Abstract
Background
The literature on botulinum neurotoxin type A (BoNT‐A) is extensive, often contradictory, and confounded by a competitive market of products and research attempting to distinguish brand individuality.
Methods
A comprehensive review of literature on the principles of BoNT‐A in aesthetics as well as clinical examples.
Results
In 2017, the Eight Key Clinical Postulates were formulated as a guide for the aesthetic practitioner in understanding BoNT‐A pharmacodynamics and to compare different toxins. These are now updated to include (a) All type A toxins act identically; (b) The mathematical relationship between toxin and receptor is the basis of efficacy, and clinical efficacy is influenced by molecular potency and patient attributes including muscle mass, gender, age, and ethnicity; (c) Efficacy, onset, and duration are functions of “molecular potency” defined as the number of active 150 kDa molecules available for binding; (d) “Molecular potency” is difficult to objectively quantify for commercially available toxins; (e) Up to a point, increased molecular potency decreases time to onset and increases duration of effect, and the “Molecular Potency Quotient” is a construct for comparing molecular potency commercial cost; (f) The area of effect of a toxin injection is dependent upon molecular potency, diffusion (passive), and spread (active); (g) Differing reconstitution volumes; and (h) Increased number of injection sites can affect spread, onset, and duration of effect.
Conclusions
The principles of BoNT‐A use in aesthetics are complex yet understandable as outlined in the framework of the updated Eight Key Clinical Postulates and serves as a useful tool for providing the most effective treatment and interpreting research on present and future toxin formulations.
Keywords: BoNT‐A, botulinum neurotoxin type A, key clinical postulates, molecular potency, review
1. INTRODUCTION
The injection of botulinum neurotoxin type A (BoNT‐A) has a myriad of clinical indications and is one of the most common procedures in aesthetics. 1 As such, staying abreast of the latest research and discoveries, understanding the clinical science and pharmacodynamics, and being aware of the myths and misconceptions in BoNT‐A use are vital for all practitioners. In doing so, clinicians will be better prepared to assess the properties of the neurotoxins they are administering so they can optimize patient care and make well‐informed decisions as new products enter the market.
The United States currently has Food and Drug Administration (FDA) approval for 4 commercially available brands of BoNT‐A: onabotulinumtoxinA (OnabotulinumtoxinA, Botox), abobotulinumtoxinA (AbobotulinumtoxinA, Dysport), incobotulinumtoxinA (IncobotulinumtoxinA, Xeomin), and the recently approved (Feb 2019) prabotulinumtoxinA (PrabotulinumtoxinA, Jeuveau), with several more currently undergoing clinical trials including daxibotulinumtoxinA (DAXI) and nivobotulinumtoxinA (NIVO, marketed as Innotox in South Korea). 2 , 3 , 4 Outside the United States, these same toxins are sold under different names such as Vistabel (Botox/OnabotulinumtoxinA), Azzalure (Dysport/AbobotulinumtoxinA), Bocouture (Xeomin/IncobotulinumtoxinA), and Nabota (Jeuveau/PrabotulinumtoxinA). Additionally, other BoNT‐A preparations exist outside the United States such as Neuronox and Botulax in South Korea, Relatox in Russia, and Chinatox/CBTX‐A in China, which is also marketed under a variety of names in different countries including Prosigne, Lantox, Liftox, and Redux. 5 , 6 , 7
Decades of research have supported the safe and efficacious use of BoNT‐A for the treatment of facial wrinkles. 8 , 9 Each commercial brand of BoNT‐A markets extensively to claim differences in efficacy, speed of onset, duration of effect, and “diffusion” characteristics; however, clinical trials have produced varied data about these defining differences. 8 , 9 Studies frequently lack the power or appropriate design to support claims in product individuality, and many differences that are found can be attributed to differences in dosing. In particular, data have been mixed regarding the equivalence and interconversion of dosing units between products, the relevance of unique manufacturing and storage processes, the impact of reconstitution volume, local spread, and diffusion. These claims can influence both consumers and practitioners yet are challenging to delineate and reconcile. Indeed, even though recent trials seem to indicate that there may be some potential advantages of some toxins over others on critical clinical attributes such as onset and duration of effect, these may be just delineating differences in toxin potency. 8 , 9
The principles of BoNT‐A use are defined by what we called the “Eight Key Clinical Postulates” and can be used as a practitioner's tool in interpreting and analyzing current and novel BoNT‐A literature for the purpose of making the most informed clinical decisions in their practice. 8 The following serves as an extensive review, expansion, and update of these postulates, incorporating new literature, novel toxins, and addendums to the foundations of BoNT‐A mechanics.
Additionally, we introduce the Molecular Potency Quotient as a new concept with which to evaluate and compare the “cost (in dollars) for a given clinical effect” of different commercially available toxins. Given that each manufacturer has its own proprietary “units” of measurement, comparing the potency of the toxins is difficult and often confusing both for providers and patients. While the potency itself is an important characteristic to define, more useful to actual clinical practice is the ratio of potency to cost. The maximum clinical effect of a toxin for facial aesthetic use is the “frozen” look with a higher degree of frozenness correlating with a greater duration of effect, but many patients desire a lesser level of paralysis that minimizes wrinkles and lines while still maintaining some natural movement. The number of units required to reach the desired endpoint divided by the cost per unit defines the “Molecular Potency Quotient.”
2. POSTULATE I
All type a toxins act identically.
2.1. Molecular structure and mechanism of action
While differences exist in manufacturing and formulation, all BoNT‐A products share an identical mechanism of action: causing muscular weakness and paralysis by preventing acetylcholine (ACh) release into the synaptic cleft at nerve endings within striated muscle, smooth muscle, and autonomic exocrine glands. Botulinum neurotoxin is produced by Clostridium botulinum, a gram‐positive, spore‐forming, anaerobic bacterium. 10 Currently, 7 major serotypes (A‐G) and over 40 subtypes (distinguished by numbers) are recognized with a hybrid serotype (H/HA/FA) described in 2013 and a new gene‐sequence‐only serotype (X) in 2017, although A1 is the only serotype approved by the FDA for aesthetic use. 11 , 12 , 13 Botulinum neurotoxins are made by the producing bacteria as a complex of various proteins. The pharmacologically active toxin is a 150 kilodalton (kDa) protein consisting of a 100 kDa heavy chain (HC) and a 50 kDa light chain (LC) connected via a single disulfide bond. 14 For some commercially available toxins, the core neurotoxin protein is noncovalently associated with a group of neurotoxin‐associated complexing proteins (NAPs) which are a combination of several hemagglutinin proteins and one nonhemagglutinin protein that act in concert, when the toxin is injected, to shield the toxin from unfavorable conditions such as stomach acid when ingested as part of spoiled food, a normal habitat of C botulinum.
The manufacturing of commercial BoNT‐A is similar but unique to each product (see Table 1). Unlike chemically synthesized drugs produced through a specific set of controllable chemical reactions, botulinum toxins are proteins produced by living strains of Clostridium bacteria. The toxin proteins are hundreds or thousands of times larger in size than most synthetic drugs and undergo twisting and folding in specific ways to produce the secondary and tertiary structures that allow for their clinical effect and specificity. 15 , 16 All FDA‐approved BoNT‐As are produced by the Hall strain of Clostridium botulinum, but from there each manufacturer takes similar but different steps to purify and prepare the toxin for clinical use. OnabotulinumtoxinA (Botox/Vistabel) consists of uniform 900 kDa toxin complexes while IncobotulinumtoxinA (Xeomin/Bocouture) undergoes purification to isolate the 150 kDa free toxin. 14 , 17 AbobotulinumtoxinA (Dysport/Azzalure) has a complex size of ~500 kDa but has not been precisely disclosed by the manufacturer. 15 , 16 The newest FDA‐approved BoNT‐A formulation, PrabotulinumtoxinA (Jeuveau) also has 900 kDa complexes. 14 , 18 DaxibotulinumtoxinA (DAXI) consists of 150 kDa toxin formulated with a stabilizing excipient peptide. 3 The diluents used in each company's manufacturing process differ from saline to human serum albumin to a gelatin phosphate buffer. The excipients used also vary including sodium chloride, lactose, sucrose, and differing volumes of human serum albumin. Finally, the methods used for finishing and drying the toxin include vacuum drying for OnabotulinumtoxinA and PrabotulinumtoxinA, freeze‐drying for AbobotulinumtoxinA, and lyophilized drying for IncobotulinumtoxinA. Included in this process is each manufacturer's own assessment of potency based on LD50 mouse toxicity assays as there is no standardized assay as of yet. 15 , 16
TABLE 1.
| OnabotulinumtoxinA (Botox) | AbobotulinumtoxinA (Dysport) | IncobotulinumtoxinA (Xeomin) | PrabotulinumtoxinA (Jeuveau) | DaxibotulinumtoxinA (DAXI) | |
|---|---|---|---|---|---|
| Company | AbbVie (Allergan) | Ipsen/Galderma | Merz | Evolus | Revance |
| Serotype | A1 | A1 | A1 | A1 | A1 |
| FDA Approval a | 2002 | 2009 | 2011 | 2019 | Expected 2020 |
| Purified product size | 900 kDa complex | ~500 kDa complex (undisclosed by manufacturer) | 150 kDa toxin only | 900 kDa complex | 150 kDa complex |
| Diluent | Saline | Gelatin phosphate buffer | Human serum albumin | Saline | Saline |
| Excipients | Sodium chloride, human serum albumin | Lactose, human serum albumin | Sucrose, human serum albumin | Sodium chloride, human serum albumin | Polysorbate‐20, buffers, single sugar, stabilizing peptide |
| Finishing | Vacuum‐dried | Freeze‐dried | Lyophilized | Vacuum‐dried | Lyophilized |
For aesthetic indications.
Complexes of BoNT‐A are most stable at pH values of 6.25 and below. At the more neutral pH levels which normally occur during reconstitution, the complexing proteins dissociate prior to injection or to reaching target neuronal cells. Studies on the dissociation of 150 kDa BoNT‐A from the attached NAPs have demonstrated that commercial preparations of BoNT‐A contain virtually no complexed neurotoxin after reconstitution. The process of dissociation is pH‐dependent and may also be salinity‐dependent. 10 , 19 After reconstitution with sterile buffered saline, one study showed that product vials contained uncomplexed neurotoxin concentrations of 89% in OnabotulinumtoxinA and 100% in AbobotulinumtoxinA and IncobotulinumtoxinA. 10 Furthermore, any complexed toxin would quickly be released into free form upon injection into the target tissue where physiologic pH conditions favor dissociation even more. 10 While the vast majority of literature suggests that the 150 kDa BoNT‐A protein remains largely disassociated with NAPs at physiologic pH, it should be noted some studies suggest that dissociation may occur even prior to or shortly after injection and not necessarily dependent on the presence of a neutral pH alone. 10 , 20 , 21
It has been the established hypothesis that the NAPs appear to play no role in the actual mechanism of action as all neurotoxin is in free form upon injection, and diffusion characteristics among all BoNT‐A products into the same muscle type and at comparable doses have not shown any significant differences. 10 , 22 However, in 1999, Cai et al published the first paper demonstrating an improvement in efficacy of BoNT‐A when complexed with NAPs. 23 Prior to this, NAPs were only suggested to play a limited role in protecting neurotoxin from gastrointestinal acidity and proteases and in the external environment. The mechanism by which these APs were suggested to enhance BoNT‐A activity was by enhancing its zinc‐dependent endopeptidase activity via reduction of the disulfide bond that links the heavy and lights chains. 23 , 24 This was exemplified in a 2004 study by Sharma and Singh who isolated one of the NAP components, specifically hemagglutinin‐33 (Hn‐33). 24 Addition of Hn‐33 separately to nonreduced BoNT‐A enhanced its endopeptidase activity 13‐fold. 24 More recent literature suggests that Hn‐33 may also enhance presentation of neurotoxin at the receptor site and facilitates internalization. 25
After injection and dissociation of the 150 kDa neurotoxin from the NAPs, the C‐terminal end of the 100 kDa heavy chain binds first to complex polysialic gangliosides that are abundantly present on the exterior surface of neuronal membranes. This is facilitated by the bipolar nature of the toxin wherein the abundance of positively charged amino acids near the binding site orient it with the negatively charged gangliosides. 26 The toxin molecules accumulate until it binds to exposed glycoprotein 2 (SV2), a protein found in the neurons of vertebrates. The heavy chain then binds to SV2 at two locations: a peptide moiety on the fourth luminal domain (SV2‐L4) and a recently discovered N‐linked glycan on the same domain. These binding points anchor and pull the toxin into the synaptic vesicle as it is endocytosed into the neuron. 27 , 28 , 29 This process has been shown to take approximately 5‐10 minutes via in vitro studies. 30
Inside the motor neuron, within the acidic environment of the endosome, the N‐terminal portion of the heavy chain inserts into the vesicle membrane creating channels that allow the 50 kDa light chain to translocate toward the cytosolic side where the disulfide bond is enzymatically cleaved by thioreductase, releasing the light chain fully into the cytosol. 31 , 32 The light chain then moves across the cytosol and binds to a member of the SNARE protein complex, specifically SNAP‐25 (synaptosomal membrane‐associated protein, 25 kDa). As a zinc‐dependent metalloproteinase enzyme, the light chain cleaves SNAP‐25 which prevents ACh‐containing synaptic vesicles from being able to fuse with the presynaptic membrane. This critical step results in blockage of ACh release into the neuromuscular junction (NMJ), thereby preventing muscle contraction. The light chain has been detected in the cytosol of rat neurons for as long as 10 months. 33 This chemodenervation is the mechanism by which all type A toxins function. In contrast, other serotypes of botulinum toxin enter the neuron via a set of membrane receptors called synaptotagmins and target different SNARE proteins such as VAMP (synaptobrevin) and syntaxin to cause chemodenervation. 34 Research involving the use of recombinant and chimeric toxins (eg, part A toxin and part B toxin) to improve clinical efficacy is ongoing. 35
The amount of active 50 kDa light chain domain in the motor neuron defines toxin longevity by cleaving SNAP‐25 and allowing toxin to persist intracellularly in neuronal cells. A number of studies have been undertaken to determine the cause of BoNT‐A’s unique longevity among the serotypes, particularly as compared to the very short‐lived BoNT‐E. Immunofluorescence techniques have demonstrated that the light chain of BoNT‐A largely localizes along the inside of the plasma membrane after cleavage from the heavy chain, whereas the light chain of BoNT‐E is seen distributed throughout the cytosol. 36 Similarly, BoNT‐A3, a subtype with shorter duration of action than A1, localizes throughout the cytosol. 33 This localization along the plasma membrane is hypothesized to be a contributing factor to duration of action where it may be less exposed to degradation by proteases leading to greater stability. The specific cause or causes of the stabilization and localization of A1 are not entirely clear, but several hypotheses include reduced susceptibility to the ubiquitination pathway via recruitment of deubiquitinating enzymes, tyrosine phosphorylation, interaction with septins on the cytoskeleton, and, notably, the presence of a dileucine motif near the C terminus which, when mutated, results in significant loss of longevity of action. 37 , 38 , 39 , 40 , 41 The BoNT‐A light chain continues to exert its lytic effects upon newly generated SNAP‐25 throughout the LC’s lengthy half‐life of several months, but once it is ultimately degraded inhibition of ACh release resolves very rapidly. 33 , 42 , 43
The binding of BoNT‐A to neurons and internalization appears to be irreversible and almost permanent, but the invoked muscle paralysis is only temporary. The onset of paralysis is typically seen within 48 hours, but has been documented clinically in as early as 6 hours, specifically among patients treated with AbobotulinumtoxinA to the frontalis muscle. 44 , 45 Time to toxin onset of action is highly influenced by muscle anatomy and thickness as well as localization of injection, particularly if it is injected in the middle of a muscle fiber as this is where the motor endplates are typically concentrated. 46 When toxin is injected into a muscle, it first resides in extracellular space until it is taken up by nerve terminal end plates. 46 Thus, injecting more centrally to the muscle body should yield an earlier clinical result; however, fibers are organized differently among different muscles. 46 This is where a clinician's anatomical knowledge may guide injection location selection for a more efficacious effect. In order to confirm the ideal location for injection, electromyography (EMG) has proven useful in demonstrating a higher intensity of endplate spikes to indicate the most efficacious location for toxin injection. 46
Glycosylation patterns may play a role in time to onset, and these vary (genetically) among individuals. 13 , 47 The same dose of BoNT‐A in one individual may exert a different time to achieve a clinical effect in another individual as different amounts of bound toxin may correspond to different numbers of light chains that enter the cytosol of nerve terminals. 47 Evidence for the relevance of glycosylation in influencing the onset of action is seen when comparing the difference in N‐glycans between vertebrates and invertebrates, as invertebrates lack sensitivity to BoNTs. 48
Muscle recovery is the least understood part of the process but appears to occur as ongoing cellular turnover at the NMJ restores contractile function which begins returning after several weeks and gradually reaches pretreatment strength in approximately 4‐6 months. Recovery of neuromuscular transmission involves replacement of the lysed proteins and proliferation of motor axon sprouts to form new synaptic contacts at the motor endplate. There is some evidence that repeated injections of BoNT‐A may result in slower functional recovery and persistent structural abnormalities of motor innervation in spite of normal function. 30 , 49 , 50 , 51
While the binding of BoNT‐A to the nerve is irreversible, the paralytic effect is temporary as motor axon sprouts form and cellular turnover in the original nerve terminal gradually allows for acetylcholine release. 49 , 50 Imaging after injection has shown a toxin distribution pattern along the long axis of muscle fibers and present for a period of about 12 hours. In mouse models, this pattern does not seem to differ regardless of the marketed brand of BoNT‐A used, given that similar volumes of injection were administered. 49
The process of recovery is divided into an aneural and neural stage. 52 The aneural stage begins from administration of BoNT‐A and ends 2‐3 weeks after injection. During this stage, nAChR genes are upregulated and postsynaptic end plates begin to form, injected muscles attain peak atrophy, and nerve axon sprouting begins which plays a key role in recovery of paralyzed motor end plates. 52 IGF‐1 signaling has been shown to be central to this recovery process. In a study of 56 rats injected with BoNT‐A to the gastrocnemius muscles, key molecules and genes targeted by the IGF‐1 signaling pathway and involved in NMJ stabilization, remodeling, and myogenesis were shown to be either upregulated or downregulated after a period of seven days. 52 Myogenic regulatory factor (MRF) proteins are activated by IGF‐1 in order to upregulate nAChR genes. 52
The neural stage begins 4‐6 weeks postinjection and is characterized by gradual re‐innervation and myogenesis of the skeletal muscle. Motor neurons are re‐innervated and nAChR clusters and NMJs begin to reform. Myogenin, an MRF that persists particularly longer than other MRFs, has been proposed to mediate myogenesis and muscle regeneration. This stage ends approximately 3‐6 months after BoNT‐A administration resulting in NMJ stabilization, regeneration, and eventually complete muscle functional recovery. 52
2.2. Modifying factors in onset and efficacy
The onset of partial paralysis typically occurs within the first 48 hours after injection of BoNT‐A (and as early as 6 hours) and commonly lasts 3‐5 months depending on dose, technique, area treated, and patient demographics. 45 On a molecular level, the time required to elicit an effect on muscle is determined by the time needed to sufficiently cleave the target SNARE proteins in order to interfere with synaptic release. 28 , 46 Nonmolecular factors may play a role in uptake and onset including temperature and activity. For example, cooling has been shown to slow toxin uptake while purposeful contractions of injected muscle have been shown to decrease time to onset, likely due to increased numbers of exocytosed synaptic vesicles providing more opportunities for toxin binding to SV2. 28 , 46 As toxins are zinc‐dependent proteases, it has been shown that zinc supplementation may shorten onset as well. 46 The average onset is typically faster among toxin‐naive patients compared with non‐naive patients.
The sites with the shortest time to onset of effect with AbobotulinumtoxinA have consistently been shown to be in the areas of the forehead and around the eyes. 53 AbobotulinumtoxinA has shown a median onset of effect of 2‐4 days for glabellar, forehead, and lateral canthal lines. 54 , 55 , 56 Response rates of AbobotulinumtoxinA by day 7 have shown to vary between 57% and 83% as measured on a 0‐3 point scale among subjects and investigators. 54 , 55 As much as 61% of subjects still show some measure of continued response up to 6 months postinjection before relapsing, although the clinical effect is minimal at that point. 49 , 57 In general, similar time to onset and response rates have been demonstrated among OnabotulinumtoxinA, IncobotulinumtoxinA, and AbobotulinumtoxinA, although one study did demonstrate slightly quicker time to onset for AbobotulinumtoxinA. 54 , 55 , 58
2.3. Future toxin developments
The future of botulinum toxin in medicine looks promising as new variants are developed with unique attributes and targets. For example, hybrid LC‐HC toxins involving subtypes A1 and A3 were demonstrated to have different potencies than either pure toxin subtype, although neither hybrid was better than pure A1 toxin (A1 is the only FDA‐approved subtype for aesthetics currently). 33 Relatedly, the joining of the LC domain of BoNT‐D with interleukin‐1 ligand for targeted inhibition of inflammatory cytokine release from macrophages shows potential as a future treatment for rheumatoid arthritis. 59
A new BoNT‐A called DaxibotulinumtoxinA (DAXI, Revance) is expected to come to market in late 2020. It uses the same A1 toxin subtype as OnabotulinumtoxinA, AbobotulinumtoxinA, IncobotulinumtoxinA, and PrabotulinumtoxinA but adds a peptide excipient (RTP004) that binds to the neurotoxin in order to stabilize and prevent aggregation of the toxin in solution. 3 The peptide is made up of two protein transduction domains consisting of a lysine chain that provides a strong electrostatic bond for the novel toxin to bind. 3 , 60 The end result is more “active” toxin available for binding upon injection and thus a higher molecular potency. 3 The peptide‐toxin interaction has the added advantage in that the final product can be formulated without human serum albumin and is stable at room temperature before reconstitution. 3 During clinical trials, the average duration of effect of DAXI was in the 24‐28 week range, significantly longer than the 12‐16 weeks typically seen with BoNT‐A products. 3 , 61
3. POSTULATE II
The mathematical relationship between toxin and receptor is the basis of efficacy, and clinical efficacy is influenced by molecular potency and patient attributes including muscle mass, gender, age, and ethnicity.
3.1. Kinetics
The clinical effects of botulinum toxin are dependent on the kinetic relationship between the toxin and its receptor, as reflected in a mathematical ligand‐receptor binding model. While there is still much to be learned about the molecular biology of botulinum toxin, the molecule clearly exhibits a classic pharmacokinetic relationship with its targeted receptors. As previously described, the mechanism of action of BoNT‐A has multiple steps. Figure 1 demonstrates this in pharmacokinetic terms. The rate and degree of reaction are dependent on both the concentration of toxin and the density of the receptors.
FIGURE 1.

Ligand‐receptor–binding model for toxin and its corresponding receptor. Bulk botulinum toxin is complexed with neurotoxin‐associated complexing Proteins and formulated with human serum albumin in the vial before becoming free type A neurotoxin (150 kDa) in a neutral to basic environment, such as upon reconstitution. Following injection, the complex‐free neurotoxin binds to 2 extraneuronal receptors, allowing bound neurotoxin to undergo endocytosis. The translocated neurotoxin is able to move intracellularly across the neuronal cytosol to the intracellular target, SNAP‐25, inducing proteolysis. Proteolysis of SNAP‐25 induces blockade of acetylcholine release into the postsynaptic neuromuscular junction, resulting in muscular paralysis. (Adapted from Simpson 62 and Lebeda et al 148 )
As stated in Postulate I, all type A toxins act identically. However, clinical differences arise due to extrinsic and intrinsic factors such as variance in molecular potency of the toxin (defined here as the number of active 150 kDa molecules available for binding), the distribution in tissue, and receptor density, which varies based on muscle type, muscle mass, gender, age, and other genetic features. 8 This relationship between the number of active 150 kDa molecules and their corresponding receptors determines the ultimate clinical effect of BoNT‐A as described below.
In this model (Figure 1), BoNT‐A is in equilibrium between a bulk state (toxin + NAPs, prior to reconstitution), free state (toxin, not yet bound to receptor, nonaggregated and available to bind), bound state (interacting with receptor), trans‐state (intermediate form between bound and active state), and lytic (biologically active) state. The rate‐limiting step in this process is the binding of the heavy chain to its corresponding receptors at the presynaptic interface. Thus, given that the free form is the only active component of BoNT‐A, it follows that the potency of the toxin is directly proportional to the absolute quantity of available, viable (active/nonaggregated) 150 kDa toxin proteins injected irrespective of the number of proprietary “units” used for each brand. 62 If optimally distributed, the quantity of viable active form that is injected is what will determine the toxin's clinical effect. 63
3.2. Molecular potency
As noted, the molecular potency of a BoNT‐A product is defined as the total number of active (ie, capable of binding) 150 kDa neurotoxin molecules present in the injected solution. In the mathematical ligand‐receptor binding model (Figure 1), an increase in molecular potency will allow a greater number of receptors to be bound, moving the reaction equilibrium toward the final lytic state, thus increasing the resultant clinical effects.
The molecular potency per unit of each BoNT‐A formulation is not identical, and units are not interchangeable between brands. Each brand's units, short for mouse units, are based upon the LD50 of the toxin (the median lethal dose that causes death in 50% of mice injected in the abdomen) found during preclinical evaluation. 64 Few well‐designed, controlled, randomized studies directly compare formulations of BoNT‐A. 65 , 66 Those that do attempt to compare brands have somewhat inconsistent and conflicting results, which may be due to a plethora of confounding variables that are difficult to control in addition to the lack of consensus regarding dose unit conversion ratios upon which they are based. 18 , 77 Generally, IncobotulinumtoxinA and OnabotulinumtoxinA may have close to a 1:1 dose equivalence ratio, but some dispute this. 78 PrabotulinumtoxinA and OnabotulinumtoxinA may have a 1:1 or higher ratio. 71 , 72 AbobotulinumtoxinA and OnabotulinumtoxinA seem to have a 2:1 or 2.5:1 equivalence ratio, although the variance of ratios used among studies ranges from 1.5:1 to 3:1. 73 , 74 , 75 , 76 , 77 , 79 The direct measurements of the quantity of the mean active 150 kDa BoNT‐A content for the FDA‐approved glabella dosing have found that IncobotulinumtoxinA, OnabotulinumtoxinA, and AbobotulinumtoxinA have 80.6 pg/20 XU, 180.8 pg/20 BU and 301.1 pg/50 DU respectively. 80 Additionally, it has been hypothesized to be due to the vacuum drying of OnabotulinumtoxinA may cause denaturation, aggregation, or otherwise inactivation of a portion of the toxin. 81
Furthermore, molecular potency can be confounded by differences in dilution solvents among products which may influence the availability and activity of the 150 kDa molecules. For example, AbobotulinumtoxinA is diluted in a phosphate buffer containing gelatin preservative while OnabotulinumtoxinA is diluted in simple saline solution. 82 These differences may alter the active amount of 150kDa molecules within each vial. As mentioned previously, the novel peptide excipient used in the upcoming toxin, DAXI, may work to prevent aggregation and thus allow for a greater amount of active toxin molecules thus increased molecular potency.
As noted, NAPs also may function as a secondary factor in influencing molecular potency, not only by preventing aggregation as in this example, but possibly by enhancing or interfering with heavy chain binding.
Finally, for toxins currently on the market, variability in potency of individual lots is allotted a range of +25% to −20% by the FDA for reasons noted above such as differences in storage and preparation of products and in the nature of specific LD50 bioassay used as well as to limit the number of mice required to conduct the assay. 83
Molecular potency is the most objective way of comparing different commercially available toxins; however, only part of the overall equation that determines an individual patient's response to BoNT‐A treatment. The muscles of facial expression vary greatly in size, mass, strength, and from individual to individual. High inter‐patient variability in response to standardized injection amounts supports this premise and adds another layer of difficulty in designing and interpreting strong clinical trials. 84 The quantity and density of receptors to which the neurotoxin binds directly impact the rate‐limiting step, and patient factors such as gender, muscle mass, facial structure, age, and genetics all affect said receptors. Additionally, given the small size of most of the targeted facial muscles, injection techniques including location, angle, and concentration all play a role in influencing clinical effect. 8
3.3. Muscle mass
Most skeletal muscles originate from and insert onto bony structures and can be grossly visualized on the outer surface of the body due to their size and strength. 85 In contrast, the muscles of facial expression have soft tissue attachments to skin. 86 When the facial muscles are contracted, they pull on the overlying skin forming dynamic wrinkles perpendicular to the direction of contraction. Furthermore, muscles of facial expression are difficult to demarcate precisely from surface anatomy due to their superficiality, intermingled borders, and overlap.
The muscles of facial expression not only differ in size from other skeletal muscles of the body but also from one another. The procerus and corrugators involved in GL have considerably more bulk than the sheet‐like orbicularis oculi involved in LCL, for example. The frontalis is large and thin yet varies in contraction strength within and between individuals. Each muscle thus requires individualized dosing regimens to attain the desired reduction in rhytides while avoiding the undesired frozen look from overdosing. While OnabotulinumtoxinA, IncobotulinumtoxinA, AbobotulinumtoxinA, and PrabotulinumtoxinA are all FDA‐approved for the treatment of glabellar lines (GL), only OnabotulinumtoxinA is thus far FDA‐approved for the treatment of lateral canthal lines (LCL) and forehead frontalis lines. 2 Yet all are used off‐label for reduction of lower facial wrinkles and even extending onto the neck and chest. 87 , 88 The facial muscle mass also varies greatly between patients based on genetics, age, and sex. 89 Correlatively, the number and density of NMJs available for BoNT‐A to bind is distinct and different for each individual and directly affects the results of neurotoxin injection.
3.4. Gender
As mentioned above, muscle mass varies greatly. Nowhere is this more evident than comparing the treatment of men and women. The number of men seeking BoNT‐A has increased significantly in the past years. 90 Although almost 20 years have passed since the FDA approved the aesthetic use of botulinum toxin, few studies have examined the role of gender in toxin dosing, efficacy, or safety. However, those that have been done have shown that men typically require higher doses to achieve the same clinical effect as women. 54 , 90 , 91
Men have a significantly greater amount of skeletal muscle than women, including in the musculature of the face and also have greater facial movement and a thinner adipose layer, further contributing to greater propensity to form more severe wrinkles. Men also have a greater density of vessels in facial skin and lower eyebrow position along the orbital rim. 91 These factors are theoretical risks for greater bruising and eyebrow ptosis, respectively. Of note, women exhibit more severe wrinkling in the perioral area than men. Studies have shown that men typically require more units for a given degree and duration of response. Again, this demonstrates that with larger muscle mass, the numbers and/or density of toxin receptors is likely to be greater. In order to bind to more receptors, more toxin is required. Men and women should not receive equal dosing in clinical practice, although more studies are needed to assess gender differencing dosing efficacy and safety of BoNT‐A. 91
Studies have shown women to have shorter response times than men regardless of BoNT‐A product, with women's response time ranging 2‐4 days and men's ranging 2‐5. 92 , 93 This is likely due to greater muscle mass and a greater number of toxin receptors available for binding among the facial structures in men thus requiring larger dosing of toxin to attain the expected clinical effect. For example, some men may require up to 80 U of OnabotulinumtoxinA for the glabellar area in order to elicit a response with a recommended starting dose of 40 U. 94 This is in comparison to females who typically require 20‐35 U. However, one study demonstrated that duration of effect among females ranged from 3 to 5 months compared with a range of 4‐6 months for males. 95 Until additional studies can delineate the cause, it is assumed this slight difference is due to either small sample sizes or relatively higher dosing among males possibly caused by “overdosing” by practitioners who automatically give men larger doses knowing they often will need more toxin.
3.5. Age
All brands of commercial BoNT‐A in the United States are approved for adults generally aged 18 to 65 years. Patients older than 65 years are very commonly treated with BoNT‐A, despite no true guidelines. Significant age‐related changes occur at the neuromuscular junction. Aging results in a progressive loss of muscle mass and strength and a decline in neurophysiologic function. 49 , 96 , 97 There is a gradual loss of motor neurons. If the motor neurons to a muscle become less efficient or even nonfunctional, the muscle fiber that they innervated becomes equally noncontributory to the dynamic movement of that muscle. Denervation by BoNT‐A is followed by a steady recovery of the original NMJ as well as by the formation of functional nerve sprouts. 49 In older patients, this compensatory process is considerably slower. Therefore, there is a progressive decline in muscle mass and strength.
There are no adequate clinical trials to prove that elderly patients over 65 respond differently to BoNT‐A than younger patients, although, again, the data are very limited in this age group. Based on limited evidence and clinical experience, it is suspected that BoNT‐A is not as effective at eliciting a clinical response among this age group. This is explained by thinner and less elastic skin, more muscle atrophy, and the existence of static wrinkles due to gravity rather than dynamic wrinkles due to muscle contraction. 98
Special considerations must also be made in the elderly. For instance, injecting the frontalis muscle can cause excessive drooping of the forehead, eyebrows, and eyelids, which could compromise vision. Thinning skin might increase diffusion distance, possibly increasing the risks of complications like ptosis in patients receiving BoNT‐A for GL. Like dosing throughout medicine, it is prudent to start with low BoNT‐A doses. Begin with the lowest possible dose for elderly patients. Many are also at an increased risk of bruising due to thin skin and common concomitant blood thinning agents. Conservative dosing, low volume injections, and proper placement of injection are crucial to avoid the unwanted spread of toxin effect. 99
3.6. Skin type and ethnicity
Comparisons of aesthetic outcomes between different racial and ethnic groups following the use of BoNT‐A have not been well documented in the literature. As patient populations become more diverse, variance in response to BoNT‐A in different racial and ethnic groups should be recognized. With the paucity of studies, recommendations based on racial differences are difficult. 99 , 100 Genetic differences in the skin of different colors could correspond with genetic differences in toxin receptor density and thus the response to BoNT‐A may be different.
Patients with skin of color have differences in skin texture and elasticity as well as the content of subcutaneous fat. These differences often result in skin with less fine lines, wrinkles, and photodamage compared to Caucasians. Asians generally have a thicker dermis, more collagen fiber, and a firmer attachment of the skin to underlying tissues resulting in fewer fine lines, wrinkles, and laxity. 101 , 102 , 103
While Asian skin may have fewer fine lines and wrinkles, Asian populations have a greater incidence of masseter hypertrophy. Bilateral masseteric hypertrophy is common and thought to be due to bruxism, jaw clenching, and overactivity of the masseter muscle. This results in a square jaw and broad‐looking face that is visually unappealing and masculinizing in women. Injection of BoNT‐A into the masseters is an effective tool for lower facial contouring, producing an improvement in bruxism and reestablishment of the triangular‐shaped face of youth. Some limitations in smiling and diminished chewing power are the most common complaints. 104 , 105 , 106
Some studies have shown that there are differences in the responses to BoNT‐A in different racial and ethnic groups. One study demonstrated that patients with skin of color exhibited a greater response rate to AbobotulinumtoxinA at 30 days compared to white patients, showing that there are important practical considerations when treating patients with skin of color. 100 Additionally, a consensus on treating patients of Asian origin has recommended a more conservative approach to dosing in, for example, the treatment of GL and perioral area. 100 , 107
4. POSTULATE III
Efficacy, onset, and duration are functions of “molecular potency” defined as the number of active 150 kDa molecules available for binding.
Efficacy, onset, and duration are all functions within the mathematical receptor binding model. Postulate I explains how all BoNT‐A products have an identical mechanism of action. Postulate II delineates the pharmacokinetic relationship of toxin and receptor in a classic ligand‐receptor model as well as patient attributes. The end result of these processes is what is observed clinically as the toxin's efficacy as measured by onset and duration of effect. Differences in the pharmacodynamic relationship between toxin and receptor can cause differences in clinical efficacy.
All brands of BoNT‐A are effective in partially paralyzing facial muscles to improve wrinkling. To obtain a competitive advantage in the commercial market, each brand markets to the public with claims of greater purity, convenience, or even efficacy over their peers on the shelf. Yet, these claims largely serve as marketing buzzwords rather than true indicators of differences between the toxins. In particular, there is no official definition of efficacy (which is probably defined as the cosmetic benefit) nor any validated scale for measuring it, rather this is done in proxy based on validated wrinkle scales.
While the efficacy of a neurotoxin has no universal definition, it can be correlated on a molecular level to the amount of viable toxin available and by the percentage of neuromuscular junctions affected. Efficacy is proportional to the number of NMJs bound by active 150 kDa BoNT‐A molecules. The more receptors bound by active toxin, the more internalization of the 150 kDa units and the stronger the clinical response will be. A highly efficacious neurotoxin will have a short onset and long duration of effect, both dependent on this ligand‐receptor relationship. While both of these clinical effects are part of efficacy, the duration is also dependent on nerve terminal and synapse recovery times. Additionally, efficacy will vary based on quantity of toxin injected, treatment area, and degree of NMJ receptors available for binding and ultimately on the desired cosmetic benefit to the patient.
5. POSTULATE IV
“Molecular potency” is difficult to objectively quantify for commercially available toxins.
Methods of comparison of molecular potency for commercially available toxins include comparing independent trial data; comparing different toxins in different subjects in a single trial (noninferiority); bilateral comparisons of different toxins in a single subject; and direct measurement of toxin pg and activity. There have been a number of trials attempting to compare molecular potency among toxins; however, the data make it difficult to form absolute conclusions. The main reason for this is that the units of toxin products are proprietary measurements and dependent on the type of assay used. This makes every toxin unique and impossible to directly compare with each other. In addition, the LD50 is manufacturer‐dependent and based on mouse models rather than human ones and the amount of 150 kDa neurotoxin, availability, and activity vary from product to product. 14 This further complicates direct methods of comparing potency among products. Keeping this in mind, the closest we can get to comparing potencies is by evaluating each toxin's clinical effect based on the FDA‐approved units, which are still confounded by differences in test subjects despite rigorous inclusion and exclusion criteria and rating scales that may not translate into actual clinical application. Each study also has its own unique endpoints. This makes comparing the efficacy of toxin brands incredibly difficult. The majority of BoNT‐A comparison studies have been focused on AbobotulinumtoxinA vs OnabotulinumtoxinA and differ in their reports of efficacy, time of onset, and duration between the two.
Some of the most often quoted comparisons of commercial toxins are onset, duration, and adverse events obtained in separate FDA approval trials. 56 , 108 , 109 However, since different FDA trials use different protocols and efficacy scales and are performed by different investigators, it is impossible to use them as accurate comparators of molecular potencies. According to the FDA Guidance to Industry, assessment scales should also be ordinal, static, reproducible, and include only a limited number of distinct and clinically meaningful categories, preferably with a photonumeric guide for patients and investigators. 110 Common assessment tools such as the facial wrinkle scale and GL severity score are 4‐point photonumeric ordinal scales that ranging from no wrinkling to severe wrinkling and have shown good inter‐ and intraobserver reproducibility. 111 , 112 The 5‐point photonumeric scale developed by Caruthers and Carruthers is a good example. By including a midpoint, it allows for grading of a continuous process such as aging. 113 , 114 , 115 Most FDA studies, however, use their own proprietary FDA‐approved, validated scales that differ from manufacturer to manufacturer.
“Side‐by‐side” methods of comparing neurotoxins are much more accurate and have suggested differences in potency between BoNT‐A products. In this scenario, one group of patients receives one particular toxin while another group receives another. In a 150‐day, multicenter, double‐blind, single‐dose (corresponding to FDA‐approved doses) noninferiority trial comparing PrabotulinumtoxinA to OnabotulinumtoxinA at the same 20U dose (approved dose for GL) and placebo, a 5:5:1 ratio of 540 patients were administered 0.1mL of the corresponding treatment to each of the 5 glabellar injection points. Although not quite reaching statistical significance, there was indication of increased duration of effect for PrabotulinumtoxinA. 71
Other side‐by‐side trials have compared potency of OnabotulinumtoxinA and AbobotulinumtoxinA. The majority of these trials are weak, present conflicting conclusions regarding potency, and often compare nonequivalent doses of drug. One study compared the FDA‐approved doses of 20 U of OnabotulinumtoxinA with 50 U of AbobotulinumtoxinA (1:2.5 dose ratio) and compared glabellar line severity at 12 and 16‐week endpoints. Results showed a 1 point or greater grade improvement in 77% and 53% of patients for weeks 12 and 16 respectively among OnabotulinumtoxinA‐treated patients and 59% and 28% improvement in AbobotulinumtoxinA‐treated patients. 68 The study, however, enrolled only a small number of patients and included mostly younger patients that may require higher doses of drug due to stronger corrugators compared to older patients. 68
Split‐face studies seem to provide the most direct and accurate method for clinically comparing toxin potency because they allow for patients to act as their own control, using reproducible, identical techniques and objective measurements. Recent studies have compared the effect of different BoNT‐A products on frontalis muscle in a split‐face design. In a randomized, double‐blind trial of 20 female subjects, 5 units of AbobotulinumtoxinA and 2 units of OnabotulinumtoxinA (reconstituted in identical 2.4 mL volumes) were injected on contralateral sides of each frontalis muscle. Results showed OnabotulinumtoxinA to have a median time to onset of effect of 3.8 days and AbobotulinumtoxinA to have a median time of onset of 1.8 days. OnabotulinumtoxinA also displayed a median duration of effect of 84 days while AbobotulinumtoxinA had a median duration of 104 days. 44 , 55 , 116 This trial is a good example of an attempt to quantify molecular potency through clinical measurement, and the differences in onset and duration are likely due to having larger quantities of active 150kDa neurotoxin molecules in 50 units of AbobotulinumtoxinA compared with 20 units of OnabotulinumtoxinA. Additionally, split muscle studies such as this one are free of subject to subject differences in facial anatomy.
The frontalis model utilized by Nestor and Ablon has been demonstrated as an effective method in comparing differences in time to onset between BoNT‐A formulations. 44 While many patients report toxin effect as early as the first day of injection, prior studies often do not capture this data until at least 1 week or more postinjection. Nestor and Ablon incorporated a novel, more sensitive and objective assessment that captured the onset of effect as early as 6 hours post–BoNT‐A injection. They utilized a Frontalis Activity Measurement Standard (FMS) and 4‐point Frontalis Rating Scale (FRS) to compare the onset of effect of AbobotulinumtoxinA to OnabotulinumtoxinA injected into contralateral sides of the frontalis muscle of the same patient. Among 20 subjects, the study demonstrated that time to onset in fact is not equivalent among the different brands of BoNT‐A. Using a dose‐unit ratio of 2.5:1 with identical injection volumes, onset of effect was measurable within the first 18 hours in 90% of frontalis sides treated with AbobotulinumtoxinA but only 20% of sides treated with OnabotulinumtoxinA. At all time points, AbobotulinumtoxinA demonstrated significantly earlier onset than OnabotulinumtoxinA, as shown in Figure 2. 44 , 116
FIGURE 2.
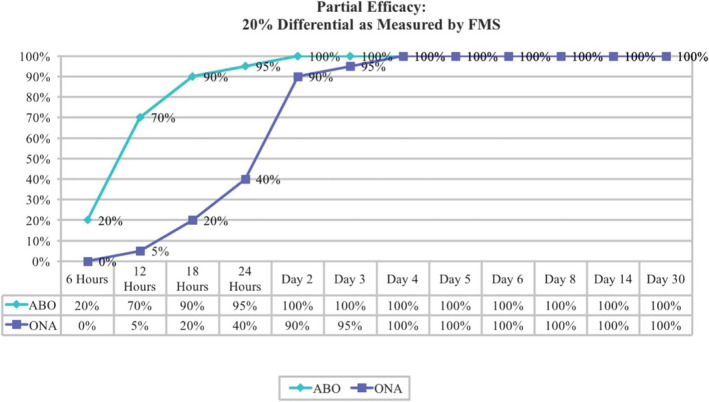
Percentage of subjects exhibiting partial efficacy from AbobotulinumtoxinA/OnabotulinumtoxinA at various time points. 44 ABO, abobotulinumtoxinA; FMS, Frontalis activity Measurement Standard; ONA, onabotulinumtoxinA
The FMS has been an effective scale for comparing different toxin products in split‐face studies. 44 , 116 It allows for direct bilateral comparison of different products, dosing, and technique on a single patient through objective quantification of changes in muscle activity because it requires investigators to measure differences in frontalis height at rest and maximum elevation. 44 , 116 The other advantage is that it allows for measurement of field of effect without having to use the Minor's test, a conventional assessment technique that compares degree of anhidrosis among products. 44 , 116 , 117 The FMS assessment includes a series of photographs using the same camera settings and lighting conditions with a rest period of 1 minute between photographs. The onset of action using this scale has been detected as early as 6 hours after injection. The FMS was utilized in a split‐face comparison of AbobotulinumtoxinA vs OnabotulinumtoxinA and allowed for precise and accurate comparison of 2 different BoNT‐A products not previously reported in the literature, as seen in Figure 3. 55 Others confirm this observation of the difference in molecular potency of AbobotulinumtoxinA compared to OnabotulinumtoxinA in regard to time of onset. One study, using dose ratios of 2.5:1 and 3:1 (AbobotulinumtoxinA:OnabotulinumtoxinA) and a generally higher dose of AbobotulinumtoxinA than OnabotulinumtoxinA, found a mean difference in glabellar lines (GL) of 0.52 days (P < .0001). 89 In this study, patients treated with AbobotulinumtoxinA reported noticeable differences in glabellar lines on Day 1 more frequently than patients treated with OnabotulinumtoxinA (28% vs 17%, respectively). The onset of effect on lateral canthal lines (LCL) was also shorter among AbobotulinumtoxinA‐treated patients compared to OnabotulinumtoxinA by a mean of 0.33 days (P = .0025). Duration of effect on GL and LCL was also shown to be superior among patients treated with AbobotulinumtoxinA rather than OnabotulinumtoxinA, with a larger proportion of patients retaining a response by 4 and 5 months. These results accounted for higher satisfaction rates among patients treated with AbobotulinumtoxinA vs OnabotulinumtoxinA.
FIGURE 3.
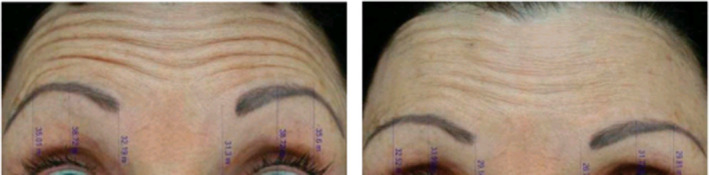
Measurement of frontalis height and wrinkle severity using the Frontalis activity Measurement Standard at baseline (left image) and 2 d following injection (right image). AbobotulinumtoxinA was injected into the patient's left frontalis (right side of images). OnabotulinumtoxinA was injected into the right frontalis (left side of image) 55
Finally, a direct molecular method of comparison is another way in which discrepancies between toxin potencies among manufacturers have been highlighted. One study compared the quantity and light chain (LC) activity of BoNT‐A in three commercial BoNT‐A products (Dysport; Botox; Xeomin). Direct measurements of the quantity of the mean active 150 kDa BoNT‐A content for the FDA‐approved glabella dosing have found that IncobotulinumtoxinA, OnabotulinumtoxinA, and AbobotulinumtoxinA have 80.6 pg/20 XU, 180.8 pg/20 BU and 301.1 pg/50 DU, respectively. These were measured with ELISA and activity measured by EndoPep assays which demonstrated equivalent light chain activity per nanogram of neurotoxin among all three products. Differences in treatment duration of action may, therefore, be due to differences in the actual quantity of neurotoxin molecules injected rather than the LD50 determined potency of the toxin. 57
5.1. Subjective scales
While objective scales have aided in determining efficacy, subjective scales such as the subjective global assessment and FACE‐Q validated, patient‐reported outcome questionnaire have been useful in assessing patient satisfaction as well. 111 , 118 These scales have been useful in identifying an improvement in patient‐reported outcomes as dosing recommendations have changed. 51 In the past, the aim of BoNT‐A administration was to achieve total muscle immobilization. This, however, compromised facial expressiveness as seen in subjective scales. Since then, ideal dosing has decreased to provide patients with a more natural and balanced look while still diminishing unwanted lines. 119 BoNT‐A treatment has also been associated with improvement in depression in depressed patients. 120 , 121 , 122 Subjective evaluations have therefore become increasingly important to achieve because patient satisfaction influences treatment choice but do not give an accurate representation of molecular potency.
5.2. Comparing duration of effect
The duration of effect is probably the most important metric of molecular potency although it comes with the caveat that increased duration is directly associated with a more frozen appearance. The frontalis model was again used in a second study which utilized both the FMS and an additional standard Frontalis Rating Scale (FRS) which quantified degree of clinical effect or level of “frozen” appearance on a scale of partial, full, to complete efficacy. Still, the FMS proved to be more sensitive in measuring effect and was able to detect changes in appearance earlier than the FRS. In correlation with the prior study, AbobotulinumtoxinA appeared to show greater molecular potency at FDA‐approved toxin ratios (50 units of AbobotulinumtoxinA and 20 units of OnabotulinumtoxinA) to OnabotulinumtoxinA in terms of maintaining duration of all degrees of efficacy among a higher proportion of frontalis sides (Table 2). 116
TABLE 2.
Median duration of partial, full, and complete efficacy (in days) after frontalis treatment with AbobotulinumtoxinA or OnabotulinumtoxinA. 116
| Measurement | Efficacy | AbobotulinumtoxinA | OnabotulinumtoxinA | Significance |
|---|---|---|---|---|
| FRS | Partial | 160 | 145 | Not significant |
| Full | 119 | 77 | P = .003 | |
| Complete | 63 | 44 | P = .01 | |
| FMS | Partial | 105 | 99 | P = .006 |
| Full | 103 | 87 | P = .003 | |
| Complete | 72 | 56 | P = .01 |
6. POSTULATE V
Up to a point, increased molecular potency decreases time to onset and increases duration of effect and the “Molecular Potency Quotient” is a construct for comparing molecular potency commercial toxin cost.
As discussed in Postulate III, efficacy is dependent on percent and degree of NMJs bound by active neurotoxin ligand. This, in turn, accounts for the degree of paralysis enacted by a toxin on muscles. Increased molecular potency can decrease the time to onset and lengthen the duration of effect. On a molecular scale, the duration is proportional to the time for nerve terminals and synaptic contacts to return to baseline upon initial binding of neurotoxin to the receptor. Onset and duration are only a function of a toxin's molecular potency to the point of total saturation of NMJs. Any number of BoNT‐A molecules past the point of saturation will then be unbound and clinically useless. Higher molecular potency may increase the number of adverse events (AEs), but this is typically dependent upon the technique of injection. Limitations of studies such as this are their small “n” values and the fact that their results may not be generalizable due to differences in volume and dose administered among practicing clinicians. 49
There is some evidence that repeated BoNT‐A injections may cause persistent structural abnormalities of innervation and slower functional recovery over time. 49 , 123 In a study of 19 females given BoNT‐A injections every 6, 9, or 12 weeks to the glabella for 2 years, all showed a significant decrease in electromyographic activity of the corrugator muscle 48 weeks post‐treatment. 123 In mouse models, recovery of neuromuscular junctions (NMJs) showed a 1.83‐2.5 times slower recovery when given 2‐3 treatments of BoNT‐A over a period of 3‐4 months versus a single injection. 49 Muscle atrophy has also been suggested as a mechanism for a long term paralytic effect of BoNT‐A on facial musculature, but the research is sparse and weak.
6.1. New toxins may have a higher molecular potency
New toxins to the market may present with a higher molecular potency. One such toxin expected to come to market in 2020 is daxibotulinumtoxinA (DAXI) (Revance). The drug has a peptide excipient used to stabilize the toxin and prevent aggregation and surface adsorption in order to increase the proportion of active toxin molecules available for binding. The high potency of this toxin was demonstrated in the Phase 3 SAKURA and Phase 2 BELMONT trials—multicenter, randomized studies in which daxibotulinumtoxinA injection showed a significantly more effective response than placebo on glabellar line severity for a median duration of response of 24 weeks. 3 DAXI was also shown to elicit a greater response rate and significantly longer duration of action on glabellar frown lines than OnabotulinumtoxinA. 61 , 124 When compared to 20U doses of DAXI, 40U and 60U of the toxin were shown to have equal efficacy. 61 DAXI has also been shown to be generally safe and well‐tolerated with the most common adverse events being a headache in 5.9%‐7.0% of subjects and injection site pain in 2.4%‐5.0% of patients. 3 , 124
6.2. Current comparisons of potency
The current general consensus is that different toxins do exhibit l differences in overall potency despite the lack of consistency in dosing and assessment measurements. Current research suggests that for the approved dosing, AbobotulinumtoxinA has a molecular potency that exceeds that of both OnabotulinumtoxinA and IncobotulinumtoxinA due to its shorter time to onset and greater duration of effect on GL in clinical trials. A 50‐unit dose of AbobotulinumtoxinA elicited an onset of effect at 2‐4 days in large populations, a median of 2.5 days, with some patients noting an effect in as little as 24 hours. 54 , 89 , 125 , 126 AbobotulinumtoxinA has shown the greatest efficacy however, in treating forehead and LCL. One study showed an onset of effect in just 24 hours with 100% of patients reporting improvement in 5 days. 89 Another study showed an onset after 2 days with full activity by Day 6. 127 Using the FMS frontalis model the median time to onset was 12‐18 hours; however, some patients exhibited an onset of effect after just 6 hours. 44 , 116
6.3. The Molecular Potency Quotient as a construct for comparing molecular potency
While prior studies have revealed some of the influences and limitations on our ability to accurately determine toxin potency, they have also demonstrated the principles derived from Postulates I and V: that all type A toxins have the same mechanism of action, and clinical effect is directly proportional to the amount of molecular potency “units,” injected. 128 This is based on the observed clinical effect and is not to be confused with the attempted quantification of molecular potency via the number of labeled units per nanogram of. 80 , 129 , 130 Putting the molecular potency into context for useful interpretation then is a simple matter of dividing the potency by the cost. We call this concept the Molecular Potency Quotient (MPQ), the formula of which is illustrated in Figure 4.
FIGURE 4.
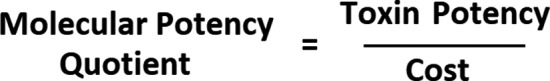
Formula for molecular potency quotient
Commercial toxin physician costs vary and are dependent on many factors including manufacturer, tier level of purchases, and even free product. In addition, each patient places his/her own value on the cost‐benefit ratio of treatment. Toxin dosing is based on the clinical anatomy as well as the needs and desires of the patient. What may be too expensive for the value‐added of a BoNT‐A treatment of one patient may not be expensive at all for the same value‐added for another patient. As such, the MPQ acts as more of a construct to help clinicians estimate a cost‐benefit ratio that may be useful in practical terms when selecting a commercially available BoNT‐A.
Additionally, this formula helps to highlight the idea that an increase in the molecular potency can be achieved by increasing the dose administered, leading to a shortened onset and enhanced duration of effect. What is important to keep in mind is enhancing molecular potency will also heighten the clinically “frozen” appearance. Enhancing potency may also potentially increase the risk of adverse events such as brow and lid ptosis.
7. POSTULATE VI
The area of effect of a toxin injection is dependent upon molecular potency, diffusion (passive) and spread (active).
A neurotoxin's efficacy can in a way be viewed in terms of its area of clinical effect on the target tissue. The area of effect of a toxin can be likened to grains of sugar dispersing into an even mound when poured onto a tabletop. With a greater number of molecules of toxin present, its molecular potency, given that all other external and confounding variables being equal, will give a greater area of effect. In the case of sugar being poured onto a tabletop, the amount and type of sugar being poured will always result in a particular area of dispersion given that the external variables are always conserved (ie, tabletop texture, humidity, table tilt.) This concept is demonstrated in Figure 5.
FIGURE 5.
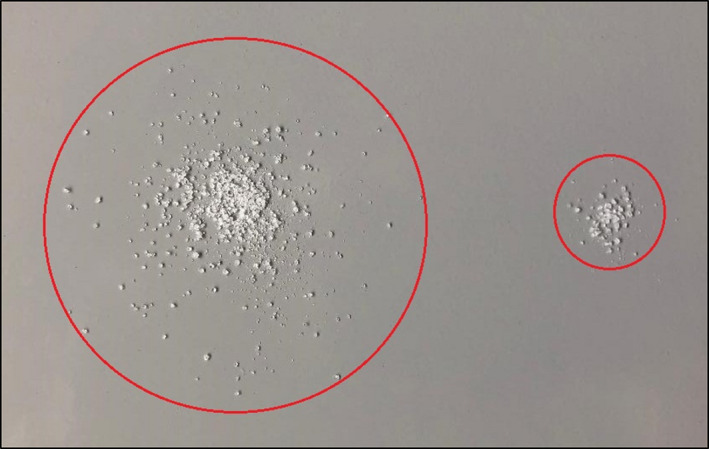
Like toxin, sugar always disperses uniformly when poured onto a flat surface given that all external variables are conserved meaning that diffusion and spread are equal. As demonstrated in the above image, a larger number of molecules of sugar akin to increased molecular potency of toxin administered (left) will yield a greater area of effect than a smaller quantity of the same “toxin” administered (right)
The area of effect is dependent upon three variables: a toxin's molecular potency, its physical spread, and its diffusion. Historically, literature has often used the terms diffusion, spread, and area of effect interchangeably, but this is misleading and inaccurate. Diffusion (D) is a passive process which is identical for all the toxins, spread (S) is an active process related to injection site, technique, and muscle activity, and molecular potency (MP) is a variable unique to each toxin and likely related to the quantity of active 150 kDa molecules present. 131 The net product of these three components is the clinical area of effect (AoE). Expressed mathematically, diffusion multiplied by spread and molecular potency equals area of effect (Figure 6).
FIGURE 6.

Formula for calculating area of effect
Diffusion is the passive kinetic dispersion of toxin beyond its original injection site and is independent of injection technique. 132 This is identical among all brands of BoNT‐A due to their shared 150 kDa core protein, and it is slow. 10 There is no evidence to suggest that diffusion is influenced by differences in the molecular weight of toxin‐NAP complexes, and all complexes dissociate entirely in the syringe or upon injection, regardless. Instead, diffusion depends on the number of toxin molecules injected and the local density of toxin receptors at NMJs. 10 , 65 , 133
Diffusion is inversely proportional to the number of receptors at the injection site and directly proportional to the dose of toxin injected. In other words, the greater the number of local receptors present in the region of injection, the greater the capacity to adsorb a larger amount of BoNT‐A molecules and to minimize their dispersion elsewhere. Once toxin is injected, it diffuses evenly out from the site. Again, this can be likened to pouring sugar out onto a tabletop. The sugar falls at the same rate and distributes itself in an evenly circumferential mound, the edges of which are equally spaced from the center, but a whole package of sugar poured onto the table will make a much larger mess than just a handful of sugar, and sugar poured onto a wet table will not tumble as far as sugar poured onto a dry table. This is corroborated by a study measuring hyperhidrosis at several locations on the back after BoNT‐A injections which found relatively smaller areas of effect (“diffusion halos” or the “Minor's test”) at the midline even when controlling for dose and depth of injection. 133 A higher quantity of toxin receptors there (a wet tabletop) would explain the decreased diffusion.
Spread is defined as the active physical distribution of toxin suspension dependent on the site of injection (muscle mass), reconstitution volume, injection volume, depth, speed of injection, and needle gauge—all elements of injection technique. Factors that may influence neurotoxin spread and diffusion are listed in Table 3. 17 A lower amount of spread is often more desirable for the purpose of accuracy and minimizing side effects in areas such as the glabella where many small muscles overlap, whereas a higher amount of spread may be desirable in areas such as the mid to upper forehead where a single, large muscle, the frontalis, controls expression. This is the reasoning behind advising patients to avoid lying flat or massaging or heating treatment areas postinjection—to prevent the excessive spread of toxin—although none of this is supported by randomized controlled trials. 134
TABLE 3.
| Factor | Does it Affect Spread? | Does it Affect Diffusion? |
|---|---|---|
| Protein Composition | No | No |
| Molecular Size | No | No |
| Neurotoxin Potency | No | No |
| Local Receptor Density | No | Yes |
| Dose | No | Yes |
| Patient Factors (age, sex, weight, etc) | Yes | No |
| Injection Technique (ie, reconstitution volume, number of injections) | Yes | No |
| Neurotoxin Concentration | Yes | No |
| Anatomic Site | Yes | Yes |
The manipulation of toxin dose and volume in both human and animal studies helps to further demonstrate the concept of toxin spread. Biopsies of rabbit muscle tissue have reported a gradient of BoNT‐A ranging from 30 to 45 mm from injection site in the latissimus dorsi, depending on dose concentration. 66 A lower concentration of toxin (higher reconstitution volume) has been shown to result in a greater amount of spread and larger field of effect. 66 This concept is discussed further in Postulate VII and may be used to a clinician's advantage, particularly when treating larger muscles, in which case the clinician may want to consider using higher dilution volumes for better efficacy. 16 The spread has also been shown to decrease with time since injection. 16
Multiple studies have measured side effect profiles in order to demonstrate differences in the area of effect (often called “diffusion” or “diffusion halo” by authors) among BoNT‐A products. For example, one study compared the effects of OnabotulinumtoxinA versus AbobotulinumtoxinA on blepharospasm among 212 patients. AbobotulinumtoxinA was associated with a significantly greater incidence of ptosis compared to OnabotulinumtoxinA, suggesting a larger area of effect. 135 Another study also showed a higher incidence of dysphagia among subjects administered AbobotulinumtoxinA rather than OnabotulinumtoxinA for cervical dystonia. 136 Assuming diffusion to be constant and spread to be equal, the variable contributing to these differences is molecular potency. This is demonstrated pictorially in Figure 7. If diffusion and spread are equal the difference of area of effect is due to molecular potency.
FIGURE 7.
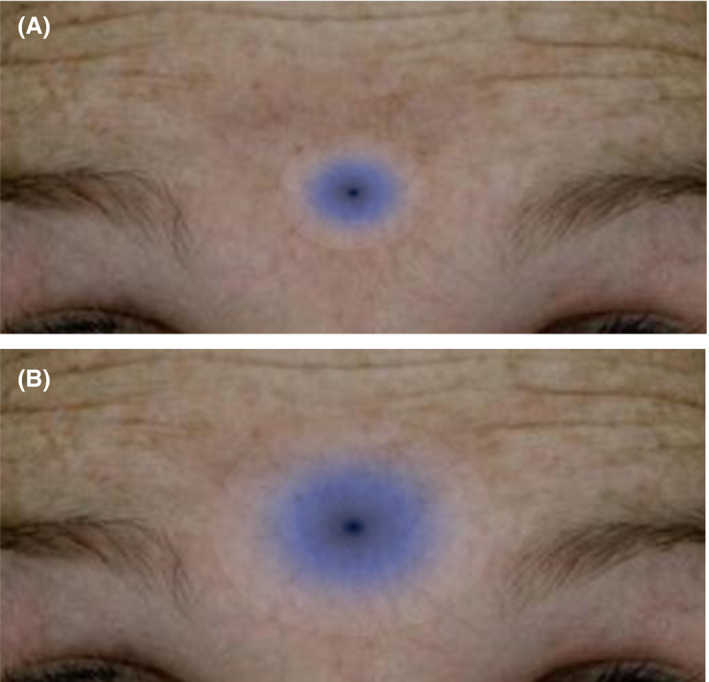
If diffusion and spread are equal, the difference in area of effect is due to molecular potency 105
8. POSTULATE VII
Optimal reconstitution volume can improve toxin distribution and thereby improve onset, efficacy, and duration of effect.
The effect of reconstitution volume on toxin spread underscores the importance of administration technique that is often underestimated. Reconstitution volumes too small do not allow for optimal spread, oversaturating a small anatomic area, while volumes too large may undersaturate a large anatomic area. A large reconstitution volume typically implies a large injection volume which naturally will affect a larger area of muscle. This is demonstrated in Figure 8. In a randomized, controlled study of 10 patients receiving BoNT‐A injections in contralateral sides of the forehead, one side received 5 U in a 0.25 mL saline solution and another received 5 U in a 0.05 mL saline solution (a fivefold difference). Results showed that injection of BoNT‐A in the lower concentration (higher volume) formulation resulted in greater spread and a larger affected area. 131
FIGURE 8.
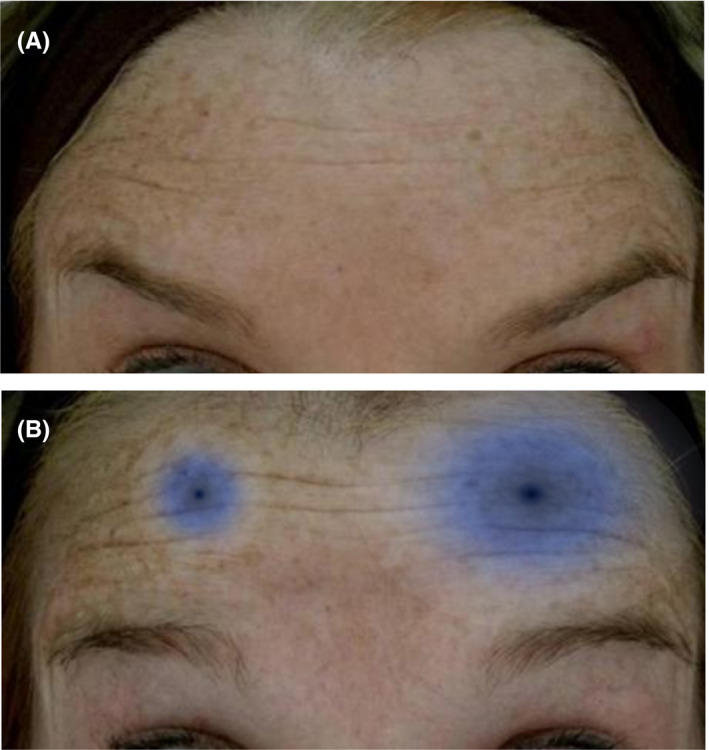
Difference in area of effect due to differing reconstitution volumes; 1.5cc (small blue circle) versus 2.5cc (large blue circle); baseline on the left, day 14 on the right 116
Clinicians each have their own “standard” method of reconstituting toxin formulations and injection techniques. Reconstitution volumes have been found to generally vary from 1 mL to 5 mL among providers. 137 Muscle size and type are also significant considerations that influence a physician's decision in the amount of volume of BoNT‐A they choose to inject to achieve the desired degree of effect. This amount varies and is subjective as current research does not provide any universally agreed‐upon guidelines to suggest optimal reconstitution volumes based on muscle type although numerous guidelines for dosing based on muscle type do exist. 17 , 87 , 88 At this point, this can only be estimated based on clinical experience or observed postinjection. In general, the optimal volume is one in which the desired effect is achieved without affecting adjacent muscles. 138
Regardless of this variability and physician preference, manufacturers of BoNT‐A preparations each provide their own instructions for reconstitution to ensure full potency of their products. AbobotulinumtoxinA and IncobotulinumtoxinA are supplied as freeze‐dried powders while OnabotulinumtoxinA is supplied as a vacuum‐dried powder, all of which must be reconstituted in normal saline (NS) prior to administration. 63 OnabotulinumtoxinA and IncobotulinumtoxinA are to be reconstituted as 100 units with 2.5 mL NS to produce 4 units per 0.1 mL and adding 3 mL NS to 300 units of AbobotulinumtoxinA will produce 10 units per 0.1 mL. 139 , 140 , 141
Although guidelines for BoNT‐A reconstitution only include unpreserved saline as a solvent, physicians often add preservatives such as benzyl alcohol for the purpose of providing mild analgesia. These preserved forms of saline have shown to improve patient comfort seemingly without compromising efficacy. 142 , 143 Although studies have not shown any differences in efficacy or onset between BoNT‐A reconstituted in NS versus preserved NS, the subjective scales that were used coupled with small numbers of patients studied make it difficult to conclusively determine how these preparations compare in regards to duration of effect.
9. POSTULATE VIII
Increased numbers of injection sites can optimize toxin distribution and thereby improve onset, efficacy, and duration of effect.
Injection technique can be adjusted in ways to influence toxin efficacy. For example, microinjections to multiple areas can optimize toxin spread and distribution rather than one larger volume injection to a single or fewer areas. 144 The goal of using this technique to achieve an ideal number of injections sites to allow for full saturation of heavy chain receptors without “wasted” toxin. The disadvantage of this technique is that the larger quantity of injections may lead to more discomfort among patients and more potential for bruising.
Lower doses over multiple injection points are desired for a more natural appearance of toxin effect among patients. Achieving this is often limited by the status quo of human freehand technique. Freehand is inaccurate in delivering precise, evenly dispersed, and equivalent amounts of neurotoxin. To achieve accuracy, physicians may often resort to using multiple syringes containing individual units or pause frequently during a procedure to take a moment to estimate the remaining volume in the syringe. Still, these techniques are burdensome, inaccurate, often tend to waste product, and add an unnecessary amount of time to patient care and the workings of their practice.
One recent study presented a viable solution to this problem through an injection‐assist device which achieved superior accuracy and precision of BoNT‐A delivery over multiple injection sites compared to freehand technique. 145 The relative percentage difference from the expected dose value was measured to be about 1% for 1‐ and 2‐unit injections and less than 1% for 4‐unit injections with the device. 145 Freehand injection accuracy was often greater than 10 times worse. 145 There was also a significant reduction in product waste. 145 This study emphasizes the benefit of achieving multiple and effectively distributed BoNT‐A injections. This concept is demonstrated in Figure 9.
FIGURE 9.
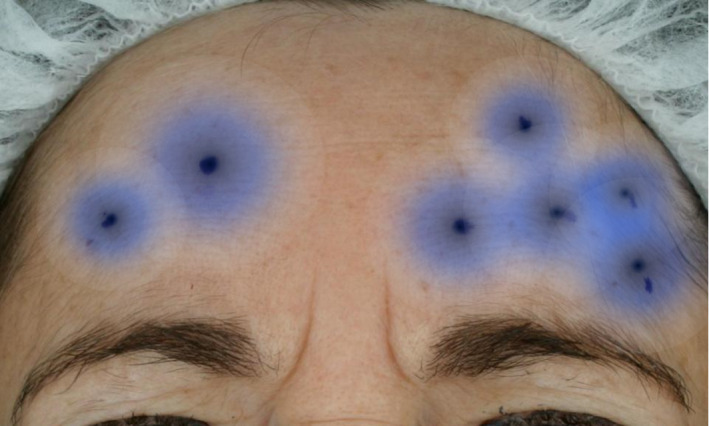
Difference in area of effect of 2 injection points versus 5 injection points 116
A greater quantity of injection sites can benefit all tissue types; however, the technique would need to be modified based on muscle type/density. Smaller, thicker muscles like the glabella may require precise, small‐volume, high‐concentration injections while thin, broad, and flat muscles like the frontalis or orbicularis oculi would benefit from more from widely spaced, high‐volume injections. In a split‐face study, the right frontalis was injected at two separate points with 12.5 units of AbobotulinumtoxinA of 0.1mL each while the left frontalis was injected at 5 separate points with 5 units of 0.04 mL each. In effect, both sides received the same total number (25 units) of AbobotulinumtoxinA however dispersed differently. The side with the higher frequency of injection points displayed a shorter onset and longer duration of effect. 116 Figure 10 demonstrates the difference in longevity of effect based on number of injection points.
FIGURE 10.
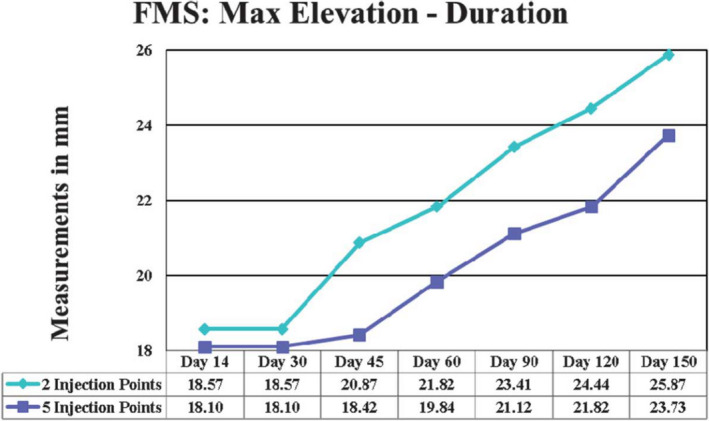
Difference in frontalis muscle elevation and longevity with 2 injection points versus 5 injection points of AbobotulinumtoxinA. Five‐point injection shows less mm of movement and thus greater efficacy at each time point vs. 2‐point injection 8
The relevance of evenly distributed, low‐dose BoNT‐A administration for maximum benefit was demonstrated by Borodic et al who identified the pattern of toxin distribution postinjection in the longissimus dorsi of rabbits. The pattern showed that BoNT‐A was distributed in a more linear fashion in injected muscle rather than in a remote muscle and that lower doses would not distribute to remote muscles. This further supports the concept that multiple, low‐dose injection sites are ideal for achieving the greatest efficacy and minimal side effects by preventing the unnecessary distribution of toxin to tissue beyond the injection site. 146
10. CONCLUSION
As clinicians, we strive to optimize patient care and offer the most efficacious treatments for patients. When selecting and administering a BoNT‐A product, it is important to understand the variables that impact a toxin's clinical effect on the patient so that we may maximize clinical efficacy and minimize adverse events. The variables we have discussed include patient factors such as muscle mass, gender, age, and ethnicity as well as toxin factors such as reconstitution volume, injection site, number of injections, injection speed, angle of injection, and importantly, molecular potency.
Though research has attempted to demonstrate differences in efficacy among the BoNT‐A products based on inherent differences in the composition and pharmacological behaviors of the toxins themselves, support for these claims remains unconvincing. Additionally, clinical studies designed to compare the toxins between patients have produced a heterogeneous pool of results as controlling for the many variables is challenging. Rather, split‐face, intra‐patient studies provide the best data for comparing the toxins’ real‐world clinical effects. Many more intra‐patient studies are needed to further delineate the three established toxins and compare the new ones on the market.
Molecular potency we define as the number of active and available 150 kDa units to be injected. It can be compared per FDA‐approved dose or per toxin vial but it is taken a step further when putting it in terms of cost. Ultimately, we want a specific clinical effect and for that clinical effect for a given muscle group the cost is the differential factor for the commercially available toxins. While skill and manipulation of the variables discussed above are critical to maximizing clinical efficacy, the value per cost of service for both the practitioner and patient may be just as important. Keeping this in mind, the MPQ may be a novel and relevant variable to consider when selecting a toxin to administer. The MPQ in turn benefits both the patient and practitioner in aesthetic outcome and cost per service.
The key clinical postulates are a valuable guide in helping aesthetic practitioners select the most appropriate toxin and injection technique specific to a patient. To review, they emphasize that all type A toxins function identically on a molecular level to inhibit Ach release by targeting SNARE complexes at nerve terminals. While all toxins act through a ligand‐receptor model, their molecular potencies are not identical. This has been a challenge to demonstrate with accuracy as most studies are typically confounded by differences in design, patient‐specific factors, and each clinician's reconstitution methods. While direct, side‐by‐side, and other types of trials have attempted to compare toxin potency, split‐face studies that utilize the FMS assessment seem to provide the most accurate means of comparison. When comparing a toxin's efficacy or potency, we look at time to onset and longevity of effect, of which the mechanisms are still being brought to light. New toxins coming to market such as DAXI seem to have higher molecular potencies due to the addition of a novel peptide. Finally, the area of effect of a toxin is determined by diffusion (a passive process), which is the same for all toxins, and spread (an active process) which we control and, ultimately, with the others being equal, molecular potency. Spread itself will influence efficacy, onset, and duration of effect and can be maximized by optimizing reconstitution volume and increasing the number of injection sites.
As administrators of BoNT‐A, it becomes our task to apply these postulates when administering current products on the market and interpreting research as new formulations emerge. In doing so, we become much more proficient in what is often our most valued service to many patients and able to more effectively provide them the most optimal aesthetic outcomes.
DISCLOSURES
Croma Pharma, GmbH: Consultant, Research Grants; Galderma: Consultant, Research Grants; Ipsen: Consultant; Allergan: Advisory Board; Merz: Research Grants.
ETHICAL STATEMENT
All work on this article was by the authors. Photographs were with permission.
Nestor MS, Arnold D, Fischer D. The mechanisms of action and use of botulinum neurotoxin type A in aesthetics: Key Clinical Postulates II. J Cosmet Dermatol. 2020;19:2785–2804. 10.1111/jocd.13702
DATA AVAILABILITY STATEMENT
Data are available upon request from the author.
REFERENCES
- 1. Cohen BE, Bashey S, Wysong A. Literature review of cosmetic procedures in men: approaches and techniques are gender specific. Am J Clin Dermatol. 2017;18(1):87‐96. [DOI] [PubMed] [Google Scholar]
- 2. drugs.com. drugs.com. Accessed January 3, 2020. drugs.com. https://www.drugs.com/international/botulinum-a-toxin.html
- 3. Carruthers JD, Fagien S, Joseph JH, et al. DaxibotulinumtoxinA in the treatment of glabellar lines: results from each of two multicenter, randomized, double‐blind, placebo‐controlled, phase 3 studies (SAKURA 1 and SAKURA 2). Plast Reconstr Surg. 2020;145(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Botulinum toxin A ‐ Allergan/Medytox ‐ AdisInsight. Available from https://adisinsight.springer.com/drugs/800021275. Accessed March 18, 2020.
- 5. Frevert J, Ahn KY, Park MY, Sunga O. Comparison of botulinum neurotoxin type A formulations in Asia. Clin Cosmet Investig Dermatol. 2018;11:327‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beware of fake toxins. Published online January 30, 2014. Available from https://www.dermatologytimes.com/dermatology-times/content/tags/aesthetic-procedures/beware-fake-toxins. Accessed March 18, 2020.
- 7. Camargo CHF, Teive HAG. Use of botulinum toxin for movement disorders. Drugs in Context. 2019;8:1‐14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nestor MS, Kleinfelder RE, Pickett A. The use of Botulinum Neurotoxin Type A in Aesthetics. Dermatol Surg. 2017;43:S344‐S362. [DOI] [PubMed] [Google Scholar]
- 9. Kane MAC, Monheit G. The practical use of AbobotulinumtoxinA in aesthetics. Aesthet Surg J. 2017;37(suppl_1):S12‐S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eisele K‐H, Fink K, Vey M, Taylor HV. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. 2011;57(4):555‐565. [DOI] [PubMed] [Google Scholar]
- 11. Samizadeh S, De Boulle K. Botulinum neurotoxin formulations: overcoming the confusion. Clin Cosmet Investig Dermatol. 2018;11:273‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dover N, Barash JR, Hill KK, Xie G, Arnon SS. Molecular characterization of a novel botulinum neurotoxin type H gene. J Infect Dis. 2014;209(2):192‐202. [DOI] [PubMed] [Google Scholar]
- 13. Pirazzini M, Rossetto O, Eleopra R, Montecucco C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69(2):200‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics. 2014;8:227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schellekens H. Follow‐on biologics: challenges of the “next generation”. Nephrol Dial Transplant. 2005;20(suppl_4):iv31‐iv36. [DOI] [PubMed] [Google Scholar]
- 16. Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics. 2014;8:227‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kerscher M, Roll S, Becker A, Wigger‐Alberti W. Comparison of the spread of three botulinum toxin type A preparations. Arch Dermatol Res. 2012;304(2):155‐161. [DOI] [PubMed] [Google Scholar]
- 18. Beer KR, Shamban AT, Avelar RL, Gross JE, Jonker A. Efficacy and safety of PrabotulinumtoxinA for the treatment of glabellar lines in adult subjects: results From 2 identical Phase III studies. Dermatol Surg. 2019;45(11):1381‐1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gu S, Rumpel S, Zhou J, et al. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science. 2012;335(6071):977‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frevert J, Dressler D. Complexing proteins in botulinum toxin type A drugs: a help or a hindrance? Biologics. 2010;4:325‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Protein Purification ‐ Principles and Practice | Robert K. Scopes | Springer. https://www.springer.com/gp/book/9780387940724. Accessed July 24, 2020.
- 22. Carli L, Montecucco C, Rossetto O. Assay of diffusion of different botulinum neurotoxin type a formulations injected in the mouse leg. Muscle Nerve. 2009;40(3):374‐380. [DOI] [PubMed] [Google Scholar]
- 23. Cai S, Sarkar HK, Singh BR. Enhancement of the endopeptidase activity of botulinum neurotoxin by its associated proteins and dithiothreitol. Biochemistry. 1999;38(21):6903‐6910. [DOI] [PubMed] [Google Scholar]
- 24. Sharma SK, Singh BR. Enhancement of the endopeptidase activity of purified botulinum neurotoxins A and E by an isolated component of the native neurotoxin associated proteins. Biochemistry. 2004;43(16):4791‐4798. [DOI] [PubMed] [Google Scholar]
- 25. Ghosal KJ, Patel K, Singh BR, Hale ML. Role of critical elements in botulinum neurotoxin complex in toxin routing across intestinal and bronchial barriers. PLoS One. 2018;13(7):e0199524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fogolari F, Tosatto SCE, Muraro L, Montecucco C. Electric dipole reorientation in the interaction of botulinum neurotoxins with neuronal membranes. FEBS Lett. 2009;583(14):2321‐2325. [DOI] [PubMed] [Google Scholar]
- 27. Yao G, Zhang S, Mahrhold S, et al. N‐linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A. Nat Struct Mol Biol. 2016;23(7):656‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rummel A. Double receptor anchorage of botulinum neurotoxins accounts for their exquisite neurospecificity. Curr Top Microbiol Immunol. 2013;364:61‐90. [DOI] [PubMed] [Google Scholar]
- 29. Mahrhold S, Bergström T, Stern D, Dorner BG, Åstot C, Rummel A. Only the complex N559‐glycan in the synaptic vesicle glycoprotein 2C mediates high affinity binding to botulinum neurotoxin serotype A1. Biochem J. 2016;473(17):2645‐2654. [DOI] [PubMed] [Google Scholar]
- 30. Colasante C, Rossetto O, Morbiato L, Pirazzini M, Molgó J, Montecucco C. Botulinum neurotoxin type A is internalized and translocated from small synaptic vesicles at the neuromuscular junction. Mol Neurobiol. 2013;48(1):120‐127. [DOI] [PubMed] [Google Scholar]
- 31. Fischer A, Mushrush DJ, Lacy DB, Montal M. Botulinum neurotoxin devoid of receptor binding domain translocates active protease. PLoS Pathog. 2008;4(12):e1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blaustein RO, Germann WJ, Finkelstein A, DasGupta BR. The N‐terminal half of the heavy chain of botulinum type A neurotoxin forms channels in planar phospholipid bilayers. FEBS Lett. 1987;226(1):115‐120. [DOI] [PubMed] [Google Scholar]
- 33. Pellett S, Bradshaw M, Tepp WH, et al. The Light Chain Defines the Duration of Action of Botulinum Toxin Serotype A Subtypes. Mbio. 2018;9(2):e00089‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tehran DA, Pirazzini M. Novel Botulinum Neurotoxins: Exploring Underneath the Iceberg Tip. Toxins. 2018;10(5):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Webb RP. Engineering of botulinum neurotoxins for biomedical applications. Toxins. 2018;10(6):231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernández‐Salas E, Steward LE, Ho H, et al. Plasma membrane localization signals in the light chain of botulinum neurotoxin. Proc Natl Acad Sci U S A. 2004;101(9):3208‐3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsai YC, Maditz R, Kuo C‐L, et al. Targeting botulinum neurotoxin persistence by the ubiquitin‐proteasome system. Proc Natl Acad Sci U S A. 2010;107(38):16554‐16559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J, Zurawski TH, Meng J, et al. A dileucine in the protease of botulinum toxin A underlies its long‐lived neuroparalysis: transfer of longevity to a novel potential therapeutic. J Biol Chem. 2011;286(8):6375‐6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toth S, Brueggmann EE, Oyler GA, Smith LA, Hines HB, Ahmed SA. Tyrosine phosphorylation of botulinum neurotoxin protease domains. Front Pharmacol. 2012;3:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vagin O, Beenhouwer DO. Septins: regulators of protein stability. Front Cell Dev Biol. 2016;4:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsai YC, Kotiya A, Kiris E, et al. Deubiquitinating enzyme VCIP135 dictates the duration of botulinum neurotoxin type A intoxication. Proc Natl Acad Sci U S A. 2017;114(26):E5158‐E5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bartels F, Bergel H, Bigalke H, Frevert J, Halpern J, Middlebrook J. Specific antibodies against the Zn(2+)‐binding domain of clostridial neurotoxins restore exocytosis in chromaffin cells treated with tetanus or botulinum A neurotoxin. J Biol Chem. 1994;269(11):8122‐8127. [PubMed] [Google Scholar]
- 43. Matak I, Bölcskei K, Bach‐Rojecky L, Helyes Z. Mechanisms of Botulinum Toxin Type A Action on Pain. Toxins. 2019;11(8):459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nestor MS, Ablon GR. Comparing the clinical attributes of abobotulinumtoxinA and onabotulinumtoxinA utilizing a novel contralateral Frontalis model and the Frontalis Activity Measurement Standard. J Drugs Dermatol. 2011;10(10):1148‐1157. [PubMed] [Google Scholar]
- 45. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full‐face injections of abobotulinum toxin type A: A randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363‐1367. [PubMed] [Google Scholar]
- 46. Hallett M. Explanation of timing of botulinum neurotoxin effects, onset and duration, and clinical ways of influencing them. Toxicon. 2015;107(Pt A):64‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lauc G, Pezer M, Rudan I, Campbell H. Mechanisms of disease: The human N‐glycome. Biochimica et Biophysica Acta. 2016;1860(8):1574‐1582. [DOI] [PubMed] [Google Scholar]
- 48. Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13(7):448‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rogozhin AA, Pang KK, Bukharaeva E, Young C, Slater CR. Recovery of mouse neuromuscular junctions from single and repeated injections of botulinum neurotoxin A. J Physiol. 2008;586(13):3163‐3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Erickson BP, Lee WW, Cohen J, Grunebaum LD. The role of neurotoxins in the periorbital and midfacial areas. Facial Plast Surg Clin North Am. 2015;23(2):243‐255. [DOI] [PubMed] [Google Scholar]
- 51. Jaspers GWC, Pijpe J, Jansma J. The use of botulinum toxin type A in cosmetic facial procedures. Int J Oral Maxillofac Surg. 2011;40(2):127‐133. [DOI] [PubMed] [Google Scholar]
- 52. Shen J, Ma J, Lee C, et al. How muscles recover from paresis and atrophy after intramuscular injection of botulinum toxin A: Study in juvenile rats. J Orthop Res. 2006;24(5):1128‐1135. [DOI] [PubMed] [Google Scholar]
- 53. Dressler D, Saberi FA. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 54. Schlessinger J, Monheit G, Kane MAC, Mendelsohn N. Time to onset of response of AbobotulinumtoxinA in the treatment of glabellar lines: a subset analysis of Phase 3 clinical trials of a new botulinum toxin Type A. Dermatol Surg. 2011;37(10):1434‐1442. [DOI] [PubMed] [Google Scholar]
- 55. Nestor MS, Ablon GR. The Frontalis Activity Measurement Standard: a novel contralateral method for assessing botulinum neurotoxin type‐A activity. J Drugs Dermatol. 2011;10(9):968‐972. [PubMed] [Google Scholar]
- 56. Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo‐controlled study of a new botulinum toxin type a for treatment of glabellar lines: efficacy and safety. Dermatol Surg. 2009;35(12):1893‐1901. [DOI] [PubMed] [Google Scholar]
- 57. Field M, Splevins A, Picaut P, et al. Correction: Field, M. et al. AbobotulinumtoxinA (Dysport®), OnabotulinumtoxinA (Botox®), and IncobotulinumtoxinA (Xeomin®) Neurotoxin Content and Potential Implications for Duration of Response in Patients. Toxins. 2019;11(2):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Azizzadeh B, Lee K. Faculty of 1000 evaluation for Split‐Face Double‐blind Study Comparing the Onset of Action of OnabotulinumtoxinA and AbobotulinumtoxinA. F1000‐ Post‐publication peer review of the biomedical literature. Published online 2012. 10.3410/f.13520958.14898060 [DOI]
- 59. Tang M, Meng J, Wang J. New engineered‐botulinum toxins inhibit the release of pain‐related mediators. Int J Mol Sci. 2019;21(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jankovic J, Truong D, Patel AT, et al. Injectable DaxibotulinumtoxinA in cervical dystonia: a phase 2 dose‐escalation multicenter study. Mov Disord Clin Pract. 2018;5(3):273‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carruthers J, Solish N, Humphrey S, et al. Injectable DaxibotulinumtoxinA for the Treatment of Glabellar Lines: A Phase 2, Randomized, Dose‐Ranging, Double‐Blind, Multicenter Comparison With OnabotulinumtoxinA and Placebo. Dermatol Surg. 2017;43(11):1321‐1331. [DOI] [PubMed] [Google Scholar]
- 62. Simpson LL. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980;212(1):16‐21. [PubMed] [Google Scholar]
- 63. Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. 2015;15(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pearce LB, Borodic GE, First ER, MacCallum RD. Measurement of botulinum toxin activity: evaluation of the lethality assay. Toxicol Appl Pharmacol. 1994;128(1):69‐77. [DOI] [PubMed] [Google Scholar]
- 65. Dover JS, Monheit G, Greener M, Pickett A. Botulinum toxin in aesthetic medicine. Dermatol Surg. 2018;44(2):249‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brodsky MA, Swope DM, Grimes D. Diffusion of botulinum toxins. Tremor Other Hyperkinet Mov. 2012;2:tre‐02‐85‐417‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lowe NJ, Shah A, Lowe PL, Patnaik R. Dosing, efficacy and safety plus the use of computerized photography for botulinum toxins type A for upper facial lines. J Cosmet Laser Ther. 2010;12(2):106‐111. [DOI] [PubMed] [Google Scholar]
- 68. Lowe PL, Patnaik R, Lowe NJ. A comparison of two botulinum type a toxin preparations for the treatment of glabellar lines: double‐blind, randomized, pilot study. Dermatol Surg. 2005;31(12):1651‐1654. [DOI] [PubMed] [Google Scholar]
- 69. Karsai S, Adrian R, Hammes S, Thimm J, Raulin C. A randomized double‐blind study of the effect of Botox and Dysport/Reloxin on forehead wrinkles and electromyographic activity. Arch Dermatol. 2007;143(11):1447‐1449. [DOI] [PubMed] [Google Scholar]
- 70. Michaels BM, Csank GA, Ryb GE, Eko FN, Rubin A. Prospective randomized comparison of onabotulinumtoxinA (Botox) and abobotulinumtoxinA (Dysport) in the treatment of forehead, glabellar, and periorbital wrinkles. Aesthet Surg J. 2012;32(1):96‐102. [DOI] [PubMed] [Google Scholar]
- 71. Rzany B‐J, Ascher B, Avelar RL, et al. A Multicenter, Randomized, Double‐Blind, Placebo‐Controlled, Single‐Dose, Phase III, Non‐Inferiority Study Comparing PrabotulinumtoxinA and OnabotulinumtoxinA for the Treatment of Moderate to Severe Glabellar Lines in Adult Patients. Aesthetic Surg J. 2019;40(4):413–429. 10.1093/asj/sjz110 [DOI] [PubMed] [Google Scholar]
- 72. Bak D‐H, Choi MJ, Lee E, et al. A comparison study of prabotulinumtoxinA vs onabotulinumtoxinA in myostatin‐deficient mice with muscle hypertrophy. Basic Clin Pharmacol Toxicol. 2019;124(4):491‐499. [DOI] [PubMed] [Google Scholar]
- 73. Karsai S, Raulin C. Current evidence on the unit equivalence of different botulinum neurotoxin A formulations and recommendations for clinical practice in dermatology. Dermatol Surg. 2009;35(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 74. Lee SH, Wee SH, Kim H‐J, et al. Abobotulinum toxin A and onabotulinum toxin A for masseteric hypertrophy: a split‐face study in 25 Korean patients. J Dermatolog Treat. 2013;24(2):133‐136. [DOI] [PubMed] [Google Scholar]
- 75. Bottet F, Peyronnet B, Boissier R, et al. Switch to Abobotulinum toxin A may be useful in the treatment of neurogenic detrusor overactivity when intradetrusor injections of Onabotulinum toxin A failed. Neurourol Urodyn. 2018;37(1):291‐297. [DOI] [PubMed] [Google Scholar]
- 76. El Kahky HM, Diab HM, Aly DG, Farag NM. Efficacy of Onabotulinum Toxin A (Botox) versus Abobotulinum Toxin A (Dysport) Using a Conversion Factor (1: 2.5) in Treatment of Primary Palmar Hyperhidrosis. Dermatol Res Pract. 2013;2013:686329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Peyronnet B, Castel‐Lacanal E, Roumiguie M, et al. Intradetrusor injections of onabotulinum toxin A (Botox®) 300 U or 200 U versus abobotulinum toxin A (Dysport®) 750 U in the management of neurogenic detrusor overactivity: A case control study. Neurourol Urodyn. 2017;36(3):734‐739. [DOI] [PubMed] [Google Scholar]
- 78. Lee JH, Park JH, Lee SK, et al. Efficacy and safety of incobotulinum toxin A in periocular rhytides and masseteric hypertrophy: side‐by‐side comparison with onabotulinum toxin A. J Dermatolog Treat. 2014;25(4):326‐330. [DOI] [PubMed] [Google Scholar]
- 79. Kranz G, Haubenberger D, Voller B, et al. Respective potencies of Botox® and Dysport® in a human skin model: A randomized, double‐blind study. Mov Disord. 2009;24(2):231‐236. [DOI] [PubMed] [Google Scholar]
- 80. Ferrari A. Pharmacological differences and clinical implications of various botulinum toxin preparations: a critical appraisal. Funct Neurol. 2018;33(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Goodnough MC, Johnson EA. Stabilization of botulinum toxin type A during lyophilization. Appl Environ Microbiol. 1992;58(10):3426‐3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wohlfarth K, Sycha T, Ranoux D, Naver H, Caird D. Dose equivalence of two commercial preparations of botulinum neurotoxin type A: time for a reassessment? Curr Med Res Opin. 2009;25(7):1573‐1584. [DOI] [PubMed] [Google Scholar]
- 83. Botulinum Toxin Type A for Injection European Pharmacopoeia Monograph. Available from https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/eur/eph_botat-508.pdf. Accessed January 2, 2020.
- 84. Abbasi NR, Durfee MA, Petrell K, Dover JS, Arndt KA. A small study of the relationship between abobotulinum toxin A concentration and forehead wrinkle reduction. Arch Dermatol. 2012;148(1):119‐121. [DOI] [PubMed] [Google Scholar]
- 85. Choi Y‐J, Won S‐Y, Lee J‐G, et al. Characterizing the Lateral Border of the Frontalis for Safe and Effective Injection of Botulinum Toxin. Aesthet Surg J. 2016;36(3):344‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lorenc ZP, Smith S, Nestor M, Nelson D, Moradi A. Understanding the functional anatomy of the frontalis and glabellar complex for optimal Aesthetic Botulinum Toxin Type A therapy. Aesthetic Plast Surg. 2013;37(5):975‐983. [DOI] [PubMed] [Google Scholar]
- 87. Ascher B, Talarico S, Cassuto D, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)–Part I: Upper facial wrinkles. J Eur Acad Dermatol Venereol. 2010;24(11):1278‐1284. [DOI] [PubMed] [Google Scholar]
- 88. Ascher B, Talarico S, Cassuto D, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)–Part II: Wrinkles on the middle and lower face, neck and chest. J Eur Acad Dermatol Venereol. 2010;24(11):1285‐1295. [DOI] [PubMed] [Google Scholar]
- 89. Kassir R, Kolluru A, Kassir M. Triple‐blind, prospective, internally controlled comparative study between AbobotulinumtoxinA and OnabotulinumtoxinA for the treatment of facial rhytids. Dermatol Ther. 2013;3(2):179‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Plastic Surgery Statistics Report. plasticsurgery.org. https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf. Accessed February 27, 2020.
- 91. Keaney TC, Alster TS. Botulinum toxin in men: review of relevant anatomy and clinical trial data. Dermatol Surg. 2013;39(10):1434‐1443. [DOI] [PubMed] [Google Scholar]
- 92. Schlessinger J, Dover JS, Joseph J, et al. Long‐term safety of abobotulinumtoxinA for the treatment of glabellar lines: results from a 36‐month, multicenter, open‐label extension study. Dermatol Surg. 2014;40(2):176‐183. [DOI] [PubMed] [Google Scholar]
- 93. Rappl T, Parvizi W, et al. Onset and duration of effect of incobotulinumtoxinA, onabotulinumtoxinA, and abobotulinumtoxinA in the treatment of glabellar frown lines: a randomized, double‐blind study. Clin Cosmetic Investig Dermatol. 2013;6:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Flynn TC. Botox in men. Dermatol Ther. 2007;20(6):407‐413. [DOI] [PubMed] [Google Scholar]
- 95. Flynn TC. Botulinum toxin: examining duration of effect in facial aesthetic applications. Am J Clin Dermatol. 2010;11(3):183‐199. [DOI] [PubMed] [Google Scholar]
- 96. Courtney J, Steinbach JH. Age changes in neuromuscular junction morphology and acetylcholine receptor distribution on rat skeletal muscle fibres. J Physiol. 1981;320:435‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gonzalez‐Freire M, de Cabo R, Studenski SA, Ferrucci L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front Aging Neurosci. 2014;6:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cheng CM. Cosmetic use of botulinum toxin type A in the elderly. Clin Interv Aging. 2007;2(1):81‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yamauchi PS. Selection and preference for botulinum toxins in the management of photoaging and facial lines: patient and physician considerations. Patient Prefer Adherence. 2010;4:345‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Taylor SC, Callender VD, Albright CD, Coleman J, Axford‐Gatley RA, Lin X. AbobotulinumtoxinA for reduction of glabellar lines in patients with skin of color:post hoc analysis of pooled clinical trial data. Dermatol Surg. 2012;38(11):1804‐1811. [DOI] [PubMed] [Google Scholar]
- 101. Wesley NO, Maibach HI. Racial (ethnic) differences in skin properties: the objective data. Am J Clin Dermatol. 2003;4(12):843‐860. [DOI] [PubMed] [Google Scholar]
- 102. Saggar V, Banker N, Wesley NO, Maibach HI. Ethnic differences in skin properties. The objective data In Barel AO, Paye M, Maibach HI. (Eds.). Handbook of cosmetic science and technology, 4th edn Boca Raton: CRC Press, Taylor & Francis Group; 2014;19‐57. [Google Scholar]
- 103. Chauhan DS, Cariappa KM, Guruprasad Y. Botulinum toxin type a for the treatment of hyperkinetic lines of the face. J Maxillofac Oral Surg. 2013;12(2):173‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Peng H‐LP, Hypertrophy M. Toxin treatment techniques, causes of complications, and prevention. Botulinum Toxin. 2018;1:73–81. [Google Scholar]
- 105. Shome D, Khare S, Kapoor R. Efficacy of botulinum toxin in treating asian indian patients with masseter hypertrophy. Plast Reconstr Surg. 2019;144(3):390e‐396e. [DOI] [PubMed] [Google Scholar]
- 106. Ahn J, Horn C, Blitzer A. Botulinum toxin for masseter reduction in Asian patients. Arch Facial Plast Surg. 2004;6(3):188‐191. [DOI] [PubMed] [Google Scholar]
- 107. Ahn BK, Kim YS, Kim HJ, Rho NK, Kim HS. Consensus recommendations on the aesthetic usage of botulinum toxin type A in Asians. Dermatol Surg. 2013;39(12):1843‐1860. [DOI] [PubMed] [Google Scholar]
- 108. Rzany B, Flynn TC, Schlöbe A, Heinz M, Harrington L. Long‐term results for incobotulinumtoxinA in the treatment of glabellar frown lines. Dermatol Surg. 2013;39(1 Pt 1):95‐103. [DOI] [PubMed] [Google Scholar]
- 109. De Boulle K, Werschler WP, Gold MH, et al. Phase 3 Study of OnabotulinumtoxinA Distributed Between Frontalis, Glabellar Complex, and Lateral Canthal Areas for Treatment of Upper Facial Lines. Dermatol Surg. 2018;44(11):1437‐1448. [DOI] [PubMed] [Google Scholar]
- 110. Food and Drug Administration . Draft Guidance for Industry on Upper Facial Lines: Developing Botulinum Toxin Drug Products. Federal Register. 2014;79:45812‐45813. [Google Scholar]
- 111. Bonaparte JP, Ellis D, Quinn JG, Ansari MT, Rabski J, Kilty SJ. A comparative assessment of three formulations of botulinum toxin A for facial rhytides: a systematic review and meta‐analyses. Syst Rev. 2013;2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Honeck P, Weiss C, Sterry W, Rzany B. Reproducibility of a four‐point clinical severity score for glabellar frown lines. Br J Dermatol. 2003;149(2):306‐310. [DOI] [PubMed] [Google Scholar]
- 113. Flynn TC, Carruthers A, Carruthers J, et al. Validated assessment scales for the upper face. Dermatol Surg. 2012;38(2ptII):309‐319. [DOI] [PubMed] [Google Scholar]
- 114. Carruthers A, Carruthers J. A validated facial grading scale: The future of facial ageing measurement tools? J Cosmetic Laser Therap. 2010;12(5):235‐241. [DOI] [PubMed] [Google Scholar]
- 115. Carruthers J, Flynn TC, Geister TL, et al. Validated assessment scales for the mid face. Dermatol Surg. 2012;38(2ptII):320‐332. [DOI] [PubMed] [Google Scholar]
- 116. Nestor MS, Ablon GR. Duration of action of abobotulinumtoxinA and onabotulinumtoxinA: a randomized, double‐blind study using a contralateral frontalis model. J Clin Aesthet Dermatol. 2011;4(9):43‐49. [PMC free article] [PubMed] [Google Scholar]
- 117. Hexsel D, Dalʼforno T, Hexsel C, Prado D. A randomized pilot study comparing the action halos of two commercial preparations of botulinum Toxin Type A. Dermatol Surg. 2008;34(1):52‐59. [DOI] [PubMed] [Google Scholar]
- 118. Chang BL, Wilson AJ, Taglienti AJ, Chang CS, Folsom N, Percec I. Patient Perceived Benefit in Facial Aesthetic Procedures: FACE‐Q as a Tool to Study Botulinum Toxin Injection Outcomes. Aesthetic Surg J. 2016;36(7):810‐820. [DOI] [PubMed] [Google Scholar]
- 119. Glogau R, Biesman B, Kane M. Assessment of Botulinum Toxin Aesthetic Outcomes. JAMA Dermatol. 2015;151(11):1177. [DOI] [PubMed] [Google Scholar]
- 120. Kruger THC, Axel WM. Depression – An emerging indication for botulinum toxin treatment. Toxicon. 2015;107:154‐157. [DOI] [PubMed] [Google Scholar]
- 121. Magid M, Finzi E, Kruger T, et al. Treating depression with Botulinum Toxin: A pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(06):205‐210. [DOI] [PubMed] [Google Scholar]
- 122. Finzi E, Rosenthal NE. Emotional proprioception: Treatment of depression with afferent facial feedback. J Psychiatr Res. 2016;80:93‐96. [DOI] [PubMed] [Google Scholar]
- 123. Boonipat T, Cohen JA, Stotland M. Abstract: Effect of Botulinum Toxin Injection Intervals on the Irreversibility of Targeted Muscle Paralysis. Plastic Reconst Surgery. 2016;4:107‐108. [Google Scholar]
- 124. Bertucci V, Humphrey S, Carruthers J, et al. Comparing injectable DaxibotulinumtoxinA and OnabotulinumtoxinA in moderate and severe glabellar lines. Dermatol Surg. 2017;43:S262‐S273. [DOI] [PubMed] [Google Scholar]
- 125. Monheit GD, Cohen JL. Reloxin Investigational Group. Long‐term safety of repeated administrations of a new formulation of botulinum toxin type A in the treatment of glabellar lines: interim analysis from an open‐label extension study. J Am Acad Dermatol. 2009;61(3):421‐425. [DOI] [PubMed] [Google Scholar]
- 126. Kane MAC, Brandt F, Rohrich RJ, Narins RS, Monheit GD, Barbara HM. Evaluation of Variable‐Dose Treatment with a New U.S. Botulinum Toxin Type A (Dysport) for Correction of Moderate to Severe Glabellar Lines: Results from a Phase III, Randomized, Double‐Blind, Placebo‐Controlled Study. Plast Reconstr Surg. 2009;124(5):1619‐1629. [DOI] [PubMed] [Google Scholar]
- 127. Yu KCY, Nettar KD, Bapna S, Boscardin WJ, Maas CS. Split‐face double‐blind study comparing the onset of action of onabotulinumtoxinA and abobotulinumtoxinA. Arch Facial Plast Surg. 2012;14(3):198‐204. [DOI] [PubMed] [Google Scholar]
- 128. Pearce LB, Bruce Pearce L, Borodic GE, Johnson EA, First ER, Maccallum R. The median paralysis unit: A more pharmacologically relevant unit of biologic activity for botulinum toxin. Toxicon. 1995;33(2):217‐227. [DOI] [PubMed] [Google Scholar]
- 129. Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, Maas C, IncobotulinumtoxinA FJ. A Highly Purified and Precisely Manufactured Botulinum Neurotoxin Type A. J Drugs Dermatol. 2019;18(1):52‐57. [PubMed] [Google Scholar]
- 130. Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D. 2010;10(2):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hsu TSJ, Dover JS, Arndt KA. Effect of volume and concentration on the diffusion of botulinum exotoxin A. Arch Dermatol. 2004;140(11):1351‐1354. [DOI] [PubMed] [Google Scholar]
- 132. Pickett A. Dysport: pharmacological properties and factors that influence toxin action. Toxicon. 2009;54(5):683‐689. [DOI] [PubMed] [Google Scholar]
- 133. Hexsel DM, Soirefmann M, Rodrigues TC, do Prado DZ. Increasing the field effects of similar doses of Clostridium botulinum type A toxin‐hemagglutinin complex in the treatment of compensatory hyperhidrosis. Arch Dermatol. 2009;145(7):837‐840. [DOI] [PubMed] [Google Scholar]
- 134. Maas C, Kane MAC, Bucay VW, et al. Current aesthetic use of abobotulinumtoxinA in clinical practice: an evidence‐based consensus review. Aesthet Surg J. 2012;32(1 Suppl):8S‐29S. [DOI] [PubMed] [Google Scholar]
- 135. Nüssgens Z, Roggenkämper P. Comparison of two botulinum‐toxin preparations in the treatment of essential blepharospasm. Graefes Arch Clin Exp Ophthalmol. 1997;235(4):197‐199. [DOI] [PubMed] [Google Scholar]
- 136. Ranoux D, Gury C, Fondarai J, Mas JL, Zuber M. Respective potencies of Botox and Dysport: a double blind, randomised, crossover study in cervical dystonia. J Neurol Neurosurg Psychiatry. 2002;72(4):459‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Kaplan JB. The dilution confusion. Plastic Surg Nurs. 2016;36(1):24‐27. [DOI] [PubMed] [Google Scholar]
- 138. Wohlfarth K, Schwandt I, Wegner F, et al. Biological activity of two botulinum toxin type A complexes (Dysport and Botox) in volunteers: a double‐blind, randomized, dose‐ranging study. J Neurol. 2008;255(12):1932‐1939. [DOI] [PubMed] [Google Scholar]
- 139. Dysport prescribing information ; 2017. Available from http://www.galdermausa.com/IFU/Dysport_IFU.pdf?_ga=2.91803420.760544459.1501421902-1680652553.1501421902. Accessed December 27, 2019.
- 140. Botox Cosmetic prescribing information; 2017. Available from https://www.allergan.com/assets/pdf/botox_cosmetic_pi.pdf. Accessed January 3, 2020.
- 141. Xeomin prescribing information; 2017. Available from http://www.xeominaesthetic.com/wp-content/uploads/2016/12/EM00451-02-Electronic-PI.pdf. Accessed January 3, 2020.
- 142. Allen SB, Goldenberg NA. Pain Difference Associated with Injection of AbobotulinumtoxinA Reconstituted with Preserved Saline and Preservative‐Free Saline: A Prospective, Randomized, Side‐by‐Side, Double‐Blind Study. Dermatol Surg. 2012;38(6):867‐870. [DOI] [PubMed] [Google Scholar]
- 143. Kwiat DM, Bersani TA, Bersani A. Increased patient comfort utilizing botulinum toxin type a reconstituted with preserved versus nonpreserved saline. Ophthal Plast Reconstr Surg. 2004;20(3):186‐189. [DOI] [PubMed] [Google Scholar]
- 144. Ramirez‐Castaneda J, Jankovic J, Comella C, Dashtipour K, Fernandez HH, Mari Z. Diffusion, spread, and migration of botulinum toxin. Mov Disord. 2013;28(13):1775‐1783. [DOI] [PubMed] [Google Scholar]
- 145. Kwolek MS, Block JE. Maximizing botulinum toxin injections for cosmetic and therapeutic applications with a single use, disposable, exact dose injection assist device. Clin Cosmet Investig Dermatol. 2019;12:35‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Borodic GE, Ferrante R, Pearce LB, Smith K. Histologic assessment of dose‐related diffusion and muscle fiber response after therapeutic botulinum A toxin injections. Mov Disord. 1994;9(1):31‐39. [DOI] [PubMed] [Google Scholar]
- 147. Thomas J, Walker SHD. Comparison and Overview of Currently Available Neurotoxins. J Clin Aesthet Dermatol. 2014;7(2):31. [PMC free article] [PubMed] [Google Scholar]
- 148. Lebeda FJ, Adler M, Erickson K, Chushak Y. Onset dynamics of type A botulinum neurotoxin‐induced paralysis. J Pharmacokinet Pharmacodyn. 2008;35(3):251‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the author.


