewer participants treated with canagliflozin versus placebo initiated any AHA (7% vs. 16%), insulin (3% vs. 9%) or any non‐insulin AHA (5% vs. 12%) (P < .001 for all); overall AHA initiation rates increased over time but were consistently lower with canagliflozin compared with placebo. During the study, the likelihood of initiating insulin was 2.7 times lower for participants treated with canagliflozin compared with placebo (hazard ratio, 0.37; 95% CI: 0.31, 0.43; P < .001). The time difference between 10% of patients in the canagliflozin and placebo groups being initiated on insulin from the beginning of the trial was about 2 years. Time to initiation of other AHAs, including metformin, dipeptidyl peptidase‐4 inhibitors, glucagon‐like peptide‐1 receptor agonists and sulphonylureas, was also delayed for canagliflozin versus placebo (P < .001 for each). Compared with placebo, canagliflozin delayed the need for initiation of other AHAs and delayed time to insulin therapy, an outcome that is important to many people with diabetes.
Keywords: antihyperglycaemic agent, insulin, SGLT2 inhibitor, type 2 diabetes
1. INTRODUCTION
Canagliflozin is a sodium glucose co‐transporter 2 (SGLT2) inhibitor that lowers blood glucose, body weight and blood pressure across a broad range of patients with type 2 diabetes. 1 In the CANagliflozin cardioVascular Assessment Study (CANVAS) Program, canagliflozin reduced the risk of the composite of cardiovascular death, non‐fatal myocardial infarction or non‐fatal stroke compared with placebo in patients with type 2 diabetes and established cardiovascular disease or high cardiovascular risk. 2 At baseline, 50% of CANVAS Program participants were on insulin, and nearly all participants (99%) were treated with at least one antihyperglycaemic agent (AHA). 3 Overall, 19% were on one AHA, 44% were on two AHAs and 36% were on ≥3 AHAs at baseline. During the course of the CANVAS Program, 22% of participants initiated new AHAs. A higher proportion of participants on placebo initiated new AHAs versus canagliflozin, but details of the timing of initiation for specific classes of AHAs have not been reported. 3 The objectives of this study were to examine the impact of canagliflozin versus placebo on initiation of insulin and other AHAs in the CANVAS Program and to quantify the extent to which canagliflozin spares the need for initiation of insulin.
2. METHODS
2.1. Ethics
CANVAS and CANVAS‐R were approved by the ethics committees at each site, and all participants provided written informed consent. All the procedures were in accordance with the principles of the Helsinki Declaration of 1964, as revised in 2013.
2.2. Study design and participants
The CANVAS Program consisted of two multicentre, double‐blind, placebo‐controlled, randomized trials: CANVAS and CANVAS‐Renal (CANVAS‐R; ClinicalTrials.gov registration no. NCT01032629, NCT01989754). The design, methods and primary results of the CANVAS Program have been published. 2 , 4 , 5 , 6 Eligible participants had type 2 diabetes (glycated haemoglobin ≥7.0% and ≤10.5%) and were either ≥30 years of age with a history of symptomatic atherosclerotic cardiovascular disease or ≥50 years of age with ≥2 risk factors for cardiovascular disease. Participants in CANVAS were randomly assigned in a 1:1:1 ratio to canagliflozin 100 or 300 mg or matching placebo; participants in CANVAS‐R were randomly assigned in a 1:1 ratio to canagliflozin 100 mg or matching placebo, with optional uptitration to canagliflozin 300 mg or matching placebo starting from week 13. During the CANVAS Program, investigators were allowed to adjust a patient's AHA therapy as they felt was appropriate in accordance with national and international guidelines, including the addition of oral and/or injectable AHAs, with the exception of any other SGLT2 inhibitors.
2.3. Outcomes
Key outcomes included new initiation of AHAs, defined as post‐randomization commencement of any AHA not taken at baseline: insulin, metformin, dipeptidyl peptidase‐4 (DPP‐4) inhibitor, glucagon‐like peptide‐1 (GLP‐1) receptor agonist and sulphonylurea.
2.4. Statistical analyses
The proportion of patients initiating new AHAs and time to initiation of new AHAs were analyzed for canagliflozin and placebo using the integrated CANVAS Program dataset. For analyses of the initiation of any AHA and any non‐insulin AHA, all participants were included. Event time was measured from randomization to the first of initiation of AHA (for those who added AHAs) or last trial contact (for those who did not), and the proportion of participants with AHA additions at 1, 3 and 5 years and 95% confidence intervals (CIs) were derived from Kaplan‐Meier survival probabilities, as was the restricted mean time to event. For the addition of specific AHAs, only participants who were not using AHAs at baseline were included in the analysis. To compare the likelihood of AHA initiation across treatment groups over the course of the trial, Cox proportional hazards models, with treatment as a fixed effect and trial as a stratification factor, were used to produce hazard ratios (HRs) and 95% CIs. If the proportional hazards assumption was violated, Peto HR and 95% CIs were calculated based on the stratified log‐rank statistic, with trial as a stratification factor. 7 , 8
Data from this study are available in the public domain via the Yale University Open Data Access Project (http://yoda.yale.edu/).
3. RESULTS
3.1. Participants
Baseline characteristics and AHA use were similar in participants treated with canagliflozin and placebo (Table S1). Participants had an average age of 63 years, the majority were men (64%) and the average length of diabetes was approximately 14 years. Approximately 77% were on metformin, 50% were on insulin, 43% were on a sulphonylurea, 12% were on a DPP‐4 inhibitor and 4% were on a GLP‐1 receptor agonist at baseline. Median follow‐up duration was 3.6 years.
3.2. Overall antihyperglycaemic agent initiation in the CANVAS Program
Kaplan‐Meier analysis showed that fewer participants in the canagliflozin arm initiated any AHA (7% vs. 16%), insulin (3% vs. 9%) or any non‐insulin AHA (5% vs. 12%) compared with participants in the placebo arm at 1 year. Similar trends were observed at 3 years (any AHA: 19% vs. 32%; insulin: 9% vs. 21%; any non‐insulin AHA: 15% vs. 26%) and 5 years (any AHA: 28% vs. 43%; insulin: 14% vs. 30%; any non‐insulin AHA: 23% vs. 36%). The proportional hazards assumption was violated (P < .05) for all AHAs with treatment effects increasing over time. Peto HRs demonstrate that the likelihood of initiating any AHA [HR, 0.47 (95% CI: 0.43, 0.51)], insulin [HR, 0.37 (95% CI: 0.31, 0.43)] or any non‐insulin AHA [HR, 0.50 (95% CI: 0.45, 0.55)] was significantly reduced compared with placebo (all P < .001; Figure S1).
3.3. Initiation of insulin in the CANVAS Program
The time difference between 10% of patients in the canagliflozin and placebo groups being initiated on insulin from the beginning of the trial was >2 years, with about 10% of patients in the placebo group on insulin at 52 weeks and 10% of patients in the canagliflozin group on insulin by 160 weeks. During the trials, the risk of initiating insulin was 2.7 times lower for participants treated with canagliflozin compared with placebo (Figure 1).
FIGURE 1.
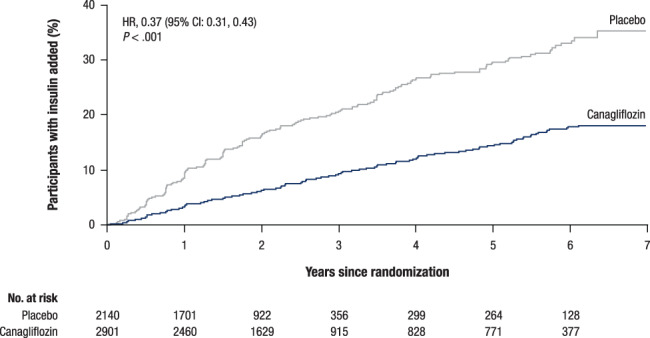
Time to initiation of insulin in the subset of participants not previously on insulin. CI, confidence interval; HR, hazard ratio
3.4. Effects on estimated glomerular filtration rate
Proportional hazards held when estimated glomerular filtration rate (eGFR) was included in the statistical model (P = .103) and the need for initiation of insulin therapy with canagliflozin compared with placebo was lower in both patients with eGFR ≥60 mL/min/1.73 m2 (HR, 0.37; 95% CI: 0.31, 0.44) and patients with eGFR <60 mL/min/1.73 m2 (HR, 0.52; 95% CI: 0.36, 0.75), with no difference in effects between subgroups (P interaction = .11). Those taking insulin at baseline had a lower eGFR than those not on insulin, but the effects of canagliflozin on attenuating the long‐term eGFR decline were qualitatively identical (Figure S2).
3.5. Initiation of other antihyperglycaemic agents
The time to initiation of other AHAs, including metformin, DPP‐4 inhibitors, GLP‐1 receptor agonists and sulphonylureas, was also delayed with canagliflozin versus placebo (Figure 2). There was strong evidence that lower proportions of participants in the canagliflozin versus placebo group initiated other AHAs at 1, 3 and 5 years of follow‐up: fewer participants treated with canagliflozin compared with placebo initiated insulin at year 1 (3% vs. 9%; P < .001), and this pattern continued through year 3 (9% vs. 21%) and year 5 (14% vs. 30%; Table S2).
FIGURE 2.
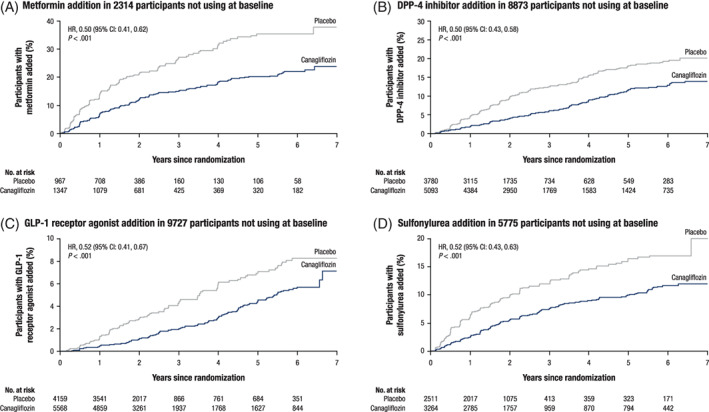
Time to initiation of other antihyperglycaemic agents in the subsets of participants not previously on these agents. A, Metformin. B, DPP‐4 inhibitors. C, GLP‐1 receptor agonists. D, Sulphonylureas. CI, confidence interval; DPP‐4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; HR, hazard ratio
3.6. Hypoglycaemia
There was a statistically lower rate of hypoglycaemia in subjects not on insulin who were taking the 300 mg dose of canagliflozin compared with placebo (χ2 P = .036). Otherwise, there were no differences in hypoglycaemia rates (Table S3).
4. CONCLUSIONS
In the CANVAS Program, participants treated with canagliflozin had fewer AHAs initiated overall and fewer insulin initiations, compared with participants treated with placebo. The extent of this benefit, with an HR of 0.37, indicates that patients were 63% less likely to go on to insulin if they were on active canagliflozin treatment. About 10% of patients in the placebo group were on insulin at 52 weeks and 10% of patients in the canagliflozin group were on insulin by 160 weeks. This pattern was consistent during years 1, 3 and 5 of follow‐up. The delayed time to initiation of insulin was consistent in both eGFR subgroups, suggesting this benefit extends to all CANVAS Program participants. Similar findings have been shown for empagliflozin (HR, 0.46; 95% CI: 0.39, 0.54). 9
Other agents also delay the need for insulin. The VERIFY trial was explicitly designed to do so and reported, in patients with newly onset type 2 diabetes, that the median time to treatment failure in a metformin monotherapy group was 36.1 months, while the median time to treatment failure for those receiving early combination therapy of vildagliptin and metformin could only be estimated to be beyond the study duration at 61.9 months. A significant reduction in the relative risk for time to initial treatment failure was observed in the early combination treatment group compared with the monotherapy group over the 5‐year study duration (HR, 0.51; 95% CI: 0.45, 0.58; P < .0001). 10 Other oral agents, such as linagliptin in the CARMELINA trial, have shown delays in the need for insulin. 11
The results reported here benefit from the rigorous design, conduct and analysis of the CANVAS Program but are limited by the post hoc nature of the analysis. In addition, information on insulin dose changes, which could also provide insight on AHA use, was not available.
Beyond reflecting improved glycaemic control, the lower use of insulin and other AHAs may give benefit as side effects such as hypoglycaemia or gastrointestinal intolerance are not a feature of the SGLT2 inhibitor class, and they are oral agents. It was not unexpected that canagliflozin use meant that other AHAs were less often used, but the delay in insulin initiation may represent additional benefits to health care systems and patients. Insulin is associated with reduced health‐related quality of life for many individuals with diabetes. 12 The costs of insulin have risen considerably for patients and health systems over the past several years, 13 and patients may experience hypoglycaemia, as well as pain associated with injection. 14 There are considerable associated costs of insulin initiation including staff costs for training, home glucose monitoring, and increased need for an ordered daily regimen. Patients and caregivers may also be reluctant to start insulin as it may be viewed as a “final” therapy and patients in some employments may be disqualified. 14 Thus, beyond reflecting improved glycaemic control, the lower use of insulin and other AHAs with canagliflozin may represent reduced utilization of health services, reduced health care costs, better adherence and improved patient satisfaction.
In conclusion, among CANVAS Program participants, canagliflozin was associated with a lower likelihood for starting insulin or other AHAs over a 5‐year period.
CONFLICT OF INTEREST
D.R.M. has served on advisory boards and as a consultant for Novo Nordisk, Novartis, Sanofi‐Aventis, and Servier and has given lectures for Novo Nordisk, Servier, Sanofi‐Aventis, Novartis, Mitsubishi Tanabe and Aché Laboratories. C.W. has served as a consultant for Janssen. M.D. has acted as consultant, advisory board member and speaker for Novo Nordisk, Sanofi‐Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca and Janssen, has served as an advisory board member for Servier and Gilead Sciences Ltd., has served as a speaker for NAPP, Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc. and has received grants in support of investigator trials from Novo Nordisk, Sanofi‐Aventis, Lilly, Boehringer Ingelheim, AstraZeneca and Janssen. A.S. is a full‐time employee of Axio Research, which received payment from Janssen for statistical support of the analyses reported in this manuscript. M.A. and M.L. were full‐time employees of Janssen Research & Development, LLC at the time of study. V.P. has received fees for advisory boards, steering committee roles or scientific presentations from AbbVie, Astellas, AstraZeneca, Bayer, Baxter, BMS, Boehringer Ingelheim, Dimerix, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pfizer, PharmaLink, Relypsa, Retrophin, Sanofi, Servier, Vifor and Tricida. K.W.M.'s disclosures can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey. B.N. is supported by an Australian National Health and Medical Research Council Principal Research Fellowship, holds a research grant for this study from Janssen and has held research grants for other large‐scale cardiovascular outcome trials from Roche, Servier and Merck Schering Plough; his institution has received consultancy, honoraria or travel support for contributions he has made to advisory boards and/or the continuing medical education programmes of Abbott, Janssen, Novartis, Pfizer, Roche, and Servier.
AUTHOR CONTRIBUTIONS
D.R.M., V.P., K.W.M. and B.N. contributed to the design and conduct of the study and interpretation of the data. C.W., M.D., M.A. and M.L. contributed to the interpretation of the data. A.S. contributed to the analysis and interpretation of data. All authors approved the final manuscript for submission. D.R.M. and B.N. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
5.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14143.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
We report these findings on behalf of the CANVAS Program Collaborative Group. We thank all investigators, study teams and patients for participating in these studies. This study was supported by Janssen Research & Development, LLC. Medical writing support was provided by Alaina Mitsch, PhD, of MedErgy, and was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
Matthews DR, Wysham C, Davies M, et al. Effects of canagliflozin on initiation of insulin and other antihyperglycaemic agents in the CANVAS Program. Diabetes Obes Metab. 2020;22:2199–2203. 10.1111/dom.14143
Trial registration: ClinicalTrials.gov identifiers NCT01032629, NCT01989754.
Funding information Janssen Research & Development, LLC; Mitsubishi Tanabe Pharma Corporation; Janssen Research & Development
REFERENCES
- 1. Rosenthal N, Meininger G, Ways K, et al. Canagliflozin: a sodium glucose co‐transporter 2 inhibitor for the treatment of type 2 diabetes mellitus. Ann N Y Acad Sci. 2015;1358:28‐43. [DOI] [PubMed] [Google Scholar]
- 2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 3. Wysham C, Davies M, Tsoukas M, Desai M, Fulcher G. Effects of canagliflozin (CANA) on HbA1c and changes in AHAs in the CANagliflozin cardioVascular Assessment Study (CANVAS) Program. Endocr Pract. 2018;24(Suppl 1):61‐62. Abstract 262. [Google Scholar]
- 4. Neal B, Perkovic V, Mahaffey KW, et al. Optimizing the analysis strategy for the CANVAS Program: a pre‐specified plan for the integrated analyses of the CANVAS and CANVAS‐R trials. Diabetes Obes Metab. 2017;19:926‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neal B, Perkovic V, Matthews DR, et al. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study‐Renal (CANVAS‐R): a randomized, placebo‐controlled trial. Diabetes Obes Metab. 2017;19:387‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, de Zeeuw D, et al. Rationale, design, and baseline characteristics of the Canagliflozin cardiovascular Assessment Study (CANVAS)—a randomized placebo‐controlled trial. Am Heart J. 2013;166:217‐223. [DOI] [PubMed] [Google Scholar]
- 7. Lin D‐Y, Dai L, Cheng G, Sailer MO. On confidence intervals for the hazard ratio in randomized clinical trials. Biometrics. 2016;72:1098‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaduganathan M, Sattar N, Fitchett DH, et al. 30‐OR: Empagliflozin delays need for insulin initiation in patients with type 2 diabetes and cardiovascular disease: Findings from EMPA‐REG OUTCOME. Diabetes. 2020;69(Suppl. 1). https://diabetes.diabetesjournals.org/content/69/Supplement_1/30-OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matthews DR, Paldanius PM, Proot P, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5‐year, multicentre, randomised, double‐blind trial. Lancet. 2019;394:1519‐1529. [DOI] [PubMed] [Google Scholar]
- 11. Hanssen NM, Jandeleit‐Dahm KA. Dipeptidyl peptidase‐4 inhibitors and cardiovascular and renal disease in type 2 diabetes: what have we learned from the CARMELINA trial? Diab Vasc Dis Res. 2019;16:303‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weng W, Liang Y, Kimball E, Hobbs T, Kong S. Trends in comorbidity burden and treatment patterns in type 2 diabetes: longitudinal data from a US cohort from 2006 to 2014. Diabetes Res Clin Pract. 2018;142:345‐352. [DOI] [PubMed] [Google Scholar]
- 13. Cefalu WT, Dawes DE, Gavlak G, et al. Insulin Access and Affordability Working Group: conclusions and recommendations. Diabetes Care. 2018;41:1299‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellis K, Mulnier H, Forbes A. Perceptions of insulin use in type 2 diabetes in primary care: a thematic synthesis. BMC Fam Pract. 2018;19:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


