Abstract
Objectives
To investigate whether xenograft EB (EndoBon) is non‐inferior to xenograft BO (Bio‐Oss) when used in reconstructive surgery of peri‐implant osseous defects.
Materials and methods
Dental patients with one implant each demonstrating peri‐implantitis were randomized to receive surgical debridement and defect fill with either BO or EB. Changes in bone level (BL) and intrabony defect depth (IDD) evaluated radiographically were the primary outcomes. The secondary outcomes included changes in probing pocket depth (PPD), bleeding on probing (BoP), and suppuration on probing (SoP). All outcomes were recorded before treatment and at 6 and 12 months post‐treatment.
Results
Twenty‐four patients (n = 11 BO, n = 13 EB) completed the study. Both groups demonstrated significant within‐group improvements in all clinical and radiographic parameters at 6 and 12 months (p ≤ .001). At 12 months, both groups presented with IDD reductions of 2.5–3.0 mm on average. The inter‐group differences were not statistically significant at all time points and for all the examined parameters (p > .05). While the radiographic defect fill in both groups exceeded > 1 mm and can be considered treatment success, successful treatment outcomes as defined by Consensus Reporting (no further bone loss, PPD ≤ 5 mm, no BOP, and no SoP) were identified in 2/11 (18%) BO and 0/13 (0%) EB individuals (Fisher's exact test, p = .199).
Conclusions
Within the limitations of this pilot study, the application of xenograft EB showed to be non‐inferior to xenograft BO when used in reconstructive surgery of peri‐implant osseous defects.
Keywords: bone substitute, defect reduction, peri‐implantitis, pilot study, radiographic evaluation, surgical treatment
1. INTRODUCTION
Peri‐implantitis is a growing concern in the dental community and a public health issue associated with high economic burden (Schwendicke, Tu, & Stolpe, 2015). The prevalence of peri‐implantitis ranges from 1% to 85% depending on the disease definition (Dreyer et al., 2018). A recent study reported that approximately 1 out of 3 patients and 1 out of 5 implants experienced peri‐implantitis (Kordbacheh Changi, Finkelstein, & Papapanou, 2019). According to the 2017 World Workshop, peri‐implantitis is defined as “a plaque‐associated pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri‐implant mucosa and subsequent progressive loss of supporting bone” (Berglundh, Armitage, et al., 2018).
Various treatment protocols for peri‐implantitis have been suggested; however, there is no consensus as to which one is the most effective intervention (Garaicoa‐Pazmino, Sinjab, & Wang, 2019). Non‐surgical therapy appears to be ineffective in reducing probing depths and eliminating bacteria from implant surfaces especially in more severe cases (Persson, Samuelsson, Lindahl, & Renvert, 2010; Renvert, Hirooka, Polyzois, Kelekis‐Cholakis, & Wang, 2019). Surgical therapy has proven to be more effective in the reduction of probing pocket depths and bleeding on probing as well as in promoting new bone fill, possibly because it provides access to the defect area for removal of the granulation tissue and debridement/decontamination of the exposed implant threads (Berglundh, Wennstrom, & Lindhe, 2018; Froum et al., 2016; Sarmiento, Norton, Korostoff, Ko, & Fiorellini, 2018). The addition of bone substitutes with or without barrier membranes has demonstrated promising results in terms of radiographic defect reduction and improvement of clinical parameters, especially in well‐contained (4‐wall and 3‐wall) intrabony defects (Jepsen et al., 2016; Renvert, Roos‐Jansaker, & Persson, 2018; Roos‐Jansaker, Persson, Lindahl, & Renvert, 2014; Schwarz, Sahm, Bieling, & Becker, 2009; Schwarz, Sahm, Schwarz, & Becker, 2010; Wiltfang et al., 2012). Nevertheless, complete resolution of the bony defect is still not predictable (Khoshkam et al., 2016).
Bovine bone substitutes have been extensively used in periodontal regeneration, socket preservation, peri‐implant reconstruction, and alveolar bone augmentation (Garaicoa‐Pazmino et al., 2019; Haugen & Lyngstadaas, 2019). Numerous preclinical and clinical histomorphometric studies have shown that bovine xenografts are biocompatible and osteoconductive, with extremely slow degradation rate, and therefore able to maintain the volume of the augmented site in the long term (Artzi, Tal, & Dayan, 2000; Cordaro et al., 2008; Mellonig, 2000; Ramirez‐Fernandez et al., 2011; Spies, Schnurer, Gotterbarm, & Breusch, 2010).
Bio‐Oss® (BO) is a well‐known deproteinized sterilized cancellous bovine bone with a porosity of 75% to 80% and small granule size of 250–1,000 μm (Degidi et al., 2004). Due to its hydrophilic properties, it facilitates the adsorption of blood cells and proteins (Jiang, Dziak, Lynch, & Stephan, 1999). This leads to reliable bone formation and implant osseointegration, which resembles the osseointegration that takes place in normally healed extraction sites (Berglundh & Lindhe, 1997). BO has been used extensively for the treatment of peri‐implantitis showing promising results in terms of reduced radiographic defect depth and improved clinical parameters (Aghazadeh, Rutger Persson, & Renvert, 2012; Matarasso, Iorio Siciliano, Aglietta, Andreuccetti, & Salvi, 2014; Roccuzzo, Pittoni, Roccuzzo, Charrier, & Dalmasso, 2017; Schwarz et al., 2010).
Endobon® (EB) is a newer bovine‐derived hydroxyapatite ceramic with small granule size (particle size 500–1,000 μm) that has been fully deproteinized by a two‐step, high temperature process for safety from bacteria, viruses, and prions (manufacturer's information at dentalwww.zimmerbiometdental.com). This processing method leads to high crystallinity and minimal resorption of graft particles (Block, 2019). The structure of EB with the interconnecting micro‐ and macropores facilitates the ingress of osteogenic cells and acceleration of bone ingrowth (Hing, Best, & Bonfield, 1999; Ramirez‐Fernandez et al., 2011). Histological and clinical data suggest that EB has similar reconstructive potential to BO when used for grafting fresh extraction sockets (Barone et al., 2013). The use of EB in the surgical treatment of peri‐implantitis has been recently reported in a clinical trial (Renvert et al., 2018).
The objective of the present study is to evaluate whether the reconstructive potential of EB is non‐inferior to BO when applied in peri‐implant intra‐osseous defects in a non‐submerged technique after 6 and 12 months of healing. We hypothesize that the peri‐implantitis defects treated with EB will not exhibit an inferior outcome compared to BO in terms of radiographic defect reduction around dental implants.
2. MATERIALS AND METHODS
2.1. Study design
The study was carried out as a randomized, controlled, single‐blinded, non‐inferiority clinical trial of 12 months of follow‐up. The study protocol was approved by the ethical committee of the VU Medical Centre, Amsterdam (NL51525.029.15), and was registered at the ISRCTN (https://www.isrctn.com/ISRCTN14347002). The study was conducted in accordance with the principles outlined in the Declaration of Helsinki (1975, revised in 2008).
2.2. Study population
The present pilot study is in compliance with the CONSORT guidelines. The study participants were recruited from patients who had been referred to the Department of Oral Implantology and Prosthodontics or the Department of Periodontology at the Academic Centre for Dentistry Amsterdam (ACTA) for treatment of peri‐implantitis. Before participation, each patient was given a detailed description of the procedure, its associated risks and benefits, and signed an informed consent. Patients who presented with a minimum of one osseo‐integrated implant, which had been in function for more than one year, were included in the study. In patients with more than one peri‐implant defect meeting the inclusion criteria, only one defect per patient was defined as the target (the most severe defect) and included in the study. All patients had received non‐surgical treatment before enrollment.
Patients were screened for the following eligibility criteria: marginal bone loss ≥3 mm detected radiographically and probing pocket depth (PPD) ≥5 mm at one or more peri‐implant sites, in combination with bleeding and/or suppuration on probing (BoP/SoP). The patients who met the initial eligibility criteria were assessed intra‐operatively for the following defect‐related inclusion criteria: intra‐osseous defect component ≥3 mm at the deepest part and presence of at least three osseous walls. The exclusion criteria included diabetes mellitus (hemoglobin A1c ≥6.5%), use of corticosteroids or other anti‐inflammatory prescription drugs, use of systemic antibiotics in the preceding month, pregnancy or lactation, implants previously surgically treated for peri‐implantitis, and implant mobility.
Dental patients were screened for eligibility between 2015 and 2018. Using a computer‐generated randomization schedule, patients who met the inclusion criteria were allocated to receive one of the two possible treatments, either BO (Bio‐Oss®, Geistlich Pharma, Wolhusen, Switzerland) or EB xenograft granules (Endobon®, Zimmer Biomet, Palm Beach Gardens, FL, USA) (Figure 1). A clinically significant difference in the effectiveness of the two graft materials was considered a difference of 1 mm in radiographic defect reduction. Therefore, a sample size calculation was performed based on the 1 mm non‐inferiority limit (standard deviation 1.2 mm) in the mean radiographic defect reduction between the two groups (Roos‐Jansaker, Lindahl, Persson, & Renvert, 2011; Roos‐Jansåker et al., 2007). The power analysis was performed using the online Sealed Envelope software (https://www.sealedenvelope.com). With a level of significance of alpha = 0.05 in a one‐sided hypothesis (or equivalently with alpha = 0.10 in a two‐sided hypothesis) and 80% power, 18 patients per group were required. A withdrawal/dropout rate of 10% was considered acceptable; therefore, it was planned to recruit a total of 40 patients.
FIGURE 1.
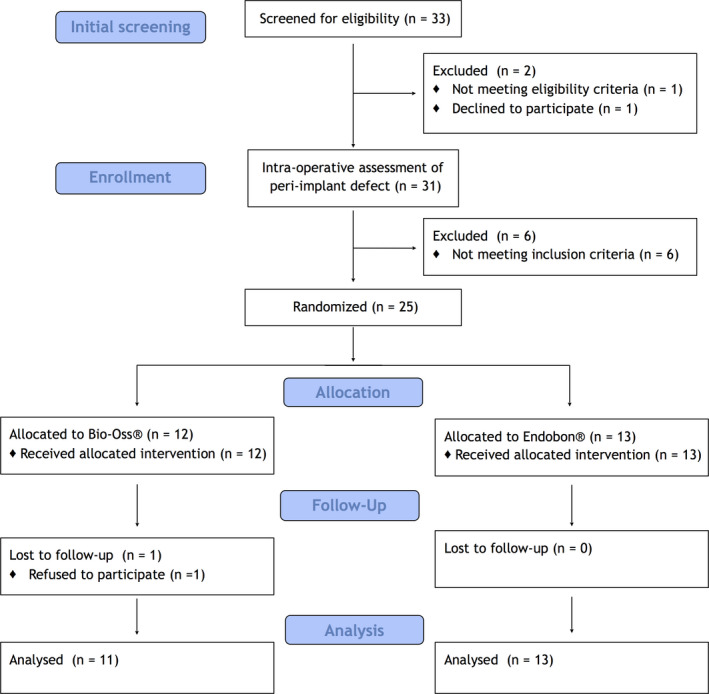
Consort diagram of patient distribution
2.3. Clinical examination
The following clinical recordings were collected at baseline and at the 6‐ and 12‐month follow‐up by an experienced, calibrated examiner who was blinded to intervention assignment (D.A.M): 1. PPD to the nearest millimeter, 2. presence/absence of BoP and SoP assessed within 30 s after probing, and 3. full‐mouth plaque score (FMPS). All measurements were performed at six sites per implant (mesiobuccal, buccal, distobuccal, distopalatal, palatal, and mesiopalatal) using the periodontal probe XP23/UNC 15 (HuFriedy, Chicago, IL, USA).
2.4. Surgical treatment and post‐operative care
Surgeries were performed by an experienced surgeon (D.W.). The surgical technique has been described previously (Jepsen et al., 2016). Briefly, following the removal of the supra‐structure whenever that was possible, intracrevicular incisions were performed around the implant. Full‐thickness mucoperiosteal flaps were raised on the buccal and lingual aspects to fully access the peri‐implant defect. Vertical releasing incisions into the vestibule at a distance of at least one tooth/implant from the target implant were performed as necessary for adequate access. Granulation tissue was removed with titanium curettes (HuFriedy, Chicago, IL, USA), and the exposed implant threads were carefully debrided and decontaminated with 3% H2O2 for 1 min, followed by rinsing with copious amounts of saline. The intrabony defect was filled with either BO or EB. Before application, both graft materials were moistened in sterile saline for 5 min. The prostheses were then reconnected, and the flaps were re‐approximated and sutured with monofilament non‐resorbable sutures (Gore‐Tex 5‐0, W.L. Gore & Associates). The wound healing was performed in a non‐submerged mode. In case the defect did not fill the inclusion criteria, the patient was excluded from the study and was treated with an open flap debridement procedure (Berglundh, Wennstrom, et al., 2018).
Detailed post‐operative instructions were given to the patients. The patients were prescribed antibiotics; amoxicillin 500 mg X 3 per day and metronidazole 500 mg X 2 per day for 8 days, starting one day before the surgery. The patients were also prescribed analgesics (paracetamol 500 mg) to use as needed. During the first 4 weeks, all participants rinsed with 0.12% chlorhexidine twice daily. Patients were recalled at 6 weeks and 3, 6, 9, and 12 months after the surgery for professional oral hygiene procedures that included supragingival debridement and polishing with a rubber cup and a low‐abrasive paste. Oral hygiene instructions were given to each patient as necessary. The study timeline is outlined in Figure S1.
2.5. Radiographic evaluation
Intra‐oral periapical radiographs of the target implant were taken using the parallel long‐cone technique and an Eggen holder (Firma Eggen, Lillehammer, Norway) at baseline, and 6 and 12 months after surgery. The evaluation of the radiographs was performed using the software ImageJ, which was designed by National Institute of Health (NIH, VA, USA) for image analysis. To compensate for the anatomic magnification and possible variation in the alignment of the films, the linear dimensions of the images were calibrated using the known length of the implant or the known distance between two implant threads.
The following radiographic measurements were recorded at the peri‐implant defect (Figure 2): (a) bone level (BL): vertical distance between the implant shoulder and the bottom of the defect and (b) intrabony defect depth (IDD): vertical distance between the alveolar crest and the bottom of the defect. Based on these measurements, changes in bone level and vertical defect depth from baseline to 6 and 12 months were calculated. The radiographic reduction of the intrabony component of the defect was calculated in mm based on the difference of the IDD between the baseline and the study end points. The supracrestal component of the defect (SC) was also evaluated based on the difference between the BL and IDD values. The most coronal contact of the implant surface with bone or bone with graft material was used to define the BL and IDD. Floating graft particles or single isles of bone or bone‐like material were not considered.
FIGURE 2.
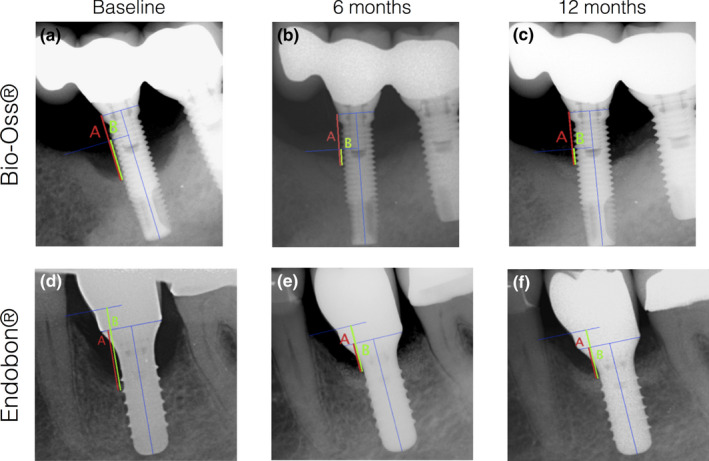
Radiographic assessment of: (a) bone level (red line) and (b) intrabony defect depth (green line) at baseline, 6 and 12 months after treatment at an implant treated with BO (a‐c) and EB (d‐f)
All radiographs were de‐identified and one examiner (A.P.) who was blinded to treatment allocations made all the radiographic measurements. In order to minimize the measurement error, the radiographic measurements at baseline, 6 and 12 months of 15 randomly selected patients we repeated by the same examiner (A.P.) after one month. The intra‐examiner agreement was evaluated by means of the intraclass correlation coefficient (ICC).
2.6. Statistical analysis
The primary outcome variables were changes in the radiographic BL and IDD. Secondary outcomes included changes in PPD, BoP, and SoP. Data were expressed as mean (SD) or percentages (%). Comparisons between the two groups were performed using the independent sample t test for quantitative variables (age, defect depth, PPD, etc) and the Chi‐square test or Fisher's exact test for qualitative variables (gender, smoking status, reason for placing implants, treatment success, etc). A repeated measures ANOVA was performed for within‐group comparisons. The level of significance was set at 5%. The statistical analyses were performed with a commercial software package (SPSS inc., IBM, Armonk, New York, USA).
3. RESULTS
3.1. Study population and baseline characteristics
The initial study design was to recruit a total of 40 peri‐implantitis patients. However, due to the relocation outside of the Academic Center for Dentistry Amsterdam (ACTA) of the clinical examiner (D.A.M.) and the surgeon (D.W.), the screening process stopped at 33 patients. Therefore, we consider the current study as a pilot study. Figure 1 outlines the flow diagram of the patient enrollment, allocation to interventions, follow‐up, and data analysis. Twenty‐five patients out of 33 fulfilled the inclusion criteria and were randomized to surgical treatment with either BO or EB. One patient refused to attend the follow‐up examinations; therefore, 24 patients completed the study and their data were analyzed.
The demographic, dental, and implant characteristics of the 24 study participants at baseline are presented in Table 1 and Table S1. The two groups were similar in terms of age, gender, smoking status, implant location, and years of functional loading. None of the participants demonstrated side effects or patient morbidity, beyond what is normally expected for similar surgical procedures.
TABLE 1.
Study population characteristics at baseline (n = 24 patients)
| Variable | BO (n = 11) | EB (n = 13) | Test value, p value |
|---|---|---|---|
| Age (years), mean (SD) | 65.5 (11.2) | 57.3 (15.1) | T = 1.479, p = .153 a |
| Gender | |||
| Male | 5 (45%) | 8 (62%) | X2 = 0.621, |
| Female | 6 (55%) | 5 (38%) | p = .431 b |
| Smoking status | |||
| Smoker | 3 (27%) | 2 (15%) | Fisher's exact test,p = .630 |
| Non‐smoker | 8 (73%) | 11 (85%) | |
| History of periodontal treatment | |||
| Yes | 4 (36%) | 6 (46%) | |
| No | 5 (46%) | 7 (54%) | |
| Unknown | 2 (18%) | 0 (0%) | ‐ |
| Type of prosthesis | |||
| Single crown | 8 (73%) | 11 (84%) | ‐ |
| Fixed partial denture | 3 (27%) | 1 (8%) | |
| Overdenture | 0 (0%) | 1 (8%) | |
| Jaw | |||
| Maxilla | 6 (55%) | 6 (46%) | X2 = 0.168, |
| Mandible | 5 (45%) | 7 (54%) | p = .682 b |
| Location | |||
| Anterior | 2 (18%) | 2 (15%) | Fisher's exact test, p = 1.000 |
| Posterior | 9 (82%) | 11 (85%) | |
| Years of function mean (SD) (range) |
7.0 (3.4) (3–13) |
8.1 (4.9) (2–20) |
T = −0.616, p = .544 a |
Abbreviations: BO, Bio‐Oss®; EB, Endobon®; SD, standard deviation.
Independent sample t test.
Chi‐square test.
3.2. Intra‐examiner reliability
The peri‐implant BL and IDD were re‐assessed by the same examiner at 1‐month interval. Fifteen patients were randomly selected, and their baseline and 6‐month and 12‐month radiographs (45 radiographs in total) were re‐evaluated in order to assess the reliability of the measurements. The intraclass correlation coefficient (ICC) values for the radiographic parameters at baseline, 6 months, and 12 months ranged from 0.948 to 0.965, indicating high agreement between repeated measurements (Table S2).
3.3. Primary and secondary outcomes
The radiographic and clinical parameters at baseline and at the 6‐ and 12‐month end points for both groups are summarized in Tables 2 and 3 and Figures 3 and 4. Both parametric (t test, repeated measures ANOVA) and non‐parametric (chi‐square, Mann–Whitney U test, Friedman) tests were used providing similar results. Here, the results of parametric tests are reported. At baseline, all radiographic and clinical parameters were similar for both groups, except the SC that was significantly different between the two groups (T = 2.405, p = .025). In the EB group, most of the implants were placed subcrestally leading to a mean SC of −0.9 (1.6) (Table 2). Radiographically assessed BL and IDD presented within‐group statistically significant reductions from baseline to 6 and 12 months. In the BO group, the mean BL decreased from 5.3 (1.2) mm to 3.3 (1.3) mm at 6 months and to 3.1 (1.3) mm at 12 months (F = 76.890, p < .001). In the EB group, the mean BL value of 4.9 (1.1) mm at baseline, decreased to 2.5 (1.1) mm at 6 months and to 2.1 (1.3) mm at 12 months (F = 46.724, p < .001). Regarding the IDD, the mean value recorded for the BO group was 4.9 (0.9) mm, 2.6 (0.6) mm, and 2.4 (0.6) mm at baseline, 6, and 12 months, respectively (F = 71.544, p < .001). The corresponding values for the EB group were 5.9 (1.8) mm, 3.1 (1.8) mm, and 2.9 (1.3) mm at baseline, 6, and 12 months, respectively (F = 49.796, p < .001) (Table 2, Figure 3). The mean changes in BL and IDD from baseline to 6 and 12 months of follow‐up were not statistically significant between the two groups (Table 3, Figure 4). The SC increased overall from baseline to 6 and 12 months; however, the within‐group differences were not statistically significant. Furthermore, the mean changes of the SC from baseline to 6 and 12 months were not significant between BO and EB (Tables 2 and 3).
TABLE 2.
Radiographic and clinical parameters (mean (SD) at baseline, 6 and 12 months of the 24 peri‐implant defects
| Parameter | BO | EB | Between‐group comparison a |
|---|---|---|---|
| BL (mm) | |||
| Baseline | 5.3 (1.2) | 4.9 (1.1) | T = 0.885, p = .386 |
| 6 months | 3.3 (1.3) | 2.5 (1.1) | T = 1.524, p = .142 |
| 12 months | 3.1 (1.3) | 2.1 (1.3) | T = 1.881, p = .073 |
| Within‐group comparison b | F = 76.890, p < .001 | F = 46.724, p < .001 | |
| IDD (mm) | |||
| Baseline | 4.9 (0.9) | 5.9 (1.8) | T = −1.763, p = .094 |
| 6 months | 2.6 (0.6) | 3.1 (1.8) | T = −0.979, p = .345 |
| 12 months | 2.4 (0.6) | 2.9 (1.3) | T = −1.385, p = .183 |
| Within‐group comparison b | F = 71.544, p < .001 | F = 49.796, p < .001 | |
| SC (mm) | |||
| Baseline | 0.4 (1.2) | −0.9 (1.6) | T = 2.405, p = .025 |
| 6 months | 0.7 (1.5) | −0.6 (1.8) | T = 1.843, p = .080 |
| 12 months | 0.7 (1.6) | −0.6 (1.6) | T = 2.359, p = .028 |
| Within‐group comparison b | F = 1.646, p = .218 | F = 0.985, p = .389 | |
| PPD (mm) | |||
| Baseline | 7.0 (1.8) | 7.1 (1.2) | T = −0.221, p = .827 |
| 6 months | 3.5 (1.0) | 3.4 (0.6) | T = 0.526, p = .604 |
| 12 months | 3.4 (0.6) | 3.4 (0.5) | T = 0.115, p = .910 |
| Within‐group comparison b | F = 42.449, p < .001 | F = 88.502, p < .001 | |
| BoP (%) | |||
| Baseline | 100 (0.0) | 100 (0.0) | T = 1.359, p = .188 |
| 6 months | 47.7 (32.5) | 32.7 (21.4) | T = −0.437, p = .670 |
| 12 months | 45.5 (33.2) | 50 (10.2) | |
| Within‐group comparison b | F = 20.331, p < .001 | F = 93.638, p < .001 | |
| SoP (%) | |||
| Baseline | 79.5 (40.0) | 86.5 (33.3) | T = −0.468, p = .645 |
| 6 months | 4.6 (15.1) | 0.0 (0.0) | T = 1.000, p = .341 |
| 12 months | 0.0 (0.0) | 1.9 (6.9) | T = −0.917, p = .369 |
| Within‐group comparison b | F = 35.552, p < .001 | F = 84.598, p < .001 | |
| Plaque (%) | |||
| Baseline | 31.7 (13.1) | 29.4 (13.0) | T = 0.390, p = .701 |
| 6 months | 15.9 (8.0) | 11.5 (6.4) | T = 1.461, p = .159 |
| 12 months | 17.5 (11.5) | 14.0 (9.3) | T = 0.776, p = .447 |
| Within‐group comparison b | F = 12.152, p = .001 | F = 12.221, p < .001 | |
Abbreviations: BL, bone level; BO, Bio‐Oss®; BoP, bleeding on probing out of six sites per implant; EB, Endobon®; IDD, intrabony defect depth; PI, full‐mouth plaque index; PPD, probing pocket depth (mean of 6 sites per implant); SC, supracrestal component; SD, standard deviation; SoP, suppuration on probing out of six sites per implant.
Independent sample t test.
Repeated measures ANOVA.
TABLE 3.
Changes in radiographic and clinical parameters (mean (SD) at 6 and 12 months, in BO and EB treatment groups
| Parameter | BO | EB | Test value, p value a |
|---|---|---|---|
| BL (mm) | |||
| Baseline to 6 months | 2.0 (0.7) | 2.4 (1.0) | T = −1.113, p = .278 |
| Baseline to 12 months | 2.2 (0.8) | 2.8 (1.3) | T = −1.233, p = .231 |
| IDD (mm) | |||
| Baseline to 6 months | 2.3 (0.9) | 2.7 (1.2) | T = −1.036, p = .312 |
| Baseline to 12 months | 2.5 (0.8) | 3.0 (1.1) | T = −1.053, p = .304 |
| SC (mm) | |||
| Baseline to 6 months | −0.3 (0.7) | −0.3 (0.8) | T = 0.117, p = .908 |
| Baseline to 12 months | −0.3 (0.7) | −0.2 (0.7) | T = −0.496, p = .625 |
| PPD (mm) | |||
| Baseline to 6 months | 3.5 (1.7) | 3.8 (1.4) | T = −0.448, p = .659 |
| Baseline to 12 months | 3.6 (1.7) | 3.8 (1.4) | T = −0.271, p = .789 |
| BoP (%) | |||
| Baseline to 6 months | 52.3 (32.5) | 67.3 (21.4) | T = −1.359, p = .188 |
| Baseline to 12 months | 54.5 (33.2) | 50.0 (10.2) | T = 0.470, p = .643 |
| SoP (%) | |||
| Baseline to 6 months | 75.0 (43.3) | 86.5 (33.3) | T = −0.738, p = .468 |
| Baseline to 12 months | 79.5 (40.0) | 84.6 (33.1) | T = −0.340, p = .737 |
| Plaque (%) | |||
| Baseline to 6 months | 15.0 (12.3) | 17.9 (11.6) | T = −0.555, p = .586 |
| Baseline to 12 months | 14.2 (8.4) | 15.4 (16.3) | T = −0.190, p = .852 |
Abbreviations: BL, bone level; BO, Bio‐Oss®; BoP, bleeding on probing out of six sites per implant; EB, Endobon®; IDD, intrabony defect depth; PI, full‐mouth plaque index; PPD, probing pocket depth (mean of 6 sites per implant); SC, supracrestal component; SD, standard deviation; SoP, suppuration on probing out of six sites per implant.
Independent sample t test.
FIGURE 3.
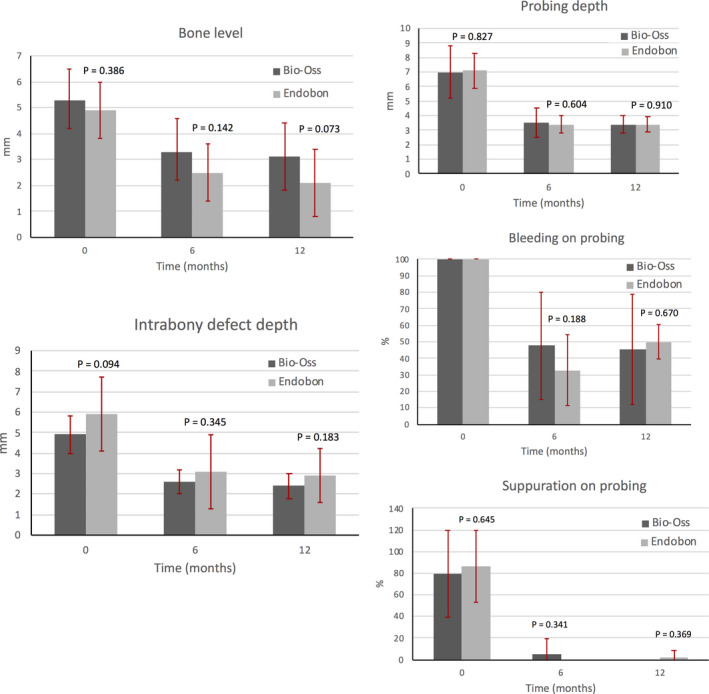
Radiographic and clinical parameters around the implants at baseline, 6 months and 12 months after treatment in both groups. There were no statistically significant differences between BO and EB in any of the parameters that were examined. The error bars represent the standard deviations (SD).
FIGURE 4.
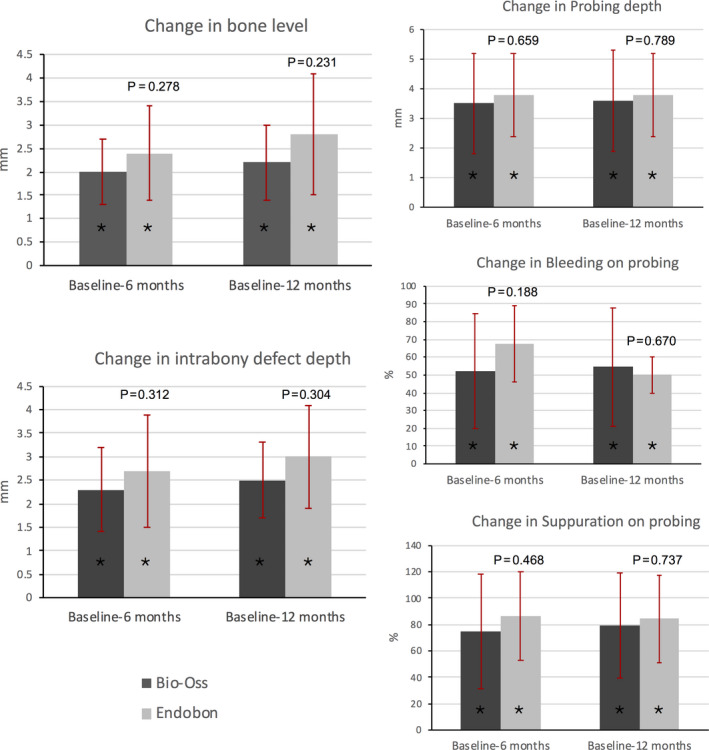
Changes in radiographic and clinical parameters around the implants from baseline to 6 and 12 months after treatment in both groups. No inter‐group differences were found in any parameter. The asterisks (*) represent statistical significant within‐group differences (p < .001) from baseline to the 6‐ and 12‐month time points in all parameters. The error bars represent the standard deviations (SD).
All clinical parameters (secondary outcomes) improved at 6 and 12 months following surgical treatment. In the BO group, the mean PPD (out of six sites per implant) decreased from 7.0 (1.8) mm to 3.5 (1.0) mm at 6 months and to 3.4 (0.6) mm at 12 months (F = 42.449, p < .001). Similarly, in the EB group PPD decreased from 7.1 (1.2) mm to 3.4 (0.6) mm at 6 months and to 3.4 (0.5) mm at 12 months (F = 88.502, p < .001). No statistically significant differences were found between the two study groups. The proportion of implant sites presenting with BoP was reduced by more than 50% at the 6‐ and 12‐month post‐operative evaluation in all patients. The proportion of implant sites with SoP was also reduced by more than 75% at the 6‐ and 12‐month post‐operative evaluation in all patients. There were no intergroup differences in BoP or SoP at any time point. Full‐mouth plaque scores were approximately 30% at baseline in both groups and were further reduced by 14%–18% after treatment. At all time points, plaque scores did not differ by study group.
3.4. Successful treatment outcome at 12 months
Successful treatment is determined by the presence of PPD ≤ 5 mm, complete absence of BoP and SoP, and no further bone loss (Heitz‐Mayfield, Needleman, Salvi, & Pjetursson, 2014; Jepsen et al., 2019). Using this strict criterion, successful treatment was found in only 2 of 11 (18%) and none of 13 (0%) individuals of the BO and EB groups, respectively (Fisher's exact test, p = .199). When less strict criteria were applied including PPD ≤ 5 mm, ≤1 site with BoP, absence of SoP, and no further bone loss (Renvert et al., 2018), 2 of 11 (18%) and 1 of 13 (8%) patients treated with BO and EB, respectively, were successfully treated (Fisher's exact test, p = .576). When it comes to regenerative therapy, reduction of the radiographic defect of >1 mm might be considered as treatment success (Renvert et al., 2018). The treatment approaches used in the present study resulted not only in no further progression of bone loss, but also in radiographic defect reduction of more than 1 mm in all patients at 12 months (Table 3).
4. DISCUSSION
The aim of the present study was to compare the reconstructive potential of two different bovine‐derived bone substitutes in contained, 3‐ or 4‐wall peri‐implant defects. The changes in bone level and intrabony defect depth (defect reduction) assessed radiographically were the primary outcome measures. Intra‐oral radiography using the parallel technique is a quick and easy way to assess the bone level around teeth or implants and is considered a reliable tool in determining the peri‐implant marginal bone level changes between different examinations (Cassetta, Di Giorgio, & Barbato, 2018). This method, however, has some inherent limitations; first of all, the X‐ray is a two dimensional examination of three‐dimensional structures and has a tendency to underestimate the amount of bone loss around implants (Serino, Sato, Holmes, & Turri, 2017). Second, the healing of the peri‐implant intra‐osseous defect and re‐osseointegration of the diseased implant surface can only be verified by means of histological imaging (Jepsen et al., 2019; Ritter et al., 2014). Third, the interpretation of radiographic defect reduction may be affected by the fact that over time, graft material may not be distinguishable from newly formed bone (Beaman et al., 2006; Tomasi et al., 2019).
Regarding the histological healing following the application of bovine‐derived xenografts into peri‐implant osseous defects, preclinical animal studies demonstrated integration of the graft particles within newly formed bone and re‐osseointegration of the previously exposed implant surfaces (Benic et al., 2016; Hammerle, Chiantella, Karring, & Lang, 1998). However, to the best of the authors’ knowledge there is a paucity of human studies regarding the histological healing of bovine xenografts in conjunction with peri‐implant related bone defects.
The present study reports no differences in the treatment outcome between the two groups. The mean radiographic defect reduction at 12 months was 2.5 (0.8) mm and 3.0 (1.1) mm for the BO and EB groups, respectively. These results are consistent with other studies where xenogenic bone grafts were used for the reconstruction of peri‐implant intrabony defects (Matarasso et al., 2014; Roccuzzo, Bonino, Bonino, & Dalmasso, 2011; Wiltfang et al., 2012). Other studies, however, reported only 1 mm reduction in bone levels after surgical treatment with bovine‐derived xenografts (Aghazadeh et al., 2012; Renvert et al., 2018). These discrepancies could be attributed to different baseline defect characteristics, as well as the use of a resorbable collagen membrane by some studies. Nevertheless, a systematic review reported that the amount of radiographic bone fill ranges from 1.46 to 3.30 mm after 3 years of healing, without achieving complete defect resolution (Khoshkam et al., 2016). In accordance with these results, complete defect resolution was not achieved in any of the cases of this study. In the present study, most implants in the EB group coincidentally appeared to be placed subcrestally leading to statistically significant between‐group difference in the SC. Following treatment however, the SC increased slightly (approximately 0.3 mm in both groups) indicating some crestal bone resorption, which was similar between the two groups. The SC is not frequently reported in studies evaluating the reconstructive treatment of peri‐implant intrabony defects. Only one study that compared the reconstructive surgery of peri‐implant defects with titanium granules to open flap debridement evaluated this parameter (Jepsen et al., 2016). Even though the mean values of the SC at baseline were greater than the values reported here, the mean change (i.e., crestal resorption) between baseline and 12 months for the group that received reconstructive treatment with titanium granules was similar to ours (0.15 mm with a standard deviation of 1.07 mm) (Jepsen et al., 2016). Therefore, we believe that although the defect configuration was different between the two groups at baseline by coincident (due to randomization), this did not affect the changes in radiographic BL and IDD at 6 and 12 months after treatment.
With regard to the secondary outcome measures, both surgical treatment modalities resulted in improvements of the clinical conditions and there were no statistically significant differences between the two groups. At 12 months, the PPD was reduced by 3.6 (1.7) mm in the group treated with BO and by 3.8 (1.4) mm in the group treated with EB. Similar reductions in PPD have been reported by other studies that used xenografts to treat peri‐implantitis (Aghazadeh et al., 2012; Matarasso et al., 2014; Renvert et al., 2018; Roccuzzo et al., 2017; Wiltfang et al., 2012). Nevertheless, if we had recruited patients with peri‐implantitis presenting with PPD ≥ 6 mm according to the new classification workshop (Berglundh, Armitage, et al., 2018), we may have had different results in PPD reductions. However, the initial planning of this study was in 2013, which prompted us to use the older definition (Zitzmann & Berglundh, 2008). At baseline, all sites bled upon probing, and at 12 months post‐treatment, the proportion of implant sites with BoP was reduced by approximately 50% in both groups. These results are in accordance with other studies; a systematic review that evaluated the long‐term outcomes of reconstructive procedures to treat peri‐implantitis reported a pooled weighted mean in the percentage of BoP reduction of 62.5% with a 95% CI of 25.2% to 89.2% (Khoshkam et al., 2016). Other clinical studies that evaluated the percentage of sites with BoP before and after reconstructive treatments with bovine xenografts reported a reduction in the proportion of sites with BoP in the range of 40%–60% (Aghazadeh et al., 2012; Roccuzzo et al., 2017; Schwarz et al., 2008). Furthermore, this study reported a reduction of approximately 80% in the proportion of sites with SoP in both groups. SoP is not frequently recorded; only few studies included it as an independent parameter using implants or implant sites as the unit of measurement, or reported it as part of a composite therapeutic index (Renvert et al., 2018; Roccuzzo et al., 2017; Wiltfang et al., 2012). Our results are therefore comparable with the study by Aghazadeh et al. who evaluated the percentage of sites with SoP at baseline and at 1 year post‐treatment and reported a mean value of 25% and 1.2%, respectively (Aghazadeh et al., 2012). We also reported that less than 2% of sites still presented SoP at 1 year; however, at baseline we recorded SoP in more than 80% of sites.
The use of composite therapeutic endpoints including information on radiographic bone levels, signs of peri‐implant soft tissue inflammation, and PPD has been published in multiple reports (Heitz‐Mayfield et al., 2014; Jepsen et al., 2019; Sanz & Chapple, 2012; Tomasi, Regidor, Ortiz‐Vigon, Regidor, Ortiz‐Vigón, & Derks, 2019). In the present study, two different versions of the composite therapeutic index were assessed based on evidence of peri‐implant tissue inflammation: (a) absolute absence of BoP and (b) allowing one site with evidence of BoP. No differences between the two groups were found regardless of the definition used. In the case of ≤1 site with BoP accepted, the success rate was 18% and 8% for the BO and EB groups, respectively. When absolute absence of BoP was the criterion, successful treatment was found in only 18% of the individuals treated with BO and none of the individuals treated with EB. Other studies that used similar criteria reported success rates up to 60% (Aghazadeh et al., 2012; Jepsen et al., 2016; Renvert et al., 2018; Roccuzzo et al., 2017). However, the reported success rates of reconstructive approaches in the literature range widely from as low as 14% up to 60% depending on the definition of the successful outcome, and possibly on the reconstructive approach used and the type of implant surface (Tomasi et al., 2019).
The low success rates reported here are associated with the fact that the treatment did not fully resolve the inflammation around the dental implants. Although there was a 50% reduction in the percentage of sites with BoP compared to baseline, at 1 year approximately 50% of sites still presented BoP. This could be attributed possibly to the fact that many implants, especially in the EB group, were placed too apically in relation to the CEJ of the adjacent teeth. It has been reported that implants placed too subcrestally are not only prone to greater peri‐implant bone loss, but also to a greater magnitude of peri‐implant inflammation with increased accumulation of neutrophils (Broggini et al., 2006; Gatti et al., 2018; Mailoa et al., 2015). Another factor that could have contributed to the lower success rate is related to the amount of keratinized tissue around the implants, which was not evaluated in this study. It has been reported that the lack of keratinized mucosa around implants impairs oral hygiene procedures, and eventually could lead to soft tissue damage, plaque accumulation, and bleeding (Monje, Insua, & Wang, 2019; Roccuzzo, Grasso, & Dalmasso, 2016). According to a recent consensus report, despite the lack of scientific evidence, the increase of non‐mobile keratinized mucosa before peri‐implant surgical approaches is recommended (Khoury et al., 2019).
An important limitation of the present study lies in the small sample size. Even though the primary objective was to recruit a total of 40 patients, the screening process had to be terminated prematurely due to the relocation of the clinical examiner (D.A.M.) and the surgeon (D.W.). The relatively short follow‐up time is another possible drawback; after 12 months, we do not know if the radiographic and clinical parameters remain stable or not.
Although this study was not designed to evaluate the effect of implant surface characteristics on the treatment outcome, this parameter cannot be ruled out (Albouy, Abrahamsson, Persson, & Berglundh, 2011). An experimental study in dogs that evaluated re‐osseointegration after the treatment of peri‐implantitis concluded that re‐osseointegration took place in implants with rough (SLA) surfaces, but failed to occur in implants with smooth (turned) surfaces (Persson, Berglundh, Lindhe, & Sennerby, 2001). A clinical study in humans that compared the outcome of a reconstructive approach between two different implant surfaces reported improved clinical and radiographic parameters, as well as higher implant survival rates after 7 years in SLA surfaces compared to TPS surfaces (Roccuzzo et al., 2017). On the other hand, Carcuac et al. reported that surgical therapy of peri‐implantitis resulted in superior outcomes at implants with non‐modified (turned) surfaces compared to implants with modified surfaces at 3 years (Carcuac et al., 2017). The present study included numerous implant types with different surface modifications (Table S1) and what is another limitation is that there was no control in the distribution of implant types and surfaces between the BO and EB group.
In the present study, a non‐submerged healing mode was applied. Although no randomized controlled trials exist comparing submerged to non‐submerged healing and favoring one versus the other, a case series of twelve patients reported favorable results in terms of radiographic defect reduction and reduced PPD using a submerged healing approach (Roos‐Jansaker et al., 2007). Nevertheless, these results should be interpreted with caution since no control group was included. Most recent studies evaluating reconstructive approaches in the treatment of peri‐implantitis used a non‐submerged healing approach and did not report any adverse events in terms of healing (Aghazadeh et al., 2012; Jepsen et al., 2016; Matarasso et al., 2014; Renvert et al., 2018; Roccuzzo et al., 2017). Despite the lack of evidence to support one mode of healing versus the other, the submerged post‐operative wound closure allows healing in a protective environment and when it is feasible, it is preferred over the non‐submerged healing (Khoury et al., 2019).
Within the limitations of this pilot study, we demonstrated that there were no differences between BO and EB for the primary or secondary outcome measures. The treatment with bovine‐derived xenografts resulted on average in radiographic defect reduction of approximately 3 mm and in PPD reduction of approximately 4 mm in both groups. Nevertheless, this study showed limited success in the resolution of inflammation. Future studies on the treatment of peri‐implantitis should include histologic analysis to evaluate the healing of the peri‐implant intra‐osseous defect and to prove true re‐osseointegration of the diseased implant surface. Longer follow‐up times are necessary to confirm the stability of the treatment outcomes.
CONFLICT OF INTEREST
The authors claim to have no financial interest, either directly or indirectly, in the products or information listed in the article.
AUTHOR CONTRIBUTION
Angeliki Polymeri: Data curation (lead); Formal analysis (lead); Writing‐original draft (lead); Writing‐review & editing (equal). David Anssari‐Moin: Conceptualization (equal); Data curation (equal); Funding acquisition (equal); Writing‐review & editing (supporting). Joyce van der Horst: Data curation (equal); Writing‐review & editing (supporting). Daniel Wismeijer: Conceptualization (equal); Writing‐review & editing (supporting). Marja L Laine: Conceptualization (equal); Writing‐review & editing (supporting). Bruno G. Loos: Conceptualization (equal); Funding acquisition (equal); Writing‐review & editing (supporting).
Supporting information
Fig S1
Table S1‐2
ACKNOWLEDGEMENTS
This work was supported through a grant to B.G.L. from Zimmer Biomet‐Dental Division, EMEA Headquarters, WTC Almeda Park, Ed. 4, Planta 2ª—C/Tirso de Molina, 40, Barcelona, Spain.
Polymeri A, Anssari‐Moin D, van der Horst J, Wismeijer D, Laine ML, Loos BG. Surgical treatment of peri‐implantitis defects with two different xenograft granules: A randomized clinical pilot study. Clin Oral Impl Res. 2020;31:1047–1060. 10.1111/clr.13651
REFERENCES
- Aghazadeh, A. , Rutger Persson, G. , & Renvert, S. (2012). A single‐centre randomized controlled clinical trial on the adjunct treatment of intra‐bony defects with autogenous bone or a xenograft: Results after 12 months. Journal of Clinical Periodontology, 39(7), 666–673. 10.1111/j.1600-051X.2012.01880.x [DOI] [PubMed] [Google Scholar]
- Albouy, J. P. , Abrahamsson, I. , Persson, L. G. , & Berglundh, T. (2011). Implant surface characteristics influence the outcome of treatment of peri‐implantitis: An experimental study in dogs. Journal of Clinical Periodontology, 38(1), 58–64. 10.1111/j.1600-051X.2010.01631.x [DOI] [PubMed] [Google Scholar]
- Artzi, Z. , Tal, H. , & Dayan, D. (2000). Porous bovine bone mineral in healing of human extraction sockets. Part 1: Histomorphometric evaluations at 9 months. Journal of Periodontology, 71(6), 1015–1023. 10.1902/jop.2000.71.6.1015 [DOI] [PubMed] [Google Scholar]
- Barone, A. , Todisco, M. , Ludovichetti, M. , Gualini, F. , Aggstaller, H. , Torres‐Lagares, D. , … Kenealy, J. (2013). A prospective, randomized, controlled, multicenter evaluation of extraction socket preservation comparing two bovine xenografts: Clinical and histologic outcomes. International Journal of Periodontics & Restorative Dentistry, 33(6), 795–802. 10.11607/prd.1690 [DOI] [PubMed] [Google Scholar]
- Beaman, F. D. , Bancroft, L. W. , Peterson, J. J. , Kransdorf, M. J. , Menke, D. M. , & DeOrio, J. K. (2006). Imaging characteristics of bone graft materials. Radiographics, 26(2), 373–388. 10.1148/rg.262055039 [DOI] [PubMed] [Google Scholar]
- Benic, G. I. , Thoma, D. S. , Munoz, F. , Sanz Martin, I. , Jung, R. E. , & Hammerle, C. H. (2016). Guided bone regeneration of peri‐implant defects with particulated and block xenogenic bone substitutes. Clinical Oral Implants Research, 27(5), 567–576. 10.1111/clr.12625 [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Armitage, G. , Araujo, M. G. , Avila‐Ortiz, G. , Blanco, J. , Camargo, P. M. , … Zitzmann, N. (2018). Peri‐implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Periodontology, 89(Suppl 1), S313–S318. 10.1002/jper.17-0739 [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , & Lindhe, J. (1997). Healing around implants placed in bone defects treated with Bio‐Oss. An experimental study in the dog. Clinical Oral Implants Research, 8(2), 117–124. 10.1034/j.1600-0501.1997.080206.x [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Wennstrom, J. L. , & Lindhe, J. (2018). Long‐term outcome of surgical treatment of peri‐implantitis. A 2–11‐year retrospective study. Clinical Oral Implants Research, 29(4), 404–410. 10.1111/clr.13138 [DOI] [PubMed] [Google Scholar]
- Block, M. S. (2019). The processing of xenografts will result in different clinical responses. Journal of Oral and Maxillofacial Surgery, 77(4), 690–697. 10.1016/j.joms.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Broggini, N. , McManus, L. M. , Hermann, J. S. , Medina, R. , Schenk, R. K. , Buser, D. , & Cochran, D. L. (2006). Peri‐implant inflammation defined by the implant‐abutment interface. Journal of Dental Research, 85(5), 473–478. 10.1177/154405910608500515 [DOI] [PubMed] [Google Scholar]
- Carcuac, O. , Derks, J. , Abrahamsson, I. , Wennström, J. , Petzold, M. , & Berglundh, T. (2017). Surgical treatment of peri‐implantitis: 3‐year results from a randomized controlled clinical trial. Journal of Clinical Periodontology, 44(12), 1294–1303. 10.1111/jcpe.12813 [DOI] [PubMed] [Google Scholar]
- Cassetta, M. , Di Giorgio, R. , & Barbato, E. (2018). Are intraoral radiographs reliable in determining peri‐implant marginal bone level changes? The correlation between open surgical measurements and peri‐apical radiographs. International Journal of Oral and Maxillofacial Surgery, 47(10), 1358–1364. 10.1016/j.ijom.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Cordaro, L. , Bosshardt, D. D. , Palattella, P. , Rao, W. , Serino, G. , & Chiapasco, M. (2008). Maxillary sinus grafting with Bio‐Oss or Straumann Bone Ceramic: Histomorphometric results from a randomized controlled multicenter clinical trial. Clinical Oral Implants Research, 19(8), 796–803. 10.1111/j.1600-0501.2008.01565.x [DOI] [PubMed] [Google Scholar]
- Degidi, M. , Piattelli, M. , Scarano, A. , Iezzi, G. , & Piattelli, A. (2004). Maxillary sinus augmentation with a synthetic cell‐binding peptide: Histological and histomorphometrical results in humans. Journal of Oral Implantology, 30(6), 376–383. 10.1563/0720.1 [DOI] [PubMed] [Google Scholar]
- Dreyer, H. , Grischke, J. , Tiede, C. , Eberhard, J. , Schweitzer, A. , Toikkanen, S. E. , … Stiesch, M. (2018). Epidemiology and risk factors of peri‐implantitis: A systematic review. Journal of Periodontal Research, 53(5), 657–681. 10.1111/jre.12562 [DOI] [PubMed] [Google Scholar]
- Froum, S. J. , Dagba, A. S. , Shi, Y. , Perez‐Asenjo, A. , Rosen, P. S. , & Wang, W. C. (2016). Successful surgical protocols in the treatment of peri‐implantitis: A narrative review of the literature. Implant Dentistry, 25(3), 416–426. 10.1097/id.0000000000000428 [DOI] [PubMed] [Google Scholar]
- Garaicoa‐Pazmino, C. , Sinjab, K. , & Wang, H.‐L. (2019). Current protocols for the treatment of peri‐implantitis. Current Oral Health Reports, 6(3), 209–217. 10.1007/s40496-019-00227-4 [DOI] [Google Scholar]
- Gatti, C. , Gatti, F. , Silvestri, M. , Mintrone, F. , Rossi, R. , Tridondani, G. , … Borrelli, P. (2018). A prospective multicenter study on radiographic crestal bone changes around dental implants placed at crestal or subcrestal level: One‐year findings. International Journal of Oral and Maxillofacial Implants, 33(4), 913–918. 10.11607/jomi.6509 [DOI] [PubMed] [Google Scholar]
- Hammerle, C. H. , Chiantella, G. C. , Karring, T. , & Lang, N. P. (1998). The effect of a deproteinized bovine bone mineral on bone regeneration around titanium dental implants. Clin Oral Implants Res, 9(3), 151–162. 10.1034/j.1600-0501.1998.090302.x [DOI] [PubMed] [Google Scholar]
- Haugen, HJ , Lyngstadaas, SP , Rossi, F , & Perale, G (2019). Bone grafts: which is the ideal biomaterial? Journal of Clinical Periodontology, 46, 92–102. 10.1111/jcpe.13058 [DOI] [PubMed] [Google Scholar]
- Heitz‐Mayfield, L. J. , Needleman, I. , Salvi, G. E. , & Pjetursson, B. E. (2014). Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. International Journal of Oral and Maxillofacial Implants, 29(Suppl), 346–350. 10.11607/jomi.2013.g5 [DOI] [PubMed] [Google Scholar]
- Hing, K. A. , Best, S. M. , & Bonfield, W. (1999). Characterization of porous hydroxyapatite. Journal of Materials Science. Materials in Medicine, 10(3), 135–145. 10.1023/a:1008929305897 [DOI] [PubMed] [Google Scholar]
- Jepsen, K. , Jepsen, S. , Laine, M. L. , Anssari Moin, D. , Pilloni, A. , Zeza, B. , … Renvert, S. (2016). Reconstruction of peri‐implant osseous defects: A multicenter randomized trial. Journal of Dental Research, 95(1), 58–66. 10.1177/0022034515610056 [DOI] [PubMed] [Google Scholar]
- Jepsen, S. , Schwarz, F. , Cordaro, L. , Derks, J. , Hammerle, C. H. F. , Heitz‐Mayfield, L. J. , Urban, I. (2019). Regeneration of alveolar ridge defects. Consensus report of group 4 of the 15th European Workshop on Periodontology on Bone Regeneration. Journal of Clinical Periodontology, 46(Suppl 21), 277–286. 10.1111/jcpe.13121 [DOI] [PubMed] [Google Scholar]
- Jiang, D. , Dziak, R. , Lynch, S. E. , & Stephan, E. B. (1999). Modification of an osteoconductive anorganic bovine bone mineral matrix with growth factors. Journal of Periodontology, 70(8), 834–839. 10.1902/jop.1999.70.8.834 [DOI] [PubMed] [Google Scholar]
- Khoshkam, V. , Suarez‐Lopez Del Amo, F. , Monje, A. , Lin, G. H. , Chan, H. L. , & Wang, H. L. (2016). Long‐term Radiographic and Clinical Outcomes of Regenerative Approach for Treating Peri‐implantitis: A Systematic Review and Meta‐analysis. International Journal of Oral and Maxillofacial Implants, 31(6), 1303–1310. 10.11607/jomi.4691 [DOI] [PubMed] [Google Scholar]
- Khoury, F. , Keeve, P. L. , Ramanauskaite, A. , Schwarz, F. , Koo, K. T. , Sculean, A. , & Romanos, G. (2019). Surgical treatment of peri‐implantitis ‐ Consensus report of working group 4. International Dental Journal, 69(Suppl 2), 18–22. 10.1111/idj.12505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordbacheh Changi, K. , Finkelstein, J. , & Papapanou, P. N. (2019). Peri‐implantitis prevalence, incidence rate, and risk factors: A study of electronic health records at a U.S. dental school. Clinical Oral Implants Research, 30(4), 306–314. 10.1111/clr.13416 [DOI] [PubMed] [Google Scholar]
- Mailoa, J. , Fu, J. H. , Chan, H. L. , Khoshkam, V. , Li, J. , & Wang, H. L. (2015). The effect of vertical implant position in relation to adjacent teeth on marginal bone loss in posterior arches: A retrospective study. International Journal of Oral and Maxillofacial Implants, 30(4), 931–936. 10.11607/jomi.4067 [DOI] [PubMed] [Google Scholar]
- Matarasso, S. , Iorio Siciliano, V. , Aglietta, M. , Andreuccetti, G. , & Salvi, G. E. (2014). Clinical and radiographic outcomes of a combined resective and regenerative approach in the treatment of peri‐implantitis: A prospective case series. Clinical Oral Implants Research, 25(7), 761–767. 10.1111/clr.12183 [DOI] [PubMed] [Google Scholar]
- Mellonig, J. T. (2000). Human histologic evaluation of a bovine‐derived bone xenograft in the treatment of periodontal osseous defects. International Journal of Periodontics & Restorative Dentistry, 20(1), 19–29. [PubMed] [Google Scholar]
- Monje, A. , Insua, A. , & Wang, H. L. (2019). Understanding peri‐implantitis as a plaque‐associated and site‐specific entity: On the local predisposing factors. Journal of Clinical Medicine, 8(2), 279 10.3390/jcm8020279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, G. R. , Samuelsson, E. , Lindahl, C. , & Renvert, S. (2010). Mechanical non‐surgical treatment of peri‐implantitis: A single‐blinded randomized longitudinal clinical study. II. Microbiological Results.Journal of Clinical Periodontology, 37(6), 563–573. 10.1111/j.1600-051X.2010.01561.x [DOI] [PubMed] [Google Scholar]
- Persson, L. G. , Berglundh, T. , Lindhe, J. , & Sennerby, L. (2001). Re‐osseointegration after treatment of peri‐implantitis at different implant surfaces. An experimental study in the dog. Clinical Oral Implants Research, 12(6), 595–603. 10.1034/j.1600-0501.2001.120607.x [DOI] [PubMed] [Google Scholar]
- Ramirez‐Fernandez, M. P. , Calvo‐Guirado, J. L. , Arcesio Delgado‐Ruiz, R. , Mate‐Sanchez Del Val, J. E. , Gomez‐Moreno, G. , & Guardia, J. (2011). Experimental model of bone response to xenografts of bovine origin (Endobon): A radiological and histomorphometric study. Clinical Oral Implants Research, 22(7), 727–734. 10.1111/j.1600-0501.2010.02052.x [DOI] [PubMed] [Google Scholar]
- Renvert, S. , Hirooka, H. , Polyzois, I. , Kelekis‐Cholakis, A. , & Wang, H. L. (2019). Diagnosis and non‐surgical treatment of peri‐implant diseases and maintenance care of patients with dental implants ‐ Consensus report of working group 3. International Dental Journal, 69(Suppl 2), 12–17. 10.1111/idj.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renvert, S. , Roos‐Jansaker, A. M. , & Persson, G. R. (2018). Surgical treatment of peri‐implantitis lesions with or without the use of a bone substitute‐a randomized clinical trial. Journal of Clinical Periodontology, 45(10), 1266–1274. 10.1111/jcpe.12986 [DOI] [PubMed] [Google Scholar]
- Ritter, L. , Elger, M. C. , Rothamel, D. , Fienitz, T. , Zinser, M. , Schwarz, F. , & Zoller, J. E. (2014). Accuracy of peri‐implant bone evaluation using cone beam CT, digital intra‐oral radiographs and histology. Dentomaxillofacial Radiology, 43(6), 20130088 10.1259/dmfr.20130088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccuzzo, M. , Bonino, F. , Bonino, L. , & Dalmasso, P. (2011). Surgical therapy of peri‐implantitis lesions by means of a bovine‐derived xenograft: Comparative results of a prospective study on two different implant surfaces. Journal of Clinical Periodontology, 38(8), 738–745. 10.1111/j.1600-051X.2011.01742.x [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Grasso, G. , & Dalmasso, P. (2016). Keratinized mucosa around implants in partially edentulous posterior mandible: 10‐year results of a prospective comparative study. Clinical Oral Implants Research, 27(4), 491–496. 10.1111/clr.12563 [DOI] [PubMed] [Google Scholar]
- Roccuzzo, M. , Pittoni, D. , Roccuzzo, A. , Charrier, L. , & Dalmasso, P. (2017). Surgical treatment of peri‐implantitis intrabony lesions by means of deproteinized bovine bone mineral with 10% collagen: 7‐year‐results. Clinical Oral Implants Research, 28(12), 1577–1583. 10.1111/clr.13028 [DOI] [PubMed] [Google Scholar]
- Roos‐Jansaker, A. M. , Lindahl, C. , Persson, G. R. , & Renvert, S. (2011). Long‐term stability of surgical bone regenerative procedures of peri‐implantitis lesions in a prospective case‐control study over 3 years. Journal of Clinical Periodontology, 38(6), 590–597. 10.1111/j.1600-051X.2011.01729.x [DOI] [PubMed] [Google Scholar]
- Roos‐Jansaker, A. M. , Persson, G. R. , Lindahl, C. , & Renvert, S. (2014). Surgical treatment of peri‐implantitis using a bone substitute with or without a resorbable membrane: A 5‐year follow‐up. Journal of Clinical Periodontology, 41(11), 1108–1114. 10.1111/jcpe.12308 [DOI] [PubMed] [Google Scholar]
- Roos‐Jansaker, A. M. , Renvert, H. , Lindahl, C. , & Renvert, S. (2007). Submerged healing following surgical treatment of peri‐implantitis: A case series. Journal of Clinical Periodontology, 34(8), 723–727. 10.1111/j.1600-051X.2007.01098.x [DOI] [PubMed] [Google Scholar]
- Roos‐Jansåker, A. M. , Renvert, H. , Lindahl, C. , & Renvert, S. (2007). Surgical treatment of peri‐implantitis using a bone substitute with or without a resorbable membrane: A prospective cohort study. Journal of Clinical Periodontology, 34(7), 625–632. 10.1111/j.1600-051X.2007.01102.x [DOI] [PubMed] [Google Scholar]
- Sanz, M. , & Chapple, I. L. (2012). Clinical research on peri‐implant diseases: Consensus report of Working Group 4. Journal of Clinical Periodontology, 39(Suppl 12), 202–206. 10.1111/j.1600-051X.2011.01837.x [DOI] [PubMed] [Google Scholar]
- Sarmiento, H. L. , Norton, M. , Korostoff, J. , Ko, K. I. , & Fiorellini, J. P. (2018). Surgical Alternatives for Treating Peri‐implantitis. The International Journal of Periodontics & Restorative Dentistry, 38(5), 665–671. 10.11607/prd.3639 [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Sahm, N. , Bieling, K. , & Becker, J. (2009). Surgical regenerative treatment of peri‐implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: A four‐year clinical follow‐up report. Journal of Clinical Periodontology, 36(9), 807–814. 10.1111/j.1600-051X.2009.01443.x [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Sahm, N. , Schwarz, K. , & Becker, J. (2010). Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri‐implantitis. Journal of Clinical Periodontology, 37(5), 449–455. 10.1111/j.1600-051X.2010.01540.x [DOI] [PubMed] [Google Scholar]
- Schwarz, F. , Sculean, A. , Bieling, K. , Ferrari, D. , Rothamel, D. , & Becker, J. (2008). Two‐year clinical results following treatment of peri‐implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane. Journal of Clinical Periodontology, 35(1), 80–87. 10.1111/j.1600-051X.2007.01168.x [DOI] [PubMed] [Google Scholar]
- Schwendicke, F. , Tu, Y. K. , & Stolpe, M. (2015). Preventing and Treating Peri‐Implantitis: A Cost‐Effectiveness Analysis. Journal of Periodontology, 86(9), 1020–1029. 10.1902/jop.2015.150071 [DOI] [PubMed] [Google Scholar]
- Serino, G. , Sato, H. , Holmes, P. , & Turri, A. (2017). Intra‐surgical vs. radiographic bone level assessments in measuring peri‐implant bone loss. Clinical Oral Implants Research, 28(11), 1396–1400. 10.1111/clr.12999 [DOI] [PubMed] [Google Scholar]
- Spies, C. K. , Schnurer, S. , Gotterbarm, T. , & Breusch, S. J. (2010). Efficacy of Bone Source and Cementek in comparison with Endobon in critical size metaphyseal defects, using a minipig model. Journal of Applied Biomaterials & Biomechanics, 8(3), 175–185. [PubMed] [Google Scholar]
- Tomasi, C. , Regidor, E. , Ortiz‐Vigón, A. , & Derks, J. (2019). Efficacy of reconstructive surgical therapy at peri‐implantitis‐related bone defects. A Systematic Review and meta‐analysis., 46(Suppl 21), 340–356. 10.1111/jcpe.13070 [DOI] [PubMed] [Google Scholar]
- Wiltfang, J. , Zernial, O. , Behrens, E. , Schlegel, A. , Warnke, P. H. , & Becker, S. T. (2012). Regenerative treatment of peri‐implantitis bone defects with a combination of autologous bone and a demineralized xenogenic bone graft: A series of 36 defects. Clinical Implant Dentistry and Related Research, 14(3), 421–427. 10.1111/j.1708-8208.2009.00264.x [DOI] [PubMed] [Google Scholar]
- Zitzmann, N. U. , & Berglundh, T. (2008). Definition and prevalence of peri‐implant diseases. Journal of Clinical Periodontology, 35(8 Suppl), 286–291. 10.1111/j.1600-051X.2008.01274.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1‐2


