Abstract
Dysregulation of the autonomic nervous system (ANS), which can be indexed by heart rate variability (HRV), has been posited to contribute to core features of autism spectrum disorder (ASD). However, the relationship between ASD and HRV remains uncertain. We assessed tonic and phasic HRV of 21 children with ASD and 21 age- and IQ-matched typically developing (TD) children and examined (1) group differences in HRV and (2) associations between HRV and ASD symptomatology. Children with ASD showed significantly lower tonic HRV, but similar phasic HRV compared to TD children. Additionally, reduced tonic HRV was associated with atypical attentional responsivity in ASD. Our findings suggest ANS dysregulation is present in ASD and may contribute to atypical attentional responses to sensory stimulation.
Keywords: autism spectrum disorder, heart rate variability, ECG, autonomic arousal
Introduction
Autism spectrum disorder (ASD) is characterized by social communication impairments, as well as the presence of atypical restricted and repetitive behaviors (RRB; American Psychiatric Association, 2013). It has been proposed that these core features of ASD can be explained, in part, by dysregulation of the autonomic nervous system (ANS), which may result in atypical reactivity to external stimuli (Porges, 2005). Behavioral manifestations of this dysregulation can include weaknesses in emotion recognition and regulation of social-emotional responses (Bal et al., 2010; Chang et al., 2012; Porges, 2005) and difficulties in tolerating environmental changes resulting in stereotyped and ritualistic behavior (Bachevalier & Loveland, 2006). Although ANS responsivity to environmental stressors has been explored widely, the relation between physiological mechanisms and ASD symptoms has not been sufficiently investigated (Benevides & Lane, 2015; Bujnakova et al., 2016). Therefore, identifying differences in physiological responses in individuals with and without ASD may offer insight into the mechanisms that may contribute to the development of the core phenotypic features of ASD.
The ANS is a division of the peripheral nervous system, which regulates the functioning of internal organs and works to maintain homeostasis, and includes the parasympathetic and sympathetic nervous systems. Generally, parasympathetic activation of the ANS regulates the body’s rest-and-digest responses, while sympathetic activity controls fight-or-flight responses (McCorry, 2007). However, activation of the parasympathetic nervous system (PNS) and sympathetic nervous system (SNS) are not only reciprocal and may be co-active or independent based on the environment (Cacioppo et al., 1994; Norris, Gollan, Berntson, & Cacioppo, 2010). One measure of ANS activity is heart rate variability (HRV; Wang et al., 2016), which is the change in the length of interval between heartbeats. During situations that do not exceed thresholds of physiological arousal, the “vagal brake” is engaged and the parasympathetic nervous system (PNS) inhibits cardiac activity by maintaining a decreased heart rate. During situations that evoke stress, the “vagal brake” is released and the sympathetic nervous system (SNS) produces physiological arousal, such as increased heart rate (Porges, 2005, 2007). As such, whether an individual can transition between low and high arousal states effectively in response to environmental stressors depends on the capacity of the ANS to efficiently vary heart rate (Appelhans & Luecken, 2006). This shift from a resting to an active state, also termed HRV reactivity, is a measure of the regulatory capacity of the ANS (Beauchaine, 2001; Porges, 2007). Hence, typical regulation of the ANS implies efficient control of the “vagal brake,” which activates PNS, slows SNS activity, and promotes social engagement with others (Porges, 2003).
Findings from studies comparing HRV in ASD and TD groups have been mixed, with some studies demonstrating that ASD is associated with lower HRV in resting state and in response to tasks (e.g., Guy, Souders, Bradstreet, Delussey, & Herringto, 2014; Neuhaus, Bernier, & Beauchaine, 2014; Van Hecke et al., 2009), while others showing a lack of difference in HRV between children with and without ASD (e.g., Bazelmans et al., 2019; Sheinkopf, Neal-Beevers, Levine, Miller-Loncar, & Lester, 2013). Thus, there appears to be no general consensus on whether there are differences in HRV between individuals with and without ASD.
Social and communication impairments in ASD have been linked to atypical ANS functioning (Porges, 1995, 2005). For example, Van Hecke and colleagues (2009) examined the neurophysiological activity of children and adolescents with ASD during rest and in response to videos of familiar and unfamiliar people and found robust differences between ASD and TD groups. Additionally, resting respiratory sinus arrhythmia (RSA) was positively correlated with better social skills in individuals with ASD. Furthermore, whereas the TD group did not respond differently to familiar and unfamiliar social stimuli, the ASD group showed decreased regulation of heart rate in response to unfamiliar people, suggesting that novel social stimuli may induce a greater degree of arousal in individuals with ASD and reflect poorer autonomic regulation. In addition, Bazelmans et al. (2019) found a positive correlation between HRV and language skills in preschool-aged children with ASD. Results from prior studies also support the idea that reduced HRV, which reflects dysregulated ANS, can serve as an indicator of social communication difficulties (e.g., Alvares et al., 2013; Kuiper, Verhoeven, & Geurts, 2017).
In addition to social impairments, neurophysiological dysregulation has also been suggested to contribute to the development of RRB, which have been classified into sensory-motor behavior and ritualistic behavior subgroups (Bishop et al., 2013). Atypical functioning of the ANS may result in over-activation or under-activation of the SNS and PNS, causing an imbalanced state of physiological arousal. Prior research has shown that children with ASD tend to be in an over-aroused state due to their hyper-responsive sympathetic system (Anderson & Colombo, 2009; Kushki, Brian, Dupuis, & Anagnostou, 2014; Kushki et al., 2013; Ming, Julu, Brimacombe, Connor, & Daniels, 2005), while others suggest that individuals with ASD may have a generally dysregulated ANS, such that they tend be either over-aroused or under-aroused depending on the environmental stimuli (Leekam, Prior, & Uljarevic, 2011; Lidstone et al., 2014). With regard to sensory-motor RRB, some research suggests that ASD symptoms are associated with atypical responsivity to sensory stimuli (e.g., Chang et al., 2012; Goodwin et al., 2008; Liss, Saulnier, Fein, & Kinsbourne, 2006), including hyposensitivity (e.g., under-reactivity to pain), hypersensitivity (e.g., over-reactivity to everyday sounds) and sensory-seeking behaviors (e.g., craving or fascination with certain stimuli; Boyd et al., 2010). With respect to ritualistic RRB, it has been suggested that they may serve the function of reducing sensory input from a state of over-arousal (Lidstone et al., 2014). In ASD, the atypical control of the “vagal brake” may lead to higher SNS activity, resulting in chronic hyperarousal (Cheshire, 2012; Ming, Patel, Kang, Chokroverty, & Julu, 2016), which in turn evokes ritualistic or rigid behaviors that serve the function of coping with the environmental demands (Thayer & Brosschot, 2005). In view of the differential severity and profile across domains of ASD characteristics, exploring the association between HRV and specific ASD symptoms holds promise in facilitating our understanding of the heterogeneity of the ASD phenotype.
Various experimental paradigms have been used by prior studies to assess differences in HRV between resting and task states. These include social tasks such as attending to pictures of faces, public speaking, and social interaction (e.g., Billeci et al., 2018; Hollocks, Howlin, Papadopoulos, Khondoker, & Simonoff, 2014; Smeekens, Didden, & Verhoeven, 2015), as well as non-social tasks such as viewing pictures of toys, drawing, and visual processing of numbers (e.g., Daluwatte et al., 2013; Hollocks et al., 2014; Kushki et al., 2014). As HRV is proposed to broadly indicate regulatory capacity of the ANS rather than specific processes (Holzman & Bridgett, 2017), the task included in this current study involved a non-social auditory attention paradigm (see Keehn, Kadlaskar, McNally Keehn, & Francis, in press).
The purpose of the current study is to (1) identify potential differences in tonic (resting) HRV and task-based phasic (reactivity and recovery) HRV in children and adolescents with ASD compared to TD children and adolescents, and (2) examine the association between HRV and social communication difficulties and restricted and repetitive behaviors, including atypical sensory responsivity. We hypothesize that (1) children with ASD will exhibit reduced resting, reactivity, and recovery HRV compared to their TD peers, and (2) lower HRV will be associated with the presence of greater ASD symptomatology.
Methods
Participants
A total of forty-two 9- to 15-year-old children and adolescents participated, including 21 individuals (4 females) with ASD and 21 TD individuals (5 females). Groups were matched on age. No group mean difference was found in IQ between ASD and TD, as determined by the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II; Wechsler, 2011; Table 1). Clinical diagnoses were confirmed using the Autism Diagnostic Observation Schedule, Second Edition, (ADOS-2; Lord et al., 2012), the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003), and expert clinical judgment based on DSM-5 criteria (APA, 2013). Children with ASD-related medical conditions (e.g., Fragile-X syndrome, tuberous sclerosis) were excluded. Participants in the TD group had no caregiver-reported clinically significant ASD symptoms or other neurological or psychiatric disorders and did not have a family history of ASD. All study procedures were conducted in accordance with protocols approved by the university’s Institutional Review Board. All parents or legal guardians and children provided informed consent and assent prior to participation.
Table 1.
Participant Characteristics
| ASD (n = 21, 4 females) |
TD (n = 21, 5 females) |
t-value | p | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | |||
| Age (years) | 11.6 | 1.3 | 9.3-14.5 | 11.2 | 1.7 | 9.0-15.0 | 0.9 | 0.39 |
| WASI-II Verbal IQ | 103 | 18 | 75-154 | 110 | 10 | 95-127 | −1.4 | 0.18 |
| WASI-II Nonverbal IQ | 103 | 17 | 59-132 | 108 | 12 | 87-134 | −1.2 | 0.24 |
| SRS-2 total score (t-score) | 76 | 11 | 57-90 | 43 | 5 | 37-55 | 12.2 | <0.001 |
| ADOS-2 | ||||||||
| Social Affect | 11 | 4 | 5-17 | |||||
| Repetitive Behavior | 2 | 1 | 0-5 | |||||
| Comparison Score | 7 | 2 | 4-10 | |||||
Note. ASD = autism spectrum disorder. TD = typically developing. WASI-II = Wechsler Abbreviated Scale of Intelligence, Second Edition. SRS-2 = Social Responsiveness Scale, Second Edition. ADOS-2 = Autism Diagnostic Observation Schedule, Second Edition.
Apparatus and Stimuli
The experiment was presented using Presentation® software (version 11.8) and conducted in a sound attenuated, darkened room. Participants were seated comfortably in a chair at approximately 1.5 m distance from five speakers (Hafler M5 Reference) and approximately 2 m from a 50-in LCD screen positioned above the center speaker. Speakers are mounted on eye-level stands and positioned in a semi-circular array at 0° (i.e., directly in front of participants), 15°, and 30° to the left and right of participants respectively. Auditory fixation at the central location was a 500Hz pure tone, whereas peripheral targets emitted from side speakers were white noise (similar to Shafiq et al., 1998). All auditory stimuli were maintained at comfortable listening level of approximately 60 dBA at the participant’s seating location.
Electrocardiogram (ECG) was recorded with a Biopac MP150 system with an ECG100C module using a modified lead II configuration with the negative electrode affixed to the right forearm, the positive to the left calf, and ground on the left forearm. Electrodes were self-adhesive 8mm Ag/AgCl disoposables (Biopac EL500) filled with conductive gel (5% NaCl, 0.85 molar NaCl). Prior to attaching the electrodes, the attachment areas on the skin were gently cleaned with a commercially available alcohol-soaked cotton pad. Hardware gain was set to 5000 (corresponding to an input gain of ±2 mV), and band-pass filtered between 1 and 35 Hz with a sampling rate of 500 Hz. Respiration was recorded using a Biopac RSP100C module coupled to a strain gauge transducer (Biopac TSD201) worn around the lower edge of the rib cage.
Procedure
Participants first completed a 5-min resting block during which time a white central crosshair was displayed on a black background. Participants were instructed to relax, remain still, and look at the crosshair. Next, participants completed the auditory gap-overlap paradigm. Prior to the start of the gap-overlap task, a black curtain was drawn in front of the speaker array approximately 1.2m from the participant, thus visually occluding speakers. Thus, rather than fixate on a specific object (e.g., central crosshair/LED in a visual gap-overlap task), participants fixated on a specific location (i.e., the source of the sound). Each trial consisted of a tone playing from the center speaker for a random duration between 1300 and 1500 ms and white noise playing from one of the side speakers either (a) with the central tone continuing to play (i.e., overlap condition), (b) 200 ms after the central tone stopped (i.e., gap condition), or (c) with the simultaneous offset of the central tone (i.e., baseline condition). Participants were instructed to look at the center location when the tone was playing, then to move only their eyes to location of the sound once the noise played (see Keehn et al., 2019, for more details).
Experimental Design
The 5-min resting block preceded three 2-min task blocks. A total of 108 trials were equally divided over three task blocks. Within each task block, the order of condition (i.e., gap, overlap, baseline) and location of noise were pseudorandomly arranged, resulting in all trial types being presented an equal number of times across blocks. This task was designed as part of a larger study investigating attentional processes in children with ASD. Given prior research indicating challenges associated with atypical attentional disengagement in ASD (e.g., Keehn et al., 2019; Sacrey, Armstrong, Bryson, & Zwaigenbaum, 2014), this task was presumed to be cognitively demanding and physiologically arousing.
Measures
Social Responsiveness Scale, Second Edition (SRS-2; Constantino, 2012).
The SRS-2 is a 65-item caregiver-report questionnaire used to measure ASD symptomatology in children aged 4 to 18 years, across the domains of Social Awareness, Social Cognition, Social Communication, Social Motivation, and Restricted Interests and Repetitive Behavior subscales. Social subscales (awareness, cognition, communication, and motivation) are combined to form the Social Communication and Interaction (SCI) scale. The SCI and Restricted and Repetitive Behavior DSM-5 Compatible scales and the Total score t-scores were used for the correlational analyses.
Child Sensory Profile, Second Edition (SP-2; Dunn, 2014).
The SP-2 is an 86-item caregiver-report questionnaire, which measures responses to various forms of sensory input (i.e., auditory, visual, touch, movement, body position, oral). Subscale raw scores used for the correlational analyses include the sensory processing quadrants (i.e., Sensitivity/Sensor, Seeking/Seeker, Avoiding/Avoider, Registration/Bystander) and behavioral responding section (i.e., Conduct, Social Emotional, and Attentional).
Data Analysis
Heart rate variability was preprocessed and analyzed using HRVanalysis software (Pichot, Roche, Celle, Barthélémy, & Chouchou, 2016). The software detects R peaks, which were manually checked and corrected by study personnel blind to group membership. The square root of the mean of the sum of the squared differences between adjacent normal RR intervals (rMSSD) was used as a temporal measure of high-frequency HRV.
Quintana and Heathers (2014) have pointed out the need for caution with respect to the widespread use of HRV as a measure of autonomic regulation and made a number of suggestions for dealing with specific issues, including respiratory monitoring. We used rMSSD as our primary measure of HRV even though it does not incorporate respiration as it is one of the most consistently used measures associated with vagal tone (Shaffer & Ginsberg, 2017), requires relatively little time to calculate accurately (as little as 1 min, cf. Laborde, Mosley, & Thayer, 2017; or even less, cf. discussion by Shaffer & Ginsberg, 2017), and is relatively robust to variation in respiration (Hill, Siebenbrock, Sollers III, & Thayer, 2009; Penttila et al., 2001). Even still, consistent with the recommendations of Laborde et al. (2017) we re-ran all analyses using a measure of RSA computed according to the peak-to-valley or P2V method (Grossman, Van Beek, & Wientjes, 1990), a technique that explicitly incorporates differences in respiration (Laborde et al., 2017). Prior to RSA calculation, all respiratory and ECG signals were examined visually by study personnel blind to group membership and respiratory and ECG cycles exhibiting movement or other artifacts were excluded from analysis. All reported statistical analyses were conducted using rMSDD, but results were unchanged when using RSA unless otherwise noted.
Statistical analyses were conducted using SPSS software (version 25). All variables were logarithm-transformed to meet normality assumptions prior to data analyses. A mixed-model repeated-measures ANOVA (RMANOVA) was used, with diagnostic classification (ASD, TD) as a between-subjects factor and blocks (resting, task block 1, 2, and 3) as a within-subjects factor. Phasic HRV was also assessed through reactivity (i.e., difference between resting block and the first task block) and recovery (i.e., difference between the first and third task blocks) using log-transformed data, based on recommendations by Laborde, Mosley, & Thayer, (2017). Independent-sample t-tests were conducted to compare reactivity and recovery measures. Lastly, for the ASD group, Pearson’s correlations were used to examine the relationship between HRV and measures of ASD symptomatology.
Results
Heart Rate Variability
There was a significant main effect of group, F(1,40) = 4.56, p = .039, ηp2 = .10, as children with ASD (M = 1.58, SD = 0.30) had significantly lower HRV compared to their TD peers (M = 1.76, SD = 0.24) (Figure 1a). There was also a significant main effect of block, F(3,120) = 4.17, p = .008, ηp2 = .09. Pairwise comparisons revealed significantly higher HRV for resting compared to task block 1, t(41) = 2.75, p = .009, and block 2, t(41) = 3.01, p = .004, but not task block 3, t(41) = 1.42, p = .16. No other blocks differed significantly from each other (p > .05). The interaction between group and block was not significant, F(3,120) = 0.11, p = .96, ηp2 = .00. Lastly, no significant group differences were found for reactivity, t(40) = 0.04, p = .97, and recovery measures, t(40) = 0.13, p = .89. Additionally, 10 participants in the ASD group were currently taking psychotropic medication (Sertraline, Ritalin, Busporin, Concerta, Lexapro, Intuniv, Strattera, Adderall, Trileptic, Vyvanse, Keppra, and Zoloft). However, there was no significant difference in overall HRV between ASD participants on and off me medication, F(1,19) = 0.1, p = .758, ηp2 = .00.
Figure 1a.
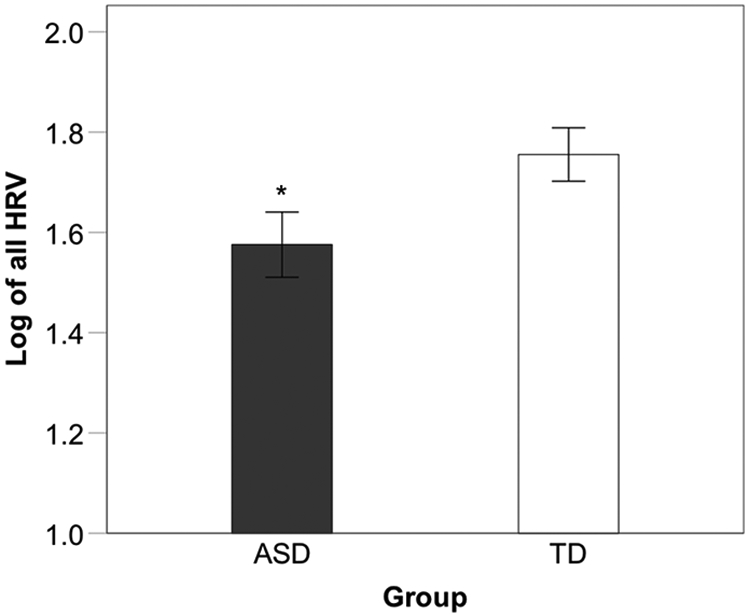
Comparison of mean HRV across all blocks between ASD and TD groups; * p < .05
Correlations with ASD Symptomatology
As there was no significant interaction between group and block, and to reduce the number of correlations, HRV was averaged across block. For the ASD group, there was a significant negative correlation between the SP-2 Attentional subscore and overall HRV, r(20) = −.51, p = .018 (Figure 1b). Additionally, a marginally significant correlation was found between the Seeking/Seeker subscale and HRV, r(20) = −.41, p = .069., which was significant when using RSA, r(20) = −.44, p = .048. No other significant correlations were found between SP-2, SRS-2, or ADOS-2 scores and HRV (all p > .05).
Figure 1b.
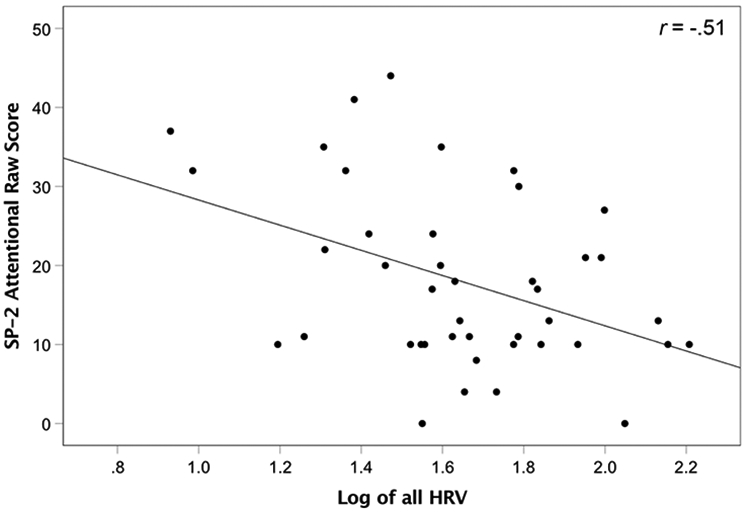
Scatterplot of correlation of SP-2 Attentional subscore and overall HRV.
Discussion
The current study sought to examine (1) the differences in tonic and phasic HRV measures between children with ASD and TD children, and (2) potential associations between HRV and ASD symptomatology. We found a significant difference in overall HRV between ASD and TD groups, with the ASD group demonstrating lower HRV, but did not find group differences in phasic HRV measures. Furthermore, there were no significant associations between measures of social communication impairments and HRV. However, a relationship was found between some domains of sensory reactivity and HRV.
Findings partially confirmed our first hypothesis that children and adolescents with ASD would demonstrate lower HRV compared to their TD peers, which is in accord with a number of existing studies (e.g., Guy, Souders, Bradstreet, Delussey, & Herringto, 2014; Neuhaus, Bernier, & Beauchaine, 2014; Van Hecke et al., 2009). Previous research has shown that higher resting HRV is an indicator that the ANS has more efficient control of physiological arousal in response to the environment, resulting in better social emotional modulation and thus more contextually-relevant and appropriate behavioral responses to environmental stimuli in TD individuals (Thayer & Brosschot, 2005; Wang et al., 2016). Conversely, lower overall HRV indicates autonomic imbalance, such as over-activation of the SNS and reduced activation of the PNS, potentially contributing to impaired regulation of social, affective, attentional, and behavioral responses to external stimuli (Thayer & Brosschot, 2005). Our finding of reduced overall HRV in ASD suggests that ANS dysregulation is present in children with ASD.
In contrast, group differences were not found in phasic HRV measures, as indicated by similar reactivity and recovery HRV across ASD and TD groups. This finding does not support our hypothesis and is inconsistent with recent research that demonstrated reduced reactivity in ASD compared to TD controls (e.g., Condy, Scarpa, & Friedman, 2017; Smeekens, Didden, & Verhoeven, 2015). It is possible that other variables may moderate reactivity to and recovery from a particular challenge, such as task type (i.e., social vs. nonsocial) and/or difficulty (Benevides & Lane, 2015). Furthermore, it should also be noted that a final resting block was not administered. Hence, our measure of recovery HRV may be more representative of task habituation rather than return to baseline.
Correlational analyses did not support our second hypothesis that lower HRV would be associated with greater ASD symptomatology, as we did not find significant correlations between HRV and social communication or RRB scores. These results diverge from prior research demonstrating associations between ASD symptoms and atypical ANS regulation (e.g., Condy et al., 2017; Matsushima et al., 2016; Van Hecke et al., 2009). However, there was a significant association between atypical attentional responses to sensory stimulation and lower HRV. This finding suggests that atypical attentional responsivity in ASD may be related to dysregulation of the ANS, as indicated by decreased HRV. The SP-2 Attentional subscale includes a combination of items from all sensory processing subscales (i.e., sensitivity, seeking, avoiding, and registration), therefore the association between HRV and the Attentional score suggests that dysregulated autonomic activity may contribute to heterogeneous profiles of sensory reactivity present in ASD. We did not find significant associations between lower HRV and individual sensory subscales, with the exception of a marginally significant association between lower HRV and increased Seeking/Seeker subscale score, which suggests that ANS dysregulation in children and adolescents with ASD is potentially associated with seeking certain sensory stimuli of interest. In agreement with the polyvagal perspective (Porges, 2007), ANS dysregulation, as evidenced by reduced HRV, may result in less control of the “vagal brake” leading to greater physiological arousal. Sensory-seeking behaviors may serve to produce more consistent and predictable sensory input and reduce the impact of existing unpredictable environmental stressors.
The results of this study contribute to a growing body of literature that attempts to explain the potential mechanisms underlying the ASD phenotype through identifying neurophysiological markers. The present study provides preliminary evidence that children with ASD have reduced HRV, which is indicative of ANS dysregulation. Additionally, the association between lower HRV and attentional responses to sensory stimulation suggests that atypical attentional modulation may be related to dysregulation of the ANS in ASD. Understanding how atypical autonomic activity may contribute to core characteristics of ASD will guide future research in identifying physiological markers of ASD and facilitate the development of effective treatments for individuals with ASD.
Limitations
This study includes a number of limitations that may influence the interpretation of our findings. First, the study sample was relatively small and thus may not be sufficiently representative of children and adolescents with or without ASD. Second, while the lack of group mean difference in IQ enhanced experimental control and allowed for a more convenient comparison of the dependent variables between ASD and TD, our ASD sample may not adequately reflect the heterogeneity in ASD with respect to cognitive functioning. Third, we did not control for medication use or comorbidity in our analyses. Future research should consider including comorbid diagnoses and the use of medication as factors of analyses.
Footnotes
Conflict of Interest
All authors report no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed assent and consent were obtained from all individual participants included in the study.
References
- Alvares GA, Quintana DS, Kemp AH, Van Zwieten A, Balleine BW, Hickie IB, & Guastella AJ (2013). Reduced heart rate variability in social anxiety disorder: Associations with gender and symptom severity. PLoS ONE, 8(7), 1–8. doi: 10.1371/journal.pone.0070468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). [Google Scholar]
- Anderson CJ, & Colombo J (2009). Larger tonic pupil size in young children with autism spectrum disorder. Developmental Psychobiology, 51(2), 207–211. doi: 10.1002/dev.20352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans BM, & Luecken LJ (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. doi: 10.1037/1089-2680.10.3.229 [DOI] [Google Scholar]
- Bachevalier J, & Loveland KA (2006). The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neuroscience and Biobehavioral Reviews, 30(1), 97–117. doi: 10.1016/j.neubiorev.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, & Porges SW (2010). Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders, 40(3), 358–370. doi: 10.1007/s10803-009-0884-3 [DOI] [PubMed] [Google Scholar]
- Bazelmans T, Jones EJH, Ghods S, Corrigan S, Toth K, Charman T, & Webb SJ (2019). Heart rate mean and variability as a biomarker for phenotypic variation in preschoolers with autism spectrum disorder. Autism Research, 12(1), 39–52. doi: 10.1002/aur.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T (2001). Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology, 13, 183–214. [DOI] [PubMed] [Google Scholar]
- Benevides TW, & Lane SJ (2015). A review of cardiac autonomic measures: Considerations for examination of physiological response in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(2), 560–575. doi: 10.1007/s10803-013-1971-z [DOI] [PubMed] [Google Scholar]
- Billeci L, Tonacci A, Narzisi A, Manigrasso Z, Varanini M, Fulceri F, … Muratori F (2018). Heart rate variability during a joint attention task in toddlers with autism spectrum disorders. Frontiers in Physiology, 9(MAY), 1–11. doi: 10.3389/fphys.2018.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Hus V, Duncan A, Huerta M, Gotham K, Pickles A, … Lord C (2013). Subcategories of restricted and repetitive behaviors in children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 43(6), 1287–1297. doi: 10.1007/s10803-012-1671-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E, & Miller H (2010). Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Research, 3(2), 78–87. doi: 10.1002/aur.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujnakova I, Ondrejka I, Mestanik M, Visnovcova Z, Mestanikova A, Hrtanek I, … Tonhajzerova I (2016). Autism spectrum disorder is associated with autonomic underarousal. Physiological Research, 65(Supplementum 5), S673–S682. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, & Fieldstone A (1994). Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology, 31, 586–598. [DOI] [PubMed] [Google Scholar]
- Chang MC, Parham LD, Blanche EI, Schell A, Chou CP, Dawson M, & Clark F (2012). Autonomic and behavioral responses of children with autism to auditory stimuli. The American Journal of Occupational Therapy, 66(5), 567. [DOI] [PubMed] [Google Scholar]
- Cheshire WP (2012). Highlights in clinical autonomic neuroscience: New insights into autonomic dysfunction in autism. Autonomic Neuroscience: Basic and Clinical, 171(1–2), 4–7. doi: 10.1016/j.autneu.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Condy EE, Scarpa A, & Friedman BH (2017). Respiratory sinus arrhythmia predicts restricted repetitive behavior severity. Journal of Autism and Developmental Disorders, 47(9), 2795–2804. doi: 10.1007/s10803-017-3193-2 [DOI] [PubMed] [Google Scholar]
- Constantino JN (2012). Social Responsiveness Scale (SRS) (2nd ed.). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Daluwatte C, Miles JH, Christ SE, Beversdorf DQ, Takahashi TN, & Yao G (2013). Atypical pupillary light reflex and heart rate variability in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 43(8), 1910–1925. doi: 10.1007/s10803-012-1741-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W (2014). Child Sensory Profile (2nd ed.). San Antonio, TX: Pearson. [Google Scholar]
- Goodwin MS, Groden J, Velicer WF, Lipsitt LP, Baron MG, Hofmann SG, & Groden G (2008). Cardiovascular arousal in individuals with autism. Focus on Autism and Other Developmental Disabilities, 21(2), 100–123. doi: 10.1177/10883576060210020101 [DOI] [Google Scholar]
- Grossman P, Van Beek J, & Wientjes C (1990). A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology, 27(6), 702–714. [DOI] [PubMed] [Google Scholar]
- Guy L, Souders M, Bradstreet L, Delussey C, & Herringto JD (2014). Brief report: Emotion regulation and respiratory sinus arrhythmia in autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(10), 2614–2620. doi: 10.1007/s10803-014-2124-8 [DOI] [PubMed] [Google Scholar]
- Hill LK, Siebenbrock A, Sollers III JJ, & Thayer JF (2009). All are measures created equal ? Heart rate variability and respiration. Biomedical Sciences Instrumentation, 45(June), 71–76. [PubMed] [Google Scholar]
- Hollocks MJ, Howlin P, Papadopoulos AS, Khondoker M, & Simonoff E (2014). Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology, 46, 32–45. doi: 10.1016/j.psyneuen.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Holzman JB, & Bridgett DJ (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neuroscience and Biobehavioral Reviews, 74, 233–255. doi: 10.1016/j.neubiorev.2016.12.032 [DOI] [PubMed] [Google Scholar]
- Keehn B, Kadlaskar G, McNally Keehn R, & Francis AL (2019). Auditory attentional disengagement in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-019-04111-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper MWM, Verhoeven EWM, & Geurts HM (2017). Heart rate variability predicts inhibitory control in adults with autism spectrum disorders. Biological Psychology, 128(July), 141–152. doi: 10.1016/j.biopsycho.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Kushki A, Brian J, Dupuis A, & Anagnostou E (2014). Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism, 5(39), 1–10. doi: 10.1186/2040-2392-5-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki Azadeh, Drumm E, Pla Mobarak M, Tanel N, Dupuis A, Chau T, & Anagnostou E (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE, 8(4), 2–9. doi: 10.1371/journal.pone.0059730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde S, Mosley E, & Thayer JF (2017). Heart rate variability and cardiac vagal tone in psychophysiological research - Recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8, 1–18. doi: 10.3389/fpsyg.2017.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Prior MR, & Uljarevic M (2011). Restricted and repetitive behaviors in autism spectrum disorders: A review of research in the last decade. Psychological Bulletin, 137(4), 562–593. doi: 10.1037/a0023341 [DOI] [PubMed] [Google Scholar]
- Lidstone J, Uljarević M, Sullivan J, Rodgers J, McConachie H, Freeston M, … Leekam S (2014). Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorders. Research in Autism Spectrum Disorders, 8(2), 82–92. doi: 10.1016/j.rasd.2013.10.001 [DOI] [Google Scholar]
- Liss M, Saulnier C, Fein D, & Kinsbourne M (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism, 10(2), 155–172. doi: 10.1177/1362361306062021 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule (2nd ed.). Torrance, CA: Western Psychological Services. [Google Scholar]
- Matsushima K, Matsubayashi J, Toichi M, Funabiki Y, Kato T, Awaya T, & Kato T (2016). Unusual sensory features are related to resting-state cardiac vagus nerve activity in autism spectrum disorders. Research in Autism Spectrum Disorders, 25, 37–46. doi: 10.1016/j.rasd.2015.12.006 [DOI] [Google Scholar]
- McCorry LK (2007). Physiology of the autonomic nervous system. American Journal of Pharmaceutical Education, 71(4), 1–11. doi: 10.1111/j.1399-6576.1964.tb00252.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Julu POO, Brimacombe M, Connor S, & Daniels ML (2005). Reduced cardiac parasympathetic activity in children with autism. Brain and Development, 27(7), 509–516. doi: 10.1016/j.braindev.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Ming X, Patel R, Kang V, Chokroverty S, & Julu PO (2016). Respiratory and autonomic dysfunction in children with autism spectrum disorders. Brain and Development, 38(2), 225–232. doi: 10.1016/j.braindev.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Neuhaus E, Bernier R, & Beauchaine TP (2014). Brief report: Social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. Journal of Autism and Developmental Disorders, 44(3), 730–737. doi: 10.1007/s10803-013-1923-7 [DOI] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson GG, & Cacioppo JT (2010). The current status of research on the structure of evaluative space. Biological Psychology, 84(3), 422–436. doi: 10.1016/j.biopsycho.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttila J, Helminen A, Jartti T, Kuusela T, Huikuri HV, Tulppo MP, … Scheinin H (2001). Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: Effects of various respiratory patterns. Clinical Physiology, 21(3), 365–376. doi: 10.1046/j.1365-2281.2001.00337.x [DOI] [PubMed] [Google Scholar]
- Pichot V, Roche F, Celle S, Barthélémy JC, & Chouchou F (2016). HRVanalysis: A free software for analyzing cardiac autonomic activity. Frontiers in Physiology, 7, 1–15. doi: 10.3389/fphys.2016.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology, 32, 301–318. [DOI] [PubMed] [Google Scholar]
- Porges SW (2003). The Polyvagal Theory: Phylogenetic contributions to social behavior. Physiology and Behavior, 79(3), 503–513. doi: 10.1016/S0031-9384(03)00156-2 [DOI] [PubMed] [Google Scholar]
- Porges SW (2005). The vagus: A mediator of behavioral and physiologic features associated with autism (Bauman ML & Kemper TL, Eds.), The Neurobiology of Autism. Baltimore: Johns Hopkins University Press. [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. doi: 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana DS, & Heathers JAJ (2014). Considerations in the assessment of heart rate variability in biobehavioral research. Frontiers in Psychology, 5, 1–10. doi: 10.3389/fpsyg.2014.00805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). Social Communication Questionnaire. Los Angeles, CA. [Google Scholar]
- Sacrey LAR, Armstrong VL, Bryson SE, & Zwaigenbaum L (2014). Impairments to visual disengagement in autism spectrum disorder: a review of experimental studies from infancy to adulthood. Neuroscience & Biobehavioral Reviews, 47, 559–577. [DOI] [PubMed] [Google Scholar]
- Shaffer F, & Ginsberg JP (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5(September), 1–17. doi: 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinkopf SJ, Neal-Beevers AR, Levine TP, Miller-Loncar C, & Lester B (2013). Parasympathetic response profiles related to social functioning in young children with autistic disorder. Autism Research and Treatment, 2013, 1–7. doi: 10.1155/2013/868396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens I, Didden R, & Verhoeven EWM (2015). Exploring the relationship of autonomic and endocrine activity with social functioning in adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(2), 495–505. doi: 10.1007/s10803-013-1947-z [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Brosschot JF (2005). Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology, 30(10), 1050–1058. doi: 10.1016/j.psyneuen.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Van Hecke AV, Lebow J, Bal E, Lamb D, Harden E, Kramer A, … Porges SW (2009). Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development, 80(4), 1118–1133. doi: 10.1111/j.1467-8624.2009.01320.x [DOI] [PubMed] [Google Scholar]
- Wang Y, Hensley MK, Tasman A, Sears L, Casanova MF, & Sokhadze EM (2016). Heart rate variability and skin conductance during repetitive TMS course in children wih autism. Applied Psychophysiology and Biofeedback, 41(1), 47–60. doi: 10.1007/s10484-015-9311-z [DOI] [PubMed] [Google Scholar]


