Significance Statement
This study indicates that CIPK23 is a phototropin‐interacting protein kinase that promotes blue light‐ and phototropin‐dependent stomatal opening in Arabidopsis thaliana. We propose that CIPK23 does not mediate plasma membrane H+‐ATPase activation but mediates inward‐rectifying K+ channel activation in stomatal opening.
SUMMARY
Phototropins (phot1 and phot2) are plant blue light receptor kinases that function to mediate phototropism, chloroplast movement, leaf flattening, and stomatal opening in Arabidopsis. Considerable progress has been made in understanding the mechanisms associated with phototropin receptor activation by light. However, the identities of phototropin signaling components are less well understood by comparison. In this study, we specifically searched for protein kinases that interact with phototropins by using an in vitro screening method (AlphaScreen) to profile interactions against an Arabidopsis protein kinase library. We found that CBL‐interacting protein kinase 23 (CIPK23) interacts with both phot1 and phot2. Although these interactions were verified by in vitro pull‐down and in vivo bimolecular fluorescence complementation assays, CIPK23 was not phosphorylated by phot1, as least in vitro. Mutants lacking CIPK23 were found to exhibit impaired stomatal opening in response to blue light but no deficits in other phototropin‐mediated responses. We further found that blue light activation of inward‐rectifying K+ (K+ in) channels was impaired in the guard cells of cipk23 mutants, whereas activation of the plasma membrane H+‐ATPase was not. The blue light activation of K+ in channels was also impaired in the mutant of BLUS1, which is one of the phototropin substrates in guard cells. We therefore conclude that CIPK23 promotes stomatal opening through activation of K+ in channels most likely in concert with BLUS1, but through a mechanism other than activation of the H+‐ATPase. The role of CIPK23 as a newly identified component of phototropin signaling in stomatal guard cells is discussed.
INTRODUCTION
Phototropins (phot1 and phot2) are plasma membrane‐associated, autophosphorylating blue light receptor kinases that induce a range of physiological responses in Arabidopsis thaliana, which help optimize photosynthetic efficiency under weak light conditions by increasing light capture and CO2 absorption in leaves (Christie et al., 1998; Takemiya et al., 2005; Inoue et al., 2010; Gotoh et al., 2018). These processes include phototropism, chloroplast photorelocation movement, leaf flattening, leaf positioning, and stomatal opening (Christie, 2007; Inoue et al., 2008a; Demarsy and Fankhauser, 2009). Despite extensive efforts by many researchers, the primary signaling events associated with each of these responses remain largely unknown (Christie et al., 2015).
Many phototropin interaction partners have been identified (Inoue et al., 2010), four of which, ATP‐BINDING CASSETTE B19 (ABCB19), PHYTOCHROME KINASE SUBSTRATE 4 (PKS4), BLUE LIGHT SIGNALING 1 (BLUS1), and CONVERGENCE OF BLUE LIGHT AND CO2 (CBC) 1, have been shown to be direct substrate targets (Christie et al., 2011; Demarsy et al., 2012; Takemiya et al., 2013; Hiyama et al., 2017). NON‐PHOTOTROPIC HYPOCOTYL 3 (NPH3), ROOT PHOTOTROPISM 2 (RPT2), and PKS1, 2, and 4 positively regulate phototropism, leaf flattening, and leaf positioning but do not regulate chloroplast movement and stomatal opening (Motchoulski and Liscum, 1999; Sakai et al., 2000; Inada et al., 2004; Lariguet et al., 2006; Inoue et al., 2008a; de Carbonnel et al., 2010; Harada et al., 2013; Tsutsumi et al., 2013). While PKS4 is phosphorylated by phot1 in a blue light‐dependent manner, phosphorylation negatively regulates its action on phototropism (Demarsy et al., 2012; Schumacher et al., 2018). In addition, the auxin efflux transporter ABCB19 has been shown to be a substrate for phot1 kinase activity (Christie et al., 2011). Phosphorylation of ABCB19 is proposed to inhibit its efflux activity and indirectly promote auxin fluxes involved in phototropism (Christie et al., 2011). The guard cell‐specific protein kinase BLUS1 is phosphorylated by both phot1 and phot2 in response to blue light, and phosphorylated BLUS1 mediates stomatal opening (Takemiya et al., 2013). CBC1 is phosphorylated by phot1 and phot2 in guard cells, and CBC1 and the closest homolog CBC2 regulate blue light‐induced stomatal opening in both positive and negative manners (Hiyama et al., 2017; Hayashi et al., 2020). The roles of CBC1 phosphorylation are not understood at the present time. Together, these findings indicate that the primary signaling events associated with different phototropin‐mediated responses are likely to be distinct, with signal propagation diverging from the phosphorylation of specific substrates.
Blue light‐driven stomatal opening is one of the most characterized phototropin signaling pathways to date (Shimazaki et al., 2007; Inoue et al., 2010). Stomatal opening, which facilitates gaseous exchange between plants and the atmosphere, is driven by the swelling of stomatal guard cells in response to blue light (Zeiger and Hepler, 1977; Shimazaki et al., 2007). This swelling is achieved by increased turgor pressure in guard cells, which is induced by hyperpolarization of the plasma membrane and subsequent K+ uptake via inward‐rectifying K+ (K+ in) channels at the membrane (Schroeder et al., 1987; Schroeder and Hedrich, 1989; Schroeder et al., 2001; Marten et al., 2010). Membrane hyperpolarization is generated by activation of the guard cell plasma membrane H+‐ATPase (Assmann et al., 1985; Shimazaki et al., 1986; Blatt, 1987; Yamauchi et al., 2016). The H+‐ATPase is activated in a blue light‐ and phototropin‐dependent manner by phosphorylation of its C‐terminus and subsequent binding of 14‐3‐3 proteins (Kinoshita and Shimazaki, 1999; Palmgren, 2001; Ueno et al., 2005; Hayashi et al., 2011). Phototropin autophosphorylation leads to H+‐ATPase activation via BLUS1 kinase activity, as well as the involvement of BLUE LIGHT‐DEPENDENT H+‐ATPASE PHOSPHORYLATION (BHP) and type 1 protein phosphatases (Kinoshita et al., 2001; Takemiya et al., 2006; Inoue et al., 2008b; Takemiya et al., 2013; Hayashi et al., 2017). However, knowledge of the signaling events coupling blue light sensing by the phototropins to activation of the H+‐ATPase is incomplete. For instance, the identity of the kinase responsible for phosphorylation of the H+‐ATPase is still lacking.
While it is well accepted that activation of the H+‐ATPase is the main driver of stomatal opening, there is additional evidence to support the involvement of two other modes of signaling and regulation. The first involves phototropin‐mediated suppression of plasma membrane anion channels that mediate stomatal closure, whereby their inhibition promotes stomatal opening through an increase in plasma membrane hyperpolarization (Marten et al., 2007). The other pathway involves phototropin modulation of K+ in channel activity, which is thought to facilitate H+‐ATPase‐driven stomatal opening through the uptake of K+ across the plasma membrane (Zhao et al., 2012). It is therefore possible that phototropin substrates besides BLUS1 could be involved in either of these signaling pathways. Here we adopted the Amplified Luminescent Proximity Homogeneous Assay Screen (AlphaScreen) methodology (PerkinElmer Life Sciences) to determine whether phototropins could interact with protein kinases other than BLUS1. In doing so, we identified CBL‐interacting protein kinase 23 (CIPK23) as a positive regulator in blue light‐dependent stomatal opening in Arabidopsis. Furthermore, we propose that CIPK23 constitutes a distinct signaling pathway involved in phototropin‐driven stomatal opening and contributes to this response through the activation of K+ in channels.
RESULTS
Identification of CIPK23 as a phototropin‐interacting protein
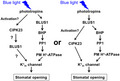
To identify novel kinase‐interacting partners of Arabidopsis phototropins, we performed an in vitro protein–protein interaction screen using AlphaScreen technology in combination with a wheat germ cell‐free protein synthesis system as reported previously (Hayashi et al., 2020). RIKEN Arabidopsis Full‐Length (RAFL) cDNA clones were used to construct an Arabidopsis protein kinase library (562 protein kinases) which were expressed individually by an in vitro transcription and translation system (Sawasaki et al., 2002; Sawasaki et al., 2004; Nemoto et al., 2011). In this system, a luminescent signal is generated when donor and acceptor beads are brought into close proximity. The donor beads generate singlet oxygen by excitation and the acceptor beads emit light by reacting with singlet oxygen. When two beads are in close proximity, the acceptor beads are able to receive singlet oxygen from the donor beads. The proximity of the beads depends on the interaction between proteins that have been conjugated onto the beads (Figure 1a). Phot1 or phot2 and each protein kinase from the library were conjugated on acceptor and donor beads as bait and prey, respectively. We then screened this library for kinases that interact with phot1 and phot2 using the AlphaScreen approach and identified CIPK23 as a candidate. A negative control protein, dihydrofolate reductase (DHFR), showed a slight interaction with phot1 and phot2 in this system that was expressed as a luminescence signal in the AlphaScreen (Figure 1b). By contrast, CIPK23 showed a high level of interaction with both phot1 and phot2. The extent of CIPK23 binding to phot1 was similar to that of NPH3 to phot1, which served as a positive control in our analysis (Motchoulski and Liscum, 1999; de Carbonnel et al., 2010).
Figure 1.
Identification of CIPK23 as an in vitro phototropin‐interacting protein.
(a) Screening of phototropins‐interacting protein kinases using the AlphaScreen luminescence system. A protein kinase from the protein kinase library and phototropins were bound onto donor and acceptor beads, respectively. The two beads can be in close proximity only when these proteins interact. Upon excitation at 680 nm, a singlet oxygen is generated from the donor beads and transferred to the acceptor beads within 200 nm, and the resultant reaction emits light at 520–620 nm.
(b) In vitro interaction of CIPK23 with phot1 and phot2 in the AlphaScreen method. All proteins used were expressed using a wheat germ cell‐free protein synthesis system. Interactions of phototropins with CIPK23 were detected by the AlphaScreen method as a luminescence signal. DHFR and NPH3 were used as negative and positive controls, respectively.
(c) In vitro pull‐down assay of phototropins and CIPK23. Recombinant FLAG‐CIPK1, FLAG‐CIPK24, and FLAG‐CIPK23 were purified from E. coli extracts using anti‐FLAG antibody‐conjugated agarose beads, and then reacted with microsomal fractions from Arabidopsis seedlings. Co‐purified proteins were detected by immunoblotting using antibodies to phot1, phot2, and FLAG.
The interaction between phototropins and CIPK23 was confirmed by in vitro pull‐down assay. FLAG‐tagged CIPK1, CIPK23, or CIPK24 was expressed and purified from Escherichia coli cells using anti‐FLAG antibody‐conjugated agarose beads. Purified FLAG‐tagged CIPK proteins were then incubated with microsomal membranes from Arabidopsis seedlings. Immunoprecipitation analysis showed that FLAG‐CIPK23 co‐purified with both phot1 and phot2 from microsomal membrane fractions, but CIPK1 and CIPK24 did not (Figure 1c). These results indicate that CIPK23 interacts with both phot1 and phot2 in vitro.
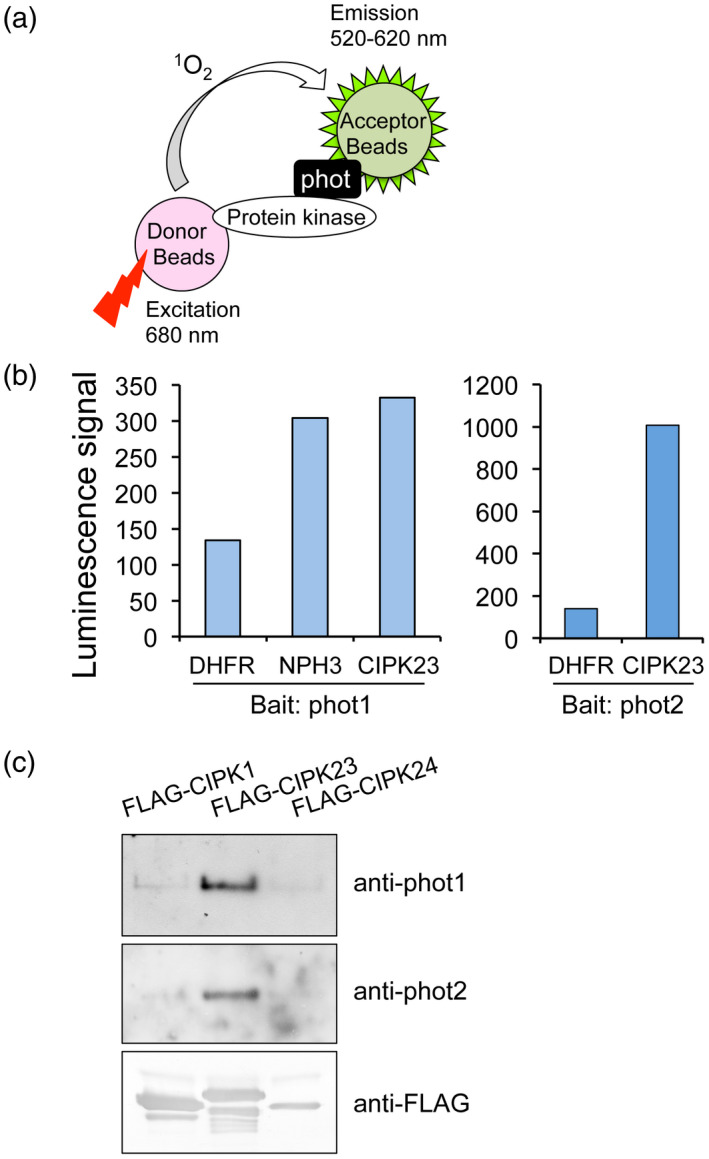
To verify the occurrence of the CIPK23–phototropin interactions in plant cells, we performed bimolecular fluorescence complementation (BiFC) in tobacco (Nicotiana benthamiana) leaves as reported previously (Kaiserli et al., 2009). Both phot1 and phot2 were found to interact with CIPK23 in the tobacco epidermal cells (Figure 2), whereas no BiFC signal was detected when CIPK24 and empty vectors were used in the series of co‐expression experiments (Figure 2; Figures S1 and S2). Taken together, these findings further demonstrate that CIPK23 specifically interacts with phot1 and phot2 in vivo.
Figure 2.
BiFC analysis showing the interactions between phototropins and CIPK23 and CIPK23 homodimerization in Nicotiana benthamiana leaf epidermal cells.
Indicated fusion proteins were transiently co‐expressed in Nicotiana cells via Agrobacterium. nYFP and cYFP represent N‐ and C‐terminal halves of the YFP protein, respectively. Phot1 homodimerization was used as a positive control. Representative confocal images show reconstitution of YFP fluorescence upon interaction. Representative images of negative controls are shown in Figure S1. Quantitative measurements of the interaction are plotted based on total YFP fluorescence in test and control infiltrations (n = 7). Scale bars = 20 μm.
CIPK23 is not required for phototropin‐mediated phototropism, chloroplast movement, leaf flattening, and promotion of plant growth
To investigate the functions of CIPK23 in phototropin‐mediated blue light responses, we obtained two transfer DNA insertional knockout mutants, cipk23‐1 (SALK_032341) and cipk23‐5 (SALK_138057) (Figure S3a; Cheong et al., 2007; Nieves‐Cordones et al., 2012). We confirmed the absence of transcripts of full‐length CIPK23 in either of these knockout mutants by reverse transcriptase (RT)‐PCR (Figure S3b).
We next confirmed the abundance of CIPK23 transcripts in various tissues from 4‐week‐old Arabidopsis plants. RT‐PCR analysis indicated that CIPK23 transcripts were expressed in guard cell protoplasts (GCPs), mesophyll cell protoplasts (MCPs), rosette leaves, petioles, inflorescence stems, and roots with a higher level being detected in GCPs (Figure S3c). Ubiquitous expression of CIPK23 was previously shown by promoter‐GUS expression assay (Cheong et al., 2007) and ubiquitous expression of phot1 and phot2 proteins was similarly confirmed (Kagawa et al., 2001; Sakamoto and Briggs, 2002). These findings suggest that CIPK23 could play a role in phototropin‐mediated responses.
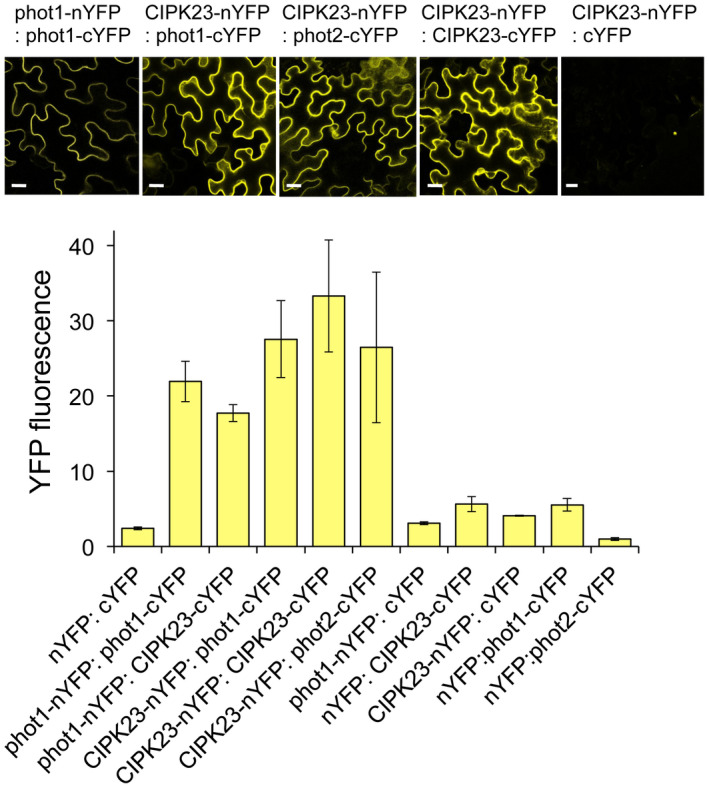
We explored whether phototropin‐mediated responses were altered in cipk23 mutants given that CIPK23 is ubiquitously expressed. First, phototropic curvature was determined using etiolated seedlings. The hypocotyls of cipk23‐1 and cipk23‐5 mutants showed phototropic bending in response to unilateral blue light (0.1 µmol m−2 sec−1) comparable to that found for wild‐type (Col) seedlings, unlike the phot1phot2 double mutant (Figure 3a). Next, we determined whether blue light‐induced chloroplast relocation movements were altered in cipk23 mutants by slit band assay (Suetsugu et al., 2005; Inoue et al., 2011) (Figure 3b). Irradiation of wild‐type leaves with weak blue light (1 µmol m−2 sec−1) through a 1‐mm slit promotes chloroplast accumulation in this region and the appearance of a darker green band (Figure 3b: upper panel). Conversely, a white band is produced when this area is irradiated with strong blue light (90 µmol m−2 sec−1) owing to chloroplast avoidance movement (Figure 3b: lower panel). Similarly, leaves of cipk23 mutants showed both green and white bands in response to weak and strong blue light, respectively. These results indicate that cipk23 mutants were not altered in chloroplast accumulation and avoidance responses.
Figure 3.
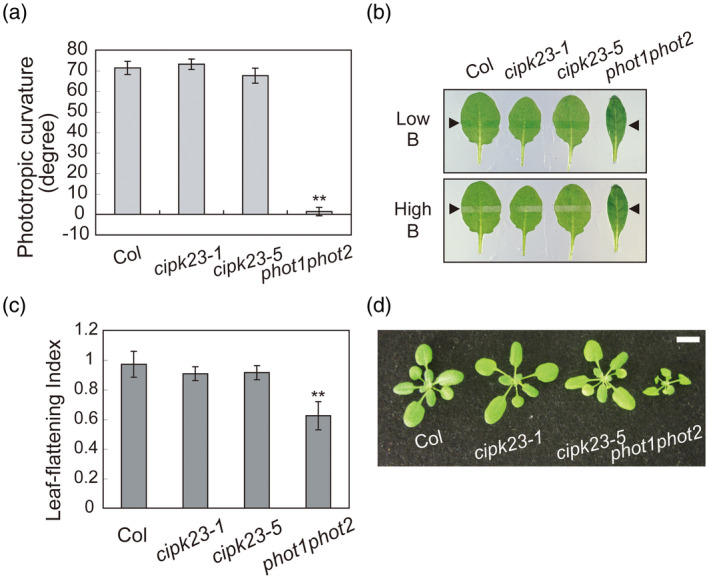
Effects of CIPK23 mutations on various phototropin‐mediated blue light responses.
(a) Phototropism of Arabidopsis thaliana wild‐type (Col), cipk23‐1, cipk23‐5, and phot1phot2. Etiolated seedlings were irradiated with unilateral blue light at 0.1 µmol m−2 sec−1 for 14 h. Values are means ± SE (n = 30–54). Differences from wild‐type plants were evaluated using Student’s t test (** P < 0.01).
(b) Chloroplast photorelocation movement in wild‐type (Col), cipk23‐1, cipk23‐5, and phot1phot2 leaves. Slit band assays were performed to observe chloroplast movement. Rosette leaves were detached and irradiated with blue light for 30 min through a 1‐mm slit. Blue light was irradiated at 1 µmol m−2 sec−1 to evaluate the accumulation response, and at 90 µmol m−2 sec−1 for the avoidance response. Upper and lower panels indicate chloroplast accumulation and avoidance responses, respectively. Black arrowheads indicate irradiated areas.
(c) Leaf flattening of wild‐type (Col), cipk23‐1, cipk23‐5, and phot1phot2 leaves. Plants were grown on soil under fluorescent light (50 µmol m−2 sec−1) for 5 weeks. The leaf‐flattening index was expressed as the ratio of projection of the leaf before and after artificial uncurling. Values are means ± SE (n = 5). Differences from wild‐type were evaluated using Student’s t test (** P < 0.01).
(d) Growth and morphology of wild‐type (Col), cipk23‐1, cipk23‐5, and phot1phot2. Plants were grown on soil for 4 weeks and photographed. The bar indicates 1 cm.
The rosette leaves of the phot1phot2 double mutant are epinastic and curl downward at the side in comparison to the leaves from wild‐type plants (Figure 3c). This leaf‐flattening response was still apparent in cipk23 mutants. Moreover, cipk23 mutants showed normal growth under our conditions, comparable to that of wild‐type plants, unlike the phot1phot2 double mutant (Figure 3d).
Taken together, the above findings demonstrate that mutants lacking CIPK23 are not altered in phototropin‐mediated phototropism, chloroplast movement, and leaf flattening in the tested conditions.
CIPK23 is required for phototropin‐mediated stomatal opening
Given the prevalence of CIPK23 expression in GCPs (Figure S3c), we determined whether blue light‐dependent stomatal opening was altered in the cipk23 mutants. Stomata in the leaf epidermis of wild‐type plants opened in response to blue light under a background of red light, but did not open in response to red light treatment (Figure 4a). This blue light response is mediated by the phototropins and is absent in the stomata of the phot1phot2 double mutant (Inoue et al., 2008b; Inoue et al., 2011; Hayashi et al., 2017). Blue light‐dependent stomatal opening was found to be impaired in epidermal peels isolated from cipk23‐1 and cipk23‐5 mutants (Figure 4a).
Figure 4.
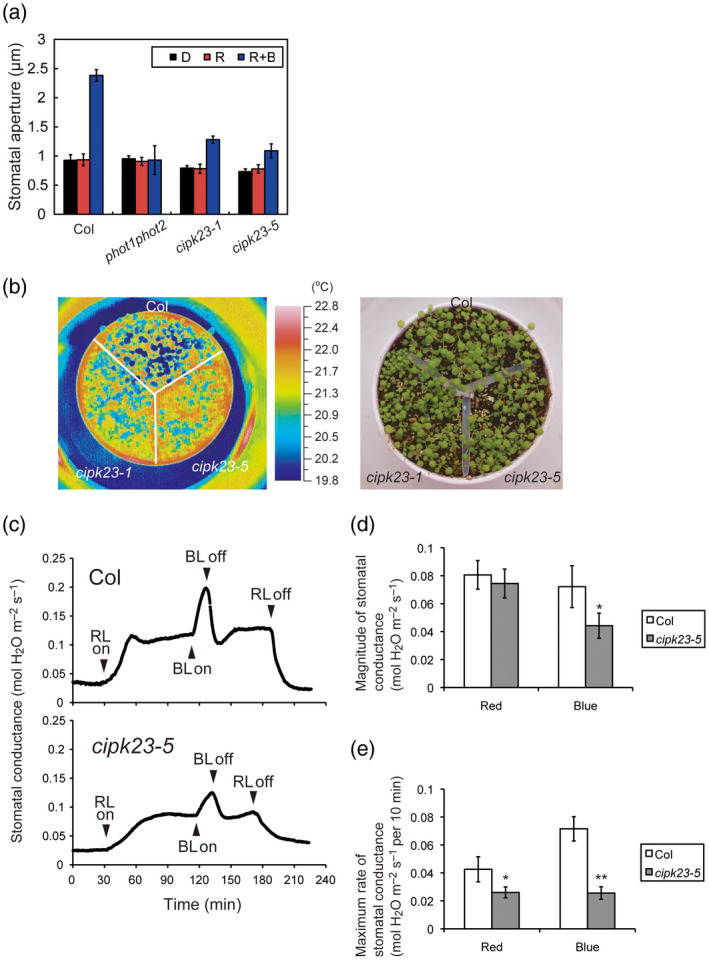
Effect of CIPK23 mutations on blue light‐dependent stomatal opening.
(a) Blue light‐dependent stomatal opening in the epidermis of wild‐type (Col), phot1phot2, cipk23‐1, and cipk23‐5 plants. Epidermal peels were isolated from dark‐adapted plants, and irradiated with red light (50 µmol m−2 sec−1; R) with or without blue light (10 µmol m−2 sec−1; R + B) for 3 h. Values are means of three independent experiments with standard deviations. In each experiment, 45 stomata were measured.
(b) Infrared thermal images of wild‐type (Col), cipk23‐1, and cipk23‐5 plants. Plants were grown in well‐watered conditions for 3 weeks under white light at 50 µmol m−2 sec−1.
(c) Stomatal conductance changes in response to red and blue light in intact leaves from wild‐type (Col) and cipk23‐5 plants. Red light (600 µmol m−2 sec−1; RL) and blue light (10 µmol m−2 sec−1; BL) were switched on/off at the times indicated by the arrowheads.
(d, e) Magnitude (d) and the maximum rate (e) of stomatal conductance in response to red and blue light. Data represent the means ± SD of four independent experiments. Differences were evaluated using Student’s t test (*P < 0.05, **P < 0.01).
The defect in blue light‐induced stomatal opening observed in cipk23 mutants was investigated in more detail. Changes in leaf temperature are known to reflect differences in stomatal aperture. For instance, water evaporation via open stomata results in a decrease in leaf temperature (Merlot et al., 2002; Hashimoto et al., 2006; Takemiya et al., 2013; Inoue et al., 2017). The leaf temperature of cipk23 mutants was higher compared to that of wild‐type leaves under our growth conditions (Figure 4b), consistent with their inability to open stomata in response to blue light (Figure 4a). The result suggests that water evaporation is reduced in cipk23 mutants since stomatal opening is reduced under the light conditions examined.
Finally, we measured the stomatal conductance of intact leaves in response to light (Figure 4c). Stomatal conductance in wild‐type leaves increased in response to strong red light (600 µmol m−2 sec−1) and reached a steady state after approximately 30 min (Figure 4c: upper graph). By comparison, this rate of increase was slightly reduced in the cipk23‐5 mutant (Figure 4c,e). Stomatal conductance in wild‐type leaves was further increased following irradiation with weak blue light (10 µmol m−2 sec−1) superimposed onto the background of red light (Figure 4c: upper graph). However, the magnitude and rate of this increase was largely reduced in the leaves of the cipk23‐5 mutant (Figure 4c–e). From the analysis of stomatal responses, we conclude that CIPK23 plays a role in mediating blue light‐dependent stomatal opening in Arabidopsis.
Blue light activation of the plasma membrane H+‐ATPase is unaltered in the guard cells of cipk23 mutants
Blue light activates the plasma membrane H+‐ATPase via phototropins through phosphorylation in guard cells. Activation of the H+‐ATPase generates the driving force for stomatal opening (Shimazaki et al., 2007; Inoue et al., 2010; Yamauchi et al., 2016; Inoue and Kinoshita, 2017). We therefore prepared GCPs from the rosette leaves of wild‐type and cipk23‐5 mutant plants, and measured H+ pumping activity in response to blue light (Figure S4a). GCPs from wild‐type or cipk23‐5 plants both showed a similar magnitude and rate of H+ pumping in response to blue light (Figure S4a; Table S1). We further determined whether blue light‐dependent phosphorylation of the H+‐ATPase was altered in GCPs isolated from cipk23‐5 through 14‐3‐3 protein binding by far‐Western blotting (Figure S4b). GCPs from the cipk23‐5 mutant exhibited similar levels of H+‐ATPase phosphorylation to those detected in GCPs from wild‐type rosette leaves. Moreover, the abundance of H+‐ATPase was unaltered in GCPs from the cipk23‐5 mutant. These results indicate that the H+‐ATPase is normally activated by blue light in the absence of CIPK23 and further suggest that signaling components downstream of the H+‐ATPase were impaired in cipk23 mutants.
The fungal toxin fusicoccin (FC) directly activates the plasma membrane H+‐ATPase and induces stomatal opening in the dark (Kinoshita and Shimazaki, 2001; Kinoshita et al., 2001). In darkness, the stomata in the epidermis of wild‐type Arabidopsis opened in response to FC treatment in a dose‐dependent manner and the aperture reached a maximum at 5 µm (Figure 5a). Stomata of the cipk23‐1 and cipk23‐5 mutants showed a similar response to 10 µm FC treatment as did those from wild‐type plants, as well as the phot1phot2 double mutant in the dark (Figure S5). Interestingly, further increases in stomatal opening were observed in the epidermis of wild‐type plants when blue light was introduced following FC treatment (Figure 5b). By contrast, stomata in the phot1phot2 double mutant did not show this increase in stomatal opening after blue light irradiation. These data therefore provide evidence for the presence of other factors in addition to the H+‐ATPase that are required for phototropin‐mediated stomatal opening. These likely include CIPK23 since the cipk23‐5 mutant also failed to show an increase in stomatal opening when irradiated with blue light following FC treatment in darkness (Figure 5b).
Figure 5.
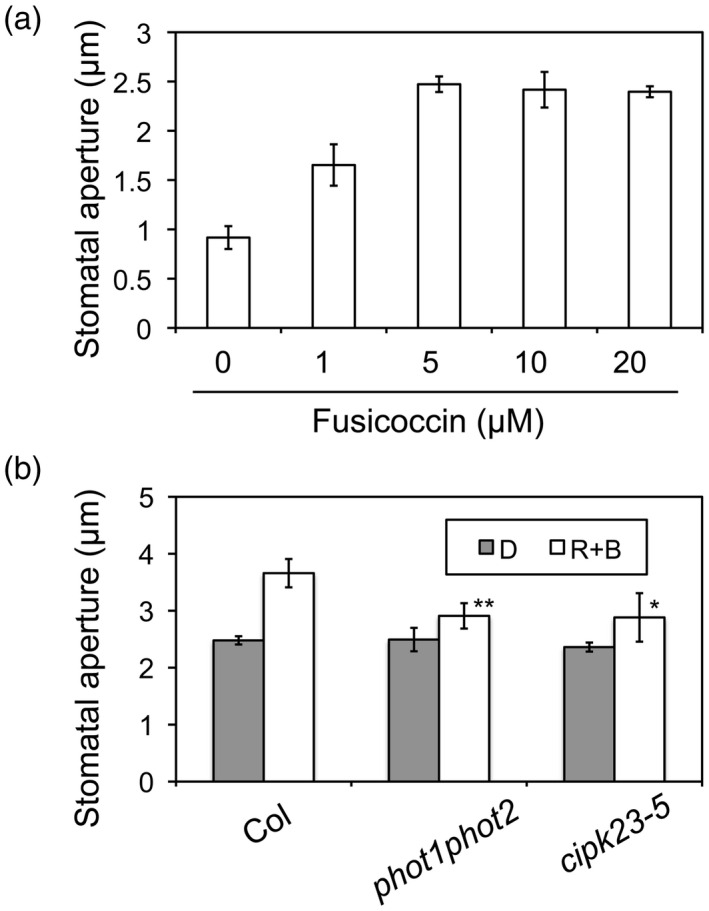
Effect of blue light on fusicoccin (FC)‐induced stomatal opening.
(a) Stomatal opening in response to FC in the epidermis of wild‐type (Col) leaves. Epidermal peels from dark‐adapted plants were incubated with FC at the indicated concentrations for 3 h in darkness. Data represent the means ± SD of three independent experiments. In each experiment, 45 stomata were measured.
(b) Effect of blue light on FC‐induced stomatal opening. Epidermal peels were incubated with FC at 10 µm with or without mixed light (R + B: red light at 50 µmol m−2 sec−1 and blue light at 10 µmol m−2 sec−1) for 3 h. Differences were evaluated using Student’s t test (*P < 0.05, **P < 0.01).
CIPK23 is required for blue light activation of K+ in channels
Recent experiments, using a voltage‐clamp technique, have demonstrated that blue light increased plasma membrane K+ in channel currents by about 50% in guard cells and this increment was completely lost in phot1phot2 guard cells (Zhao et al., 2012). In addition, CIPK23 promotes K+ uptake through direct activation of the AKT1 K+ in channel in roots (Li et al., 2006; Xu et al., 2006). Given our present findings, we rationalized that CIPK23 could be involved in mediating the signaling between the phototropins and K+ in channels. To test this possibility, we determined the activity of K+ in channels in GCPs isolated from the cipk23‐5 mutant by monitoring the K+ current by whole‐cell patch clamping. No difference in K+ in channel activity was observed between wild‐type GCPs and those from the cipk23‐5 mutant under dark conditions (Figure 6a). A short pulse (30 sec) of blue light led to a rapid (within 5 min), 2‐fold increase in K+ in channel activity in wild‐type GCPs, as reported previously (Figure 6a; Zhao et al., 2012). By contrast, this change in K+ current in response to blue light was highly diminished in GCPs from the cipk23‐5 mutant (Figure 6a). Steady‐state current–voltage curves also showed that K+ in currents increased in response to blue light in wild‐type GCPs, whereas this was decreased in the case of cipk23‐5 (Figure 6b,c; Figure S6a,b). These findings therefore suggest that blue light‐induced changes in guard cell K+ in currents are impaired in the cipk23‐5 mutant. Thus, CIPK23 likely couples phototropin activation to changes in K+ in currents at the guard cell plasma membrane.
Figure 6.
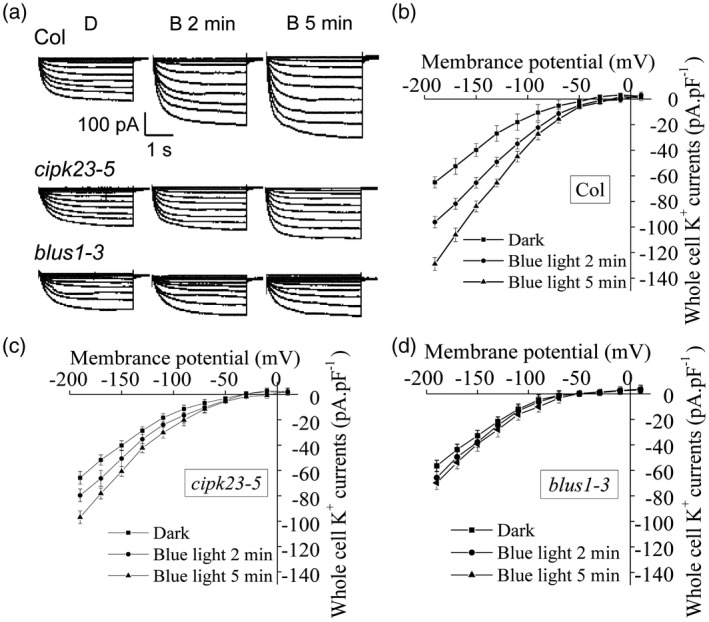
Effect of CIPK23 and BLUS1 mutations on blue light‐induced activation of K+ in currents in guard cell protoplasts.
(a) Effect of blue light on voltage‐dependent K+ in currents in GCPs from wild‐type (Col), cipk23‐5, and blus1‐3 plants. Whole‐cell K+ in currents (pA) were measured 2 and 5 min after the pulse of blue light (100 μmol m−2 sec−1, 30 sec). Measurements were performed under dim red light (0.2 μmol m−2 sec−1) as a control.
(b–d) The relationship between the whole‐cell inward‐rectifying K+ currents (pA pAF−1) and the membrane potential (mV) in GCPs from wild‐type (Col) (b), cipk23‐5 (c), and blus1‐3 (d) plants. Each value is the mean current from six or eight independent experiments, and the error bars denote SE.
Recent studies have shown that the guard cell‐specific kinase BLUS1, which mediates blue light‐dependent stomatal opening, acts as a substrate for phototropin kinase activity and forms a complex with phot1 and phot2 (Takemiya et al., 2013; Schnabel et al., 2018). Thus, similar electrophysiological experiments were performed using GCPs from the blus1‐3 mutant. Blue light‐induced changes in guard cell K+ in currents were found to be impaired in the guard cells of blus1‐3 (Figure 6a,b,d; Figure S6a,c). We therefore conclude that BLUS1 also participates in the signaling for the K+ in channel activation by blue light.
We have no evidence that phototropin kinases phosphorylate CIPK23
Phototropins may directly phosphorylate CIPK23 in response to blue light since we found that CIPK23 interacts with both phot1 and phot2 (Figures 1 and 2). To determine whether or not CIPK23 is a direct substrate for phototropin kinase activity, we performed in vitro thio‐phosphorylation assays using the gatekeeper‐engineered variant of phot1, phot1T740G, and N6‐benzyl‐ATPγS as a phosphodonor, as reported recently (Schnabel et al., 2018). Blue light‐dependent autophosphorylation of phot1 was clearly observed as a consequence of thio‐phosphorylation (Figure S7: white arrowhead). By contrast, a thio‐phosphorylation signal corresponding to CIPK23 phosphorylation by phot1 was not observed (Figure S7: black arrowhead). From these experiments, we conclude that CIPK23 is not a substrate for phototropin kinase, at least in the gatekeeper/thio‐phosphorylation system.
DISCUSSION
CIPK23 is a positive regulator of phototropin‐mediated stomatal opening
Mutants lacking CIPK23 showed impaired blue light‐dependent stomatal opening in both experiments using epidermal fragments and intact leaves (Figure 4). FC‐induced stomatal opening in darkness was unaffected in the cipk23‐5 mutant but was affected under blue light conditions (Figure S5; Figure 5b). Furthermore, the plasma membrane H+‐ATPase was normally activated in response to blue light in cipk23‐5 guard cells (Figure S4). Thus, we assume that CIPK23 is involved in a signaling pathway which is different from that associated with phototropin‐dependent activation of the plasma membrane H+‐ATPase for stomatal opening. Finally, we found that blue light activation of the K+ in channel current was impaired in the guard cells of the cipk23‐5 mutant compared to wild‐type guard cells (Figure 6; Figure S6). From these results, we conclude that CIPK23 acts as a positive regulator in stomatal opening and mediates blue light signaling from phototropins to the K+ in channels in guard cells (Figure S10). However, previous studies have indicated that CIPK23 functions as a negative regulator of ABA or water stress signaling in guard cells (Cheong et al., 2007; Nieves‐Cordones et al., 2012). In this case, mutations in CIPK23 were found to enhance ABA‐ or water stress‐dependent inhibition of light‐induced stomatal opening. It is therefore reasonable to interpret that CIPK23 acts as a positive regulator of phototropin‐mediated stomatal opening through the activation of K+ in channels, and this action appears to antagonize ABA‐induced stomatal closure.
CIPK23 is a member of the SnRK3 subfamily that mainly acts in adaptive responses through the regulation of ion transporters (Hrabak et al., 2003; Luan, 2008; Sanyal et al., 2015). CIPK23 stimulates K+ and nitrate uptake through phosphorylation‐dependent activation of the high‐affinity K+ transporter 5 (HAK5), the K+ in channel AKT1, and the nitrate transporter CHL1/NRT1.1 in the plasma membrane of roots under low K+ and N conditions (Li et al., 2006; Xu et al., 2006; Ho et al., 2009; Ragel et al., 2015). Since uptake of K+ and nitrate into guard cells contributes to stomatal opening (Schroeder et al., 2001; Guo et al., 2003; Shimazaki et al., 2007), these studies provide further support that CIPK23 acts as a positive regulator of stomatal opening through the activation of ion transporters downstream of the phototropins. The work presented here highlights an additional level of complexity associated with phototropin signaling events required for transporter regulation and stomatal opening.
A recent electrophysiological study using transient expression in Xenopus oocytes suggested that CIPK23 potentially functions as a positive regulator in ABA‐induced stomatal closure through activation of the S‐type anion channel SLAC1 (Maierhofer et al., 2014). There is a conclusion discrepancy between Maierhofer et al. (2014) and this study. However, slac1 mutants show large stomatal opening and impairment of ABA‐induced stomatal closure (Negi et al., 2008; Vahisalu et al., 2008). In contrast, cipk23 mutants did not show such open stomata phenotypes but showed closed stomata phenotypes (Cheong et al., 2007; Nieves‐Cordones et al., 2012; Figure 4). From these findings, we conclude that SLAC1 activation by CIPK23 may not strongly contribute to regulation of stomatal aperture in our experimental conditions. Further electrophysiological measurements of anion channel activity in cipk23 guard cells are therefore needed to clarify how CIPK23 regulates SLAC1 under our experimental conditions.
Regulation of K+ in channel activity by CIPK23 in guard cells
It is well established that blue light activates the guard cell H+‐ATPase via the phototropins, resulting in hyperpolarization of the plasma membrane, which in turn activates voltage‐dependent K+ in channels that are required for K+ uptake (Schroeder et al., 2001; Shimazaki et al., 2007; Inoue et al., 2010). However, in addition to this single‐scheme signaling, recent findings have demonstrated that blue light also enhances the K+ in channel current in guard cells via phototropins (Zhao et al., 2012). Since this enhancement is observed under the membrane voltage‐clamped conditions, it is thought that the effect of blue light on the K+ in channel current is independent from plasma membrane H+‐ATPase activity. In the present study, we found that cipk23‐5 exhibited impaired stomatal opening and lacked K+ in channel activation in response to blue light (Figures 4, 5, 6; Figure S6). In addition, activation and phosphorylation of the H+‐ATPase in response to blue light were not affected in cipk23‐5 guard cells (Figure S4). From these results, we conclude that CIPK23 does not mediate signaling from the phototropins to H+‐ATPase activation but activates K+ in channels via the phototropins (Figure S10). Blue light‐dependent stomatal opening is completely abolished in the phot1phot2 mutant but not completely in cipk23 mutants (Figure 4), suggesting that the blue light activation of K+ in channels partially contributes to stomatal opening and phototropin‐mediated activation of both the H+‐ATPase and K+ in channels is needed for full stomatal opening.
AKT1 is a guard cell‐expressing K+ in channel that functions in stomatal opening together with other K+ in channels, including KAT1, KAT2, and AKT2 (Véry and Sentenac, 2003; Gambale and Uozumi, 2006; Harada and Shimazaki, 2009; Takahashi et al., 2013). The kincless mutant was generated by expressing a dominant‐negative variant of KAT2 in the kat2 mutant background. Guard cell K+ in currents are lost in the kincless mutant (Lebaudy et al., 2008), which shows a strong impairment in blue light‐dependent stomatal opening (Takahashi et al., 2013). CIPK23 has been shown to activate AKT1 by direct phosphorylation to promote K+ uptake in roots under low K+ conditions (Li et al., 2006; Xu et al., 2006; Sanchez‐Barrena et al., 2020). On the basis of these findings, we propose that CIPK23 could also activate guard cell AKT1 by direct protein phosphorylation in response to blue light. Indeed, CIPK23 has been shown to specifically interact with AKT1 among other plant K+ in channels in yeast (Li et al., 2006). Alternatively, CIPK23 could phosphorylate and regulate the activity of other K+ in channels in guard cells either directly or indirectly via scaffold proteins. Further investigation will therefore be required to clarify the role of CIPK23 in regulating K+ in channel activity in phototropin signaling.
The CIPK protein family consists of 26 members in Arabidopsis. Phylogenetic tree analysis indicates that CIPK23 is localized in the clade containing CIPK3, CIPK9, and CIPK26 (Figure S8). According to a public microarray database eFP browser (http://bar.utoronto.ca/efp/cgi‐bin/efpWeb.cgi?dataSource=Guard_Cell), CIPK3 and CIPK9 are expressed in guard cells at similar levels to CIPK23 (Figure S9). These expression data suggest that CIPK3, CIPK9, and CIPK26 may function redundantly with CIPK23 in the regulation of stomatal opening. However, Luan’s group has demonstrated that members outside this clade, namely, CIPK6 and CIPK16, have similar functions to CIPK23 with respect to AKT1 activation (Lee et al., 2007). Expression of CIPK6 is also apparent in guard cells (Figure S9), suggesting that it may also have a similar function to CIPK23 in stomatal opening.
We found that blue light increased K+ in channel currents 2‐fold in wild‐type guard cells (Zhao et al., 2012; Figure 6a,b), and this enhancement in K+ in channel activity appears to contribute to stomatal opening. However, it has already been reported that Arabidopsis guard cells possess sufficient K+ in channel activity required for stomatal opening (Szyroki et al., 2001; Lebaudy et al., 2008; Takahashi et al., 2013). Stomatal opening is affected only when K+ in channel activity is largely reduced (by 70–80%). It is therefore difficult to attribute the impairment in stomatal opening in cipk23 mutants solely to a failure in K+ in channel activation. Other ion transporters, such as nitrate transporters activated through CIPK23 (Guo et al., 2003; Ho et al., 2009), could also play an important role in stomatal opening in response to blue light.
Activation of CIPK23 via the phototropins
One question arising from our findings is how the phototropins activate CIPK23 in response to blue light. Structural and biochemical analyses suggest that CIPK23 is activated by binding of the calcium sensor calcineurin B‐like (CBL) 1/9 in a Ca2+‐dependent manner (Luan, 2008) and/or phosphorylation by another protein kinase (Chaves‐Sanjuan et al., 2014). The activation of CIPK23 in guard cells may therefore require an increase in cytosolic Ca2+ or phosphorylation of CIPK23 by an upstream protein kinase. At least for the former, previous reports have demonstrated that cytosolic Ca2+ increases in response to ABA or high concentrations of CO2 in guard cells contribute to stomatal closure (McAinsh et al., 1990; Schroeder and Hagiwara, 1990; Allen et al., 1999, 2001; Kim et al., 2010). Indeed, guard cell K+ in channel currents have shown to be blocked by cytosolic Ca2+ increases in electrophysiological experiments (Grabov and Blatt, 1998, 1999; Wang et al., 2013). In addition, the plasma membrane‐anchoring of the CIPK23 kinase domain strongly increased K+ in channel currents in the absence of CBL interactions (Lee et al., 2007). These observations suggest that CIPK23 activity for stomatal opening may be independent from cytosolic Ca2+ increases and the function of CBL1/9 may be required for the recruitment of CIPK23 to the plasma membrane.
A guard cell‐specific kinase BLUS1, which acts as a substrate for phototropins, mediates blue light‐dependent stomatal opening through H+‐ATPase activation (Takemiya et al., 2013). In addition, our electrophysiological experiments indicated that blue light‐dependent activation of K+ in channel currents was severely impaired in the blus1‐3 guard cells (Figure 6a,d; Figure S6a,c). These results suggest that BLUS1 activates both the plasma membrane H+‐ATPase and the K+ in channel, but CIPK23 only activates the K+ in channel in stomatal opening. On the basis of these findings, we speculate that CIPK23 may regulate BLUS1 in blue light activation of the K+ in channels (Figure S10: left model). In the present study, phot1 did not phosphorylate CIPK23 in vitro (Figure S7), although these proteins were shown to physically interact in vitro and in vivo (Figures 1 and 2). Since CIPK23 is likely to form a protein complex with phototropins and BLUS1, another potential signaling scenario could involve CIPK23 acting as a substrate for BLUS1 (Figure S10: right model). However, we have not been successful to date in determining whether or not CIPK23 is a substrate for BLUS1 kinase activity because of the difficulty in producing active recombinant BLUS1 (Hayashi et al., 2017). Further experiments are now needed to clarify these points and improve our understanding of how CIPK23 regulates phototropin signaling.
EXPERIMENTAL PROCEDURES
Plant materials and growth conditions
Arabidopsis thaliana and N. benthamiana were grown on soil with a 16‐h light/8‐h dark photoperiod under white fluorescent light (50 µmol m−2 sec−1) at 22–24°C. Four‐ to five‐week‐old plants were used for stomatal bioassays, isolation of GCPs, and preparation of total RNA.
We obtained cipk23‐1 (SALK_032341) and cipk23‐5 (SALK_138057) from the Arabidopsis Biological Resource Center and isolated homozygous mutants by PCR using genomic DNA according to the SIGnAL website (http://signal.salk.edu). We used both mutants for phenotypic analyses after confirmation of knockout mutants by RT‐PCR (Figure S3b).
Protein kinases screening by using the AlphaScreen
All proteins for the AlphaScreen method were expressed using a wheat germ cell‐free protein synthesis system (Sawasaki et al., 2002, 2004, 2005). Full‐length cDNAs of PHOT1 and PHOT2 were amplified by RT‐PCR from the wild‐type (Col) cDNAs using the following oligonucleotide primers: 5′‐CCCAAGCTTATGGAACCAACAGAAAAACCATCG‐3′ and 5′‐CCCAAGCTTTCAAAAAACATTTGTTTGCAGATCTTC‐3′ for PHOT1 and 5′‐ATGGAGAGGCCAAGAGCCCCTCC‐3′ and 5′‐TTAGAAGAGGTCAATGTCCAAGTCC‐3′ for PHOT2. The amplified PHOT1 and PHOT2 fragments were cloned into the pFLAG‐MAC vector (Sigma‐Aldrich) via HindIII and SmaI sites, respectively. The coding regions of FLAG‐tagged phototropins were amplified by PCR from these vectors using the following primers: 5′‐GGGGTACCATGGACTACAAGGACGACGATGAC‐3′ and 5′‐GGGGTACCTCAAAAAACATTTGTTTGCAGATCTTC‐3′ for FLAG‐PHOT1 and 5′‐GGGGTACCATGGACTACAAGGACGACGATGAC‐3′ and 5′‐GGGGTACCTTAGAAGAGGTCAATGTCCAAGTCC‐3′ for FLAG‐PHOT2. The DNA fragments were cloned into the KpnI site of the pEU3‐NII vector, and resulting plasmids were used for the protein synthesis of FLAG‐phot1 and FLAG‐phot2 as baits for the protein–protein interaction screening. Each of the full‐length cDNAs of Arabidopsis protein kinases (562 members) was amplified from the RAFL cDNA library and added to the DNA regions of the SP6 promoter and N‐terminal biotin ligation site by split‐primer PCR as described previously (Sawasaki et al., 2002, 2004; Nemoto et al., 2011). In vitro transcription, translation, and biotin labeling were performed according to previous methods (Sawasaki et al., 2005; Takahashi et al., 2009; Nemoto et al., 2011). FLAG‐tagged phot1 and phot2 were synthesized in the presence of 100 µm flavin mononucleotide as a chromophore (Sawasaki et al., 2004).
To detect the interactions between FLAG‐tagged phototropins and protein kinases, 5 µl of each protein‐synthesized mixture was mixed and reacted in a 20 µl reaction mixture containing 50 mm Tris‐HCl (pH 7.6), 100 mm potassium acetate, 10 mm MgCl2, 0.1 mm DTT, 5 µg ml−1 anti‐FLAG antibody (Sigma‐Aldrich), 1 mg ml−1 BSA, 0.1 µl streptavidin‐coated donor beads, and 0.1 µl anti‐IgG acceptor beads at 23°C for 1 h. Luminescent signals were analyzed by the AlphaScreen detection program (PerkinElmer Life and Analytical Sciences; Takahashi et al., 2009).
In vitro pull‐down assays
Pull‐down assays were carried out using recombinant CIPKs expressed and purified from E. coli in combination with microsomal membranes from Arabidopsis seedlings. Full‐length cDNAs of CIPK1, CIPK23, and CIPK24 were amplified by RT‐PCR using the following oligonucleotide primers: 5′‐CCCAAGCTTATGGTGAGAAGGCAAGAGGAGG‐3′ and 5′‐CCCAAGCTTCTAAGTTACTATCTCTTGCTCCGGCG‐3′ for CIPK1, 5′‐CCCAAGCTTATGGCTTCTCGAACAACGCCTTCAC‐3′ and 5′‐CCCAAGCTTTTATGTCGACTGTTTTGCAATTGTCCG‐3′ for CIPK23, and 5′‐CCCAAGCTTATGACAAAGAAAATGAGAAGAGTGGGC‐3′ and 5′‐CCCAAGCTTTCAAAACGTGATTGTTCTGAGAATCTC‐3′ for CIPK24. The amplified DNA fragment was cloned into the HindIII site of the pFLAG‐MAC Expression Vector (Sigma‐Aldrich). The resulting plasmid vectors were transformed into the E. coli BL21 strain. The recombinant CIPK proteins were expressed as a fusion protein with FLAG‐tag and purified from E. coli extracts using anti‐FLAG agarose (Sigma‐Aldrich) according to the manufacturer’s instructions (https://www.sigmaaldrich.com/content/dam/sigma‐aldrich/docs/Sigma/Bulletin/f2426bul.pdf). The agarose beads were then incubated with microsomal proteins (100 µg) for 2 h at 4°C. Proteins were solubilized from the beads by adding a half volume of SDS sample buffer containing 4.5% SDS, 30% sucrose, 22.5% β‐mercaptoethanol, 0.018% Coomassie Brilliant Blue, 4.5 mm EDTA, and 45 mm Tris‐HCl (pH 8.0) and subjected to SDS‐PAGE. Proteins were immunodetected by using anti‐phot1, anti‐phot2, and anti‐FLAG monoclonal antibodies (Sigma‐Aldrich) according to a previously described method (Inoue et al., 2011).
BiFC assays
Full‐length cDNAs of CIPK23, CIPK24, and PHOT2 were amplified by RT‐PCR using the following oligonucleotide primers: 5′‐GCCTCTAGAATGGCTTCTCGAACAACGCCTTCAC‐3′ and 5′‐ATCCCGGGTGTCGACTGTTTTGCAATTGTCCG‐3′ for CIPK23, 5′‐GCCTCTAGAATGACAAAGAAAATGAGAAGAGTGGGC‐3′ and 5′‐ATCCCGGGAAACGTGATTGTTCTGAGAATCTC‐3′ for CIPK24, and 5′‐GCCTCTAGAATGGAGAGGCCAAGAGCCCCT‐3′ and 5′‐ATCCCGGGGAAGAGGTCAATGTCCAAGTCCG‐3′ for PHOT2. The amplified DNA fragments were cloned into the binary vectors pSPYNE‐35S and pSPYCE‐35S (Walter et al., 2004) via XbaI and SmaI sites. The pSPYNE‐35S and pSPYCE‐35S vectors bearing PHOT1 cDNA were used as reported previously (Kaiserli et al., 2009). Agrobacterium tumefaciens (GV3101) was transformed with the resulting plasmid vectors and used for transformation of N. benthamiana. Agrobacteria‐mediated co‐infiltration of N. benthamiana leaves with pSPYNE and pSPYCE containing the indicated inserts was performed as previously described (Walter et al., 2004; Kaiserli et al., 2009). Detection of reconstituted YFP fluorescence was monitored 2.5 days post‐infiltration using a confocal microscope (Zeiss LSM510 and Leica SP8) (Walter et al., 2004; Kaiserli et al., 2009). Total YFP fluorescence from seven separate images and three independent experiments was quantified using Fiji (ImageJ) (Schindelin et al., 2012).
Expression of CIPK23
RT‐PCR was performed as described previously (Inoue et al., 2008a). GCPs and MCPs were enzymatically prepared from rosette leaves as reported by Ueno et al. (2005) with slight modifications. Total RNAs were extracted from GCPs, MCPs, rosette leaves, petioles, inflorescence stems, and roots of 4‐week‐old wild‐type (Col) plants or from the aerial parts of cipk23‐1 and cipk23‐5 mutants with ISOGEN (Nippon Gene). First‐strand cDNAs were synthesized from 2 µg of each total RNA by SuperScript III reverse transcriptase using oligo(dT)12‐18 primer (Invitrogen). Full‐length CIPK23 cDNAs were amplified by PCR using the following oligonucleotide primers: 5′‐ATGGCTTCTCGAACAACGCCTTCAC‐3′ and 5′‐TTATGTCGACTGTTTTGCAATTGTCCG‐3′ (Figure S3b). For amplification of the fragment of CIPK23 cDNA, PCR was performed using the following oligonucleotide primers: 5′‐AGTTTCAAACTGCTTCTGCTCCAC‐3′ and 5′‐ACGAGGATTACATTTGCTGAGGTC‐3′ (Figure S3c). As an internal standard, a fragment of ACT8 was used with the primers 5′‐ACTTTACGCCAGTGGTCGTACAAC‐3′ and 5′‐AAGGACTTCTGGGCACCTGAATCT‐3′.
Measurement of stomatal aperture
Stomatal aperture in the leaf abaxial epidermis was measured according to previous methods (Inoue et al., 2008b; de Carbonnel et al., 2010) with a modification. To determine the stomatal opening, the epidermal fragments were incubated in 2 ml buffer containing 5 mm MES/bistrispropane (pH 6.5), 50 mm KCl, and 0.1 mm CaCl2, and illuminated with light for 3 h at room temperature (Figure 4a). To determine the stomatal opening by FC, the epidermis were incubated in 2 ml of buffer containing 5 mm MES/bistrispropane (pH 6.5), 10 mm KCl, 0.1 mm CaCl2, and FC at indicated concentrations for 3 h (Figure 5; Figure S5).
Phenotypic analyses of phototropin‐mediated responses
Phototropic curvature of etiolated seedlings, chloroplast relocations and leaf flattening of rosette leaves, and stomatal conductance in intact leaves were determined according to previous methods (Doi et al., 2004; Inoue et al., 2008a, 2008b, 2011, 2017; Takemiya et al., 2013).
H+ pumping and H+‐ATPase phosphorylation in guard cell protoplasts
GCPs were enzymatically prepared from rosette leaves and used for the measurements of H+ pumping, immunoblotting, and far‐Western blotting according to previously described methods (Ueno et al., 2005; Inoue et al., 2008b; Takemiya et al., 2013). Immunoblotting and far‐Western blotting were performed using anti‐H+‐ATPase antibodies and GST‐14‐3‐3 protein (GF14phi) to determine the amount of H+‐ATPase and the levels of H+‐ATPase phosphorylation, respectively.
Isolation of guard cell protoplasts and whole‐cell K+ current recordings
GCPs for whole‐cell K+ current recordings were isolated as described previously (Zhao et al., 2012). Prior to each experiment, epidermal peels were obtained carefully from the abaxial surface of the youngest and fully expanded leaves of 2‐week‐old Arabidopsis and cut into pieces of 5 mm length. The epidermal strips were exposed to enzyme buffer (1.3% cellulase RS, 0.0075% pectolyase Y‐23, 0.25% BSA, 0.5 mm ascorbate (pH 5.5), and osmolality at 460 mOmol kg−1 adjusted with sorbitol) for approximately 20 min.
Whole‐cell K+ current recordings patch‐clamp pipettes were pulled with a vertical puller (model PC‐10; Narishige), modified for two‐stage pulls, and fire‐polished by a microforge (model MF‐90; Narishige) before using. The pipette solution typically contained 100 mm K‐glutamate, 2 mm MgCl2, 1.1 mm Mg‐ATP, 10 mm Hepes (4‐[2‐hydroxyethyl]‐1‐piperazineethanesulfonic acid)‐KOH (pH 7.2). Osmolality of the pipette solution was adjusted at 510 mOsmol kg−1 with d‐sorbitol. Bath solutions contained 10 mm K‐glutamate, 2 mm MgCl2, 0.5 mm CaCl2, and 10 mm Mes‐KOH (pH 5.5), and the osmolarity was adjusted to 460 mOsmol kg−1 with d‐sorbitol. Whole‐cell currents were measured in response to 3‐sec voltage pulses from −190 to −10 mV in 20 mV steps for the K+ in channel currents, using an EPC‐9 patch‐clamp amplifier (HEKA Elektronik, Lambrecht, Germany).
At the onset of each patch‐clamp experiment, the room was illuminated with a dim red light (0.2 µmol m−2 sec−1). After the whole‐cell configuration was obtained, the membrane was clamped to −52 mV (holding potential). When the recorded currents appeared stable, the patch‐clamped cells were illuminated with 100 µmol m−2 sec−1 blue light for 30 sec. A fiber optic halogen light source (Nikon, Tokyo) and Plexiglas filters were used for light treatments. Whole‐cell data were low‐pass filtered with a cutoff frequency of 2.9 kHz and analyzed with the software PLUSEFIT (version 8.7) and IGOR 3.0. The final whole‐cell currents were expressed in pA (Figure S6; Yin et al., 2013) or as currents per unit capacitance (pA pF−1) (Figure 6b–d) to account for variations in the cell surface area. Data are presented as means ± SE.
Thio‐phosphorylation in in vitro phosphorylation assay
Detection of thio‐phosphorylation derived from phot1 kinase activity was performed as previously described (Schnabel et al., 2018). The cDNA fragments of GST‐PHOT1‐T740G and GST‐CIPK23 were cloned into the pSP64 vector. GST‐phot1T740G and GST‐CIPK23 were expressed using the TNT SP6 High‐Yield Wheat Germ Protein Expression System (Promega), with 2 µg of each vector for a 20 µl reaction, and 10 µm flavin mononucleotide as a chromophore. Protein expression was performed at room temperature in the dark for 2 h. The in vitro kinase assay was performed as previously described (Sakai et al., 2001) with modifications. Ten µl of in vitro protein expression extract and N6‐benzyl‐ATPγS (500 µm) were mixed in each reaction volume (20 µl) with phosphorylation buffer (37.5 mm Tris‐HCl (pH 7.5), 5.3 mm MgSO4, 150 mm NaCl, and 1 mm EGTA). Light was irradiated to the samples for 20 sec at a total fluence of 60 000 µmol m−2. Reactions were incubated in the dark at room temperature for 5 min and terminated by adding EDTA (pH 8.0) to a final concentration of 20 mm. Thio‐phosphorylated molecules were alkylated by adding p‐nitrobenzyl mesylate at a final concentration of 2.5 mm and incubated for 2 h. Then, protein samples were subjected to SDS‐PAGE and Western blotting as described previously (Schnabel et al., 2018). Protein thio‐phosphorylation and GST protein were detected using a rabbit anti‐thiophosphoester monoclonal antibody and a goat anti‐GST monoclonal antibody, respectively, as primary antibodies. HRP‐conjugated anti‐rabbit or anti‐goat secondary antibody and Pierce ECL Plus Western blotting substrate (Thermo Fisher Scientific) were used to develop the signals.
ACCESSION NUMBERS
PHOT1 (AT3G45780), PHOT2 (AT5G58140), CIPK23 (AT1G30270), BLUS1 (AT4G14480), CIPK1 (AT3G17510), CIPK24 (AT5G35410), ACT8 (AT1G49240).
CONFLICT OF INTEREST
The authors of the manuscript declare no conflict of interest.
AUTHOR CONTRIBUTIONS
SI and KS conceived the original screening and research plans; SI, MS, KS, YE, TS, TK, JMC, XZ, and KS designed and supervised the experiments; SI, EK, XZ, TW, and AT performed experiments; SI, EK, MO, HT, TS, XZ, JMC, and KS analyzed the data; SI, EK, TW, XZ, JMC, and KS wrote the article.
Supporting information
Figure S1. Representative confocal images of reconstitution of YFP fluorescence upon interaction between phot1 and CIPK23 and corresponding negative controls.
Figure S2. BiFC analysis showing the interactions between phototropins and CIPK24 and CIPK24 homodimerization in Nicotiana benthamiana leaf epidermal cells.
Figure S3. Transfer DNA insertional mutants used in this study and expression of CIPK23 in various tissues.
Figure S4. Blue light‐induced activation of the plasma membrane H+‐ATPase in GCPs from wild‐type (Col) and cipk23‐5 plants.
Figure S5. Stomatal opening in response to fusicoccin in darkness.
Figure S6. Effects of blue light on whole‐cell inward‐rectifying K+ channel currents in wild‐type (Col), cipk23‐5, and blus1‐3 GCPs.
Figure S7. CIPK23 is not phosphorylated by phot1 in the in vitro kinase assay.
Figure S8. Phylogenetic relationships among Arabidopsis thaliana CIPK family members.
Figure S9. Gene expression levels of CIPK23, CIPK3, CIPK9, CIPK26, CIPK6, and CIPK16 in guard cells.
Figure S10. Proposed blue light signalings in guard cells.
Table S1. Blue light‐dependent H+‐pumping in GCPs from wild‐type (Col) and cipk23‐5 plants.
ACKNOWLEDGMENTS
We thank the ABRC for providing seed stocks and Y. Tomokiyo, Y. Iwashita, M. Aibe, N. Nishihara‐Seki, and A. Kawajiri in our laboratory for technical assistance. This work was supported, in part, by Japan Society for the Promotion of Science (JSPS) KAKENHI grants (nos. JP15K07101 and JP25840105 to S.I.), Grants‐in‐Aid for Scientific Research on Priority Areas (nos. JP17084005 and JP21227001 to K.S.), and the Hori Sciences And Arts Foundation (to S.I.). We acknowledge funding support from the UK Biotechnology and Biological Sciences Research Council (BB/M023079/1 to E.K.; BB/M002128/1 and BB/R001499/1 to J.M.C). E.K. acknowledges the University of Glasgow for the award of a Lord Kelvin Adam Smith Fellowship. T.W. was supported by an MVLS DTP PhD studentship at the University of Glasgow.
Contributor Information
Shin‐Ichiro Inoue, Email: shin@bio.nagoya-u.ac.jp.
Ken‐Ichiro Shimazaki, Email: kenrcb@kyushu-u.org.
DATA AVAILABILITY STATEMENT
All relevant data are included in the manuscript and its supporting materials.
References
- Allen, G.J. , Chu, S.P. , Harrington, C.L. , Schumacher, K. , Hoffmann, T. , Tang, Y.T. , Grill, E. and Schroeder, J.I. (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature, 411, 1053–1057. [DOI] [PubMed] [Google Scholar]
- Allen, G.J. , Kwak, J.M. , Chu, S.P. , Llopis, J. , Tsien, R.Y. , Harper, J.F. and Schroeder, J.I. (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19, 735–747. [DOI] [PubMed] [Google Scholar]
- Assmann, S.M. , Simoncini, L. and Schroeder, J.I. (1985) Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba . Nature, 318, 285–287. [Google Scholar]
- Blatt, M.R. (1987) Electrical characteristics of stomatal guard cells: the contribution of ATP‐dependent, “electrogenic” transport revealed by current‐voltage and difference‐current‐voltage analysis. J. Membr. Biol. 98, 257–274. [Google Scholar]
- Chaves‐Sanjuan, A. , Sanchez‐Barrena, M.J. , Gonzalez‐Rubio, J.M. , Moreno, M. , Ragel, P. , Jimenez, M. , Perdo, J.M. , Martinez‐Ripoll, M. , Quintero, F.J. and Albert, A. (2014) Structural basis of the regulatory mechanism of the plant CIPK family of protein kinases controlling ion homeostasis and abiotic stress. Proc. Natl Acad. Sci. USA, 111, E4532–E4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong, Y.H. , Pandey, G.K. , Grant, J.J. , Batistic, O. , Li, L. , Kim, B.‐G. , Lee, S.‐C. , Kudla, J. and Luan, S. (2007) Two calcineurin‐B‐like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 52, 223–239. [DOI] [PubMed] [Google Scholar]
- Christie, J.M. , Reymond, P. , Powell, G. , Bernasconi, P. , Railbekas, A.A. , Liscum, E. and Briggs, W.R. (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science, 282, 1698–1701. [DOI] [PubMed] [Google Scholar]
- Christie, J.M. (2007) Phototropin blue‐light receptors. Annu. Rev. Plant Biol. 58, 21–45. [DOI] [PubMed] [Google Scholar]
- Christie, J.M. , Yang, H. , Richter, G.L. et al (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol. 9, e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, J.M. , Blackwood, L. , Petersen, J. and Sullivan, S. (2015) Plant flavoprotein photoreceptors. Plant Cell Physiol. 56, 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carbonnel, M. , Davis, P. , Roelfsema, M.R. , Inoue, S. , Schepens, I. , Lariguet, P. , Geisler, M. , Shimazaki, K. , Hangarter, R. and Fankhauser, C. (2010) The Arabidopsis PHYTOCHROME KINASE SUBSTRATE 2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 152, 1391–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarsy, E. and Fankhauser, C. (2009) Higher plants use LOV to perceive blue light. Curr. Opin. Plant Biol. 12, 69–74. [DOI] [PubMed] [Google Scholar]
- Demarsy, E. , Schepens, I. , Okajima, K. , Hersch, M. , Bergmann, S. , Christie, J.M. , Shimazaki, K. , Tokutomi, S. and Fankhauser, C. (2012) Phytochrome kinase substrate 4 is phosphorylated by the phototropin 1 photoreceptor. EMBO J. 31, 3457–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi, M. , Shigenaga, A. , Emi, T. , Kinoshita, T. and Shimazaki, K. (2004) A transgene encoding a blue‐light receptor, phot1, restores blue‐light responses in the Arabidopsis phot1 phot2 double mutant. J. Exp. Bot. 55, 517–523. [DOI] [PubMed] [Google Scholar]
- Gambale, F. and Uozumi, N. (2006) Properties of shaker‐type potassium channels in higher plants. J. Membr. Biol. 210, 1–19. [DOI] [PubMed] [Google Scholar]
- Gotoh, E. , Suetsugu, N. , Yamori, W. , Ishishita, K. , Kiyabu, R. , Fukuda, M. , Higa, T. , Shirouchi, B. and Wada, M. (2018) Chloroplast accumulation response enhances leaf photosynthesis and plant biomass production. Plant Physiol. 178, 1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov, A. and Blatt, M.R. (1998) Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA, 95, 4778–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov, A. and Blatt, M.R. (1999) A steep dependence of inward‐rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol. 119, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, F.Q. , Young, J. and Crawford, N.M. (2003) The nitrate transporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell, 15, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada, A. and Shimazaki, K. (2009) Measurement of changes in cytosolic Ca2+ in Arabidopsis guard cells and mesophyll cells in response to blue light. Plant Cell Physiol. 50, 360–373. [DOI] [PubMed] [Google Scholar]
- Harada, A. , Takemiya, A. , Inoue, S. , Sakai, T. and Shimazaki, K. (2013) Role of RPT2 in leaf positioning and flattening and a possible inhibition of phot2 signaling by phot1. Plant Cell Physiol. 54, 36–47. [DOI] [PubMed] [Google Scholar]
- Hashimoto, M. , Negi, J. , Young, J. , Israelsson, M. , Schroeder, J.I. and Iba, K. (2006) Arabidopsis HT1 kinase controls stomatal movements in response to CO2 . Nat. Cell Biol. 8, 391–397. [DOI] [PubMed] [Google Scholar]
- Hayashi, M. , Inoue, S. , Takahashi, K. and Kinoshita, T. (2011) Immonohistochemical detection of blue light‐induced phosphorylation of the plasma membrane H+‐ATPase in stomatal guard cells. Plant Cell Physiol. 52, 1238–1248. [DOI] [PubMed] [Google Scholar]
- Hayashi, M. , Inoue, S.I. , Ueno, Y. and Kinoshita, T. (2017) A Raf‐like protein kinase BHP mediates blue light‐dependent stomatal opening. Sci Rep. 7, 45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, M. , Sugimoto, H. , Takahashi, H. , Seki, M. , Shinozaki, K. , Sawasaki, T. , Kinoshita, T. and Inoue, S. (2020) Raf‐like kinases CBC1 and CBC2 negatively regulate stomatal opening by negatively regulating plasma membrane H+‐ATPase phosphorylation in Arabidopsis. Photochem Photobiol Sci. 19, 88–98. [DOI] [PubMed] [Google Scholar]
- Hiyama, A. , Takemiya, A. , Munemasa, S. , Okuma, E. , Sugiyama, N. , Tada, Y. , Murata, Y. and Shimazaki, K. (2017) Blue light and CO2 signals converge to regulate light‐induced stomatal opening. Nat Commun. 8, 1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, C.H. , Lin, S.H. , Hu, H.C. and Tsay, Y.F. (2009) CHL1 functions as a nitrate sensor in plants. Cell, 138, 1184–1194. [DOI] [PubMed] [Google Scholar]
- Hrabak, E.M. , Chan, C.W. , Gribskov, M. et al (2003) The Arabidopsis CDPK‐SnRK superfamily of protein kinases. Plant Physiol. 132, 666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada, S. , Ohgishi, M. , Mayama, T. , Okada, K. and Sakai, T. (2004) RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin1 in Arabidopsis thaliana . Plant Cell, 16, 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, S. and Kinoshita, T. (2017) Blue light regulation of stomatal opening and the plasma membrane H+‐ATPase. Plant Physiol. 174, 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, S. , Kinoshita, T. , Takemiya, A. , Doi, M. and Shimazaki, K. (2008a) Leaf positioning of Arabidopsis in response to blue light. Mol. Plant, 1, 15–26. [DOI] [PubMed] [Google Scholar]
- Inoue, S. , Kinoshita, T. , Matsumoto, M. , Nakayama, K.I. , Doi, M. and Shimazaki, K. (2008b) Blue light‐induced autophosphorylation of phototropin is a primary step for signaling. Proc. Natl Acad. Sci. USA, 105, 5626–5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, S. , Takemiya, A. and Shimazaki, K. (2010) Phototropin signaling and stomatal opening as a model case. Curr. Opin. Plant Biol. 13, 587–593. [DOI] [PubMed] [Google Scholar]
- Inoue, S. , Matsushita, T. , Tomokiyo, Y. , Matsumoto, M. , Nakayama, K.I. , Kinoshita, T. and Shimazaki, K. (2011) Functional analyses of the activation loop of phototropin2 in Arabidopsis . Plant Physiol. 156, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, S. , Iwashita, N. , Takahashi, Y. et al (2017) Brassinosteroid involvement in Arabidopsis thaliana stomatal opening. Plant Cell Physiol. 58, 1048–1058. [DOI] [PubMed] [Google Scholar]
- Kagawa, T. , Sakai, T. , Suetsugu, N. , Oikawa, K. , Ishiguro, S. , Kato, T. , Tabata, S. , Okada, K. and Wada, M. (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high‐light avoidance response. Science, 291, 2138–2141. [DOI] [PubMed] [Google Scholar]
- Kaiserli, E. , Sullivan, S. , Jones, M.A. , Feeney, K.A. and Christie, J.M. (2009) Domain swapping to assess the mechanistic basis of Arabidopsis phototropin 1 receptor kinase activation and endocytosis by blue light. Plant Cell, 21, 3226–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T.H. , Böhmer, M. , Hu, H. , Nishimura, N. and Schroeder, J.I. (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61, 561–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, T. and Shimazaki, K. (1999) Blue light activates the plasma membrane H+‐ATPase by phosphorylation of the C‐terminus in stomatal guard cells. EMBO J. 18, 5548–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, T. and Shimazaki, K. (2001) Analysis of the phosphorylation level in guard‐cell plasma membrane H+‐ATPase in response to fusicoccin. Plant Cell Physiol. 42, 424–432. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T. , Doi, M. , Suetsugu, N. , Kagawa, T. , Wada, M. and Shimazaki, K. (2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature, 414, 656–660. [DOI] [PubMed] [Google Scholar]
- Lariguet, P. , Schepens, I. , Hodgson, D. et al (2006) PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl Acad. Sci. USA, 103, 10134–10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaudy, A. , Vavasseur, A. , Hosy, E. , Dreyer, I. , Leonhardt, N. , Thibaud, J.B. , Véry, A.A. , Simonneau, T. and Sentenac, H. (2008) Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl Acad. Sci. USA, 105, 5271–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S.C. , Lan, W.‐Z. , Kim, B.‐G. , Li, L. , Cheong, Y.H. , Pandey, G.K. , Lu, G. , Buchanan, B.B. and Luan, S. (2007) A protein phosphorylation/dephosphorylation network regulates a plant potassium channel. Proc. Natl Acad. Sci. USA, 104, 15959–15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Kim, B.‐G. , Cheong, Y.H. , Pandey, G.K. and Luan, S. (2006) A Ca2+ signaling pathway regulates a K+ channel for low‐K response in Arabidopsis . Proc. Natl Acad. Sci. USA, 103, 12625–12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, S. (2008) The CBL‐CIPK network in plant calcium signaling. Trends Plant Sci. 14, 37–42. [DOI] [PubMed] [Google Scholar]
- Maierhofer, T. , Diekmann, M. , Offenborn, J.N. , Lind, C. , Bauer, H. , Hashimoto, K. , Al‐Rasheid, S. , Luan, S. , Kudla, J. , Geiger, D. and Hedrich, R. (2014) Site‐ and kinase‐specific phosphorylation‐mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Sci. Signal. 7, ra86. [DOI] [PubMed] [Google Scholar]
- Marten, H. , Hedrich, R. and Roelfsema, M.R. (2007) Blue light inhibits guard cell plasma membrane anion channels in a phototropin‐dependent manner. Plant J. 50, 29–39. [DOI] [PubMed] [Google Scholar]
- Marten, I. , Deeken, R. , Hedrich, R. and Roelfsema, M.R. (2010) Light‐induced modification of plant plasma membrane ion transport. Plant Biol. 12(s1), 64–79. [DOI] [PubMed] [Google Scholar]
- McAinsh, M. , Brownlee, C. and Hetherington, A. (1990) Abscisic acid‐induced elevation of cytosolic free Ca2+ precedes stomatal closure. Nature, 343, 186–188. [Google Scholar]
- Merlot, S. , Mustilli, A.C. , Genty, B. , North, H. , Lefebvre, V. , Sotta, B. , Vavasseur, A. and Giraudat, J. (2002) Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 30, 601–609. [DOI] [PubMed] [Google Scholar]
- Motchoulski, A. and Liscum, E. (1999) Arabidopsis NPH3: a NPH1 photoreceptor‐interacting protein essential for phototropism. Science, 286, 961–964. [DOI] [PubMed] [Google Scholar]
- Negi, J. , Matsuda, O. , Nagasawa, T. , Oba, Y. , Takahashi, H. , Kawai‐Yamada, M. , Uchimiya, H. , Hashimoto, M. and Iba, K. (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature, 452, 483–486. [DOI] [PubMed] [Google Scholar]
- Nemoto, K. , Seto, T. , Takahashi, H. , Nozawa, A. , Seki, M. , Shinozaki, K. , Endo, Y. and Sawasaki, T. (2011) Autophosphorylation profiling of Arabidopsis protein kinases using the cell‐free system. Phytochemistry, 72, 1136–1144. [DOI] [PubMed] [Google Scholar]
- Nieves‐Cordones, M. , Caballero, F. , Martínez, V. and Rubio, F. (2012) Disruption of the Arabidopsis thaliana inward‐rectifier K+ channel AKT1 improves plant responses to water stress. Plant Cell Physiol. 53, 423–432. [DOI] [PubMed] [Google Scholar]
- Palmgren, M.G. (2001) PLANT PLASMA MEMBRANE H+‐ATPases: powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 817–845. [DOI] [PubMed] [Google Scholar]
- Ragel, P. , Ródenas, R. , García‐Martín, E. et al (2015) The CBL‐interacting protein kinase CIPK23 regulates HAK5‐mediated high‐affinity K+ uptake in Arabidopsis roots. Plant Physiol. 169, 2863–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, T. , Kagawa, T. , Kasahara, M. , Swartz, T.E. , Christie, J.M. , Briggs, W.R. , Wada, M. and Okada, K. (2001) Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA, 98, 6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, T. , Wada, T. , Ishiguro, S. and Okada, K. (2000) RPT2: a signal transducer of the phototropic response in Arabidopsis . Plant Cell, 12, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto, K. and Briggs, W.R. (2002) Cellular and subcellular localization of phototropin 1. Plant Cell, 14, 1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Barrena, M.J. , Chaves‐Sanjuan, A. , Raddatz, N. , Mendoza, I. , Cortes, A. , Gago, F. , Gonzalez‐Rubio, J.M. , Benavente, J.L. , Quintero, F.J. , Pardo, J.M. and Albert, A. (2020) Recognition and activation of the plant AKT1 potassium channel by the kinase CIPK23. Plant Physiol. 182, 2143–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal, S.K. , Pandey, A. and Pandey, G.K. (2015) The CBL‐CIPK signaling module in plants: a mechanistic perspective. Physiol Plant., 155, 89–108. [DOI] [PubMed] [Google Scholar]
- Sawasaki, T. , Ogasawara, T. , Morishita, R. and Endo, Y. (2002) A cell‐free protein synthesis system for high‐throughput proteomics. Proc. Natl Acad. Sci. USA, 99, 14652–14657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawasaki, T. , Hasegawa, Y. , Morishita, R. , Seki, M. , Shinozaki, K. and Endo, Y. (2004) Genome‐scale, biochemical annotation method based on the wheat germ cell‐free protein synthesis system. Phytochemistry, 65, 1549–1555. [DOI] [PubMed] [Google Scholar]
- Sawasaki, T. , Gouda, M.D. , Kawasaki, T. , Tsuboi, T. , Tozawa, Y. , Takai, K. and Endo, Y. (2005) The wheat germ cell‐free expression system: methods for high‐throughput materialization of genetic information. Methods Mol. Biol. 310, 131–144. [DOI] [PubMed] [Google Scholar]
- Schindelin, J. , Arganda‐Carreras, I. , Frise, E. et al (2012) Fiji: an open‐source platform for biological‐image analysis. Nat. Methods, 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel, J. , Hombach, P. , Waksman, T. , Giuriani, G. , Petersen, J. and Christie, J.M. (2018) A chemical genetic approach to engineer phototropin kinases for substrate labeling. J Biol Chem. 293, 5613–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I. , Raschke, K. and Neher, E. (1987) Voltage dependence of K+ channels in guard‐cell protoplasts. Proc. Natl Acad. Sci. USA, 84, 4108–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I. and Hedrich, R. (1989) Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem. Sci. 14, 187–192. [DOI] [PubMed] [Google Scholar]
- Schroeder, J.I. and Hagiwara, S. (1990) Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid: activation of nonselective Ca2+ permeable channels. Proc. Natl Acad. Sci. USA, 87, 9305–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I. , Allen, G.J. , Hugouvieux, V. , Kwak, J.M. and Waner, D. (2001) Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627–658. [DOI] [PubMed] [Google Scholar]
- Schumacher, P. , Demarsy, E. , Waridel, P. , Petrolati, L.A. , Trevisan, M. and Fankhauser, C. (2018) A phosphorylation switch turns a positive regulator of phototropism into an inhibitor of the process. Nat. Commun. 9, 2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki, K. , Iino, M. and Zeiger, E. (1986) Blue light‐dependent proton extrusion by guard‐cell protoplasts of Vicia faba . Nature, 319, 324–326. [Google Scholar]
- Shimazaki, K. , Doi, M. , Assmann, S.M. and Kinoshita, T. (2007) Light regulation of stomatal movement. Annu. Rev. Plant Biol. 58, 219–247. [DOI] [PubMed] [Google Scholar]
- Suetsugu, N. , Kagawa, T. and Wada, M. (2005) An auxilin‐like J‐domain protein, JAC1, regulates phototropin‐mediated chloroplast movement in Arabidopsis. Plant Physiol. 139, 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyroki, A. , Ivashikina, N. , Dietrich, P. et al (2001) KAT1 is not essential for stomatal opening. Proc. Natl Acad. Sci. USA, 98, 2917–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H. , Nozawa, A. , Seki, M. , Shinozaki, K. , Endo, Y. and Sawasaki, T. (2009) A simple and high‐sensitivity method for analysis of ubiquitination and polyubiquitination based on wheat cell‐free protein synthesis. BMC Plant Biol. 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y. , Ebisu, Y. , Kinoshita, T. , Doi, M. , Okuma, E. , Murata, Y. and Shimazaki, K. (2013) bHLH transcription factors that facilitate K+ uptake during stomatal opening are repressed by abscisic acid through phosphorylation. Sci. Signal. 6, ra48. [DOI] [PubMed] [Google Scholar]
- Takemiya, A. , Inoue, S. , Doi, M. , Kinoshita, T. and Shimazaki, K. (2005) Phototropins promote plant growth in response to blue light in low light environments. Plant Cell, 17, 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya, A. , Kinoshita, T. , Asanuma, M. and Shimazaki, K. (2006) Protein phosphatase 1 positively regulates stomatal opening in response to blue light in Vicia faba . Proc. Natl Acad. Sci. USA, 103, 13549–13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemiya, A. , Sugiyama, N. , Fujimoto, H. , Tsutsumi, T. , Yamauchi, S. , Hiyama, A. , Tada, Y. , Christie, J.M. and Shimazaki, K. (2013) Phosphorylation of BLUS1 kinase by phototropins is a primary step in stomatal opening. Nat. Commun. 4, 2094. [DOI] [PubMed] [Google Scholar]
- Tsutsumi, T. , Takemiya, A. , Harada, A. and Shimazaki, K. (2013) Disruption of ROOT PHOTOTROPISM2 gene does not affect phototropin‐mediated stomatal opening. Plant Sci. 201–202, 93–97. [DOI] [PubMed] [Google Scholar]
- Ueno, K. , Kinoshita, T. , Inoue, S. , Emi, T. and Shimazaki, K. (2005) Biochemical characterization of plasma membrane H+‐ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol. 46, 955–963. [DOI] [PubMed] [Google Scholar]
- Vahisalu, T. , Kollist, H. , Wang, Y.F. et al (2008) SLAC1 is required for plant guard cell S‐type anion channel function in stomatal signalling. Nature, 452, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry, A. and Sentenac, H. (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 54, 575–603. [DOI] [PubMed] [Google Scholar]
- Walter, M. , Chaban, C. , Schütze, K. et al (2004) Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Chen, Z.H. , Zhang, B. , Hills, A. and Blatt, M.R. (2013) PYR/PYL/RCAR abscisic acid receptors regulate K+ and Cl‐ channels through reactive oxygen species‐mediated activation of Ca2+ channels at the plasma membrane of intact Arabidopsis guard cells. Plant Physiol. 163, 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Li, H.‐D. , Chen, L.‐Q. , Wang, Y. , Liu, L.‐L. , He, L. and Wu, W.‐H. (2006) A protein kinase, interacting with two calcineurin B‐like proteins, regulates K+ transporter AKT1 in Arabidopsis . Cell, 125, 1347–1360. [DOI] [PubMed] [Google Scholar]
- Yamauchi, S. , Takemiya, A. , Sakamoto, T. , Kurata, T. , Tsutsumi, T. , Kinoshita, T. and Shimazaki, K. (2016) Plasma membrane H+‐ATPase (AHA1) plays a major role in Arabidopsis thaliana for stomatal opening in response to blue light. Plant Physiol. 171, 2731–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Y. , Adachi, Y. , Ye, W. , Hayashi, M. , Nakamura, Y. , Kinoshita, T. , Mori, I.C. and Murata, Y. (2013) Difference in abscisic acid perception mechanisms between closure induction and opening inhibition of stomata. Plant Physiol. 163, 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger, E. and Hepler, P.K. (1977) Light and stomatal function: blue light stimulates swelling of guard cell protoplasts. Science, 20, 887–889. [DOI] [PubMed] [Google Scholar]
- Zhao, X. , Qiao, X. , Yuan, J. , Ma, X. and Zhang, X. (2012) Nitric oxide inhibits blue light‐induced stomatal opening by regulating the K+ influx in guard cells. Plant Sci. 184, 29–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Representative confocal images of reconstitution of YFP fluorescence upon interaction between phot1 and CIPK23 and corresponding negative controls.
Figure S2. BiFC analysis showing the interactions between phototropins and CIPK24 and CIPK24 homodimerization in Nicotiana benthamiana leaf epidermal cells.
Figure S3. Transfer DNA insertional mutants used in this study and expression of CIPK23 in various tissues.
Figure S4. Blue light‐induced activation of the plasma membrane H+‐ATPase in GCPs from wild‐type (Col) and cipk23‐5 plants.
Figure S5. Stomatal opening in response to fusicoccin in darkness.
Figure S6. Effects of blue light on whole‐cell inward‐rectifying K+ channel currents in wild‐type (Col), cipk23‐5, and blus1‐3 GCPs.
Figure S7. CIPK23 is not phosphorylated by phot1 in the in vitro kinase assay.
Figure S8. Phylogenetic relationships among Arabidopsis thaliana CIPK family members.
Figure S9. Gene expression levels of CIPK23, CIPK3, CIPK9, CIPK26, CIPK6, and CIPK16 in guard cells.
Figure S10. Proposed blue light signalings in guard cells.
Table S1. Blue light‐dependent H+‐pumping in GCPs from wild‐type (Col) and cipk23‐5 plants.
Data Availability Statement
All relevant data are included in the manuscript and its supporting materials.


