Abstract
Eicosanoids modulate both innate and adaptive immune responses in Mycobacterium tuberculosis (Mtb) infection and have been suggested as possible Host Directed Therapy (HDT) targets, but more knowledge of eicosanoid dynamics in Mtb infection is required. We investigated the levels and ratios of eicosanoid mediators and their cellular sources, monocyte subsets and CD4 T cells in Tuberculosis (TB) patients with various clinical states of Mtb infection. Patients consenting to prospective enrolment in a TB quality registry and biorepository, 16 with pulmonary TB (before and at-end-of treatment), 14 with extrapulmonary TB and 17 latently infected (LTBI) were included. Plasma levels of Prostaglandin E2 (PGE2), Lipoxin A4 (LXA4), and Leukotriene B4 (LTB4) were measured by enzyme-linked immunosorbent assay. Monocyte subsets and CD4 T cells and their expression of Cyclooxygenase-2 (COX-2), Prostaglandin receptor EP2 (EP2), and 5-Lipoxygenase (5-LOX) were analyzed by flow cytometry with and without Purified Protein Derivate (PPD)-stimulation. Pulmonary TB patients had elevated levels of the anti-inflammatory mediator LXA4 at diagnosis compared to LTBI (p < 0.01), while levels of PGE2 and LTB4 showed no difference between clinical states of Mtb infection. LTB4 was the only mediator to be reduced upon treatment (p < 0.05), along with the ratio LTB4/LXA4 (p < 0.01). Pulmonary TB patients had higher levels of total monocytes at diagnosis compared to end-of-treatment and LTBI (both p < 0.05), and a relative increase in the classical monocyte subset. All monocyte subsets had low basal expression of COX-2 and 5-LOX, which were markedly increased upon PPD stimulation. By contrast, the expression of EP2 was reduced upon stimulation. CD4 T cells expressed low basal COX-2 activity that increased modestly upon stimulation, whereas their basal expression of 5-LOX was considerable. In conclusion, the level of eicosanoids in plasma seem to vary between clinical states of Mtb infection. Mediators in the eicosanoid system are present in monocytes and CD4 T cells. The expression of eicosanoids in monocytes are responsive to mycobacterial stimulation independent of Mtb disease state, but subsets are heterogeneous with regard to eicosanoid-mediator expression. Further exploration of eicosanoid mediators as targets for HDT in TB are warranted.
Keywords: tuberculosis, eicosanoids, prostaglandins, leukotrienes, lipoxins, monocytes, T cells, host-directed therapy
Introduction
Globally, nearly 10 million new cases of tuberculosis (TB) resulting in 1.3 million deaths, are reported annually despite intensive global strategies to fight the TB epidemic (1, 2). The sustained success of the causative agent, Mycobacterium Tuberculosis (Mtb), is partly explained by complex and multifactorial mechanisms to avoid, evade and subvert host immune responses (3–7).
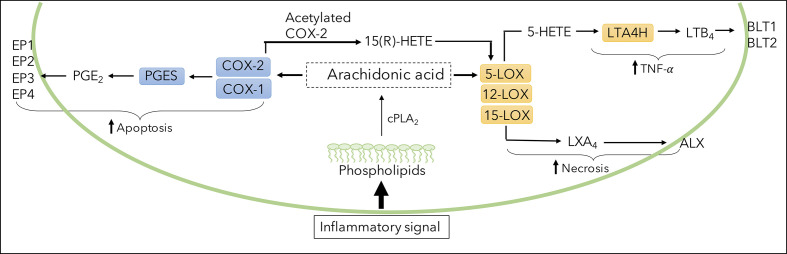
Host-directed therapy (HDT), aiming to enhance host immune responses and modulate Mtb-induced inflammation, is a possible approach to improve treatment outcomes and contribute to shorter treatment regimens (8–10). Lipid mediators of the eicosanoid family are suggested as possible HDT targets (11–14). Eicosanoid biosynthesis consists of several pathways ( Figure 1 ). Breakdown of Arachidonic Acid (AA), an integral part of all cell membranes, leads to production of lipid mediators through the gate-keeping enzymes Cyclooxygenase-2 (COX-2) and 5-lipooxygenase (5-LOX). Generated through COX-2, Prostaglandin E2 (PGE2) acts through four distinct receptors, of which Prostaglandin E2 receptor 2 (EP2) seems to play a role in host susceptibility to Mtb infection (15, 16). In parallel, 5-LOX generates pro-inflammatory leukotrienes and anti-inflammatory lipoxins, of which Leukotriene B4 (LTB4) and Lipoxin A4 (LXA4) seem to modulate innate and adaptive immune responses by exerting pro-inflammatory or pro-resolution effects (17–19). In Mtb infection, the dynamics between eicosanoids may impact on protective host immune responses important for containment or progression of TB disease (6, 14, 20). Disease severity in TB has been reported to be associated with increased ratio of LXA4/LTB4 and/or reduced ratio of PGE2/LXA4, rather than changes in absolute levels of specific metabolites (21, 22). Still, it is unclear whether eicosanoids can act as biomarkers, separating latent from active TB cases or reflecting responses to TB therapy (22).
Figure 1.
Illustration of eicosanoid biosynthesis. Formation of PGE2, LTB4, and LXA4 through breakdown of arachidonic acid (AA) by cyclooxygenase (COX) and lipoxygenase (LOX) pathways. COX enzymes (constitutive COX-1 or inducible COX-2) and downstream PGES generates PGE2 which binds to various receptors (EP1-EP4). 5-LOX-derived LTB4 is produced via the precursor 5-HETE, converted by enzyme LTA4H and exerts effects through receptors BLT1 and BLT2. LXA4 is derived from LOX enzymes 5,12 and 15-LOX and binds to the ALX receptor.
Macrophages, innate immune cells of critical importance in TB pathogenesis and disease, are major producers of eicosanoids (8, 23). Monocytes are macrophage precursors circulating in peripheral blood and are divided into different subsets; classical monocytes (CD14++CD16-), intermediate monocytes (CD14++, CD16+), and non-classical monocytes (CD14+CD16++) (24). These subsets harbor diverging biological roles in antigen presentation, phagocytosis and production of inflammatory mediators during Mtb infection (4) suggesting an influence on innate and adaptive host responses (25), of which effector CD4 T-cells exert crucial functions in anti-Mtb host defense by facilitating macrophage activation (5, 26, 27).
We hypothesized an imbalance in the levels of eicosanoids secondary to Mtb-induced increased COX-2 activity. We therefore explored absolute levels of lipid mediators and their ratios in plasma in different clinical states of Mtb infection. Further, we mapped the expression and function of relevant eicosanoid enzymes and receptors in monocyte subsets and T cells in active TB compared to latently infected individuals (LTBI). We also analyzed changes during TB treatment to better understand eicosanoid-mediated immunomodulatory mechanisms in Mtb infection and disease.
Materials and Methods
Study Population
Participants aged 18–70 years diagnosed with pulmonary TB (PTB), extrapulmonary TB (EPTB) or latent TB infection (LTBI) were recruited from the Dep. of Infectious Diseases and Dep. of Pulmonary Medicine at the Oslo University Hospital into a prospective observational cohort study (Prognostic Immunological markers in Tuberculosis). The TB diagnosis was based on positive sputum smears for acid fast bacilli, radiological findings, and/or Mtb positive cultures. MDR-TB and disseminated TB cases were excluded. All patients were treated with standard TB therapy following WHO guidelines: intensive phase of 2 months with 4 anti-TB drugs (isoniazid (INH), rifampicin (RIF), ethambutol (EMB), pyrazinamide (PZA)) and a continuation phase of 4 months with 2 anti-TB drugs. All patients received and responded to standard TB therapy for 6–12 months. LTBI were defined by positive QuantiFERON®-TB In Tube assay (> 0.70 IU/ml), absence of TB suspected clinical symptoms and, if relevant, negative Mtb cultures. Blood samples and clinical information was collected at diagnosis and, for patients with PTB disease, also at the end of anti-TB therapy after 6–12 months. To obtain an immunologically more homogenous patient cohort, exclusion criteria for all participants were HIV infection, diabetes, immunosuppressive diseases, and/or use of immunosuppressive medication.
Eicosanoid Measurements in Plasma
Blood samples collected in EDTA vacutainers were centrifuged immediately for 20 min at 2,000 g, snap-frozen and stored at -80°C until analysis. Using a competitive parameter immunoassay, human plasma concentrations of LTB4 and PGE2 were quantified using commercial EIA kits (Cayman chemical, Ann Harbour, MI). Plasma concentrations of LXA4 were assessed using enzyme immunoassay (EIA) kits (Oxford Biomedical Research, Oxford, MI). All assays were performed according to manufacturer’s instructions. Briefly, samples underwent extraction protocols using C18-SPE Cartridges (Waters inc) or extraction using acetone precipitation, acidification and ethyl acetate extraction prior to analysis. Samples were then run in duplicates and optical density was determined at 450 or 650 nm using a Spectramax Abs plus microplate reader (Molecular devices Corporation). Lipid concentrations were calculated based on a sigmoidal standard curve using a 4-Parameter logistic fit. Inter-assay and intra-assay controls were included in all experiments.
Cell Preparation and Stimulation
Peripheral blood mononuclear cells (PBMC) drawn on cell preparation tubes, CPT (BD Biosciences, San Jose, CA), were isolated by centrifugation and cryopreserved in 20% DMSO/80% fetal calf serum at -150 °C until analysis. Cryopreserved PBMCs were thawed in a water bath at 37°C and washed with pre-warmed RPMI 1640 (Sigma-Aldrich) media supplemented with 1% L-glutamine and 10% fetal calf serum and rested for 2h prior to cell counts and viability check (Tryphan blue microscopy). Only samples with >80% viability were included in analysis. PBMC (8x105 cells) were either unstimulated or stimulated with 10 ug/ml Purified protein derivative (PPD, SSI, Denmark) and placed in a 37°C degree (with 5% CO2) incubator overnight (18h). Cells were subsequently stained with either a monocyte or a T cell antibody panel (See Supplementary Tables 1 and 2 ).
Flow Cytometry Analyses
Cells were washed, stained and incubated for 30 min at 4°C. The monocyte panel consisted of Fixable Viability Dye 660, HLA-DR Alexa 700, Lineage cocktail 1 (CD3, CD20, CD56, and CD19) APC, CD14 PerCP, CD16 BV605, and EP2 PE. The T cell panel consisted of Fixable Viability Dye 660, CD3 PerCP, CD4 Alexa 700, EP2 PE. Then, cells were fixed and permeabilized according to the manufacturer’s instructions (T cell panel with eBioscience FoxP3 transcriptional factor staining kit and monocyte panel with Cytofix/cytoperm staining kit, BD Bioscience) and subsequently stained for intracellular markers with conjugated antibodies (COX-2 FITC). Unconjugated antibody for 5-LOX were stained for 30 min at 4°C, followed by 2x wash with PBS and an additional incubation with Alexa Fluor® 405-conjugated secondary antibody (Abcam) was performed for another 30 min at 4°C. Flow cytometry (FACS Canto II, BD Biosciences) was performed in a blinded random order to remove any analysis bias. Fluorescence minus one (FMO) were used for gating of COX-2, EP2, 5-LOX ( Supplementary Figure 1C ). Total monocytes (HLA-DR+ cells gated from the monocyte cloud) were further gated into the monocyte subsets classical monocytes (CD14++CD16-), Intermediate monocytes (CD14++, CD16+) and non-classical monocytes (CD14+CD16++) ( Supplementary Figure 1A ) (24). T cells were gated from live CD3 and CD4 positive cells with measurement of COX-2, EP2 and 5-LOX ( Supplementary Figure 1B ). Data are given as frequencies or mean fluorescent intensity (MFI). Flow analysis was performed using FlowJo software (Tree Star Inc.).
Statistical Analysis
All data are expressed with median and interquartile range (IQR). Non-parametrical statistical methods were applied. For ELISA quantification, p-values were calculated using the Kruskal-Wallis test with Dunn’s post-hoc for multiple comparisons and Wilcoxon signed rank test was used for paired samples. For flow cytometry data, Mann-Whitney U test was used for groupwise comparison of unpaired data and Wilcoxon for matched pair test by using Graphpad Prism version 8.0 (Graphpad Software, LA Jolla, CA) and SPSS (IBM).
Ethical Considerations
The study was approved by the Regional Committees for Ethics in Medical Research (REK-Sør-Øst 2016/2123). Biobank samples were collected and stored in the “Research Biobank Infectious Diseases” (“Forskningsbiobank Infeksjonssykdommer” (REK 1.2006.181-S-0885, SHDNR. 09/513), Department of Infectious Diseases, OUS, Ullevål. Written informed consent was obtained from all participants before inclusion.
Results
Study Participants Characteristics
Thirty patients with TB disease (14 patients with EPTB and 16 patients with PTB) and 17 patients with LTBI, were included. Demographic and clinical variables of Mtb infected patients are listed in Table 1 .
Table 1.
Demographic and clinical variables in patients with Mtb infection and disease.
| EPTB n = 14 | PTB N = 16 | LTBI N = 17 | |
|---|---|---|---|
| Gender, male (n, %) | 9 (64) | 13 (81) | 6 (35) |
| Age (median, range) | 30 (18–40) | 32 (18–64) | 29 (20–54) |
| Ethnicity (n, %) | |||
| Caucasian | 3 (21) | 6 (37) | 0 (0) |
| African | 8 (57) | 3 (19) | 5 (29) |
| Asian | 2 (14) | 6 (37) | 9 (52) |
| Unknown | 1 (7) | 1 (6) | 3 (18) |
| Previous TB treatment (n, %) | |||
| Yes | 1 (7) | 2 (13) | 0 (0) |
| Unknown | 2 (14) | 1 (6) | 0 (0) |
| Confirmed Mtb complex in culture/PCR (%) | |||
| Yes | 8 (57) | 13 (81) | 0 (0) |
| Resistance (%) | |||
| Monoresistant TB1 | 5 (36) | 2 (13) | |
| No of TB localizations (%) | |||
| 1 | 12 (86) | 13 (81) | |
| 2 | 2 (14) | 3 (19) | |
| Low: High symptom score2 | 7:7 | 5:11 | 16:1 |
| QuantiFERON-TB Gold (positive: negative: no data) | 12:0:2 | 9:3:4 | 17:0:0 |
| ESR3 at baseline (mm/hour, range) | 32 (5–82) | 35 (3–109) | 21 (13–40) |
| CRP3 at baseline (mg/L, range) | 17 (1–89) | 19 (0.6–97) | 3 (0.7–10) |
1Monoresistance is defined as resistance to one first-line anti-TB drug only.
2High symptom score is defined as 2 or more of the following symptoms: Fever (>38.0°), weight loss, cough, lymphadenopathy, night sweat. Low symptom score is defined as one of the symptoms listed or asymptomatic.
3ESR, erythrocyte sedimentation rate; CRP, C- reactive protein.
Levels of Plasma Eicosanoids During Different Stages of Mtb Infection
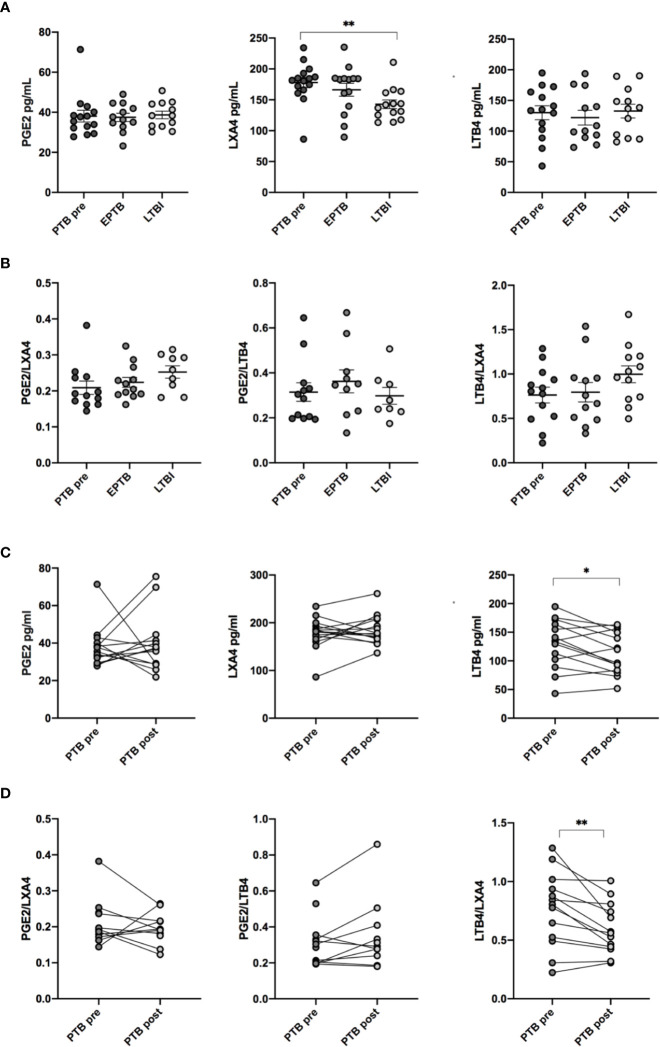
Plasma levels of PGE2, LXA4, and LTB4 were analyzed at diagnosis of TB disease (PTB and EPTB) and compared to LTBI. No significant differences in either PGE2 or LTB4 levels between the groups were observed ( Figure 2A ). In contrast, concentrations of the anti-inflammatory LXA4 were elevated in PTB compared to LTBI (p < 0.01). Concentrations of PTB and EPTB were comparable. Next, the effects of eicosanoids may depend on their relative contribution rather than absolute levels. We therefore investigated the following eicosanoid ratios: PGE2:LXA4, PGE2:LBT4 and LTB4:LXA4 ( Figure 2B ). However, there were no significant differences in either PGE2:LXA4, PGE2:LTB4 or LTB4/LXA4 ratios between the various groups at baseline.
Figure 2.
Concentration and ratio of LXA4, PGE2 and LTB4 in plasma. (A) Concentrations of eicosanoid metabolites in pg/ml in plasma measured by ELISA at different stages of Mtb infection: EPTB (N = 14), PTB at time of diagnosis (PTB pre, N = 16), and LTBI (N = 17) (B) Ratio of eicosanoid metabolites in plasma in different stages of Mtb infection. (C) Levels of eicosanoid metabolites before (PTB pre, N = 16) and after treatment (PTB post, N = 16). (D) Ratios of eicosanoids before and after treatment. Data presented as median with interquartile ranges. P-values were calculated using the Kruskal-Wallis test with Dunn’s post-hoc for multiple comparisons and Wilcoxon signed rank test was used for paired samples. Statistical significance represented by asterisk: ns, not significant; *p < 0.05; **p < 0.01.
During TB treatment, LTB4 levels decreased significantly in PTB patients (p < 0.05), whereas LXA4 and PGE2 levels did not change ( Figure 2C ). LTB4/LXA4 ratio was reduced during treatment (p < 0.01), while PGE2/LXA4 and PGE2/LTB4 ratio showed no difference ( Figure 2D ).
Eicosanoid balance may be affected by host genomic background and genetic polymorphism (28), we therefore investigated levels and ratios of eicosanoids by stratifying patients according to ethnicity (Caucasian, African, and Asian), but no differences were detected ( Supplementary Figure 2 ).
Monocytes, Monocyte Subsets, and Expression of COX-2, EP2, and 5-LOX
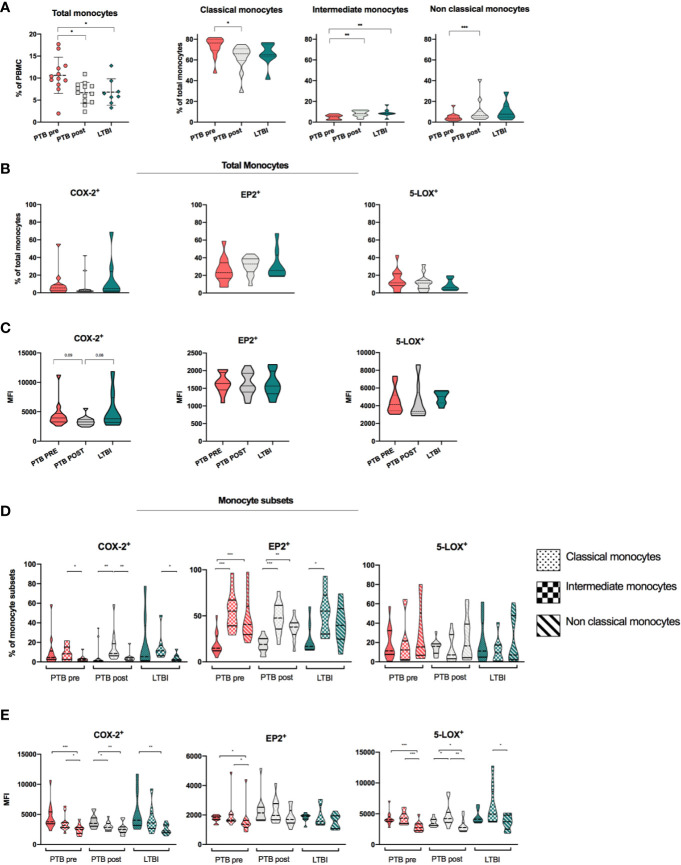
We found increased frequencies of total monocytes in PTB at diagnoses compared to LTBI (11 vs 6.5%, p < 0.05) and with a significant reduction at end-of-treatment (p < 0.05) ( Figure 3A ). The distribution of monocyte subsets revealed higher frequencies of CD14++CD16- classical monocytes (CM) in PTB at diagnosis compared to LTBI, with a significant reduction during treatment (p <0.05, median 76% in PTB at time of diagnosis vs median 66% in PTB at end-of –treatment). Frequencies of both CD14++CD16+ intermediate monocytes (IM) and CD14+CD16++non-classical monocytes (NCM) were low in PTB at diagnosis, however with a significant increase in both IM and NCM at end-of-treatment (p <0.01 and p <0.001, respectively). LTBI had significantly higher frequencies of IM compared to PTB at diagnosis (p < 0.01) ( Figure 3A ).
Figure 3.
Expression of monocytes, monocyte subsets and eicosanoid expression in stages of Mtb infection. (A) Distribution and frequencies of monocytes and monocyte subsets in different clinical stages of Mtb infection. PTB at time of diagnosis (PTB pre, pink, N = 13), PTB at end- of- treatment (PTB post, grey, N = 13), and LTBI (green, n = 8). Monocyte subsets were defined as classical monocytes (CD14++CD16-), Intermediate monocytes (CD14++CD16+) and non-classical monocytes (CD14+CD16++) (Gating strategy Supplementary Figure 1A ) (29). (B) Frequencies of COX-2, EP2, and 5-LOX in unstimulated samples in total monocytes. (C) Mean fluorescence intensity (MFI) of COX-2, EP2, and 5-LOX in unstimulated samples in total monocytes. Frequencies (D) and MFI (E) of COX-2, EP2, and 5-LOX in unstimulated classical, intermediate, and non-classical monocyte subsets, presented by fill pattern, stratified by patient group (color). Mann-Whitney test was used for unpaired samples, Wilcoxon signed rank test used for paired samples. Statistical significance represented by asterisk: *p < 0.05; **p < 0.01; ***p < 0.001.
We further analyzed the expression of COX-2, 5-LOX, and EP2 in total monocytes in different states of Mtb infection ( Figure 3B ). %COX-2 expression was low and comparable in PTB at diagnosis (5.5%) and LTBI (4.8%), and with no significant changes during TB treatment (PTB post: 2.5%). Levels of EP2 were higher (PTB Pre: 23.1%, PTB post: 32.9%, and LTBI 25.6%), while levels of 5-LOX were comparable to COX-2 (PTB Pre: 11.55%, PTB post: 11.2%, and LTBI 5.9%), with no significant differences between patient groups ( Figure 3B ). When comparing MFI levels, PTB at diagnosis and LTBI showed a trend of increased levels of COX-2 compared to PTB at end-of treatment (p = 0.09 and p = 0.08, respectively), while there were no differences in levels of EP2 and 5-LOX ( Figure 3C ).
As expression of eicosanoid enzymes may be unevenly distributed in different monocyte subsets, we further explored the eicosanoid expression between the CM, IM, NCM monocyte subsets in the different stages of Mtb infection. Overall, as shown for total monocytes, COX-2 expressing cells were low in all monocyte subsets, but significantly higher in IM subsets (8.3%) compared to NCM (2.3%) (P < 0.05) in PTB at diagnosis ( Figure 3D ). Expression of EP2 was modest in CM in all patient groups (PTB pre: 14.9, PTB post: 19.0, LTBI: 16.8). Interestingly, levels of EP2 were significantly elevated in both the IM and NCM subsets compared to CM in PTB at diagnosis and at end-of treatment (p < 0.001 and p < 0.01) ( Figure 3D ). There were no differences in frequencies of COX-2, EP2 and 5-LOX-expressing cells between patient groups ( Supplementary Figure 3 ). When investigating MFI levels, COX-2 levels were higher in CM compared to NCM in all groups (p <0.001, p <0.01, and p <0.01 for PTB pre, PTB post, and LTBI), while levels of 5-LOX were higher in CM and IM subsets compared to NCM in PTB at diagnosis and at end-of treatment (p < 0.001 and p < 0.05) ( Figure 3E ).
Dynamics of COX-2, EP2, and 5-LOX in Monocytes Upon In Vitro PPD Stimulation
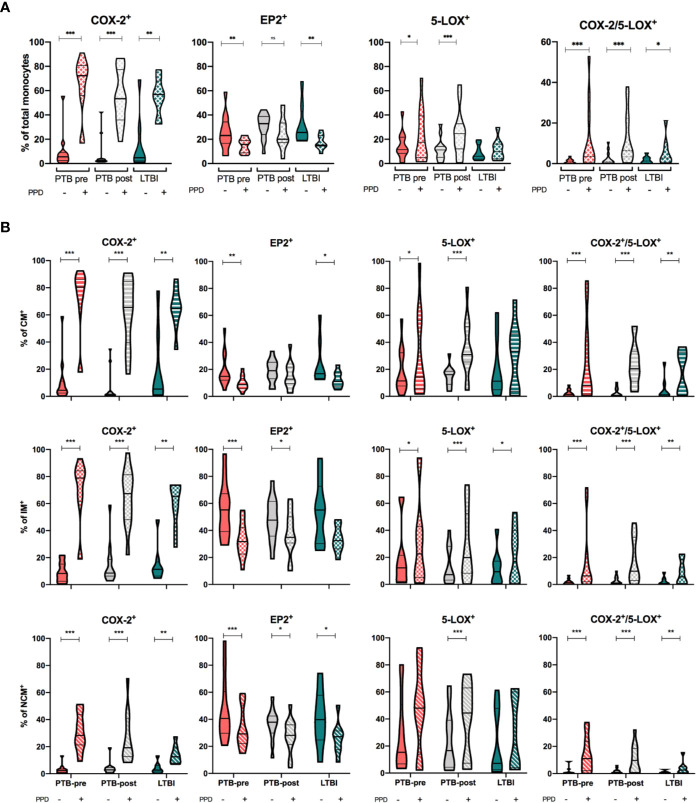
We then investigated how PPD-stimulation affected the expression of COX-2, 5-LOX and EP2 in monocytes and monocyte subsets. In total monocytes, COX-2-expression was significantly increased by PPD (p < 0.01), while EP2-expression was significantly downregulated for all groups (p < 0.01) except in PTB at end-of- treatment ( Figure 4A and Supplementary Figure 4 ). 5-LOX mimicked COX-2 responses and was significantly up-regulated in PTB at diagnosis and at end- of- treatment (p < 0.05 and p < 0.001) ( Figure 4A and Supplementary Figure 4 ). Next, we investigated co-expression of COX-2 and 5-LOX in unstimulated and stimulated monocytes. Frequency of double positive monocytes were low in all groups at baseline, but with significant upregulation by PPD in all groups (p <0.001, p<0.001, p <0.05 for PTB pre, PTB post, and LTBI, respectively) ( Figure 4A ).
Figure 4.
Eicosanoid expression in unstimulated and PPD-stimulated monocytes. (A) Expression of COX-2, EP2, 5-LOX and double positive (COX-2/5-LOX) expression in HLA-DR+ Total monocytes in unstimulated (filled bar) and stimulated (hatched bar) samples, stratified by patient group: PTB-pre (PTB at diagnosis, pink, n = 13), PTB post (PTB at end- of- treatment, grey, n = 13), and LTBI (latent TB, green, n = 8). (B) Expression of COX-2, EP2, 5-LOX, and double positive (COX-2/5-LOX) expression in unstimulated (filled bar) and stimulated (hatched bar) Classical (CD14++CD16-), Intermediate (CD14++CD16+) and non-classical monocytes (CD14+CD16++) stratified by patient group (color). Gating strategy is shown in Supplementary Figure 1 . Mann-Whitney test was used for unpaired samples, Wilcoxon signed rank test used for paired samples. Statistical significance represented by asterisk: *p < 0.05; **p < 0.01; ***p < 0.001.
Within the various monocyte subsets, PPD stimulation induced a significant increase in COX-2 expression regardless of group (p < 0.01), however most pronounced for CM and IM monocytes (p < 0.05) ( Figure 4B and Supplementary Figure 4 ). Downregulation of EP2 upon PPD stimulation was seen for all monocyte subsets in all patient groups, however most pronounced for the intermediate monocytes in PTB at diagnosis (p < 0.001) ( Figure 4B and Supplementary Figure 4 ). Although 5-LOX was upregulated upon PPD stimulation in several of the monocyte subsets, there were no differences in induced 5-LOX expression between subsets in any of the clinical states of Mtb infection ( Figure 4B and Supplementary Figure 4 ). Co-expression of COX-2/5-LOX was significantly upregulated in all subsets by PPD (p = 0.001, p = 0.001, and p = 0.01 in PTB pre, PTB post, and LTBI respectively) ( Figure 4B and Supplementary Figure 4 ).
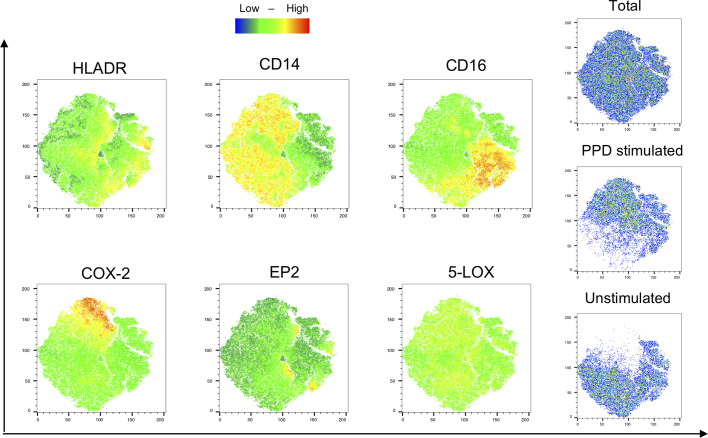
Next, we evaluated the monocyte population in unstimulated and stimulated samples from PTB patients at diagnosis by the t-distributed stochatic neighbouring embedding algorithm (t-SNE) for unbiased visualization of COX-2, EP2, and 5-LOX-expressing subpopulations ( Figure 5 ). This showed that EP2 is expressed by small clusters of cells in both unstimulated and stimulated regions, while 5-LOX was widely expressed throughout the monocyte populations. COX-2 is highly expressed in stimulated regions that also expressed high levels of CD14, and low levels of CD16. HLA-DR and CD14 were found in both stimulated and unstimulated regions of the monocyte population, while CD16 is mostly present in unstimulated samples ( Figure 5 ).
Figure 5.
Visualization of phenotypic changes with T-sne. Visualization of monocyte-related markers (HLA-DR, CD14, CD16) and the eicosanoid enzymes/proteins (COX-2, EP2, and 5-LOX) were analyzed with an unbiased approach using t-Distributed Stochastic Neighbor Embedding (tSNE) algorithm. Unstimulated and PPD-stimulated samples from 1 patient with PTB at diagnosis were concatenated and downsampled to 3*105 cells/group. Color scale denotes low (blue) to high (red) density of the markers.
Expression of Eicosanoid Enzymes in CD4 T Cells
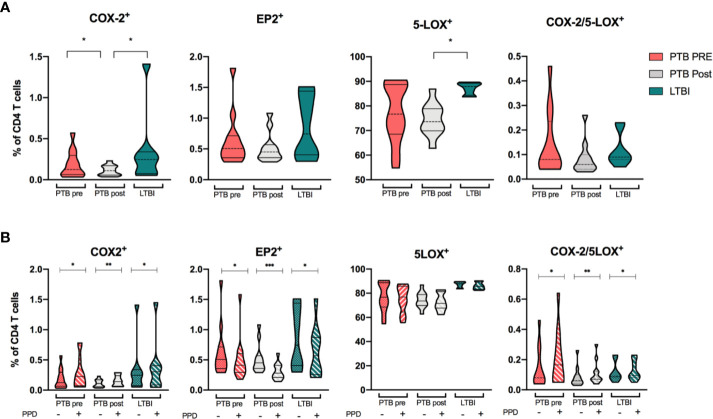
Since effector CD4 T-cells are important in anti-Mtb host defense we explored the relevance of this cell subset in eicosanoid biosynthesis by analyzing their expression of COX-2, EP2, and 5-LOX. Basal levels of COX-2 and EP2 expression in CD4 T cells were generally low for both PTB at diagnosis and LTBI, with even lower frequencies in the PTB group at end-of-treatment (p < 0.05). In contrast, 5-LOX–expression was high in all clinical groups, especially in LTBI ( Figure 6A ). Basal co-expression of COX-2/5-LOX was low in all groups with no significant differences between patient groups ( Figure 6A ). Next, we investigated the capacity of T cells to induce eicosanoid enzymes upon PPD-stimulation ( Figure 6B and Supplementary Figure 5 ). For all groups, COX-2 was modestly, but significantly induced (p <0.05, p <0.01 and p <0.05 for PTB pre, PTB post and LTBI, respectively), while EP2 expression decreased in all groups (p <0.05, p <0.001, and p <0.05). PPD-stimulation did not affect 5-LOX expression, but double positive COX-2/5-LOX T cells were significantly induced by stimulation (p <0.05, p <0.01, and p <0.05 for PTB pre, PTB post, and LTBI, respectively) ( Figure 6B ).
Figure 6.
Eicosanoid expression in CD4 T cells. (A) COX-2, EP2, 5-LOX, and double positive (COX-2/5-LOX) expression in unstimulated samples from patients with PTB at diagnosis (pink, n = 13), PTB at end-of-treatment (grey, n = 13), and LTBI (green, n = 8) in CD4 T cells. (B) Unstimulated (filled bar) and PPD-induced expression (hatched bar) of COX-2, EP2, 5-LOX, and COX-2/5-LOX in CD4 T cells. Wilcoxon matched-pairs signed rank test was used for paired data, Mann-Whitney for unpaired group comparisons. Statistical significance represented by asterisk: ns, not significant; *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
Several studies have emphasized the role of eicosanoids and lipid mediators in TB pathogenesis (30–32). We hypothesized that the AA-derived metabolites PGE2, LXA4, and LTB4 reflects the ongoing inflammation and resolution processes orchestrated by eicosanoids in TB. We report absolute levels and ratios of eicosanoids, and the expression of key enzymes and proteins within the eicosanoid network in monocyte subsets and CD4 T cells, in different states of Mtb infection of interest to future HDT strategies.
We show that patients with TB disease have elevated plasma levels of the anti-inflammatory mediator LXA4 compared to LTBI. Levels of LTB4 decreased during treatment, whereas LXA4-levels remained unchanged and consequently the ratio of LTB4/LXA4 was lower after anti-TB therapy. Further, patients with TB disease had higher frequencies of predominately classical monocytes. The monocyte subsets in all patient groups expressed low basal levels of eicosanoid proteins, but while COX-2 and 5-LOX increased upon PPD stimulation, EP2 was reduced. Our data demonstrates dynamic changes and an imbalance in eicosanoids in TB that is not fully restored after treatment.
Pulmonary TB Patients Exhibit a Relative Increase in Anti-Inflammatory Eicosanoids
We report that PTB patients exhibit a relative increase in the anti-inflammatory eicosanoid LXA4 compared to LTBI at diagnosis, which extends beyond treatment. This finding has also been reported in previous studies and may propose a role for LXA4 as a potential biomarker for TB disease (21, 22). Moreover, LXA4 has been linked to increased bacterial survival due to abrogated host immunity caused by promoted necrosis of infected macrophages and suppressed production of TNF-α (14, 18), suggesting a role for LXA4 in TB progression. By contrast, the pro-inflammatory mediator PGE2 has been suggested to confer protective responses during TB disease, by acting in a cross-regulatory network with IL-1 and promoting apoptosis of infected macrophages (20). Strict classification of PGE2 with regard to anti- or pro-inflammatory capacity is however less evident as PGE2 has been shown to harbor both pro- and anti-inflammatory effects depending on concentration and timing at local sites of infection (33–35). In contrast to other reports showing increased levels of PGE2 in TB disease (20–22), we observed no difference in PGE2-levels or PGE2:LXA4 ratio between patient groups. However, we observed reduced ratios of LTB4:LXA4 upon treatment, likely reflecting the reduced levels of LTB4 while LXA4 remained unchanged. In TB, an optimal balance of LTB4 is considered preferable, as high levels of this mediator tips the balance towards excess production of TNF-α, macrophage necrosis and enhanced growth of Mtb (18). Our data with no significant differences in levels of LXA4 and PGE2 during treatment of PTB, may be linked to treatment induced inflammatory imprinting of eicosanoid profiles, as shown in other studies (36, 37): Even though the inciting stimulus is cleared, the production of anti-inflammatory mediators may be extended and occur months after initial exposure (33, 37). Hence, future studies on dynamics of eicosanoids in Mtb infection should preferably secure samples 6–12 months after finalized TB treatment.
Monocyte Heterogeneity and Eicosanoid Signaling in Mtb Infection
We report increased frequencies of total monocytes in PTB at diagnosis compared to end-of-treatment and LTBI. Classical monocytes constituted the major subset regardless of clinical state, while frequencies of intermediate monocytes and non-classical monocytes were low but increase moderately during treatment. Monocytes are reported to expand during TB progression (38), and our data suggests that this expansion is mostly due to increased frequency of classical monocytes. However data are conflicting, and some report an association between CD16+ expansion (i.e. intermediate and non-classical monocytes) and disease severity (25, 38–40), whilst others have shown higher levels of CD16+ monocytes in LTBI patients (41). Notably, our study did not assess severity within PTB patients.
Although functions of monocyte subsets in Mtb infection are reported by others (42), evidence is scarce concerning their contribution in eicosanoid regulation. We report low basal expression of COX-2 in all monocyte subsets, but significantly higher expression in intermediate monocytes, possibly indicating higher production of PGE2 in this subset. Other studies have shown that intermediate monocytes produce high levels of TNF-α, IL-6 and IL-1, but reduced levels of IL-10 (42–44). Thus, the finding that intermediate monocytes express high frequencies of COX-2, as well as elevated expression of EP2 compared to classical subsets, may support the notion that these cells are important producers of inflammatory mediators in general. Further, high levels of EP2 in intermediate and non-classical monocytes suggests that these cells are more susceptible to PGE2 signaling, compared to classical monocytes, indicating a pivotal role for intermediate and non-classical monocytes in eicosanoid signaling.
COX-2 and 5-LOX in total monocytes and monocyte subsets are clearly increased upon mycobacterial stimulation, while expression of EP2 is reduced. As classical monocytes and intermediate monocytes are considered superior phagocytes and important for secreting pro-inflammatory cytokines, it is not surprising that these subsets show elevated levels of COX-2 upon stimulation (24, 45). However, the finding that 5-LOX mimics the upregulated response of COX-2, indicates that mycobacterial antigens promote parallel induction of both enzymes also reflected in the increased levels of the anti-inflammatory LXA4 in PTB patients, as shown in our study. Our results therefore support suggestions of mycobacterial antigens manipulating the host response toward further bacterial spread through eicosanoid imbalance resulting from 5-LOX-induction and generation of LXA4 (14). Further, we found that basal levels of monocytes co-expressing COX-2 and 5-LOX were low, albeit with a significant upregulation by PPD. This strengthens our findings that monocyte subsets differ in their contribution to eicosanoid production. Reduced EP2 expression upon PPD stimulation may indicate a negative feedback loop in Mtb infection resulting in reduced PGE2-effects, as suggested by others describing a strong regulatory connection between COX-2 and EP2 in PGE2 synthesis (46). EP2 is reported to be distinctly upregulated upon LPS stimulation (47), and is normally not internalized or desensitized upon stimulation (48, 49). Thus, our findings of a marked reduction in EP2 receptor upon PPD stimulation raise questions regarding the potential for COX inhibitors as HDT in TB as the natural increase in EP2 expression observed in other bacterial infections seems to be abrogated by Mtb.
Eicosanoid Expression in Cells of the Adaptive Immune Response
In accordance with others (50, 51), we demonstrate that eicosanoid enzymes are also expressed by CD4 T cells. The COX-2-gene is transcriptionally upregulated in human T cells during T cell receptor signaling in vitro (48). Although CD4 T cells express low basal COX-2 activity, expression is modestly induced upon stimulation with PPD, indicating a functional role in response to Mtb infection with CD4 T cells capable of PGE2 production. Surprisingly, CD4 T cells show very high levels of basal 5-LOX activity, which is seemingly unaffected by PPD stimulation. Although it has been shown that 5-LOX is expressed by T cells (51), to our knowledge, this has not previously been shown in the context of TB. 5-LOX expression in T cells may possibly suggest a capacity for CD4 T cells to produce the anti-inflammatory mediator LXA4 or the pro-inflammatory LTB4. The unexpectedly high levels of 5-LOX in CD4 T cells should be explored in future studies.
There are some limitations to our study. First, a small number of patients give reduced power in statistical calculations and small differences may not have been detected. Further, we have not analyzed genes important in eicosanoid biosynthesis, e.g lta4h locus polymorphism as shown in previous reports (18). Future studies on eicosanoid enzyme and receptor polymorphism, as well as expression of other key markers in eicosanoid biosynthesis involved in TB pathogenesis (e.g. LTA4H, EP1-3, 15-LOX, and PTGES), are warranted. Changes and dynamics in peripheral blood may not reflect immune responses at local sites of Mtb infection. Thus, our study design limits our possibility to conclude on causative factors for TB progression, but still highlights important aspects of eicosanoid biosynthesis in Mtb infection.
Concluding Remarks
In this study, we show that levels of eicosanoids in plasma vary between clinical states of Mtb infection with an increased anti-inflammatory profile in active TB disease. Imbalance of eicosanoids extends beyond treatment. Monocytes and CD4 T cells express mediators involved in eicosanoid signaling, but monocyte subgroups differ with regard to their responsiveness and contribution to eicosanoid mediators. PPD-stimulation induced changes in eicosanoid mediators that could represent a Mtb strategy to divert host immune responses, but this need further investigation. Causative or not, monocyte heterogeneity is likely to impact on TB pathogenesis by influencing the balance of mediators of the eicosanoid pathway, but more mechanistic in-depth studies are warranted to understand the full network interaction. Therefore, our data support future studies exploring the role of eicosanoid mediators in immunopathogenesis in Mtb infection and as potential targets for HDT strategies in TB.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Regional Committees for Ethics in Medical Research (REK-Sør-Øst 2016/2123). Biobank samples were collected and stored in the “Research Biobank Infectious Diseases” (“Forskningsbiobank Infeksjonssykdommer” (REK 1.2006.181-S-0885, SHDNR. 09/513), Department of Infectious Diseases, OUS, Ullevål. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
Conceived and designed the experiments: KN, MJ, KT, SJ, and AD-R. Recruited patients and collected clinical data: SJ, AD-R, and KT. Data acquisition: KN and MJ. Analyzed the data: KN, MJ, SJ, AD-R, and KT. Drafted and reviewed the manuscript: KN, MJ, SJ, AD-R, and KT. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by The Research Council of Norway through the Medical Student Research Program at the University of Oslo, and the Department of Infectious diseases, Oslo University Hospital.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all study participants. The staff at the laboratory and clinical wards of Dept. of Infectious Diseases and the TB diagnostic unit at Dept. of Pulmonary Medicine, OUH. In particular thanks to Birgitte Stiksrud, Mette Sannes, Kjerstin Røstad, Linda G. Skeie, Hallgeir Tveiten, Sarah Nur, Kjersti Sellæg, Helene Gjelsaas, Ida Østebø. Also, thanks to Ingebjørg Seljeflot at the Center for Clinical Heart Research, Dept. of Cardiology and Christian Thorstensen at Dept. of Pharmacology, OUH.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.579849/full#supplementary-material
Abbreviations
AA, Arachidonic acid; CM, Classical monocytes; COX-2, Cyclooxygenase 2; EP2, Prostaglandin E2 receptor 2; HDT, Host-directed therapy; IFN-γ, Interferon-γ; IM, Intermediate monocytes; IL-1, Interleukin 1; LTB4, Leukotriene B4; LXA4, Lipoxin A4; Mtb, Mycobacterium tuberculosis; NCM, Non-classical monocytes; PGE2, Prostaglandin E2; PBMC, Peripheral blood mononuclear cells; TB, Tuberculosis; TNF-α, tumor necrosis factor α; 5-LOX, 5-lipoxygenase.
References
- 1. Global tuberculosis report 2018 (2018). Licence: CCBY-NC-SA3.0IG.
- 2. Glaziou P, Floyd K, Raviglione MC. Global Epidemiology of Tuberculosis. Semin Respir Crit Care Med (2018) 39(3):271–85. 10.1055/s-0038-1651492 [DOI] [PubMed] [Google Scholar]
- 3. Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest (2005) 115(6):1601–6. 10.1172/JCI23949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marakalala MJ, Martinez FO, Pluddemann A, Gordon S. Macrophage heterogeneity in the immunopathogenesis of tuberculosis. Front Microbiol (2018) 9(1028). 10.3389/fmicb.2018.01028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mayer-Barber KD, Barber DL. Innate and Adaptive Cellular Immune Responses to Mycobacterium tuberculosis Infection. Cold Spring Harb Perspect Med (2015) 5(12):9. 10.1101/cshperspect.a018424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat Immunol (2010) 11(8):751–8. 10.1038/ni.1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Behar SM, Divangahi M, Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol (2010) 8(9):668–74. 10.1038/nrmicro2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med (2008) 14(10):461–9. 10.1016/j.molmed.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 9. Tobin DM. Host-Directed Therapies for Tuberculosis. Cold Spring Harb Perspect Med (2015) 5(10). 10.1101/cshperspect.a021196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Connor G, Gleeson LE, Fagan-Murphy A, Cryan SA, O’Sullivan MP, Keane J. Sharpening nature’s tools for efficient tuberculosis control: A review of the potential role and development of host-directed therapies and strategies for targeted respiratory delivery. Adv Drug Deliv Rev (2016) 102:33–54. 10.1016/j.addr.2016.04.024 [DOI] [PubMed] [Google Scholar]
- 11. Marakalala MJ, Raju RM, Sharma K, Zhang YJ, Eugenin EA, Prideaux B, et al. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat Med (2016) 22(5):531–8. 10.1038/nm.4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tobin DM, Ramakrishnan L. TB: the Yin and Yang of lipid mediators. Curr Opin Pharmacol (2013) 13(4):641–5. 10.1016/j.coph.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Behar SM, Martin CJ, Nunes-Alves C, Divangahi M, Remold HG. Lipids, apoptosis, and cross-presentation: links in the chain of host defense against Mycobacterium tuberculosis. Microbes Infect (2011) 13(8-9):749–56. 10.1016/j.micinf.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen M, Divangahi M, Gan H, Shin DS, Hong S, Lee DM, et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J Exp Med (2008) 205(12):2791–801. 10.1084/jem.20080767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaul V, Bhattacharya D, Singh Y, Van Kaer L, Peters-Golden M, Bishai WR, et al. An important role of prostanoid receptor EP2 in host resistance to Mycobacterium tuberculosis infection in mice. J Infect Dis (2012) 206(12):1816–25. 10.1093/infdis/jis609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang L, Zhang Q, Luo LL, Yue J, Zhao YL, Han M, et al. Polymorphisms in the prostaglandin receptor EP2 gene confers susceptibility to tuberculosis. Infect Genet Evol (2016) 46:23–7. 10.1016/j.meegid.2016.10.016 [DOI] [PubMed] [Google Scholar]
- 17. Dietzold J, Gopalakrishnan A, Salgame P. Duality of lipid mediators in host response against Mycobacterium tuberculosis: good cop, bad cop. F1000Prime Rep (2015) 7:29. 10.12703/P7-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tobin DM, Vary JC, Jr., Ray JP, Walsh GS, Dunstan SJ, Bang ND, et al. The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell (2010) 140(5):717–30. 10.1016/j.cell.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol (2015) 15(8):511–23. 10.1038/nri3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature (2014) 511(7507):99–103. 10.1038/nature13489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pavan Kumar N, Moideen K, Nancy A, Viswanathan V, Shruthi BS, Shanmugam S, et al. Plasma Eicosanoid Levels in Tuberculosis and Tuberculosis-Diabetes Co-morbidity Are Associated With Lung Pathology and Bacterial Burden. Front Cell Infect Microbiol (2019) 9:335. 10.3389/fcimb.2019.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JY, Jung YW, Jeong I, Joh JS, Sim SY, Choi B, et al. Immune parameters differentiating active from latent tuberculosis infection in humans. Tuberculosis (2015) 95(6):758–63. 10.1016/j.tube.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 23. Cadena AM, Flynn JL, Fortune SM. The importance of first impressions: Early events in mycobacterium tuberculosis infection influence outcome. mBio (2016) 7(2):(e00342–16). 10.1128/mBio.00342-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res (2012) 53(1-3):41–57. 10.1007/s12026-012-8297-3 [DOI] [PubMed] [Google Scholar]
- 25. Balboa L, Barrios-Payan J, Gonzalez-Dominguez E, Lastrucci C, Lugo-Villarino G, Mata-Espinoza D, et al. Diverging biological roles among human monocyte subsets in the context of tuberculosis infection. Clin Sci (Lond) (2015) 129(4):319–30. 10.1042/CS20150021 [DOI] [PubMed] [Google Scholar]
- 26. Khan N, Vidyarthi A, Amir M, Mushtaq K, Agrewala JN. T-cell exhaustion in tuberculosis: pitfalls and prospects. Crit Rev Microbiol (2017) 43(2):133–41. 10.1080/1040841X.2016.1185603 [DOI] [PubMed] [Google Scholar]
- 27. Zhai W, Wu F, Zhang Y, Fu Y, Liu Z. The Immune Escape Mechanisms of Mycobacterium Tuberculosis. Int J Mol Sci (2019) 20(2):340. 10.3390/ijms20020340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell (2012) 148(3):434–46. 10.1016/j.cell.2011.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood (2010) 116(16):e74–80. 10.1182/blood-2010-02-258558 [DOI] [PubMed] [Google Scholar]
- 30. Divangahi M, Behar SM, Remold H. Dying to live: how the death modality of the infected macrophage affects immunity to tuberculosis. Adv Exp Med Biol (2013) 783:103–20. 10.1007/978-1-4614-6111-1_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sorgi CA, Soares EM, Rosada RS, Bitencourt CS, Zoccal KF, Pereira PAT, et al. Eicosanoid pathway on host resistance and inflammation during Mycobacterium tuberculosis infection is comprised by LTB4 reduction but not PGE2 increment. Biochim Biophys Acta Mol Basis Dis (2019) 1866(3):165574. 10.1016/j.bbadis.2019.165574 [DOI] [PubMed] [Google Scholar]
- 32. Rito T, Ferreira J, Cavadas B, Soares P, Oliveira O, Richards MB, et al. Association of Leukotriene A4 Hydrolase with Tuberculosis Susceptibility Using Genomic Data in Portugal. Microorganisms (2019) 7(12):650. 10.3390/microorganisms7120650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruiz A, Sarabia C, Torres M, Juarez E. Resolvin D1 (RvD1) and maresin 1 (Mar1) contribute to human macrophage control of M. tuberculosis infection while resolving inflammation. Int Immunopharmacol (2019) 74:105694. 10.1016/j.intimp.2019.105694 [DOI] [PubMed] [Google Scholar]
- 34. Allard B, Panariti A, Martin JG. Alveolar Macrophages in the Resolution of Inflammation, Tissue Repair, and Tolerance to Infection. Front Immunol (2018) 9:1777. 10.3389/fimmu.2018.01777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kroon EE, Coussens AK, Kinnear C, Orlova M, Moller M, Seeger A, et al. Neutrophils: Innate effectors of TB resistance? Front Immunol (2018) 9(2637). 10.3389/fimmu.2018.02637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vinhaes CL, Oliveira-de-Souza D, Silveira-Mattos PS, Nogueira B, Shi R, Wei W, et al. Changes in inflammatory protein and lipid mediator profiles persist after antitubercular treatment of pulmonary and extrapulmonary tuberculosis: A prospective cohort study. Cytokine (2019) 123:154759. 10.1016/j.cyto.2019.154759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Newson J, Motwani MP, Kendall AC, Nicolaou A, Muccioli GG, Alhouayek M, et al. Inflammatory Resolution Triggers a Prolonged Phase of Immune Suppression through COX-1/mPGES-1-Derived Prostaglandin E2. Cell Rep (2017) 20(13):3162–75. 10.1016/j.celrep.2017.08.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. La Manna MP, Orlando V, Dieli F, Di Carlo P, Cascio A, Cuzzi G, et al. Quantitative and qualitative profiles of circulating monocytes may help identifying tuberculosis infection and disease stages. PloS One (2017) 12(2):e0171358. 10.1371/journal.pone.0171358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Srivastava S, Ernst JD, Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev (2014) 262(1):179–92. 10.1111/imr.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Norris BA, Ernst JD. Mononuclear cell dynamics in M. tuberculosis infection provide opportunities for therapeutic intervention. PloS Pathog (2018) 14(10):e1007154. 10.1371/journal.ppat.1007154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barcelos W, Sathler-Avelar R, Martins-Filho OA, Carvalho BN, Guimaraes TM, Miranda SS, et al. Natural killer cell subpopulations in putative resistant individuals and patients with active Mycobacterium tuberculosis infection. Scand J Immunol (2008) 68(1):92–102. 10.1111/j.1365-3083.2008.02116.x [DOI] [PubMed] [Google Scholar]
- 42. Sampath P, Moideen K, Ranganathan UD, Bethunaickan R. Monocyte Subsets: Phenotypes and Function in Tuberculosis Infection. Front Immunol (2018) 9:1726. 10.3389/fimmu.2018.01726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee J, Tam H, Adler L, Ilstad-Minnihan A, Macaubas C, Mellins ED. The MHC class II antigen presentation pathway in human monocytes differs by subset and is regulated by cytokines. PloS One (2017) 12(8):e0183594. 10.1371/journal.pone.0183594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McClean CM, Tobin DM. Macrophage form, function, and phenotype in mycobacterial infection: lessons from tuberculosis and other diseases. Pathog Dis (2016) 74(7). 10.1093/femspd/ftw068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abeles RD, McPhail MJ, Sowter D, Antoniades CG, Vergis N, Vijay GK, et al. CD14, CD16 and HLA-DR reliably identifies human monocytes and their subsets in the context of pathologically reduced HLA-DR expression by CD14(hi) /CD16(neg) monocytes: Expansion of CD14(hi) /CD16(pos) and contraction of CD14(lo) /CD16(pos) monocytes in acute liver failure. Cytometry Part A J Int Soc Analyt Cytol (2012) 81(10):823–34. 10.1002/cyto.a.22104 [DOI] [PubMed] [Google Scholar]
- 46. Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood (2011) 118(20):5498–505. 10.1182/blood-2011-07-365825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Katsuyama M, Ikegami R, Karahashi H, Amano F, Sugimoto Y, Ichikawa A. Characterization of the LPS-stimulated expression of EP2 and EP4 prostaglandin E receptors in mouse macrophage-like cell line, J774.1. Biochem Biophys Res Commun (1998) 251(3):727–31. 10.1006/bbrc.1998.9540 [DOI] [PubMed] [Google Scholar]
- 48. Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes? Immunol Cell Biol (2012) 90(6):579–86. 10.1038/icb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lone AM, Tasken K. Proinflammatory and immunoregulatory roles of eicosanoids in T cells. Front Immunol (2013) 4:130. 10.3389/fimmu.2013.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pablos JL, Santiago B, Carreira PE, Galindo M, Gomez-Reino JJ. Cyclooxygenase-1 and -2 are expressed by human T cells. Clin Exp Immunol (1999) 115(1):86–90. 10.1046/j.1365-2249.1999.00780.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cook-Moreau JM, El-Makhour Hojeij Y, Barriere G, Rabinovitch-Chable HC, Faucher KS, Sturtz FG, et al. Expression of 5-lipoxygenase (5-LOX) in T lymphocytes. Immunology (2007) 122(2):157–66. 10.1111/j.1365-2567.2007.02621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.