Abstract
We aimed to investigate the association of iron and polyunsaturated fatty acid (PUFA) intake with diabetic peripheral neuropathy (DPN) in individuals with type 2 diabetes. This cross-sectional study included 147 individuals with type 2 diabetes. Dietary intake was assessed using three-day food records. DPN was diagnosed on the basis of a Michigan Neuropathy Screening Instrument—Physical Examination score ≥2.5. Adjusted for total energy intake, iron intake was significantly higher in individuals with DPN than in those without DPN (10.9 ± 4.0 mg vs. 9.9 ± 3.6 mg, p = 0.041). In addition, the iron/PUFA ratio was significantly higher in individuals with DPN (1.4 ± 0.8 vs. 1.1 ± 0.4, p = 0.005). Logistic regression analyses showed that iron intake (odds ratio (OR): 1.152; 95% confidence interval (CI): 1.012, 1.311) and iron/PUFA ratio (OR: 2.283; 95% CI: 1.066, 4.887) were associated with DPN after adjustment for total energy intake, sex, age, body mass index, systolic blood pressure, diabetes duration, estimated glomerular filtration rate, glycated hemoglobin, low-density lipoprotein cholesterol, and smoking. In conclusion, high dietary iron intake and an elevated iron/PUFA ratio were associated with the presence of DPN. The present study suggests the importance of the dietary pattern of iron and PUFA intake in individuals with type 2 diabetes.
Keywords: diabetic peripheral neuropathy, dietary intake, iron, polyunsaturated fatty acid, type 2 diabetes
1. Introduction
Diabetic peripheral neuropathy (DPN) is the most common form of diabetic neuropathy [1]. It is an important cause of foot ulceration and a major contributor to falls and fractures [2]. Long-duration diabetes, old age, hyperglycemia, hypertension, dyslipidemia, obesity, alcohol, smoking, and insulin resistance are known risk factors for DPN [3,4]. From a pathophysiologic point of view, oxidative stress is a key contributor to DPN [5]. However, alpha-lipoic acid, a currently available antioxidant treatment, showed a clinically relevant effect on symptomatic DPN when administered by intravenous infusion [6]. Furthermore, there is no approved preventive or curative treatment for DPN other than risk factor management. Therefore, the identification of additional modifiable factors is crucial for developing a new strategy to treat DPN.
Diet plays a critical role in the development of type 2 diabetes and its complications [7,8]. The Mediterranean [9] and vegetarian [10] diets reduce diabetes risk. In addition, a low-carbohydrate, high-unsaturated and low-saturated fat diet improved glycemic control and cardiovascular disease risk factors [11]. Several studies showed that the blood concentration of micronutrients was decreased in individuals with DPN. For example, vitamin D deficiency is associated with DPN [12] and with painful DPN even after adjusting for confounding factors [13]. Among metformin users, vitamin B12 deficiency was commonly detected and might have a detrimental effect on DPN [14]. The blood levels of other vitamins B such as vitamin B1 and B6 [15] were also associated with a high frequency of DPN. Therefore, assessing these micronutrients might be useful for the stratification of DPN risk [16]. However, from the perspective of clinical practice, the measurement of the blood levels of micronutrients might not be feasible in terms of cost and complexity of the procedure. In this regard, nutritional assessment might be a solution to evaluate an individual’s risk of DPN.
Iron, a trace element, participates in a variety of cellular processes, including oxygen delivery, mitochondrial electron transport, DNA synthesis, and gene regulation [17]. However, excess iron can generate oxidative stress and cause tissue damage [18]. Interestingly, previous studies have shown an association between dietary iron intake and diabetes risk [19,20]. Several rodent models of DPN have shown that iron deficiency rather than iron overload was associated with the risk of DPN [21,22,23]. However, no study has evaluated the association between dietary iron intake and DPN in humans.
Polyunsaturated fatty acids (PUFA), especially, omega-3 PUFA, are antioxidants [24]. Intake of PUFA and replacement of saturated fatty acids (SFA) with PUFA reduced the risk of type 2 diabetes [25,26]. In addition, a high dietary PUFA intake was associated with a lower risk of DPN [27]. Despite these findings, few studies have evaluated the association between these nutrients and DPN. Therefore, in this study, we examined the association of iron intake and of the ratio between iron intake and PUFA intake (iron/PUFA) with DPN in individuals with type 2 diabetes.
2. Materials and Methods
2.1. Study Population
The original prospective observational study was designed to discover reliable screening tools and biomarkers for DPN in type 2 diabetes. The present study analyzed data from individuals who were enrolled during the initial 2-year period (2017–2019) of the prospective observational study at the Seoul National University Bundang Hospital (SNUBH). We recruited 200 individuals with type 2 diabetes regardless of the presence of DPN. The inclusion criteria were: age ≥19 years, diagnosis of type 2 diabetes, and no change in glucose-lowering drugs in the last 3 months. The exclusion criteria were: other causes of neuropathy such as heavy alcohol consumption (alcohol consumption >30 g/day for men and >20 g/day for women), chronic kidney disease (estimated glomerular filtration rate (eGFR) <30 mL min−1 (1.73 m)−2), pregnancy, and severe foot ulcers requiring hospital admission. The study was approved by the Institutional Review Board of the SNUBH (no. B-2007-627-309), and each participant provided written informed consent.
2.2. Assessment of Dietary Intake
Dietary intake was assessed using 3-day food records. Energy and nutrient intake for each participant was calculated based on the Korean Food Composition Table, ninth revision, which was developed by the Korean National Rural Resources Development Institute [28]. The proportion of energy from carbohydrate, protein, and fat and the ratios of PUFA to SFA (PUFA/SFA), monounsaturated fatty acids (MUFA) to SFA (MUFA/SFA), iron to PUFA (iron/PUFA), iron to omega-6 PUFA (iron/omega-6 PUFA), and iron to omega-3 PUFA (iron/omega-3 PUFA) were also calculated.
2.3. Assessment of DPN
DPN was assessed using the Michigan Neuropathy Screening Instrument (MNSI), which includes two separate assessments: a 15-item, self-administered questionnaire (MNSI-Q) and a lower-extremity physical examination (MNSI-PE) [29]. The MNSI was validated in individuals with type 2 diabetes in Korea [30]. Trained healthcare providers performed all neuropathic examinations, and participants were diagnosed as having DPN when their MNSI-PE score was ≥2.5.
2.4. Anthropometric and Biochemical Measurements
Anthropometric indices were measured in barefoot participants wearing light clothing by a trained research nurse. Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m). Systolic and diastolic blood pressure (BP) were measured using an electronic BP measuring device after 10 min rest in a sitting position. We defined drinkers as those who drink any alcoholic beverage more than once a month. Smoking status was classified as never smoker (<100 cigarettes in lifetime and currently a nonsmoker), ex-smoker (≥100 cigarettes in lifetime and currently a nonsmoker), and current smoker (≥100 cigarettes in lifetime and currently a smoker). Blood samples were collected after an overnight fast. Plasma glucose levels were measured by the hexokinase method, and glycated hemoglobin (HbA1c) levels were measured by high-performance liquid chromatography (Bio-Rad, Hercules, CA, USA). Serum insulin levels were measured by an immunoradiometric assay (DIAsource ImmunoAssays, Nivelles, Belgium). Total cholesterol, triglycerides, high-density lipoprotein (HDL)–cholesterol and low-density lipoprotein (LDL)–cholesterol were measured by an enzymatic colorimetric assay. Liver functions tests, renal function tests, serum transferrin, ferritin, iron, total iron-binding capacity (TIBC), and neuron-specific enolase (NSE) were measured using established protocols by the Central Laboratory of SNUBH. Homeostatic model assessment for insulin resistance (HOMA-IR) and homeostatic model assessment for beta-cell function (HOMA-B) were calculated using the approximation equation of Matthews et al. [31]. Serum hepcidin and nitrotyrosine were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (Intrinsic Life Sciences, La Jolla, CA, USA; Hycult Biotech, Uden, The Netherlands).
2.5. Statistical Analysis
Data were expressed as mean ± standard deviation (SD) or number (%). Variables that were not normally distributed were natural log-transformed prior to analysis. Comparisons of continuous variables between individuals with DPN and those without DPN were performed using Student’s unpaired t-tests. Categorical variables were compared using chi-square tests. Nutrient intakes of individuals with and without DPN were compared by analysis of covariance with adjustment for total energy intake. After adjustment for total energy intake, Pearson’s correlation coefficient and partial correlation coefficient were used to evaluate the correlations between MNSI-PE scores and the mean daily intakes of energy and nutrients. The association of dietary iron intake, iron/PUFA ratio, iron/omega-6 PUFA ratio, and iron/omega-3 PUFA ratio with DPN was analyzed using logistic regression models. Multivariable logistic regression analyses were performed using significantly different variables between individuals with DPN and those without DPN and known risk factors for DPN. In all cases, p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 25.0 (IBM, SPSS, Armonk, NY, USA).
3. Results
3.1. Characteristics of the Participants
The present study analysis included data from 147 of 200 individuals with type 2 diabetes, while the remaining 53 individuals did not complete their three-day food records. There were no significantly different demographic or biochemical data between individuals included and excluded in the analysis (Table S1). Among the participants, 46.3% were diagnosed as having DPN. There was no difference in sex or age between individuals with or without DPN. BMI and HOMA-IR were numerically higher in individuals with DPN than in those without DPN, but the difference was not significant. The eGFR was lower in individuals with DPN than in those without DPN. Total cholesterol and LDL–cholesterol levels were lower in individuals with DPN than in their counterparts without DPN. There was no difference in the frequency of usage of lipid-lowering drugs, but the percentage of moderate-intensity statin users was numerically higher in individuals with DPN than in those without DPN (Table 1, Table S2). The frequency of antidiabetic drugs’ use, smoking, and alcohol consumption was comparable between groups (Table 1, Table S3).
Table 1.
Clinical and biochemical data of individuals according to the presence of diabetic peripheral neuropathy (DPN).
| Characteristic | DPN (–) (n = 79) | DPN (+) (n = 68) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Male, n (%) | 45 (56.9) | 41 (60.3) | 0.683 | ||||
| Age (years) | 57.5 | ± | 9.0 | 60.1 | ± | 9.8 | 0.090 |
| Height (cm) | 163.4 | ± | 8.1 | 163.3 | ± | 9.2 | 0.938 |
| Body weight (kg) | 66.1 | ± | 11.0 | 68.7 | ± | 11.1 | 0.158 |
| BMI (kg/m2) | 24.7 | ± | 3.2 | 25.7 | ± | 2.9 | 0.053 |
| Systolic BP (mmHg) | 128 | ± | 13 | 132 | ± | 15 | 0.155 |
| Diastolic BP (mmHg) | 75 | ± | 9 | 75 | ± | 9 | 0.971 |
| Diabetes duration (years) | 9.4 | ± | 7.2 | 10.4 | ± | 7.0 | 0.377 |
| FPG (mmol/L) | 7.5 | ± | 1.6 | 7.9 | ± | 2.3 | 0.222 |
| HbA1c (mmol/mol) | 54.6 | ± | 13.1 | 57.9 | ± | 15.3 | 0.154 |
| HbA1c (%) | 7.1 | ± | 1.2 | 7.4 | ± | 1.4 | 0.153 |
| Total cholesterol (mmol/L) | 4.3 | ± | 1.0 | 3.9 | ± | 0.8 | 0.002 |
| Triglyceride (mmol/L) a | 1.3 | ± | 0.0 | 1.4 | ± | 0.0 | 0.283 |
| HDL–cholesterol (mmol/L) a | 1.2 | ± | 0.0 | 1.2 | ± | 0.0 | 0.070 |
| LDL–cholesterol (mmol/L) | 2.5 | ± | 0.7 | 2.2 | ± | 0.6 | 0.006 |
| Urea nitrogen (mmol/L) | 5.6 | ± | 1.5 | 5.9 | ± | 2.3 | 0.378 |
| Creatinine (μmol/L) | 68.1 | ± | 18.6 | 74.3 | ± | 23.0 | 0.094 |
| eGFR (mL min−1 (1.73 m)−2) | 97.4 | ± | 21.1 | 90.1 | ± | 22.2 | 0.042 |
| AST (U/L)a | 26.0 | ± | 1.4 | 27.2 | ± | 1.4 | 0.437 |
| ALT (U/L) | 28.9 | ± | 17.1 | 27.1 | ± | 12.3 | 0.469 |
| Insulin (pmol/L) | 58.1 | ± | 32.2 | 63.9 | ± | 33.0 | 0.293 |
| HOMA-IR | 2.6 | ± | 1.6 | 3.2 | ± | 1.9 | 0.065 |
| HOMA-B a | 38.8 | ± | 2.3 | 40.9 | ± | 2.0 | 0.670 |
| MNSI-Q (score) | 1.7 | ± | 2.0 | 2.9 | ± | 2.1 | <0.001 |
| MNSI-PE (score) | 1.3 | ± | 0.6 | 3.5 | ± | 0.7 | <0.001 |
| Smoking status, n (%) | 0.350 | ||||||
| Never smoker | 33 (41.8) | 36 (52.9) | |||||
| Ex-smoker | 30 (38.0) | 19 (27.9) | |||||
| Current smoker | 16 (20.3) | 13 (19.1) | |||||
| Alcohol, n (%) | 43 (54.4) | 34 (50.0) | 0.592 | ||||
Data are expressed as mean ± SD or geometric mean ± geometric SD or number (%). a Variable was natural log-transformed before statistical analysis and expressed as geometric mean ± geometric SD. DPN, diabetic peripheral neuropathy; BMI, body mass index; BP, blood pressure; HbA1, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; eGFR, estimated glomerular filtration rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HOMA-IR, homeostatic model assessment for insulin resistance; HOMA-B, homeostatic model assessment for beta cell function; FPG, fasting plasma glucose; MNSI-Q, Michigan Neuropathy Screening Instrument-questionnaire; MNSI-PE, Michigan Neuropathy Screening Instrument-physical examination.
3.2. Iron Intake, Iron/PUFA Ratio, and DPN
Total energy intake and the percentage of energy from carbohydrates, protein, and fat were comparable between individuals with DPN and those without DPN. After adjustment for total energy intake, iron intake was significantly higher in individuals with DPN (10.9 ± 4.0 mg vs. 9.9 ± 3.6 mg, p = 0.041). Intakes of total PUFA, omega-6 PUFA, and omega-3 PUFA and the omega-6/omega-3 PUFA ratio were comparable between groups. Interestingly, the iron/PUFA ratio was significantly higher in individuals with DPN (1.4 ± 0.8 vs. 1.1 ± 0.4, p = 0.005). In addition, both iron/omega-6 PUFA ratio and iron/omega-3 PUFA ratio were significantly higher in individuals with DPN (Table 2).
Table 2.
Mean daily intake of energy and nutrients estimated from three-day food records according to the presence of DPN.
| Variable | DPN (–) (n = 79) | DPN (+) (n = 68) | p Value | p Value a | ||||
|---|---|---|---|---|---|---|---|---|
| TE intake (kJ) | 6255.9 | ± | 1812.5 | 6218.3 | ± | 1412.6 | 0.889 | NA |
| Carbohydrate (% TE) | 61.5 | ± | 12.2 | 63.4 | ± | 10.7 | 0.327 | NA |
| Protein (% TE) | 16.5 | ± | 3.5 | 16.5 | ± | 3.2 | 0.934 | NA |
| Fat (% TE) | 23.7 | ± | 9.2 | 22.9 | ± | 9.3 | 0.612 | NA |
| SFA (g) | 13.4 | ± | 8.1 | 12.6 | ± | 7.1 | 0.500 | 0.434 |
| MUFA (g) | 13.0 | ± | 7.9 | 13.0 | ± | 7.9 | 0.987 | 0.887 |
| PUFA (g) | 10.2 | ± | 5.2 | 9.2 | ± | 4.7 | 0.211 | 0.155 |
| Omega-6 PUFA (g) | 8.6 | ± | 4.5 | 7.6 | ± | 3.9 | 0.144 | 0.092 |
| Omega-3 PUFA (g) | 1.4 | ± | 0.8 | 1.3 | ± | 1.0 | 0.637 | 0.653 |
| Omega-6/Omega-3 PUFA ratio | 7.2 | ± | 2.7 | 7.3 | ± | 3.1 | 0.811 | NA |
| PUFA/SFA ratio | 0.9 | ± | 0.6 | 0.9 | ± | 0.5 | 0.698 | NA |
| MUFA/SFA ratio | 1.0 | ± | 0.2 | 1.0 | ± | 0.2 | 0.253 | NA |
| Iron (mg) | 9.9 | ± | 3.6 | 10.9 | ± | 4.0 | 0.130 | 0.041 |
| Iron/PUFA ratio (mg/g) | 1.1 | ± | 0.4 | 1.4 | ± | 0.8 | 0.005 | NA |
| Iron/omega-6 PUFA ratio (mg/g) | 1.3 | ± | 0.5 | 1.7 | ± | 1.1 | 0.006 | NA |
| Iron/omega-3 PUFA ratio (mg/g) | 9.3 | ± | 5.5 | 12.1 | ± | 7.8 | 0.016 | NA |
| Vitamin B1 (mg) | 0.9 | ± | 0.4 | 0.8 | ± | 0.3 | 0.635 | 0.625 |
| Vitamin B6 (mg) | 0.4 | ± | 0.2 | 0.4 | ± | 0.2 | 0.922 | 0.871 |
| Vitamin B12 (µg) | 3.7 | ± | 3.1 | 3.7 | ± | 3.5 | 0.988 | 0.967 |
| Vitamin D (µg) | 4.2 | ± | 4.2 | 3.1 | ± | 4.0 | 0.116 | 0.118 |
| Dietary fiber (g) | 21.7 | ± | 8.2 | 23.3 | ± | 8.1 | 0.240 | 0.156 |
Data are expressed as mean ± SD. a p value for ANCOVA adjusted for total energy intake. DPN, diabetic peripheral neuropathy; TE, total energy; NA, not applicable; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; PUFA/SFA, PUFA intake to SFA intake; MUFA/SFA, MUFA intake to SFA intake.
MNSI-PE scores were positively correlated with iron intake after adjustment for total energy intake (r = 0.262, p = 0.001) and with the iron/PUFA ratio (r = 0.276, p = 0.001) (Table 3). In addition, MNSI-PE scores were also positively correlated with the iron/omega-6 PUFA ratio and the iron/omega-3 PUFA ratio. However, other nutrients did not display any significant correlation with MNSI-PE scores. After adjustment for total energy intake, sex, age, BMI, systolic BP, diabetes duration, eGFR, HbA1c, LDL–cholesterol, and smoking, logistic regression analyses showed that iron intake was associated with DPN (OR: 1.152; 95% CI: 1.012, 1.311). However, the level of significance was diminished after adjustment for HOMA-IR or HOMA-B. After full adjustment, the iron/PUFA ratio and the iron/omega-6 PUFA ratio were consistently associated with DPN, but the iron/omega-3 PUFA ratio was not significantly associated with DPN (Table 4).
Table 3.
Correlation analysis between MNSI-PE scores and mean daily intake of energy and nutrients.
| Variable | Coefficient | p Value |
|---|---|---|
| Carbohydrate (% TE) | 0.095 | 0.250 |
| Protein (% TE) | 0.014 | 0.870 |
| Fat (% TE) | −0.059 | 0.480 |
| SFA (g) a | −0.078 | 0.347 |
| MUFA (g) a | −0.037 | 0.661 |
| PUFA (g) a | −0.118 | 0.155 |
| Omega-6 PUFA a | −0.146 | 0.079 |
| Omega-3 PUFA a | 0.003 | 0.976 |
| Omega-6/Omega-3 PUFA ratio | −0.023 | 0.780 |
| PUFA/SFA ratio | 0.003 | 0.969 |
| MUFA/SFA ratio | 0.029 | 0.731 |
| Iron (mg) a | 0.262 | 0.001 |
| Iron/PUFA ratio (mg/g) | 0.276 | 0.001 |
| Iron/omega-6 PUFA ratio (mg/g) | 0.271 | 0.001 |
| Iron/omega-3 PUFA ratio (mg/g) | 0.204 | 0.013 |
| Vitamin B1 (mg) a | −0.075 | 0.368 |
| Vitamin B6 (mg) a | 0.021 | 0.806 |
| Vitamin B12 (µg) a | 0.056 | 0.501 |
| Vitamin D (µg) a | −0.089 | 0.285 |
| Dietary fiber (g) a | 0.151 | 0.068 |
Pearson’s correlation analysis was conducted. a Partial correlation analysis was conducted after adjusting for total energy intake. MNSI-PE, Michigan Neuropathy Screening Instrument-physical examination; TE, total energy; SFA, saturated fatty acids; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; PUFA/SFA, PUFA intake to SFA intake; MUFA/SFA, MUFA intake to SFA intake.
Table 4.
Odds ratios (ORs, 95% CI) between iron intake, iron/PUFA, and DPN.
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Iron intake | |||
| Model 1 | 1.126 | 1.003, 1.264 | 0.044 |
| Model 2 | 1.147 | 1.014, 1.298 | 0.029 |
| Model 3 | 1.152 | 1.012, 1.311 | 0.032 |
| Model 4 | 1.136 | 0.995, 1.297 | 0.059 |
| Model 5 | 1.139 | 0.998, 1.301 | 0.053 |
| Iron/PUFA ratio | |||
| Model 1 | 2.628 | 1.324, 5.216 | 0.006 |
| Model 2 | 2.375 | 1.168, 4.830 | 0.017 |
| Model 3 | 2.283 | 1.066, 4.887 | 0.034 |
| Model 4 | 2.215 | 1.032, 4.757 | 0.041 |
| Model 5 | 2.214 | 1.034, 4.742 | 0.041 |
| Iron/omega-6 PUFA ratio | |||
| Model 1 | 2.321 | 1.287, 4.186 | 0.005 |
| Model 2 | 2.136 | 1.160, 3.934 | 0.015 |
| Model 3 | 2.096 | 1.089, 4.032 | 0.027 |
| Model 4 | 2.037 | 1.058, 3.922 | 0.033 |
| Model 5 | 2.046 | 1.063, 3.935 | 0.032 |
| Iron/omega-3 PUFA ratio | |||
| Model 1 | 1.069 | 1.012, 1.130 | 0.018 |
| Model 2 | 1.051 | 0.992, 1.112 | 0.090 |
| Model 3 | 1.054 | 0.995, 1.117 | 0.073 |
| Model 4 | 1.055 | 0.995, 1.118 | 0.074 |
| Model 5 | 1.052 | 0.993, 1.115 | 0.082 |
Model 1 is adjusted for total energy intake; Model 2 is additionally adjusted for sex, age, BMI, systolic BP, diabetes duration, and eGFR; Model 3 is additionally adjusted for HbA1c, LDL–cholesterol, and smoking; Model 4 is additionally adjusted for HOMA-IR from Model 3; Model 5 is additionally adjusted for HOMA-B from Model 3. PUFA, polyunsaturated fatty acids; DPN, diabetic peripheral neuropathy; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HbA1, glycated hemoglobin; LDL, low-density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; HOMA-B, homeostatic model assessment for beta cell function.
3.3. Biochemical Markers of Iron Status and Oxidative Stress Markers of DPN
Biochemical markers of iron status such as serum transferrin, ferritin, iron, TIBC, and hepcidin were comparable between individuals with and those without DPN. The levels of oxidative stress markers of DPN, such as NSE and nitrotyrosine, were not different between individuals with and without DPN (Table 5).
Table 5.
Laboratory biochemical data of individuals according to the presence of DPN.
| Variable | DPN (–) (n = 79) | DPN (+) (n = 68) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Transferrin (μmol/L) | 31.6 | ± | 4.5 | 32.1 | ± | 4.3 | 0.494 |
| Ferritin (μg/L) | 150.2 | ± | 120.4 | 140.8 | ± | 109.5 | 0.627 |
| Iron (μmol/L) | 19.2 | ± | 7.0 | 19.2 | ± | 6.8 | 0.975 |
| TIBC (μmol/L) | 60.6 | ± | 8.2 | 61.9 | ± | 7.9 | 0.325 |
| TSAT (%) | 31.9 | ± | 10.5 | 31.7 | ± | 11.9 | 0.897 |
| Hepcidin (ng/mL) | 3.2 | ± | 1.7 | 3.0 | ± | 1.7 | 0.525 |
| NSE (ng/mL) | 12.6 | ± | 3.6 | 12.3 | ± | 2.6 | 0.573 |
| Nitrotyrosine (nmol/L) a | 10.0 | ± | 2.2 | 9.0 | ± | 2.0 | 0.276 |
Data are expressed as mean ± SD or geometric mean ± geometric SD. a Variable was natural log-transformed before statistical analysis and expressed as geometric mean ± geometric SD. DPN, diabetic peripheral neuropathy; TIBC, total iron-binding capacity; TSAT, transferrin saturation NSE, neuron-specific enolase.
4. Discussion
In this cross-sectional study, we observed that iron intake and iron intake relative to PUFA levels were higher in participants with DPN than in participants without DPN. Furthermore, the presence of DPN or the severity of DPN assessed by the MNSI-PE was positively associated with iron intake and the iron/PUFA ratio.
Prospective cohort studies of the Japanese and Chinese populations reported that iron intake was associated with an increased risk of diabetes [20,32]. In a case–control study of Europids with type 2 diabetes, a hemochromatosis-causing mutation C282Y was associated with a higher risk of diabetic retinopathy [33]. In a rat model, iron caused renal tubular injury due to the formation of free hydroxyl radicals [34]. A prospective intervention study revealed that a low-iron diet delayed the progression of diabetic nephropathy [35]. In regard to neuropathy, an in vitro study demonstrated that iron overload aggravated the oxidative stress injury of neurons in the presence of high glucose concentrations [36]. In our study, iron intake was associated with DPN, and this association was no longer significant after adjustment for HOMA-IR or HOMA-B. Therefore, insulin resistance and pancreatic beta cell dysfunction might be an important factor promoting the association between iron and DPN.
Oxidative stress causes pancreatic beta cell dysfunction, insulin resistance, and diabetic complications [37,38]. Meanwhile, iron can generate oxidative stress by the formation of hydroxyl radicals through the Fenton reaction [39]. Therefore, we need to measure reactive oxygen species (ROS) to understand the possible mechanism underlying the role of iron in DPN. However, the measurement of ROS is very tricky due to their reactivity and unstable properties [40]. Therefore, it might be more reasonable to measure oxidation target products of ROS. Previous cross-sectional studies reported that serum NSE and nitrotyrosine levels, which are oxidation target products of ROS, were closely associated with DPN [41,42]. In this background, we tested whether these indices were associated with DPN and useful for identifying DPN. However, we did not observe any differences in the levels of these biomarkers between individuals with DPN and those without DPN. This negative result might relate to the characteristics of the study subjects. We enrolled participants with relatively well-controlled type 2 diabetes, in contrast to the earlier study which enrolled subjects with both type 1 and type 2 diabetes [41], and with a more severe degree of hyperglycemia; the mean HbA1c was up to 9.4% [42]. In fact, there are other oxidative markers of ROS-induced modifications of lipids, proteins, and DNA or RNA that we need to measure further [43]. For example, serum malondialdehyde and urinary 8-hydroxy-2′-deoxy-guanosine might be good candidates, because their levels were shown to be higher in individuals with diabetic nephropathy [44] and in individuals with diabetic microvascular complications [45]. However, we did not have available samples to test these molecules, which is one of the limitations of our study.
Hepcidin is an established master regulator of iron metabolism and an index of the iron pool in the body [46] that predicted the progression of diabetic nephropathy, one of the microvascular complications of type 2 diabetes [47]. However, in the present study, we did not observe differences in the levels of serum transferrin, ferritin, iron, and hepcidin linked to the presence of DPN. Therefore, we cautiously infer that a dietary pattern including high-iron-containing food might be a more important risk factor for DPN than the actual amount of iron. In addition, there are two types of dietary iron: heme and non-heme iron. Heme iron is present in red meat, poultry, and seafood, while non-heme iron is present in both plant and animal foods. Heme iron contributes 10–15% of total iron intake, but because of its higher absorption, it can contribute over 40% of the total absorbed iron [48]. As it is thought that the gut microbiota can influence the absorption capacity of iron [49], it might be necessary to consider both the amount and the quality of iron intake, as well as gut environmental factors, to best assess iron absorption.
Our results are different from results of rodent models of DPN which showed that iron deficiency rather than iron overload was associated with the risk of DPN [21,22,23]. It is hard to compare the results from human and rodent studies. In addition, cross-sectional studies and the intervention studies are different in terms of interpreting cause and effect. Even after consideration of the aforementioned points, we suggest that following might lead to different results in rodent models of DPN. First, in streptozotocin-diabetic rats, a single high dose of streptozotocin could induce nonspecific toxicity, which affects neurons directly [50]. In that circumstance, iron deficiency might cause impairment of iron-containing repair enzymes. Second, in ob/ob and db/db mice, iron deficiency can cause iron-deficiency anemia. The hemoglobin level in db/db mice on a high-iron diet was 19.4 g/dL, while it was 10.7 g/dL in db/db mice on a low-iron diet. This difference in hemoglobin might cause ischemia in the peripheral limbs. In contrast, considering that the individuals in our study did not have iron-deficiency anemia, iron deficiency might not have influenced our study results.
PUFA can be divided into two subclasses: omega-6 and omega-3. Omega-6 PUFA include linoleic acid and arachidonic acid [51]. Omega-3 PUFA include alpha-linolenic acid, eicosapentaenoic acid, and docosahexaenoic acid [52]. Omega-3 PUFA is an antioxidant able to produce a direct superoxide scavenging effect [24] and an indirect reactive oxygen species reduction effect via upregulation of antioxidant molecules [53]. Previous cross-sectional studies demonstrated that PUFA intake was associated with a lower odds ratio (OR) for the presence of diabetic retinopathy [54] and that linolenic acid intake was associated with lower odds of peripheral neuropathy [27]. A meta-analysis revealed that omega-3 fatty acid supplementation reduced the amount of proteinuria in individuals with type 2 diabetes [55]. For the cardiovascular risk, a few randomized controlled trials [56,57] and a meta-analysis [58] did not show a benefit of omega-3 PUFA, but the Japan EPA Lipid Intervention Study (JELIS) [59] and the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) [60] have shown a benefit of omega-3 PUFA [60]. Until now, there is controversy about the role of omega-3 PUFA supplements for individuals with diabetes in the prevention of cardiovascular events [7]. To our best knowledge, there is a lack of studies investigating the role of omega-3 PUFA supplements for DPN. Considering the high prevalence of DPN and the limited treatment options for DPN, it is valuable to investigate the association between omega-3 PUFA and the risk of DPN. In this study, we observed a lower trend of PUFA and omega-3 PUFA intake in individuals with DPN compared to those without DPN. Meanwhile, omega-6 PUFA is considered pro-inflammatory by some researchers because linoleic acid, the representative of omega-6 PUFA, is converted into arachidonic acid. In addition, a few studies suggested that omega-6 PUFA is related to chronic inflammatory diseases such as obesity, nonalcoholic fatty liver disease, cardiovascular disease [61,62]. However, other studies showed that high consumption of omega-6 PUFA did not increase cardiovascular events [63,64]. In this study, we observed a lower, but not significant, omega-6 PUFA intake in individuals with DPN. Considering the significant correlation between omega-6 PUFA intake and omega-3 PUFA intake in this study (r = 0.713, p < 0.001), it is not possible to interpret the results of omega-6 PUFA and omega-3 PUFA separately.
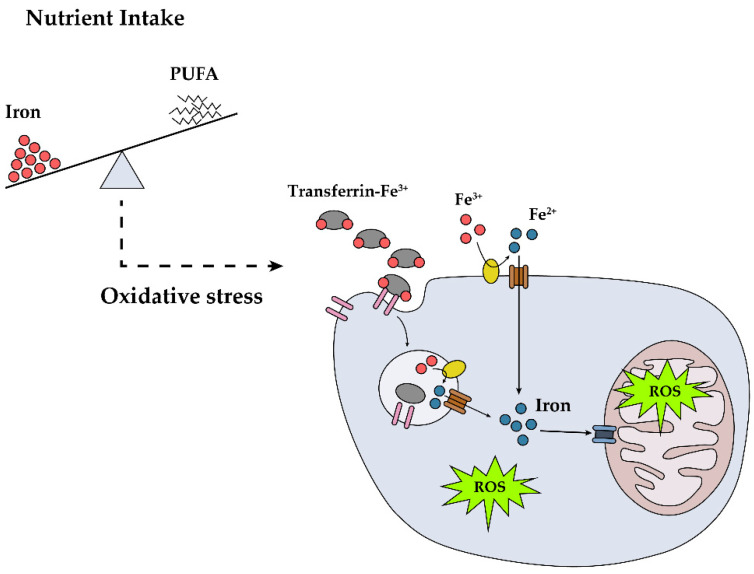
In light of the in vitro [36] and animal model [65] data, we postulated that increased body iron can damage neurons or Schwann cells via direct or indirect pathways. Considering the PUFA-related antioxidant effect observed in an iron-related, pro-oxidant environment, we calculated the iron/PUFA ratio and found that a higher iron/PUFA ratio was associated with a higher OR of DPN. This finding suggests that the ratio of iron to PUFA might be an important marker of DPN (Figure 1) and can be used as an indicator to screen for or prevent DPN in individuals with type 2 diabetes. In addition, even though the ratio iron/omega-6 PUFA, rather than the ratio iron/omega-3 PUFA, showed a statistically significant association with DPN after adjusting for confounders, we need to be cautious in interpreting these data. A relatively small amount of omega-3 PUFA compared with omega-6 PUFA might bring about these non-significant results.
Figure 1.
Potential role of dietary iron in the development of diabetic peripheral neuropathy (DPN). High dietary iron and iron intake relative to polyunsaturated fatty acids (PUFA) lead to DPN through reactive oxygen species (ROS) formation.
Our study has several limitations. First, because of its cross-sectional nature, we could not establish a causal relationship. Second, our study was based on a relatively small sample size, which may have affected the assessment of significant differences in known risk factors for DPN, such as age, BMI, and diabetes duration. Studies with a larger sample size or a prospective or intervention study would be of interest to confirm or reassess these findings. Third, neurophysiologic studies were not used to confirm the DPN diagnosis. Fourth, the Korean Food Composition Table does not contain data regarding haem iron content, so we could not analyze the intake of heme iron and non-heme iron separately. Lastly, among various oxidative stress markers, we measured only NSE and nitrotyrosine levels, which were comparable between groups.
Despite these limitations, this study has several strengths. First, we obtained dietary nutrient intake estimates with the use of three-day food records. In addition, we controlled for a number of dietary and nondietary covariates to reduce possible confounding effects. Above all, this is the first study to examine the association between dietary iron intake and DPN. In addition, we suggest the iron/PUFA ratio as a new index associated with DPN.
5. Conclusions
Dietary iron intake and the iron/PUFA ratio were associated with DPN. The present study suggests the importance of the dietary pattern of iron and PUFA intake in individuals with type 2 diabetes, which might be an intervention target for preventing or treating DPN.
Acknowledgments
The authors sincerely thank all the participants for their cooperation. In addition, we thank Yoojung Song (Seoul National University Bundang Hospital, Seongnam, Korea) for assistance with data registration and analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/11/3365/s1, Table S1: Clinical and biochemical data of individuals according to the presence of DPN. Table S2: Use of lipid-lowering drugs in individuals according to the presence of DPN. Table S3: Use of antidiabetic drugs in individuals according the the presence of DPN.
Author Contributions
Conceptualization, S.H.C.; methodology, Y.S.; software, K.K. and Y.S.; validation, K.K. and T.J.O.; formal analysis, K.K. and Y.S.; investigation, T.J.O. and S.H.C; resources, S.H.C. and H.C.J.; data curation, K.K.; writing—original draft preparation, K.K.; writing—review and editing, K.K.; visualization, K.K.; supervision, T.J.O.; project administration, T.J.O. and H.C.J.; funding acquisition, T.J.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the SNUBH Research Fund (14-2020-025).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cameron N.E., Eaton S.E., Cotter M.A., Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 2.Pop-Busui R., Boulton A.J., Feldman E.L., Bril V., Freeman R., Malik R.A., Sosenko J.M., Ziegler D. Diabetic neuropathy: A position statement by the American diabetes association. Diabetes Care. 2017;40:136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papanas N., Ziegler D. Risk factors and comorbidities in diabetic neuropathy: An update 2015. Rev. Diabet. Stud. 2015;12:48–62. doi: 10.1900/RDS.2015.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh T.J., Lee J.E., Choi S.H., Jang H.C. Association between body fat and diabetic peripheral neuropathy in middle-aged adults with type 2 diabetes mellitus: A preliminary report. J. Obes. Metab. Syndr. 2019;28:112–117. doi: 10.7570/jomes.2019.28.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent A.M., Russell J.W., Low P., Feldman E.L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr. Rev. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- 6.Mijnhout G.S., Kollen B.J., Alkhalaf A., Kleefstra N., Bilo H.J. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: A meta-analysis of randomized controlled trials. Int. J. Endocrinol. 2012;2012:456279. doi: 10.1155/2012/456279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evert A.B., Dennison M., Gardner C.D., Garvey W.T., Lau K.H.K., MacLeod J., Mitri J., Pereira R.F., Rawlings K., Robinson S., et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care. 2019;42:731–754. doi: 10.2337/dci19-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies M.J., D’Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G., Rossing P., Tsapas A., Wexler D.J., Buse J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61:2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 9.Salas-Salvado J., Bullo M., Estruch R., Ros E., Covas M.I., Ibarrola-Jurado N., Corella D., Aros F., Gomez-Gracia E., Ruiz-Gutierrez V., et al. Prevention of diabetes with mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014;160:1–10. doi: 10.7326/M13-1725. [DOI] [PubMed] [Google Scholar]
- 10.Chiu T.H.T., Pan W.H., Lin M.N., Lin C.L. Vegetarian diet, change in dietary patterns, and diabetes risk: A prospective study. Nutr. Diabetes. 2018;8:12. doi: 10.1038/s41387-018-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tay J., Thompson C.H., Luscombe-Marsh N.D., Wycherley T.P., Noakes M., Buckley J.D., Wittert G.A., Yancy W.S., Jr., Brinkworth G.D. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes. Metab. 2018;20:858–871. doi: 10.1111/dom.13164. [DOI] [PubMed] [Google Scholar]
- 12.Lv W.S., Zhao W.J., Gong S.L., Fang D.D., Wang B., Fu Z.J., Yan S.L., Wang Y.G. Serum 25-hydroxyvitamin d levels and peripheral neuropathy in patients with type 2 diabetes: A systematic review and meta-analysis. J. Endocrinol. Invest. 2015;38:513–518. doi: 10.1007/s40618-014-0210-6. [DOI] [PubMed] [Google Scholar]
- 13.Shillo P., Selvarajah D., Greig M., Gandhi R., Rao G., Wilkinson I.D., Anand P., Tesfaye S. Reduced vitamin d levels in painful diabetic peripheral neuropathy. Diabet. Med. 2019;36:44–51. doi: 10.1111/dme.13798. [DOI] [PubMed] [Google Scholar]
- 14.Gupta K., Jain A., Rohatgi A. An observational study of vitamin b12 levels and peripheral neuropathy profile in patients of diabetes mellitus on metformin therapy. Diabetes Metab. Syndr. 2018;12:51–58. doi: 10.1016/j.dsx.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Gorson K.C., Ropper A.H. Additional causes for distal sensory polyneuropathy in diabetic patients. J. Neurol. Neurosurg. Psychiatry. 2006;77:354–358. doi: 10.1136/jnnp.2005.075119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W., Zhou Q., Jia Y., Xu J. Cluster and factor analysis of elements in serum and urine of diabetic patients with peripheral neuropathy and healthy people. Biol. Trace Elem. Res. 2020;194:48–57. doi: 10.1007/s12011-019-01747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairo G., Bernuzzi F., Recalcati S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006;1:25–39. doi: 10.1007/BF02829934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simcox J.A., McClain D.A. Iron and diabetes risk. Cell Metab. 2013;17:329–341. doi: 10.1016/j.cmet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Z., Zhou M., Yuan B., Qi L., Dai Y., Luo Y., Holmboe-Ottesen G. Iron intake and body iron stores, anaemia and risk of hyperglycaemia among Chinese adults: The prospective Jiangsu Nutrition Study (JIN) Public Health Nutr. 2010;13:1319–1327. doi: 10.1017/S1368980009991868. [DOI] [PubMed] [Google Scholar]
- 20.Eshak E.S., Iso H., Maruyama K., Muraki I., Tamakoshi A. Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: A large population-based prospective cohort study. Clin. Nutr. 2018;37:667–674. doi: 10.1016/j.clnu.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Baum P., Kosacka J., Estrela-Lopis I., Woidt K., Serke H., Paeschke S., Stockinger M., Kloting N., Bluher M., Dorn M., et al. The role of nerve inflammation and exogenous iron load in experimental peripheral diabetic neuropathy (PDN) Metabolism. 2016;65:391–405. doi: 10.1016/j.metabol.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Kosacka J., Woidt K., Toyka K.V., Paeschke S., Kloting N., Bechmann I., Bluher M., Thiery J., Ossmann S., Baum P., et al. The role of dietary non-heme iron load and peripheral nerve inflammation in the development of peripheral neuropathy (PN) in obese non-diabetic leptin-deficient ob/ob mice. Neurol. Res. 2019;41:341–353. doi: 10.1080/01616412.2018.1564191. [DOI] [PubMed] [Google Scholar]
- 23.Paeschke S., Baum P., Toyka K.V., Bluher M., Koj S., Kloting N., Bechmann I., Thiery J., Kosacka J., Nowicki M. The role of iron and nerve inflammation in diabetes mellitus type 2-induced peripheral neuropathy. Neuroscience. 2019;406:496–509. doi: 10.1016/j.neuroscience.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Richard D., Kefi K., Barbe U., Bausero P., Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008;57:451–455. doi: 10.1016/j.phrs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Meyer K.A., Kushi L.H., Jacobs D.R., Jr., Folsom A.R. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care. 2001;24:1528–1535. doi: 10.2337/diacare.24.9.1528. [DOI] [PubMed] [Google Scholar]
- 26.Salmeron J., Hu F.B., Manson J.E., Stampfer M.J., Colditz G.A., Rimm E.B., Willett W.C. Dietary fat intake and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2001;73:1019–1026. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 27.Tao M., McDowell M.A., Saydah S.H., Eberhardt M.S. Relationship of polyunsaturated fatty acid intake to peripheral neuropathy among adults with diabetes in the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care. 2008;31:93–95. doi: 10.2337/dc07-0931. [DOI] [PubMed] [Google Scholar]
- 28.Park S., Kim S., Lee S.H., Choe J., Choi Y. Development of 9th revision Korean food composition table and its major changes. Korean J. Community Nutr. 2018;23:352–365. doi: 10.5720/kjcn.2018.23.4.352. [DOI] [Google Scholar]
- 29.Feldman E.L., Stevens M.J., Thomas P.K., Brown M.B., Canal N., Greene D.A. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 30.Won J.C., Im Y.J., Lee J.H., Kim C.H., Kwon H.S., Cha B.Y., Park T.S. Clinical phenotype of diabetic peripheral neuropathy and relation to symptom patterns: Cluster and factor analysis in patients with type 2 diabetes in Korea. J. Diabetes Res. 2017;2017:5751687. doi: 10.1155/2017/5751687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.He J., Fang A., Yu S., Shen X., Li K. Dietary nonheme, heme, and total iron intake and the risk of diabetes in adults: Results from the China Health and Nutrition Survey. Diabetes Care. 2020;43:776–784. doi: 10.2337/dc19-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterlin B., Globocnik Petrovic M., Makuc J., Hawlina M., Petrovic D. A hemochromatosis-causing mutation c282y is a risk factor for proliferative diabetic retinopathy in Caucasians with type 2 diabetes. J. Hum. Genet. 2003;48:646–649. doi: 10.1007/s10038-003-0094-3. [DOI] [PubMed] [Google Scholar]
- 34.Alfrey A.C., Froment D.H., Hammond W.S. Role of iron in the tubulo-interstitial injury in nephrotoxic serum nephritis. Kidney Int. 1989;36:753–759. doi: 10.1038/ki.1989.259. [DOI] [PubMed] [Google Scholar]
- 35.Facchini F.S., Saylor K.L. A low-iron-available, polyphenol-enriched, carbohydrate-restricted diet to slow progression of diabetic nephropathy. Diabetes. 2003;52:1204–1209. doi: 10.2337/diabetes.52.5.1204. [DOI] [PubMed] [Google Scholar]
- 36.Zhao S., Zhang L., Xu Z., Chen W. Neurotoxic effects of iron overload under high glucose concentration. Neural Regen. Res. 2013;8:3423–3433. doi: 10.3969/j.issn.1673-5374.2013.36.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newsholme P., Keane K.N., Carlessi R., Cruzat V. Oxidative stress pathways in pancreatic beta-cells and insulin-sensitive cells and tissues: Importance to cell metabolism, function, and dysfunction. Am. J. Physiol. Cell Physiol. 2019;317:C420–C433. doi: 10.1152/ajpcell.00141.2019. [DOI] [PubMed] [Google Scholar]
- 38.Pitocco D., Tesauro M., Alessandro R., Ghirlanda G., Cardillo C. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013;14:21525–21550. doi: 10.3390/ijms141121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emerit J., Beaumont C., Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed. Pharmacother. 2001;55:333–339. doi: 10.1016/S0753-3322(01)00068-3. [DOI] [PubMed] [Google Scholar]
- 40.Dalle-Donne I., Rossi R., Colombo R., Giustarini D., Milzani A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 41.Li J., Zhang H., Xie M., Yan L., Chen J., Wang H. NSE, a potential biomarker, is closely connected to diabetic peripheral neuropathy. Diabetes Care. 2013;36:3405–3410. doi: 10.2337/dc13-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards J.F., Casellini C.M., Parson H.K., Obrosova I.G., Yorek M., Vinik A.I. Role of peroxynitrite in the development of diabetic peripheral neuropathy. Diabetes Care. 2015;38:e100–e101. doi: 10.2337/dc14-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigagli E., Lodovici M. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid. Med. Cell Longev. 2019;2019:5953685. doi: 10.1155/2019/5953685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta S., Gambhir J.K., Kalra O., Gautam A., Shukla K., Mehndiratta M., Agarwal S., Shukla R. Association of biomarkers of inflammation and oxidative stress with the risk of chronic kidney disease in type 2 diabetes mellitus in north Indian population. J. Diabetes Complications. 2013;27:548–552. doi: 10.1016/j.jdiacomp.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Tatsch E., De Carvalho J.A., Hausen B.S., Bollick Y.S., Torbitz V.D., Duarte T., Scolari R., Duarte M.M., Londero S.W., Vaucher R.A., et al. Oxidative DNA damage is associated with inflammatory response, insulin resistance and microvascular complications in type 2 diabetes. Mutat. Res. 2015;782:17–22. doi: 10.1016/j.mrfmmm.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Ganz T., Nemeth E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta. 2012;1823:1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enko D., Wagner H., Kriegshauser G., Kimbacher C., Stolba R., Worf E., Halwachs-Baumann G. Hepcidin-25 vs. Conventional clinical biomarkers in the diagnosis of functional iron deficiency. Eur. J. Haematol. 2015;95:507–513. doi: 10.1111/ejh.12523. [DOI] [PubMed] [Google Scholar]
- 48.Hurrell R., Egli I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010;91:1461S–1467S. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- 49.Das N.K., Schwartz A.J., Barthel G., Inohara N., Liu Q., Sankar A., Hill D.R., Ma X., Lamberg O., Schnizlein M.K., et al. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 2020;31:115–130. doi: 10.1016/j.cmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brien P.D., Sakowski S.A., Feldman E.L. Mouse models of diabetic neuropathy. ILAR J. 2014;54:259–272. doi: 10.1093/ilar/ilt052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saini R.K., Keum Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance — A review. Life Sci. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 52.Sokola-Wysoczanska E., Wysoczanski T., Wagner J., Czyz K., Bodkowski R., Lochynski S., Patkowska-Sokola B. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders — A review. Nutrients. 2018;10:1561. doi: 10.3390/nu10101561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakai C., Ishida M., Ohba H., Yamashita H., Uchida H., Yoshizumi M., Ishida T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE. 2017;12:e0187934. doi: 10.1371/journal.pone.0187934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki M., Kawasaki R., Rogers S., Man R.E., Itakura K., Xie J., Flood V., Tsubota K., Lamoureux E., Wang J.J. The associations of dietary intake of polyunsaturated fatty acids with diabetic retinopathy in well-controlled diabetes. Invest. Ophthalmol. Vis. Sci. 2015;56:7473–7479. doi: 10.1167/iovs.15-17485. [DOI] [PubMed] [Google Scholar]
- 55.Chewcharat A., Chewcharat P., Rutirapong A., Papatheodorou S. The effects of omega-3 fatty acids on diabetic nephropathy: A meta-analysis of randomized controlled trials. PLoS ONE. 2020;15:e0228315. doi: 10.1371/journal.pone.0228315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ORIGIN Trial Investigators N-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 57.ASCEND Study Collaborative Group Effects of n-3 fatty acid supplements in diabetes mellitus. N. Engl. J. Med. 2018;379:1540–1550. doi: 10.1056/NEJMoa1804989. [DOI] [PubMed] [Google Scholar]
- 58.Aung T., Halsey J., Kromhout D., Gerstein H.C., Marchioli R., Tavazzi L., Geleijnse J.M., Rauch B., Ness A., Galan P., et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: Meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 60.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., Doyle R.T., Jr., Juliano R.A., Jiao L., Granowitz C., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl J. Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 61.Patterson E., Wall R., Fitzgerald G.F., Ross R.P., Stanton C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012;2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naughton S.S., Mathai M.L., Hryciw D.H., McAinch A.J. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016;125:90–99. doi: 10.1016/j.prostaglandins.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Chowdhury R., Warnakula S., Kunutsor S., Crowe F., Ward H.A., Johnson L., Franco O.H., Butterworth A.S., Forouhi N.G., Thompson S.G., et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 64.Hooper L., Al-Khudairy L., Abdelhamid A.S., Rees K., Brainard J.S., Brown T.J., Ajabnoor S.M., O’Brien A.T., Winstanley L.E., Donaldson D.H., et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018;7:CD011094. doi: 10.1002/14651858.CD011094.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cameron N.E., Cotter M.A. Effects of an extracellular metal chelator on neurovascular function in diabetic rats. Diabetologia. 2001;44:621–628. doi: 10.1007/s001250051669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.