Abstract
Aim
The aim of this prospective cohort study was to assess the effect of a pocket irrigator/evacuator device (IED) in the non‐surgical treatment of peri‐implantitis.
Material and Methods
In total 24 patients having 38 implants diagnosed with peri‐implantitis were included in this study. Peri‐implant pockets were irrigated six times in three consecutive weeks. The primary outcome was bleeding on probing (BoP). Secondary outcome parameters included plaque index (Pl), suppuration on probing (SoP), probing pocket depth (PPD), marginal bone loss (MBL), presence and numbers of periodontal pathogens. Parameters were assessed at baseline and 3 months after the last treatment. Treatment pain perception was scored using the visual analog scale (VAS) after the first and last treatment.
Results
At 3 months, IED treatment revealed significant reduction of peri‐implant BoP (71% [±20] vs 57% [±28] [P = .014]) and peri‐implant plaque scores (10 [±14] to 5 [±9] [P = .039] [T0 vs T3 respectively]). Significant reduction in mean peri‐implant PPD from 4.92 mm (SD ± 1.28) to 4.66 mm (SD ± 1.35) (P = .041) was observed. In addition, a reduction in VAS pain score between the first and the last (6th) treatment was found (P = .039). No reduction in SoP (P = .088) was found. No changes in mean periodontal full mouth plaque, BOP, SOP and PPD levels, MBL and microbiological outcomes were found.
Conclusion
Beneficial clinical effects in terms of BoP, PPD and PI were found at 3 months after IED treatment. However, the IED does not seem to effectively treat peri‐implantitis in terms of disease resolution.
Keywords: clinical trial, dental implants, microbiology, peri‐implantitis, pocket irrigation
1. INTRODUCTION
Treatment of peri‐implant inflammation, that is, peri‐implantitis and peri‐implant mucositis, is mainly focused on decontamination of the implant surface in order to create peri‐implant health and prevent peri‐implant bone loss. Various non‐surgical peri‐implantitis treatment modalities, including different mechanical debridement methods (curettes, air polishing and/or ultrasonic), laser (Er;YAG) and/or photodynamic treatments or pharmaceutical therapies (chlorhexidine, local or systemic antibiotics), have been described in the literature. 1 Although studies show satisfying results regarding the treatment of peri‐implant mucositis, incomplete resolution of disease in the treatment of peri‐implantitis is seen in the majority of non‐surgical studies. 2 , 3 A screw‐shaped implant design, an over contoured prosthetic design or pain during a treatment intervention may limit the effect of conventional mechanical treatment modalities. The use of alternative therapies could therefore be advocated. Recently, a new pocket irrigator/evacuator device (IED), the Fluxion® (GumCareCompanion, Gum Irrigator BV), based on a high‐frequency change in evacuation and irrigation, has been introduced in a study by van Dijk et al 4 to improve flushing of the subgingival area. Decreased probing pocket depths and reduced signs of inflammation (bleeding/suppuration) 3 months after treatment 4 were found in their study on the treatment of periodontal pockets, using this IED in two treatment sessions per week during a 3‐week period. Whether the IED can be used as an effective device in the treatment of peri‐implant diseases is yet unknown. Therefore, the aim of this prospective cohort study was to evaluate the clinical, microbial and radiographical effects of non‐surgical peri‐implantitis treatment using the IED. The null hypothesis formulated was that the use of the IED in the treatment of peri‐implantitis has no effect on clinical, microbial and radiographical outcomes.
2. METHODOLOGY AND STUDY POPULATION
2.1. Methodology
Patients participating in this study were recruited from patient populations of the Center of Dentistry and Oral Hygiene and the Department of Oral and Maxillofacial Surgery of the University Medical Center Groningen (UMCG), Groningen, The Netherlands. Patients who were referred to one of these departments by external dentists were also screened for participation. The study was conducted in full accordance with the principles of the Declaration of Helsinki as stated in 64th WMA General Assembly, Fortaleza, Brazil, October 2013 and in accordance with the Medical Research Involving Human Subjects Act (WMO). Approval by the Institutional Review Board of the University Medical Center Groningen, the Netherlands (METc2017.644) was given. A written informed consent was obtained from all participants before entering the trial.
2.1.1. Inclusion criteria
Patients who met the following criteria were found eligible to participate in this study:
Age ≥ 18 years;
At least one endosseous implant in the oral cavity with clinical and radiographical signs of peri‐implantitis, which was defined as: progressive marginal bone loss (MBL) of ≥2 mm, as compared to the initial bone level after implant placement, in combination with a peri‐implant probing pocket depth (PPD) ≥5 mm and bleeding and/or suppuration on probing (SoP);
Implants had been in function for at least two years;
Good understanding of the treatment protocol and able to give informed consent.
2.1.2. Exclusion criteria
A patient was excluded from participation in this study if any of the following criteria was met:
Medical and general contraindications for the procedures;
A history of local radiotherapy to the head and neck region;
Uncontrolled diabetes (HbA1c > 7% or > 53 mmol/mol) 5 ;
Use of antibiotics during the last 3 months;
Long‐term use of anti‐inflammatory drugs;
Smoking
Active periodontitis of the remaining dentition (PPD > 5 mm);
Incapability of performing basic oral hygiene measures as a result of physical or mental disorders;
Implants with bone loss exceeding 2/3 of the length of the implant;
Implant mobility;
Implants at which no pocket probing measurements could be performed that is, no probing site could be identified
Mechanical peri‐implantitis treatment in the previous 3 months.
2.1.3. Sample size determination
Since no data were available for estimating the effect size, a pilot study design was chosen. In total, 24 patients were included. 6
2.2. Study population
The guideline for reporting an observational study was followed as suggested by The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement (guideline found online at http://www.equator-network.org/reporting-guidelines/strobe/).
2.2.1. Study product
A detailed product description of the IED (the Fluxion®; GumCareCompanion, Gum Irrigator BV) is given by van Dijk et al. 4 Briefly, the apparatus consists of a base‐station and a connected hand piece (see Figure 1). The hand piece contains a disposable nozzle (suction cup) with an internal hose and on/off button in the middle of the hand piece which (de)activates a vacuum pump. Through the nozzle hose, an alternated interplay between water flushing (high repeated frequency of 250 milliseconds per cycle) and evacuation (negative pressure of 0.35 mm Hg) supposedly ensures irrigation after placement of the suction cup on the approximal/interdental area/papilla (see Figure 2).
Figure 1.
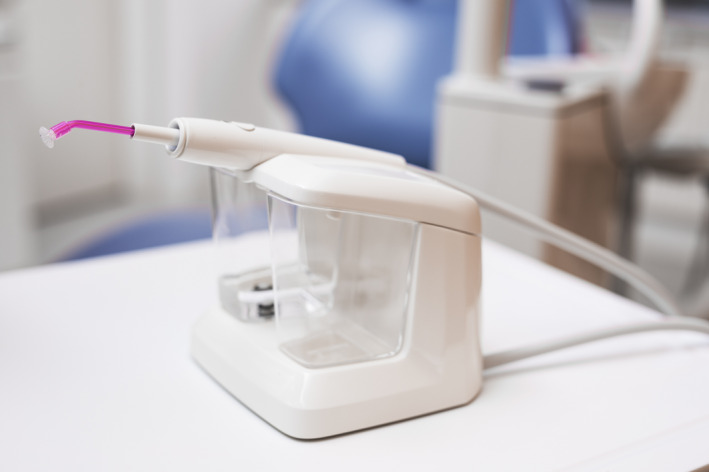
The Fluxion [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
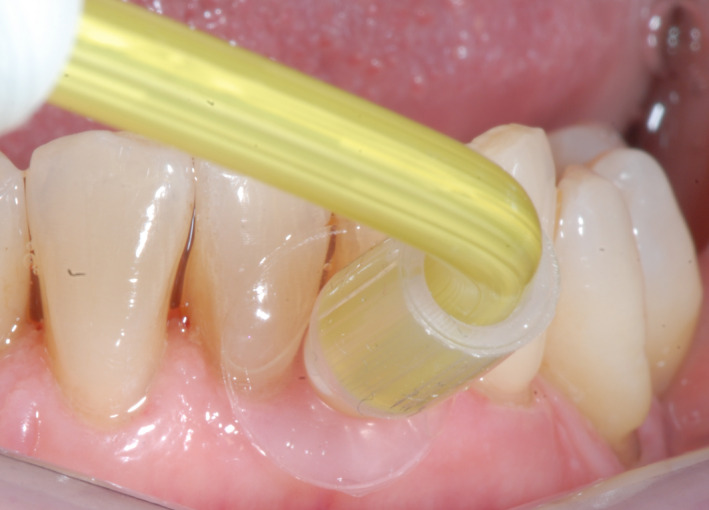
Placement of the suction cup on the approximal/interdental area/papilla [Colour figure can be viewed at wileyonlinelibrary.com]
2.2.2. Study design and setting
The study was performed between June 2018 and March 2019 at the department of Oral and Maxillofacial surgery, at the University Medical Center Groningen (UMCG), The Netherlands. Peri‐implant pockets were irrigated twice a week during a period of three consecutive weeks (six times in total). 4 Two trained dental hygienists treated all sites with peri‐implantitis sub‐ and supragingivally. During the first treatment appointment, the remaining dentition was cleaned using an ultrasonic device (EMS®) and if needed manually with periodontal curettes (Gracey curettes; Hu‐Friedy®). Patients received extensive oral hygiene instructions prior to submucosal irrigation using an electric toothbrush and interdental brushes and reinforcement took place every appointment. To ensure irrigation, care was taken to position the IED tip perpendicular to the implant axis. The submucosal sites with peri‐implantitis were irrigated with water using the IED for 14 seconds (two intervals of 7 seconds; default setting IED) per site (four sites per implant, mesiobuccal, mesiolingual, distobuccal, distolingual). Clinical outcomes (bleeding on probing [BoP, %], plaque score [%], suppuration score [%], PPD [mm]), peri‐apical radiographs and the presence and numbers of eight bacterial marker species were assessed at baseline (T0) and at 3 months (T3) after the last (6th) treatment. The patient‐centred outcomes of treatment pain perception were evaluated using a Visual Analog Scale (VAS) (score 0‐10) immediately after the first and last (6th) treatment.
2.2.3. Outcome measurements
Primary outcome parameter
The main study parameter was change in mean peri‐implant bleeding score (%).
Secondary outcome parameters
Secondary outcome parameters were mean peri‐implant plaque score (%), mean peri‐implant SoP score (%) and mean peri‐implant PPD. Other secondary parameters were mean full mouth periodontal bleeding score, mean full mouth periodontal plaque score (%), mean full mouth periodontal suppuration score (%), mean periodontal PPD, marginal soft tissue level at the midbuccal implant site (recession), width of keratinized epithelium at the buccal implant site, mean peri‐implant MBL, microbiological composition of the peri‐implant and periodontal area, complications and adverse events, and patient‐reported pain outcome. A successful treatment outcome was defined as: peri‐implant PPD < 5 mm, BoP ≤ 20% (maximum of one out of six sites bleeding) and no progressive bone loss at T3 compared to T0.
2.2.4. Clinical measurements
Full mouth periodontal and peri‐implant chart
A full mouth periodontal and peri‐implant chart were made prior to initial peri‐implant and periodontal treatment and at T3 by one and the same examiner (DFMH). At six sites per tooth and implant (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, distolingual), BoP, presence of plaque (Pl), SoP, and PPD were assessed (1 = present, 0 = not present for BoP, SoP and presence of plaque; absolute values measured to the nearest millimetre, using a Hu‐Friedy® PCPUNC156 periodontal probe for PPD). If the profile of the implant and/or the contour of the prosthesis hindered probing at six sites per implant, at least one position was identified where proper probing measurements could be performed. To assess the marginal soft tissue level, a partial Vinyl Polysiloxane (VPS) impression (EXABITE™ II NDS; GC America Inc) of the suprastructure was made at the implant site. This individual VPS mould was buccally trimmed to half way the suprastructure. The distance from the midbuccal marginal mucosa to the cervical margin of the VPS mould was assessed at the implant site at T0 and T3 (see Figure 3). In case of a solitary attachment (locator) or bar suprastructure present, the top of these suprastructures were taken as fixed reference point.
Figure 3.
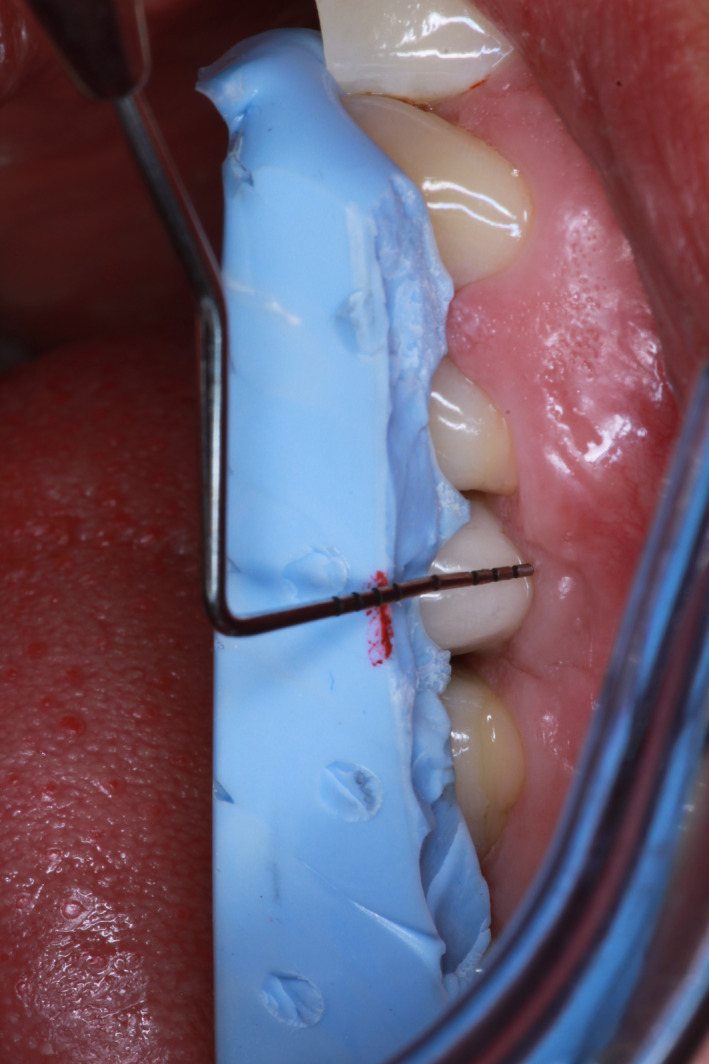
Measurement of distance from mid‐buccal marginal mucosa to Vinyl Polysiloxane (VPS) mold [Colour figure can be viewed at wileyonlinelibrary.com]
Radiographic analysis
Peri‐apical radiographs were taken at baseline and 3 months after the last treatment (Planmeca Intra X‐ray unit; Planmeca). An individualized X‐ray holder and paralleling technique were used to standardize radiographs and assure perpendicularity (ie positioning of the film parallel to the long axis of the implant). 7 Peri‐implant bone loss was measured on peri‐apical radiographs taken at baseline and 3 months after the last treatment using DICOM software (DicomWorks 1.5). Calibration of each radiograph took place on a 3‐point reference scale using the known implant length or diameter (see Figure 4). Bone level difference was calculated at the mesial and distal implant site. Radiographic examination of 10 randomly selected implants was done twice by DFMH and HJAM in order to calculate the inter‐observer agreement.
Figure 4.

DICOM measurement of mesial and distal marginal bone loss [Colour figure can be viewed at wileyonlinelibrary.com]
Microbiological sampling
The microflora residing in the peri‐implant sulcus was sampled at T0 and T3 using four sterile paper points. Samples were taken at four sites around the implant (mesiobuccal, distobuccal, mesiolingual and distolingual). In patients with more than one implant included, sampling was divided over the implants and the collected samples were pooled in an empty vial. In dentate patients, bacterial samples were also taken from the deepest tooth site in each quadrant. If no deepened pockets were present, samples were taken from the mesiobuccal pockets of the 16, 26, 36 and 46. Outcome variables were presence and numbers of Aggregatibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg), Prevotella intermedia (Pi), Tannerella forsythia (Tf), Fusobacterium nucleatum (Fn), Parvimonas micra (Pm), Treponema denticola (Td) and Filifactor alocis (Fa). Microbial samples were sent to Laboral Diagnostics, Houten, the Netherlands and analysed using quantitative polymerase chain reaction (qPCR) technique.
Other study parameters
History of periodontitis, alcohol use, implant function time, implant type and implant surface topography, medical condition and medication intake were other study parameters assessed.
2.2.5. Statistical analysis
The primary and secondary clinical outcome variables were analysed at patient level. Variables were assessed at implant/tooth‐level by computing the mean value of the 6 scores per implant and tooth per parameter, at T0 and T3. Outcomes in patients having more than one implant were combined. A Wilcoxon signed‐rank test (given the non‐normal distributed data) was used to analyse for within‐patient differences between baseline and follow‐up (3 months) for the periodontal and peri‐implant clinical parameters PI and SoP, microbiological outcomes and patient‐centred outcomes (VAS score 0‐10). A paired sampled t test (given the normal distributed data) was used to analyse the difference in mean periodontal and peri‐implant BoP, PPD and MBL between T0 and T3. A Cohen's kappa was calculated for the inter‐observer agreement of peri‐apical radiograph analysis. For all other study parameters, quantitative descriptive analysis was performed. Statistical Package for the Social Sciences (SPSS Statistics for Windows, IBM, Version 23.0 Armonk, NY, US) was used for all analyses.
3. RESULTS
In total, 24 adult patients with 73 implants of which 38 implants diagnosed with peri‐implantitis were included in the study. No patients were lost to follow‐up, and no implants were lost. No peri‐implant MBL occurred, and no mobility of implants was detected during or at completion of the study. An overview of the patient and implant characteristics is presented in Table 1 and 2. Outcomes on clinical parameters PI and SOP showed a non‐normal distribution and are therefore presented with median values and corresponding interquartile range (IQR) and p‐values (see Table 3 and 4). The means of BoP and PPD are presented with corresponding standard deviation (SD).
Table 1.
Characteristics of included patients
| Characteristics | N |
|---|---|
| Total number of patients | 24 |
| Age (y; mean [SD]) | 60 (12.51) |
| Gender; M (male), F (female) | M 11, F 13 |
| Smoking | |
| n subjects at baseline (%) | 0 (0%) |
| n subjects at 3 mo (%) | 3 (13%) |
| History of periodontitis; n subjects (%) | 6 (25%) |
| Dental status; n subjects (%) | |
| Partially edentulous | 22 (92%) |
| Fully edentulous | 2 (8%) |
| Medication intake; n subjects (%) | 13 (54%) |
| ≥3 medicines, n of total subjects taking medication (%) | 6 (46%) |
| <3 medicines, n of total subjects taking medication (%) | 7 (54%) |
| Alcohol user a ; n subjects (%) | 6 (25%) |
| Mean marginal bone level (T0; T3) | 3.67 mm; 3.64 mm |
| Mean recession b (T0; T3) | 7.91 mm; 8.03 mm |
| Mean VAS score (after 1st, after 6th treatment) | 0.41; 0.05 |
| Deepest pocket per implant at baseline; n implants (%) | |
| 5 mm | 5 (13%) |
| 6 mm | 12 (32%) |
| 7 mm | 13 (34%) |
| ≥8 mm | 8 (21%) |
| Treatment success; n subjects (%) | 5 (21%) |
| meanPPD (T0; T3) | 3.89 mm; 3.13 mm |
| MBL c (T0; T3) | 2.78 mm; 2.75 mm |
Patients were considered alcohol users if their daily intake exceeded 10 g; equivalent to a quarter litre of beer (5%). 31
Distance from the midbuccal marginal mucosa to the cervical margin of the individualized VPS mould.
Marginal bone loss at implant level.
Table 2.
Included implants characteristics and prosthetic factors
| Characteristics | N |
|---|---|
| Total number of implants (range) | 73 (1‐9) |
| Number of implants with peri‐implantitis (range) | 38 (1‐5) |
| Implant brand, implant surface; n implants (%) | |
| Nobel Biocare | |
| Porous anoidized surface, TiUnite® | 21 (55%) |
| Straumann | |
| Sandblasted large grit acid‐etched, SLAactive® | 6 (16%) |
| Astra Tech | |
| Fluoride‐treated TiOBlast, Osseospeed® | 5 (13%) |
| Biomet 3i | |
| Calcium‐phosphate DCD OSSEOTITE®, NanoTite® | 3 (8%) |
| Camlog | |
| Sandblasted acid‐etched, Promote® | 2 (5%) |
| IMZ | |
| Titanium plasma sprayed | 1 (3%) |
| Implant function time (years; mean [range]) | 9 (2.2;17.6) |
| Implant position; n implants (%) | |
| Maxilla | 29 (76%) |
| Mandibula | 9 (24%) |
| Implant restoration; n implants (%) | |
| Screw‐retained | 24 (63%) |
| Cement‐retained | 14 (22%) |
| Splinted/non‐splinted; n implants (%) | 18 (47%)/20 (53%) |
Table 3.
Overview of median and mean peri‐implant clinical parameters at baseline (T0) and 3 mo after (T3) treatment
| Parameters | T0 | T3 | T0 | T3 | P‐value a |
|---|---|---|---|---|---|
| Median [IQR] | Median [IQR] | Mean (SD) | Mean (SD) | ||
| BoP (%) b | — | — | 71 (±20) | 57 (±28) | .014**, b |
| PI (%) c | 0 [0,17] | 0 [0,8] | 10 (±14) a | 5 (±9) a | .039*, a |
| SoP (%) c | 31 [0,50] | 12 [0,28] | — | — | .088 |
| PPD (mm) b | — | — | 4.92 (±1.28) | 4.66 (±1.35) | .041**, b |
Abbreviations: BoP, peri‐implantitis bleeding on probing; IQR, Interquartile Range; Pl, peri‐implantitis plaque score; PPD, probing pocket depth; SD, standard deviation; SoP, peri‐implantitis suppuration on probing.
Mean values of PI are presented to improve understanding of the significant median reduction.
Paired sampled t test for T0 vs T3.
Wilcoxon signed‐rank test for T0 vs T3.
Statistically significant difference between median levels at baseline and 3 mo after the last (6th) treatment (P < .05, power 95%).
Statistically significant difference between mean levels at baseline and 3 mo after the last (6th) treatment (P < .05, power 95%).
Table 4.
Overview of median and mean periodontal full mouth clinical parameters at baseline (T0) and 3 mo after (T3) treatment
| Parameters | T0 | T3 | T0 | T3 | P‐value |
|---|---|---|---|---|---|
| Median [IQR] | Median [IQR] | Mean (SD) | Mean (SD) | ||
| BoP (%) * , a | — | — | 14 (±9) | 13 (±13) | .676 |
| PI (%) ** , b | 18 [8,43] | 14 [7,29] | — | — | .322 |
| SoP (%) ** , b | 0 [0,0] | 0 [0,0] | — | — | 1.000 |
| PPD (mm) * , a | — | — | 1.94 (±0.67) | 1.97 (±0.68) | .547 |
Abbreviations: BoP, full mouth periodontal bleeding on probing; IQR, Interquartile Range; Pl, full mouth periodontal plaque score; PPD, full mouth periodontal probing pocket depth; SD, standard deviation; SoP, full mouth periodontal suppuration on probing.
Paired sampled t test for T0 vs T3.
Wilcoxon signed‐rank test for T0 vs T3.
3.1. Primary outcome
A reduction in mean peri‐implant BoP between T0 and T3 (71% [±20]; 57% [±28], respectively) was found (P = .014) (see Table 3).
3.2. Secondary outcomes
At the three‐month evaluation, the mean peri‐implant plaque scores had dropped from 10 (±14) to 5 (±9) (P = .039), and the peri‐implant SoP reduced from a median of 31 [IQR; 0, 50] at T0 to a median of 12 [IQR; 0, 28] at T3 (P = .088) (see Table 3). A reduction in mean peri‐implant PPD was found between T0 (4.92 [SD ± 1.28]) and T3 (4.66 [SD ± 1.35]) (P = .041). No differences in mean periodontal full mouth clinical parameters were found between T0 and T3 (Table 4). No complications occurred and no adverse events were recorded during the study. Pain scores of 0.41 ± 0.91 (VAS pain score scale 0‐10) and 0.05 ± 0.21 were found after the first treatment and the last (6th) treatment, respectively (P = .039). A sub‐analysis for mean pocket depth initially measuring: <4 mm, 4‐5 mm, ≥5 mm is presented in Table 5.
Table 5.
Subdivided mean peri‐implant probing pocket depth and range [min‐max] in mm with total number of implants (n)
| T0 | T3 | ∆T0‐T3 | |
|---|---|---|---|
| Mean probing pocket depth | 4.92 [2.46; 8.17] | 4.66 [2.33; 7.67] | 0.26 [−0.13; −0.50] |
| n implants (number of sites) | 38 (228) | ||
| Mean probing pocket depth < 4 mm | 3.26 [2.46; 3.83] | 3.00 [2.33; 4.00] | 0.26 [−0.13; 0.17] |
|
P‐value a P = .541 n implants (number of sites) |
5 (30) | ||
| Mean probing pocket depth 4‐5 mm | 4.38 [4.00; 4.83] | 4.17 [ 3.75; 5.33] | 0.21 [−0.25; 0.50] |
|
P‐value a P = .071 n implants (number of sites) |
20 (120) | ||
| Mean probing pocket depth ≥ 5 mm | 6.12 [5.30; 8.17] | 5.82 [3.83; 7.67] | 0.30 [−1.47; −0.50] |
|
P‐value a P = .228 n implants (number of sites) |
13 (78) | ||
Statistical analysis between T0 and T3.
3.2.1. Peri‐implant bone loss
A correlation coefficient for the inter‐observer agreement for MBL measurements was calculated (Cronbach's a). An agreement of α = 0.989 was found. No difference in mean peri‐implant bone loss between T0 and T3 (3.67 mm (SD ± 1.93) vs 3.64 mm (SD ± 2.02) respectively) was observed (P = .558).
3.2.2. Microbiological outcomes
A total of 46 submucosal samples (23 patients with one peri‐implant sample and one periodontal sample) were available for microbiological analysis. One microbiological patient sample got lost in mail transport. Peri‐implant microbiological outcomes did not show a significant difference between T0 and T3 (see Table 6). High detection frequencies were found at implant sites for Tf, Pm, Fn (found in respectively 20, 22 and 23 patients). The lowest frequencies were found for Aa, Pi and Td which were found in 3, 6 and 8 patients at baseline, respectively. No significant difference between T0 and T3 for periodontal microbiological outcome was found.
Table 6.
Log‐transformed median and mean peri‐implant bacterial counts before (T0) and 3 mo after (T3) treatment, number of patients (n) with counts above detection level (DL) at T0 and T3
| T0 | T3 | T0 | T3 | T0 | T3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Mean | SD | Mean | SD | n > DL | n > DL | |
| Aa | 0 | 0.00, 0.00 | 0 | 0.00, 0.00 | 0.57 | ±1.53 | 0.56 | ±1.58 | 3 | 3 |
| Pg | 0 | 0.00, 6.49 | 0 | 0.00, 6.42 | 2.71 | ±3.23 | 2.93 | ±3.16 | 10 | 11 |
| Pi | 0 | 0.00, 2.30 | 0 | 0.00, 2.45 | 1.13 | ±2.04 | 1.12 | ±2.08 | 6 | 6 |
| Tf | 5.04 | 3.40, 5.54 | 4.14 | 3.30, 5.63 | 4.27 | ±1.95 | 4.14 | ±1.95 | 20 | 20 |
| Pm | 3.87 | 3.20, 4.85 | 3.43 | 2.64, 5.04 | 3.72 | ±1.39 | 3.43 | ±1.95 | 22 | 20 |
| Fn | 4.76 | 4.34, 5.28 | 4.7 | 4.20, 5.43 | 4.7 | ±0.88 | 4.7 | ±0.87 | 23 | 23 |
| Td | 0 | 0.00, 4.04 | 0 | 0.00, 3.11 | 1.59 | ±2.32 | 1.33 | ±2.02 | 8 | 8 |
| Fa | 0 | 0.00, 4.90 | 0 | 0.00, 4.61 | 2.17 | ±2.49 | 2.1 | ±2.36 | 11 | 11 |
Outcomes based on 23 patient samples; 1 patient without baseline sample excluded from analysis. No significant reduction between T0 and T3 for any of the pathogens was found (Wilcoxon signed‐rank test).
Abbreviations: Aa, Aggregatibacter actinomycetemcomitans; Fa, Filifactor alocis; Fn, Fusobacterium nucleatum; IQR, interquartile range; Pg, Porphyromonas gingivalis; Pi, Prevotella intermedia; Pm, Parvimonas micra; Td, Treponema denticola; Tf, Tannerella forsythia.
3.2.3. Success outcome
Five patients (21%) having seven implants (18%) were treated successfully (PPDs < 5 mm, BoP ≤ 20%, no progressive bone loss). Probing pocket depths corresponding with the successfully treated patients are presented in Table 1. A difference of 0.76 mm was found between baseline and 3 months after treatment.
4. DISCUSSION
4.1. Principal findings
The aim of this prospective cohort study was to evaluate the clinical and microbial effects of non‐surgical peri‐implantitis treatment using an IED. Statistically significant reductions were found for the primary outcome BoP and secondary outcomes Pl and PPD at 3 months after the last (6th) IED treatment. In addition, a significant treatment pain reduction between pain outcomes scored during the first and last (6th) treatment appointment was observed. No significant differences were found regarding the secondary peri‐implant clinical parameters: SoP, marginal bone level, microbiological load or any of the mean periodontal full mouth levels. Therefore, the formulated null hypothesis of no effect could be partly rejected.
4.2. Comparison with current literature
A direct comparison of the results with the current literature is not possible since no other studies on peri‐implantitis with repeated peri‐implant treatment sessions in consecutive weeks have been described in the literature. A split mouth study by van Dijk et al 2018 on inflamed periodontal sites is the single study that describes the results of repeated use of an IED in consecutive weeks. 4 Outcomes of the present study seem in line with the study by van Dijk et al 2018, showing a significant reduction in BoP, PPD and PI after IED treatment. However, compared to the incremental PPD difference and mean BoP and PI reduction described in the study by van Dijk et al 2018, smaller differences in outcomes of those parameters were found in the present study. This could be explained by the fact that decontamination of the implant surface is more challenging than decontamination of tooth surfaces due to differences in accessibility between the peri‐implant and periodontal pocket. 8 Exposure of the roughened implant surface and in more severe sites exposure of implant threads could impair surface decontamination. When using an IED, one could imagine that implant treads create resistance against the intended flush out of the subgingival biofilm. A complete distortion of the attached biofilm might therefore be hindered, maintaining a contaminated peri‐implant surface and ongoing peri‐implant inflammation.
Despite some overall clinical improvements, only 18% of the treated implants in the present study obtained clinically healthy peri‐implant tissues. The majority of patients (57%) still showed BoP after therapy, which might indicate ongoing disease progression. It should be kept in mind; however; there may also be other factors that influence the bleeding tendency of the peri‐implant mucosa, such as implant position, gender and PPD. 9 In general, peri‐implant tissues seem more prone to BoP than periodontal tissues. 10 , 11 In addition, probing forces exceeding 0.25 N can cause traumatic bleeding 12 making that we have to interpret BoP values with caution. Considering this, the outcomes of the present study, with regard to BoP, do not differ much from other non‐surgical peri‐implantitis “one time treatment” studies, showing final bleeding scores of >50%. 13 Studies on treatment modalities such as ultrasonic device, an air‐abrasive device, the Vector® system or Er;YAG laser combined with chemotherapeutics or as single therapy, on the other hand, have shown larger reductions in BoP. 14 , 15 , 16 , 17 , 18 , 19 , 20 To improve treatment success rates of the IED, adjunctive measures (eg chlorhexidine alone or in conjunction with systemic delivery of antibiotics) seem to be indicated. 21 , 22 , 23 , 24 , 25
Concerning PPD, a comparable PPD reduction (0.2 mm) was found in a study by Renvert et al 2009 when an ultrasonic scaler was used with three‐month follow‐up. 18 In contrast, Karring et al 2005 showed a slight increase in pocket depth (0.2 mm). 17 Greater PPD reductions were observed in studies when mechanical therapies were combined with the use of local antibiotics (0.49 mm). 26 In the present study, greater PPD reductions (0.76 mm) were found for the successfully treated patients (Table 1). These implants appeared to have a mean lower PPD and a mean lower MBL at baseline. Successful non‐surgical peri‐implantitis treatment outcomes might therefore depend on the mean PPD and mean MBL at baseline. A sub‐analysis on pocket probing depth categories in our study revealed the highest reduction (0.30 mm) in the subgroup with mean pocket depth ≥ 5 mm at baseline (Table 5). Presumably, penetration into deep pockets may be easier than into moderate pockets. 27
A sub‐analysis of smoking status on clinical parameters was also performed by the group of van Dijk et al 2018. The largest incremental difference in PPD was observed in non‐smokers and no significant difference between the BOP scores of smokers and non‐smokers was observed. Although all patients in the current study presented themselves as non‐smokers on inclusion, three patients reported positive on active smoking at the 3‐month evaluation. A sub‐analysis of the data, taking into account these active smokers, did not influence clinical outcomes.
Despite the intense treatment protocol of six repeated treatments in a period of 3 weeks, the results of the present study do not seem better than “one time treatment” studies. Although peri‐implant plaque levels appeared lower at T3 than T0 (change in interquartile range, see Table 3), no difference in full mouth plaque index was found. It could be therefore questioned if the repeated treatment approach provides a substantial benefit and further improves the level of self‐care. Future research on consecutive treatment appointments should confirm this finding.
No significant reduction of any of the microbiological pathogens studied at the 3‐month evaluation was found. This is comparable to other studies reporting on microbiological outcome after single debridement therapy. 15 , 28 Even studies reporting on the adjunctive use of a topical antibiotic (minocycline) or antiseptic (chlorhexidine) did not observe significant differences in levels of bacterial species. 29 , 30
To the best of our knowledge, this is the first study which evaluated how much pain patients experienced during non‐surgical peri‐implantitis therapy. Patients reported low levels of pain (0.41 on VAS scale 0‐10) during the first treatment and even lower levels during the last treatment (6th) (0.05). It might be speculated that the lower levels of pain during the last treatment are linked to less inflamed peri‐implant tissues but future studies should elucidate on this finding.
4.3. Limitations of the study and future recommendations
The single arm study design, limited sample size and short follow‐up should be considered as drawbacks of our study. In addition, the influence of the IED on soft tissue damage, the true ability of subgingival fluid penetration, the appropriate size of the suction tip and angulation of tip placement during treatment remain unclear. Future research on the IED should focus on the effect of treatment in incipient peri‐implantitis lesions, peri‐implant mucositis or in supportive peri‐implant therapy, preferably combined with using an antiseptic solution as irrigating agent in a randomized clinical trial with longer follow‐up.
4.4. Conclusion
The IED does not effectively treat peri‐implantitis in terms of disease resolution. However, some beneficial effects were found such as reduced BoP (%), reduced plaque score (%) and reduced PPD at 3 months after treatment. The repeatedly applied therapy does not seem to result in significantly better treatment outcomes compared to the “one time treatment” outcomes found in the literature.
5. CLINICAL RELEVANCE
5.1. Scientific rationale for the study
The most effective approach to treat peri‐implantitis remains to be found. Recently, a new pocket IED based on an alternated interplay between flushing and evacuation has been introduced.
5.2. Principal findings
This prospective cohort study indicates that the IED does not seem to be suitable to treat peri‐implantitis in terms of disease resolution, but some beneficial effects may be found such as reduced clinical parameters and reduced pain perception.
5.3. Practical implications
Future research on the IED is needed, focussing on the effect of treatment in incipient peri‐implantitis lesions, peri‐implant mucositis or in supportive peri‐implant therapy.
CONFLICT OF INTEREST
All other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors were involved conceiving the study; DH collected, processed and analysed the data; DH led the writing; Y.d.W., AJv.W., GR and HM critically revised the manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank Sanne Scholten and Barbara van Eijkelenborg for their enthusiastic contribution to the study and outstanding patient care. The study was sponsored by GumCareCompanion, Gum Irrigator BV (Groningen, the Netherlands).
Hentenaar DFM, De Waal YCM, Van Winkelhoff AJ, Meijer HJA, Raghoebar GM. Non‐surgical peri‐implantitis treatment using a pocket irrigator device; clinical, microbiological, radiographical and patient‐centred outcomes—A pilot study. Int J Dent Hygiene. 2020;18:403–412. 10.1111/idh.12462
Clinical trial registration was done at the Netherlands National Trial Register (www.trialregister.nl, trial number NL6806).
Funding information
AJ van Winkelhoff is co‐owner of Laboral Diagnostics, a company that provides clinical oral microbiology for dental professionals.
REFERENCES
- 1. Heitz‐Mayfield L, Needleman I, Salvi GE, Pjetursson BE. Consensus statements and clinical recommendations for prevention and management of biologic and technical implant complications. Int J Oral Maxillofac Implants. 2014;29:346‐350. [DOI] [PubMed] [Google Scholar]
- 2. Heitz‐Mayfield L, Salvi GE. Peri‐implant mucositis. J Periodontol. 2018;89:257‐266. [DOI] [PubMed] [Google Scholar]
- 3. Schwarz F, Derks J, Monje A, Wang HL. Peri‐implantitis. J Periodontol. 2018;89:267‐290. [DOI] [PubMed] [Google Scholar]
- 4. Dijk V, Lie MA, Van den Heuvel ER, Van der Weijden GA Adult periodontitis treated with a new device for subgingival lavage ‐ a randomized controlled clinical trial using a split‐mouth design. Int J Dent Hyg. 2018;16:559‐568. [DOI] [PubMed] [Google Scholar]
- 5. Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:95‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287‐291. [Google Scholar]
- 7. Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360‐363. [PubMed] [Google Scholar]
- 8. Lang NP. Nonsurgical therapy for teeth and implants – when and why? Periodontology 2000 2000;79:15‐21. [DOI] [PubMed] [Google Scholar]
- 9. Farina R, Filippi M, Brazzioli J, Tomasi C, Trombelli L. Bleeding on probing around dental implants: a retrospective study of associated factors. J Clin Periodontol. 2017;44:115‐122. [DOI] [PubMed] [Google Scholar]
- 10. Ericsson I, Lindhe J. Probing depth at implants and teeth. An experimental study in the dog. J Clin Periodontol. 1993;20:623‐627. [DOI] [PubMed] [Google Scholar]
- 11. Cionca N, Hashim D, Cancela J, Giannopoulou C, Mombelli A. Pro‐inflammatory cytokines at zirconia implants and teeth. A cross‐sectional assessment. Clin Oral Invest. 2016;20:2285‐2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashim D, Cionca N, Combescure C, Mombelli A. The diagnosis of peri‐implantitis: A systematic review on the predictive value of bleeding on probing. Clin Oral Implants Res. 2018;29:276‐293. [DOI] [PubMed] [Google Scholar]
- 13. Figuero E, Graziani F, Sanz I, Herrera D, Sanz M. Management of peri‐implant mucositis and peri‐implantitis. Periodontology 2000. 2014;66:255‐273. [DOI] [PubMed] [Google Scholar]
- 14. Muthukuru M, Zainvi A, Esplugues EO, Flemmig TF. Non‐surgical therapy for the management of peri‐implantitis: a systematic review. Clin Oral Implants Res. 2012;23:77‐83. [DOI] [PubMed] [Google Scholar]
- 15. Persson G, Roos‐Jansåker AM, Lindahl C, Renvert S. Microbiologic results after non‐surgical erbium‐doped:yttrium, aluminum, and garnet laser or air‐abrasive treatment of peri‐implantitis: a randomized clinical trial. J Periodontol. 2011;82:1267‐1278. [DOI] [PubMed] [Google Scholar]
- 16. Schwarz F, Bieling K, Nuesry E, Sculean A, Becker J. Clinical and histological healing pattern of peri‐implantitis lesions following non‐surgical treatment with an Er:YAG laser. Lasers Surg Med. 2006;38:663‐671. [DOI] [PubMed] [Google Scholar]
- 17. Karring ES, Stavropoulos A, Ellegaard B, Karring T. Treatment of peri‐implantitis by the Vector system. Clin Oral Implants Res. 2005;16:288‐293. [DOI] [PubMed] [Google Scholar]
- 18. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non‐surgical treatment of peri‐implantitis: a double‐blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009;36:604‐609. [DOI] [PubMed] [Google Scholar]
- 19. Bassetti M, Schär D, Wicki B, et al. Anti‐infective therapy of peri‐implantitis with adjunctive local drug delivery or photodynamic therapy: 12‐month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res. 2014;25:279‐287. [DOI] [PubMed] [Google Scholar]
- 20. John G, Sahm N, Becker J, Schwarz F. Nonsurgical treatment of peri‐implantitis using an air‐abrasive device or mechanical debridement and local application of chlorhexidine. Twelve‐month follow‐up of a prospective, randomized, controlled clinical study. Clin Oral Investig. 2015;19:1807‐1814. [DOI] [PubMed] [Google Scholar]
- 21. Estefanía‐Fresco R, García‐de‐la‐Fuente AM, Egaña‐Fernández‐Valderrama A, Bravo M, Aguirre‐Zorzano LA. One‐year results of a non‐surgical treatment protocol for peri‐implantitis. A retrospective case series. Clin Oral Implants Res. 2019;7:702‐712. [DOI] [PubMed] [Google Scholar]
- 22. Mombelli A, Feloutzis A, Brägger U, Lang NP. Treatment of peri‐implantitis by local delivery of tetracycline. Clinical, microbiological and radiological results. Clin Oral Implants Res. 2001;12:287‐294. [DOI] [PubMed] [Google Scholar]
- 23. Salvi GE, Persson GR, Heitz‐Mayfield LJ, Frei M, Lang NP. Adjunctive local antibiotic therapy in the treatment of peri‐implantitis II: clinical and radiographic outcomes. Clin Oral Implants Res. 2007;18:281‐285. [DOI] [PubMed] [Google Scholar]
- 24. Büchter A, Meyer U, Kruse‐Lösler B, Joos U, Kleinheinz J. Sustained release of doxycycline for the treatment of peri‐implantitis: randomised controlled trial. Br J Oral and Maxillofac Surg. 2004;42:439‐444. [DOI] [PubMed] [Google Scholar]
- 25. Persson GR, Salvi GE, Heitz‐Mayfield LJ, Lang NP. Antimicrobial therapy using a local drug delivery system (Arestin) in the treatment of peri‐implantitis. I: Microbiological outcomes. Clin Oral Implants Res. 2006;17:386‐393. [DOI] [PubMed] [Google Scholar]
- 26. Faggion CM, Listl S, Frühauf N, Chang H‐J, Tu Y‐K. A systematic review and Bayesian network meta‐analysis of randomized clinical trials on non‐surgical treatments for peri‐implantitis. J Clin Periodontol. 2014;10:1015‐1025. [DOI] [PubMed] [Google Scholar]
- 27. Eakle WS, Ford C, Boyd RL. Depth of penetration in periodontal pockets with oral irrigation. J Clin Periodontol. 1986;13:39‐44. [DOI] [PubMed] [Google Scholar]
- 28. Persson GR, Samuelsson E, Lindahl C, Renvert S. Mechanical non‐surgical treatment of peri‐implantitis: a single‐blinded randomized longitudinal clinical study. II. Microbiological results. J Clin Periodontol. 2010;37:563‐573. [DOI] [PubMed] [Google Scholar]
- 29. Renvert S. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri‐implant infections: a randomized clinical trial. J Clin Periodontol. 2006;33:362‐369. [DOI] [PubMed] [Google Scholar]
- 30. Renvert S, Roos‐Jansåker AM, Claffey N. Non‐surgical treatment of peri‐implant mucositis and peri‐implantitis: a literature review. J Clin Periodontol. 2008;35:305‐315. [DOI] [PubMed] [Google Scholar]
- 31. Galindo‐Moreno P, Fauri M, Ávila‐Ortiz G, Fernández‐Barbero JE, Cabrera‐León A, Sánchez‐Fernández E. Influence of alcohol and tobacco habits on peri‐implant marginal bone loss: a prospective study. Clin Oral Implants Res. 2005;16:579‐586. [DOI] [PubMed] [Google Scholar]


