Abstract
Campylobacter is one of the most important microorganisms responsible for foodborne diseases in the EU. In this study, we investigated resistance to tetracycline in 139 Campylobacter jejuni and Campylobacter coli samples isolated from human clinical cases. From these, 110 were resistant to tetracycline, with MIC (minimal inhibitory concentration) varying in a range of 1 to >512 μg/mL, and 109 (78.4%) carried tet(O), a gene that confers resistance to tetracycline through the expression of a protein that confers protection to the ribosome. Amongst the tetracycline-resistant isolates, one C. jejuni (HCC30) was the only tet(O)-negative sample, presenting an MIC of 256 μg/mL. Instead, the mosaic gene tet(O/M/O) was found in HCC30 and, as far as we know, this is the first description of this chimeric gene originating from homologous recombination between tet(O) and tet(M). The previously described mosaic gene tet(O/32/O), also found in Campylobacter, presents a chimeric structure very similar to that of tet(O/M/O), affecting domains II and III of encoded proteins distantly related to the elongation factor G (EF-G). The tet(O/M/O) mosaic gene has been found in nucleotide databases in several genomes of Campylobacter isolated from different origins, indicating its frequent acquisition, even though it can be undetected through screening by PCR with specific tet(O) primers. In this work, we address the improvement of classical PCR to efficiently diagnose the most prevalent tetracycline resistance determinants in Campylobacter, including tet(O/M/O), which should be taken into account in the optimization of campylobacteriosis treatments.
Keywords: Campylobacter coli, Campylobacter jejuni, tetracycline resistance, tet genes, mosaic tet(O/M/O)
1. Introduction
Campylobacter spp. infections have been the most dominant gastrointestinal disease reported in the EU since 2005. Generally, this infection is self-limiting but can be the trigger for severe illness, such as Guillain–Barré syndrome and autoimmune inflammatory conditions [1]. The level of antimicrobial resistance of this zoonosis, in European member states, according to the European Committee on Antimicrobial Susceptibility Testing EUCAST ecological cut-off values, varies from 0.5% to gentamicin up to 45.4% to tetracycline, in Campylobacter jejuni isolated from human infections, whilst in Campylobacter coli, 1.8% were resistant to gentamicin and 68.3% were resistant to tetracycline [1]. In isolates obtained from animals, the same pattern of resistance occurs in both C. coli and C. jejuni isolated from fattening pigs and calves [1]. The Campylobacter genus, being both a zoonotic and enteric microorganism, has acquired several antimicrobial resistances due to its exposure to antimicrobials used in the treatment/prophylaxis of disease in food-producing animals, companion animals and humans [2]. Some of the most used antimicrobials in the treatment of campylobacteriosis are macrolides and fluoroquinolones [3,4], and occasionally aminoglycosides and oral beta-lactams, however, tetracycline, fluoroquinolones, macrolides, florfenicol and trimethoprim–sulfamethoxazole are the drugs against which Campylobacter presents the greatest resistance [4].
Tetracyclines are a group of antibiotics extensively used in the treatment of both animal and human infections, but also as prophylaxis agents and as growth promotors in animal husbandry [5]. This antimicrobial presents a broad spectrum of activities and is low cost, making it suitable for incorporation in animal feeds at subtherapeutic doses to act as a growth promotor which can be a practice responsible for the development of bacterial resistance [6,7]. The first tetracycline resistance genes discovered were genes tet(A) to tet(E), in Gram-negative bacteria, mediating efflux pumps and genes tet(L) to tet(N), in Gram-positive cocci, conferring resistance by encoding ribosomal protection proteins [8]. tet(O) was found in a self-transmissible plasmid from C. coli and it was thought to have diverged from the gene tet(M) [9]. Today, there are more than 60 tetracycline resistance genes described and besides the already mentioned mechanisms of action, resistance mediated by enzymatic inactivation of the molecule was also reported [7,10,11,12,13]. The improvement of diagnostic techniques and the elimination of false positives corroborate the knowledge that the tet(O) sequence is the only resistance determinant to tetracycline in C. jejuni and C. coli [4,14], especially associated with the acquisitions of plasmids containing the gene [15,16,17], although it can also be found in the chromosome [18,19,20]. Mosaic genes are common amongst genes responsible for ribosomal protection proteins and several of these elements have been described, with the majority deriving from tet(O), tet(W) and tet32, including tet(O/32/O) recently found in C. coli and C. jejuni isolated from humans [7].
In this work, we describe the detection of a tet(O/M/O) mosaic gene in C. jejuni, analyze its relevance for the molecular diagnosis of antimicrobial resistance and discuss implications for structure–function relationships of ribosomal protection factors.
2. Materials and Methods
2.1. Microbial Growth And Antibiotic Resistance Testing
Campylobacter spp. of human origin were isolated between 2010 and 2012 (139 strains; 132 C. jejuni and seven C. coli) and were described previously [21,22,23]. Cultivation procedures of the isolates included culture on blood agar in a microaerophilic atmosphere (CampyGen™; Thermo Scientific, Lenexa, KS, USA) at 42 °C for 24–48 h [21,22]. Tetracycline minimal inhibitory concentrations (MICs) of the isolates were determined by agar dilution methods according to the Clinical and Laboratory Standards Institute CLSI [24].
2.2. PCR Screening of Resistance Determinants
The presence of tet(O) genes was investigated using PCR with primers and conditions already described. Primers specific for tet(O), tetOF (5’-gcgttttgtttatgtgcg) and tetOR (5’-atggacaacccgacagaag) were modified from primers previously described by Bacon et al. [25] and degenerated primers, designed to amplify all tet genes, tetDF (5’-GCTCA(T/C)GTTGA(T/C)GCAGGAA) and tetDR (5’-AGGATTTGGCGG(C/G)ACTTC(G/T)A) [26], were used in PCR with the following conditions: initial melting temperature of 95 °C for 5 min; 30 cycles of 95 °C for 30 s, 55 °C or 50 °C (for specific and degenerated primers, respectively) for 30 s and 72 °C for 1.5 min and a final extension step of 72 °C for 2 min. Reagents used in the PCR reactions contained 0.2 mmol/L dNTPs (Takara-Clontech, Kusatsu, Shiga, Japan), 0.5 μmol/L of each primer, 0.025 U/μL Taq polymerase (Biotools, Madrid, Spain), 1 × PCR buffer with 1.5 mmol/L MgCl2 (Biotools), and 5 μL of DNA template in a total volume of 50 μL. PCR products (558 or 1293 bp, for specific or degenerated primers, respectively) were purified with a Speedtools PCR clean-up kit (Biotools, Madrid, Spain) according to the manufacturer’s instructions and Sanger sequencing was performed by the facilities of the Universidad de Extremadura, Spain (STAB).
2.3. Bioinformatic Tools
Comparison of DNA and protein sequences was performed by the basic local alignment search tool (BLAST) using the nucleotide collection nr/nt or the non-redundant protein sequence (nr) databases [27]. Clustal X 2.0 software was used for multiple sequence alignments.
3. Results
Seventy-nine percent of the tested Campylobacter isolates (104 C. jejuni and six C. coli out of 139, Supplementary Table S1), were resistant to tetracycline according to the CLSI [24] breakpoint (MIC ≥ 16 mg/L) and the presence of the tet(O) gene, determined by PCR, almost matched the resistance phenotype of the isolates with the unique exception of C. jejuni HCC30, which presented a tetracycline MIC of 256 mg/L (Table 1). Thus, among the analyzed isolates, the tet(O) gene was the main resistance determinant carried by 99.1% (109/110) of tetracycline-resistant isolates.
Table 1.
Involvement of tet(O) in tetracycline resistance.
| MIC # (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Tet(O) * | ≤4 | 8 | 16 | 32 | 64 | 128 | 256 | ≥512 | Sum |
| C. jejuni | + | 0 | 0 | 3 | 3 | 24 | 50 | 9 | 14 | 103 |
| - | 26 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 29 | |
| C. coli | + | 0 | 0 | 0 | 1 | 1 | 0 | 3 | 1 | 6 |
| - | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
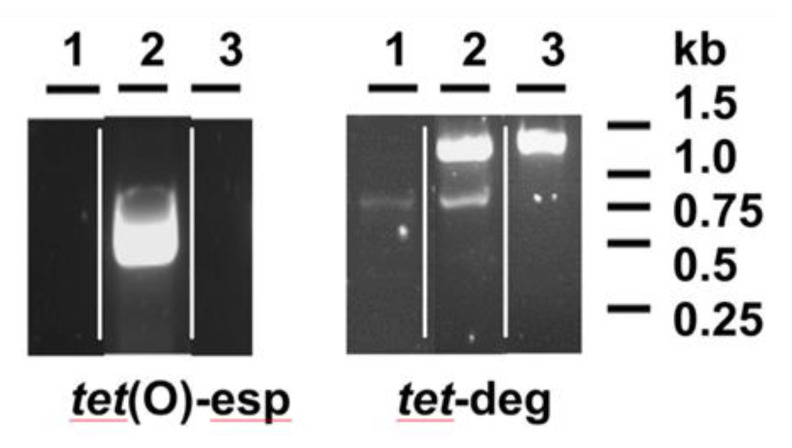
The specific primers used in this work to detect the tet(O) gene [25] had been extensively and successfully used [19] and amplified the expected 0.5 Kb DNA fragment in most tetracycline-resistant isolates as HSA1 (Figure 1). The same was not possible with isolate HCC30, for which PCR fragment amplification failed, in a similar way to the susceptible background of isolate HCC69 (Figure 1). However, degenerated primers, designed based on available sequences for ribosomal protection proteins [26], made possible the production of a PCR fragment with the expected size (1 to 1.5 Kb) in both tetracycline-resistant isolates, HCC30 and HSA1, but not in HCC69 (Figure 1). The DNA fragment amplified in HCC30 proved to be 100% identical to three sequences present in C. coli (accession no. AY394560.1 and MF134831) and C. jejuni (CP023446), and closely related (presenting up to three single nucleotide polymorphisms) to nine sequences from C. jejuni (CP048769.1, CP048767.1, CP048765.1, CP059964.1, CP048764.1, CP048763.1, CP048756.1, CP048771.1, KF864551.1), five from C. coli (MF037584.1, KC876752.1, KC876751.1, KC876749.1, CP044164.1) and one from C. fetus (CP027287.1), all of them annotated as tetO.
Figure 1.
PCR detection of tet(O) sequences from Campylobacter. DNA samples were amplified by using specific primers (tet(O)-esp) or degenerated primers (tet-deg), according to conditions explained in the Material and Methods section and analyzed by agarose gel electrophoresis. Strains analyzed are: 1, HCC69 (susceptible to tetracycline); 2, HSA1 (resistant to tetracycline); 3, HCC30 (resistant to tetracycline). The figure presented is composed of images obtained from different gels.
Analysis of these sequences revealed it to correspond to a not yet described class of tetracycline-resistant gene that presents the replacement of the 776–1127 bp internal fragment of tet(O) by the homologous sequence from tet(M), a new mosaic structure that will be named hereafter as tet(O/M/O) (Figure 2). Strains of C. jejuni and C. coli are known to present the mosaic tet(O/32/O) [7] and, as far as we know, this is the first report describing the existence of the tet(O/M/O) mosaic sequence mobilized among different species of Campylobacter, although a recent report mentioned its occurrence in particular sequence types of C. jejuni [28]. Interestingly, both tet(O/32/O) and tet(O/M/O) mosaic genes from Campylobacter share the same chimeric structure, with a DNA fragment of similar length (about 300 bp) from tet(32) or tet(M) inserted in nearly the same position of tet(O). Encoded proteins are thus chimeras with TetM or Tet32 insertions with corresponding polymorphisms mapped to structural domains II and III of the ribosomal protection proteins (Figure 3) and their distantly related homolog, translation elongation factor G (EF-G) from which Tet proteins have obtained the capacity to bind to the ribosome and release the tetracycline molecule [29], resulting in resistance to this antimicrobial.
Figure 2.
Chimeric structure of tet(O/M/O) mosaic gene. Nucleotide sequences shown are: tet(M), X04388; tet(O), CP044175 and tet(O/M/O), this work (100% identical to AY394560.1). Identity color code: black, homology between three sequences; blue, homology between tet(O) and tet(O/M/O); red, homology between tet(M) and tet(O/M/O). Primers used in this work are shown below the sequences and indicated in green.
Figure 3.
Chimeric structure of Tet(O/M/O) and Tet(O/32/O) mosaic proteins. Amino acid sequence shown are: Tet(M), WP_004632336; Tet(O), EAI7795628; Tet(32), WP_002602099.1; Tet(O/32/O), AINH01000038; Tet(O/M/O), this work (100% identical to WP_002872163, encoded by AY394560.1). Identity color code: black, homology ≥ 80% among sequences; blue, homology ≥ 60% among tet(O), tet(O/M/O) and/or Tet(O/32/O); red, tet(M) and tet(O/M/O); green, tet(32) and tet(O/32/O).
4. Discussion
The percentage of resistance to tetracycline showed by the isolates studied was much higher compared to the resistance found in other works with Campylobacter spp. isolated from humans. Elhadidy et al [30], found 49.7% resistance to tetracycline, much in accordance with the level of resistance found by Marotta et al. [31], with 49.0% resistant isolates, even though this last study used the epidemiological cut-off values (ECOFFs) defined by EUCAST [32]. Considering the same resistance definition, the study carried out in Spain by Ocejo et al. [33] showed that the level of resistance in Campylobacter spp., isolated from animals, was 76.5%. Tetracyclines were extensively used in Spain in animal husbandry, being 40% of all the antibiotics consumed in 2013 [34] and the high results of resistance found in our work might reflect this practice.
The use of degenerated primers for the amplification of tet genes originated the amplification of a fragment already present in nucleotide databases, which was described in a wide spectrum of Campylobacter species and origins, such as C. coli isolated from humans, turkeys and pigs, C. fetus isolated from humans and C. jejuni isolated from chickens. Analysis of the sequence revealed it to correspond to a not yet described class of tetracycline-resistant gene that presents a mosaic structure with the replacement of the 776-1127 bp internal fragment of tet(O) by the homologous sequence from tet(M) (Figure 2). This tet(O/M/O) mosaic gene might have evolved from a homologous recombination event between tet(O) and tet(M), encoding a ribosomal protection factor widely spread in Firmicutes from the Bacilli class, such as enterococci and streptococci [26,27,35,36], many of which share the mammalian gastrointestinal tract environment with Campylobacter, which, together with Megasphaera and Riemerella, are the only three Gram-negative genera in which tet mosaic genes have been reported [13]. Curiously, all these genera are common inhabitants of the gastrointestinal tract of both animals and humans.
tet(O/W/O) mosaic genes were the first reported in [37] and since then several others, including different chimeras between tet(M), tet(O), tet(S), tet(W) and tet(32), have been described and proved to be functional with resistance levels comparable to the non-mosaic genes [7], also including tet(O/M/O), since plasmid pCC31 carrying an identical sequence to that found in HCC30 mobilized tetracycline resistance among Campylobacter strains [38]. Taking into account that the National Center of Biotechnology Information (NCBI) nucleotide collection (nr/nt) database presents 126 full coding sequences for tet(O) genes from Campylobacter, among which 17 and five correspond to the tet(O/M/O) and tet(O/32/O) mosaic genes, respectively (data available on 21st October 2020), and that both chimeras present similar domain organization, makes it advisable to use the degenerated primers previously described [26] in PCR for an accurate identification of tetracycline-resistant determinants of strains producing negative results in conventional PCR by specific primers.
The mechanism of action of tetracycline is based on the binding of the molecule at the A site of the 30S subunit of the ribosome, a connection that involves the 16S rRNA [39]. The presence of tetracycline blocks tRNA binding and consequently the inhibition of protein synthesis [40]. tet(O) and tet(M) are paralogs of EF-G, a translation GTPase, and are able to remove tetracycline from the ribosome dependent on the GTP hydrolysis [41]. There is not much available information about the functional role of domains II and III of EF-G, since ribosomal binding determinants are located in domain IV and CTE, the C-terminal extension that is lacking in EF-G, whereas GTPase activity centers are in domain I, the G-domain [29]. The fact that, during evolution of ribosomal protection proteins, in Campylobacter, all tet(O) mosaic sequences present insertions of genes tet(32) and tet(M) in regions encoding domains II and III (Figure 2 and Figure 3), strongly suggests that still unknown structural and/or functional determinants might have driven the selection of these chimeric sequences, under exposure to the antibiotic used for infection treatment in humans or prophylaxis/growth enhancement in animals [42].
5. Conclusions
This work describes the identification of a new class of tetracycline-resistant determinants in Campylobacter, the tet(O/M/O) mosaic gene. The occurrence of this genetic element suggests that recombination exchange could have taken place within the same bacterium carrying co-existing tet(O) and tet(M) resistance determinants. Whether this mosaic gene was transferred to Campylobacter by means of a plasmid, by conjugative transposons, or if recombination occurred with a resident tet(O) gene after natural transformation of Campylobacter, remains unknown. The relevant fact is that the co-existence of closely related sequences in the same environment might provide bacteria with genetic tools to accelerate the evolution of antimicrobial resistance determinants. Thus, besides recognizing the tet(O/M/O) mosaic gene for the first time, this work provides a new PCR strategy to detect this resistance determinant, preventing it from occurring as a false negative, a finding that might be relevant for clinicians and/or biochemists interested in diagnosing antimicrobial resistance and/or understanding the evolution of ribosomal protection proteins.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/11/1710/s1, Table S1: Tetracycline phenotype and genotype of Campylobacter isolates.
Author Contributions
Conceptualization, A.Q.; Formal analysis, M.J.C., S.V. and A.Q.; Methodology, L.H.; Writing—original draft, M.J.C. and A.Q.; Writing—review and editing, M.J.C. and A.Q. All authors have read and agreed to the published version of the manuscript.
Funding
M.J.C. wishes to thank Fundação para a Ciência e Tecnologia (FCT), through the strategic project UID/MAR/04292/2013 granted to MARE and the Integrated Programme of SR&TD “Smart Valorization of Endogenous Marine Biological Resources Under a Changing Climate” (reference Centro-01-0145-FEDER- 000018), co-funded by the Centro 2020 program, Portugal 2020, European Union, through the European Regional Development Fund. Works in the A.Q. laboratory have been funded by projects AGL2012-39028-C03-03 and AGL2016-74882-C3-2-R (“Ministerio de Ciencia e Innovación, Gobierno de España”) and IB16073 (“Consejería de Economía e Infraestructuras, Junta de Extremadura” and FEDER).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Food Safety Authority, European Centre for Disease Prevention and Control The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019;17:e05598. doi: 10.2903/j.efsa.2019.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai L., Sahin O., Grover M., Zhang Q. New and alternative strategies for the prevention, control, and treatment of antibiotic-resistant Campylobacter. Transl. Res. 2020;223:76–88. doi: 10.1016/j.trsl.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa Y. Systematic research to overcome newly emerged multidrug-resistant bacteria. Microbiol. Immunol. 2020;64:231–251. doi: 10.1111/1348-0421.12781. [DOI] [PubMed] [Google Scholar]
- 4.Tang Y., Fang L., Xu C., Zhang Q. Antibiotic resistance trends and mechanisms in the foodborne pathogen, Campylobacter. Anim. Health Res. Rev. 2017;18:87–98. doi: 10.1017/S1466252317000135. [DOI] [PubMed] [Google Scholar]
- 5.Chopra I., Roberts M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granados-Chinchilla F., Rodríguez C. Tetracyclines in Food and Feedingstuffs: From Regulation to Analytical Methods, Bacterial Resistance, and Environmental and Health Implications. J. Anal. Methods Chem. 2017;2017:1315497. doi: 10.1155/2017/1315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warburton P.J., Amodeo N., Roberts A.P. Mosaic tetracycline resistance genes encoding ribosomal protection proteins. J. Antimicrob. Chemother. 2016;71:3333–3339. doi: 10.1093/jac/dkw304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zilhao R., Papadopoulou B., Courvalin P. Occurrence of the Campylobacter resistance gene tetO in Enterococcus and Streptococcus spp. Antimicrob. Agents Chemother. 1988;32:1793–1796. doi: 10.1128/AAC.32.12.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sougakoff W., Papadopoulou B., Nordmann P., Courvalin P. Nucleotide sequence and distribution of gene tetO encoding tetracycline resistance in Campylobacter coli. FEMS Microbiol. Lett. 1987;44:153–159. doi: 10.1111/j.1574-6968.1987.tb02260.x. [DOI] [Google Scholar]
- 10.Leski T.A., Bangura U., Jimmy D.H., Ansumana R., Lizewski S.E., Stenger D.A., Taitt C.R., Vora G.J. Multidrug-resistant tet(X)-containing hospital isolates in Sierra Leone. Int. J. Antimicrob. Agents. 2013;42:83–86. doi: 10.1016/j.ijantimicag.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Marosevic D., Kaevska M., Jaglic Z. Resistance to the tetracyclines and macrolide-lincosamide-streptogramin group of antibiotics and its genetic linkage—A review. Ann. Agric. Environ. Med. 2017;24:338–344. doi: 10.26444/aaem/74718. [DOI] [PubMed] [Google Scholar]
- 12.Park J., Gasparrini A., Reck M.R., Symister C.T., Elliott J.L., Vogel J.P., Wencewicz T.A., Dantas G., Tolia N.H.M. Plasticity, dynamics, and inhibition of emerging tetracycline resistance enzymes. Nat. Chem. Biol. 2017;13:730–736. doi: 10.1038/nchembio.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marilyn C. Roberts. [(accessed on 30 October 2020)]; Available online: http://faculty.washington.edu/marilynr/
- 14.Lynch C., Hawkins K., Lynch H., Egan J., Bolton D., Coffey A., Lucey B. Investigation of molecular mechanisms underlying tetracycline resistance in thermophilic Campylobacter spp. suggests that previous reports of tet(A)-mediated resistance in these bacteria are premature. Gut Pathog. 2019;11:56. doi: 10.1186/s13099-019-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabre A., Oleastro M., Nunes A., Santos A., Sifré E., Ducournau A., Bénéjat L., Buissonnière A., Floch P., Mégraud F., et al. Whole-Genome Sequence Analysis of Multidrug-Resistant Campylobacter Isolates: A Focus on Aminoglycoside Resistance Determinants. J. Clin. Microbiol. 2018;56:e00390-18. doi: 10.1128/JCM.00390-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French N.P., Zhang J., Carter G.P., Midwinter A.C., Biggs P.J., Dyet K., Gilpin B.J., Ingle D.J., Mulqueen K., Rogers L.E., et al. Genomic Analysis of Fluoroquinolone- and Tetracycline-Resistant Campylobacter jejuni Sequence Type 6964 in Humans and Poultry, New Zealand, 2014–2016. Emerg. Infect. Dis. 2019;25:2226–2234. doi: 10.3201/eid2512.190267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenover F.C., Williams S., Gordon K.P., Nolan C., Plorde J.J. Survey of plasmids and resistance factors in Campylobacter jejuni and Campylobacter coli. Antimicrob. Agents Chemother. 1985;27:37–41. doi: 10.1128/AAC.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crespo M.D., Olson J.W., Altermann E., Siletzky R.M., Kathariou S. Chromosomal tet(O)-harboring regions in Campylobacter coli isolates from turkeys and swine. Appl. Environ. Microbiol. 2012;78:8488–8491. doi: 10.1128/AEM.02258-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratt A., Korolik V. Tetracycline resistance of Australian Campylobacter jejuni and Campylobacter coli isolates. J. Antimicrob. Chemother. 2005;55:452–460. doi: 10.1093/jac/dki040. [DOI] [PubMed] [Google Scholar]
- 20.Tang Y., Meinersmann R.J., Sahin O., Wu Z., Dai L., Carlson J., Plumblee Lawrence J., Genzlinger L., LeJeune J.T., Zhang Q. Wide but Variable Distribution of a Hypervirulent Campylobacter jejuni Clone in Beef and Dairy Cattle in the United States. Appl. Environ. Microbiol. 2017;83:e01425-17. doi: 10.1128/AEM.01425-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hormeño L., Palomo G., Ugarte-Ruiz M., Porrero M.C., Borge C., Vadillo S., Píriz S., Domínguez L., Campos M.J., Quesada A. Identification of the main quinolone resistance determinant in Campylobacter jejuni and Campylobacter coli by MAMA-DEG PCR. Diagn. Microbiol. Infect. Dis. 2016;84:236–239. doi: 10.1016/j.diagmicrobio.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Hormeño L., Ugarte-Ruiz M., Palomo G., Florez-Cuadrado D., Vadillo S., Píriz S., Domínguez L., Campos M.J., Quesada A. ant(6)-I Genes Encoding Aminoglycoside O-Nucleotidyltransferases Are Widely Spread Among Streptomycin Resistant Strains of Campylobacter jejuni and Campylobacter coli. Front. Microbiol. 2018;9:2515. doi: 10.3389/fmicb.2018.02515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mourkas E., Florez-Cuadrado D., Pascoe B., Calland J.K., Bayliss S.C., Mageiros L., Méric G., Hitchings M.D., Quesada A., Porrero C., et al. Gene pool transmission of multidrug resistance among Campylobacter from livestock, sewage and human disease. Environ. Microbiol. 2019;21:4597–4613. doi: 10.1111/1462-2920.14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standard Institute (CLSI) Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. 3rd ed. Clinical and Laboratory Standard Institute; Wayne, PA, USA: 2016. CLSI guideline M45. [Google Scholar]
- 25.Bacon D.J., Alm R.A., Burr D.H., Hu L., Kopecko D.J., Ewing C.P., Trust T.J., Guerry P. Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect. Immun. 2000;68:4384–4390. doi: 10.1128/IAI.68.8.4384-4390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbosa T.M., Scott K.P., Flint H.J. Evidence for recent intergeneric transfer of a new tetracycline resistance gene, tet(W), isolated from Butyrivibrio fibrisolvens, and the occurrence of tet(O) in ruminal bacteria. Environ. Microbiol. 1999;1:53–64. doi: 10.1046/j.1462-2920.1999.00004.x. [DOI] [PubMed] [Google Scholar]
- 27.National Center for Biotechnology Information U.S. National Library of Medicine 8600 Rockville Pike, Bethesda MD, 20894 USA
- 28.Lopes B.S., Strachan N., Ramjee M., Thomson A., MacRae M., Shaw S., Forbes K.J. Nationwide Stepwise Emergence and Evolution of Multidrug-Resistant Campylobacter jejuni Sequence Type 5136, United Kingdom. Emerg. Infect. Dis. 2019;25:1320–1329. doi: 10.3201/eid2507.181572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dönhöfer A., Franckenberg S., Wickles S., Berninghausen O., Beckmann R., Wilson D.N. Structural basis for TetM-mediated tetracycline resistance. Proc. Natl. Acad. Sci. USA. 2012;109:16900–16905. doi: 10.1073/pnas.1208037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elhadidy M., Ali M.M., El-Shibiny A., Miller W.G., Elkhatib W.F., Botteldoorn N., Dierick K. Antimicrobial resistance patterns and molecular resistance markers of Campylobacter jejuni isolates from human diarrheal cases. PLoS ONE. 2020;15:e0227833. doi: 10.1371/journal.pone.0227833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marotta F., Garofolo G., di Marcantonio L., di Serafino G., Neri D., Romantini R., Sacchini L., Alessiani A., Di Donato G., Nuvoloni R., et al. Antimicrobial resistance genotypes and phenotypes of Campylobacter jejuni isolated in Italy from humans, birds from wild and urban habitats, and poultry. PLoS ONE. 2019;14:e0223804. doi: 10.1371/journal.pone.0223804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Committee on Antimicrobial Susceptibility Testing. [(accessed on 30 October 2020)]; Available online: https://eucast.org.
- 33.Ocejo M., Oporto B., Hurtado A. Occurrence of Campylobacter jejuni and Campylobacter coli in Cattle and Sheep in Northern Spain and Changes in Antimicrobial Resistance in Two Studies 10-years Apart. Pathogens. 2019;8:98. doi: 10.3390/pathogens8030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Medicines Agency (EMA) European Surveillance of Veterinary Antimicrobial Consumption. Sales of Veterinary Antimicrobial Agents in 30 European Countries in 2015 (EMA/387934/2015) European Medicines Agency; Amsterdam, The Netherlands: 2018. [Google Scholar]
- 35.Marasini D., Karki A.B., Buchheim M.A., Fakhr M.K. Phylogenetic Relatedness Among Plasmids Harbored by Campylobacter jejuni and Campylobacter coli Isolated From Retail Meats. Front. Microbiol. 2018;9:2167. doi: 10.3389/fmicb.2018.02167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spigaglia P., Barbanti F., Mastrantonio P. Tetracycline resistance gene tet(W) in the pathogenic bacterium Clostridium difficile. Antimicrob. Agents Chemother. 2008;52:770–773. doi: 10.1128/AAC.00957-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanton T.B., Humphrey S.B. Isolation of tetracycline-resistant Megasphaera elsdenii strains with novel mosaic gene combinations of tet(O) and tet(W) from swine. Appl. Environ. Microbiol. 2003;69:3874–3882. doi: 10.1128/AEM.69.7.3874-3882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batchelor R.A., Pearson B.M., Friis L.M., Guerry P., Wells J.M. Nucleotide sequences and comparison of two large conjugative plasmids from different Campylobacter species. Microbiology. 2004;150:3507–3517. doi: 10.1099/mic.0.27112-0. [DOI] [PubMed] [Google Scholar]
- 39.Speer B.S., Shoemaker N.B., Salyers A.A. Bacterial resistance to tetracycline: Mechanisms, transfer, and clinical significance. Clin. Microbiol. Rev. 1992;5:387–399. doi: 10.1128/CMR.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu A.M., Choi Y.H., Tu M.J. RNA Drugs and RNA Targets for Small Molecules: Principles, Progress, and Challenges. Pharmacol. Rev. 2020;72:862–898. doi: 10.1124/pr.120.019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W., Atkinson G.C., Thakor N.S., Allas U., Lu C.C., Chan K.Y., Tenson T., Schulten K., Wilson K.S., Hauryliuk V., et al. Mechanism of tetracycline resistance by ribosomal protection protein Tet(O) Nat. Commun. 2013;4:1477. doi: 10.1038/ncomms2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thaker M., Spanogiannopoulos P., Wright G.D. The tetracycline resistome. Cell. Mol. Life Sci. 2010;67:419–431. doi: 10.1007/s00018-009-0172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.